Abstract
Aims
C‐reactive protein (CRP) is used for monitoring postoperative inflammation (POI) and detecting infectious complications. The aim of this study was to assess the effect of visceral obesity (VO) on acute POI measured through CRP after elective laparoscopic colorectal resection.
Methods
Pre‐operative Computed tomography images of 357 patients who underwent laparoscopic colorectal resection were analyzed. Visceral adipose tissue (VAT) area was measured for each patient. VO was defined as VAT area >163.8 cm2 in men and >80.1 cm2 in women according to accepted sex‐specific cut‐offs. Postoperative outcomes and CRP values were compared between VO and non‐VO groups. The most appropriate CRP value for identifying infectious complications in the two groups was assessed with receiver operating characteristic (ROC) curves. Univariate and multivariate analyses were conducted for factors affecting POI including VO.
Results
No differences in postoperative outcomes and infectious complications were found in VO patients (62.2% of the overall population). Both in the overall cohort and in patients without infectious complications, VO was associated with higher CRP values on postoperative day (POD) 1, POD2, POD3, and POD5. A positive correlation was found between VAT and CRP on all PODs. VO independently predicted higher CRP on POD1‐3 in patients without infectious complications but not in those who developed complications. ROC curves analysis showed optimal accuracy for detection of infectious complications for CRP on POD3 in both groups, though the optimal cut‐off value was higher in VO group (154 vs 136 mg/L).
Conclusions
VO is not associated to increased complications after laparoscopic colorectal resection. Nevertheless, it is independently associated to higher CRP in the overall population and in patients without infectious complications. Consequently, CRP values on POD3 higher than cut‐offs commonly adopted in the clinical practice should be carefully evaluated in VO patients to assess the occurrence of infectious complications.
What's known?
The degree of postoperative inflammation (POI) in the surgical stress response can be assessed in clinical practice through bioumoral markers such as C‐reactive protein (CRP). In colorectal surgery, several factors affect CRP levels such as surgical approach, extent of surgery, and occurrence of postoperative complications. Among patient‐related factors, low skeletal muscle mass is a well‐known risk factor for increased POI.
What's new?
This is the first paper which assessed the role of visceral adipose tissue on CRP levels as a marker of POI degree. After laparoscopic colorectal resection, visceral obese (VO) patients present higher CRP values compared with normal patients, independently from postoperative complications. In patients affected by VO, high CRP in the postoperative values could not represent a signal of complications and should be carefully evaluated. Specific cut‐off values of CRP should be considered for VO and non‐VO patients to detect clinically significant infectious complications.
1. INTRODUCTION
Hospitalization and surgical procedures affect normal homeostasis and trigger metabolic stress response. This process is commonly called systemic inflammatory response or surgical stress response, and involves both immune and neuro‐endocrine systems. 1 Several approaches have been proposed for measuring the degree of surgical stress response in clinical practice, and C‐reactive protein (CRP) remains the most used and affordable method despite it reflects only the amount of postoperative inflammation (POI) which can be used as a surrogate of the surgical stress response. In colorectal surgery, several studies confirmed the usefulness of CRP for safe early discharge, aiming at early detection of adverse events. 2 , 3 , 4 , 5 Conversely, several procedure‐ and patient‐related factors could influence POI, so that specific subgroups of patients may need the estimation and application of specific cut‐off values. Among these factors, surgical approach and nutritional status have been recently found to be associated with inflammation. 6 , 7 , 8 In particular, low skeletal muscle mass has been associated with increased POI, 7 while little is known about the postoperative pro‐inflammatory role of visceral obesity (VO) and its possible clinical implications.
In this study, we assessed the relationship between visceral adipose tissue (VAT) area measured at CT scan and POI defined according to postoperative CRP levels. We assumed that VO, defined as VAT excess, could intensify POI following laparoscopic colorectal surgery, thus requiring specific cut‐off values for detecting postoperative infectious complications in VO patients.
2. PATIENTS AND METHODS
2.1. Patients and study design
From January 2012 to February 2020, 476 patients underwent elective laparoscopic colorectal resection for benign disease (adenomas and diverticular disease) or colorectal adenocarcinoma at the Division of General and Hepatobiliary Surgery, University of Verona Hospital Trust. Exclusion criteria were inflammatory bowel disease (n = 21), familial adenomatous polyposis (n = 6), and mortality within postoperative day (POD) 5 (n = 2). All patients with missing data or pre‐operative CT imaging in our Picture Archiving and Communication System (PACS) were also excluded from analysis (n = 90). Since no differences in pre‐operative CRP values were found between patients undergoing surgery for diverticular disease and colorectal neoplasia (2, 1‐5 mg/L vs 2, 1‐7 mg/L, P = .53), all patients were considered together for postoperative analysis purposes.
This study was approved by the Ethics Committee of the University Hospital of Verona, Italy (reference number “58642 ‐ CRINF‐1560CESC”). Written informed consent for anonymized data collection and analysis was obtained from all the patients included in the study.
2.2. Clinical and demographic data
Clinical and pathological data collected included age, sex, and Charlson's comorbidity index (CCI). Patient height, weight, and body mass index (BMI) were recorded from pre‐operative assessment. All tumors were confirmed histologically and staged according to the 8th Edition of the American Joint Committee on Cancer (AJCC) TNM Classification. Intraoperative variables were tumor location, type of resection, duration of surgical procedure, blood loss, and need for conversion. All patients who followed the enhanced recovery after surgery (ERAS) protocol and achieved all the previously described goals 9 were registered as “ERAS” while patients excluded from fast‐track protocol or with failed ERAS were registered as “non‐ERAS.”
Postoperative complications were graded according to the Clavien–Dindo Classification and divided into minor (grade I‐II) and major grade (grade III‐V). When several adverse events occurred in the same patient, the highest grade was adopted. The rate of infectious complications, which included anastomotic leak, pneumonia, and surgical site infections was analyzed. 10 , 11 Postoperative length of stay (LOS), 30‐day readmission, and reoperation rates were registered together with postoperative mortality.
2.3. Laboratory tests
Pre‐operative systemic inflammatory condition was assessed through serum CRP within 3 weeks before surgery (Pre‐Op CRP). Serum CRP levels in the postoperative period were routinely measured on POD1‐3 for all the patients in the study group and, additionally, on POD4‐5 if patients remained in hospital and according to clinical indication. The concentration of CRP was measured using a validated immunoturbidimetric assay on a Roche Cobas 8000 (Roche Diagnostics, Basel, Switzerland). The analytical characteristics of this method are as follows: limit of detection, 0.3 mg/L; linearity, 0.3‐350 mg/L; intra‐assay imprecision, 1.2%–3.6%; upper limit of the normal reference range: <5 mg/L.
2.4. Body composition analysis
Computed tomography (CT) images were retrieved from digital PACS and analyzed using ImageJ software (ImageJ; The National Institutes of Health, Washington, MD, USA; version 1.47) as previously described. 12 Briefly, VAT area (cm2) was measured on a single axial CT image at the third lumbar vertebra (L3) using Hounsfield unit thresholds of −190 to −30, in line with accepted methodology. 13 , 14 This parameter is highly correlated with total body adipose tissue. 15 Images analysis was performed by a single researcher specifically trained for the task. VO was defined using sex‐specific VAT cut‐off values 14 , 16 as follows: VO group with VAT >163.8 cm2 in men and VAT >80.1 cm2 in women and non‐visceral obese (non‐VO) group with VAT below these thresholds.
2.5. Statistical analysis
Results are presented as percentages (%) and means (±SD) or medians and interquartile range (IQR) according to normal distribution assessed using the Kolmogorov–Smirnov test. Chi‐square and Fisher exact tests were used for categorical variables while Student's t test or Mann–Whitney U test was used for quantitative variables as appropriate. Relationships between continuous variables were assessed using Spearman's rank correlation coefficient analysis and the correlation coefficient (ρ) was calculated.
Univariate analysis was conducted to assess the factors affecting CRP values in the overall population. Subgroup analysis was performed separately for patients who presented infectious complications. The factors analyzed were VO (vs non‐VO), ERAS (vs standard care), men (vs women), CCI >4 (vs ≤4), cancer (vs benign disease), extent of resection (right hemicolectomy vs left hemicolectomy vs rectal resection), need for conversion (vs no conversion), and surgical time >median (vs ≤ median). A screened P‐value of <.10 at univariate analysis was then considered for entering the multivariate model after validating the absence of multicollinearity. In multivariate logistic regression, a P < .05 was considered statistically significant.
Since POD3 CRP value is used in our clinical practice as one of the discharge criteria, we tested it as a predictor of infectious complications by receiver operating characteristic (ROC) curves analysis. The area under the curve (AUC) is a direct measure of diagnostic accuracy of a test. A test with an AUC between 0.7 and 0.8 is considered as having a good diagnostic accuracy. Cut‐off values with the highest sensitivity and specificity were determined by using the Youden's index. To compare accuracy between ROC curves in subgroup analysis, the DeLong method was applied. 17
Results were considered statistically significant when P value was found to be <.05. SPSS software (version 23, SPSS, Inc) was used for statistical analysis. Graphics were outlined using GraphPad Prism version 9.0.
3. RESULTS
3.1. Cohort under study
After the selection process, a cohort of 357 patients was included for study purpose. Median (IQR) BMI in the overall population was 25.0 (22.9‐28.1) kg/m2. Median (IQR) VAT area was 150.4 (95.7‐212.6) cm2 and, as predictable, was higher in men than in women (183.8 [125.4‐251.7] cm2 vs 103.3 [57.5‐167.2] cm2; P < .001). After application of sex‐specific cut‐offs, 222 patients (62.2%) were classified as VO and 135 patients (37.8%) as non‐VO, with no differences in VO incidence between men and women (60.3% vs 64.6%; P = .41). According to BMI criteria, 178 patients (49.9%) were classified as normal weight (BMI <25 kg/m2), 126 patients (35.3%) as overweight (BMI 25‐29.9 kg/m2) and 53 patients (14.8%) as obese (BMI ≥30 kg/m2). Within these three BMI categories, VO prevalence was 41%, 77%, and 98.1% respectively (P < .001). Relationship between BMI and VAT is depicted in Figure 1. BMI and VAT showed a positive correlation in normal (ρ = 0.40, P < .001) and overweight (ρ = 0.24, P = .01) patients, but not in obese patients (ρ=−0.04, P = .78).
FIGURE 1.
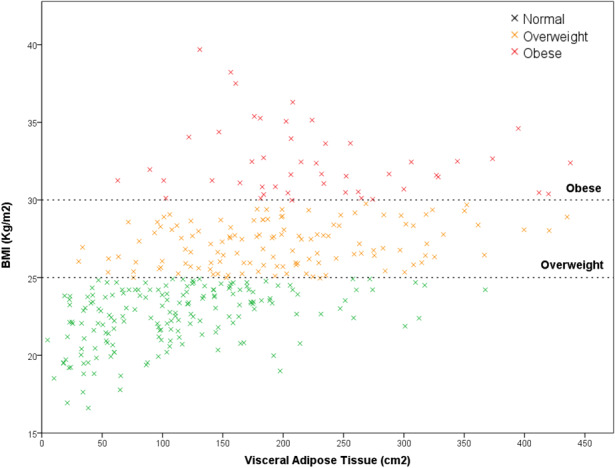
Correlation between body mass index (BMI) and visceral adipose tissue (VAT) in normal (BMI <25 kg/m2), overweight (BMI 25‐29.9 kg/m2), and obese (BMI ≥30 kg/m2) patients
Baseline clinical and pathological characteristics are presented in Table 1. VO patients were older and had higher BMI (P < .001) and CCI (P = .01). No relevant differences were found in terms of extent of surgery or staging of malignant disease.
TABLE 1.
Clinical and pathological characteristics in the groups analyzed
| Parameter |
VO n = 222 |
Non‐VO n = 135 |
P |
|---|---|---|---|
| Men | 120 (54.1) | 79 (58.5) | .41 |
| Age, y | 68.9 ± 11.3 | 63.3 ± 12.7 | <.001 |
| BMI, kg/m2 | 26.5 (24.2‐29.4) | 23.6 (21.6‐24.8) | <.001 |
| ERAS | 150 (67.6) | 99 (73.3) | .25 |
| Charlson's comorbidity index | 4 (3‐5) | 3 (2‐5) | .01 |
| Surgical indication | .93 | ||
| Colon cancer | 116 (52.3) | 72 (53.3) | |
| Rectal cancer | 54 (24.3) | 29 (21.5) | |
| Adenoma | 18 (8.1) | 11 (8.1) | |
| Diverticular disease | 34 (15.3) | 23 (17) | |
| Neoadjuvant therapy a | 25 (14.7) | 13 (12.9) | .67 |
| TNM stage a | .17 | ||
| TNM stage I/II | 111 (65.3) | 57 (56.4) | |
| TNM stage III/IV | 59 (34.7) | 44 (43.6) | |
| Surgical procedure | .84 | ||
| Right hemicolectomy | 73 (32.9) | 43 (31.9) | |
| Left hemicolectomy | 79 (35.6) | 51 (37.8) | |
| Rectal resection | 70 (31.6) | 41 (30.4) | |
| Associated resections | 47 (21.2) | 23 (17) | .34 |
| Surgical time, min | 237 (190‐297) | 220 (191‐270) | .18 |
| Blood loss, mL | 50 (30‐100) | 40 (30‐90) | .16 |
| Conversion | 18 (8.1) | 6 (4.4) | .18 |
| Overall complications | 94 (42.3) | 57 (42.2) | .98 |
| Minor grade | 77 (34.7) | 44 (32.6) | .78 |
| Major grade | 17 (7.7) | 13 (9.6) | .52 |
| Infectious complications | 34 (15.3) | 23 (17) | .67 |
| Pneumonia | 8 (3.6) | 7 (5.2) | .47 |
| Anastomotic leak | 12 (5.4) | 8 (5.9) | .84 |
| Surgical site infections | 11 (5) | 7 (5.2) | .92 |
| Time to infectious complication, d | 4 (2‐5.25) | 4 (3‐7) | .44 |
| Mortality | 1 (0.5) | 1 (0.7) | .72 |
| Readmission | 7 (3.2) | 5 (3.7) | .78 |
| Redo surgery | 10 (4.5) | 9 (6.7) | .38 |
| Length of stay, d | 6 (5‐8) | 6 (4‐8) | .30 |
Abbreviations: BMI, body mass index; ERAS, enhanced recovery after surgery; VO, visceral obesity.
Percentage on 271 colon and rectal cancers.
Postoperative outcomes are presented in Table 1. In the overall population, 158 patients (42.3%) developed postoperative complications, and 30 patients (8.4%) suffered a severe complication according to the Clavien–Dindo classification. No differences were found between the VO and non‐VO cohorts, as for overall, major grade, and infectious complications. Postoperative LOS was also comparable between groups.
Thirty‐three patients (9.2%) were discharged before POD4, and CRP values were measured only on POD1‐3. In the 209 cases (58.5%) discharged after POD5, 219 patients (61.3%) had CRP measured on POD4 and 160 (44.8%) on POD5 according to clinical needs.
3.2. VO and POI
Baseline pre‐operative CRP values were significantly associated with VAT both in women (ρ = 0.36, P = .001) and men (ρ = 0.19; P = .04). A positive correlation between VAT and postoperative CRP values was found both in women and men on POD1 (ρ = 0.24, P = .001, and ρ = 0.20, P = .04), POD2 (ρ = 0.32, P < .001 and ρ = 0.15, P = .03), and POD3 (ρ = 0.31, P < .001, and ρ = 0.17, P = .02), but not on POD4 and POD5. No significant correlation between BMI and CRP values was found on each POD.
VO patients displayed higher baseline CRP levels compared with non‐VO patients (3, 1‐6 mg/L vs 1, 1‐3 mg/L; P < .001). Higher CRP values were also observed in the VO group on POD1, POD2, POD3, and POD5 (Figure 2 and Table 2).
FIGURE 2.
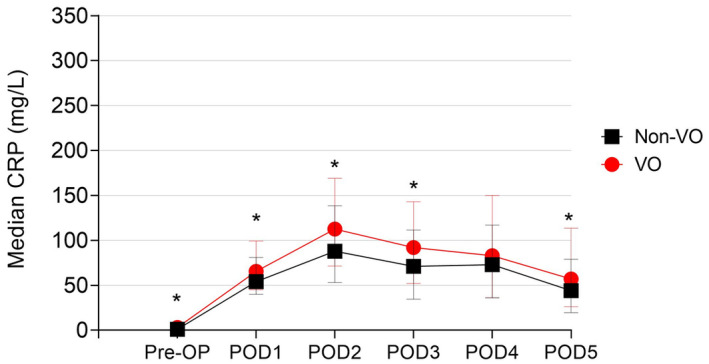
Trend in postoperative C‐reactive protein in the overall population according to visceral obesity (VO) status (*P < .05)
TABLE 2.
Surgical stress response expressed as C‐reactive protein values in the overall population and according to visceral obesity
| Parameter |
Overall population n = 357 |
Visceral obesity | ||
|---|---|---|---|---|
|
VO n = 222 |
Non‐VO n = 125 |
P | ||
| Pre‐operative CRP | 2 (1‐5) | 3 (1‐6) | 1 (1‐3) | <.001 |
| CRP POD1 | 62 (43‐95) | 65 (45‐99) | 54 (40‐81) | .002 |
| CRP POD2 | 102 (61‐156) | 112 (71‐169) | 88 (53‐138) | .001 |
| CRP POD3 | 85 (45‐132) | 92 (52‐143) | 71 (34‐111) | .003 |
| CRP POD4 | 74 (36‐135) | 83 (36‐150) | 73 (36‐117) | .50 |
| CRP POD5 | 52 (23‐103) | 57 (26‐113) | 44 (19‐79) | .04 |
Abbreviations: CRP, C‐reactive protein; POD, postoperative day; VO, visceral obesity.
Linear regression analysis showed that VO was positively correlated with CRP from POD 1 to 3, with the highest coefficient on POD2 (β = 0.164; P < .001). As given in Table 3, conversion to open surgery and surgical time were positively associated with CRP levels. Conversely, ERAS protocol displayed a negative correlation.
TABLE 3.
Univariate analysis for factors affecting C‐reactive protein values in the overall population
| Factor | CRP POD1 | CRP POD2 | CRP POD3 | CRP POD4 | CRP POD5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| VO | 0.151 | .004 | 0.164 | .003 | 0.125 | .02 | 0.029 | .74 | 0.068 | .34 |
| ERAS | −0.151 | .004 | −0.292 | <.001 | −0.332 | <.001 | −0.281 | .001 | −0.247 | <.001 |
| Men | 0.047 | .38 | 0.081 | .14 | 0.043 | .39 | 0.074 | .39 | 0.092 | .20 |
| CCI | 0.075 | .16 | 0.136 | .01 | 0.154 | .004 | 0.017 | .84 | 0.116 | .11 |
| Cancer | 0.089 | .09 | 0.128 | .02 | 0.126 | .02 | 0.011 | .90 | 0.068 | .34 |
| Specimen length | −0.006 | .91 | 0.034 | .53 | 0.103 | .05 | 0.137 | .11 | 0.064 | .97 |
| Conversion | 0.170 | .001 | 0.143 | .01 | 0.085 | .11 | 0.098 | .25 | −0.049 | .50 |
| Surgical time | 0.208 | <.001 | 0.261 | <.001 | 0.245 | <.001 | 0.176 | .04 | 0.069 | .36 |
Abbreviations: CCI, Charlson's comorbidity index; CRP, C‐reactive protein; ERAS, enhanced recovery after surgery; POD, postoperative day; VO, visceral obesity.
In multivariate analysis (Table 4), VO was confirmed as an independent risk factor for increased CRP on POD1 and POD2, but not on POD3 (β = 0.062; P = .23). ERAS enrollment was independently associated with reduced CRP values from POD2 to POD5, while conversion to open surgery or duration of surgery was associated with increased CRP values on POD1 and POD1, or with POD 2 and POD3, respectively.
TABLE 4.
Multivariate analysis for factors affecting C‐reactive protein values in the overall population
| Factor | CRP POD1 | CRP POD2 | CRP POD3 | CRP POD4 | CRP POD5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| VO | 0.119 | .02 | 0.121 | .02 | — | — | ||||
| ERAS | — | — | −0.144 | .01 | −0.279 | <.001 | −0.255 | .004 | −0.247 | <.001 |
| Men | ||||||||||
| CCI | — | — | — | — | ||||||
| Cancer | — | — | — | — | — | — | ||||
| Specimen length | — | — | ||||||||
| Conversion | 0.146 | .01 | — | — | ||||||
| Surgical time | 0.137 | .01 | 0.177 | .01 | 0.193 | <.001 | — | — | ||
Abbreviations: CCI, Charlson's comorbidity index; CRP, C‐reactive protein; ERAS, enhanced recovery after surgery; POD, postoperative day; VO, visceral obesity.
3.3. Infectious complications and POI
No differences in CRP levels according to the presence of VO were observed in patients with infectious complications (Figure 3A). Univariate analysis in this subgroup of patients did not reveal any association with abnormal CRP values in the postoperative period (Table 5), so that multivariate analysis was not performed. Further analysis was therefore conducted only for patients without infectious complications. In this subgroup, CRP values were significantly higher in VO compared with non‐VO patients on POD1, POD2, POD3, and POD5 (Figure 3B). Univariate analysis showed a positive correlation between VO and CRP on POD 1 to 3 (Table 6), with the highest coefficient on POD2 (β = 0.200; P = .001). In multivariate analysis (Table 7), VO was confirmed as an independent risk factor for increased CRP values between POD1 and 3. Other factors associated with high CRP values were conversion to open surgery (POD1‐2) and time of surgery (POD 1‐4). ERAS enrollment was independently associated with lower CRP values on POD2, POD3, and POD5.
FIGURE 3.
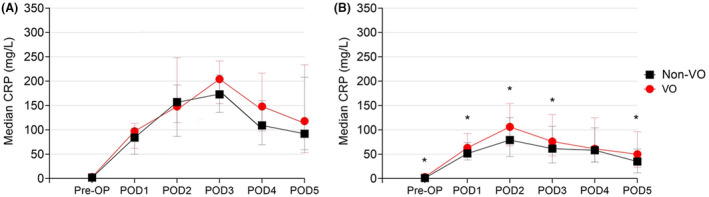
Trend in postoperative C‐reactive protein values in (A) patients with infectious complications and (B) patients without postoperative infectious complications according to visceral obesity (VO) status (*P < .05)
TABLE 5.
Univariate analysis for factors affecting C‐reactive protein values in patients with infectious complications
| Factor | CRP POD1 | CRP POD2 | CRP POD3 | CRP POD4 | CRP POD5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| VO | 0.113 | .40 | 0.113 | .42 | 0.092 | .50 | 0.146 | .45 | 0.041 | .79 |
| ERAS | 0.041 | .76 | −0.038 | .79 | −0.259 | .05 | −0.211 | .27 | −0.147 | .34 |
| Men | 0.197 | .14 | 0.294 | .03 | 0.301 | .02 | 0.326 | .10 | 0.092 | .55 |
| CCI | 0.021 | .88 | −0.027 | .85 | −0.005 | .97 | −0.117 | .55 | −0.197 | .20 |
| Cancer | 0.148 | .27 | 0.082 | .59 | 0.159 | .24 | 0.113 | .56 | −0.003 | .99 |
| Specimen length | −0.085 | .53 | −0.132 | .35 | 0.026 | .85 | −0.005 | .98 | −0.015 | .92 |
| Conversion | −0.075 | .58 | −0.230 | .10 | −0.200 | .14 | −0.081 | .68 | −0.210 | .17 |
| Surgical time | 0.028 | .85 | −0.052 | .72 | −0.052 | .72 | −0.142 | .47 | −0.119 | .47 |
Abbreviations: CCI, Charlson's comorbidity index; CRP, C‐reactive protein; ERAS, enhanced recovery after surgery; POD, postoperative day; VO, visceral obesity.
TABLE 6.
Univariate analysis for factors affecting C‐reactive protein values in patients without infectious complications
| Factor | CRP POD1 | CRP POD2 | CRP POD3 | CRP POD4 | CRP POD5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| VO | 0.168 | .003 | 0.200 | .001 | 0.173 | .003 | 0.081 | .40 | 0.074 | .36 |
| ERAS | −0.144 | .01 | −0.293 | <.001 | −0.296 | <.001 | −0.197 | .04 | −0.220 | .01 |
| Men | 0.018 | .75 | 0.040 | .50 | 0.060 | .31 | −0.029 | .77 | 0.083 | .31 |
| CCI | 0.055 | .34 | 0.139 | .02 | 0.168 | .004 | 0.065 | .51 | 0.227 | .01 |
| Cancer | 0.082 | .16 | 0.153 | .01 | 0.138 | .02 | 0.040 | .68 | 0.117 | .15 |
| Specimen length | −0.014 | .80 | 0.035 | .56 | 0.097 | .10 | 0.095 | .33 | 0.061 | .45 |
| Conversion | 0.220 | <.001 | 0.250 | <.001 | 0.196 | .001 | 0.185 | .05 | 0.020 | .81 |
| Surgical time | 0.220 | <.001 | 0.321 | <.001 | 0.334 | <.001 | 0.329 | .001 | 0.124 | .15 |
Abbreviations: CCI, Charlson's comorbidity index; CRP, C‐reactive protein; ERAS, enhanced recovery after surgery; POD, postoperative day; VO, visceral obesity.
TABLE 7.
Multivariate analysis for factors affecting C‐reactive protein values in patients without infectious complications
| Factor | CRP POD1 | CRP POD2 | CRP POD3 | CRP POD4 | CRP POD5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| VO | 0.141 | .01 | 0.156 | .01 | 0.124 | .03 | ||||
| ERAS | — | — | −0.165 | .01 | −0.199 | .001 | — | — | −0.220 | .01 |
| Men | ||||||||||
| CCI | — | — | — | — | — | — | ||||
| Cancer | — | — | — | — | ||||||
| Specimen length | ||||||||||
| Conversion | 0.218 | <.001 | 0.163 | .004 | — | — | — | — | ||
| Surgical time | 0.195 | .001 | 0.251 | <.001 | 0.267 | <.001 | 0.289 | .01 | ||
Abbreviations: CCI, Charlson's comorbidity index; CRP, C‐reactive protein; ERAS, enhanced recovery after surgery; POD, postoperative day; VO, visceral obesity.
3.4. Assessment of POD3 CRP cut‐off values
Considering the outcome of infectious complications, ROC curve analysis revealed an AUC of 0.86 (95% CI: 0.78‐0.95, P < .001), thus confirming the clinical significance of measuring CRP on POD3 as a reliable predictor of infectious complications. In the overall population, the optimal cut‐off was 134 mg/L, with 80% sensitivity and 78.9% specificity. Even considering VO and non‐VO separately, CRP value on POD3 was an efficient predictor of infectious complications, displaying an AUC value >0.85 (Figure 4). According to the Youden's index, the optimal cut‐off value of CRP on POD3 was 136 mg/L (87.5% sensitivity and 88% specificity) for non‐VO patients, and 154 mg/L (83.3% sensitivity and 81.2% specificity) for VO patients, respectively.
FIGURE 4.
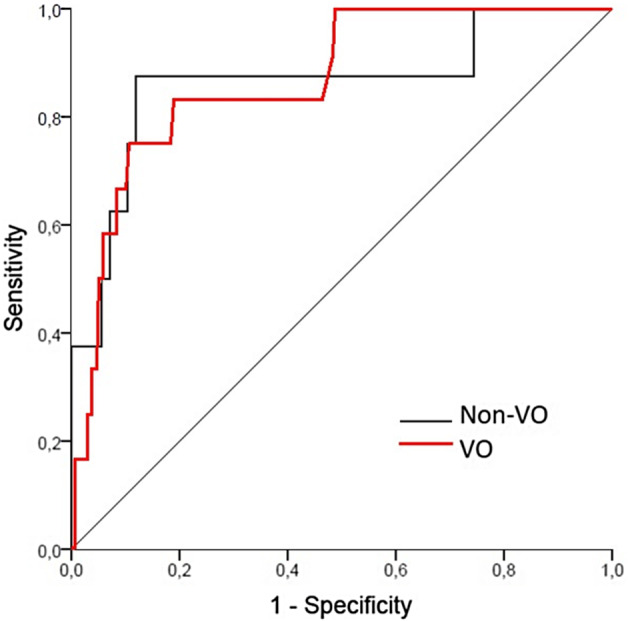
ROC curves for C‐reactive protein levels on postoperative day 3 show good sensitivity and specificity for detection of infectious complications both in visceral obesity (VO) and Non‐VO patients
4. DISCUSSION
Body composition assessment in surgical patients has recently gained attention because of possible clinical implications in complications and long‐term survival. For example, low skeletal muscle mass seems to be strongly associated with poor postoperative outcomes, especially in cancer patients. 18 , 19 , 20 Reduced skeletal muscle mass may promote the development of a pro‐inflammatory environment, basically through the onset of insulin resistance, 21 which is responsible for enhanced POI after colorectal resection. 7 Similarly, insulin resistance and higher levels of pro‐inflammatory cytokines such as IL‐6 and TNF‐α are associated with abnormal adipose tissue accumulation, in particular excess of visceral adipocytes. 22 Although the chronic systemic inflammation which characterizes VO is a well‐known risk factor for development of several diseases associated with metabolic syndrome, 22 , 23 the role of VO in worsening surgical outcomes is still debated. 22 , 24 , 25 Little is known about the role of VO on acute inflammatory response after colorectal resection. Our study aimed to characterize the magnitude of POI after laparoscopic colorectal resection according to VO, trying to assess its role in development of infectious complications. We hypothesized that VO patients presented higher degree of POI, thus requiring separate cut‐offs for predicting safe discharge on POD3.
Although procalcitonin presents higher specificity than CRP in differentiating between inflammation and infection, 26 its use in the clinical practice after colorectal surgery is still limited, and mostly influenced the development of severe bacterial infections. 26 , 27 , 28 We therefore chose CRP as an objective marker of POI because of its widespread use in clinical practice and its role as predictor of complications. 2 In uncomplicated surgery, CRP is generally low on POD1, then exhibits a maximal increase on POD2 and decreases by POD3. Nonetheless, CRP further increases after POD2 in patients developing adverse events. Consequently, CRP on POD3 is widely considered a reliable marker of severe postoperative adverse events, especially infectious complications. Combined with negative clinical findings, a CRP value below the specific cut‐offs on POD3 was found as an important marker for allowing safe discharge. 2
In our study, we highlighted a significant correlation between VAT measured at pre‐operative CT images and CRP values on POD1‐3. Higher values of VAT were associated to increased POI. On the contrary, BMI values, which are commonly used in the clinical practice for evaluation of the degree of patients' adiposity, showed no correlation with CRP values on each POD. We can hypothesize that although BMI is associated to VAT amount (Figure 1), BMI evaluation is insufficient for the assessment of the amount of visceral fat which is responsible for the increase in inflammatory response in the postoperative period. The results of this study confirm our original hypothesis of an association between VO and a pro‐inflammatory environment, as shown by higher baseline and postoperative CRP values in the overall population. Multivariate analysis confirmed VO as an independent risk factor for increased POI, with higher CRP values on POD1 and 2. Interestingly, the increase in POI was stronger in patients who did not develop infectious complications. Our results are in line with recent evidence of low POI because of reduced surgical stress from the synergistic effect of laparoscopy and ERAS protocol. 1 , 29 Nevertheless, while conversion to open surgery and surgical time are well‐known factors associated with increased POI, we demonstrate here for the first time that VO may be responsible for a larger inflammatory response after laparoscopic colorectal resection. However, this association was not confirmed in patients who developed infectious complications. We can speculate that whether VO is associated with higher POI even in complicated cases, its effect on CRP may be concealed by the greater inflammatory response fostered by infection.
Previous studies evaluating the role of VO on POI were carried out in patients who underwent minimally invasive esophagectomy. Doyle et al reported altered patterns of cytokine expression in VO patients both pre‐ and postoperatively. Despite a heightened immune and inflammatory response, this appeared to have no clinical adverse sequelae for VO subjects. 30 In accordance with these findings, Okamura et al showed that VAT quartiles were significantly associated with CRP levels both in the overall population and in patients who did not develop postoperative infectious complications. 31 Following the results of these studies, our findings confirm that VO could intensify POI following laparoscopic colorectal resection, though VO was not responsible for worse postoperative course, since the complication rates appeared to be similar between VO and non‐VO patients. Whether increased POI in VO patients is related to higher magnitude of tissue damage, enhanced inflammatory response, or both these elements would coexist, this cannot be elucidated from the present study.
Infectious complications after colorectal surgery have a major clinical impact as they increase LOS, treatment costs, and worsen long‐term survival in cancer patients. 32 , 33 When early diagnosed, they can be treated effectively, and their impact is minimized. In the era of fast‐track protocols, several CRP cut‐off values have been proposed to ensure safe discharge. Different thresholds have been used depending on surgical procedure and surgical approach, since the amount of normal POI varies between open 34 , 35 , 36 and laparoscopic surgery. 2 , 37 In our opinion, all parameters that could influence POI should be considered when proposing CRP cut‐off values for safe discharge, so that its diagnostic efficiency could be increased. Our study, following previous evidences in minimally invasive esophageal surgery, 30 , 31 demonstrates increased POI after laparoscopic colorectal resection in patients with increased VAT. As an example, a CRP value on POD3 of 150 mg/L could lead to postpone hospital discharge and to look for a source of infection according to our standard fast‐track protocol. According to the present results, if this patient was VO the risk of infectious complications would be overestimated being CRP <154 mg/L while it would be a correct management for a non‐VO patient. The use of CRP cut‐off values plays a more interesting role in asymptomatic patients who can present an aspecific increased or non‐decreasing CRP value on POD3. In these patients, the use of tailored cut‐off values which consider VO status would improve diagnostic performance (ie, both sensitivity and specificity), allowing clinicians to better identify patients who require further diagnostics and those who can be safely discharged without risks. Our analysis with ROC curves demonstrated that the CRP cut‐off value at POD3 after minimally invasive colorectal surgery should be differentiated according to patient's body composition, with VO patients presenting higher threshold for safe discharge.
Our study has some limitations, which shall be mentioned. First, the sample size was relatively small since we focused on patients undergoing elective surgery with minimally invasive approach. Despite this decision allowed us to analyze a more homogeneous population, the low incidence of severe complications such as anastomotic leak prevented from specific ROC curve analysis. Second, this is a retrospective observational study conducted at a single institution. The findings presented in this paper need to be validated in larger and prospective cohorts. Third, since introduction of ERAS protocol, more and more patients have been discharged before POD4, thus reducing the availability of data on CRP values on POD4 and 5.
Our paper also has many strengths. The analysis of CT images and VAT was conducted by a single researcher, with large experience in body composition analysis, who was blinded to postoperative outcomes. Then, as previously mentioned, we selected a homogeneous cohort that limited the differences in clinical, pathological, and surgical variables between the two groups. Moreover, to our knowledge, this is one of the few studies analyzing the impact of VAT and VO on POI, and it is the sole to consider patients undergoing minimally invasive colorectal resections.
In conclusion, our findings confirm the presence of a pro‐inflammatory environment before surgery and highlight an enhanced POI in VO patients. This increased inflammatory response was significant in the overall population and in patients without infectious complications, while our analysis failed to find significant difference in those who developed infectious complications. Interestingly, despite the POI was increased in VO patients, no differences in postoperative complications could be found. We also confirmed that CRP measured on POD3 may present high sensitivity and specificity in predicting infectious complications, though different cut‐off values should be considered for VO and non‐VO. Future studies in larger cohorts should hence aim at elucidating the relationship between VAT, increased POI, and incidence of postoperative complications with specific interest for anastomotic leak.
DISCLOSURES
Conti C., Pedrazzani C., Turri G., Gecchele G., Valdegamberi A., Ruzzenente A., Zamboni GA, Lippi G., Guglielmi A. have no conflict of interests or funding to declare.
AUTHORS’ CONTRIBUTIONS
Conti C., Pedrazzani C., Lippi G., Ruzzenente A., and Guglielmi A., provided study concept and design; Conti C., Turri G., Zamboni GA, Gecchele G., and Valdegamberi A. collected data; Pedrazzani C., Conti C. Ruzzenente A., Lippi G., and Valdegamberi A. performed data analysis and interpretation; Pedrazzani C., Conti C., and Turri G. drafted the manuscript; Turri G., Gecchele G., Zamboni GA, Valdegamberi A., Ruzzenente A., Lippi G., and Guglielmi A. provided critical revision of the paper. All authors read and approved the final version of this manuscript.
Conti C, Pedrazzani C, Turri G, et al. Visceral obesity enhances inflammatory response after laparoscopic colorectal resection. Int J Clin Pract. 2021;75:e14795. 10.1111/ijcp.14795
Funding information
Open Access Funding provided by Universita degli Studi di Verona within the CRUI‐CARE Agreement.
WOA Institution: Universita degli Studi di Verona
Blended DEAL: CARE
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mari G, Costanzi A, Crippa J, et al. Surgical stress reduction in elderly patients undergoing elective colorectal laparoscopic surgery within an ERAS protocol. Chirurgia. 2016;111:476‐480. doi: 10.21614/chirurgia.111.6.476 [DOI] [PubMed] [Google Scholar]
- 2. Pedrazzani C, Moro M, Mantovani G, et al. C‐reactive protein as early predictor of complications after minimally invasive colorectal resection. J Surg Res. 2017;210:261‐268. doi: 10.1016/j.jss.2016.11.047 [DOI] [PubMed] [Google Scholar]
- 3. Facy O, Paquette B, Orry D, et al. Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery. Results from the IMACORS study. Ann Surg. 2016;263:961‐966. doi: 10.1097/SLA.0000000000001303 [DOI] [PubMed] [Google Scholar]
- 4. Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C‐reactive protein: a diagnostic meta‐analysis of 1832 patients. Ann Surg. 2012;256:245‐250. doi: 10.1097/SLA.0b013e31825b60f0 [DOI] [PubMed] [Google Scholar]
- 5. Adamina M, Warschkow R, Näf F, et al. Monitoring c‐reactive protein after laparoscopic colorectal surgery excludes infectious complications and allows for safe and early discharge. Surg Endosc. 2014;28:2939‐2948. doi: 10.1007/s00464-014-3556-0 [DOI] [PubMed] [Google Scholar]
- 6. Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362‐380. doi: 10.1016/j.surg.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 7. Reisinger KW, Derikx JPM, van Vugt JLA, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35:924‐927. doi: 10.1016/j.clnu.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Veenhof AAFA, Vlug MS, van der Pas MHGM, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216‐221. doi: 10.1097/SLA.0b013e31824336e2 [DOI] [PubMed] [Google Scholar]
- 9. Pedrazzani C, Conti C, Mantovani G, et al. Laparoscopic colorectal surgery and Enhanced Recovery after Surgery (ERAS) program: experience with 200 cases from a single Italian center. Medicine. 2018;97:4‐9. doi: 10.1097/MD.0000000000012137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ling ML, Apisarnthanarak A, Abbas A, et al. APSIC guidelines for the prevention of surgical site infections. Antimicrob Resist Infect Control. 2019;8:97‐134. doi: 10.1186/s13756-019-0638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339‐351. doi: 10.1016/j.surg.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 12. Pedrazzani C, Conti C, Zamboni GA, et al. Impact of visceral obesity and sarcobesity on surgical outcomes and recovery after laparoscopic resection for colorectal cancer. Clin Nutr. 2020;39:3763‐3770. doi: 10.1016/j.clnu.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 13. Black D, Mackay C, Ramsay G, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017;24:2241‐2251. doi: 10.1245/s10434-017-5829-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629‐635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Punyanitya M, Wang ZM, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol. 2004;97:2333‐2338. doi: 10.1152/japplphysiol.00744.2004 [DOI] [PubMed] [Google Scholar]
- 16. Doyle SL, Bennett AM, Donohoe CL, et al. Establishing computed tomography‐defined visceral fat area thresholds for use in obesity‐related cancer research. Nutr Res. 2013;33:171‐179. doi: 10.1016/j.nutres.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 18. Richards CH, Roxburgh CSD, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7:e41883. doi: 10.1371/journal.pone.0041883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223‐226. doi: 10.1097/MCO.0b013e32832a7902 [DOI] [PubMed] [Google Scholar]
- 20. Laird BJA, Fallon M, Hjermstad MJ, et al. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol. 2016;34:2769‐2775. doi: 10.1200/JCO.2015.65.7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997‐1006. doi: 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- 22. Rickles AS, Iannuzzi JC, Mironov O, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg. 2013;17:133‐143. doi: 10.1007/s11605-012-2045-9 [DOI] [PubMed] [Google Scholar]
- 23. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881‐887. doi: 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 24. Martin L, Hopkins J, Malietzis G, et al. Assessment of computed tomography (CT)‐defined muscle and adipose tissue features in relation to short‐term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol. 2018;25:2669‐2680. doi: 10.1245/s10434-018-6652-x [DOI] [PubMed] [Google Scholar]
- 25. Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41:186‐196. doi: 10.1016/j.ejso.2014.10.056 [DOI] [PubMed] [Google Scholar]
- 26. Giaccaglia V, Salvi PF, Cunsolo GV, et al. Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care. 2014;29:528‐532. doi: 10.1016/j.jcrc.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 27. Silvestre J, Rebanda J, Lourenҫo C, Póvoa P. Diagnostic accuracy of C‐reactive protein and procalcitonin in the early detection of infection after elective colorectal surgery ‐ a pilot study. BMC Infect Dis. 2014;14:444. doi: 10.1186/1471-2334-14-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lagoutte N, Facy O, Ravoire A, et al. C‐reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg. 2012;149:e345‐e349. doi: 10.1016/j.jviscsurg.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 29. Wang G, Jiang Z, Zhao K, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379‐1388. doi: 10.1007/s11605-012-1880-z [DOI] [PubMed] [Google Scholar]
- 30. Doyle SL, Mongan AM, Donohoe CL, et al. Impact of visceral obesity and metabolic syndrome on the postoperative immune, inflammatory, and endocrine response following surgery for esophageal adenocarcinoma. Dis Esophagus. 2017;30:1‐11. doi: 10.1093/dote/dox008 [DOI] [PubMed] [Google Scholar]
- 31. Okamura A, Watanabe M, Fukudome I, et al. Relationship between visceral obesity and postoperative inflammatory response following minimally invasive esophagectomy. World J Surg. 2018;42:3651‐3657. doi: 10.1007/s00268-018-4675-x [DOI] [PubMed] [Google Scholar]
- 32. Krarup PM, Nordholm‐Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long‐term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930‐938. doi: 10.1097/SLA.0b013e3182a6f2fc [DOI] [PubMed] [Google Scholar]
- 33. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269‐278. doi: 10.1016/j.jamcollsurg.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 34. Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long‐term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44‐52. doi: 10.1016/S1470-2045(08)70310-3 [DOI] [PubMed] [Google Scholar]
- 35. Forsmo HM, Pfeffer F, Rasdal A, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2016;18:603‐611. doi: 10.1111/codi.13253 [DOI] [PubMed] [Google Scholar]
- 36. Ortega‐Deballon P, Radais F, Facy O, et al. C‐reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010;34:808‐814. doi: 10.1007/s00268-009-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh PP, Zeng ISL, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta‐analysis of use of serum C‐reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101:339‐346. doi: 10.1002/bjs.9354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


