Abstract
Prediction of the risk of cardiovascular events (CVE's) is important to optimize outcomes after kidney transplantation. Aortoiliac stenosis is frequently observed during pre‐transplant screening. We hypothesized that these patients are at higher risk of post‐transplant CVE's due to the joint underlying atherosclerotic disease. Therefore, we aimed to assess whether aortoiliac stenosis was associated with post‐transplant CVE's. This retrospective, single‐center cohort study included adult kidney transplant recipients, transplanted between 2000 and 2016, with contrast‐enhanced imaging available. Aortoiliac stenosis was classified according to the Trans‐Atlantic Inter‐Society Consensus (TASC) II classification and was defined as significant in case of ≥50% lumen narrowing. The primary outcome was CVE‐free survival. Eighty‐nine of 367 patients had significant aortoiliac stenosis and were found to have worse CVE‐free survival (median CVE‐free survival: stenosis 4.5 years (95% confidence interval (CI) 2.8–6.2), controls 8.9 years (95% CI 6.8–11.0); log‐rank test P < .001). TASC II C and D lesions were independent risk factors for a post‐transplant CVE with a hazard ratio of 2.15 (95% CI 1.05–4.38) and 6.56 (95% CI 2.74–15.70), respectively. Thus, kidney transplant recipients with TASC II C and D aortoiliac stenosis require extensive cardiovascular risk management pre‐, peri,‐ and post‐transplantation.
Keywords: cardiovascular diseases, kidney transplantation, mortality, risk factors
1. INTRODUCTION
Kidney transplantation is the preferred treatment for end‐stage renal disease because of improved survival and quality of life compared to dialysis. 1 , 2 , 3 The most important cause of mortality among patients with end‐stage renal disease is cardiovascular disease. 4 The risk of dying from a cardiovascular cause increases shortly after transplantation and decreases thereafter when compared to waitlisted patients. 5 However, following transplantation, cardiovascular events (CVE's) remain an important cause of morbidity and mortality with a 3–4 times higher incidence of CVE's compared to the general population. 6 , 7 , 8 , 9 The etiology is complex and consists of traditional risk factors, such as diabetes, advanced age and smoking, and disease‐specific factors, such as dialysis. 6 , 10 , 11 , 12 , 13 , 14 , 15 Identification of these risk factors is important to predict the patient‐specific risk of a cardiovascular event in the post‐transplantation period.
Aortoiliac stenosis could be an additional risk factor for CVE's considering the joint underlying atherosclerotic disease. Patients are often screened for the presence of aortoiliac stenosis because it can cause technical difficulties during graft arterial anastomoses, 16 , 17 , 18 insufficient blood perfusion of the graft, 17 , 18 and exacerbation of lower extremity ischemia in the perioperative period. 18 Technical difficulties can occur in a twofold manner. In case of unforeseen conditions, arterial repair may be required during transplantation. 16 This additional intervention may lead to prolonged cold and warm ischemia time, which is associated with an increased risk of complications and inferior graft outcomes. 6 , 19 In case severe aortoiliac stenosis is diagnosed before transplantation, vascular surgery may be indicated. 16 , 20 However, an additional operation may lead to an increased risk of cardiac morbidity and mortality in these high‐risk patients. 21
Besides the role of these intermediate factors, there is evidence for the association between aortoiliac stenosis itself and CVE's. Aortoiliac stenosis as well as systemic vascular disease could lead to an increased risk of CVE's, 22 , 23 limiting post‐transplant success. A previous study from our own center found an independent association between Trans‐Atlantic Inter‐Society Consensus (TASC) II C and D aortoiliac stenosis and mortality. 24 Due to liberalization of acceptance criteria for kidney transplantation, we are confronted with increasing age of transplant candidates and with an increasing burden of comorbidities. Although it pays to transplant patients with high comorbidity scores, as they are far better off compared to remaining on the waiting list, 25 this may also lead to a growing number of kidney transplant candidates presenting with aortoiliac stenosis. 26 Thus, it is important to investigate whether this vascular condition is associated with a higher risk of CVE's in order to optimize preexisting cardiovascular disease and cardiovascular monitoring after transplantation. 27 Therefore, we performed a retrospective cohort study with the following objectives: firstly, to evaluate whether preexisting aortoiliac stenosis is associated with CVE's after kidney transplantation and secondly, to identify other factors associated with post‐transplant CVE's.
2. MATERIALS AND METHODS
2.1. Study design and population
A retrospective, single‐center, cohort study was conducted at the Erasmus University Medical Center in Rotterdam, The Netherlands. The cohort included adult kidney transplant recipients, transplanted between January 1, 2000 and December 31, 2016, who had undergone contrast‐enhanced imaging in the pre‐transplantation work‐up to assess the presence of aortoiliac stenosis. The indication for contrast‐enhanced imaging was not determined by a standardized protocol, but the decision was made by the transplant surgeon based on coexistence of risk factors for vascular disease. High‐risk patients had, for example, a long history of smoking or dialysis, symptoms of systemic vascular disease or multiple comorbidities such as diabetes and cerebrovascular disease. Grading of aortoiliac stenosis was standardized according to the TASC II classification. This classification takes the extent of aortoiliac stenosis into account with TASC II A being the least and TASC II D being the most extensive aortoiliac disease. 28 A significant stenosis was defined as a stenosis with a minimum of 50% lumen narrowing. In order to achieve a satisfactory anastomosis of the graft artery with the iliac arterial system, some patients with significant aortoiliac stenosis required a pre‐transplant intervention. Based on the TASC II classification, the decision was made for percutaneous transluminal angioplasty with or without stenting, endarterectomy or vascular bypass as described in our previous study regarding aortoiliac stenosis. 24 If the stenosis was unilateral and asymptomatic, the donor kidney was often transplanted on the contralateral side without further treatment. Patients without aortoiliac stenosis or with less than 50% lumen narrowing were used as controls.
2.2. Data sources and ethical approval
A clinical database containing all patients who received contrast‐enhanced imaging together with electronic patient records containing health administrative data were used to obtain patient characteristics and survival data. Contrast‐enhanced abdominal magnetic resonance angiography, abdominal computed tomography, and conventional angiography were used for the assessment of aortoiliac stenosis. The presence of a significant aortoiliac stenosis was determined by a trained researcher and checked and classified according to the TASC II classification by an interventional radiologist. The following pre‐transplant patient characteristics were collected to correct for potential confounders in the association between aortoiliac stenosis and CVE's based on literature: age, sex, body mass index (BMI), race, smoking status, type of donor, total duration of dialysis (including previous time on dialysis), a history with a cerebrovascular accident (CVA) or transient ischemic attack (TIA), ischemic cardiac disease, dyslipidemia (a positive history or lipid‐lowering therapy prior to transplantation), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), history of peripheral arterial disease (PAD) and obstructive sleep apnea syndrome (OSAS). 6 , 8 , 10 , 11 , 12 , 13 , 14 , 23 , 29 , 30 , 31 , 32 A positive history of PAD included Fontaine stage II‐IV. 33 Ethical approval for this study was obtained from the Ethics Committee of the Erasmus Medical Center University in Rotterdam (MEC‐2019‐0373).
2.3. Outcome measures
The primary outcome was CVE‐free survival, defined as the time between transplantation and the first event, with CVE as a composite endpoint of ischemic heart disease, non‐hemorrhagic cerebrovascular disease and PAD. Ischemic heart disease included myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft and angina pectoris with coronary angiography showing stenosis or with medication as treatment for coronary artery disease which resolved the angina pectoris. Non‐hemorrhagic cerebrovascular disease included non‐hemorrhagic stroke, TIA and amaurosis fugax. Ischemic cardiovascular death or death with an unknown cause also counted as a CVE. Secondary outcomes were the risk of a CVE within 90 days and patient survival.
2.4. Statistical analyses
Descriptive statistics consisted of demographic and clinical characteristics. Continuous variables were presented with mean and standard deviation (SD) since means were normally distributed in case of a sample size greater than thirty according to the central limit theorem. Frequencies and corresponding percentages were used for categorical variables. The unpaired t‐test was used for differences between continuous variables and chi‐square test for categorical variables. If categorical variables had an expected count of less than five, Fisher's exact test was used. Fisher‐Freeman‐Halton exact test was used in case variables had more than two categories and an expected count below five. Cumulative CVE‐free survival and patient survival were illustrated with Kaplan‐Meier curves and these were compared with the log‐rank test. To correct for confounding, multivariate cox proportional hazards regression analysis was used for CVE‐free survival. Patients without an event were censored at the last follow‐up date. The proportional hazard assumption for variables in the final model was tested visually with log minus log plots for categorical variables and scatterplots of residuals for continuous variables. For the risk of a CVE within 90 days, logistic regression analysis was used. Patients without an event and with the last follow‐up date within 90 days were excluded from this analysis. For model development of both the cox regression model and logistic regression model, potential confounders were first tested in univariate analysis and were then included in multivariate analysis if the P‐value was ≤.2. To reach the final model, backward stepwise selection of variables was used and models were compared with likelihood ratio tests. Screening for two‐way interactions between each of the variables took place with Bonferroni correction. Missing values were handled with a complete case analysis. IBM SPSS statistics software version 25 was used for the statistical analyses. A P value less than .05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
In the period studied, 373 kidney transplant recipients had undergone pre‐transplant contrast‐enhanced imaging with a median time between imaging and transplantation of 4.7 months (interquartile range (IQR) 1.4–10.4). Median follow‐up time after transplantation was 7.2 years (IQR 5.1–11.1). Complete case analysis for missing values excluded six patients reducing the total number of patients included to 367. Eighty‐nine patients (24.3%) had significant aortoiliac stenosis and were classified according to the TASC II classification: 52 TASC II A, 19 TASC II B, 11 TASC II C and 7 TASC II D. A pre‐transplant vascular intervention was needed in 20 patients with aortoiliac stenosis. Two hundred seventy‐eight patients (75.7%) without significant stenosis were used as controls. Table 1 gives an overview of patient characteristics. Patients with aortoiliac stenosis were significantly older at the time of transplantation (stenosis 63.9 (SD 8.5) years, controls 58.3 (SD 13.4) years; P < .001), had more often a smoking history (never smoked: stenosis 13.5%, controls 35.3%; P < .001), a history of symptomatic PAD (stenosis 46.1%, controls 15.5%; P < .001) and ischemic cardiac disease (stenosis 48.3%, controls 32.4%; P = .006). Other baseline characteristics were not significantly different.
TABLE 1.
Characteristics of kidney transplant recipients
| Overall (N = 367) | TASC II stenosis (N = 89) | Control (N = 278) | |||||
|---|---|---|---|---|---|---|---|
| Variables | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | P‐value |
|
Year of KTx 2000–2008 2009–2016 |
96 (26.2) 271 (73.8) |
26 (29.2) 63 (70.8) |
70 (25.2) 208 (74.8) |
.451 | |||
| Age at KTx in years | 59.7 (12.6) | 63.9 (8.5) | 58.3 (13.4) | < .001 a | |||
|
Sex Male Female |
250 (68.1) 117 (31.9) |
64 (71.9) 25 (28.1) |
186 (66.9) 92 (33.1) |
.378 | |||
| BMI at KTx in kg/m2 | 26.4 (4.7) | 25.9 (4.7) | 26.5 (4.6) | .266 | |||
|
Race Europe South America Middle East North Africa Africa Asia North America |
263 (71.7) 53 (14.4) 17 (4.6) 14 (3.8) 10 (2.7) 9 (2.5) 1 (.3) |
65 (73.0) 13 (14.6) 5 (5.6) 2 (2.2) 3 (3.4) 1 (1.1) 0 (.0) |
198 (71.2) 40 (14.4) 12 (4.3) 12 (4.3) 7 (2.5) 8 (2.9) 1 (.4) |
.905 | |||
|
Smoking status Never smoked Currently smoking Quit smoking |
110 (30.0) 83 (22.6) 174 (47.4) |
12 (13.5) 29 (32.6) 48 (53.9) |
98 (35.3) 54 (19.4) 126 (45.3) |
< .001 a | |||
| COPD | 36 (9.8) | 11 (12.4) | 25 (9.0) | .353 | |||
| CVA/TIA | 54 (14.7) | 18 (20.2) | 36 (12.9) | .092 | |||
| DM | 133 (36.2) | 36 (40.4) | 97 (34.9) | .342 | |||
| OSAS | 16 (4.4) | 2 (2.2) | 14 (5.0) | .376 | |||
| Dyslipidemia | 164 (44.7) | 46 (51.7) | 118 (42.4) | .127 | |||
| Peripheral arterial disease | 84 (22.9) | 41 (46.1) | 43 (15.5) | < .001 a | |||
| Ischemic cardiac disease | 133 (36.2) | 43 (48.3) | 90 (32.4) | .006 a | |||
| Time between imaging and KTx in years | .60 (.64) | .51 (.60) | .63 (.65) | .118 | |||
|
Cause of ESRD Diverse b Hypertension DM Polycystic kidney disease Glomerulonephritis Autoimmune |
117 (31.9) 89 (24.3) 87 (23.7) 42 (11.4) 19 (5.2) 13 (3.5) |
22 (24.7) 29 (32.6) 22 (24.7) 11 (12.4) 5 (5.6) 0 (.0) |
95 (34.2) 60 (21.6) 65 (23.4) 31 (11.2) 14 (5.0) 13 (4.7) |
.069 | |||
|
Type of donor Living Deceased |
226 (61.6) 141 (38.4) |
54 (60.7) 35 (39.3) |
172 (61.9) 106 (38.1) |
.840 | |||
| KTx in the past | 35 (9.5) | 13 (14.6) | 22 (7.9) | .061 | |||
|
Dialysis Preemptive HD PD Both; HD and PD |
64 (17.4) 190 (51.8) 62 (16.9) 51 (13.9) |
11 (12.4) 48 (53.9) 20 (22.5) 10 (11.2) |
53 (19.1) 142 (51.1) 42 (15.1) 41 (14.7) |
.203 | |||
| Time on dialysis, inclusive the past in months | 30.1 (29.9) | 33.3 (31.5) | 29.0 (29.4) | .243 | |||
Abbreviations: TASC, Trans‐Atlantic Inter‐Society Consensus; SD, standard deviation; KTx, kidney transplantation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; TIA, transient ischemic attack; DM, diabetes mellitus; OSAS, obstructive sleep apnea syndrome; ESRD, end‐stage renal disease; HD, hemodialysis; PD, peritoneal dialysis.
P‐value < .05.
Diverse includes IgA nephropathy, vesico‐ureteral reflux, congenital disorders, vascular disease and infections.
3.2. Cardiovascular event‐free survival
The last follow‐up date was June 30, 2020. A total of 174 first events had occurred, with 55 events in the stenosis group (61.8% of all patients with stenosis) and 119 in the control group (42.8% of all patients without stenosis). Figure 1A illustrates the cumulative CVE‐free survival of patients with and without pre‐transplant aortoiliac stenosis. Patients with aortoiliac stenosis had worse CVE‐free survival compared to patients without stenosis (log‐rank test P < .001). Median CVE‐free survival was 4.5 years (95% confidence interval (CI) 2.8‐6.2) in patients with any stenosis and 8.9 years (95% CI 6.8–11.0) in patients without stenosis. Figure 1B shows the Kaplan‐Meier curve stratified according to the TASC II classification, which showed a clear effect of severity with inferior CVE‐free survival in patients with TASC II B (median CVE‐free survival 4.9 years (95% CI 0.00–10.23); P = .007), C (median CVE‐free survival 1.9 years (95% CI 0.20–3.55); P < .001) and D (median CVE‐free survival 0.11 years (95% CI .00–.30); P < .001) compared to the controls. Significance remained after Bonferroni correction for four tests (significance level set at .013). Outcomes of the composite cardiovascular endpoint are presented in Table 2, showing that ischemic heart disease was the most frequent cardiovascular event in both groups.
FIGURE 1.
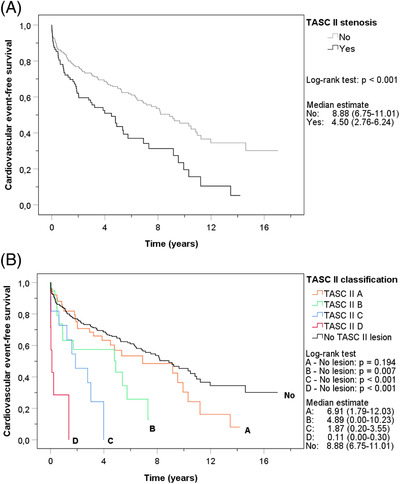
Kaplan‐Meier curves of cumulative cardiovascular event‐free survival. (A). Cardiovascular event‐free survival of patients with stenosis and patients without stenosis. (B). Cardiovascular event‐free survival of patients with different TASC II lesions and patients without stenosis
TABLE 2.
Separate outcomes of the composite cardiovascular endpoint
| Cardiovascular events | Overall (N = 174) N (%) | TASC II stenosis (N = 55) N (%) | Control (N = 119) N (%) | P‐value |
|---|---|---|---|---|
|
Ischemic heart disease |
92 (52.6) |
26 (46.4) |
66 (55.5) |
.311 |
| Non‐hemorrhagic cerebrovascular disease | 25 (14.3) | 7 (12.5) | 18 (15.1) | |
| Peripheral arterial disease | 58 (33.1) | 23 (41.1) | 35 (29.4) |
3.3. Cox proportional hazards regression model
To investigate whether the association of aortoiliac stenosis with CVE's would remain significant when corrected for various confounders, multivariate cox regression analysis was performed (Table 3). The following potential confounders were selected based on a P value ≤.2 on univariate analysis: the presence of aortoiliac stenosis, age, sex, race, smoking status, COPD, CVA/TIA, DM, dyslipidemia, PAD and ischemic cardiac disease. In the final model, having TASC II C or D aortoiliac stenosis was a significant, independent risk factor for a CVE with a hazard ratio (HR) of 2.15 (95% CI 1.05–4.38) and 6.56 (95% CI 2.74–15.70), respectively. Other risk factors that remained significant in the final model were age (HR 1.03; 95% CI 1.01–1.04), smoking status (currently smoking: HR 2.01; 95% CI 1.27–3.18), CVA/TIA (HR 1.56; 95% CI 1.05–2.31), PAD (HR 1.57; 95% CI 1.09–2.25) and ischemic cardiac disease (HR 1.63; 95% CI 1.18–2.24). None of these variables in the final model were time‐dependent (Figures S1 and S2). Interaction terms were not significant after correction for multiple testing.
TABLE 3.
Univariate and multivariate cox proportional hazards regression model analysis of cardiovascular event‐free survival in kidney transplant recipients (N = 367)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P‐value | HR | 95% CI | P‐value |
|
Stenosis No TASC II A TASC II B TASC II C TASC II D |
Ref 1.33 2.23 3.73 11.52 |
– (.87–2.01) (1.25–3.97) (1.87–7.44) (4.92–26.99) |
< .001 b .188 a .007 b < .001 b < .001 b |
Ref .82 1.06 2.15 6.56 |
– (.53–1.28) (.57–1.99) (1.05–4.38) (2.74–15.70) |
< .001 b – .382 .853 .036 b < .001 b |
| Age at KTx per year | 1.04 | (1.02–1.05) | < .001 b | 1.03 | (1.01–1.04) | < .001 b |
|
Sex Female Male |
Ref 1.42 |
– (1.01–1.98) |
– .041 b |
– – |
– – |
– – |
| BMI per kg/m2 | .99 | (.96–1.02) | .514 | – | – | – |
|
Race Caucasian Non‐Caucasian |
Ref .68 |
– (.48–.98) |
– .037 b |
– – |
– – |
– – |
|
Smoking status Never smoked Currently smoking Quit smoking |
Ref 2.24 1.94 |
– (1.44–3.47) (1.31–2.86) |
.001 b – < .001 b .001 b |
Ref 2.01 1.39 |
– (1.27–3.18) (.92–2.09) |
.011 b – .003 b .118 |
| COPD | 1.89 | (1.22–2.92) | .004 b | – | – | – |
| CVA/TIA | 1.90 | (1.29–2.81) | .001 b | 1.56 | (1.05–2.31) | .027 b |
| DM | 1.56 | (1.14–2.13) | .006 b | – | – | – |
| OSAS | .85 | (.35–2.08) | .724 | – | – | – |
| Dyslipidemia | 1.53 | (1.13–2.06) | .006 b | – | – | – |
| Peripheral arterial disease | 2.36 | (1.71–3.24) | < .001 b | 1.57 | (1.09–2.25) | .015 b |
| Ischemic cardiac disease | 1.98 | (1.46–2.68) | < .001 b | 1.63 | (1.18–2.24) | .003 b |
|
Type of donor Living Deceased |
Ref 1.19 |
– (.87–1.63) |
– .284 |
– – |
– – |
– – |
| Time on dialysis, inclusive the past per month | 1.00 | (1.00–1.01) | .968 | – | – | – |
Abbreviations: HR, hazard ratio; CI, confidence interval; TASC, Trans‐Atlantic Inter‐Society Consensus; KTx, kidney transplantation; Ref, reference; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; TIA, transient ischemic attack; DM, diabetes mellitus; OSAS, obstructive sleep apnea syndrome.
P‐value ≤ .2.
P‐value < .05.
3.4. Logistic regression model
A total number of 36 events occurred in the 90‐day postoperative period. Eight patients were lost to follow‐up in the 90‐day postoperative period without the occurrence of an event and were therefore excluded. Univariate and multivariate logistic regression analysis were performed to identify risk factors for a CVE within 90 days (Table 4). The presence of a stenosis, age, sex, race, CVA/TIA, dyslipidemia, PAD, ischemic cardiac disease, and type of donor were included in the multivariate analysis due to a P value ≤.2 on univariate analysis. The final model consisted of TASC II D stenosis as a risk factor for a CVE within 90 days after transplantation with an odds ratio (OR) of 17.70 (95% CI 3.16–99.19). Other significant risk factors in the final model were age (OR 1.05; 95% CI 1.00–1.09), male sex (OR 3.45; 95% CI 1.13–10.56), dyslipidemia (OR 2.51; 95% CI 1.14–5.55), and type of donor (deceased: OR 2.74; 95% CI 1.25–5.98). No significant interaction terms were found.
TABLE 4.
Univariate and multivariate logistic regression analysis for 90‐days cardiovascular event (N = 359)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P‐value | OR | 95% CI | P‐value |
|
Stenosis No TASC II A TASC II B TASC II C TASC II D |
Ref .90 .57 2.30 25.83 |
– (.30–2.71) (.07–4.49) (.47–11.24) (4.76–140.35) |
.003 b – .849 .597 .305 < .001 b |
Ref .68 .47 2.43 17.70 |
– (.21–2.14) (.06–3.82) (.46–12.99) (3.16–99.19) |
.010 b – .504 .476 .298 .001 b |
| Age at KTx per year | 1.06 | (1.02–1.10) | .003 b | 1.05 | (1.00–1.09) | .041 b |
|
Sex Female Male |
Ref 4.13 |
– (1.43–11.98) |
– .009 b |
Ref 3.45 |
– (1.13–10.56) |
– .030 b |
| BMI per kg/m2 | .97 | (.90–1.05) | .495 | |||
|
Race Caucasian Non‐Caucasian |
Ref .47 |
– (.19–1.17) |
– .106 a |
– – |
– – |
– – |
|
Smoking status Never smoked Currently smoking Quit smoking |
Ref 1.00 1.46 |
– (.36–2.82) (.64–3.34) |
.564 – .996 .368 |
– – – |
– – – |
– – – |
| COPD | 1.14 | (.38–3.42) | .820 | – | – | – |
| CVA/TIA | 1.77 | (.76–4.12) | .188 a | – | – | – |
| DM | 1.28 | (.63–2.57) | .497 | – | – | – |
| OSAS | 2.17 | (.59–8.00) | .245 | – | – | – |
| Dyslipidemia | 2.75 | (1.33–5.69) | .006 b | 2.51 | (1.14–5.55) | .023 b |
| Peripheral arterial disease | 2.34 | (1.14–4.82) | .021 b | – | – | – |
| Ischemic cardiac disease | 1.94 | (.97–3.87) | .062 a | – | – | – |
|
Type of donor Living Deceased |
Ref 2.17 |
– (1.08–4.35) |
– .029 b |
Ref 2.74 |
– (1.25–5.98) |
– .011 b |
| Time on dialysis, inclusive the past per month | 1.00 | (.98–1.01) | .535 | – | – | – |
Abbreviations: OR, odds ratio; CI, confidence interval; TASC, Trans‐Atlantic Inter‐Society Consensus; KTx, kidney transplantation; Ref, reference; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; TIA, transient ischemic attack; DM, diabetes mellitus; OSAS, obstructive sleep apnea syndrome.
P‐value ≤ .2.
P‐value < .05.
3.5. Patient survival
A total of 158 patients died; 55 in the stenosis group and 103 in the control group. Figure 2A shows the Kaplan‐Meier survival curves of patients with and without stenosis. Kidney transplant recipients with any stenosis had significantly inferior survival compared to patients with no TASC II lesion (log‐rank test P < .001). The median patient survival of patients with stenosis was 6.4 years (95% CI 4.3–8.6), whereas the median survival of the control group was almost twice as long (median survival 12.0 years (95% CI 10.3–13.6). Figure 2B demonstrates the differences between the Kaplan‐Meier patient survival curves of the various TASC II lesions. Patient survival of patients with a TASC II A stenosis did not differ from the survival of patients without a TASC II lesion (P = .303). Patients with TASC II B, C or D stenosis had a significantly inferior survival compared to the controls (P = .003, P < .001, and P < .001, respectively). The results remained significant after Bonferroni correction for four tests (significance level .013).
FIGURE 2.
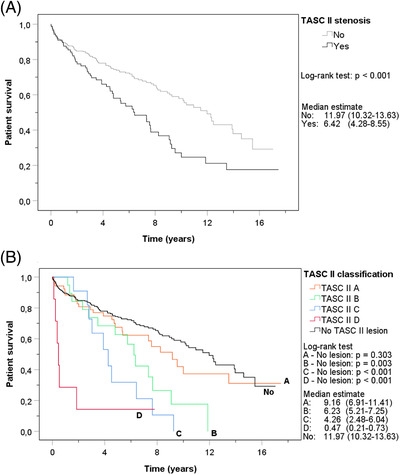
Kaplan‐Meier curves of cumulative patient survival with all‐cause mortality as event. (A). Patient survival for stenosis and no stenosis. (B). Patient survival for different TASC II lesions and no stenosis
4. DISCUSSION
This study demonstrated that the presence of a TASC II C or D stenosis was a risk factor for cardiovascular events after kidney transplantation. The association remained significant even after adjustment for various confounders, proving an independent association between TASC II C/D lesions and cardiovascular events. The increased cardiovascular risk could be explained by the presence of systemic atherosclerosis in these patients. 22 Other significant independent risk factors associated with post‐transplant CVE were increasing age, smoking, CVA/TIA, PAD and ischemic cardiac disease; these have been demonstrated in other studies to be well‐known risk factors for CVE's. 6 , 10 , 11 , 12 , 13 , 14 , 29 , 30 The presence of a TASC II D stenosis was also found to be associated with a short‐term risk of CVE within 90 days post‐transplantation. In addition, age, male sex, dyslipidemia and deceased donor transplantation were significant risk factors for a CVE within 90 days.
One unexpected finding was that neither the univariate nor the multivariate analysis showed time on dialysis to be associated with CVE's, independent of including dialysis time as a continuous or categorical parameter within the model. Time on dialysis has proven to be an important non‐traditional risk factor for CVE's in kidney transplant recipients in prior studies. 6 , 10 , 11 , 12 The reason why we did not observe this may be due to a low incidence of preemptive transplanted patients combined with a high incidence of CVE's in this high‐risk cohort, which could have affected the association. An interesting predictor of 90‐day CVE was a deceased donor transplant compared to a living donor kidney transplantation. Consistent with Ponticelli et al., 12 type of donor was not a significant predictor of CVE's on the long term after transplantation. The increased risk of deceased donor transplantation on short‐term CVE has been shown in previous studies without explanation. 30 , 31 , 34 One explanation for this finding could be that unfavorable clinical and socioeconomic factors were overrepresented in patients who received a deceased donor kidney transplant. 35 Our present study corrected only partially for these factors. Also, post‐transplant factors may have contributed to an increased CVE risk after deceased donor kidney transplantation. Graft survival 24 , 36 and renal function 36 are worse after deceased donor kidney transplantation compared to living donor transplantation, which are associated with cardiovascular mortality and events. 6 , 37 , 38 Finally, it may be that recipients of a deceased donor kidney transplantation could have had their pre‐transplant cardiovascular screening less recently than recipients of living donor transplantation, which could have resulted in more CVE's shortly after transplantation.
Different authors found an association between the prevalence of CVE's and PAD in kidney transplant recipients or patients on dialysis, 23 , 30 , 39 but studies about aortoiliac stenosis in particular are lacking. We recently showed that the presence of TASC II C or D stenosis was an independent risk factor for mortality. 24 Other studies on aortoiliac vascular disease mainly focused on aortoiliac calcification instead of stenosis, possibly because patients with stenosis are less often transplanted. A meta‐analysis indicated inferior prognosis in patients with any degree of aortoiliac calcification. 40 A recently published article investigated the association between aortoiliac calcification score and cardiovascular events. 41 They found a significant and independent association between calcification score and cardiovascular events with a HR of 2.04 (95% CI, 1.20–3.45). Five year cardiovascular event‐free survival was about 75% in the group with the highest calcification score. Cardiovascular event‐free survival in our study was worse, which probably means that stenotic vascular disease indicates a more advanced stage of disease.
The strength of our study, compared to other studies on aortoiliac vascular disease, was that our analysis was stratified on severity of stenosis. This way, we could show that severe TASC II lesions have more impact on cardiovascular risk. A disadvantage of the retrospective design of this study was documentation source–if cardiovascular events were not registered or not registered comprehensively enough, the event could not be included in the outcome. Furthermore, post‐transplant risk factors were not included, because this study aimed to find pre‐transplant cardiovascular risk factors. Nonetheless, exclusion of these variables does not mean post‐transplant risk factors did not influence the risk of CVE's in our cohort. Delayed graft function, graft failure and rejection episodes after transplantation are associated with CVE's 8 , 10 , 30 , 42 as well as post‐transplant DM, which has been described as a cardiovascular risk factor in multiple studies. 11 , 14 , 15 , 29 , 30 , 43 Additionally, immunosuppressive agents used after transplantation, in particular calcineurin inhibitors and steroids, increase cardiovascular risk. 44 However, the same immunosuppression regimen including tacrolimus, mycophenolate mofetil and steroids was used for all patients. Another limitation of this study was selection bias. As mentioned before, patients included in the study had undergone contrast‐enhanced imaging because of a high a priori probability of aortoiliac stenosis, which means our study population consisted of patients with high numbers of comorbidities and cardiovascular risk factors. In addition, it is important to realize that our control group did not only consist of patients without stenosis, but also of patients with less than 50% stenosis, such as calcifications. The 5‐year patient survival rate of our control group was worse than the 5‐year survival rate of all Erasmus MC patients transplanted between 2010 and 2015, namely 75% compared to 86%. 45 This finding supports the hypothesis that selection bias played an important role leading to overrepresentation of cardiovascular risk factors in our control cohort. Despite our high‐risk population, the independent risk of TASC II C and D stenosis could be determined because of our large cohort with enough events making adjustment possible for many confounders.
Currently, the Framingham Risk Score is often used to predict cardiac events, 46 but the usability in kidney transplant recipients has been controversial. 47 , 48 We believe that transplant‐specific risk factors should be added to risk models for the kidney transplant population in order to prevent underestimation of cardiovascular risk. Improvement of prognostic powers have been achieved by studies moving beyond the Framingham Risk Score, but some refinement is still needed. 47 In our study, the presence of a TASC II C or D lesion had the highest hazard ratio in the multivariate model, indicating the strength of the association and the importance of this additional risk factor in pre‐transplantation risk prediction.
Our previous article found that aortoiliac stenosis with TASC II C or D lesions was associated with uncensored graft loss, although not with death‐censored graft loss. 24 According to our current study findings, TASC II C and D lesions are also significant risk factors for post‐transplant CVE's, but TASC II A and B lesions, and on short‐term also TASC II C, are not independently associated with CVE's. Yet, aortoiliac vascular disease is the main reason for kidney transplant ineligibility. 49 A survey of Rijkse et al. 50 among transplant surgeons found that 29.8% of all respondents considered the increased mortality risk because of cardiovascular comorbidity as the most important concern in case of transplanting a patient with severe aortoiliac vascular disease. However, the present study showed that aorto‐iliac stenosis is only an independent risk factor for CVE's in case of TASC II C or D disease. Therefore, the intention should be to accept patients with TASC II A or B disease for kidney transplantation if there are no technical limitations. Close collaboration with vascular surgeons could help in accepting these patients for transplantation. On the other hand, patients with TASC II C or D stenosis deserve extra attention in the cardiovascular work‐up because of worse CVE‐free survival as well as patient survival. Especially the serious impaired patient survival raises ethical concerns and doubts about proceeding with transplantation. Factors that may contribute to the inferior outcomes of this subgroup of kidney transplant recipients are the cardiovascular toxicity of various immunosuppressive agents and the high prevalence of frailty (17.1%), which is associated with increased mortality, morbidity and hospital costs. 51 , 52 Comprehensive cardiovascular screening with multiple procedures 27 as well as changes in immunosuppression regimen such as avoidance or withdrawal of steroids 53 or replacement of tacrolimus by belatacept 54 are options which could be considered for patients with extensive aortoiliac stenosis, although long‐term evidence is currently lacking. Strategies to improve pre‐transplant frailty status should be investigated with randomized controlled trials to assess whether these could improve outcomes in this specific group of high‐risk transplant patients. Multidisciplinary team meetings including surgeons, nephrologists and cardiologists are recommended for these patients at high risk. Discussion of surgical‐technical aspects as well as the possibilities of pre‐, peri,‐ or post‐transplant risk management could lead to correct decision‐making concerning patients with TASC II C or D stenosis.
5. CONCLUSIONS
Preexisting aortoiliac TASC II A and B lesions were not independently associated with inferior cardiovascular outcomes after kidney transplantation. Therefore, these patients do not need additional cardiovascular monitoring. The presence of TASC II C or D aortoiliac stenosis was independently associated with post‐transplant CVE's. Successful cardiovascular risk management is needed pre‐, peri,‐ and post‐transplantation for patients with TASC II C or D aortoiliac stenosis.
AUTHOR CONTRIBUTIONS
Shabnam Babakry: participated in research design, data collection, performance of the research, data analysis and writing of the manuscript. Elsaline Rijkse: participated in research design, data collection, performance of the research, data analysis and writing of the manuscript. Joke I. Roodnat: participated in critical review of the manuscript. Diederik C. Bijdevaate: participated in data collection and critical review of the manuscript. Jan N.M. IJzermans: participated in critical review of the manuscript. Robert C. Minnee: participated in research design, critical review of the manuscript and supervision of the project.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
SUPPORTING INFORMATION
Babakry S, Rijkse E, Roodnat JI, Bijdevaate DC, IJzermans JNM, Minnee RC. Risk of post‐transplant cardiovascular events in kidney transplant recipients with preexisting aortoiliac stenosis. Clin Transplant. 2022;36:e14515. 10.1111/ctr.14515
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093‐2109. [DOI] [PubMed] [Google Scholar]
- 2. Jansz TT, Bonenkamp AA, Boereboom FTJ, et al. Health‐related quality of life compared between kidney transplantation and nocturnal hemodialysis. PLoS One. 2018;13(9):e0204405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schold JD, Buccini LD, Goldfarb DA, et al. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol. 2014;9(10):1773‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rangaswami J, Mathew RO, Parasuraman R, et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant. 2019;34(5):760‐773. [DOI] [PubMed] [Google Scholar]
- 5. Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol. 2006;17(3):900‐907. [DOI] [PubMed] [Google Scholar]
- 6. Pilmore H, Dent H, Chang S, et al. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89(7):851‐857. [DOI] [PubMed] [Google Scholar]
- 7. Aakhus S, Dahl K, Wideroe TE. Cardiovascular disease in stable renal transplant patients in norway: morbidity and mortality during a 5‐yr follow‐up. Clin Transplant. 2004;18(5):596‐604. [DOI] [PubMed] [Google Scholar]
- 8. Kasiske BL. Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med. 1988;84(6):985‐992. [DOI] [PubMed] [Google Scholar]
- 9. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16‐S23. [PubMed] [Google Scholar]
- 10. de Mattos AM, Prather J, Olyaei AJ, et al. Cardiovascular events following renal transplantation: role of traditional and transplant‐specific risk factors. Kidney Int. 2006;70(4):757‐764. [DOI] [PubMed] [Google Scholar]
- 11. Vanrenterghem YF, Claes K, Montagnino G, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 12. Ponticelli C, Villa M, Cesana B, et al. Risk factors for late kidney allograft failure. Kidney Int. 2002;62(5):1848‐1854. [DOI] [PubMed] [Google Scholar]
- 13. Kasiske BL. Epidemiology of cardiovascular disease after renal transplantation. Transplantation. 2001;72:S5‐S8. [DOI] [PubMed] [Google Scholar]
- 14. Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82(5):603‐611. [DOI] [PubMed] [Google Scholar]
- 15. Gill JS. Cardiovascular disease in transplant recipients: current and future treatment strategies. Clin J Am Soc Nephrol. 2008;3:S29‐S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Droupy S, Eschwege P, Hammoudi Y, et al. Consequences of iliac arterial atheroma on renal transplantation. J Urol. 2006;175:1036‐1039. [DOI] [PubMed] [Google Scholar]
- 17. Nanmoku K, Watarai Y, Narumi S, et al. Surgical techniques and procedures for kidney transplant recipients with severe atherosclerosis. Exp Clin Transplant. 2017;15(6):594‐601. [DOI] [PubMed] [Google Scholar]
- 18. Yuan XP, Gao W, Jiao WH, et al. Kidney transplantation in diabetic recipients with iliac atherosclerosis: arterial anastomosis with nakayama's ring pin stapler after endarterectomy. Int J Urol. 2012;19(4):336‐342. [DOI] [PubMed] [Google Scholar]
- 19. Cheng H, Clymer JW, Po‐Han Chen B, et al. Prolonged operative duration is associated with complications: a systematic review and meta‐analysis. J Surg Res. 2018;229:134‐144. [DOI] [PubMed] [Google Scholar]
- 20. Tozzi M, Franchin M, Soldini G, et al. Treatment of aortoiliac occlusive or dilatative disease concomitant with kidney transplantation: how and when?. Int J Surg. 2013;11:S115‐S119. [DOI] [PubMed] [Google Scholar]
- 21. Devereaux PJ, Goldman L, Cook DJ, et al. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura E, Sato Y, Iwakiri T, et al. Asymptomatic plaques of lower peripheral arteries and their association with cardiovascular disease: an autopsy study. J Atheroscler Thromb. 2017;24(9):921‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y, Ning Y, Shang W, et al. Association of peripheral arterial disease with all‐cause and cardiovascular mortality in hemodialysis patients: a meta‐analysis. BMC Nephrol. 2016;17(1):195‐016‐0397‐0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rijkse E, Kimenai HJAN, Roodnat JI, et al. Impact of aortoiliac stenosis on graft and patient survival in kidney transplant recipients using the TASC II classification. Transplantation. 2019;103(10):2164‐2172. [DOI] [PubMed] [Google Scholar]
- 25. Laging M, Kal‐van Gestel JA, van de Wetering J, et al. A high comorbidity score should not be a contraindication for kidney transplantation. Transplantation. 2016;100(2):400‐406. [DOI] [PubMed] [Google Scholar]
- 26. Lindner A, Charra B, Sherrard DJ, et al. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290(13):697‐701. [DOI] [PubMed] [Google Scholar]
- 27. Palepu S, Prasad GV. Screening for cardiovascular disease before kidney transplantation. World J Transplant. 2015;5(4):276‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norgren L, Hiatt WR, Dormandy JA, et al. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33:S1‐S75. [DOI] [PubMed] [Google Scholar]
- 29. Seoane‐Pillado MT, Pita‐Fernández S, Valdés‐Cañedo F, et al. Incidence of cardiovascular events and associated risk factors in kidney transplant patients: a competing risks survival analysis. BMC Cardiovasc Disord. 2017;17(1):72‐017‐0505‐0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496‐506. [DOI] [PubMed] [Google Scholar]
- 31. Yazbek DC, de Carvalho AB, Barros CS, et al. Cardiovascular disease in early kidney transplantation: comparison between living and deceased donor recipients. Transplant Proc. 2012;44(10):3001‐3006. [DOI] [PubMed] [Google Scholar]
- 32. Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta‐analysis of prospective cohort studies. Atherosclerosis. 2013;229(2):489‐495. [DOI] [PubMed] [Google Scholar]
- 33. Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21(5‐6):499‐533. [PubMed] [Google Scholar]
- 34. Guimarães J, Araújo AM, Santos F, et al. Living‐donor and deceased‐donor renal transplantation: differences in early outcome–A single‐center experience. Transplant Proc. 2015;47(4):958‐962. [DOI] [PubMed] [Google Scholar]
- 35. Roodnat JI, Laging M, Massey EK, et al. Accumulation of unfavorable clinical and socioeconomic factors precludes living donor kidney transplantation. Transplantation. 2012;93(5):518‐523. [DOI] [PubMed] [Google Scholar]
- 36. Schwarz A, Scheffner I, Catzikyrkou C, et al. Differences between live and deceased‐donor kidney transplantation in a centre performing protocol biopsies. Transpl Int. 2013;26:44‐44. [Google Scholar]
- 37. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde M, et al, Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375(9731):2073‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296‐1305. [DOI] [PubMed] [Google Scholar]
- 39. Patel SI, Chakkera HA, Wennberg PW, et al. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc Med. 2017;22(3):225‐230. [DOI] [PubMed] [Google Scholar]
- 40. Rijkse E, van Dam JL, Roodnat JI, et al. The prognosis of kidney transplant recipients with aorto‐iliac calcification: a systematic review and meta‐analysis. Transpl Int. 2020;33(5):483‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benjamens S, Rijkse E, Te Velde‐Keyzer CA, et al. Aorto‐iliac artery calcification prior to kidney transplantation. J Clin Med. 2020;9(9):E2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jardine AG, Fellström B, Logan JO, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the assessment of lescol in renal transplantation (ALERT) study. Am J Kidney Dis. 2005;46(3):529‐536. [DOI] [PubMed] [Google Scholar]
- 43. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early‐diagnosed new‐onset post‐transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69(3):588‐595. [DOI] [PubMed] [Google Scholar]
- 44. Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol. 2019;32(3):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eurotransplant . Patient survival analysis application V1.5 2017. Eurotransplant Web site. https://prd.txnet.eu/SurvivalCurves/index.jsp Accessed: 10/12, 2020.
- 46. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837‐1847. [DOI] [PubMed] [Google Scholar]
- 47. Mansell H, Stewart SA, Shoker A. Validity of cardiovascular risk prediction models in kidney transplant recipients. ScientificWorldJournal. 2014;2014:750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herzog AL, Kalogirou C, Wanner C, et al. Comparison of different algorithms for the assessment of cardiovascular risk after kidney transplantation by the time of entering waiting list. Clin Kidney J. 2019;13(2):150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kianda MN, Wissing KM, Broeders NE, et al. Ineligibility for renal transplantation: prevalence, causes and survival in a consecutive cohort of 445 patients. Clin Transplant. 2011;25(4):576‐583. [DOI] [PubMed] [Google Scholar]
- 50. Rijkse E, Kimenai HJAN, Dor FJMF, et al. Screening, management, and acceptance of patients with aorto‐iliac vascular disease for kidney transplantation: a survey among 161 transplant surgeons. Eur Surg Res. 2021: 1–8. [DOI] [PubMed] [Google Scholar]
- 51. Quint EE, Zogaj D, Banning LBD, et al. Frailty and kidney transplantation: a systematic review and meta‐analysis. Transplant Direct. 2021;7(6):e701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng XS, Myers JN, Chertow GM, et al. Prehabilitation for kidney transplant candidates: is it time?. Clin Transplant. 2017;31(8). [DOI] [PubMed] [Google Scholar]
- 53. Haller MC, Royuela A, Nagler EV, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016(8):CD005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Masson P, Henderson L, Chapman JR, et al. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014;2014(11):CD010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


