Abstract
Over the past decade, single‐use tangential flow filtration (TFF) technologies have emerged to reduce system preparation time, promote fast and flexible product change over, and ultimately shorten process development and manufacturing time/cost. In this study, the performance of a recently developed Pellicon® single‐use TFF capsule was compared against traditional Pellicon® cassettes by assessing TFF process performance (such as flux, residuals clearance, and yield) and post‐purification product attributes (such as concentration and mass‐weighted average molecular weight). Good scaling was shown by comparing process performance and product attributes across different scales and formats. Additionally, similar TFF process performance and post‐purification product attributes were observed for the single‐use capsule compared to the reusable TFF cassettes. The capsule requires a smaller flush than the cassette, and it is easier to use since it does not require a compression holder or pre‐sanitization. The results provide insight into the application of the single‐use TFF capsule and scalability of TFF processes for the purification of conjugate vaccines.
Keywords: scale up, single use, TFF, vaccines
1. INTRODUCTION
Single‐use technologies can transform biopharmaceutical manufacturing by increasing speed to commercialization and flexibility of facility design while decreasing capital requirements, manufacturing cost, contamination risk, and environmental impact. 1
Tangential flow filtration (TFF) is used in almost every biopharmaceutical manufacturing downstream process to provide gentle, fast concentration, and diafiltration. The current trend toward smaller bioreactors and multiproduct manufacturing facilities has increased the demand for plug‐and‐play single‐use TFF devices. A recent advance is the introduction of a spiral TFF device (Pellicon® Capsule) that has the high performance of conventional cassettes with the added benefit of self‐containment and operating without a compression holder. Since the Pellicon® Capsule comes pre‐sterilized and preservative‐free, it can eliminate up to 10 operational steps in the total TFF process and reduce auxiliary solution volume consumption by up to 85% when used with a single‐use flow path‐compared to multi‐use cassette systems. 2
Highly effective vaccines against diseases caused by Neisseria meningitidis, Streptococcus pneumoniae, and H. influenza can be produced by chemical conjugation of the capsular polysaccharide from these bacteria to an immunogenic protein such as tetanus toxoid, CRM197 or diphtheria toxoid. 3 , 4 The conjugation process typically involves a polysaccharide activation step in preparation for covalent linkage to the immunogenic protein. 5 , 6 , 7 TFF is widely used for buffer exchange and purification of activated and conjugated polysaccharides against reaction byproducts or residuals. 8 , 9 , 10
Although earlier studies have demonstrated the feasibility of using ultrafiltration for the concentration, diafiltration, and purification of polysaccharide vaccines, 8 , 9 , 10 there are no systematic studies available to demonstrate the use of single use TFF technologies for the purification of conjugate vaccines. The primary objective of this work was to evaluate a novel, gamma‐sterilized Pellicon® single‐use TFF capsule against traditional reusable Pellicon® cassettes with respect to performance, flush requirement, product recovery and ease‐of‐use; whereas the secondary objective was to assess the scalability of Pellicon® TFF cassettes and capsule for the purification of activated polysaccharides.
2. EXPERIMENTAL
2.1. Materials and methods
General procedure for preparation of activated polysaccharide has been reported in the literature. 6 , 7 For this study, the pneumococcal polysaccharide is synthesized on the cell wall of bacterium S. pneumoniae (S. pneumoniae) and goes through fermentation and recovery process. The resulting recovered pneumococcal polysaccharide (hereinafter referred to as native pneumococcal polysaccharide) is diluted with potassium phosphate, pH 6.0 dilution buffer and water for injection (WFI) to the target phosphate and polysaccharide concentration. If required, the pH of the diluted pneumococcal polysaccharide is adjusted to the target range. The native pneumococcal polysaccharide oxidation is started by adding a quantity of periodate solution (sodium meta‐periodate in WFI) sufficient to achieve the desired degree of oxidation. The oxidation is then allowed to mix for the target time at the target temperature. After the completion of the oxidation reaction, a quantity of quenching reagent solution (quench reagent in WFI) is added to consume any unreacted sodium periodate. After addition, the quench reaction proceeds for the target time range while agitating, maintaining temperature at the target range to produce the crude activated polysaccharide. The resulting activated polysaccharide was used for subsequent purifications using different MilliporeSigma TFF modules (MilliporeSigma, Burlington, MA) listed in Table 1. All modules were flushed with WFI for ≥20 L/m2 to remove storage solution. Next, all modules were equilibrated with the feed matrix buffer and then, diafiltration was performed for buffer exchange/purification. Additional information on TFF operation conditions can be found in Table 2. Once diafiltration was done, the purified activated polysaccharide was recovered by pumping the product out of the tank/line via retentate line. Then rinse/flush was performed by adding 1 system hold‐up volume of DF buffer (WFI), recirculating, and collecting the flush volume. The flowrate during recirculation was maintained at 3.5 L/min/m2 (LMM).
TABLE 1.
Summary of TFF modules used for purification of activated polysaccharide
| Module name | Surface area (cm2) | Membrane chemistry | Nominal molecular weight cut‐off (kDa) | Screen type | Catalog number |
|---|---|---|---|---|---|
|
Pellicon® TFF Cassette (small scale) Biomax® membrane |
88 | Polyethersulfone | 30 | A | P3B030A00 |
|
Pellicon® TFF Cassette (small scale) Ultracel® membrane |
88 | Regenerated cellulose | 30 | C | P3C030C00 |
|
Pellicon® TFF Cassette (large scale) Ultracel® membrane |
1100 | Regenerated cellulose | 30 | C | P3C030C01 |
|
Pellicon® TFF Capsule (large scale) Ultracel® membrane |
1000 | Regenerated cellulose | 30 | C | PCC030C01 |
TABLE 2.
Summary of TFF operating conditions
| Module name | Diafiltration buffer | Diafiltration concentration (g/L) | Cross flow rate (L/min/m2) | Number of diavolumes | Membrane loading (g/m2) |
|---|---|---|---|---|---|
| All modules | WFI | 4.0 | 3.5 | 20 | 20 |
The hydrodynamics and flux performances of four TFF modules (88 cm2 Biomax® cassette, 88 cm2 Ultracel® cassette, 1100 cm2 Ultracel® cassette, and 1000 cm2 Ultracel® single‐use capsule) for purification of an activated polysaccharide were compared. Biomax® and Ultracel® membranes were included to investigate the impact of membrane chemistry on activated polysaccharide purification while 88, 1000, 1100 cm2 modules were studied to assess scalability within the Pellicon® family. The performance of the cassettes and capsule membranes were assessed by the diafiltration of the polysaccharide under the same cross flow rate and optimal transmembrane pressure (TMP) across the membrane surface.
TMP excursion studies were performed to determine the optimal operating TMP condition for the UFDF operation. For each module listed under Table 1, TMP was ramped up from 2 psi to greater than 23 psi and the corresponding permeated flux at intermediate TMP target was measured. In these studies, the permeate was returned to the retentate vessel to ensure the concentration in the retentate vessel remains constant.
High performance size exclusion chromatography, coupled with refractive index and multiangle laser light scattering (MALLS) detectors (Wyatt Technology, Santabarbara, CA), was used for sample concentration and molecular weight measurements. A gas chromatography (GC) method was used to determine the amount of quenching reagent in the crude and purified polysaccharide mixtures. External standard solution was used to quantitate the residuals quenching reagent concentration.
A mixed‐mode HPLC assay is used to separate an iodate peak mainly based on its ionic interaction with a positively charged stationary phase. Periodate is quantitatively converted to iodate by pre‐treating test samples with a reducing agent, glucose. The pre‐treated samples are then injected into a mixed mode weak anion exchange chromatography column and iodate peak is eluted by acetonitrile and buffer. The eluted peaks are detected by UV absorption at 223 nm.
3. RESULTS AND DISCUSSION
3.1. Transmembrane pressure excursion
Figure 1 demonstrates the TMP excursion profile obtained with all modules considered in this study. At low TMP values, the osmotic pressure is negligible and the permeate flux increases linearly with TMP. As flux increases the concentration of retained solutes at the membrane surface ‐and resultant osmotic pressure potential‐ increases due to the formation of the polarization layer. At elevated TMPs, both the osmotic pressure and transmembrane pressure increase at the same rate causing the permeate flux to plateau at its maximum. 11 The Biomax® 88 cm2 cassette reached its maximum flux at a lower TMP, because of higher permeability. Therefore, to obtain a comparable maximum flux, the target TMP was set to 20 psi for Biomax® 88 cm2 cassette and 25 psi for other modules during the diafiltration process. The pressure drop (feed pressure minus retentate pressure) during diafiltration was the same in 88 cm2 cassettes (Pellicon® Cassette Ultracel® and Biomax® 88 cm2). Additionally, a similar diafiltration pressure drop profile was observed for the Pellicon® TFF Cassette 1100 cm2 and Pellicon® TFF Capsule 1000 cm2.
FIGURE 1.
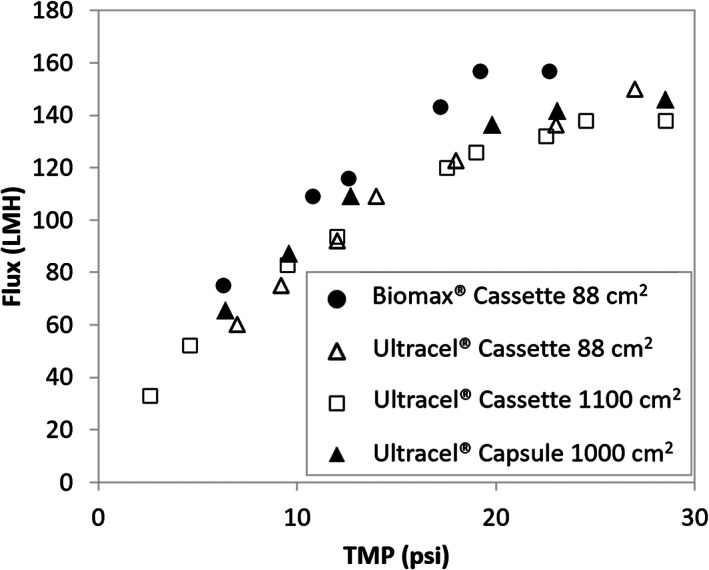
TMP excursion profile obtained with all modules considered in this study
The results illustrate very similar TMP‐excursion profiles upon scale up from 88 to 1100 cm2. Furthermore, flux versus TMP behavior for the single‐use capsule and multi‐use cassette are nearly identical. While module geometry and flow hydrodynamics are known to impact TFF performance, 10 , 12 the results indicate similar hydrodynamic and mass transfer behaviors despite the modules format and scale differences.
3.2. Clearance of reaction residuals/byproducts
As mentioned earlier, one of the objectives of the activation UFDF is to reduce the concentration of reaction byproducts and residuals (i.e. periodate/iodate and quench reagent).
The sieving coefficient (S), the ratio of residual concentration in the filtrate to the bulk, is the slope of semi‐log plot of the normalized residual concentration, C/C0 versus number of diavolumes (N):
| (1) |
Here, C and C0 represent the residual concentration at different time points in the diafiltration and the residual concentration at the beginning of the diafiltration process, respectively. The graphs in Figure 2 and Figure 3 present the clearance of the quench reagent and residual periodate/iodate during the diafiltration process plotted in semi‐log plot format, respectively. For quench reagent, linear regression was performed to fit the normalized concentration versus diavolume data to Equation 1 and the resulting sieving coefficient for each module is summarized in Table 3. The sieving coefficients are all in the range of 0.58 to 0.69 with the corresponding ranges overlapping across all modules. Therefore, the quenching reagent residual clearance characteristics are very similar in these modules.
FIGURE 2.
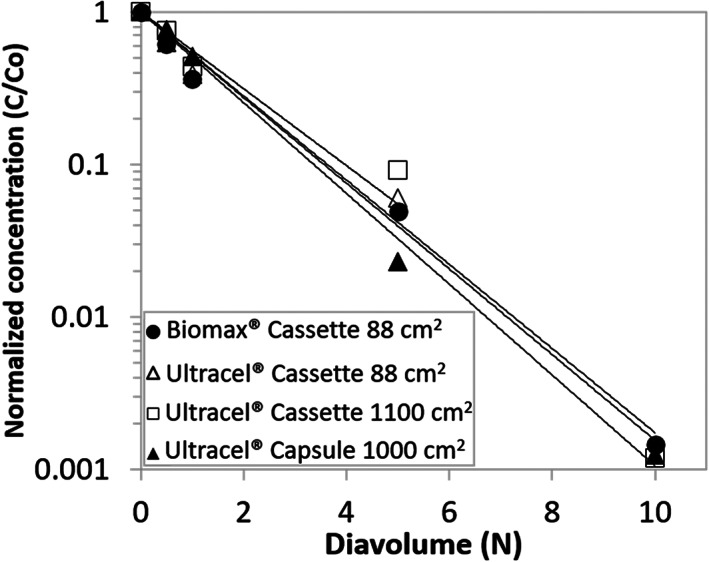
Residual quench reagent clearance during diafiltration
FIGURE 3.
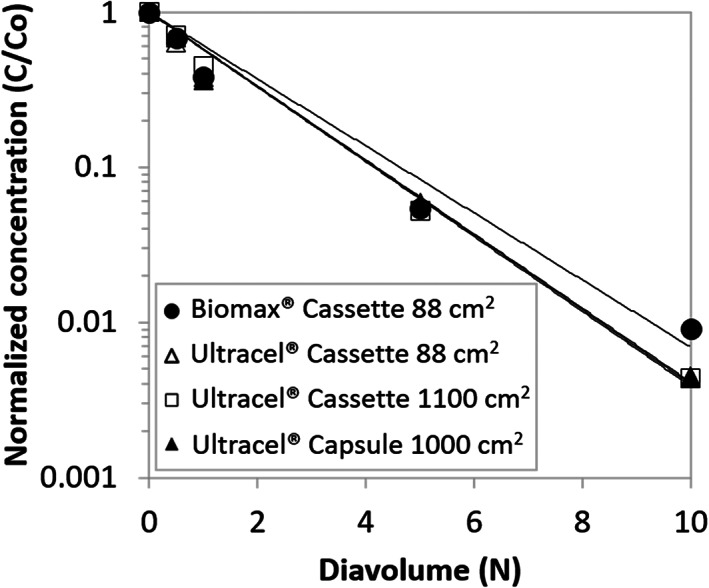
Residual periodate/iodate clearance during diafiltration
TABLE 3.
Sieving coefficient for clearance of quenching reagent for modules considered in this study
| Module name | Sieving coefficient ± Error a | R2 |
|---|---|---|
|
Pellicon® TFF Cassette (small scale) Biomax® membrane |
0.65 ± 0.03 | 0.99 |
|
Pellicon® TFF Cassette (small scale) Ultracel® membrane |
0.58 ± 0.06 | 0.96 |
|
Pellicon® TFF Cassette (large scale) Ultracel® membrane |
0.64 ± 0.04 | 0.97 |
|
Pellicon® TFF Capsule (large scale) Ultracel® membrane |
0.69 ± 0.04 | 0.99 |
The errors in the sieving coefficient were calculated through error propagation analyses considering 20% relative error in concentration assay results.
For periodate/iodate, linear regression was performed to fit the normalized concentration vs diavolume data to Equation 1, a similar approach to the quench reagent. The resulting sieving coefficient for each module is summarized in Table 4. The sieving coefficients are all in the range of 0.50 to 0.56 with the corresponding ranges overlapping across all modules. The retention of periodate is most likely due to electrostatic interaction with the membrane, ions, or molecules in the system. Similar to the quench reagent, the periodate/iodate residual clearance characteristics are similar in all modules. Note that normalized concentration beyond 10 DV is not included in the Figure 2 and Figure 3 since the concentration of residuals (residual periodate/iodate and quench reagent) in the bulk was below the level of quantification of the analytical assays.
TABLE 4.
Sieving coefficient for clearance of periodate/iodate for modules considered in this study
| Module name | Sieving coefficient ± Error a | R2 |
|---|---|---|
|
Pellicon® TFF Cassette (small scale) Biomax® membrane |
0.50 ± 0.03 | 0.97 |
|
Pellicon® TFF Cassette (small scale) Ultracel® membrane |
0.55 ± 0.02 | 0.99 |
|
Pellicon® TFF Cassette (large scale) Ultracel® membrane |
0.56 ± 0.02 | 0.99 |
|
Pellicon® TFF Capsule (large scale) Ultracel® membrane |
0.55 ± 0.02 | 0.99 |
The errors in the sieving coefficient were calculated through error propagation analyses considering 20% relative error in concentration assay results.
3.3. Molecular weight of activated polysaccharide
The molecular weight (MW) of the activated polysaccharide is measured with size exclusion chromatography with MALLS during the diafiltration process for each device considered in this study. After the molecular weight was analyzed, the normalized molecular weight of the activated polysaccharide was calculated as:
| (2) |
Figure 4 describes the normalized activated polysaccharide molecular weight calculated using Equation 2. A slight increase in molecular weight across all modules is observed, which is most likely due to the transmission of small molecular weight activated saccharides toward the permeate. Periodate oxidation of polysaccharides is known to generate small fragments, which can get washed out during UFDF. 13 Considering the SEC‐MALLS assay variability, the difference in molecular weight between different devices is not considered significant.
FIGURE 4.
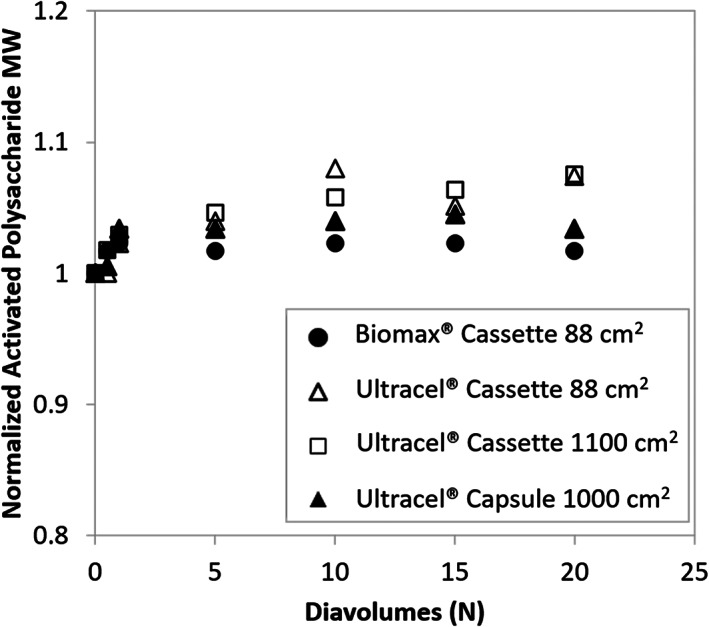
Normalized activated polysaccharide molecular weight during diafiltration
3.4. TFF yield
Figure 5 demonstrates the UFDF yield for all runs considered in this study. The yield values at large scale are comparable (90% for single‐use capsule vs 91% for multi‐use cassette) which again suggest comparable UFDF performance for cassette and capsule device format. The yields for the small‐scale devices with 88 cm2 surface area are both 84%, indicating the membrane chemistry and TFF channel configuration do not impact purification yield. The lower yield for the small scale modules may be an artifact of having a greater proportion of process volume in the system hold‐up (~26% at small scale vs 5% ‐ 6% at large scale), hence a larger fraction of unrecoverable product for given flushing efficiency.
FIGURE 5.
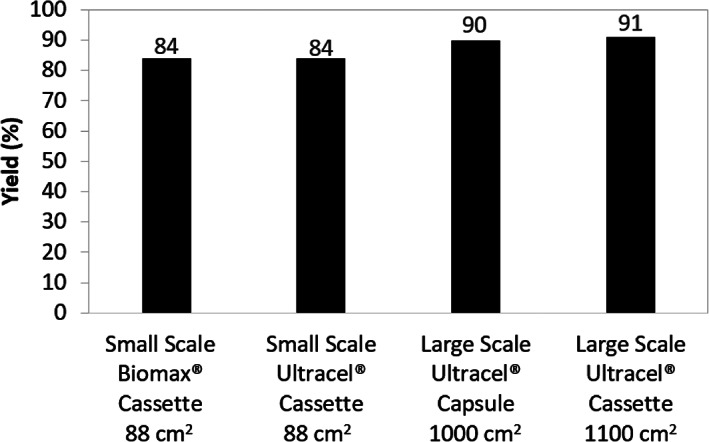
TFF yield for all modules
3.5. Auxiliary solution consumption and ease‐of‐use
The capsule was simpler to use than the cassette as it does not require loading/unloading from a compression holder and it comes pre‐sterilized by gamma irradiation, reducing installation time, and initial flush volume as well as eliminating the need for a sanitization step before processing. The goal of this study was to evaluate the filtration performance comparability of the capsule and cassette at bench scale to assess the suitability of the single‐use capsule as an alternative to re‐usable cassettes. For scale‐up considerations, savings in auxiliary solutions and time are desirable benefits in pilot and manufacturing scales as the multi‐use cassette operation requires additional pre‐ and post‐use steps as well as larger volumes for an initial water flush, sanitization step before processing the activated polysaccharide solution, and post‐process cleaning steps required for re‐use. For a typical TFF operation, using the single‐use capsule conserves time and resources associated with these extra steps necessary for cassette re‐use, which can add up to 110 L of auxiliary solution for every square meter of installed cassette membrane area, and up to 5 hrs extra for a 2‐hr process. 2
4. CONCLUSIONS
Single‐use TFF technologies promote fast and flexible product change over, reduce system preparation time, and ultimately shorten manufacturing time/cost. In this study, the performance of a recently developed Pellicon® single‐use TFF capsule was compared against traditional Pellicon® cassettes by assessing TFF process performance and post‐purification product attributes for purification of an activated polysaccharide. Similar TFF process performance and post‐purification product attributes were observed for the single‐use capsule compared to the reusable TFF cassettes. In particular, the flux vs TMP behavior for the single use capsule and multi‐use cassettes are nearly identical indicating similar hydrodynamics and mass transfer behaviors despite the modules format and scale differences. Activation reaction residuals clearance profiles and their corresponding sieving coefficients are similar and the TFF yield with large‐scale multi‐use cassette and single‐use capsule were comparable. The results provide insight into the application of the single‐use TFF technology and scalability of TFF processes for the purification of activated polysaccharides used in conjugate vaccine manufacturing.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Seyed Pouria Motevalian: Conceptualization (equal); formal analysis (supporting); methodology (equal); supervision (equal); validation (supporting); visualization (equal); writing – original draft (lead); writing – review and editing (lead). Jonathan Steen: Conceptualization (equal); formal analysis (lead); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (lead); visualization (equal); writing – original draft (supporting); writing – review and editing (supporting). Janine De Leon: Data curation (equal); investigation (equal); writing – original draft (supporting). Verl Sriskanda: Data curation (equal); formal analysis (supporting); investigation (equal). Ivette Carino: Data curation (equal); investigation (equal); project administration (equal). Amar Prashad: Conceptualization (equal); supervision (equal). Brenda Carrillo Conde: Resources (equal); supervision (equal). Bo Arve: Conceptualization (equal); supervision (equal).
ACKNOWLEDGMENT
The authors would like to acknowledge Xi Hu, Nataliya Bazhina, Frank Kotch, Louane Hann, and Leo Letendre for their significant contribution to this work. The author would like to acknowledge Latha Dhandapani, Xu Liang, Hetal Patel, and Yan Zhao for analytical and Brad Evans for statistical analyses support. The author would like to thank Ranga Godavarti, Steve Kolodziej, Sal Giglia, and Jessica Torres for their review of the manuscript.
Motevalian SP, Steen J, De Leon J, et al. Evaluation of single‐use tangential flow filtration technology for purification of activated polysaccharides used in conjugate vaccine manufacturing. Biotechnol Progress. 2021;37(6):e3204. doi: 10.1002/btpr.3204
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ken Wong JJ, Kline S, Repetto R, Mahajan E. BPOG five‐year vision for single‐use technologies. American Pharmaceutical Review. 2017;20. [Google Scholar]
- 2. EMDMillipore. Innovative single‐use tangential flow filtration devices for streamlined bioprocessing.
- 3. Emini EA, Watson WJ, Prasad AK, et al. Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof. In: Google Patents; 2016.
- 4. Hausdorff W, Siber G, Paradiso P. Multivalent pneumococcal polysaccharide‐protein conjugate composition. In: Google Patents; 2006.
- 5. Anderson PW, Eby RJ. Immunogenic conjugates. In: Google Patents; 1990.
- 6. Bozzano LG, Lee YC, Lee RT. Synthesis of Neoglycoconjugates. Neoglycoconjugates: Preparation and Applications. San Diego, California: Academic Press; 2012;53‐117. [Google Scholar]
- 7. Mäkelä PH, Käyhty H. Evolution of conjugate vaccines. Expert Rev Vaccines. 2002;1(3):399‐410. [DOI] [PubMed] [Google Scholar]
- 8. Hadidi M, Buckley JJ, Zydney AL. Ultrafiltration behavior of bacterial polysaccharides used in vaccines. J Membr Sci. 2015;490:294‐300. [Google Scholar]
- 9. Hadidi M, Buckley JJ, Zydney AL. Effect of electrostatic interactions on the ultrafiltration behavior of charged bacterial capsular polysaccharides. Biotechnol Prog. 2016;32(6):1531‐1538. [DOI] [PubMed] [Google Scholar]
- 10. Emami P, Motevalian SP, Pepin E, Zydney AL. Purification of a conjugated polysaccharide vaccine using tangential flow diafiltration. Biotechnol Bioeng. 2019;116(3):591‐597. [DOI] [PubMed] [Google Scholar]
- 11. Van Reis R, Goodrich E, Yson C, Frautschy L, Whiteley R, Zydney A. Constant Cwall ultrafiltration process control. J Membr Sci. 1997;130(1–2):123‐140. [Google Scholar]
- 12. Motevalian SP, Borhan A, Zhou H, Zydney A. Twisted hollow fiber membranes for enhanced mass transfer. J Membr Sci. 2016;514:586‐594. [Google Scholar]
- 13. Dyatlov V, Kruppa I, Mamaeva S, et al. Change of polysaccharide molecular‐weight distribution and fraction homogeneity after periodate oxidation. Chem Nat Compd. 2014;50(6):973‐977. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


