Abstract
Objective
The aim of this study is to determine the factors that affect patients’ ability to carry out high dose of massed practice.
Methods
Patients with stroke were included in the study if they had no severe impairment in motor and cognitive functions. Dose of massed practice, motor function, perceived amount and quality of use of the arm in the real world, wrist and elbow flexors spasticity, dominant hand stroke, presence of shoulder pain, and central post-stroke pain were assessed on the first day. Dose of massed practice was assessed again on the second day. The data were analyzed using descriptive statistics and linear multiple regression.
Results
Only motor function (β = –0.310, r = 0.787, P < 0.001), perceived amount of use (β = 0.300, r = 0.823; 95% CI = 0.34–107.224, P = 0.049), severity of shoulder pain (β = –0.155, r = –0.472, P = 0.019), wrist flexors spasticity (β = –0.154, r = –0.421, P = 0.002), age (β = –0.129, r = –0.366, P = 0.018), dominant hand stroke (β = –0.091, r = –0.075, P = 0.041), and sex (β = –0.090, r = –0.161, P = 0.036) significantly influenced patients’ ability to carry out high dose of massed practice.
Conclusion
Many factors affect patients’ ability to carry out high dose of massed practice. Understanding these factors can help in designing appropriate rehabilitation.
Keywords: dose, motor recovery, activities of daily living, quality of life
1. Introduction
Good function of the motor system is essential for carrying out activities of daily living (ADL) effectively. For instance, moving one’s upper limb is essential for eating, buttoning one’s shirt, breast feeding a baby, opening the door, bathing, cooking, and wearing shoes or clothes. However, the function of the motor system which is essential for movements that allow us to carry out our ADL may be impaired following stroke [1]. Consequently, people with stroke may not be able to use the affected limb in carrying out ADL. In addition, ADL such as bathing, cooking, and buttoning one’s shirts that require the use of both hands may not be carried out effectively. Therefore, rehabilitation to improve movement is important to regain the aforementioned functions. This can be achieved through the use of a number of rehabilitation techniques such as the constraint-induced movement therapy (CIMT) [2–5].
The CIMT is a neurorehabilitation technique used to improve functions of the motor system in people with disorders of the brain, particularly stroke [5–7]. The technique has many components; however, the chief among them include massed practice, constraint, and transfer package [8]. The massed practice involves repetitive practice of functional tasks with the affected limb. It is being regarded as the main driver for recovery, as it is essential for inducing use-dependent plasticity [9,10]. The constraint involves restraint of the unaffected limb with a mitt or sling for hours during the day to help maximize the use of the affected limb in the real world or laboratory or clinic. However, it is important to note that, the constraint does not have to be physical. Behaviural constraint wherein the patients consciously limit the use of the unaffected limb is also used [11,12]. The transfer package involves a contract that is designed for the patient to use the affected limb in daily activities in the real world in order to maximize recovery of function [8].
The neuroscientific basis for these three major components of CIMT (massed practice, constraint, and transfer package) is to help reverse learned non-use phenomenon that occurs after stroke [13,14]. This is said to be achieved through its influence on molecular activities, anatomical structures, and neurophysiological functions of the brain such as increased expression of brain-derived neurotrophic factor, increased cortical activation, and increased gray matter in sensory and motor areas [15–18]. Consequently, use of the limb in carrying out ADL is improved [5,19]. However, a pre-requisite for massed practice to effect recovery of brain’s functions is that, its dose needs to be as high as practical [9,20]. In essence, high dose of massed practice is required for recovery of motor function. In addition, what is very interesting is that, as long as patients performed high-dose massed practice, it does not matter whether the tasks carried out are specific or non-specific [21]. Furthermore, this high dose of massed practice required for motor recovery has been reported in the literature to range between 300 and 600 repetitions per day [7,22].
Interestingly, Birkenmeier and colleagues reported that, patients with stroke can carry out about 300 repetitions of massed practice within 1 h [22]. This seems to suggest that, high dose of massed practice during CIMT is possible. However, whether the ability to carry out high dose of massed practice is influenced by the clinical and personal characteristics of the patients, seems not to be determined. This is because in the study by Birkenmeier and colleagues, the patients were within chronic stage of stroke and the study participants had moderate movement ability of the upper limb. Therefore, the aim of this study is to determine the personal and clinical characteristics or factors that can influence patients’ ability to carry out high dose of massed practice during CIMT. Similarly, factors that could predict perceived amount and quality of use of the arm in the real world were also investigated.
2. Method
2.1. Study design
This study is a cross-sectional (observational) study, approved by the Research Ethics Committee of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria (Approval number, 954524802).
2.2. Study population
The study population is inpatient and outpatient stroke survivors in Ahmadu Bello University Teaching Hospital (Shika and Tudun Wada sites) in Kaduna state, Nigeria.
The inclusion criteria used for the selection of the study participants are: participants with clinical diagnosis of stroke, who met ICD-9 criteria, with no very severe impairment in motor function as indicated by a score of 1–3 on the motor arm item of the National Institutes of Health Stroke Scale (NIHSS) and a score of three or more on the upper arm item of the motor assessment scale. Additionally, participants were included if they had no severe cognitive impairment as indicated by a score of one or less on the consciousness and communication items of the NIHSS, had ability to perform two-step commands, and had a score of less than eight on the Short Blessed Memory Orientation and Concentration Scale [23,24]. However, participants were excluded if they had neglect indicated by more than three errors on the star cancellation test and sensory loss of two or more points on the sensory item of NIHSS [24].
2.3. Sample size estimation
The minimum sample size estimated for the study was 131 patients with stroke. The sample size was estimated (for linear multiple regression) using G-power software [25]. The parameters used for the estimation are effect size f 2 = 0.15, P = 0.05, power = 80% and the number of independent variable = 13 (age, sex, side affected, dominant hand stroke [before stroke], type of stroke, time since stroke, upper limb motor function, perceived amount and quality of the use of the arm in the real world, elbow and wrist flexors spasticity, presence of shoulder pain, and central post-stroke pain [CPSP]). However, 10% attrition rate (13) was added to make the total sample, 144.
The sampling technique used in the study was convenience sampling technique based on the above study inclusion and exclusion criteria.
2.4. Data collection procedure
Screening of the study participants for eligibility was carried out by qualified physiotherapists (one in each of the study sites), who were blinded to the aims of the study. For the patients who are eligible for inclusion in the study, their demographic and personal characteristics such as age, sex, time since stroke, and the types of stroke were recorded using a data capture sheet prepared purposely for the study.
Following this, the participants were asked to sit in a chair that has arm rest with a wooden table in front. An empty cup made of plastic with a fixed handle was placed on the table very close to the chair. The participants were made to carry out massed practice by picking up the cup from the table and taking it to their mouths. The massed practice was carried out for 1 h. Picking up a cup from the table and taking it to the mouth was chosen because it is commonly done in the real world. In addition, following stroke, performing tasks such as picking up a cup and taking it to the mouth can be challenging [7]; though, it can be more challenging to some patients compared to others depending on their motor ability.
During the practice, arm slings were worn by the participants in the unaffected upper limbs to ensure constraint of the limb. Stop watch was used to determine the time the participants started and completed performing massed practice. In addition, pen and paper were used to record the number of times (dose) the massed practice was performed by each participant. The massed practice was carried on the first and the second days. Thereafter, the average of the number of times (dose) the massed practice was carried out on the first and second days was calculated for each of the participants. The massed practice was timed using a stop watch.
Other outcomes that were assessed are motor function assessed using Wolf motor function test (WMFT), perceived amount and quality of use of the arm in the real world assessed using motor activity log (MAL), wrist and elbow flexors spasticity assessed using modified Ashworth scale (MAS), dominant hand stroke (before stroke) assessed using Oldfield Handedness Questionnaire, severity of shoulder pain assessed using visual analogue scale (VAS), and CPSP assessed using Douleur Neuropathique 4 Questionnaire (DNQ4). The WMFT consists of 17 items that are scored from zero to five. The higher the score, the better the motor function [3]. The measure has been reported to have good construct and criterion-related validity and inter-rater reliability [26]. The MAL has two parts that measure perceived amount and quality of use of arm in the real world [4]. In total, it consists of 30 items in which each of the items is scored from zero to five. A higher score indicates that, the perceived amount or quality of use of arm in the real world is good. The scale has been reported to be valid and reliable [27,28].
The MAS is a reliable measure of spasticity scored as 0, 1, +1, 2, 3, or 4, with 0 indicating absence of spasticity [29]. However, for the sake of statistical analysis, we considered a score of +1 as 2, a score of 2 as 3, a score of 3 as 4 and a score of 4 as 5. The VAS is a reliable instrument that consists of a 0–10 cm horizontal or vertical line used to assess patients’ report of the severity of their pain, with 0 denoting least pain and 10 denoting highest pain [30,31]. The DNQ4 is a reliable instrument that consists of four items capable of differentiating between neuropathic pain and non-neuropathic pain [32,33]. It is used in the assessment of CPSP. CPSP is a neuropathic pain syndrome following stroke that is characterized by pain and sensory abnormalities in the affected body part [34]. The Oldfield Handedness Questionnaire is a 20 items inventory that is rated by direct observation of the individuals’ behavior to help identify handedness [35]. All the study outcomes were assessed by well trained and blinded assessors (blinded to the aims of the study) who are qualified physiotherapists (one in each of the study sites).
2.5. Data analysis procedure
Participants’ demographic characteristics were summarized using descriptive statistics. Linear multiple regression analysis was used to determine which of the independent variables could significantly predict the participants’ ability to carry out high-dose massed practice required for recovery of motor function, and perceived amount and quality of use of the arm in the real world. The level of significance was set at <0.05. All the analyses were performed using SPSS version 20.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance the tenets of the Helsinki Declaration, and has been approved by the Research Ethics Committee of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria (Approval number, 954524802).
Informed consent: Informed consent has been obtained from all individuals included in this study.
3. Results
3.1. Characteristics of the study participants
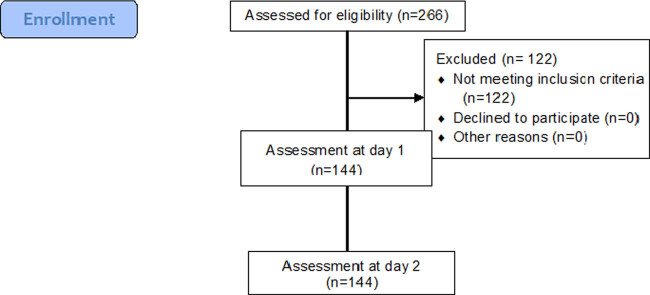
One hundred and forty four patients with stroke with age range, 21–101 years and time since stroke range, 1–208 weeks participated in the study. Eighty eight of the participants were men, while 56 were women. Details of the characteristics of the study participants are presented in Table 1. See also Figure 1 for the study flowchart.
Table 1.
Characteristics of the study participants
| Variable | Mean ± SD/Median (Interquartile range) | n | % |
|---|---|---|---|
| Sex (male = 1; female = 2) | 88/56 | 61.1/38.9 | |
| Type of stroke (ischemic = 1; hemorrhagic = 2) | 75/69 | 52.1/47.9 | |
| Dominant hand stroke (before stroke) (right = 1; left = 2) | 126/18 | 87.5/12.5 | |
| Side affected (right = 1; left = 2) | 101/43 | 70.1/29.9 | |
| Age (years) | 58.71 ± 19.90 | ||
| Time since stroke (weeks) | 36.38 ± 39.99 | ||
| Perceived amount of use (MAL [AOU], 0–5) | 3.00 ± 0.57 | ||
| Perceived quality of use (MAL [QOU], 0–5) | 3.05 ± 0.59 | ||
| Motor function ([WMFT], 0–5) | 1.96 ± 0.74 | ||
| Dose of massed practice (number of repetition of the task per hour) | 437.50 ± 99.18 | ||
| Star cancellation | 0.70 ± 0.84 | ||
| Star cancellation error | 1.00(1.00) | ||
| Number of rest | 3.80 ± 1.28 | ||
| Severity of shoulder pain ([VAS], 0–10 cm) | 1.31 ± 1.32 | ||
| Wrist flexors spasticity ([MAS], 0–5) | 0.00(0.00) | ||
| Elbow flexors spasticity ([MAS], 0–5) | 0.50(1.00) | ||
| CPSP | 1.00(2.00) | ||
| Severity of arm paresis | 0.50(1.00) | ||
| Severity of sensory loss | 0.00(0.00) | ||
| Cognitive ability | 2.00(3.50) |
MAL [AOU], motor activity log [amount of use]; MAL [QOU], motor activity log [quality of use]; WMFT, Wolf motor function test; VAS, visual analogue scale; MAS, modified Ashworth scale.
Figure 1.
Study flowchart.
3.2. Predictors of ability to carry out high dose of massed practice
The participants performed an average of 437.50 ± 99.18 dose of massed practice with a range 220–634. However, the mean and standard deviation of the residuals for dose of massed practice was 433.14 ± 89.62.
The result of the linear multiple regression showed that, the total variance explained by the whole model was significant, 88.4% (R = 0.884), F(13, 144) = 35.931, R 2 = 0.782, P < 0.001. See Figure 2 for the scatter plot illustrating the regression line.
Figure 2.
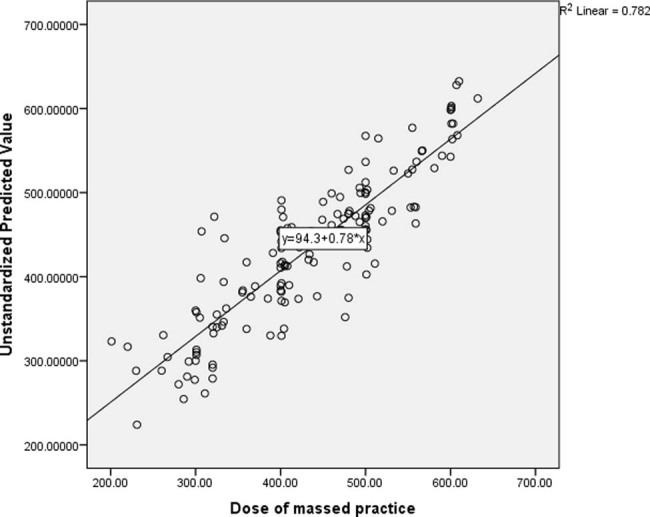
Scatter plot illustrating the regression line of predictors of ability to carry out high dose of massed practice during CIMT.
In the final model, the only independent variables that significantly predicted patients’ ability to carry out high dose of massed practice were motor function (β = –0.31, P < 0.001), perceived amount of use of the arm in the real world (β = 0.30, P = 0.049), severity of shoulder pain (β = –0.16, P = 0.019), wrist flexors spasticity (β = –0.15, P = 0.002), age (β = –0.129, P = 0.018), dominant hand stroke [before stroke] (β = –0.09, P = 0.041), and sex (β = –0.09, P = 0.036). See Table 2 for the details of the result.
Table 2.
Predictors of high dose of massed practice
| Variables | β | r | 95% | P |
|---|---|---|---|---|
| Age (years) | –0.129 | –0.366 | –1.198 to 0.114 | 0.018* |
| Time since stroke (weeks) | –0.049 | –0.162 | –0.34 to 0.093 | 0.260 |
| Type of stroke (ischemic = 1; hemiplegic = 2) | –0.004 | –0.332 | –21.458 to 20.00 | 0.945 |
| Dominant hand stroke (before stroke) (right = 1; left = 2) | –0.091 | –0.075 | –54.259 to –1.175 | 0.041* |
| Severity of shoulder pain (VAS, 0–10 cm) | –0.155 | –0.472 | –21.843 to –1.946 | 0.019* |
| Wrist flexors spasticity ([MAS], 0–5) | –0.154 | –0.421 | –52.708 to –11.597 | 0.002* |
| Elbow flexors spasticity ([MAS], 0–5) | –0.050 | –0.556 | –33.432 to 14.897 | 0.449* |
| CPSP | 0.102 | –0.548 | –3.639 to 18.931 | 0.812 |
| Side affected (Right = 1; Left = 2) | –0.041 | 0.010 | –27.876 to 9.936 | 0.350 |
| Perceived amount of use (MAL [AOU], 0–5] | 0.300 | 0.823 | 0.34 to 107.224 | 0.049* |
| Perceived quality of movement (MAL [QOU], 0–5] | 0.132 | 0.979 | –22.831 to 68.102 | 0.326 |
| Motor function (WMFT, 0–5) | 0.310 | 0.787 | 19.830 to 64.513 | <0.001* |
| Sex (male=1; female=2) | –0.090 | –0.161 | –35.862 to –1.268 | 0.036* |
VAS, visual analogue scale; MAS, modified Ashworth scale; MAL [AOU], motor activity log [amount of use]; MAL [QOU], motor activity log [quality of use]; WMFT, Wolf motor function test.
*Significance at p < 0.05.
3.3. Predictors of perceived amount of use of the arm in the real world
The observed mean perceived amount of use of the arm in the real world was 3.05 ± 0.59. However, the mean and standard deviation of the residuals for perceived amount of use of the arm was 3.00 ± 0.50.
The result of the multiple regression analysis showed that, the total variance explained by the whole model was significant, 88.6% (R = 0.886), F(11, 144) = 43.875, R 2 = 0.785, P < 0.001. See Figure 3 for the scatter plot illustrating the regression line.
Figure 3.
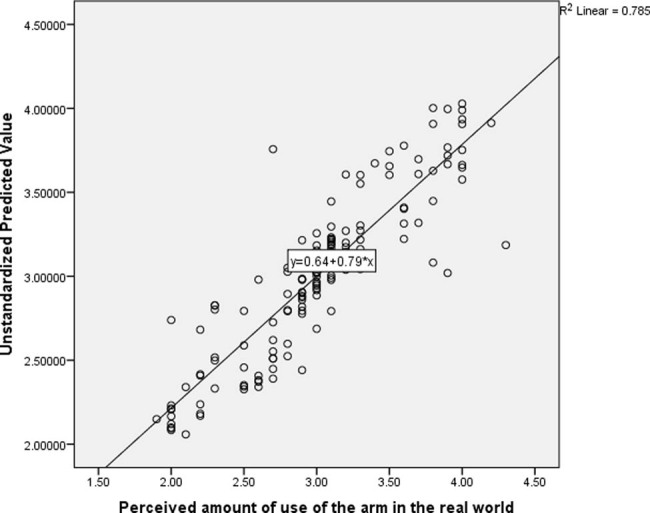
Scatter plot illustrating the regression line of predictors of perceived amount of use of the arm in the real limb following CIMT.
In the final model, the only independent variables that significantly predicted perceived amount of use of the arm in the real world were motor function (β = 0.699, P < 0.001) and CPSP (β = –0.159, P = 0.034). See Table 3 for the details of the result.
Table 3.
Predictors of perceived amount of use of the arm in the real world
| Variables | β | r | 95% | P |
|---|---|---|---|---|
| Age (years) | –0.079 | –0.275 | –0.005 to 0.001 | 0.131 |
| Time since stroke (weeks) | –0.012 | –0.094 | –0.001 to 0.001 | 0.773 |
| Type of stroke (ischemic = 1; hemiplegic = 2) | 0.046 | –0.295 | –0.061 to 0.165 | 0.364 |
| Dominant hand stroke (before stroke) (right = 1; left = 2) | 0.055 | 0.048 | –0.050 to 0.236 | 0.199 |
| Severity of shoulder pain (VAS, 0–10 cm) | 0.029 | –0.440 | –0.042 to 0.067 | 0.653 |
| Wrist flexors spasticity ([MAS], 0–5) | –0.092 | –0.361 | –0.219 to 0.004 | 0.059 |
| Elbow flexors spasticity ([MAS], 0–5) | –0.110 | –0.618 | 0.245 to 0.015 | 0.082 |
| CPSP | –0.159 | –0.601 | –0.127 to –0.005 | 0.034* |
| Side affected (right = 1; left = 2) | –0.071 | 0.064 | –0.190 to 0.016 | 0.097 |
| Motor function (WMFT, 0–5) | 0.699 | 0.850 | 0.450–0.611 | <0.001* |
| Sex (right = 1; left = 2) | 0.006 | –0.090 | 0.087–0.102 | 0.876 |
VAS, visual analogue scale; MAS, modified Ashworth scale; WMFT, Wolf motor function test.
*Significance at p < 0.05.
3.4. Predictors of perceived quality of use of the arm in the real world
The observed mean perceived quality of use of the arm in the real world was 3.00 ± 0.57. However, the mean and standard deviation of the residuals for perceived quality of use of the arm in the real world was 3.05 ± 0.51.
The result of the multiple regression analysis showed that, the total variance explained by the whole model was significant, 85.4% (0.854), F(11, 144) = 32.418, R 2 = 0.73, P < 0.001. See Figure 4 for the scatter plot illustrating the regression line.
Figure 4.
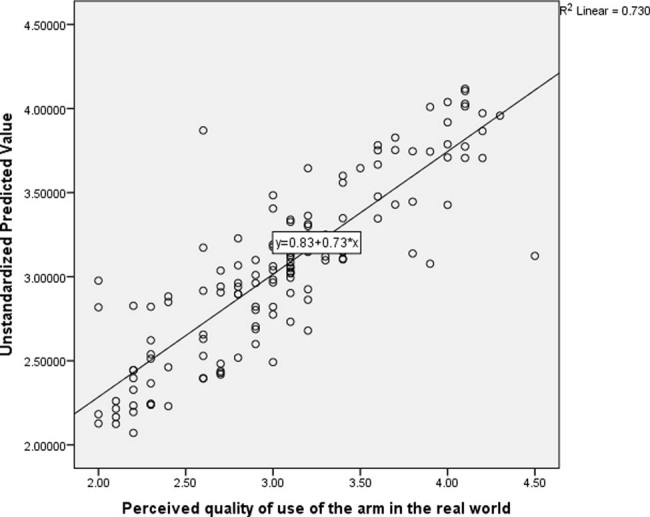
Scatter plot illustrating the regression line of predictors of perceived quality of use of the arm in the real limb following CIMT.
In the final model, the only independent variables that significantly predicted perceived quality of use of the arm in the real world were motor function (β = 0.714, P < 0.001) and dominant hand stroke (before stroke) (β = 0.105, P = 0.029). See Table 4 for the details of the result.
Table 4.
Predictors of perceived quality of use of the arm in the real world
| Variables | β | r | 95% | P |
|---|---|---|---|---|
| Age (years) | –0.109 | –0.299 | –0.007 to 0.000 | 0.066 |
| Time since stroke (weeks) | –0.012 | –0.019 | –0.002 to 0.001 | 0.695 |
| Type of stroke (ischemic = 1; hemiplegic = 2) | 0.068 | 0.281 | –0.053 to 0.213 | 0.238 |
| Dominant hand stroke (before stroke) (right = 1; left = 2) | 0.105 | 0.083 | –0.019 to 0.355 | 0.029* |
| Severity of shoulder pain (VAS, 0–10 cm) | –0.022 | –0.411 | –0.055 to 0.074 | 0.766 |
| Wrist flexors spasticity ([MAS], 0–5) | –0.082 | –0.312 | –0.232 to 0.030 | 0.131 |
| Elbow flexors spasticity ([MAS], 0–5) | –0.026 | –0.549 | –0.181 to 0.124 | 0.712 |
| CPSP | –0.156 | –0.566 | –0.140 to 0.044 | 0.062 |
| Side affected (right = 1; left = 2) | –0.059 | 0.066 | –0.197 to 0.045 | 0.215 |
| Motor function (WMFT, 0–5) | 0.714 | 0.822 | 0.473 to 0.662 | <0.001* |
| Sex (right = 1; left = 2) | 0.006 | –0.119 | 0.148 to 0.074 | 0.513 |
VAS, visual analogue scale; MAS, modified Ashworth scale; WMFT, Wolf motor function test.
*Significance at p < 0.05.
4. Discussion
The result showed that, only seven independent variables (motor function, perceived amount of use of the arm in the real world, severity of shoulder pain, wrist flexors spasticity, age, dominant hand stroke, and sex) significantly influenced the participants’ ability to carry out high dose of massed practice per day, required for the recovery of motor function. For the real world arm use, only motor function and CPSP; and motor function and dominant hand stroke significantly influenced perceived amount and quality of use of the arm, respectively. However, the mean scores for motor function and perceived amount of use of the arm indicate mild motor ability and moderate perceived amount of use of the arm, respectively. In addition, severity of shoulder pain was mild, there was little or no wrist flexors spasticity, the patients were middle aged, most of the participants had dominant hand stroke and the majority were men. Good motor function is the evidence for the integrity of the motor system that controls human movement. Ability to move is strongly related to the ability to carry out ADL [36].
Similarly, ability to carry out ADL such as washing, bathing, feeding, cutting meat or a loaf of bread, and walking is also a predictor of good quality of life [37]. Achieving good quality of life is the ultimate goal of rehabilitation. Furthermore, there is a strong positive correlation between how frequently the arm is used in carrying out tasks in the real world (perceived amount of use of the arm) and motor function [38]. Therefore, it is important to encourage people with stroke to use their affected arms in the real world. This is the rationale for transfer package, one of the most important components of CIMT. Transfer package is a set of behavioral techniques used to help patients transfer the therapeutic gains following CIMT to real daily life situations [39]. Thus, CIMT protocols should include transfer package and tasks that are related to the patient’s everyday tasks that consider their cultural practices or norms.
Presence of pain especially pain in the shoulder during the first and third months after the stroke can limit movement [40]. Therefore, pain management especially shoulder pain should be a priority in order to help patients with stroke carry out high-dose massed practice during CIMT. In addition, it is important for the therapists to determine the dose of massed practice patients with pain in the arm can carry out during CIMT. Accordingly, in a child with severe shoulder pain and motor impairment following cerebral malaria, 75 repetitions of massed practice per day for 6 weeks resulted in marked improvement in motor function [41]. This can serve as a model for the dose of massed practice to be used in people with stroke who have pain that could limit their ability to carry out high dose of massed practice.
Spasticity can affect movement pattern [42], and this can slow down movement speed and efficiency. Consequently, this may have downstream effects on the patients’ quality of life [43]. Therefore, in patients with wrist and/or elbow flexors spasticity, it is important to ask patients to carry out a specific dose of massed practice in terms of how many times the practice is done rather than asking them to carry it out in for example 2 h. This is because the patients may not be able to achieve the dose of massed practice required for improvement within those recommended hours. In addition, spasticity may be associated with pain [44]. Both pain and spasticity can limit function. As such, for patients to be able to carry out high dose of massed practice, spasticity and pain as the case may be, need to be managed promptly. Furthermore, as we chronologically age, the structures of the nervous system (both the central and peripheral nervous systems) age too [45]. This may lead to motor performance deficits such as impaired coordination and speed [46].
In addition, older people may suffer fatigue quite readily compared to younger people. All these can hinder the patients’ ability to carry out high dose of massed practice. However, use of distributed tasks practice in which practice is done in sessions per day to help with preventing the effects of fatigue, can help older patients to achieve high-dose massed practice required for recovery. Distributed practice has been used with success [47].
For sex influence, women tend to perform daily tasks less than men after stroke [48,49]. However, this can be attributed to old age and lower pre-stroke physical function level [48]. Similarly, it may also be related to loss of internal locus of control. Thus, in designing CIMT protocol for female patients, measures such as motivational interviewing should be used to motivate them. Such measures of motivation have been used to improve stroke patients’ performance in physical function [50]. In addition, women tend to be more depressed and have anxiety more than men after stroke [49]. Depressive symptoms are negatively correlated with all variables of motor skills [50,51]. Therefore, it is important to improve the mental health of patients especially women undergoing CIMT. This will enable them achieve the desired goal.
Individuals with dominant hand stroke demonstrate less impairment than those with non-dominant hand stroke [52]. Thus, they may be able to perform high-dose massed practice during CIMT. Massed practice with the dominant hand results in better improvement in motor function [53]. Consequently, therapists should devise means through which patients with non-dominant hand stroke can be able to practice high-dose massed practice. This can be done through the use of distributed practice whereby practice sessions in a day can be spread to suit the patients’ capability. In addition, transfer package, one of the components of CIMT can be used to help such patients achieve high-dose massed practice. Transfer package helps with adherence and extension of therapy beyond the laboratory or clinic. Adherence and increasing dose of therapy improves outcomes [54]. Furthermore, although side affected may influence dose of massed practice because of hemisphere-specific motor deficit [55]; in this study, side affected did not significantly influence dose of massed practice. This is probably because the patients included in the study had mild to moderate impairment in motor function.
Although this study has some strengths such as its relatively large sample size; it however has some key limitations such as lack of follow-up of the participants. This limitation can affect the reliability of the findings, and therefore it needs to be factored in when conducting future studies on the subject matter. In addition, motivation and enriched environment were not assessed in this study. Motivation and enriched environment are some of the factors that can affect recovery of motor function [56].
5. Conclusion
Many personal and clinical characteristics of patients with stroke can affect their ability to carry out high-dose massed practice during rehabilitation. Therefore, it is important that these factors are factored in during rehabilitation in order to help patients achieve the dose of massed practice required for recovery following stroke. In particular, distributed practice and transfer package can be used to help patients with stroke achieve high-dose massed practice during CIMT.
Acknowledgments
We would like thank the patients who participated in the study and the therapists who helped in the collection of the data.
Footnotes
Author contributions: A.A. conceived the idea. A.A. and B.S. designed the study with inputs from U.U., S.T., and W.S. B.S. monitored and actively involved in the data collection. A.A. analyzed and interpreted the data. B.S., U.U., S.T., and W.S. reviewed the data analysis and interpretation. A.A. drafted the manuscript. B.S., U.U., S.T., and W.S. critically reviewed the drafted manuscript. All authors approved the manuscript for submission.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Raghavan P. Upper limb motor impairment post stroke. Phys Med Rehabil Clin N Am. 2015;26(4):599–610. 10.1016/j.pmr.2015.06.008. [DOI] [PMC free article] [PubMed]
- [2].Ostendorf CG, Wolf SL. Effect of forced use of the upper extremity of a hemiplegic patient on changes in function: a single-case design. Phys Ther. 1981;61(7):1022–8. [DOI] [PubMed]
- [3].Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104(2):125–32. [DOI] [PubMed]
- [4].Taub E, Miller NE, Novack TA, Cook IEW, Fleming WC, Nepomuceno CS. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347–54. [PubMed]
- [5].Etoom E, Hawamdeh M, Hawamdeh Z. Constraint induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: systematic review and metaanalysis. Int J Rehabil Res. 2016;39(3):197–210. [DOI] [PubMed]
- [6].Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D. Effect of constraint induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–104. [DOI] [PubMed]
- [7].Abdullahi A. Effects of number of repetitions and number of hours of shaping practice during constraint-induced movement therapy: a randomized controlled trial. Neurol Res Int. 2018;2018:5496408. 10.1155/2018/5496408. [DOI] [PMC free article] [PubMed]
- [8].Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterising the intervention protocol. Eura Medicophys. 2006;42:257–68. [PubMed]
- [9].Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75(5):2144–9. [DOI] [PubMed]
- [10].Mawase F, Uchera S, Brutian AS. Motor relay enhances use dependent plasticity. S Neurosci. 2017;37(10):2673–85. [DOI] [PMC free article] [PubMed]
- [11].Abdullahi A, Umar NA, Ushotanefe U, Abba MA, Akindele MO, Truijen S, et al. Comparing two different modes of tasks practice during lower limbs constraint-induced movement therapy in people with stroke: a randomized clinical trial. Neural Plast. 2021;2021:6664058. 10.1155/2021/6664058. [DOI] [PMC free article] [PubMed]
- [12].Brogårdh C, Vestling M, Sjölund BH. Shortened constrained induced movement therapy in subacute stroke-no effect of using a restraint: a randomized controlled study with independent observers. J Rehabil Med. 2009;41:231–6. [DOI] [PubMed]
- [13].Taub E, Uswatt G. Constraint-induced movement therapy: answers and questions after two decades of research. Neurorehabilitation. 2006;21(2):93–5. [PubMed]
- [14].Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42(3):241–56. [PubMed]
- [15].Wang W, Wang A, Yu L. Constraint-induced movement therapy promotes brain functional reorganization in stroke patients with hemiplegia. Neural Regen Res. 2012;7(32):2548–53. 10.3969/j.issn.1673-5374.2012.32.010. [DOI] [PMC free article] [PubMed]
- [16].Sawaki L, Butler AJ, Leng X. Constraint induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505–13. [DOI] [PMC free article] [PubMed]
- [17].Gauthier LV, Tamb E, Perkins C, Ostemann M, Mark VW, Uswatte G. Remodeling the brain plastic structural brain changes produced by different motor therapies afterstroke. Stroke. 2008;30(5):1520–5. 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed]
- [18].Cunningham DA, Varnerin N, Machado A, Bonnetta C, Janini D, Roelle S. Stimulation targeting higher motor areasin stroke rehabilitation: a proof-of-concept, randomized, double-blinded placebo-controlled study of effectiveness and underlying mechanisms. Restor Neurol Neurosci. 2015;33(6):911–26. 10.3233/RNN-150574. [DOI] [PMC free article] [PubMed]
- [19].Dromerick AW, Lang CE, Birkienmeire RL. Very early CIMT during stroke rehabilitation (VECTOR): a single-center RCT. Neurology. 2009;73(3):195–201. 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed]
- [20].Abdullahi A, Shehu S, Dantani B. Feasibility of high repetitive task practice during CIMT in acute stroke. Int J Ther Rehabil. 2014;21(4):190–5. 10.12968/ijtr.2014.21.4.190. [DOI]
- [21].Classer J, Lieperi J, Wise SP. Rapid plasticition of human cortical mout presentation induced by practice. J Neurophysiol. 1998;79(2):1117–23. [DOI] [PubMed]
- [22].Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–35. 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed]
- [23].Poole JL, Whitney SL. Motor assessment scale for stroke patients: concurrent validity and interrater reliability. Arch Phys Med Rehabil. 1988;69:195–7. [PubMed]
- [24].Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989;334(8668):908–11. [DOI] [PubMed]
- [25].Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39(2):175–91. [DOI] [PubMed]
- [26].Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32(7):1635–9. [DOI] [PubMed]
- [27].Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke. 2005;36(11):2493–6. [DOI] [PubMed]
- [28].Vander Lee JH, Beckerman H, Knol DL, DeVet HCW, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410–4. [DOI] [PubMed]
- [29].Kaya T, Karatepe AG, Gunaydin R, Koc A, AltundalErcan U. Inter-rater reliability of the modified ashworth scale and modified modified ashworth scale in assessing poststroke elbow flexor spasticity. Int J Rehabil Res. 2011;34(1):59–64. 10.1097/MRR.0b013e32833d6cdf. [DOI] [PubMed]
- [30].Pomeroy VM, Frames C, Faragher EB, Hesketh A, Hill E, Watson P, et al. Reliability of a measure of post-stroke shoulder pain in patients with and without aphasia and/or unilateral spatial neglect. Clin Rehabil. 2000;144(6):584–91. 10.1191/0269215500cr365oa. [DOI] [PubMed]
- [31].McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue: a critical review. Psychol Med. 1988;18:1007–19. [DOI] [PubMed]
- [32].Bouhassira D, Attal N, Alchaar H. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. [DOI] [PubMed]
- [33].Benzon HT. The neuropathic pain scales. Reg Anesth Pain Med. 2005;30:417–21. [DOI] [PubMed]
- [34].Klit H, Nielsen J, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8(9):857–68. 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed]
- [35].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed]
- [36].Tanaka T, Hashimoto K, Kobayashi K, Sugawara H, Abo M. Revised version of the ability for basic movement scale (ABMS II) as an early predictor of functioning related to activities of daily living in patients after stroke. J Rehabil Med. 2010;42:179–81. 10.2340/16501977-0487. [DOI] [PubMed]
- [37].Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sorenson P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2(52):52. 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed]
- [38].Franck JA, Smeets RJ, Seelen HAM. Changes in actual arm-hand use in stroke patients during and after clinical rehabilitation involving a well-defined arm-hand rehabilitation program: a prospective cohort study. PLoS One. 2019;14(4):e0214651. 10.1371/journal.pone.0214651. [DOI] [PMC free article] [PubMed]
- [39].Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, et al. Method for enhancing real-world use of a more-affected arm in chronic stroke: the transfer package of CI therapy. Stroke. 2013;44(5):1383–8. 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed]
- [40].Hamzat TK, Osundiya OC. Musculoskeletal pain and its impact on motor performance among stroke survivors. Hong Kong Physiother J. 2010;28(1):11–5. 10.1016/j.hkpj.2010.11.001. [DOI]
- [41].Abdullahi A, Shehu S, Abdurrahman Z, Bello B. Determination of optimal dose of tasks practice during constraint induced movement therapy in a stroke patient with severe upper limb pain. Indian J Physiother Occup Ther. 2015;9(1):198. 10.5958/0973-5674.2015.00039.8. [DOI]
- [42].Mochizuki G, Centen A, Resnick M, Lowrey C, Dukelow SP, Scott SH. Movement kinematics and proprioception in post-stroke spasticity: assessment using the Kinarm robotic exoskeleton. J Neuro Eng Rehabil. 2019;16:146. 10.1186/s12984-019-0618-5. [DOI] [PMC free article] [PubMed]
- [43].Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013;80(3 Suppl 2):S45–52. 10.1212/WNL.0b013e3182764c86. [DOI] [PubMed]
- [44].Baricich A, Picelli A, Molteni F, Guanziroli E, Santamato A. Post-stroke spasticity as a condition: a new perspective on patient evaluation. Funct Neurol. 2016;31(3):179–80. 10.11138/FNeur/2016.31.3.179. [DOI] [PMC free article] [PubMed]
- [45].Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, BennettA D, et al. Aging, the central nervous system, and mobility. J Gerontol. 2013;68(11):1379–86. 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed]
- [46].Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Mot Control. 2002;6(1):19–31. 10.1123/mcj.6.1.19. [DOI] [PubMed]
- [47].Dettmers C, Teske U, Hamzei F, Uswatte G, Taub E, Weiller C. Distributed form of constraint-induced movement therapy improves functional outcome and quality of life after stroke. Arch Phys Med Rehabil. 2005;86(2):204–9. 10.1016/j.apmr.2004.05.007. [DOI] [PubMed]
- [48].Sue-Min L, Duncan PW, Dew P, Keighley J. Research. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2(3):A13. [PMC free article] [PubMed]
- [49].Espuela FL, Cuenca JCP, Díaza CL, Sánchez JMP, Gamez-Leyva G, Naranjo IC. Sex differences in long-term quality of life after stroke: influence of mood and functional status. Neurología. 2019;35:470–8. 10.1016/j.nrleng.2017.10.002. [DOI] [PubMed]
- [50].Rapolienė J, Endzelytė E, Jasevičienė I, Savickas R. Stroke patients motivation influence on the effectiveness of occupational therapy. Rehabil Res Pract. 2018;2018:9367942. 10.1155/2018/9367942. [DOI] [PMC free article] [PubMed]
- [51].Yoshida HM, Lima FO, Barreira J, Appenzeller S, Fernandes PT. Is there a correlation between depressive symptoms and motor skills in post-stroke patients? Arq Neuropsiquiatr. 2019;77(3):155–60. 10.1590/0004-282X20190012. [DOI] [PubMed]
- [52].Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the non-dominant hand affected. Neurorehabil Neural Repair. 2006;20(3):380–9. 10.1177/1545968305284528. [DOI] [PMC free article] [PubMed]
- [53].Khan MA, Ahmad F, Moiz JA, Nooho MM. Effects of task related training and hand dominance on upper limb motor function in subjects with stroke. Iran Rehabil J. 2011;9(2):55–9.
- [54].Gunnes M, Indredavik B, Langhammer B, Lydersen S, Ihle-Hansen H, Dahl AE, et al. LAST Collaboration group. Associations between adherence to the physical activity and exercise program applied in the LAST study and functional recovery after stroke. Arch Phys Med Rehabil. 2019;100(12):2251–9. 10.1016/j.apmr.2019.04.023. [DOI] [PubMed]
- [55].Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47(13):2953–66. 10.1016/j.neuropsychologia. [DOI] [PMC free article] [PubMed]
- [56].Solomon-Avnon D, Mawase F. The dose and intensity matter for chronic stroke. J Neurol Neurosurg Psychiatry. 2019;90:1187–8. [DOI] [PubMed]