Dear Editor
We herein present the case of a 77‐year‐old Caucasian male patient treated with Dupilumab for chronic rhinosinusitis with bilateral nasal polyposis (CRswNP) with concomitant Grover disease. For his chronic rhinosinusitis, he was unsuccessfully treated with oral and intranasal corticosteroid and received two endoscopic sinus surgery (ESS) followed by multiple relapses. Five years ago, he was diagnosed with Grover disease and unsuccessfully treated with topical corticosteroids and Acitretin. The severity of his CRswNP was documented by a nasal polyps score (NPS, a score attesting the degree of nasal obstruction via endoscopy) of 5, a sino‐nasal outcome test (SNOT22, a 22‐item health‐related quality of life evaluation) of 55, the presence of severe smell impairment (VAS‐hyposmia = 10), his history of relapses and the need of continuous use of intranasal corticosteroids. He was therefore eligible to be treated with Dupilumab at standard doses (600 mg the initial dose and then 300 mg every other week). Dupilumab is a human monoclonal IgG4 antibody that inhibits interleukin‐4 (IL‐4) and interleukin‐13 (IL‐13) signaling by specifically binding to the IL‐4Rα subunit shared by the IL‐4 and IL‐13 receptor complexes and it is the first biological available for the treatment of CRswNP since 2019. 1 , 2 Dupilumab was used as monotherapy and was well tolerated, with no secondary adverse effects. After 14 weeks of treatment, the chronic rhinosinusitis did not improve (SNOT22 = 54, NPS = 8, VAS‐Hyposmia = 9) thus requiring the administration of one cycle of oral corticosteroid, but a complete regression of the dermatological signs and pruritus was observed. Thus, even though the ETN's therapeutic failure (40 weeks after the treatment: SNOT22 = 54, NPS = 8, VAS‐Hyposmia = 5, after a second cycle of oral corticosteroid), given the excellent dermatological response, the treatment with Dupilumab was not discontinued and after 44 weeks the patient remained completely clear (Figure 1).
FIGURE 1.
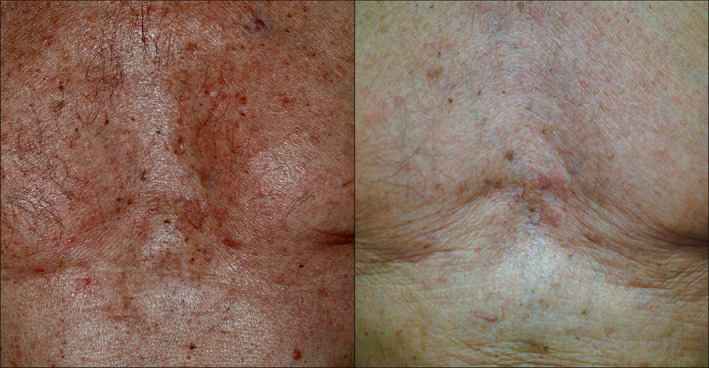
Clinical examination before treatment (on the left side) and after 40 weeks of treatment with Dupilumab at standard dose (on the left side)
Grover disease is a relatively common skin condition that generally affects Caucasian men older than 40 years and is characterized by an acute‐to‐subacute highly pruritic skin eruption of slightly flesh‐colored to erythematous, edematous papules and/or papulovesicles that commonly affects the trunk. Although the pathogenic mechanism of the disease remains unknown, it has been associated with heat, sweating, renal failure, malignancy and several drugs. There is currently no standard approach: as first‐line therapy topical steroids and vitamin D analogues are frequently used in association with adjuvant antihistamines; in severe, prolonged or refractory cases, oral retinoids and oral corticosteroids should be considered as well as phototherapy. 3 Butler et al. recently described three cases of Grover disease, initially unsuccessfully treated with traditional drugs, that markedly improved with Dupilumab at standard doses in 6–8 weeks. 4 The positive response to Dupilumab suggests that type‐2 inflammation may play a role in the pathogenesis of Grover disease, as shown by previous studies. Among them, a case of Grover disease after receiving intravenous recombinant human IL4, a known Th2‐inducer, and a second case induced by Ipilumab, an anti‐CTLA‐4 monoclonal antibody. 5 , 6 In the latter report, given that the same patient did not develop the same clinical lesions under treatment with Pembrolizumab (an anti‐PD1 monoclonal antibody), is consistent with preclinical data demonstrating that PD‐1 inhibition induces a Th1/Th17 responses while producing less Th2 type inflammation. One knows that age‐related changes of the immune system may induce a pro‐inflammatory status with dysregulation in the Th1/Th2‐system skewed toward a Th2‐inflammation profile. The higher production of Th2 cytokines documented in elderly results, in fact, in an enhanced unspecific B cell activation and IgE antibody production. 7 , 8 , 9 Since type‐2‐inflammation seems to contribute to the pathogenesis, the phenomenon illustrated before could explain why the incidence of Grover disease increases with age. Precisely because older patients, often dealing with multiple comorbidities, are the most affected from Grover disease, novel treatment options, safer and better tolerated than traditional medicines, need to be found. In the light of our case and the report of Butler et al, 4 Dupilumab seems to provide a safe and effective treatment for patients recalcitrant to traditional medications and oral drugs. Certainly, further studies are necessary to confirm our hypothesis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Written informed consent from the patient, for the use of image and publication of his case detail, has been obtained. F. Barei, N. Morini and S. Torretta wrote the paper while S. Ferrucci supervised this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Fokkens WJ, Lund VJ, Hopkins C, et al. Epos 2020. Off J Eur Int Rhinol Soc Confed Eur ORL‐HNS. 2020;29 suppl:1‐464. [Google Scholar]
- 2. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of Dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394:1638‐1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 3. Aldana PC, Khachemoune A. Grover disease: review of subtypes with a focus on management options. Int J Dermatol. 2020;59:543‐550. [DOI] [PubMed] [Google Scholar]
- 4. Butler DC, Kollhoff A, Berger T. Treatment of Grover disease with Dupilumab. JAMA Dermatol. 2021;153(3):353‐356. doi: 10.1001/jamadermatol.2020.5097 [DOI] [PubMed] [Google Scholar]
- 5. Mahler SJ, De Villez RL, Pulitzer DR. Transient acantholytic dermatosis induced by recombinant human interleukin 4. J Am Acad Dermatol. 1993;29(2 Pt 1):206‐209. doi: 10.1016/0190-9622(93)70169-t [DOI] [PubMed] [Google Scholar]
- 6. Uemura M, Fa'ak F, Haymaker C, et al. A case report of Grover's disease from immunotherapy‐a skin toxicity induced by inhibition of CTLA‐4 but not PD‐1. J Immunother Cancer. 2016;4:55. doi: 10.1186/s40425-016-0157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Martinis M, Sirufo MM, Viscido A, Ginaldi L. Food allergies and ageing. Int J Mol Sci. 2019;20(22):5580. doi: 10.3390/ijms20225580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015. Jan;29:24‐30. doi: 10.1016/j.jtemb.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 9. Alberti S, Cevenini E, Ostan R, et al. Age‐dependent modifications of type 1 and type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech Ageing Dev. 2006;127(6):560‐566. doi: 10.1016/j.mad.2006.01.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


