Abstract
The error‐related negativity (ERN) and the error positivity (Pe) are electrophysiological components associated with error processing that are thought to exhibit distinctive developmental trajectories from childhood to adulthood. To investigate the age and age moderation effects on the ERN and the Pe strength during development, we conducted a preregistered three‐level meta‐analysis synthesizing 120 and 41 effect sizes across 18 group comparison studies and 19 correlational studies, respectively. The meta‐analysis included studies with mean age between 3.6 and 28.7 (min‐max age range: 3.5 and 49.8) years for age‐group comparisons and 6.1 to 18.7 (min‐max age range: 4.0–35.7) years for age correlations. Results showed that age was associated with a more negative ERN (SMD = −.433, r = −.230). No statistically significant association between age and the Pe was found (SMD = .059, r = −.091), except for in a group comparison between younger and older adolescents. The age effects were not significantly moderated by whether a Flanker or a Go/No‐Go task was used, whereas a probabilistic learning task moderated the age effect on the Pe. Moreover, the Fz and Cz electrode sites yielded stronger negative associations between age and the ERN and the Pe, respectively. The results confirm that the ERN and the Pe show differential development courses and suggest that sample and methodological characteristics influence the age effects, and lay the foundation for investigations of developmental patterns of the ERN and the Pe in relation to psychopathology and early genetic and environmental risk factors.
Keywords: adolescence, childhood, development, error‐related negativity, error positivity, meta‐analysis
1. INTRODUCTION
Childhood and adolescence are periods of dramatic developmental changes in body and behavior, including changes in the brain and cognitive functions. A hallmark feature of neurocognitive development during this period is that various cognitive functions develop at different speeds, with the continued development of complex cognitive control subprocesses into young adulthood (Crone & Steinbeis, 2017; Downes et al., 2017; Overbye et al., 2021). These include implicit and explicit subprocesses, such as error processing, that become increasingly efficient during childhood and adolescence (Overbye et al., 2019). Making mistakes is an ideal opportunity to adjust our behavior and to learn. Thus, error signals play an important role in many models of learning, where operations such as detection and reaction to errors are essential parts of an efficient learning system (Cavanagh & Frank, 2014; Rumelhart et al., 1986; Schultz, 2015).
From a neural perspective, specific electrophysiological signals are produced when a person makes an error. These signals have been found to strengthen across childhood and adolescence (Grammer et al., 2014; Overbye et al., 2019; Tamnes et al., 2013), which are developmental periods that are important for learning and adaptation (Dahl et al., 2018; Peters & Crone, 2017). However, childhood and adolescence also represent windows of vulnerability for the onset of many neurodevelopmental and mental disorders (Dalsgaard et al., 2020). Characterizing and understanding age‐related differences in neural error processes signals may deepen our understanding of typical cognitive neurodevelopment and inform studies of atypical developmental patterns implicated in psychopathology or associated with risk factors. The present meta‐analysis aimed to examine the effects of age on two specific electrophysiological components associated with error processing, the error‐related negativity (ERN), and the error positivity (Pe), and to investigate to what extent sample characteristics and methodological differences between studies influence the age effects during typical development. Two distinct event‐related potentials (ERPs) can be detected using electroencephalography (EEG) by time‐locking the averaged electrocortical response of the brain to errors of commission on a cognitive task. The ERN and the Pe are reliable electrophysiological components that can be detected within milliseconds following an error, such as pushing the wrong button during a cognitive task (Davies et al., 2001; Gehring et al., 1993, 2018; Nieuwenhuis et al., 2001). The ERN is a sharp negative deflection in the EEG that can be observed 50–100 ms after an error of commission. Its maximum voltage is found at frontocentral electrodes and it is reported to originate from a network of regions that include posterior and anterior cingulate cortex, (pre‐) supplementary motor area, and medial prefrontal areas (Agam et al., 2011; Edwards et al., 2012; Herrmann et al., 2004; Ladouceur et al., 2007; Miltner et al., 2003; van Veen & Carter, 2002a; see Gehring et al., 2012 for an overview). There is an ongoing debate regarding the functional significance of the ERN. Some posit that it is involved in processing cognitive conflict (Botvinick et al., 2001, 2004; Carter & van Veen, 2007), while others consider the ERN to be part of action evaluation when the outcome of an action is worse than expected (Holroyd & Coles, 2002), or as a neural index of a trait reflecting sensitivity to internal threat (Weinberg et al., 2012). In addition, the ERN has also been linked to frontal midline theta activity (4–8 Hz), possibly indicating that the ERN (time‐domain) and the increased power in the theta frequency band (time‐frequency domain) observed during error processing reflect the same process (Yeung et al., 2007), related processes (Luu et al., 2004), or complementary neural signatures of error processing (Munneke et al., 2015).
The Pe is a positive deflection that peaks around 200–500 ms after an erroneous response. Its maximum voltage is found at centroparietal electrodes and it is reported to originate from the cingulate cortex (Herrmann et al., 2004; Ladouceur et al., 2007; van Veen & Carter, 2002b; Vocat et al., 2008), and has been suggested to reflect error awareness (Ficarella et al., 2019; Nieuwenhuis et al., 2001). The functional role of the Pe is, however, also debated, with some arguing that the Pe might also reflect the motivational significance (Overbeek et al., 2005) or subjective/emotional evaluation of making an error (Falkenstein et al., 2000).
Despite the debate regarding the exact functions of the ERN and the Pe, both are likely to be involved in an error processing system by signaling a need to adjust behavior to adapt or improve future performance. Arguably, this makes the ERN and the Pe important neural markers of cognitive development during periods of rapid learning and adaptation, such as childhood and adolescence. Indeed, a larger ERN amplitude has been associated with better academic grades in undergraduates (Hirsh & Inzlicht, 2010), whereas a larger Pe amplitude has been associated with better academic achievement in children (Kim et al., 2016). Concomitantly, atypical error processing has been reported in autism (e.g., Henderson et al., 2006), schizophrenia (e.g., Simmonite et al., 2012), ADHD (e.g., Liu et al., 2020), anxiety (e.g., Hanna et al., 2020), and obsessive‐compulsive disorder (e.g., Carrasco, Harbin, et al., 2013). Although the role of the Pe in anxiety is unclear (Ladouceur et al., 2006; McDermott et al., 2009; Meyer et al., 2012), the ERN seems to be particularly connected to anxiety disorders (Meyer, 2016, 2017; Olvet & Hajcak, 2008; Riesel, 2019), possibly reflecting variation in internal threat sensitivity (Weinberg et al., 2016). Specifically, the ERN is increased in children and adolescents with anxiety (Hanna et al., 2020; Hum et al., 2013; Ladouceur et al., 2006; Meyer et al., 2013, 2017) and obsessive‐compulsive disorder (Carrasco, Harbin, et al., 2013; Carrasco, Hong, et al., 2013; Hanna et al., 2012). Furthermore, recent studies have also demonstrated the importance of examining the development of error processing in relation to psychopathology, as internalizing symptoms could partially mediate age‐related differences of the ERN (Meyer et al., 2018). In addition, a longitudinal study showed that greater ERN in adolescence moderates higher levels of adult internalizing psychopathology among individuals who were behaviorally inhibited as infants (Tang et al., 2020). Taken together, the links between the ERN and the Pe and both academic performance and psychopathology in childhood and adolescence emphasize the importance of characterizing the typical developmental patterns of these ERP components and their individual variation.
During development, the ERN and the Pe get stronger with age. Whereby the ERN shows prolonged development across adolescence (Lo, 2018; Tamnes et al., 2013), and the Pe plateaus before adolescence (Davies et al., 2004a, 2004b; Overbye et al., 2019). However, some developmental studies have reported findings that deviate from these patterns (Eppinger et al., 2009; Richardson et al., 2011), and it remains unclear whether such inconsistent findings are due to differences in sample characteristics, such as age or sex, or methodological differences. For example, the experimental tasks used in these developmental studies vary, which could explain variation in latency and/or amplitude of the ERN and the Pe. Furthermore, there is also considerable variability in how the ERN and the Pe are measured, including variation in electrode site or brain topography (e.g., using one electrode, averaging across multiple electrodes, or decomposing brain activity). Finally, the strength of the ERP components might also be subject to different quantification methods (e.g., peak, mean amplitude, residuals, mean difference between correct, and error trials). Thus, sample and methodological differences between studies may potentially moderate the age effects on ERN and Pe.
To better characterize and understand age‐related differences in ERN and Pe strength, we conducted a preregistered meta‐analysis to examine the effects of age and potential moderating factors of the age effects on the ERN and the Pe. As stated in our preregistration (Boen et al., 2020), we expected that the ERN magnitude would increase with age, but not to be moderated by age, which would indicate a linear and continuous development across childhood and adolescence. We also expected that the Pe magnitude increased with age, whereas this would be moderated by age, reflecting developmental increases during middle childhood, followed by stabilization around late childhood. Moreover, we aimed to explore the moderating effects of sex, experimental task, measurement, and quantification method on the age effect sizes on the ERN and the Pe magnitude.
2. METHOD
A protocol for this systematic literature search and meta‐analysis was published on August 6, 2020 (Boen et al., 2020) following the Preferred Reporting Items Reviews and Meta‐Analyses Protocols (PRISMA‐P) guidelines (Moher et al., 2015). The systematic search was conducted on August 10, 2020 using PubMed and Scopus. A completed PRISMA 2020 checklist (Page et al., 2021) for the current meta‐analysis can be found in Supplementary Appendix S1.
2.1. Search strategy and selection criteria
A systematic literature search included the following search phrase: (“ERN” OR “error‐ related negativity” OR “error negativity” OR “Pe” OR “error positivity”) AND (“child*” OR “adolescen*” OR “youth”) AND (“EEG” OR “electroencephalography” OR “ERP” OR “event‐related potential”). The eligibility criteria were: (1) original studies in English, (2) published in peer‐reviewed journals, (3) that reports the ERN/Pe in at least two cross‐sectional or longitudinal age groups or the correlation coefficient with age, (4) in children and/or adolescents (with participants <18 years of age), and (5) with no reported mental or neurodevelopmental disorders. As the eligible studies include studies reporting age‐group differences and studies reporting correlations with age, we will refer to these as group studies and correlational studies, respectively.
The systematic search resulted in 480 hits in PubMed and 481 hits in Scopus, adding up to a total of 961 articles. After the removal of duplicates, 559 were left, and 4 more were removed as they were non‐articles (e.g., conference poster). Thus, 555 abstracts were included for screening. From these, 184 full‐text articles were screened for inclusion, whereby 147 articles were excluded, and 37 articles were included in the quantitative analyses in this meta‐analysis. The studies that were excluded and placed in the “not typical development/methodology” category reported: (1) age correlations across both typical and non‐typically developing children and/or adolescents; (2) age included as a covariate in a multiple regression analysis or multifactorial ANOVA – and not the raw correlation between age and the ERN/Pe or mean and standard deviation of the ERN/Pe in each age group; (3) effects of age not tested despite including a healthy control group within the developmental age‐range. Studies that included both typical and atypical participants were excluded from analyses unless they reported separate results for the typically developing group only. The authors of the studies that were excluded were not contacted. Authors (R.B. and C.K.T.) evaluated the studies for inclusion in collaboration. R.B. collected and coded the data from each study. See Figure 1 for a flowchart of the selection process.
FIGURE 1.
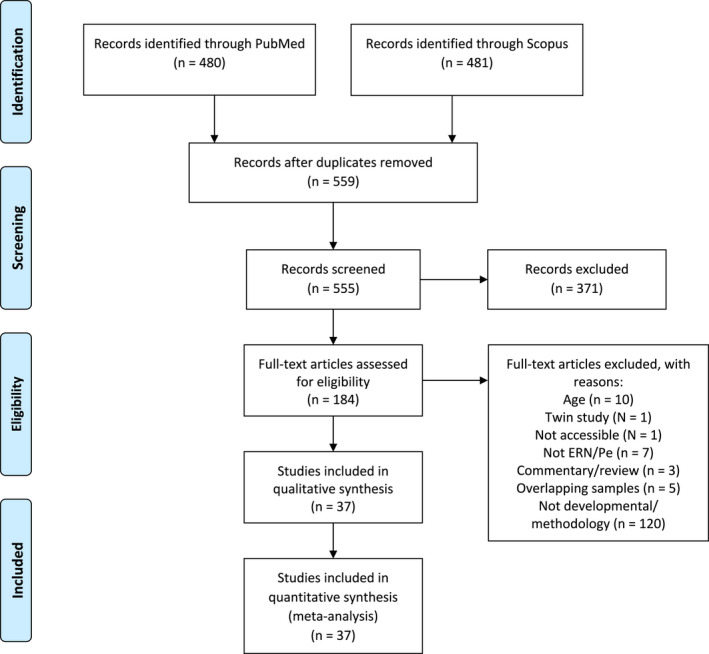
Flow diagram of the selection process
2.2. Data extraction
For the age group studies, the mean value and standard deviation for each ERP component (i.e., either peak values, mean amplitude, difference wave form, or residuals) and N in each group were extracted. For correlation studies, mean age and the r correlation coefficient between the ERP component and age were extracted. As we also wanted to investigate the moderating roles of sex, experimental task, electrode site (s), quantification method of the ERP components, we also extracted proportion of male participants included, experimental task used, electrodes used for estimation, and quantification method of the ERP components. The moderating variables were extracted from all eligible studies. For the included studies, we also contacted the corresponding authors to request data that could not be found in the publication.
2.3. Data quality, small study bias, and publication bias
Each eligible study underwent a modified version of the checklist for EEG research (Keil et al., 2014). The checklist included 17 items that were scored 0, 1, or 2 if the items were either not, partially, or fully explained in the study. The checklist and scoring procedure are provided in Supplementary Appendix S2. We calculated the statistical power of each study and reported the median statistical power to assess the evidential value of the eligible studies using the “metameta” R package (Quintana, 2020) along with the overall summary effect size estimate. To assess the possibility for small study bias, which can include publication bias, we created and inspected contour‐enhanced Funnel plot and performed Egger’s regression test. The effect sizes underwent a correction for publication bias to get an estimate of corrected effect size using the “PublicationBias” package (Mathur & VanderWeele, 2020), under the assumption that significant results are five times more likely to get published compared to non‐significant results and that the effects of age on the ERN group and correlational studies and the Pe group studies favor negative and positive estimates, respectively. For the Pe correlational studies, the model was conducted favoring negative estimates as the model did not converge favoring positive estimates. For the significant point estimates, we conducted a sensitivity analysis to get an estimate of the amount of publication bias that needs to be present in order to get a point estimate with confidence intervals that include zero.
2.4. Deviation from protocol
Initially, we did not determine which type of experimental conditions should be included in the meta‐analysis. Some of the reviewed studies reported data from social conditions (Barker et al., 2018) and audience conditions (Kim et al., 2005). These studies also reported data from non‐social and alone conditions; we included data from the latter studies to reduce heterogeneity across the included studies. We also initially aimed to account for experimental design (i.e., cross‐sectional vs. longitudinal studies), however, the included studies that reported longitudinal data were few (four group studies and one correlational study) and the inclusion of this factor did not improve model fit (Supplementary Tables S1–S3). As such, this random factor was not included in the final multilevel analytic model. Thus, the final three‐level meta‐analytic model accounted for (i) variance of the effect sizes, (ii) between effect sizes from the same study, and (iii) between studies (Assink & Wibbelink, 2016).
The moderating effect of sex was estimated based on the proportion of males (in %), and not by calculating effect sizes separately for each sex as originally planned because ERP measures for male and female participants were typically not reported. The reported statistics for task difficulty were also highly heterogeneous across studies. That is, the studies reported different estimations of task accuracy (e.g., mean error rate, false alarm rates, overall accuracy, and accuracy on specific trials). This precluded any meaningful estimation of task difficulty or its potential moderating effects. Finally, we aimed to use the puniform package to assess publication bias (van Aert & van Assen, 2018), however, due to the multilevel structure of the current data, we used the “PublicationBias” R package to allow for clustering of point estimates (Mathur & VanderWeele, 2020). Here, this involves a sensitivity analysis to get an estimate of how much the point estimate could be attenuated given an estimated publication bias (e.g., significant results are five times more likely to be published compared to non‐significant results). Moreover, it will also provide an estimate of how severe the publication bias might be before the point estimates are non‐significant.
2.5. Statistical analysis
All statistical analyses were performed in R statistical software version 4.0.3 using the meta (Schwarzer, 2007) and metafor (Viechtbauer, 2010) packages. Due to variation in samples and methodology, we used random‐effects models with a restricted maximum‐likelihood estimator. For the group studies, the raw effect sizes were estimated from the mean and standard deviation for each age group. Here, we used standardized mean difference (SMD) as measure of effect size. In this case, the younger group was compared to the older group. Thus, a negative SMD indicates that the older group had a lower mean value than the younger group. To illustrate, a negative SMD will indicate that the older group had a more negative ERN or a less positive Pe amplitude compared to the younger group. This will also apply for the main effect (i.e., β0) in the moderation analysis. For the correlation studies, the Pearson’s r underwent a Fisher’s z‐transformation and were back‐transformed to Pearson’s r when reporting the overall age effect. The effect sizes were calculated using the “escalc” function in the metaphor R package (Viechtbauer, 2010) to get estimates of Hedges’ g (Hedges, 1981) for the group studies and z‐scores (Fisher, 1921) for the correlational studies. To assess heterogeneity, which is inter‐study variation in the true effect sizes, the I 2 ‐statistic was estimated to indicate how much of the observed variation could be attributed to the true effect sizes. As many of the studies contributed to the quantitative synthesis with more than one effect size, several of the effect sizes are likely to be correlated. To account for the statistical dependency in the data, we computed a three‐level meta‐analytic model to account for both within and between study variance (Assink & Wibbelink, 2016). The use of a three‐level meta‐analytical model provides three variance levels; that is, within‐study sampling variation (), between effect size variation () and between‐study variation (). The estimated variance of the true effects will be estimated and referred to as for the second level variance and for the third level variance. Finally, the Q‐statistic was estimated to indicate if the effect sizes between studies are statistically different from each other.
2.6. Moderation analysis
The moderators were defined a priori (Boen et al., 2020), and included age, sex, experimental task, electrode site, and quantification method. The group studies provided mean estimates of the ERN and the Pe for each age group. Thus, to estimate the effect size across age, the mean value for the ERN and the Pe must be compared between two different age groups. To investigate the effect of age during development, the age groups were categorized and compared against each other, where the younger age group was always compared against the older age group (i.e., to examine how the ERN and the Pe amplitudes changes with increasing age). Further, to examine the moderating effect of age, the age groups had to be divided into age categories that reflects increasing age. Thus, for the age group comparisons, the age groups were placed into three age categories: Children <12 years of age, Adolescents 12–18 years of age, adults >18 years of age. The age groups were placed into their respective age category based on the mean age of the study group that was used for group comparisons in each study. For instance, if a study reported the ERN and/or the Pe for a group with a mean age below 12 years of age, this group was placed in the child category. Moreover, for the age group comparisons, the effect sizes were estimated for children (difference between an older child group and a younger child group), children, and adolescents (difference between a child group and an adolescent group), children and adults (difference between a child group and an adult group), adolescents (difference between a younger adolescent group and an older adolescent group), and adolescents and adults (difference between an adolescent group and an adult group). In the moderation analysis, we tested whether the age‐effects obtained for each of the age groups deviated from that of a reference category (i.e., the mean effect obtained from comparing children versus adults). For the correlation analyses, the mean age of the respective sample was used in the moderation analysis. Few studies reported the ERN and/or the Pe separately for male and female participants, thus it was impossible to include a categorical variable of sex in the analysis. However, to investigate the moderation effect of sex, we computed sex as a continuous measure where the proportion of male participants in the study was included in the analysis. The majority of the studies used either the flanker task or the go/no‐go task to measure the ERN and the Pe (96.97% of the effect sizes for group studies; 80.49% of the correlational studies). We therefore dummy coded the experimental task for Flanker task, Go/No‐Go‐task, and other. The “other” category included tasks such as the probabilistic learning task, lexical decision task, letter discrimination task, choice reaction time task, and the Simon task. Electrode site (including brain topography) was dummy coded for Fz, FCz, Cz, Pz, multiple electrodes, and principal component analysis (PCA). The quantification method was dummy coded as mean amplitude (estimated across several time points within a defined time window for the component of interest), residuals (residuals after regressing out the data obtained from correct trials), delta (mean difference waveform estimation is based on the difference in correct and error trials, commonly reported as ΔERN and ΔPe) and peak amplitude (either peak to peak, baseline to peak, or the most negative/positive peak within a defined time window for the component of interest). The dummy coding is mutually exclusive and will result in one redundant variable, one of which will be used as the reference group (i.e., the intercept). In a meta‐regression where only dummy coding applies, a significant effect of the intercept (i.e., reference) will indicate that the mean effect of the reference group significantly deviates from zero, while a significant effect of the other included categories indicates that the mean effect deviates from that of the reference condition.
3. RESULTS
Of the 37 eligible studies, 18 studies included group difference data in ERN/Pe (with a total of 120 effect sizes) and 19 studies included correlation data between ERN/Pe and age (with a total of 41 effect sizes). An overview of the included studies is shown in Table 1.
TABLE 1.
Overview of included studies
| Study | n | Age range | % males | Experimental task | Electrode site | Quantification method |
|---|---|---|---|---|---|---|
| Group Studies | ||||||
| Brooker (2018) | 119 | 3.5–4.5 | 42 | Go/No‐Go | FCz | Delta |
| Checa et al. (2014) | 50 | 4.0–25.5 | 50 | Flanker | FCz, Cz | Mean Amplitude |
| Clawson et al. (2017) | 97 | 8.0–28.0 | 58 | Flanker | PCA | Mean Amplitude |
| DuPuis et al. (2015) | 234 | 5.2–7.5 | 65 | Go/No‐Go | Fz | Peak |
| Eppinger et al. (2009) | 35 | 10.0–24.0 | 47 | Probabilistic Learning Task (Other) | FCz, Cz | Peak |
| Mean Amplitude | ||||||
| Grammer et al. (2018) | 49 | 5.0–6.0 | 51 | Go/No‐Go | FCz, CZ, Pz, | Mean Amplitude |
| Horowitz‐Kraus (2011) | 46 | 13.0–26.0 | 52 | Lexical Decision Task (Other) | Cz, Fz | Peak |
| Kim et al. (2005) | 20 | 7.0–11.0 | 35 | Go/No‐Go | Fz, Cz, Pz | Peak |
| Kim et al. (2007) | 22 | 7.0–25.0 | 50 | Go/No‐Go | Cz | Peak |
| Ladouceur et al. (2004) | 11 | 9.0–17.0 | 36 | Flanker | Cz | Peak |
| Ladouceur et al. (2007) | 46 | 8.7–49.8 | 39 | Flanker | Cz, Pz | Peak, Delta |
| Meyer et al. (2014) | 70 | 8.0–15.0 | 57 | Flanker | Fz, Cz | Mean Amplitude, Delta |
| Richardson et al. (2011) | 77 | 7.0–9.0 | 44 | Flanker | FCz | Peak |
| Santesso and Segalowitz (2008) | 74 | 15.0–20.0 | 100 | Flanker, Go/No‐Go | Fz, FCz, Cz, Pz | Peak |
| Santesso et al. (2006) | 67 | 10.0–30.0 | 40 | Flanker | Fz, FCz, Cz, Pz | Peak |
| Meel et al. (2012) | 63 | 6.0–26.0 | 56 | Flanker | Fz, FCz, Cz | Mean Amplitude |
| Wiersema et al. (2007) | 44 | 7.0–24.0 | 59 | Go/No‐Go | Cz, Fz, Pz, Cz | Peak |
| Zhang et al. (2009) | 31 | 7.0–37.0 | NA | Go/No‐Go | Fz, Cz, Pz | Peak |
| Correlational Studies | ||||||
| Barker et al. (2018) | 62 | 8.7–17.1 | 0 | Flanker | Multiple | Mean Amplitude |
| Buzzell et al. (2017) | 43 | 9.9–35.1 | 47 | Flanker | CPz | Delta |
| Danovitch et al. (2019) | 124 | 6.0–8.3 | 49 | Go/No‐Go | FCz, Pz | Mean Amplitude, Delta |
| Gavin et al. (2019)* | 240 | 7.1–25.8 | 46 | Flanker | Cz | Peak |
| Hajcak et al. (2008) | 18 | 8.0–16.0 | 44 | Simon Task (Other) | Fz | Peak |
| Gorday and Meyer (2018) | 99 | 8.0–14.0 | 0 | Go/No‐Go | Fz | Delta |
| Hanna et al. (2012) | 44 | 10.0–18.0 | 50 | Flanker | Cz | Mean Amplitude |
| Hogan et al. (2005) | 23 | 12.0–22.1 | 48 | Choice Reaction Time Task (Other) | FCz | Peak |
| Ip et al. (2019) | 49 | 4.0–9.0 | 47 | Go/No‐Go | FCz | Mean Amplitude |
| Kamijo et al. (2016) | 42 | 8.8–12.6 | 55 | Flanker | FCz | Mean Amplitude |
| Kessel et al. (2016) | 304 | 5.2–7.5 | 57 | Go/No‐Go | Cz | Mean Amplitude, Delta |
| Kessel et al. (2019) | 74 | 8.8–10.7 | 49 | Flanker | Multiple | Delta |
| Ladouceur et al. (2012) | 14 | 7.0–35.7 | 36 | Flanker | FCz | Delta |
| Ladouceur et al. (2018) † | 30 | 9.0–14.0 | 50 | Flanker | FCz | Mean Amplitude, Residual, Delta |
| Liu et al., 2020 | 77 | 8.0–18.0 | 61 | Flanker | FCz, CPz | Mean Amplitude, Delta |
| Overbye et al. (2019) | 98 | 8.3–19.7 | 51 | Flanker | Multiple | Peak, Residual, Delta |
| Padilla et al. (2014) | 38 | 11.0–18.0 | 53 | Letter Discrimination Task (Other) | Fz | Mean Amplitude |
| Taylor et al. (2018) | 69 | 12.0–18.0 | 54 | Flanker | FCz | Mean Amplitude |
| Weinberg et al. (2016) | 515 | 13.6–15.5 | 0 | Flanker | FCz | Delta |
The effect sizes for the ERN and the Pe were derived from the age correlation after latency correction, and includes unpublished data.
Includes ERN‐age correlations for the typically developing group not reported in the published manuscript.
3.1. Checklist for EEG research
All included studies underwent scoring on an EEG checklist (modified from Keil et al., 2014). The percentage of the studies that obtained either “1” or “2” for each item are presented in Figure 2. Full scoring of each study is reported in Supplementary Appendix S2.
FIGURE 2.
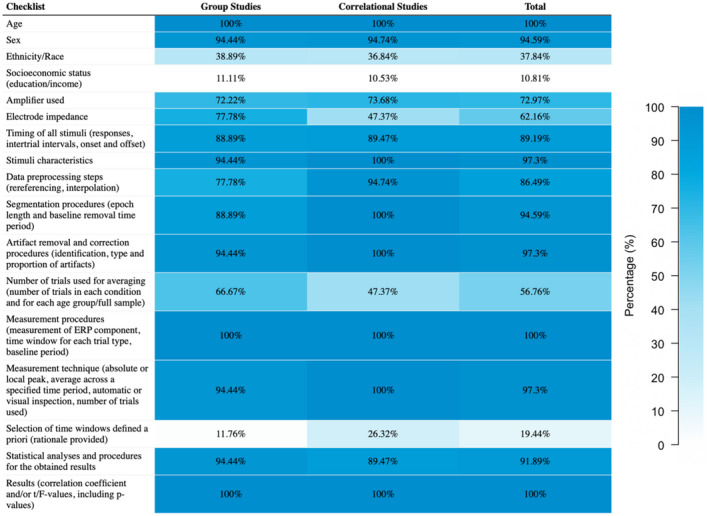
Checklist for the included studies
3.2. Assessment of statistical power
To assess the statistical power of the current meta‐analysis, the median statistical power of the included studies was estimated for a range of true effect sizes and the obtained summary effect size and visualized using a firepower plot (Quintana, 2020). The results showed that the power for ERN group studies and correlation studies was 0.21 and 0.44, respectively, assuming that the summary effect size is the true effect size. The power for the Pe estimates was 0.05 for group studies and 0.13 for correlation studies (Figure 3). The results indicate that most of the included studies that investigate the age‐effect on the ERN and the Pe are underpowered to reliably detect a wide range of age‐effects, especially in studies that are comparing age‐groups. The results of these analyses are also provided in Supplementary Table S4.
FIGURE 3.
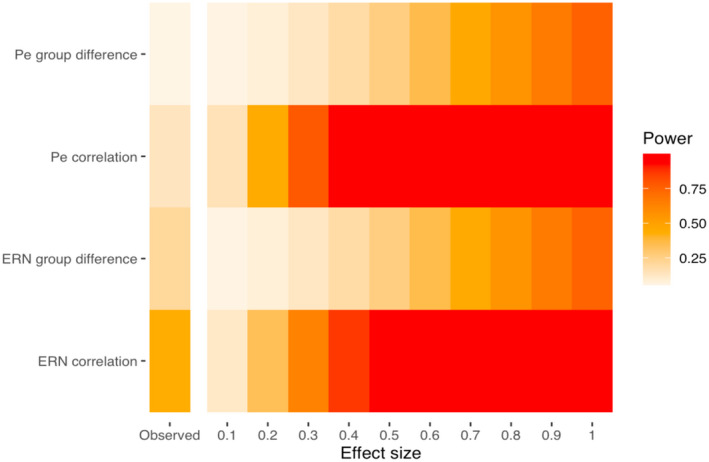
Firepower plot showing the statistical power for each meta‐analysis, assuming a range of effect sizes, and the observed effect size of meta‐analysis is the true effect size
3.3. Test for asymmetry
Contour‐enhanced Funnel plots were created to visually inspect and assess the existence of publication bias (Supplementary Figures S1–S4). Eggers test for asymmetry was not significant for the ERN group studies (z = 1.35, p = .18) nor the Pe group studies (z = −0.09, p = .93). However, the correlational studies for both the ERN (z = 3.02, p = .003) and the Pe showed evidence for asymmetry (z = −3.66, p = .0003), indicating that the studies do exhibit evidence for a small study bias, which may be due to publication bias. In this case, the small study bias indicates that smaller studies among the correlational studies exhibit a different effect size than the larger studies. Although the test for asymmetry was significant only for the correlational studies, the adjusted effect sizes will be presented for both the group and correlational studies to also obtain a more conservative estimate of the age‐effect.
3.4. Dependency and heterogeneity in the data
To account for dependency in the data (i.e., effect sizes drawn from the same study), we computed a three level meta‐analytical model. The variance components were = .317 and = .073 for the ERN group studies and = .000 and = .009 for ERN correlational studies, and = .059 and = .040 for Pe group studies and = .000 and = .065 for Pe correlational studies. The overall variances of the models are shown in Table 2. For the overall three level meta‐analytic model, the Q‐statistic were significant for ERN group studies (Q [df = 70] = 318.34, p < .001) and correlation studies (Q [df = 29] = 46.63, p = .0203), and for the Pe group studies (Q [df = 48] = 99.50, p < .001) and correlation studies (Q [df = 11] = 76.98, p < .0001). The results from the Q‐statistics indicate substantial variability across age‐related effect sizes of the ERN and the Pe. Due to the large amount of dependent effect sizes in the current dataset, a three level meta‐analytic model (i.e., accounting for both within and between study variance) was used in all subsequent analyses for both ERN and Pe.
TABLE 2.
Overall variance
| Variance | Error‐related negativity (ERN) | Error positivity (Pe) | |||
|---|---|---|---|---|---|
| Group studies | Correlational studies | Group studies | Correlational studies | ||
|
|
14.90% | 54.34% | 45.65% | 15.69% | |
|
|
69.16% | 0.00% | 32.44% | 0.00% | |
|
|
15.94% | 45.66% | 21.91% | 84.31% | |
Note: = within study sampling variation, = between effect size variation and = between study variation.
3.5. Overall effect of age
Across all studies reporting age group differences, there was a significant overall effect of age on the ERN (SMD = −.433, CI: −.654, −.212, p < .001; SMD adjusted effect size = −.17, CI = −.34, −.00, p = .048), but not for the Pe (SMD = .059, CI: −.122, .240, p = .515; SMD adjusted effect size = −.04, CI: −.17, .10, p = .539). Forest plots for the ERN and the Pe are shown in Figures 4 and 5, respectively. Similarly, for the correlation data, there was a significant overall effect of age on the ERN (z′ = −.234 CI: −.302, −.166, p < .0001, r = −.230; z′ adjusted effect size = −.16, CI = −.22, −.11, p < .001), but not for the Pe (z′ = −.091, CI: −.334, .153, p = .430, r = −.091; z′ adjusted effect size = .005, CI = −.07, .08, p = .858). Sensitivity analyses indicated that significant results needed to be 5.07‐fold more likely to get published compared to non‐significant results for the confidence interval of the ERN group age‐effect to include zero, whereas no amount of publication bias could attenuate the confidence intervals enough to include zero for the ERN correlational studies. In sum, the sensitivity analyses provide strong evidence favoring an effect of age on the ERN. Thus, the results indicate that the ERN becomes larger with age across childhood and adolescence (i.e., more negative), whereas the Pe does not show a consistent change in magnitude with age across development. Raw effect sizes for each study can be found in Supplementary Tables S5–S8.
FIGURE 4.
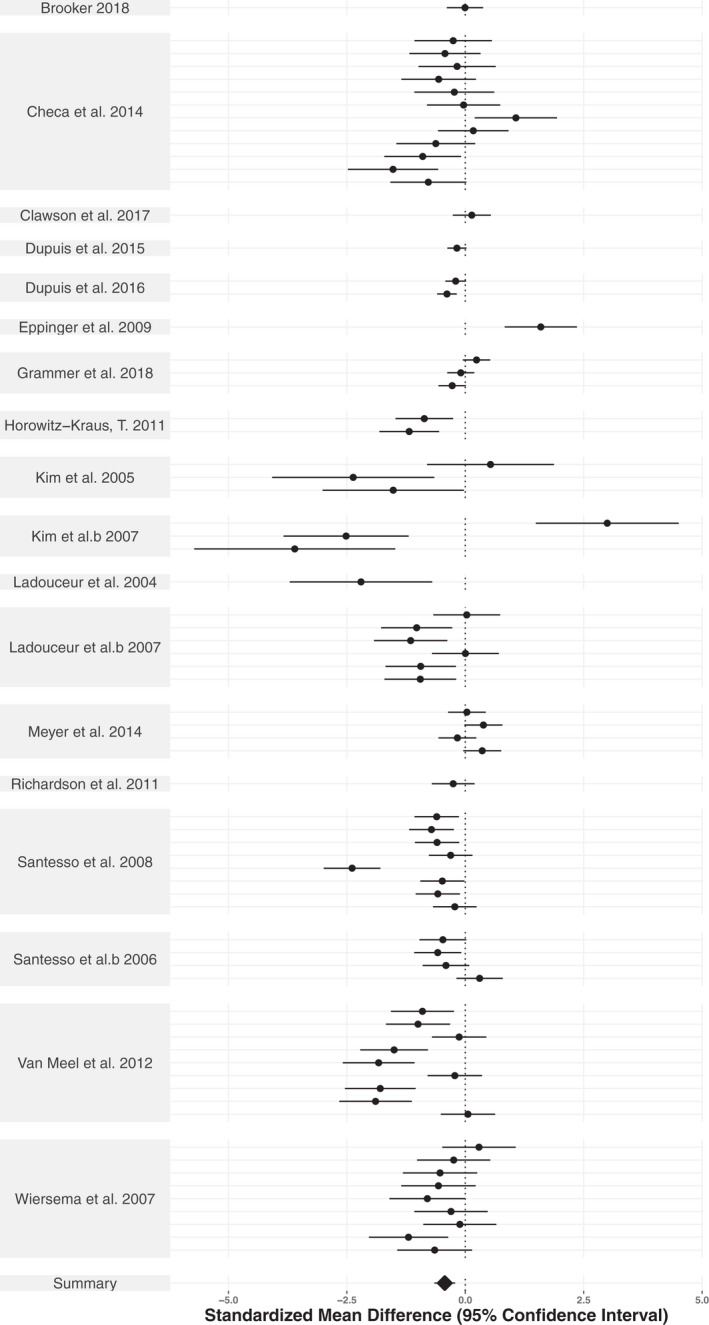
Forest plot illustrating the age‐group effect sizes for the ERN. Standardized mean difference(s) per study is represented by the black dots. The summary standardized mean difference from the three‐level meta‐analytic model is represented as a black diamond. The 95% confidence interval is represented as horizontal lines
FIGURE 5.
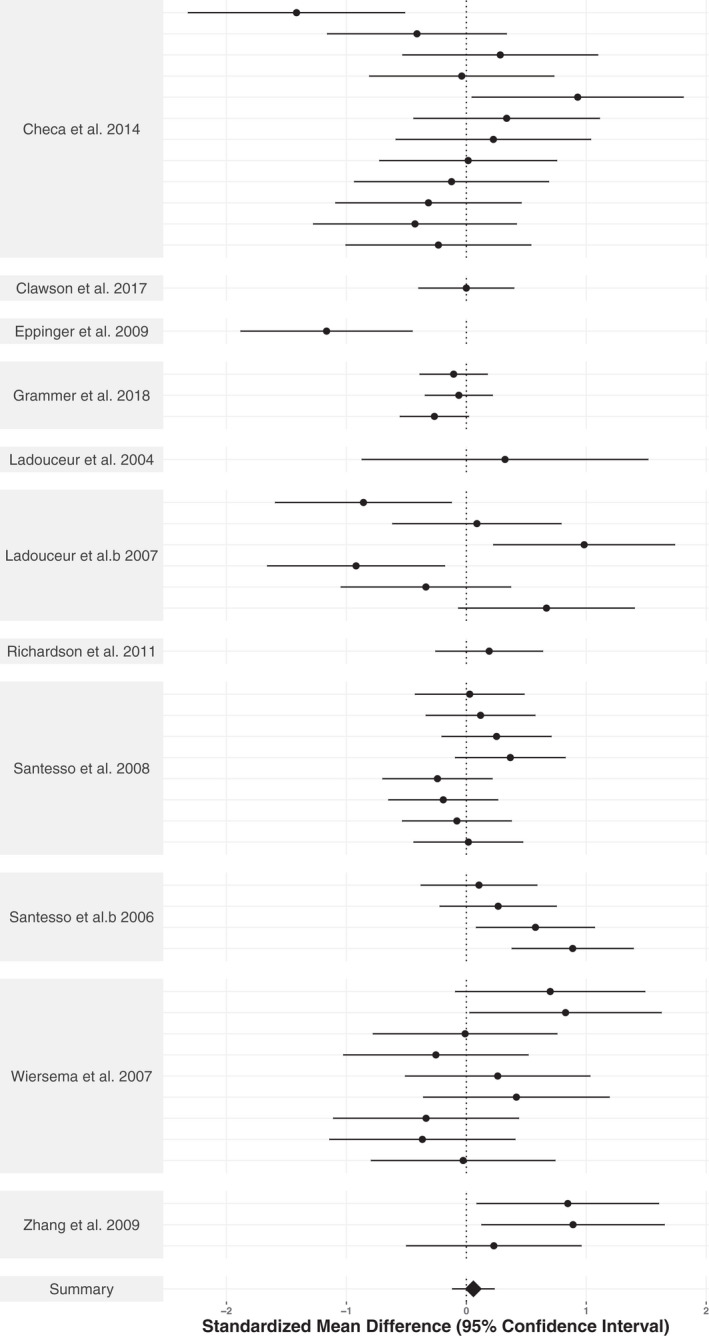
Forest plot illustrating the age‐group effect sizes for the Pe. Standardized mean difference(s) per study is represented by the black dots. The summary standardized mean difference from the three‐level meta‐analytic model is represented as a black diamond. The 95% confidence interval is represented as horizontal lines
3.6. Moderating effects of age and sex
The age effects on the ERN were significant between age groups, where the mean age‐effect of children compared to adults (estimate = −0.498 CI = −.848, −.148), younger compared to older adolescents (estimate = −1.312, CI = −2.234, −.390), and adolescents compared to adults (estimate = −.507 CI = −.888, −.126) deviated from zero. Further, using the mean age‐effect between children and adults as the reference, none of the other age‐group comparisons deviated significantly from the reference (Table 3). For the Pe, only the adolescent group showed a mean age‐effect that deviated from zero (estimate = .947 CI = .291, 1.604) and exhibited a significantly stronger age effect compared to the mean age‐effect between children and adults (Table 4), indicating that group studies that consist of adolescents could moderate the age effect on the Pe. The distributions of the effect sizes across age groups for the ERN and the Pe are shown in Figure 6. For correlational data, the sample mean age did not moderate the age‐effect for the ERN (estimate = −.011, CI = −.032, .009, p = .254), nor for the Pe (estimate = −.025, CI = −.110, .059, p = .521). The inclusion of an age2 term did not reach significance in either of the correlation age models.
TABLE 3.
Moderating age‐group effects on the ERN
| Age groups | β0 (95% CI) | β1 (95% CI) | F (df1, df2) |
|---|---|---|---|
| F (4,66) = 1.355, p = .259 | |||
| Children versus Adults (ref) | −.498 (−.848, −.148)** | ||
| Children | −.329 (−.671, .013) | .168 (−.276, .613) | |
| Children versus Adolescents | −.136 (−.708, .436) | .362 (−.293, 1.016) | |
| Adolescents | −1.312 (−2.234, −.390)** | −.814 (−1.799, .170) | |
| Adolescents versus Adults | −.507 (−.888, −.126)** | −.009 (−.517, .498) |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviation: Ref, reference category.
p < .01.
TABLE 4.
Moderating age‐group effects on the Pe
| Age groups | β0 (95% CI) | β1 (95% CI) | F (df1, df2) |
|---|---|---|---|
| F(4, 44) = 3.952, p = .008 | |||
| Children versus Adults (ref) | −.034 (−.394, .325) | ||
| Children | .210 (−.215, .634) | .244 (−.163, .651) | |
| Children versus Adolescents | −.082 (−.697, .533) | −.048 (−.651, .555) | |
| Adolescents | .947 (.291, 1.604)** | .981 (.269, 1.694)** | |
| Adolescents versus Adults | −.193 (−.595, .210) | −.158 (−.622, .305) |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviation: Ref, reference category.
p < .01.
FIGURE 6.
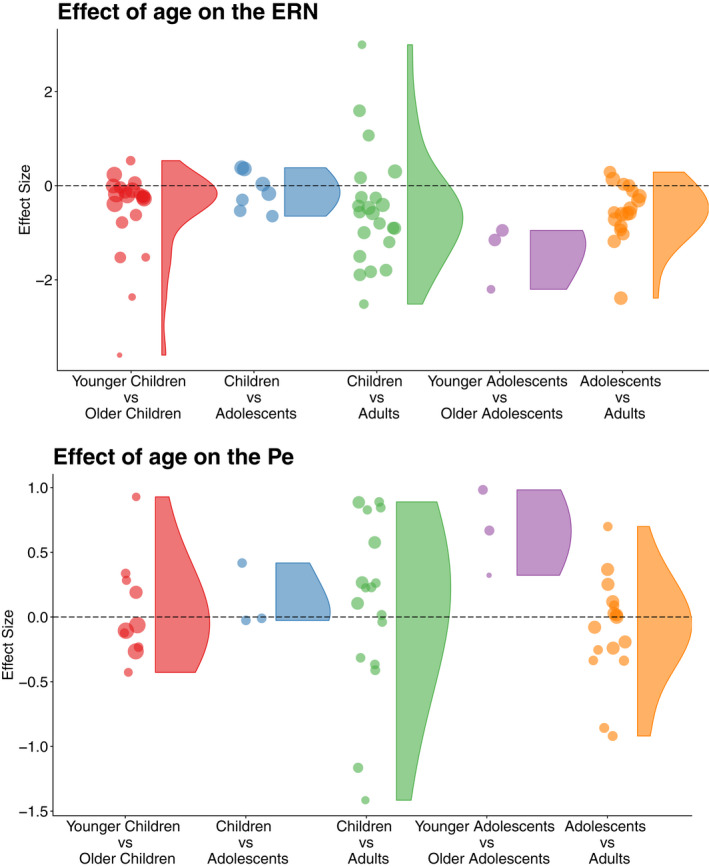
Violin plot of the effect sizes across age groups for the ERN (top) and the Pe (bottom). The dots represent individual effect sizes and the size represents the variation (i.e., larger dots indicate less variation). Age ranges, ERN: Younger Children (3.6–8.1) versus Older Children (4.6–11.0), Children (7.9–11.0) versus Adolescence (12.7–13.4), Children (5.1–11.4) versus Adults (20.8–26.5), Younger Adolescents (12.2–12.4) versus Older Adolescents (15.8–16.5), Adolescence (12.4–16.5) versus Adults (18.5–28.7). Pe: Younger Children (5.1–8.1) versus Older Children (6.3–11.0), Children (7.9) versus Adolescents (13.4), Children (5.1–11.4) versus Adults (20.8–26.5), Younger Adolescents (12.2–12.4) versus Older Adolescents (15.8–16.5), Adolescence (12.4–16.5) versus Adults (18.5–28.7). The age ranges are based on the mean age from the age group that was derived from each of the included studies
Finally, the results did not show any significant moderating effect of sex (i.e., proportion of males) for the ERN group studies (estimate = −.001, CI = −.013, .011, p = .859), ERN correlational studies (estimate = .001, CI = −.003, .004, p = .677), Pe group studies (estimate = −.001, CI = −.009, .008, p = .864), or the Pe correlational studies (estimate = 0.00, CI = −.014, .014, p = .990). The z‐transformed correlations for the ERN and the Pe are shown in Figure 7.
FIGURE 7.
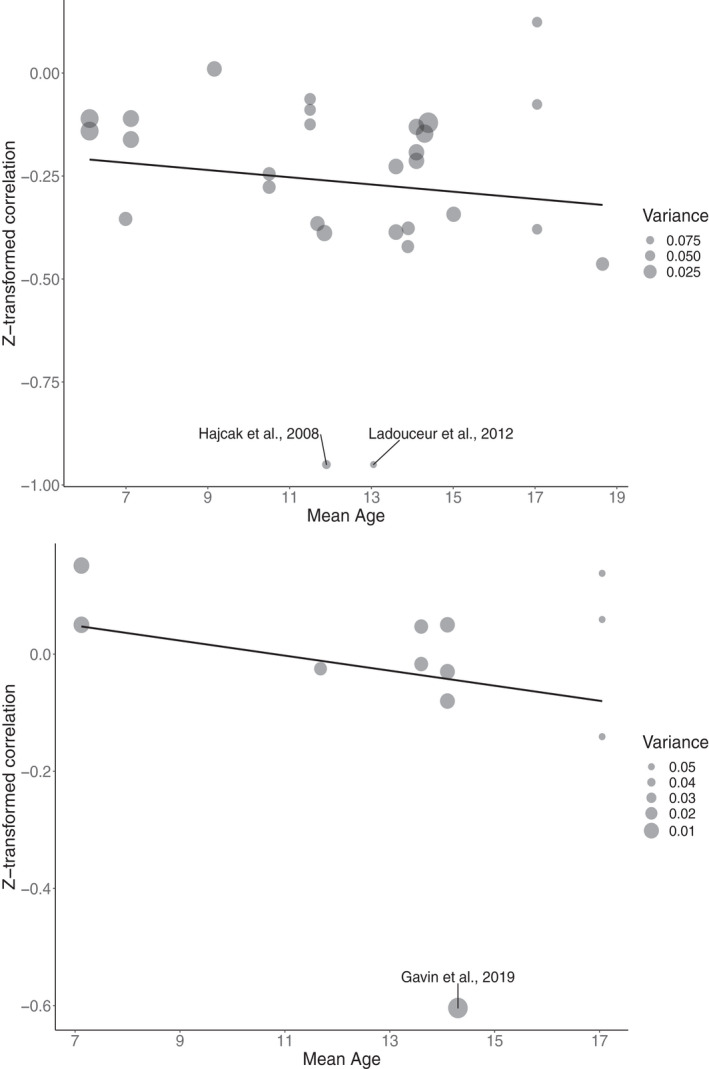
Bubble plot illustrating the Z‐transformed correlations between mean age and ERN (top) and the Pe (bottom). Larger dots indicate less variation. Effect sizes that exceed 2.5 standard deviations from the mean are labeled with the study name. Regression line is included for visualization purposes only as it was estimated using linear regression that does not account for dependency in the data. Mean age range: 6.1–18.7
3.7. Moderating effects of task
For the ERN group studies, there were mean effects of the Go/No‐Go and the Flanker task, however, none of the tasks moderated the age‐effect (Table 5). For the ERN correlational data, the mean age‐effect of the other experimental tasks, Go/No‐Go, and the Flanker task were significantly different from zero, however, none of the effects were significantly different from each other (Table 6).
TABLE 5.
Moderating methodological effects on the ERN: Group studies
| β0 (95% CI) | β1 (95% CI) | F (df1, df2) | |
|---|---|---|---|
| Task | F (2, 68) = .471, p = .626 | ||
| Other (Ref) | −.099 (−.991, .794) | ||
| Flanker | −.508 (−.818, −.197)** | −.409 (−1.354, .536) | |
| Go/No‐go | −.370 (−.737, −.003)* | −.272 (−1.236, .693) | |
| Electrode Site | F (4, 66) = .312, p = .869 | ||
| Fz (Ref) | −.420 (−.801, −.038)* | ||
| FCz | −.383 (−.771, .005) | .037 (−.477, .551) | |
| Cz | −.530 (−.842, −.218)** | −.110 (−.564, .344) | |
| Pz | −.435 (−.968, .097) | −.015 (−.632, .601) | |
| PCA | .138 (−1.221, 1.497) | .558 (−.854, 1.969) | |
| Quantification | F (2, 68) = .249, p = .781 | ||
| Delta (Ref) | −.397 (−1.003, .210) | ||
| Mean Amplitude | −.321 (−0.722, .080) | .076 (−.621, .772) | |
| Peak | −.498 (−.810, −.186)** | −.101 (−.759, .556) |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviations: Ref, reference category. PCA, principal component analysis.
p < .05;
p < .01.
TABLE 6.
Moderating methodological effects on the ERN: Correlational studies
| β0 (95% CI) | β1 (95% CI) | F (df1, df2) | |
|---|---|---|---|
| Task | F (2, 26) = 0.632, p = .539 | ||
| Other (Ref) | −.365 (−.608, −.122)** | ||
| Flanker | −.231 (−.323, −.139)*** | .134 (−.126, .394) | |
| Go/No‐go | −.218 (−.361, −.074)** | .148 (−.134, .430) | |
| Electrode Site | F (4, 24) = 2.72, p = .054 | ||
| Fz (Ref) | −.467 (−.660, −.274)*** | ||
| FCz | −.204 (−.285, −.123)*** | .263 (.053, .472)* | |
| Cz | −.160 (−.273, −.047)** | .307 (.083, .531)** | |
| CPz | −.464 (−.815, −.112)* | .003 (−.398, .405) | |
| Multiple | −.172 (−.307, −.038)* | .295 (.059, .530)* | |
| Quantification | F (3, 25) = 0.071, p = .975 | ||
| Delta (Ref) | −.231 (−.324, −.138)*** | ||
| Mean Amplitude | −.250 (−.343, −.157)*** | −.018 (−.120, .083) | |
| Peak | −.226 (−.418, −.034)* | .006 (−.208, .219) | |
| Residual | −.216 (−.433, .002) | .016 (−.205, .237) |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviation: Ref, reference category.
p < .05;
p < .01;
p < .001.
For the Pe group data, the mean age‐effect from the category of other types of experimental tasks did significantly deviate from zero, which yielded a larger effect size compared to the mean age‐effect obtained from the Go/No‐Go task and the Flanker task (Table 7). Finally, none of the tasks showed any mean age‐effects deviating from zero nor moderation effects on the Pe correlational data (Table 8).
TABLE 7.
Moderating methodological effects on the Pe: Group studies
| β0 (95% CI) | β1 (95% CI) | F (df1, df2) | |
|---|---|---|---|
| Task | F (2, 46) = 4.453, p = .017 | ||
| Other (Ref) | −1.165 (−2.081, −.249)* | ||
| Flanker | .035 (−.180, .250) | 1.200 (.260, 2.141)* | |
| Go/No‐go | .204 (−.043, .451) | 1.369 (.421, 2.317)** | |
| Electrode Site | F (4, 44) = 1.024, p = .406 | ||
| Cz (Ref) | −.076 (−.296, .145) | ||
| Fz | .298 (−.045, .642) | .374 (−.034, .782) | |
| FCz | .009 (−.261, .278) | .085 (−.264, .433) | |
| Pz | .150 (−.122, .422) | .226 (−.125, .576) | |
| PCA | .000 (−.764, .764) | .076 (−.719, .870 | |
| Quantification | F (1, 47) = 5.472, p = .024 | ||
| Mean Amplitude (Ref) | −.183 (−.441, .075) | ||
| Peak | .190 (−.000, .381) | −.373 (−.694, −.052)* |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviations: Ref, reference category. PCA, principal component analysis.
p < .05;
p < .01.
TABLE 8.
Moderating methodological effects on the Pe: Correlational studies
| β0 (95% CI) | β1 (95% CI) | F (df1, df2) | |
|---|---|---|---|
| Task | F (2, 9) = 0.376, p = .697 | ||
| Other (Ref) | .019 (−.705, .743) | ||
| Flanker | −.164 (−.509, .182) | −.182 (−.985, .620) | |
| Go/No‐go | .101 (−.578, .779) | .082 (−.910, 1.074) | |
| Electrode Site | F (4, 7) = 18.640, p < .001 | ||
| Cz (Ref) † | −.604 (−.758, −.451)*** | ||
| FCz | .019 (−.287, .324) | .623 (.281, .965)** | |
| Pz | .101 (−.051, .253) | .705 (.489, .921)*** | |
| CPz | .015 (−.179, .209) | .619 (.371, .867)*** | |
| Multiple | −.021 (−.148, .107) | .583 (.384, .783)*** | |
| Quantification | F (3, 8) = 1.345, p = .327 | ||
| Delta (Ref) | .030 (−.243, .304) | ||
| Mean Amplitude | .027 (−.232, .287) | −.003 (−.192, .186) | |
| Peak | −.332 (−.691, .027) | −.362 (−.814, .089) | |
| Residual | −.064 (−.423, .295) | −.094 (−.395, .208) |
Note: β0 represents the mean age‐effect, whereas the β1 represents the moderation effect compared to the mean effect of the reference category.
Abbreviation: Ref, reference category.
p < .01;
p < .001.
The point estimate was from the Gavin et al., 2019 dataset using latency filtering, post‐hoc analysis using the data from before the latency jittering filter yielded similar, albeit a weaker main effect of the Cz (i.e., β0 (95% CI) = −.228 (−.381, −.074), p = .01).
3.8. Moderating effects of electrode site
For the ERN group studies, significant mean age‐effect for electrode site was identified for the Fz and Cz. However, none of the mean age‐effects moderated the age‐effect (Table 5). For the ERN correlational studies, there were mean age‐effects of Fz, FCz, Cz, CPz, and across multiple electrodes. The moderation analysis showed that multiple electrodes, FCz, and Cz resulted in lower estimated age‐effect of the ERN compared to the Fz (Table 6). For the Pe group studies, none of the electrode sites were found to have a mean age‐effect that significantly deviated from zero, nor any that deviated from each other (Table 7). However, the data from the correlational studies indicated that the Cz was the only electrode site that exhibited an effect that deviated significantly from zero and yielded significantly larger age‐effect compared to the other electrode sites, including FCz, Pz, CPz, and averaging across multiple electrodes (Table 8).
3.9. Moderating effects of quantification method
For the ERN group studies, there was a significant mean effect of peak, however, it did not differ significantly from the other quantification methods (Table 5). For the ERN correlational data, delta, mean amplitude, and peak, but not the residual method, showed significant mean age‐effect deviating from zero, however, none of the quantification methods were significantly different from each other (Table 6). For the Pe group data, the quantifying the Pe using the peak amplitude did significantly deviate from the age‐effect obtained with the mean amplitude quantification method (Table 7), However, none of the quantification methods were significant nor exhibit moderation effects for the Pe correlational data (Table 8).
4. DISCUSSION
The current meta‐analysis investigated the effects of age on ERN and Pe strength in children and adolescents, and whether the age‐effects were moderated by sample and methodological differences. Across all age groups and ages, spanning from childhood to adulthood (mean age range; group studies: 3.6–28.7 years old, correlational studies: 6.1–18.7 years old), the overall effect size of age on the ERN was small‐to‐medium (SMD = −.433, r = −.230), while the overall effect of age on the Pe was negligible and non‐significant (SMD = .059, r = −.091). Group studies showed that the age effect on Pe differed by age grouping, where the age effect obtained from comparing younger versus older adolescents yielded a larger positive mean age effect compared to the mean age effect obtained comparing children versus adults. However, we did not observe any significant effect of linear or non‐linear age terms on the Pe from the correlational data. Furthermore, we did not observe a significant moderating effect of sex on the age‐effects. Regarding methodological differences, we found that studies using the Flanker and the Go/No‐Go tasks showed no age‐effects of the Pe, whereas there was a negative mean effect on age in for the category of other experimental tasks. Moreover, the age‐effects on the ERN and the Pe differed according to electrode site, where the Fz yielded the largest negative association between the ERN and age, and the Cz electrode yielded the largest negative association between the Pe and age.
4.1. Effects of age
In line with a previous review (Tamnes et al., 2013), we found evidence for a more negative ERN with increasing age across childhood and adolescence. The results support our hypothesis that the ERN has a prolonged developmental trajectory extending into young adulthood. However, we did not find sufficient evidence for development of the Pe, except a significant mean age effect when comparing younger versus older adolescents. This was in contrast to our hypothesis, as we expected the Pe to develop during childhood, and not during adolescence. Although speculative, these age‐related differences in the ERN relative to the Pe could reflect differential maturation of bottom‐up and top‐down mechanisms in the error processing system. Thus, the development of the ERN could reflect improvement in sorting out relevant sensory information that compete for cognitive resources, whereas the Pe may reflect top‐down processes that occur after the sensory information has passed a cognitive bottleneck. For instance, incongruent trials in a Flanker task (i.e., where a target arrow is pointing in the opposite direction of the flanked arrows) could activate multiple responses leading to a response conflict. Thus, as children develop and improve in selective attention or attentional control (e.g., ignoring the Flankers), changes in the ERN may be reflected by changes in the early activation of competing responses. The Pe, however, has been linked to error awareness (e.g., Nieuwenhuis et al., 2001). Thus, it may be possible that the Pe reflects conscious detection of the behavioral result of the competing responses (i.e., a behavioral error). However, it should also be noted that the differential maturation patterns in the ERN and the Pe could be due to other aspects of the ERP components than development in strength. For instance, a recent study suggested that the age‐effects on the ERN amplitude might be confounded by age‐related changes in latency variability, as the variability can attenuate the averaged EEG signal (Gavin et al., 2019). Thus, the age‐effect on the ERN (and the Pe) in the current meta‐analysis could partly be explained by decreasing latency variability and not changes (or lack of changes) in the mean amplitude per se. Interestingly, others have argued that the ERN is better time‐locked to motoric responses rather than button presses, which may result in shorter latencies (Burle et al., 2008; Śmigasiewicz et al., 2020). Indeed, most developmental studies response‐locked the EEG signal to incorrect button presses, which might influence latency variability. Thus, future studies could benefit from accounting for latency variability and/or use electromyography to capture the onset of the incorrect response. Moreover, relying on measures solely in the temporal domain precludes the ability to disentangle error‐related activity from activity associated with other cognitive processes such as conflict monitoring, as such cognitive processes typically overlap in time. In contrast, a study examining the frequency domain showed that frequency bands relate differentially to error detection and conflict monitoring (Cohen & van Gaal, 2014). Thus, we also urge researchers to more broadly examine age‐related differences in error‐related EEG signals, including developmental changes in both the time domain and the frequency domain (but see DuPuis et al., 2015; Gavin et al., 2019).
Finally, although we found an overall effect size of age on the ERN across the studies included in the current meta‐analysis, it is important to note that several studies reported non‐linear age‐effects on the ERN in a developmental sample, with stronger effects in children compared to adolescents (Davies et al., 2004b; Gavin et al., 2019). This is especially relevant when considering correlational studies that vary in their age‐range. That is, the age‐effect could actually be suppressed in studies with an age‐range that includes developmental periods where the true age‐effect on the ERN starts to decelerate or stabilize. Moreover, using mean age as the predictor variable on the ERN age effect could potentially underestimate the age‐effect. Thus, depending on the sample characteristics (i.e., mean age, age‐range, age distribution, and the number of participants), the bivariate correlations between ERN and age may not fully capture the maturational pattern of the ERN nor the Pe component. Future studies might benefit from including non‐linear age terms in their regression models, or alternatively, use other analytical approaches that allow for non‐linear patterns. This could further aid our understanding of the developmental trajectories of the ERN and the Pe, as well as provide insight into periods of accelerated or decelerated development.
4.2. Sex differences
Results from studies examining sex differences in the ERN and the Pe during development are mixed (Davies et al., 2004b; DuPuis et al., 2015; Lo, 2018; Torpey et al., 2012). In the current meta‐analysis, we did not find any moderating effect of sex on the age effects on ERN or Pe. However, it is important to note that we used a continuous measure of sex (i.e., proportion of males) as we were unable to include a dichotomous variable of sex (e.g., to investigate potential mean differences in the age‐effect on the ERN and the Pe components for male and female participants, separately). This is a similar procedure as used in a former meta‐analytical review on the ERN (Lo, 2018) that did find that higher proportion of males were related to increases in the ERN (see also Fischer et al., 2016), whereas our results did not indicate sufficient evidence for a differential age‐effect on the ERN and the Pe. It should also be noted that sex differences might interact with age (e.g., girls having larger ERN compared to boys in younger age groups, whereas exhibiting a reverse pattern in older age groups). Thus, depending on the age range included in the study, only testing for a main effect of sex could be suppressed or fail to detect true sex effects. Future studies might benefit from investigating the developmental patterns of the ERN and the Pe in girls and boys separately or test for interaction effects between sex and age. However, depending on the age‐range, an interaction effect between sex and age might also be influenced by puberty‐related effects as girls tend to mature earlier than boys.
4.3. Experimental task
The vast majority of the included studies used the Flanker or the Go/No‐Go task to measure the ERN and the Pe. It is important to consider the possibility that the experimental task might influence the ERN and the Pe, both in combination with and independently from the amounts of error trials included in the analysis. This is especially important as the numbers of errors of commission influence the stability of the ERP components, where at least six to eight error trials are required to quantify the ERN and the Pe (Olvet & Hajcak, 2009) and since ERN correlates with amounts of errors committed in the task independent of trials used to estimate the ERN in the EEG analysis (Fischer et al., 2017). In the current meta‐analysis, we found that both the Flanker and the Go/No‐Go tasks are suitable experimental tasks to detect age‐effects on the ERN, while we did not find evidence for this for the Pe. Indeed, it was the category for other experimental tasks (i.e., age effect derived from a probabilistic learning task) that yielded a significant negative mean effect on the Pe (i.e., decreased Pe with increasing age), possibly indicating that other types of experiments than the Flanker and the Go/No‐Go tasks could be used to further examine age‐related changes in the Pe. To speculate, it is possible that the motivational significance of making an error in a probabilistic learning task is different from that of making an error in a Flanker or a Go/No‐Go task. In addition, a probabilistic learning task might also lead the participants to recruit other cognitive processes or to increase cognitive load compared to Flanker or Go/No‐Go task. However, we also urge caution when interpreting the results from the category for other experimental tasks as these were based on very few studies and effect sizes (i.e., three effect sizes for the ERN and one effect size for the Pe).
4.4. Electrode site and quantification method
The included studies varied in their methodological approach to quantify the ERN and the Pe, using different electrode sites and quantification methods, all of which might influence the age effect on error processing. Indeed, one study that quantified the ERN using different methodological approaches, including electrode sites and quantification methods, showed 72 unique estimates of the ERN, which also influenced the strength of associations to behavioral measures and sex (Sandre et al., 2020). This is interesting as the current study showed that methodological choices moderated the age effect on the ERN and the Pe, emphasizing the importance of sufficiently describing and considering methodological choices when estimating ERPs. In addition, it also emphasizes the importance of being consistent in the methodological choices when comparing age groups (or other groups) when comparing ERP components to rule out the potential confounding effect of methodological choices. Thus, in line with previous recommendations (Luck & Gaspelin, 2017), we urge developmental researchers who are using EEG to consider their methodological choices a priori. Based on the moderation analysis and the quality control checklist in the current study, we recommend building on existing research when choosing electrode sites, quantification method, and time window to estimate the error component of interest.
4.5. Limitations
Some limitations of the current meta‐analysis should be noted. First, the group studies were dummy coded, and as such, we were not able to provide an accurate estimation of non‐linear age trends. Second, as discussed above, methodological variability could influence the moderation analysis. It is also possible that there exists a systematic skew in methodological choices for studies that report significant age effects. For instance, some studies may report age effects when using the Cz electrode to estimate the ERN in children, but Fz in adolescents, or when using specific experimental tasks to measure the ERN and the Pe in children, and other tasks in adolescents. However, much of the variation in effect sizes are within the same study as 161 effect sizes are distributed across 37 studies, thus much of the variation in moderation variables investigated was also within studies that were similar in many other aspects. Of note, we urge caution when interpreting the mean and moderating effect of the variables that are based on few studies. Finally, most of the included studies are based on cross‐sectional studies, whereas longitudinal studies are required to directly characterize the developmental trajectories of the ERN and the Pe.
4.6. Conclusion
In conclusion, we found meta‐analytic evidence for a more negative ERN with increasing age across childhood and adolescence, whereas a similar age‐effect on the Pe was not found, indicating differential maturation of these elements of the error processing system. Further, we also found moderation effects of age group, task, and electrode site on the age effect on the Pe, such that the adolescent age group, other experimental task, and electrode Cz categories yielded the largest age effects on the Pe. For the ERN, the electrode site was found to moderate the age effect, such that the Fz yielded the largest age effects on the ERN. These findings provide new insight into typical age‐related developmental differences in ERN and Pe strength and their moderators as well as foundational evidence for further investigations of developmental patterns of error processing in relation to psychopathology, early risk factors, or interventions.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Rune Boen: Conceptualization; data curation; formal analysis; investigation; methodology; software; visualization; writing – original draft. Daniel S. Quintana: Funding acquisition; methodology; resources; software; visualization; writing – review and editing. Cecile D. Ladouceur: Investigation; methodology; resources; writing – review and editing. Christian Tamnes: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; validation; writing – review and editing.
Supporting information
FIGURE S1 Funnel plot for the ERN group studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S2 Funnel plot for the Pe group studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S3 Funnel plot for the ERN correlational studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S4 Funnel plot for the Pe correlational studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
TABLE S1
TABLE S2
TABLE S3
TABLE S4
TABLE S5 Effect sizes for the group studies on the ERN
TABLE S6 Effect sizes for the group studies on the Pe
TABLE S7 Effect sizes for the correlational studies on the ERN
TABLE S8 Effect sizes for the correlational studies on the Pe
Appendix S1
Appendix S2
Boen, R. , Quintana, D. S. , Ladouceur, C. D. , & Tamnes, C. K. (2022). Age‐related differences in the error‐related negativity and error positivity in children and adolescents are moderated by sample and methodological characteristics: A meta‐analysis. Psychophysiology, 59, e14003. 10.1111/psyp.14003
REFERENCES
- Agam, Y. , Hämäläinen, M. S. , Lee, A. K. C. , Dyckman, K. A. , Friedman, J. S. , Isom, M. , Makris, N. , & Manoach, D. S. (2011). Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proceedings of the National Academy of Sciences, 108(42), 17556–17561. 10.1073/pnas.1103475108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assink, M. , & Wibbelink, C. J. M. (2016). Fitting three‐level meta‐analytic models in R: A step‐by‐step tutorial. The Quantitative Methods for Psychology, 12(3), 154–174. 10.20982/tqmp.12.3.p154 [DOI] [Google Scholar]
- Barker, T. V. , Troller‐Renfree, S. V. , Bowman, L. C. , Pine, D. S. , & Fox, N. A. (2018). Social influences of error monitoring in adolescent girls. Psychophysiology, 55(9), e13089. 10.1111/psyp.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen, R. , Quintana, D. , Ladouceur, C. , & Tamnes, C. K. (2020). Age‐related differences in the error‐related negativity and error positivity in children and adolescents: A systematic review and meta‐analysis protocol. PsyArXiv. 10.31234/osf.io/57ae2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M. M. , Braver, T. S. , Barch, D. M. , Carter, C. S. , & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. 10.1037/0033-295x.108.3.624 [DOI] [PubMed] [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Brooker, R. J. (2018). Maternal behavior and socioeconomic status predict longitudinal changes in error‐related negativity in preschoolers. Child Development, 89(3), 725–733. 10.1111/cdev.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle, B. , Roger, C. , Allain, S. , Vidal, F. , & Hasbroucq, T. (2008). Error negativity does not reflect conflict: A reappraisal of conflict monitoring and anterior cingulate cortex activity. Journal of Cognitive Neuroscience, 20(9), 1637–1655. 10.1162/jocn.2008.20110 [DOI] [PubMed] [Google Scholar]
- Buzzell, G. A. , Richards, J. E. , White, L. K. , Barker, T. V. , Pine, D. S. , & Fox, N. A. (2017). Development of the error‐monitoring system from ages 9–35: Unique insight provided by MRI‐constrained source localization of EEG. Neuroimage, 157, 13–26. 10.1016/j.neuroimage.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco, M. , Harbin, S. M. , Nienhuis, J. K. , Fitzgerald, K. D. , Gehring, W. J. , & Hanna, G. L. (2013). Increased error‐related brain activity in youth with obsessive–compulsive disorder and unaffected siblings. Depression and Anxiety, 30(1), 39–46. 10.1002/da.22035 [DOI] [PubMed] [Google Scholar]
- Carrasco, M. , Hong, C. , Nienhuis, J. K. , Harbin, S. M. , Fitzgerald, K. D. , Gehring, W. J. , & Hanna, G. L. (2013). Increased error‐related brain activity in youth with obsessive–compulsive disorder and other anxiety disorders. Neuroscience Letters, 541, 214–218. 10.1016/j.neulet.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, C. S. , & van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 367–379. 10.3758/CABN.7.4.367 [DOI] [PubMed] [Google Scholar]
- Cavanagh, J. F. , & Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa, P. , Castellanos, M. C. , Abundis‐Gutiérrez, A. , & Rosario Rueda, M. (2014). Development of neural mechanisms of conflict and error processing during childhood: Implications for self‐regulation. Frontiers in Psychology, 5, 326. 10.3389/fpsyg.2014.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson, A. , Clayson, P. E. , Keith, C. M. , Catron, C. , & Larson, M. J. (2017). Conflict and performance monitoring throughout the lifespan: An event‐related potential (ERP) and temporospatial component analysis. Biological Psychology, 124, 87–99. 10.1016/j.biopsycho.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Cohen, M. X. , & van Gaal, S. (2014). Subthreshold muscle twitches dissociate oscillatory neural signatures of conflicts from errors. Neuroimage, 86, 503–513. 10.1016/j.neuroimage.2013.10.033 [DOI] [PubMed] [Google Scholar]
- Crone, E. A. , & Steinbeis, N. (2017). Neural perspectives on cognitive control development during childhood and adolescence. Trends in Cognitive Sciences, 21(3), 205–215. 10.1016/j.tics.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Dahl, R. E. , Allen, N. B. , Wilbrecht, L. , & Suleiman, A. B. (2018). Importance of investing in adolescence from a developmental science perspective. Nature, 554(7693), 441–450. 10.1038/nature25770 [DOI] [PubMed] [Google Scholar]
- Dalsgaard, S. , Thorsteinsson, E. , Trabjerg, B. B. , Schullehner, J. , Plana‐Ripoll, O. , Brikell, I. , Wimberley, T. , Thygesen, M. , Madsen, K. B. , Timmerman, A. , Schendel, D. , McGrath, J. J. , Mortensen, P. B. , & Pedersen, C. B. (2020). Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry, 77(2), 155–164. 10.1001/jamapsychiatry.2019.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovitch, J. H. , Fisher, M. , Schroder, H. , Hambrick, D. Z. , & Moser, J. (2019). Intelligence and neurophysiological markers of error monitoring relate to children’s intellectual humility. Child Development, 90(3), 924–939. 10.1111/cdev.12960 [DOI] [PubMed] [Google Scholar]
- Davies, P. L. , Segalowitz, S. J. , Dywan, J. , & Pailing, P. E. (2001). Error‐negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biological Psychology, 56(3), 191–206. 10.1016/S0301-0511(01)00080-1 [DOI] [PubMed] [Google Scholar]
- Davies, P. L. , Segalowitz, S. J. , & Gavin, W. J. (2004a). Development of error‐monitoring event‐related potentials in adolescents. Annals of the New York Academy of Sciences, 1021(1), 324–328. 10.1196/annals.1308.039 [DOI] [PubMed] [Google Scholar]
- Davies, P. L. , Segalowitz, S. J. , & Gavin, W. J. (2004b). Development of response‐monitoring ERPs in 7‐ to 25‐year‐olds. Developmental Neuropsychology, 25(3), 355–376. 10.1207/s15326942dn2503_6 [DOI] [PubMed] [Google Scholar]
- Downes, M. , Bathelt, J. , & Haan, M. D. (2017). Event‐related potential measures of executive functioning from preschool to adolescence. Developmental Medicine & Child Neurology, 59(6), 581–590. 10.1111/dmcn.13395 [DOI] [PubMed] [Google Scholar]
- DuPuis, D. , Ram, N. , Willner, C. J. , Karalunas, S. , Segalowitz, S. J. , & Gatzke‐Kopp, L. M. (2015). Implications of ongoing neural development for the measurement of the error‐related negativity in childhood. Developmental Science, 18(3), 452–468. 10.1111/desc.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, B. G. , Calhoun, V. D. , & Kiehl, K. A. (2012). Joint ICA of ERP and fMRI during error‐monitoring. Neuroimage, 59(2), 1896–1903. 10.1016/j.neuroimage.2011.08.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger, B. , Mock, B. , & Kray, J. (2009). Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology, 46(5), 1043–1053. 10.1111/j.1469-8986.2009.00838.x [DOI] [PubMed] [Google Scholar]
- Falkenstein, M. , Hoormann, J. , Christ, S. , & Hohnsbein, J. (2000). ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology, 51(2), 87–107. 10.1016/S0301-0511(99)00031-9 [DOI] [PubMed] [Google Scholar]
- Ficarella, S. C. , Rochet, N. , & Burle, B. (2019). Becoming aware of subliminal responses: An EEG/EMG study on partial error detection and correction in humans. Cortex, 120, 443–456. 10.1016/j.cortex.2019.07.007 [DOI] [PubMed] [Google Scholar]
- Fischer, A. G. , Danielmeier, C. , Villringer, A. , Klein, T. A. , & Ullsperger, M. (2016). Gender influences on brain responses to errors and post‐error adjustments. Scientific Reports, 6(1), 24435. 10.1038/srep24435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. G. , Klein, T. A. , & Ullsperger, M. (2017). Comparing the error‐related negativity across groups: The impact of error‐ and trial‐number differences. Psychophysiology, 54(7), 998–1009. 10.1111/psyp.12863 [DOI] [PubMed] [Google Scholar]
- Fisher, R. A. (1921). On the «probable error» of a coefficient of correlation deduced from a small sample. Metron, 1, 1–32. [Google Scholar]
- Gavin, W. J. , Lin, M.‐H. , & Davies, P. L. (2019). Developmental trends of performance monitoring measures in 7‐ to 25‐year‐olds: Unraveling the complex nature of brain measures. Psychophysiology, 56(7), e13365. 10.1111/psyp.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, W. J. , Goss, B. , Coles, M. G. H. , Meyer, D. E. , & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. 10.1111/j.1467-9280.1993.tb00586.x [DOI] [Google Scholar]
- Gehring, W. J. , Goss, B. , Coles, M. G. H. , Meyer, D. E. , & Donchin, E. (2018). The error‐related negativity. Perspectives on Psychological Science, 13(2), 200–204. 10.1177/1745691617715310 [DOI] [PubMed] [Google Scholar]
- Gehring, W. J. , Liu, Y. , Orr, J. M. , & Carp, J. (2012). The error‐related negativity (ERN/Ne). In Kappenman I. E. S. & Luck S. J. (Eds.), The Oxford handbook of event‐related potentials. Oxford University Press. [Google Scholar]
- Gorday, J. Y. , & Meyer, A. (2018). Linking puberty and error‐monitoring: Relationships between self‐reported pubertal stages, pubertal hormones, and the error‐related negativity in a large sample of children and adolescents. Developmental Psychobiology, 60(4), 483–490. 10.1002/dev.21625 [DOI] [PubMed] [Google Scholar]
- Grammer, J. K. , Carrasco, M. , Gehring, W. J. , & Morrison, F. J. (2014). Age‐related changes in error processing in young children: A school‐based investigation. Developmental Cognitive Neuroscience, 9, 93–105. 10.1016/j.dcn.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer, J. K. , Gehring, W. J. , & Morrison, F. J. (2018). Associations between developmental changes in error‐related brain activity and executive functions in early childhood. Psychophysiology, 55(3), e13040. 10.1111/psyp.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak, G. , Franklin, M. E. , Foa, E. B. , & Simons, R. F. (2008). Increased error‐related brain activity in pediatric obsessive–compulsive disorder before and after treatment. American Journal of Psychiatry, 165(1), 116–123. 10.1176/appi.ajp.2007.07010143 [DOI] [PubMed] [Google Scholar]
- Hanna, G. L. , Carrasco, M. , Harbin, S. M. , Nienhuis, J. K. , LaRosa, C. E. , Chen, P. , Fitzgerald, K. D. , & Gehring, W. J. (2012). Error‐related negativity and tic history in pediatric obsessive–compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 51(9), 902–910. 10.1016/j.jaac.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, G. L. , Liu, Y. , Rough, H. E. , Surapaneni, M. , Hanna, B. S. , Arnold, P. D. , & Gehring, W. J. (2020). A diagnostic biomarker for pediatric generalized anxiety disorder using the error‐related negativity. Child Psychiatry and Human Development, 51(5), 827–838. 10.1007/s10578-020-01021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, L. V. (1981). Distribution theory for glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- Henderson, H. , Schwartz, C. , Mundy, P. , Burnette, C. , Sutton, S. , Zahka, N. , & Pradella, A. (2006). Response monitoring, the error‐related negativity, and differences in social behavior in autism. Brain and Cognition, 61(1), 96–109. 10.1016/j.bandc.2005.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, M. J. , Römmler, J. , Ehlis, A.‐C. , Heidrich, A. , & Fallgatter, A. J. (2004). Source localization (LORETA) of the error‐related‐negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20(2), 294–299. 10.1016/j.cogbrainres.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Hirsh, J. B. , & Inzlicht, M. (2010). Error‐related negativity predicts academic performance. Psychophysiology, 47(1), 192–196. 10.1111/j.1469-8986.2009.00877.x [DOI] [PubMed] [Google Scholar]
- Hogan, A. M. , Vargha‐Khadem, F. , Kirkham, F. J. , & Baldeweg, T. (2005). Maturation of action monitoring from adolescence to adulthood: An ERP study. Developmental Science, 8(6), 525–534. 10.1111/j.1467-7687.2005.00444.x [DOI] [PubMed] [Google Scholar]
- Holroyd, C. B. , & Coles, M. G. H. (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error‐related negativity. Psychological Review, 109(4), 679–709. 10.1037/0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Horowitz‐Kraus, T. (2011). Does development affect the error‐related negativity of impaired and skilled readers?: An ERP study. Developmental Neuropsychology, 36(7), 914–932. 10.1080/87565641.2011.606415 [DOI] [PubMed] [Google Scholar]
- Hum, K. M. , Manassis, K. , & Lewis, M. D. (2013). Neural mechanisms of emotion regulation in childhood anxiety. Journal of Child Psychology and Psychiatry, 54(5), 552–564. 10.1111/j.1469-7610.2012.02609.x [DOI] [PubMed] [Google Scholar]
- Ip, K. I. , Liu, Y. , Moser, J. , Mannella, K. , Hruschak, J. , Bilek, E. , Muzik, M. , Rosenblum, K. , & Fitzgerald, K. (2019). Moderation of the relationship between the error‐related negativity and anxiety by age and gender in young children: A preliminary investigation. Developmental Cognitive Neuroscience, 39(100), 702. 10.1016/j.dcn.2019.100702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo, K. , Bae, S. , & Masaki, H. (2016). The association of childhood fitness to proactive and reactive action monitoring. PLOS ONE, 11(3), e0150691. 10.1371/journal.pone.0150691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil, A. , Debener, S. , Gratton, G. , Junghöfer, M. , Kappenman, E. S. , Luck, S. J. , Luu, P. , Miller, G. A. , & Yee, C. M. (2014). Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology, 51(1), 1–21. 10.1111/psyp.12147 [DOI] [PubMed] [Google Scholar]
- Kessel, E. M. , Meyer, A. , Hajcak, G. , Dougherty, L. R. , Torpey‐Newman, D. C. , Carlson, G. A. , & Klein, D. N. (2016). Transdiagnostic factors and pathways to multifinality: The error‐related negativity predicts whether preschool irritability is associated with internalizing versus externalizing symptoms at age 9. Development and Psychopathology, 28(4 pt1), 913–926. 10.1017/S0954579416000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel, E. M. , Nelson, B. D. , Finsaas, M. , Kujawa, A. , Meyer, A. , Bromet, E. , Carlson, G. A. , Hajcak, G. , Kotov, R. , & Klein, D. N. (2019). Parenting style moderates the effects of exposure to natural disaster‐related stress on the neural development of reactivity to threat and reward in children. Development and Psychopathology, 31(4), 1589–1598. 10.1017/S0954579418001347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. Y. , Iwaki, N. , Imashioya, H. , Uno, H. , & Fujita, T. (2007). Error‐related negativity in a visual go/no‐go task: Children versus adults. Developmental Neuropsychology, 31(2), 181–191. 10.1080/87565640701190775 [DOI] [PubMed] [Google Scholar]
- Kim, E. Y. , Iwaki, N. , Uno, H. , & Fujita, T. (2005). Error‐related negativity in children: Effect of an observer. Developmental Neuropsychology, 28(3), 871–883. 10.1207/s15326942dn2803_7 [DOI] [PubMed] [Google Scholar]
- Kim, M. H. , Grammer, J. K. , Marulis, L. M. , Carrasco, M. , Morrison, F. J. , & Gehring, W. J. (2016). Early math and reading achievement are associated with the error positivity. Developmental Cognitive Neuroscience, 22, 18–26. 10.1016/j.dcn.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Dahl, R. E. , Birmaher, B. , Axelson, D. A. , & Ryan, N. D. (2006). Increased error‐related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47(10), 1073–1082. 10.1111/j.1469-7610.2006.01654.x [DOI] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Dahl, R. E. , & Carter, C. S. (2004). ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences, 1021(1), 329–336. 10.1196/annals.1308.040 [DOI] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Dahl, R. E. , & Carter, C. S. (2007). Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science, 10(6), 874–891. 10.1111/j.1467-7687.2007.00639.x [DOI] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Slifka, J. S. , Dahl, R. E. , Birmaher, B. , Axelson, D. A. , & Ryan, N. D. (2012). Altered error‐related brain activity in youth with major depression. Developmental Cognitive Neuroscience, 2(3), 351–362. 10.1016/j.dcn.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Tan, P. Z. , Sharma, V. , Bylsma, L. M. , Silk, J. S. , Siegle, G. J. , Forbes, E. E. , McMakin, D. L. , Dahl, R. E. , Kendall, P. C. , Mannarino, A. , & Ryan, N. D. (2018). Error‐related brain activity in pediatric anxiety disorders remains elevated following individual therapy: A randomized clinical trial. Journal of Child Psychology and Psychiatry, 59(11), 1152–1161. 10.1111/jcpp.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Hanna, G. L. , Hanna, B. S. , Rough, H. E. , Arnold, P. D. , & Gehring, W. J. (2020). Behavioral and electrophysiological correlates of performance monitoring and development in children and adolescents with attention‐deficit/hyperactivity disorder. Brain Sciences, 10(2), 1–14. 10.3390/brainsci10020079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, S. L. (2018). A meta‐analytic review of the event‐related potentials (ERN and N2) in childhood and adolescence: Providing a developmental perspective on the conflict monitoring theory. Developmental Review, 48, 82–112. 10.1016/j.dr.2018.03.005 [DOI] [Google Scholar]
- Luck, S. J. , & Gaspelin, N. (2017). How to get statistically significant effects in any ERP experiment (and why you should not). Psychophysiology, 54(1), 146–157. 10.1111/psyp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu, P. , Tucker, D. M. , & Makeig, S. (2004). Frontal midline theta and the error‐related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(8), 1821–1835. 10.1016/j.clinph.2004.03.031 [DOI] [PubMed] [Google Scholar]
- Mathur, M. B. , & VanderWeele, T. J. (2020). Sensitivity analysis for publication bias in meta‐analyses. Journal of the Royal Statistical Society. Series C, Applied Statistics, 69(5), 1091–1119. 10.1111/rssc.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, J. M. , Perez‐Edgar, K. , Henderson, H. A. , Chronis‐Tuscano, A. , Pine, D. S. , & Fox, N. A. (2009). A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry, 65(5), 445–448. 10.1016/j.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meel, C. S. van, Heslenfeld, D. J. , Rommelse, N. N. , Oosterlaan, J. , & Sergeant, J. A. (2012). Developmental trajectories of neural mechanisms supporting conflict and error processing in middle childhood. Developmental Neuropsychology, 37(4), 358–378. 10.1080/87565641.2011.653062 [DOI] [PubMed] [Google Scholar]
- Meyer, A. (2016). Developing psychiatric biomarkers: A review focusing on the error‐related negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry, 3(4), 356–364. 10.1007/s40501-016-0094-5 [DOI] [Google Scholar]
- Meyer, A. (2017). A biomarker of anxiety in children and adolescents: A review focusing on the error‐related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience, 27, 58–68. 10.1016/j.dcn.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Bress, J. N. , & Proudfit, G. H. (2014). Psychometric properties of the error‐related negativity in children and adolescents. Psychophysiology, 51(7), 602–610. 10.1111/psyp.12208 [DOI] [PubMed] [Google Scholar]
- Meyer, A. , Carlton, C. , Crisler, S. , & Kallen, A. (2018). The development of the error‐related negativity in large sample of adolescent females: Associations with anxiety symptoms. Biological Psychology, 138, 96–103. 10.1016/j.biopsycho.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Hajcak, G. , Glenn, C. R. , Kujawa, A. J. , & Klein, D. N. (2017). Error‐related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion (Washington, D.C.), 17(3), 487–496. 10.1037/emo0000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Hajcak, G. , Torpey, D. C. , Kujawa, A. , Kim, J. , Bufferd, S. , Carlson, G. , & Klein, D. N. (2013). Increased error‐related brain activity in six‐year‐old children with clinical anxiety. Journal of Abnormal Child Psychology, 41(8), 1257–1266. 10.1007/s10802-013-9762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Weinberg, A. , Klein, D. N. , & Hajcak, G. (2012). The development of the error‐related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year‐olds. Developmental Cognitive Neuroscience, 2(1), 152–161. 10.1016/j.dcn.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner, W. H. R. , Lemke, U. , Weiss, T. , Holroyd, C. , Scheffers, M. K. , & Coles, M. G. H. (2003). Implementation of error‐processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error‐related negativity. Biological Psychology, 64(1), 157–166. 10.1016/S0301-0511(03)00107-8 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Shamseer, L. , Clarke, M. , Ghersi, D. , Liberati, A. , Petticrew, M. , Shekelle, P. , Stewart, L. A. , & PRISMA‐P Group . (2015). Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic Reviews, 4(1), 1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke, G.‐J. , Nap, T. S. , Schippers, E. E. , & Cohen, M. X. (2015). A statistical comparison of EEG time‐ and time–frequency domain representations of error processing. Brain Research, 1618, 222–230. 10.1016/j.brainres.2015.05.030 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis, S. , Ridderinkhof, K. R. , Blom, J. , Band, G. P. H. , & Kok, A. (2001). Error‐related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38(5), 752–760. 10.1111/1469-8986.3850752 [DOI] [PubMed] [Google Scholar]
- Olvet, D. M. , & Hajcak, G. (2008). The error‐related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review, 28(8), 1343–1354. 10.1016/j.cpr.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet, D. M. , & Hajcak, G. (2009). The stability of error‐related brain activity with increasing trials. Psychophysiology, 46(5), 957–961. 10.1111/j.1469-8986.2009.00848.x [DOI] [PubMed] [Google Scholar]
- Overbeek, T. J. M. , Nieuwenhuis, S. , & Ridderinkhof, K. R. (2005). Dissociable components of error processing. Journal of Psychophysiology, 19(4), 319–329. 10.1027/0269-8803.19.4.319 [DOI] [Google Scholar]
- Overbye, K. , Walhovd, K. B. , Fjell, A. M. , Tamnes, C. K. , & Huster, R. J. (2021). Electrophysiological and behavioral indices of cognitive conflict processing across adolescence. Developmental Cognitive Neuroscience, 48(100), 100929. 10.1016/j.dcn.2021.100929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbye, K. , Walhovd, K. B. , Paus, T. , Fjell, A. M. , Huster, R. J. , & Tamnes, C. K. (2019). Error processing in the adolescent brain: Age‐related differences in electrophysiology, behavioral adaptation, and brain morphology. Developmental Cognitive Neuroscience, 38(100), 100665. 10.1016/j.dcn.2019.100665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla, M. L. , Pfefferbaum, A. , Sullivan, E. V. , Baker, F. C. , & Colrain, I. M. (2014). Dissociation of preparatory attention and response monitoring maturation during adolescence. Clinical Neurophysiology, 125(5), 962–970. 10.1016/j.clinph.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. , & Crone, E. A. (2017). Increased striatal activity in adolescence benefits learning. Nature Communications, 8(1), 1983. 10.1038/s41467-017-02174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana, D. S. (2020). The metameta R package (Version 0.1.1). Zenodo. 10.5281/zenodo.3944098 [DOI] [Google Scholar]
- Richardson, C. , Anderson, M. , Reid, C. L. , & Fox, A. M. (2011). Neural indicators of error processing and intraindividual variability in reaction time in 7 and 9 year‐olds. Developmental Psychobiology, 53(3), 256–265. 10.1002/dev.20518 [DOI] [PubMed] [Google Scholar]
- Riesel, A. (2019). The erring brain: Error‐related negativity as an endophenotype for OCD—A review and meta‐analysis. Psychophysiology, 56(4), e13348. 10.1111/psyp.13348 [DOI] [PubMed] [Google Scholar]
- Rumelhart, D. E. , Hinton, G. E. , & Williams, R. J. (1986). Learning representations by back‐propagating errors. Nature, 323(6088), 533–536. 10.1038/323533a0 [DOI] [Google Scholar]
- Sandre, A. , Banica, I. , Riesel, A. , Flake, J. , Klawohn, J. , & Weinberg, A. (2020). Comparing the effects of different methodological decisions on the error‐related negativity and its association with behavior and gender. International Journal of Psychophysiology, 156, 18–39. 10.1016/j.ijpsycho.2020.06.016 [DOI] [PubMed] [Google Scholar]
- Santesso, D. L. , & Segalowitz, S. J. (2008). Developmental differences in error‐related ERPs in middle‐ to late‐adolescent males. Developmental Psychology, 44(1), 205–217. 10.1037/0012-1649.44.1.205 [DOI] [PubMed] [Google Scholar]
- Santesso, D. L. , Segalowitz, S. J. , & Schmidt, L. A. (2006). Error‐related electrocortical responses in 10‐year‐old children and young adults. Developmental Science, 9(5), 473–481. 10.1111/j.1467-7687.2006.00514.x [DOI] [PubMed] [Google Scholar]
- Schultz, W. (2015). Neuronal reward and decision signals: From theories to data. Physiological Reviews, 95(3), 853–951. 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite, M. , Bates, A. T. , Groom, M. J. , Jackson, G. M. , Hollis, C. , & Liddle, P. F. (2012). Error processing‐associated event‐related potentials in schizophrenia and unaffected siblings. International Journal of Psychophysiology, 84(1), 74–79. 10.1016/j.ijpsycho.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Śmigasiewicz, K. , Ambrosi, S. , Blaye, A. , & Burle, B. (2020). Inhibiting errors while they are produced: Direct evidence for error monitoring and inhibitory control in children. Developmental Cognitive Neuroscience, 41(100), 100742. 10.1016/j.dcn.2019.100742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes, C. K. , Walhovd, K. B. , Torstveit, M. , Sells, V. T. , & Fjell, A. M. (2013). Performance monitoring in children and adolescents: A review of developmental changes in the error‐related negativity and brain maturation. Developmental Cognitive Neuroscience, 6, 1–13. 10.1016/j.dcn.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, A. , Crawford, H. , Morales, S. , Degnan, K. A. , Pine, D. S. , & Fox, N. A. (2020). Infant behavioral inhibition predicts personality and social outcomes three decades later. Proceedings of the National Academy of Sciences, 117(18), 9800–9807. 10.1073/pnas.1917376117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. B. , Visser, T. A. W. , Fueggle, S. N. , Bellgrove, M. A. , & Fox, A. M. (2018). The error‐related negativity (ERN) is an electrophysiological marker of motor impulsiveness on the Barratt Impulsiveness Scale (BIS‐11) during adolescence. Developmental Cognitive Neuroscience, 30, 77–86. 10.1016/j.dcn.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey, D. C. , Hajcak, G. , Kim, J. , Kujawa, A. , & Klein, D. N. (2012). Electrocortical and behavioral measures of response monitoring in young children during a go/no‐go task. Developmental Psychobiology, 54(2), 139–150. 10.1002/dev.20590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aert, R. C. M. , & van Assen, M. A. L. M. (2018). Correcting for publication bias in a meta‐analysis with the P‐uniform* method. MetaArXiv. 10.31222/osf.io/zqjr9 [DOI]
- van Veen, V. , & Carter, C. S. (2002a). The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiology & Behavior, 77(4), 477–482. 10.1016/S0031-9384(02)00930-7 [DOI] [PubMed] [Google Scholar]
- van Veen, V. , & Carter, C. S. (2002b). The timing of action‐monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14(4), 593–602. 10.1162/08989290260045837 [DOI] [PubMed] [Google Scholar]
- Vocat, R. , Pourtois, G. , & Vuilleumier, P. (2008). Unavoidable errors: A spatio‐temporal analysis of time‐course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia, 46(10), 2545–2555. 10.1016/j.neuropsychologia.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Weinberg, A. , Meyer, A. , Hale‐Rude, E. , Perlman, G. , Kotov, R. , Klein, D. N. , & Hajcak, G. (2016). Error‐related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. 10.1111/psyp.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, A. , Riesel, A. , & Hajcak, G. (2012). Integrating multiple perspectives on error‐related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 36(1), 84–100. 10.1007/s11031-011-9269-y [DOI] [Google Scholar]
- Wiersema, J. R. , van der Meere, J. J. , & Roeyers, H. (2007). Developmental changes in error monitoring: An event‐related potential study. Neuropsychologia, 45(8), 1649–1657. 10.1016/j.neuropsychologia.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Yeung, N. , Bogacz, R. , Holroyd, C. B. , Nieuwenhuis, S. , & Cohen, J. D. (2007). Theta phase resetting and the error‐related negativity. Psychophysiology, 44(1), 39–49. 10.1111/j.1469-8986.2006.00482.x [DOI] [PubMed] [Google Scholar]
- Zhang, J.‐S. , Wang, Y. , Cai, R.‐G. , & Yan, C.‐H. (2009). The brain regulation mechanism of error monitoring in impulsive children with ADHD‐‐an analysis of error related potentials. Neuroscience Letters, 460(1), 11–15. 10.1016/j.neulet.2009.05.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Funnel plot for the ERN group studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S2 Funnel plot for the Pe group studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S3 Funnel plot for the ERN correlational studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
FIGURE S4 Funnel plot for the Pe correlational studies. The shaded bands correspond to p‐values at p < .05 (yellow) and p < .01 (red)
TABLE S1
TABLE S2
TABLE S3
TABLE S4
TABLE S5 Effect sizes for the group studies on the ERN
TABLE S6 Effect sizes for the group studies on the Pe
TABLE S7 Effect sizes for the correlational studies on the ERN
TABLE S8 Effect sizes for the correlational studies on the Pe
Appendix S1
Appendix S2


