Abstract
Objectives
Research on effectiveness and cost‐effectiveness of longstanding exercise therapy in patients with axial SpondyloArthritis (axSpA) or Rheumatoid Arthritis (RA) is scarce, and mainly concerned patients with a relatively favorable health status. We aim to evaluate the effectiveness and cost‐effectiveness of longstanding exercise therapy compared to usual care in the subgroup of patients with axSpA or RA and severe limitations in functioning.
Methods
In two separate, parallel randomized controlled trials the effectiveness and cost‐effectiveness of longstanding, active exercise therapy (52 weeks) compared with usual care (1:1) will be evaluated. The longstanding, active exercise therapy will focus on improving individual limitations in daily activities and participation and will be given by a trained physical therapist in the vicinity of the participant. For each diagnosis, 215 patients with severe limitations in activities and participation will be included. Assessments are performed at baseline, 12, 26, and 52 weeks. The primary outcome measure of effectiveness is the individual level of functioning (activities and participation), as measured with the Patient‐Specific Complaints instrument at 52 weeks. For cost‐effectiveness analyses, the EuroQol (EQ‐5D‐5L) and questionnaires on healthcare use and productivity will be administered. The economic evaluation will be a cost‐utility analysis from a societal perspective. After 52 weeks, the patients in the usual care group are offered longstanding, active exercise therapy as well. Follow‐up assessments are done at 104, 156, and 208 weeks.
Conclusion
The results of these studies will provide insights in the effectiveness and cost‐effectiveness of longstanding exercise therapy in the subgroup of axSpA and RA patients with severe functional limitations.
Keywords: axial spondyloarthritis, exercise therapy, physical therapy, randomized controlled trial, rheumatoid arthritis
1. INTRODUCTION
Axial SpondyloArthritis (axSpA) and Rheumatoid Arthritis (RA) are chronic rheumatic diseases often with an progressive course, defined by chronic inflammation of the joints, tendons and synovial joint lining (Dougados & Baeten, 2011; Smolen et al., 2016). AxSpA is mainly characterized by inflammation of the spine and sacroiliac joints and ankylosis of the spine, and RA by arthritis of the peripheral joints (Dougados & Baeten, 2011; Smolen et al., 2016). Joint pain, stiffness and fatigue are major and common symptoms in both diseases, whereas extra‐articular manifestations in for example skin, blood vessels or inner organs occur less frequently (Mielants & Van den Bosch, 2009; Sepriano et al., 2017).
The prevalence of ankylosing spondylitis (major subtype of axSpA) varies worldwide from <0.01% to 1.8% (Stolwijk et al., 2012), whereas RA affects about 1%–1.5% of the Western population (Smolen et al., 2016; Turesson & Matteson, 2004). AxSpA, occurs equally in men and women, whereas RA is more frequent in women. Treatment of both diseases is primarily pharmacological, consisting of non‐steroidal anti‐inflammatory drugs, conventional biologicals or targeted synthetic disease modifying anti rheumatic drugs and/or glucocorticosteroids (Guo et al., 2018; Hurkmans et al., 2011; Rausch Osthoff et al., 2018). In addition, non‐pharmacological treatment is given in the majority of patients, of which patient education and exercise therapy constitute the cornerstones.
With respect to exercise therapy in axSpA, multiple systematic reviews concluded that supervised exercise therapy is an effective and safe treatment option, resulting in small to modest improvements in pain, disease activity, functional ability and axial mobility (Benatti & Pedersen, 2015; Dagfinrud et al., 2008; O'Dwyer et al., 2014; Regel et al., 2017; Sveaas et al., 2017). In RA, systematic reviews concluded a moderate, positive effect on aerobic capacity, muscle strength and overall functional ability (Hurkmans et al., 2009; Mewes, 2016a, 2016b; Swardh & Brodin, 2016).
In general, most of the studies included in these reviews concerned programs of a relatively short duration (≤12 weeks) and mostly concerned patients with stable disease, no co‐morbid conditions and relatively favorable functional ability (Bakker et al., 1994; Gaujoux‐Viala & Fautrel, 2012; Mewes, 2016a). Patients with active disease, irreversible joint damage, multiple joint replacements and/or severe comorbidity hampering participation in exercise therapy programs are underrepresented in research so far.
Only one trial in RA patients specifically included patients with active disease. Yet that study concerned a short‐term program, whereas it is conceivable that patients with severe limitations in activities and participation, due to persistent high disease activity, joint damage or complications of the disease and/or comorbidity are in need of long‐term treatment (van den Ende et al., 2000). Consequently, cost‐effectiveness studies on physical therapy are also lacking in these specific subgroups. Economic analyses are rare at all in studies on effectiveness on supervised exercise therapy in rheumatic and musculoskeletal diseases (Bakker et al., 1994; Gaujoux‐Viala & Fautrel, 2012).
Thus, there is a lack of knowledge on the effect of long‐term exercise therapy in the subgroup of patients with severe functional disability. We aim to evaluate the effectiveness and cost‐effectiveness of longstanding exercise therapy compared to usual care in the subgroup of patients with axSpA or RA and severe limitations in functioning. We hypothesize that longstanding exercise therapy, tailored to individual patients' needs and optimized according to the latest scientific insights, in the defined subgroups of patients with axSpA or RA and severe functional limitations is effective and cost‐effective compared to usual care.
2. METHODS
2.1. Study design
In two parallel nationwide RCTs, including either axSpA or RA patients with severe functional limitations in activities and participation, longstanding (≥52 weeks), active exercise therapy will be compared with usual care to evaluate its effectiveness and cost‐effectiveness (L‐EXSPA/L‐EXTRA; Longstanding‐EXercise therapy in patients with axSpA/Longstanding‐EXercise Therapy in patients with RA). Both RCTs are registered at the Netherlands National Trial Register: L‐EXSPA (NL8235) and L‐EXTRA (NL8238). The reporting of these studies is done in line with the CONSORT extension non‐pharmacological studies (Boutron et al., 2017).
The total duration of the studies is 208 weeks, with the duration of the inclusion period being 104 weeks. Assessments take place at baseline, 12, 26, and 52 weeks (primary endpoint). After the primary endpoint, patients in the usual care group are offered longstanding, active exercise therapy as well. In both groups, follow‐up assessments take place at 104, 156, and 208 weeks or end of study. Participants are followed for a minimal period of 52 weeks and a maximum of 208 weeks. For participants entering the study in a later stage of the inclusion period, the follow‐up will end before the maximum follow‐up duration of 208 weeks. An overview of the studies is provided in the flowchart (Figure 1).
FIGURE 1.
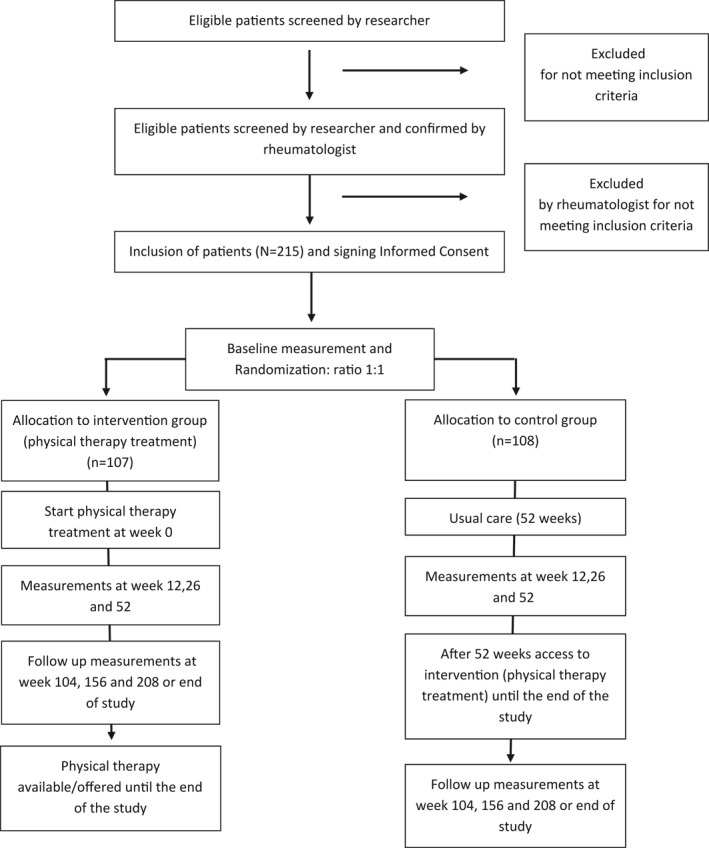
Study flowchart of two parallel studies for long‐term exercise therapy in axial SpondyloArthritis (axSpA) and Rheumatoid Arthritis (RA) patients
2.2. Participants
The study populations consist of axSpA and RA patients with severe limitations in functioning (activities and participation). The definition of these populations was established in 2014 by an expert group of patient representatives, rheumatologists, health professionals and researchers, in collaboration with National Health Care Institute of the Netherlands. Our inclusion criteria are based on this definition.
Inclusion criteria:
Consenting adult patients diagnosed with axSpA or RA by a rheumatologist.
One or more severe functional disabilities despite adequate medical treatment of the rheumatic condition resulting in limitations in daily activities involving self‐care (e.g., dressing, washing), transfers (e.g., getting in and out of bed, rising from a chair or using the toilet), and/or mobility indoors or outdoors.
Functional disability directly or indirectly related to the rheumatic condition, and caused by for example persisting or progressive high disease activity despite optimal medical treatment and/or severe joint damage and/or deformities and/or severe comorbidity (e.g., pulmonary or cardiovascular disease, depression, morbid obesity).
Functional disability can or could not be stopped or improved by a short, intermittent exercise therapy intervention.
Exclusion criteria:
Patients who received individual treatment of a physical therapist or a multidisciplinary team in the setting of a rehabilitation center or rheumatology clinic or center the last three months.
Patients in need of admission to a hospital, rehabilitation center or rheumatology clinic or other forms of intensive, multidisciplinary care.
Patients who are unable to give informed consent.
2.3. Study procedures
During the inclusion period (104 weeks), potential participants are informed by means of various media: websites, digital newsletters, flyers and (digital) posters. The Dutch Arthritis Society and the Dutch rheumatologists are involved in the recruitment of potential participants. Potential participants can register for the study via a registration link or the treating rheumatologists can register participants by contacting the researchers (MT, MvW; https://forms.lumc.nl/lumc2/aanmeldingsformulier‐patient). As part of the procedure to screen the eligibility, the researcher first conducts a telephone interview with every patient that has registered. During that interview, a standardized list of relevant activities of daily living and the nature and extent of difficulties the patient experiences and their impact is discussed. The patient is then reviewed in a weekly conference with 4 members of the team present. In case of doubt, additional questions are posed during another telephone interview. If it is concluded that the patient fulfills this and the other eligibility criteria, the rheumatologist is contacted to confirm the diagnosis and agree with the inclusion of the patient. After consent of the participant the treating rheumatologist is contacted to confirm the participants diagnosis. Participants meeting all inclusion criteria and with a written informed consent are included in the study.
2.4. Randomization and blinding
Randomization is executed by a research co‐worker (WP, SvW), who is not involved in the assessments or data analysis. The randomization takes place in blocks of varying sizes (4‐6‐8 participants, size randomized) in a ratio of 1:1 and is stratified by gender and health care insurance status (Castor EDC. [2019]. Castor Electronic Data Capture). Randomization is stratified by gender and insurance status (0–11 vs. 12 or more sessions covered by additional health insurance), to make sure there will be no imbalance between the groups. For gender, the course of the disease and the effect of the treatment could be different between males and females (Nilsson et al., 2021; Rusman et al., 2020). For insurance status, it is relevant that since 2012 exercise therapy is no longer covered in the basic health insurance for axSpA and RA patients in The Netherlands. The majority of patients has however an additional insurance to be able to cover the costs of physical therapy, with coverage varying with respect to the number of sessions that is reimbursed. An over‐representation or under‐representation of patients with (an extensive) coverage of exercise therapy in their additional health insurance in the control group could lead to relatively high or low usage of exercise therapy in that group and thus either decrease or increase the contrast with the intervention group. Given the nature of the intervention, participants and healthcare professionals involved in the treatment cannot be blinded to the treatment allocation and are instructed not to reveal information to the researchers regarding treatment allocation. The researchers are blinded to the allocation status, which is only revealed to them after the final statistical analysis. Participants are informed about their assigned condition after the baseline assessment by a research co‐worker who is not involved in the assessments or analysis.
2.5. Intervention
2.5.1. Recruitment and training of therapists
The intervention is delivered by trained physical therapists (PTs) working in the surroundings of the participant's home and are recruited by a research co‐worker (WP, SvW) who is not involved in the assessments. Recruitment mainly takes place through an existing national network of PTs with specific expertise regarding the treatment of patients with rheumatic and musculoskeletal diseases (RMDs; https://reumanetnl.nl/). To apply the treatment protocol, PTs are encouraged to comply with national recommendations for the physical therapy management of RA and axSpA (Hurkmans et al., 2018; van Weely et al., 2019) and receive an 8‐h training (combination of e‐learning [4‐h]), a scheduled, live online or face‐to‐face training (individual or small groups; 4‐h and self‐study). The training is provided by expert PTs in the project group (WP, SvW). The training contains specific information about the study protocol, the treatment procedures and how to tailor the treatment to the participant. Every PT has access to an e‐learning app and receives a manual with similar information. Treating PTs may contact PTs with extensive expertise in this subpopulation with questions about the treatment protocol, tailoring the intervention, managing co‐morbidities or other participant health problems. These experts PTs work in collaborating centers and have ample experience in treating patients with RMDs and severe limitations. In addition, interactive education sessions are held regularly to evaluate the intervention and to improve the treatment fidelity.
2.5.2. Content of the intervention
The intervention consists of longstanding (≥52 weeks), active exercise therapy aimed at improving individual limitations in daily activities and participation. Within 52 weeks, 64 treatments are planned, with two supervised treatments per week in the first 12 weeks. From week nine on participants are instructed and motivated to perform home‐based exercises and increase physical activities in addition to the supervised treatments. An overview of the intervention is provided in Tables 1a and 1b. The intervention is reported in accordance with the Consensus on Exercise Reporting Template (CERT; Slade et al., 2016).
TABLE 1a.
Structure of the exercise therapy intervention
| Week | Session 1 | Session 2 |
|---|---|---|
| 1 | Anamnesis & physical examination | |
| 2 | Physical examination (if not finished yet) and goal setting | |
| 3–8 | Treatment | Treatment |
| 9–12 | Treatment and structural education/guidance in self‐management of physical activity | Treatment and structural education/guidance in self‐management of physical activity |
| 13–52 a , b | Treatment, exercise planning and education and self‐management of physical activity | Optional, 14 additional treatments sessions can be scheduled in agreement with the participant |
Treatment can continue until 208 weeks or the end of the study.
Evaluation and if necessary, adaptation of treatment plan and ‐goals.
TABLE 1b.
Content of the exercise therapy intervention
| Individual active exercise training adapted to individual treatment goals. Exercise functions and activities, including: |
| Type: |
| ‐ Aerobic training |
| Walking, biking, cross trainer, rowing and other (rhythmic) movements in which large muscle groups are used. |
| ‐ Strength training |
| With use of own weight, attributes or devices. |
| ‐ Functional training |
| Exercises that train motor skills such as balance or coordination, and activities of daily living; e.g. transfers, self‐ care, wash and dress oneself, housekeeping, and gait. |
| Timing: |
| First 12 weeks, two times a week. After 12 weeks, one time per week with an option of 14 extra treatment sessions in the first year. |
| Dose of exercise: |
| Duration of a training session is 30 min and intensity are based on the ACSM a guidelines. The training can be structured with increasing frequency, timing and intensity until the goal is achieved in steps of 5%–10% increase each week. |
| Guidance by physical therapist: |
| Instructing, demonstrating and giving feedback. |
| Training location: |
| The training will take place at a training center close to the participants home. Or at the home of the participant, depending on the physical limitations and ability to travel of the participant. |
| Individual counseling physical activity, informing, advising and educating: |
| Personal factors: |
| Lifestyle/healthy behavior focusing on physical activity and optimal exercise level. |
| External factors: |
| Exercises at home (execution, time and place). |
| Assistive product: |
| Device that monitors the physical activity for motivation and behavioral change. |
| Homework exercise program. |
American College of Sports Medicine (ACSM).
The therapy includes a combination of functional exercises, aerobic exercises, muscle strengthening and flexibility/joint range of motion exercises, education and self‐management and promotion of physical activity. All exercises are tailored to the individual participants' disability, health status, needs and goals by adjusting the type of exercises, their intensity, frequency, duration, progression and site of delivery (practice or at home). The treatment is adapted to the individual participant, using a framework (based on 3iS strategy and Coach2Move program (de Rooij et al., 2017; de Vries et al., 2016)) to standardize the methods of initial assessment, setting treatment goals, clinical reasoning in monitoring participants' health status and treatment adjustment. Every treatment session must consist of either a combination of functional training and aerobic training or functional training and strength training. These training sessions must meet the dosage (frequency, intensity and duration) and progression based on the American College of Sport Medicine guidelines for exercise prescription (Garber et al., 2011). From week nine onwards, participants receive an activity tracker to monitor daily physical activity. Approximately every 12 weeks the treatment goals and the treatment plan are evaluated and adjusted accordingly. After a minimum of 52 weeks of therapy, the participant can continue the intervention until the end of the study. For each treatment session, PTs register process parameters, including the content of the applied treatment, training intensity, participant adherence, and side effects. These process parameters are used to tailor the treatment to participants' individual capabilities and are registered in OnlinePROMs® (2020, Interactive Studios BV).
2.6. Control (care as usual)
In the control group, the participants receive the usual care, to be determined by their treating physician(s) and participants themselves. After 52 weeks, the control group also has access to the intervention until the end of the study.
2.7. Outcome measures
The primary outcome is the difference between the intervention and control groups in changes in participants' reported limitations in functioning assessed by the Patient Specific Complaints Numeric Rating Scale (PSC NRS; Beurskens et al., 1999; Stevens et al., 2017) at 52 weeks. The secondary outcomes of the two studies are divided into four categories: Daily Functioning (Function); Quality of life; Health care usage and costs (from the societal perspective) and Perceived effect and satisfaction with treatment. A detailed description of all outcome measures and their timepoints are shown in Tables 2a and 2b.
TABLE 2a.
Outcome measures at the different timepoints
| Measures | Trial period | Follow‐up | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 c | T6 c | ||
| 0 weeks | 12 weeks | 26 weeks | 52 weeks | 104 weeks | 156 weeks | 208 weeks | ||
| General characteristics | X | |||||||
| Primary outcome | X | X | X | X | X | |||
| Secondary outcomes | ||||||||
| (a) Function | X | X | X | X | X a | X a | ||
| (b) Quality of life | X | X | X | X | X | X | ||
| (c) Health care usage and costs | X | X | X | X | X | X | X | |
| (d) Perceived effect and satisfaction with treatment | X b | X b | X b | X b | ||||
The 6MWT will not be measured at T5 and T6.
In control group only if physical therapy has been used.
Or end of study for participants included after 12 months after start of the study.
TABLE 2b.
Outcome measures and their description
| Measures | Description |
|---|---|
| General characteristics | |
| Sociodemographic and disease characteristics; comorbidity; | Age, gender, weight and height to calculate the body Mass index, status of living, level of education, insurance status, smoking, affected joints, joint surgery history, drugs and alcohol consumption and physical activity. |
| Primary outcome | |
| PSC NRS (Patient Specific Complaints Numeric Rating Scale) | The PSC NRS is an individualized outcome measure designed to detect changes in a client's perception of functioning and/or participation over time (Beurskens et al., 1999; Stevens et al., 2017). It consists of three scales (NRS) indicating the level of difficulty patients encounter while executing activities that are most relevant for them ranging from 0 = easy, to 10 = impossible to do. |
| Secondary outcomes | |
| Function | |
| PROMIS‐10 (Patient Reported Outcome Measurement Information System‐10) | PROMIS is a standardized metric for measuring health across chronic diseases, developed using the item response theory (Bartlett et al., 2015; Fries et al., 2009, 2011; Terwee et al., 2014). The PROMIS Short Form v2.0—Physical Function 10a will be used in this study to measure the patient reported physical function. It is a short questionnaire consisting of 10 questions. All questions have five answer options ranging from 1 = easy to 5 = impossible to do. From the raw score a T‐score is derived, with the Dutch/Flemish population mean and a standard deviation. A high score indicates a poor patient reported physical function. |
| BASFI (Bath Ankylosing Spondylitis Functional Index) b | BASFI is a validated instrument to assess the degree of functional limitation in patients with axial spondyloarthritis (Calin et al., 1994; van Tubergen et al., 2015). It comprises 10 questions on how well activities went in the past week. The questions are answered by a NRS, ranging from 0 = easy to 10 = impossible to do. The BASFI score is calculated by taking the mean of the score of the 10 individual questions. Scores can range from 0 to 10, with a high score referring to severe limitations. |
| HAQ‐DI (Health Assessment Questionnaire‐Disability Index) c | The HAQ measures functional ability in RA patients and comprises 20 questions regarding eight domains of activities of daily living with the total score ranging from 0 (no functional limitations) to 3 (serious functional limitations) (Boers et al., 2017; Bruce & Fries, 2003; Fries et al., 1980; Siegert et al., 1984). |
| 6‐Minute Walk Test a | The 6‐min walk test is a performance‐based test, in which the patient is requested to walk at a comfortable speed for 6 min, with the distance measured in meters. Patients are allowed to use a walking aid (Butland et al., 1982; K. de Jong, 2000). According to the practice guideline for this instrument, the test is not used in case a patient cannot walk at all or needs a lot of support from another person in order to be able to walk. |
| Quality of Life | |
| RA‐QoL (Rheumatoid Arthritis Quality of Life questionnaire) c | The RA‐QoL is a 30‐item patient‐based quality of life instrument specific for patients with RA. It was developed by researchers in the United Kingdom and The Netherlands and proved to be unidimensional, reliable and have good construct validity (Z. de Jong et al., 1997; Tijhuis et al., 2001; Whalley et al., 1997). The RAQol comprises 30 statements, each with a yes/no response format. The overall score ranges from 0 to 30, with a high score indicating a poor QoL. |
| SF‐36 (Short Form‐36) | The Short Form‐36 for Quality of life is a generic quality of life instrument (Aaronson et al., 1998; Brazier et al., 1992; Z. de Jong et al., 1997). The 36 items are divided over 8 dimensions, from which 2 summary scales can be computed: The Physical Component and Mental Component Summary Scales (PCS and MCS), both with a score ranging from 0 (worst health status) to 100 (best health status). |
| EuroQol (EQ‐5D‐5L) | The EuroQol (Dolan, 1997; EuroQol‐Group., 1990) is a standardized instrument including 5 dimensions of health (mobility, selfcare, daily activities, pain/complaints and anxiety/depression), resulting in a score anchored at 0–1, with a higher score indicating better health. It also includes a visual analog scale with a score ranging from 0 (worst possible health) to 100 (perfect health). |
| Health care usage and costs | |
| Health care usage and patient costs in the past months | Including General Practitioner visits, outpatient visits, hospital days, rehabilitation center, nursing home, home care, medication use, informal care, patient costs and productivity. Similar questionnaires have been used in previous studies on physical therapy in inflammatory arthritis (van den Hout et al., 2005). |
| Work status (paid and unpaid labor) | This questionnaire is constructed by the research group, including a health economist, containing questions regarding the current work status, the number of hours of work or volunteer work and the effect of the disease on the work of the participants. The questionnaire is based on questionnaires that were previously used in the RAPIT trial (van den Hout et al., 2005). |
| Perceived effect and satisfaction with treatment | |
| Perceived effect anchor question | Contains the anchor question on the perceived effect: “Has the exercise therapy changed your daily functioning?” |
| Satisfaction with longstanding exercise therapy | Short questionnaire on patient satisfaction with treatment, based on the Consumer Quality Index for physical therapy (CQ‐Index) will be administered (Sixma et al., 2008). The questionnaire consists of questions regarding the satisfaction with the physical therapist, the treatment plan. Questions are open and multiple choice. A high score indicates a high satisfaction with the exercise therapy. |
| Perceived side effects of longstanding exercise therapy | A short‐constructed questionnaire on patient satisfaction with treatment. The patient describes the perceived effect on for instance pain, functioning, daily activities on a 7‐point Likert scale. Scores can range from 1 to 7 ranging, 1 = very much deteriorated to 7 = very much improved. A high score indicates an improved perceived effect. |
| Content of longstanding exercise therapy | A short questionnaire constructed by the research group to ask the patient about the content of the therapy he or she received. |
Performance measure.
Measured only in the study population of axial spondyloarthritis patients.
Measured only in the study population of rheumatoid arthritis patients.
2.7.1. Data collection
At baseline, general characteristics (e.g., age, gender, education level, length, and weight) and disease specific characteristics are collected (e.g., the relevant medical history of the participant and exercises behavior). The individual level of functioning is measured with the PSC NRS (Beurskens et al., 1999; Stevens et al., 2017) and the 6MWT (Butland et al., 1982; K. de Jong, 2000; de Vries et al., 2016) and will be assessed at baseline, 52 weeks (primary endpoint), and at 104, 156, and 208 weeks or end of study. The 6MWT will be assessed by the researchers at baseline, 26, 52 weeks and at 104 weeks. The data will be stored in the online database OnlinePROMs©.
2.7.2. (Serious) adverse events
We defined a serious adverse event (SAE) as an untoward occurrence that results in death or is life threatening (at the time of the event), requires hospitalization or prolongation of existing in participants' hospitalization, or results in significant or permanent disability or incapacity. The SAE should be directly related to the exercise therapy treatment. All other untoward symptoms or complaints related to the exercise therapy treatment are defined as non‐serious adverse events (AEs). Examples of non‐serious AEs may include: falls without injuries, muscle injuries or any new occurrence of an unwanted unfavorable AE that is not defined as a SAE.
Serious and non‐serious AEs are recorded and followed until they have abated, or until a stable situation has been reached. The assessors will report all SAEs to the sponsor without undue delay after obtaining knowledge of the events. All participants and therapists are asked to immediately and proactively report any AE or SAE to the assessors/researchers.
2.8. Sample size calculation
The primary measure of effectiveness is the PSC NRS at 52 weeks (Beurskens et al., 1999; Stevens et al., 2017). The threshold for discrimination for changes in patient reported outcomes in chronic diseases is an effect size of 0.5. Using a population effect size of 0.5 (alpha = 0.05, power of 0.90, two‐sided, two‐sample equal‐variance t‐test) 86 patients are required per group. Taking into account a 20% drop‐out rate, we aim to recruit 215 patients per study.
2.9. Statistical analysis
2.9.1. Primary analysis
2.9.1.1. Effect on functioning
All primary analyses will be done based on the intention‐to‐treat principle. Using linear mixed models, for the primary outcome measure, changes on the PSC NRS at 52 weeks will be calculated (change in PSC NRS as dependent variable and treatment condition [intervention or control] as independent variable). Adjustments will take place for baseline values, and if necessary, for unbalanced covariates. The assumptions of constant variance and linear relationships will be assessed. Transformations will be used when appropriate. Similar analyses will be done for the secondary outcome measures.
2.9.1.1.1. Cost‐effectiveness
The economic evaluation will be a cost‐utility analysis (CUA) from a societal perspective, with a 1‐year time horizon, consistent with the Dutch guidelines (https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline‐for‐economic‐evaluations‐in‐healthcare) and following the methodology of a previous CUA on long‐term, dynamic exercise in RA (van den Hout et al., 2005). Costs will be estimated from a societal perspective, including healthcare costs, patient costs, and productivity costs. Other costs will be calculated from cost questionnaires, with prices of healthcare Dutch standard prices and charges, as described in the Dutch guidelines. In the CUA, the impact on disease burden will be measured using quality‐adjusted life years (QALYs) estimated from the Dutch tariff for the EQ‐5D‐5L at 0, 12, 26, and 52 weeks (Versteegh et al., 2016). In the cost‐effectiveness analyses, mean costs and patient outcome will be statistically compared, with multiple imputation to account for missing data. Costs will be related to patient outcomes using net‐benefit analysis. Sensitivity analysis will be performed on the perspective of the cost analysis (societal vs. health care only) and the utility measure (Dutch EQ‐5D‐5L vs. Visual Analog Scale).
2.9.2. Secondary analysis
Secondary analyses include a per protocol analysis. Moreover, an analysis with only those participants in the control group who did not or only to a small extent (8 sessions or less) used physical therapy will be performed. In addition, a mixed model analysis will be done taking into account all time points up to and including 52 weeks in order to compare the primary and all secondary outcome measures over time. The research question of the trials, determination of primary outcomes and the ensuing power calculation are all based on analysis of the whole group and not on specific subgroups.
2.9.3. Follow‐up analysis
A follow‐up is executed in both the intervention group and usual care group at 104 weeks and at 156 and 208 weeks after randomization or at the end of the study. This follow‐up is done in order to monitor the longer‐term effectiveness in the intervention group.
2.9.4. Adverse events
The absolute number and the relative frequency of the SAEs and the AEs will be reported for both allocation groups. Also, a description of every occurred (S)AE is provided to give a complete overview of the events that occurred during the study.
2.10. Data management
All the data of the participants will be anonymized with assignment of a study number to every participant. The collected data will be stored for 15 years on a local drive at Leiden University Medical Center and a backup of the data will be stored at Data Archiving and Networked Services‐The Royal Netherlands Academy of Arts and Sciences (DANS‐KNAW; https://dans.knaw.nl/nl).
3. DISCUSSION
There is a subgroup of patients with axSpA and RA (5%) with severe limitations in activities and participation despite medical treatment of the rheumatic condition, resulting from joint damage or persistent high disease activity, complications of the disease, its treatment or comorbidity. Despite the observed need for exercise therapy, research on effectiveness of longstanding, active exercise therapy in this particular subgroup is absent. By conducting two parallel RCTs, we aim to evaluate the effectiveness and cost‐effectiveness of longstanding exercise therapy compared to usual care in the subgroup of patients with axSpA or RA and severe limitations in functioning. These two studies are first to investigate the effectiveness and cost‐effectiveness of longstanding exercise therapy in this subgroup and the outcomes of these studies could lead to new knowledge and further improvement of the treatment. We hypothesize that longstanding, active exercise therapy in the described subgroups of patients with severe limitations is effective and cost‐effective compared to usual care.
4. IMPLICATIONS ON PHYSIOTHERAPY PRACTICE
The results of this research will result in new knowledge about the effectiveness and cost‐effectiveness of longstanding exercise therapy for these specific subgroups, which should be implemented in physiotherapy guidelines. Physical therapists may use this knowledge in daily practice to improve the treatment of this subgroup.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
Substantial contributions to the conception (C. H. M. van den Ende, M. G. J. Gademan, S. F. E. van Weely (SvW) and T. P. M. Vliet Vlieland) or design of the work (A. M. van Tubergen, A. A. den Broeder, C. H. M. van den Ende, D. van Schaardenburg, M. G. J. Gademan, S. F. E. van Weely and T. P. M. Vliet Vlieland, W. F. Peter (WP) and W. B. van den Hout); AND Drafting the article or revising it critically for important intellectual content (A. A. den Broeder, A. M. van Tubergen, C. H. M. van den Ende, D. van Schaardenburg, M. G. J. Gademan, M. M. H. Teuwen (MT), M. A. T. van Wissen (MvW), S. F. E. van Weely and T. P. M. Vliet Vlieland, W. F. Peter and W. B. van den Hout); AND Final approval of the version to be published (A. A. den Broeder, A. M. van Tubergen, C. H. M. van den Ende, D. van Schaardenburg, M. G. J. Gademan, M. M. H. Teuwen, M. A. T. van Wissen, S. F. E. van Weely and T. P. M. Vliet Vlieland, W. F. Peter and W. B. van den Hout); AND Agreement to be accountable for appropriate portions of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (A. A. den Broeder, A. M. van Tubergen, C. H. M. van den Ende, D. van Schaardenburg, M. G. J. Gademan, M. M. H. Teuwen, M. A. T. van Wissen, S. F. E. van Weely and T. P. M. Vliet Vlieland, W. F. Peter and W. B. van den Hout).
ETHICS AND DISSEMINATION
The studies have been approved by the Medical Ethical Committee of the Leiden‐Den Haag‐Delft (METC LDD; L‐EXSPA: NL70093.058.19, L‐EXTRA: NL69866.058.19) and the two studies will be conducted in agreement with the declaration of Helsinki (2013) and in compliance with the General Data Protection Regulations and the Dutch Medical Research Involving Human Subjects Act. Participants will have considerable time to decide whether they are willing to engage in the study. Written informed consent will be obtained from all engaged participants in the study. This study was classified as a low risk study by the METC LDD so that no Data Safety Monitoring Board was put in place. The safety of the participants will be monitored by the online submission system of the METC LDD to report SAEs. SAEs will be reported within 24 h after notification. The METC LDD will decide whether the safety of the participant is threatened and based on their judgment, the study can be continued or not. We intend to submit the results of the two studies in separate publications in peer‐reviewed journals. Furthermore, we aim to present these results at international congresses and to disseminate the results to guideline committees.
ACKNOWLEDGMENTS
The authors would like to thank the Dutch Arthritis Society (ReumaNederland) and the Royal Dutch Society for Physical Therapy (Koninklijk Nederlands Genootschap voor Fysiotherapie) for their cooperation in the preliminary phase of these studies. Besides, the authors would especially like to thank N. Lopuhaä and S. de Jong (both Dutch Arthritis Society) for their contribution to informing potential participants and support in the recruitment process. This project is financially supported by Ministry of Health, Welfare and Sport, ZonMw (Project number L‐EXTRA: 852004018, Project number L‐EXSPA: 852004019), the Dutch Arthritis Society (ReumaNederland) and the Royal Dutch Society for Physical Therapy (KNGF).
van Wissen, M. A. T. , Teuwen, M. M. H. , van den Ende, C. H. M. , Vliet Vlieland, T. P. M. , den Broeder, A. A. , van den Hout, W. B. , Peter, W. F. , van Schaardenburg, D. , van Tubergen, A. M. , Gademan, M. G. J. , & van Weely, S. F. E. (2022). Effectiveness and cost‐effectiveness of longstanding exercise therapy versus usual care in patients with axial spondyloarthritis or rheumatoid arthritis and severe limitations: The protocols of two parallel randomized controlled trials. Physiotherapy Research International, 27(1), e1933. 10.1002/pri.1933
van Wissen and Teuwen shared first co‐authorship.
Contributor Information
Maria. A. T. van Wissen, Email: m.a.t.van_wissen@lumc.nl.
Max. M. H. Teuwen, Email: m.m.h.teuwen@lumc.nl.
DATA AVAILABILITY STATEMENT
Not applicable, as this study concerns a protocol study.
REFERENCES
- Aaronson, N. K. , Muller, M. , Cohen, P. D. A. , Essink‐Bot, M. L. , Fekkes, M. , Sanderman, R. , Sprangers, M. A. G. , te Velde, A. , & Verrips, E. (1998). Translation, validation, and norming of the Dutch language version of the SF‐36 Health Survey in community and chronic disease populations. Journal of Clinical Epidemiology, 51(11), 1055–1068. 10.1016/s0895-4356(98)00097-3 [DOI] [PubMed] [Google Scholar]
- Bakker, C. , Hidding, A. , van der Linden, S. , & van Doorslaer, E. (1994). Cost effectiveness of group physical therapy compared to individualized therapy for ankylosing spondylitis. A randomized controlled trial. Journal of Rheumatology, 21(2), 264–268. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8182635 [PubMed] [Google Scholar]
- Bartlett, S. J. , Orbai, A. M. , Duncan, T. , DeLeon, E. , Ruffing, V. , Clegg‐Smith, K. , & Bingham, C. O. (2015). Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PLoS One, 10(9), e0138543. 10.1371/journal.pone.0138543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti, F. B. , & Pedersen, B. K. (2015). Exercise as an anti‐inflammatory therapy for rheumatic diseases‐myokine regulation. Nature ReviewsRheumatology, 11(2), 86–97. 10.1038/nrrheum.2014.193 [DOI] [PubMed] [Google Scholar]
- Beurskens, A. J. , de Vet, H. C. , Köke, A. J. , Lindeman, E. , van der Heijden, G. J. , Regtop, W. , & Knipschild, P. G. (1999). A patient‐specific approach for measuring functional status in low back pain. Journal of Manipulative Physiological Therapeutics, 22(3), 144–148. 10.1016/s0161-4754(99)70127-2 [DOI] [PubMed] [Google Scholar]
- Boers, M. , Jacobs, J. W. G. , van Vliet Vlieland, T. P. M. , & van Riel, P. L. C. M. (2017). Consensus Dutch health assessment questionnaire. Annals of the Rheumatic Diseases, 66(1), 132–133. 10.1136/ard.2006.059451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutron, I. , Altman, D. G. , Moher, D. , Schulz, K. F. , & Ravaud, P. (2017). CONSORT statement for randomized trials of nonpharmacologic treatments: A 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Annals of Internal Medicine, 167(1), 40–47. 10.7326/m17-0046 [DOI] [PubMed] [Google Scholar]
- Brazier, J. E. , Harper, R. , Jones, N. M. , O'Cathain, A. , Thomas, K. J. , Usherwood, T. , & Westlake, L. (1992). Validating the SF‐36 health survey questionnaire: New outcome measure for primary care. BMJ, 305(6846), 160–164. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, B. , & Fries, J. F. (2003). The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health and Quality of Life Outcomes, 1, 20. 10.1186/1477-7525-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland, R. J. , Pang, J. , Gross, E. R. , Woodcock, A. A. , & Geddes, D. M. (1982). Two‐, six‐, and 12‐minute walking tests in respiratory disease. British Medical Journal, 284(6329), 1607–1608. 10.1136/bmj.284.6329.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin, A. , Garrett, S. , Whitelock, H. , Kennedy, L. G. , O'Hea, J. , Mallorie, P. , & Jenkinson, T. (1994). A new approach to defining functional ability in ankylosing spondylitis: The development of the bath ankylosing spondylitis functional index. Journal of Rheumatology, 21(12), 2281–2285. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7699629 [PubMed] [Google Scholar]
- Dagfinrud, H. , Hagen, K. B. , & Kvien, T. K. (2008). Physiotherapy interventions for ankylosing spondylitis. Cochrane Database of Systematic Reviews, 1, CD002822. 10.1002/14651858.CD002822.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, K. (2000). Richtlijn 6‐minutes timed walking test. Revalidatiecentrum de Hoog‐straat. https://meetinstrumentenzorg.nl/wp‐content/uploads/instrumenten/6MWT‐form‐1.pdf [Google Scholar]
- de Jong, Z. , van der Heijde, D. , McKenna, S. P. , & Whalley, D. (1997). The reliability and construct validity of the RAQoL: A rheumatoid arthritis‐specific quality of life instrument. British Journal of Rheumatology, 36(8), 878–883. 10.1093/rheumatology/36.8.878 [DOI] [PubMed] [Google Scholar]
- de Rooij, M. , van der Leeden, M. , Cheung, J. , van der Esch, M. , Häkkinen, A. , Haverkamp, D. , Roorda, L. D. , Twisk, J. , Vollebregt, J. , Lems, W. F. , & Dekker, J. (2017). Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: A randomized controlled trial. Arthritis Care & Research, 69(6), 807–816. Retrieved from https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/acr.23013?download=true [DOI] [PubMed] [Google Scholar]
- de Vries, N. M. , Staal, J. B. , van der Wees, P. J. , Adang, E. M. M. , Akkermans, R. , Olde Rikkert, M. G. M. , & Nijhuis‐van der Sanden, M. W. G. (2016). Patient‐centred physical therapy is (cost‐) effective in increasing physical activity and reducing frailty in older adults with mobility problems: A randomized controlled trial with 6 months follow‐up. Journal of Cachexia, Sarcopenia and Muscle, 7(4), 422–435. 10.1002/jcsm.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan, P. (1997). Modeling valuations for EuroQol health states. Medical Care, 35(11), 1095–1108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- Dougados, M. , & Baeten, D. (2011). Spondyloarthritis. Lancet, 377(9783), 2127–2137. 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- EuroQol‐Group . (1990). EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy, 16(3), 199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- Fries, J. F. , Cella, D. , Rose, M. , Krishnan, E. , & Bruce, B. (2009). Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. Journal of Rheumatology, 36(9), 2061–2066. 10.3899/jrheum.090358 [DOI] [PubMed] [Google Scholar]
- Fries, J. F. , Krishnan, E. , Rose, M. , Lingala, B. , & Bruce, B. (2011). Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Research and Therapy, 13(5), R147. 10.1186/ar3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries, J. F. , Spitz, P. , Kraines, R. G. , & Holman, H. R. (1980). Measurement of patient outcome in arthritis. Arthritis & Rheumatism, 23(2), 137–145. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- Garber, C. E. , Blissmer, B. , Deschenes, M. R. , Franklin, B. A. , Lamonte, M. J. , Lee, I. M. , Nieman, D. C. , & Swain, D. P. (2011). American College of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine & Science in Sports & Exercise, 43(7), 1334–1359. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Gaujoux‐Viala, C. , & Fautrel, B. (2012). Cost effectiveness of therapeutic interventions in ankylosing spondylitis: A critical and systematic review. PharmacoEconomics, 30(12), 1145–1156. 10.2165/11596490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Guo, Q. , Wang, Y. , Xu, D. , Nossent, J. , Pavlos, N. J. , & Xu, J. (2018). Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Research, 6, 15. 10.1038/s41413-018-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkmans, E. , van der Giesen, F. J. , Bloo, H. , Boonman, D. C. , van der Esch, M. , Fluit, M. , Hilberdinks, W. K. , Peter, W. F. , van der Stegen, H. P. , Veerman, E. A. , Vermeulen, H. M. , Hendriks, H. M. , Schoones, J. W. , & Vliet Vlieland, T. P. (2011). Physiotherapy in rheumatoid arthritis: Development of a practice guideline. Acta Reumatologica Portuguesa, 36(2), 146–158. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21841734 [PubMed] [Google Scholar]
- Hurkmans, E. , van der Giesen, F. J. , Vliet Vlieland, T. P. M. , Schoones, J. , & Van den Ende, E. C. H. M. (2009). Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews, 4, CD006853. 10.1002/14651858.CD006853.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkmans, E.J. , Peter, W.F.H. , Swart, N.M. , Meerhoff, G.A. , & Vliet Vlieland, T. P. M. (2018). Herziene KNGF‐richtlijn reumatoïde artritis 2018. Retrieved from https://www.kngf.nl/binaries/content/documents/kennisplatform/producten/richtlijnen/reumatoide‐artritis/reumatoide‐artritis/kngfextranet%3Adownload [Google Scholar]
- Mewes, J. , de Bekker, P. , Bossen, D. , & Steuten, L. (2016a). Systematic review of supervised phyiscal exercise therapy for patients with ankylosing spondylitis. Panaxea. [Google Scholar]
- Mewes, J. , de Bekker, P. , Bossen, D. , & Steuten, L. (2016b). Systematic review of supervised physical exercise therapy for patients with rheumatoid arthritis. Panaxea. [Google Scholar]
- Mielants, H. , & Van den Bosch, F. (2009). Extra‐articular manifestations. Clinical & Experimental Rheumatology, 27(4 Suppl 55), S56–S61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19822047 [PubMed] [Google Scholar]
- Nilsson, J. , Andersson, M. L. E. , Hafstrom, I. , Svensson, B. , Forslind, K. , Ajeganova, S. , Leu Agelii, M. , & Gjertsson, I. (2021). Influence of age and sex on disease course and treatment in rheumatoid arthritis. Open Access Rheumatology, 13, 123–138. 10.2147/OARRR.S306378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dwyer, T. , O'Shea, F. , & Wilson, F. (2014). Exercise therapy for spondyloarthritis: A systematic review. Rheumatology International, 34(7), 887–902. 10.1007/s00296-014-2965-7 [DOI] [PubMed] [Google Scholar]
- Rausch Osthoff, A. K. , Niedermann, K. , Braun, J. , Adams, J. , Brodin, N. , Dagfinrud, H. , Duruoz, T. , Esbensen, B. A. , Günther, K.‐P. , Hurkmans, E. , Juhl, C. B. , Kennedy, N. , Kiltz, U. , Knittle, K. , Nurmohamed, M. , Pais, S. , Severijns, G. , Swinnen, T. W. , Pitsillidou, I. A. , Warburton, L. , Yankov, Z. , & Vliet Vlieland, T. P. M. (2018). 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Annals of the Rheumatic Diseases, 77(9), 1251–1260. 10.1136/annrheumdis-2018-213585 [DOI] [PubMed] [Google Scholar]
- Regel, A. , Sepriano, A. , Baraliakos, X. , van der Heijde, D. , Braun, J. , Landewe, R. , Van den Bosch, F. , Falzon, L. , & Ramiro, S. (2017). Efficacy and safety of non‐pharmacological and non‐biological pharmacological treatment: A systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open, 3(1), e000397. 10.1136/rmdopen-2016-000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusman, T. , van Bentum, R. E. , & van der Horst‐Bruinsma, I. E. (2020). Sex and gender differences in axial spondyloarthritis: Myths and truths. Rheumatology (Oxford), 59(Suppl4), iv38–iv46. 10.1093/rheumatology/keaa543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepriano, A. , Regel, A. , van der Heijde, D. , Braun, J. , Baraliakos, X. , Landewe, R. , Van den Bosch, F. , Falzon, L. , & Ramiro, S. (2017). Efficacy and safety of biological and targeted‐synthetic DMARDs: A systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open, 3(1), e000396. 10.1136/rmdopen-2016-000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert, C. E. H. , Vleming, L. J. , Vandenbroucke, J. P. , & Cats, A. (1984). Measurement of disability in Dutch rheumatoid arthritis patients. Clinical Rheumatology, 3(3), 305–309. 10.1007/BF02032335 [DOI] [PubMed] [Google Scholar]
- Sixma, H. , Hendriks, M. , de Boer, D. (2008). Handboek CQI Ontwikkeling: Richtlijnen en voorschriften voor de ontwikkeling van een CQI meetinstrument (manual for development of a CQ Index measurement instrument). Centrum Klantervaring Zorg. Retrieved from https://www.nivel.nl/sites/default/files/bestanden/Handboek‐CQI‐Ontwikkeling.pdf [Google Scholar]
- Slade, S. C. , Dionne, C. E. , Underwood, M. , & Buchbinder, R. (2016). Consensus on Exercise Reporting Template (CERT): Explanation and elaboration statement. British Journal of Sports Medicine, 50(23), 1428–1437. 10.1136/bjsports-2016-096651 [DOI] [PubMed] [Google Scholar]
- Smolen, J. S. , Aletaha, D. , & McInnes, I. B. (2016). Rheumatoid arthritis. Lancet, 388(10055), 2023–2038. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- Stevens, A. , Köke, A. , van der Weijden, T. , & Beurskens, A. (2017). Ready for goal setting? Process evaluation of a patient‐specific goal‐setting method in physiotherapy. BMC Health Services Research, 17(1), 618. 10.1186/s12913-017-2557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolwijk, C. , Boonen, A. , van Tubergen, A. , & Reveille, J. D. (2012). Epidemiology of spondyloarthritis. Rheumatic Disease Clinics of North America, 38(3), 441–476. 10.1016/j.rdc.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveaas, S. H. , Smedslund, G. , Hagen, K. B. , & Dagfinrud, H. (2017). Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: A systematic review and meta‐analysis. British Journal of Sports Medicine, 51(14), 1065–1072. 10.1136/bjsports-2016-097149 [DOI] [PubMed] [Google Scholar]
- Swardh, E. , & Brodin, N. (2016). Effects of aerobic and muscle strengthening exercise in adults with rheumatoid arthritis: A narrative review summarising a chapter in physical activity in the prevention and treatment of disease (FYSS 2016). British Journal of Sports Medicine, 50(6), 362–367. 10.1136/bjsports-2015-095793 [DOI] [PubMed] [Google Scholar]
- Terwee, C. B. , Roorda, L. D. , de Vet, H. C. , Dekker, J. , Westhovens, R. , van Leeuwen, J. , Cella, D. , Correia, H. , Arnold, B. , Perez, B. , & Boers, M. (2014). Dutch‐Flemish translation of 17 item banks from the Patient‐Reported Outcomes Measurement Information System (PROMIS). Quality of Life Research, 23(6), 1733–1741. 10.1007/s11136-013-0611-6 [DOI] [PubMed] [Google Scholar]
- Tijhuis, G. J. , de Jong, Z. , Zwinderman, A. H. , Zuijderduin, W. M. , Jansen, L. M. A. , Hazes, J. M. W. , & Vliet Vlieland, T. P. M. (2001). The validity of the Rheumatoid Arthritis Quality of Life (RAQoL) questionnaire. Rheumatology (Oxford), 40(10), 1112–1119. 10.1093/rheumatology/40.10.1112 [DOI] [PubMed] [Google Scholar]
- Turesson, C. , & Matteson, E. L. (2004). Management of extra‐articular disease manifestations in rheumatoid arthritis. Current Opinion in Rheumatology, 16(3), 206–211. 10.1097/00002281-200405000-00007 [DOI] [PubMed] [Google Scholar]
- van den Ende, C. H. M. , Breedveld, F. C. , le Cessie, S. , Dijkmans, B. A. C. , de Mug, A. W. , & Hazes, J. M. W. (2000). Effect of intensive exercise on patients with active rheumatoid arthritis: A randomised clinical trial. Annals of the Rheumatic Diseases, 59(8), 615–621. 10.1136/ard.59.8.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hout, W. B. , de Jong, Z. , Munneke, M. , Hazes, J. M. W. , Breedveld,F. C. , & Vliet Vlieland, T. P. M. (2005). Cost‐utility and cost‐effectiveness analyses of a long‐term, high‐intensity exercise program compared with conventional physical therapy in patients with rheumatoid arthritis. Arthritis & Rheumatism, 53(1), 39–47. 10.1002/art.20903 [DOI] [PubMed] [Google Scholar]
- van Tubergen, A. , Black, P. M. , & Coteur, G. (2015). Are patient‐reported outcome instruments for ankylosing spondylitis fit for purpose for the axial spondyloarthritis patient? A qualitative and psychometric analysis. Rheumatology (Oxford), 54(10), 1842–1851. 10.1093/rheumatology/kev125 [DOI] [PubMed] [Google Scholar]
- van Weely, S. F. E. , van der Giesen, F. J. , van Gaalen, F. A. , van der Horst‐Bruinsma, I. E. , Ramiro, S. , Weel, A. E. A. M. , Lopuhaä, N. , & Vlieland, T. P. M. (2019). Aanbevelingen fysiotherapie bij mensen met axiale spondyloartritis. Retrieved from https://reumanetnl.nl/wp‐content/uploads/2019/06/Aanbevelingen‐Fysiotherapie‐bij‐mensen‐met‐AxialeSpondyloartritus.pdf [Google Scholar]
- Versteegh, M. M. , Vermeulen, K. M. , Evers, S. M. A. A. , de Wit, G. A. , Prenger, R. , & Stolk, E. A. (2016). Dutch tariff for the five‐level version of EQ‐5D. Value in Health, 19(4), 343–352. 10.1016/j.jval.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Whalley, D. , McKenna, S. P. , de Jong, Z. , & van der Heijde, D. (1997). Quality of life in rheumatoid arthritis. Rheumatology, 36(8), 884–888. 10.1093/rheumatology/36.8.884 [DOI] [PubMed] [Google Scholar]
- World Medical Association . (2013). Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, as this study concerns a protocol study.


