Abstract
Context:
Recurrent caries are the leading cause of composite resin failure.
Aims:
The purpose of this pilot study was to test the efficacy of a novel copper iodide (CuI) containing dental adhesive in an in vitro caries model.
Subjects and Methods:
Streptococcus mutans and Lactobacillus acidophilus were grown individually on the complex medium for 48 h at 37°C. The pH of the mixed medium was 7.0 initially and tested every 24 h. 40 extracted teeth were prepared with standardized cavity preparations and coated with control or experimental CuI adhesives and imaged using a micro-computed tomography (microCT). Four study groups were evaluated: (1) control (2) 0.5 μg/ml CuI (3) 1.0 μg/ml CuI, 4) 5.0 μg/ml CuI. After incubation, the teeth were re-imaged using the microCT. Utilizing AnalyzePro software the three-dimensional data sets were overlaid and demineralization was measured and statistics were run.
Statistics:
Stratified ANOVA models were run to determine if there were differences between the control and experimental adhesive groups. Similarly, pH and bacterial concentrations were evaluated to ensure the viability of polymicrobial specimen.
Results and Conclusions:
Significant differences were found between the control group and the 1.0 and 5.0 CuI adhesive groups. No differences in pH were noted between the groups. Overlaid changes in demineralization were recorded as volume loss. CuI adhesives with 5 mg/ml or higher have the potential to limit tooth demineralization after bacterial penetration of a dental restoration in an in vitro caries model. Further testing is needed.
Keywords: Caries, composite resin, dental bonding, microbiology
Introduction
Resin-based composites (RBC) have become one of the most widely utilized dental materials in the world. In the United States alone over 166 million direct restorations were placed in 2005 with over half of those being RBC.[1,2] More recently, a study that surveyed Canadian and American pediatric dentists, found an average of 79% prevalence for composite resin for direct restorations of permanent molars in healthy children.[3] RBC popularity has increased due to the perceived ease of placement, life-like esthetics, wear-resistance, and ability for conservative tooth preparations.[4,5,6,7]
However, the durability of RBC restorations is not ideal with the number one cause of failure being secondary caries under the adhesive layer at the restorative tooth interface.[8,9,10,11,12,13,14,15] Over 50% of replacement restorations are due to recurrent carries with the cost in the USA totaling over 5 billion dollars annually.[16,17,18,19] Despite this evident weakness, the emphasis of material improvements for RBC materials has concentrated on aspects pertaining to the filler particle size, shrinkage stress, wear resistance, curing depth, and esthetics.[20,21,22] While these advancements have likely improved shrinkage stress values and durability, they have done little to nothing to address the main mechanism of failure, which is more related to the adhesive required to bond these restorations to tooth structure.
A pivotal aspect to consider in the longevity of RBC restorations is the adhesive interface. Preserving the integrity of the zone of resin interdiffusion is essential to RBC longevity and subsequent prevention of bacterial penetration at the restorative-tooth interface.[23] Interestingly, research developments in the field of adhesives have concentrated on creating more simplified, easy-to-use systems as opposed to improving their durability. As a result, the newer so-called universal adhesive systems perform poorly compared to the older multiple-step adhesive systems.[24,25,26,27,28]
Three primary mechanisms work synergistically to cause premature restoration failure at the adhesive layer; enzymatic degradation of collagen in the hybrid layer by endogenous proteases, biodegradation of ester linkages in the adhesive resins by salivary esterase, and bacterial penetration and proliferation.[23,27,29,30,31,32,33,34,35] These three mechanisms work together to contribute to the ongoing degradation of adhesive restorations. For example, matrix metalloproteinases (MMPs) and cysteine cathepsins degrade collagen in the hybrid layer allowing penetration and proliferation of bacteria under the adhesive.[36,37,38,39] Bacteria then produce acids which further activate MMPs and salivary esterase, which in turn degrade ester linkages in the adhesive.[35,40] These biodegradation byproducts such as bishydroxypropoxyphenyl-propane (BisHPPP) act as transcription factors to turn on gene expression of virulence factors such as glucan expression in caries causing bacteria which further allows for adherence and protection of the biofilm.[35,40,41,42,43]
Copper and silver nanoparticles have been evaluated as additives to dental materials to increase their longevity.[44,45,46,47,48,49] Recently, adhesive materials containing particles of poly-acrylic acid-coated copper iodide (PAA-CuI) have demonstrated strong long-term antibacterial properties with a 99.99% reduction in viable cell counts of Streptococcus mutans at 1 year.[46] Furthermore, it was found that CuI also prevents collagen degradation due to proteolytic activity.[47] ALGhanem et. al found that PAA-CuI doped adhesives prevented degradation of the adhesive interface after 1 year.[48] Copper has also been shown to delay adhesive interface degradation while increasing bond strengths and reducing nanoleakage.[49,50]
Despite the many benefits of copper-doped adhesives, no studies have yet evaluated copper-containing adhesives in a caries model to determine its effect on the formation of recurrent caries. Many models have been developed to evaluate caries formation around dental composite restorations each with a set of advantages and disadvantages.[51] The in vitro methods typically involve chemical or biological challenges that lower global pH for a defined time.[51] One promising model is the in vivo method which involves in situ models where dental composite-tooth interfaces are fixated on dentures or removable appliances and worn by volunteers.[51] Additional models exist that have incorporated engineered composite-tooth gaps of controlled size to introduce a weak spot for bacterial penetration and proliferation.[51] Likewise, this model sought to simulate a small break in the adhesive layer and determine how bacterial infiltration might affect the microenvironment directly adjacent to that weak point of the adhesive layer. Through this model, antimicrobial capacity of the adhesive can be tested to determine if sufficient bacterial inhibition occurred. Bacterial inhibition would lead to decreased demineralization of the tooth structure exposed to the biofilm. Likewise, different adhesive generations were tested to determine which might make a better carrier for such an additive.
The purpose of this study was to evaluate copper-iodide doped adhesive resins in a new in vitro caries model. The primary aim was to see if demineralization at the adhesive tooth interface could be measured more precisely than with a global pH-based caries model. The primary null hypothesis was that PAA-CuI doped adhesives would not inhibit tooth demineralization at the adhesive-tooth interface. The secondary aim was to look at platonic bacterial counts and global pH. The secondary null hypothesis was that global pH of the test environment would not differ nor would platonic cell counts between the control adhesives and adhesives with CuI.
Subjects and Methods
Both synthesis of the PAA-CuI particles and generation of PAA-CuI adhesives was conducted according to Sabatini et al.[46] Briefly, 10 mg of PAA-CuI powder was admixed with 1 ml of one of two adhesives, Optibond Solo (Kerr, Orange, CA) and Optibond XTR (Kerr, Orange, CA), to yield a concentrated solution (10 mg/ml). To ensure uniform dissolution of the particles, a probe tip sonicator (Sonic Dismebrator 100, Fisher Scientific) was used for 15 s under dark conditions in an iced-water bath. Immediately after, 10, 50, or 500 μL of the concentrated solution was micro-pipetted into amber vials containing either 990, 950, or 500 μL, respectively of the appropriate stock adhesive to yield a final working concentration of 0.1, 0.5, or 5 mg/ml of PAA-CuI adhesive. The following four study groups were evaluated: (1) Control (2) 0.1 CuI, (3) 0.5 CuI, 4) 5.0 CuI. Different adhesives were used to determine how easily the particles would disperse into solution utilizing different generations of adhesives within the same brand family. In this way, the basic components are the same, just divided into different bottles with different viscosities.
Forty extracted, soft tissue impacted 3rd molars were collected and stored under a protocol approved by the Medical University of South Carolina (IRB ID No. PRO43691). An a-priori power analysis was preformed from pilot data to determine the appropriate sample size. The teeth were sanded down using 600-grit silicon carbide abrasive paper (EcoMet 250, Buehler, Lake Bluff, IL) on the occlusal surfaces to create a completely flat surface. The root surfaces were similarly reduced to 3 mm apical to the cementoenamel junction. Standardized hemisphere well preparations were created on the flattened occlusal surfaces using a #35 diamond round bur (801.31.035, Brasseler USA) with the following specifications: 6 mm diameter and 3 mm depth as shown in Figure 1. The teeth were then numbered and randomly divided into eight study groups as mentioned above with a sample size of five for each group. Each bonding agent was then applied to the appropriate tooth well and polymerized following the manufacturer's recommendations [Table 1] with LED light-curing unit (VALO, Ultradent) with a power density of 1400 mW/cm2. Next, a small break in the adhesive was made at the apex of the well using a custom jig and a quarter round carbide bur (Brassler USA) with a high speed, electric handpiece (TiMax Z95 L, NSK) with water [Figure 2]. This generated a defect in the adhesive with a depth and width of 0.5 mm. The teeth were then micro-computed tomography (microCT) scanned (Scanco μCT40, Scanco Medical) at a 10 μm resolution. After prescanning, the teeth were sterilized using ethylene oxide (EtOH) according to ISO 9001:2000 and EN 13485:2003. EtOH was chosen because it was found to have no effect on caries models whereas other sterilization methods can alter the rate of demineralization.[50]
Figure 1.
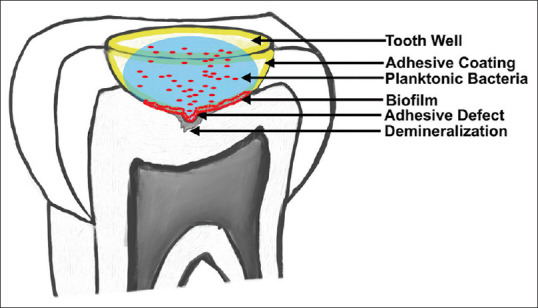
Graphic depiction of the caries model
Table 1.
Adhesive systems used and the manufacturer’s instructions for each
| Adhesive resin product | Lot number | Manufacturer’s instructions |
|---|---|---|
| Optibond Solo Plus | 5807385 | Etch enamel and dentin for 15 s with 37.5% phosphoric acid and rinse thoroughly. Dry lightly without desiccating. Apply product to tooth surfaces, air thin for 3 s and light cure for 20 s |
| Optibond XTR Primer | 6171908 | Apply self-etching primer to tooth surfaces using a scrubbing motion for 20 s and air thin with medium pressure for 5 s |
| Optibond XTR Adhesive | 5143490 | Apply adhesive layer to tooth surfaces using a brushing motion for 15 s and air thin for 5 s with medium pressure. Light cure for 10 s |
Figure 2.

Graphic depiction of the jig used to uniformly create the defect in the adhesive layer
For caries bacterial models mixed biofilms ≥2 species are more desirable for biofilm formation.[51] To form a mixed culture for the caries model, two common acid-forming oral bacteria were chosen. S. mutans (ATCC 25175) and Lactobacillus acidophilus (4356) were grown individually on the following medium for 48 h at 37°C; Tryptic soy broth 30 g/L (Hardy Diagnostics), Yeast Extract 5 g/L (Difco), and Sucrose 20 g/L (Sigma). The 48-hour cultures of both organisms were individually diluted to 0.100 OD600 in fresh medium and mixed to form the inoculum for the teeth. This mixed inoculum had a bacterial content of 6.9 × 105 viable L. acidophilus per ml and 4.4 × 106 viable S. mutans per ml [Figure 3]. The pH of the medium was 7.0 initially and tested every 24 h [Figure 4].
Figure 3.
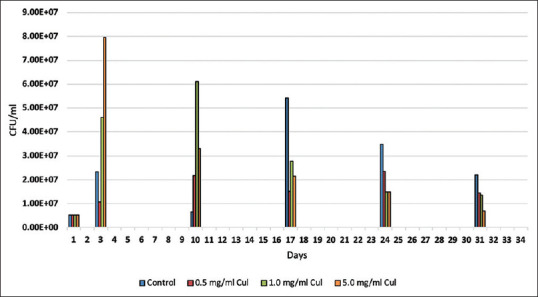
Average planktonic bacterial concentration over 31 days
Figure 4.
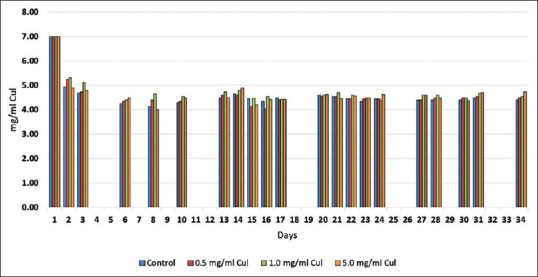
Average pH values over 31 days
The teeth were aseptically placed individually in sterile six-well tissue culture plates (Greiner Bio-one). Each tooth's well preparation received 75 μL of the inoculum mix, which was replaced with fresh medium daily, and the teeth incubated at 37°C. The liquid in the tooth preparation was drawn up and down 5 times to get a homogeneous liquid mix. The pH of the spent medium was determined using pH strips (EMD Millipore Corp.). Every 7 days, the spent medium from each tooth was plated onto Lactobacillus MRS agar (MRS) (hardy diagnostics) and brain heart infusion agar (Hardy Diagnostics) to quantify L. acidophilus and S. mutans.
After 31 days of incubation, the teeth were EtOH sterilized again to stop any progression of decay. The teeth were immediately re-scanned using the microCT with the same settings used for the prescans. The resulting images were exported from the ScanCo software into Dicom data sets. Utilizing Analyze Pro, Analyze Inc, Overland Park, KA, USA, the pre-scan and the post-scan were then density calibrated and the three-dimensional Dicom data sets were overlaid in AnalyzePro using a best-fit algorithm to align the different three-dimensional data sets. A blinded operator analyzed the data, which was then reconfirmed by a second operator. Threshold values were set and utilized for all samples. The sample area size and location were determined and repeated for all samples. This location was three-dimentionally centered on the small break in the adhesive. The software creates a color pseudo-map illustrating the differences between the prescan and postincubation scan where volume loss in contrasting colors. For clarity in the figures, the control was color-coded blue and yellow and the experimental was red and orange [Figure 5]. This computer software allows for the overlaying of 3-dimensional DICOM files for the purpose of analyzing subtle changes in mineral density. This nondestructive process allows for visualization of demineralization and repetition of analysis to confirm the accuracy of the measurements
Figure 5.
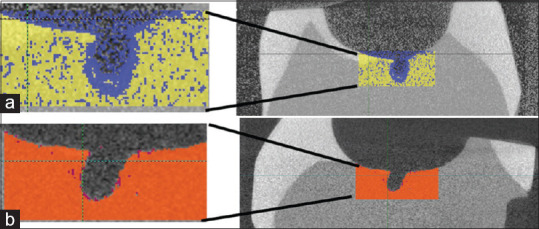
(a) Adhesive control sample showing demineralization in blue at the defect location. Here yellow represents the prestudy scan data and blue represents the post-study scan data. This particular sample had a mean volume loss of 2.12 mm3. (b) Experimental adhesive with 5 mg/ml copper iodide particles. Here, orange represents the prestudy scan data and dark red represents post-study scan data. Notice almost no red color is visible with data showing a mass loss of only 0.05 mm3
Stratified ANOVA models were run to determine if there were differences in viable planktonic bacterial concentrations and bacterial type between control adhesives and adhesives modified with varying concentration of CuI. If the P value was significant, post hoc comparisons were run. Generalized linear models were run with adhesive material, PAA-Cul treatment, and their interaction in the model. Each outcome was looked at to see if they were normally distributed. Log transformations were needed for each of the outcomes so they could meet the normality assumption therefore models were run on transformed data. If Cul treatment or the interaction term was significant, post hoc comparisons were run to see where specific differences were. Post hoc adjustments for multiple comparisons were made using a Tukey adjustment.
The sample size was much lower than expected due to damage occurring throughout the experiment to the teeth. To increase the statistical power of the pilot study, adhesive groups were combined to include both samples created with Optibond XTR and Optibond Solo Plus.
Results
The caries model tests the demineralization at the restorative/tooth interface at the apex of the tooth well. This is testing the microenvironment at a small defect in the adhesive layer to determine if antimicrobial ability of CuI would inhibit demineralization from the polymicrobial biofilm. Overlaid changes in demineralization were recorded as volume loss from the pre-scan to the post-scan in mm3 [Figure 5]. 1.0 μg/ml and 5 μg/ml of CuI incorporated into dental adhesive was able to significantly reduce demineralization volume loss compared to the control group (P = 0.019 and 0.005, respectively). Likewise, significant differences were found when comparing. 5 μg/ml and 5.0 μg/ml experimental groups (P = 0.018) [Figure 6].
Figure 6.
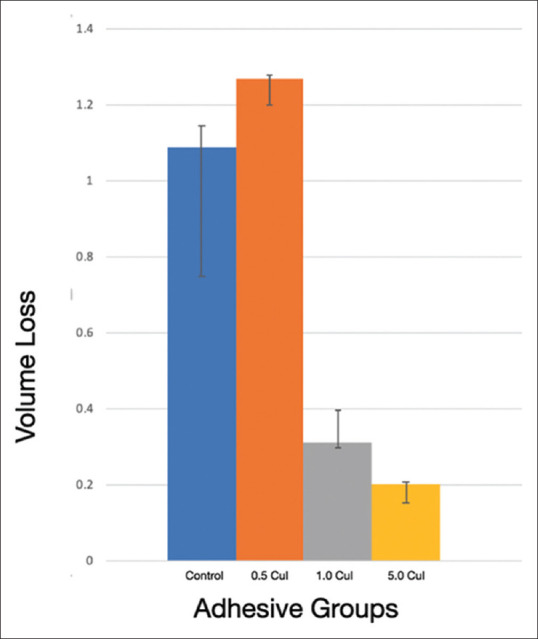
Volume loss from demineralization across adhesive groups
The pH and viable cell count test the macroenvironment within the tooth well. There were no statistical differences in the planktonic bacterial concentrations throughout the 31-day time span of this test [Figure 3]. Likewise, pH was tested regularly and stayed consistent throughout the 31-day span [Figure 4].
Discussion
The goal of this in vitro caries model was to test how adhesive resin responds in a polymicrobial environment once the adhesive layer is no longer intact. This model was designed to mimic the clinical scenario of marginal bond failure and recurrent decay, where a fairly large (0.5 mm) break in the adhesive was made and then inoculated with cariogenic bacteria. This model demonstrated that PAA-CuI doped adhesives had little effect on global pH, and only minimal effects on planktonic bacterial cell count. However, PAA-CuI was able to prevent demineralization at the adhesive tooth interface, creating a zone of inhibition even at the break area which is superficially unprotected. Therefore, our observations indirectly suggest that biofilm deposition near the adhesive was affected by the PAA-CuI in such a way that it was locally inhibiting bacterial colonization and therefore minimizing the acidification and demineralization of the microenvironment. Contrastingly, global planktonic bacteria viability was essentially unaltered by the antimicrobial adhesive additive CuI when compared to the controls. In retrospect, this is expected as the bactericidal mechanism of action of CuI is via contact rather than release. Many models fail to mimic a true cariogenic environment and cause total solution acidification, and likewise illustrate cariostatic phenomena by introducing bacteriostatic or bactericidal drugs into solution.[51,52] Therapeutic agents that make demineralization more difficult such as fluoride-containing materials and bioactive glasses are better suited for these types of models by simply raising the acid-sensitive threshold. However, in our model, we show that pH levels in the media were stable, suggesting that the observed demineralization was a localized function at the biofilm level.
Copper has a known antimicrobial effect and the PAA coated CuI has been shown in vitro to inhibit the growth of streptococcus mutans, the pathogen most associated with dental caries.[46] Dental caries are known to be a polymicrobial process and with the addition of lactobacillus to the microbial makeup, the intent was to create a more realistic clinical scenario to truly test the effectiveness of this antimicrobial additive. However, it was discovered that to maintain this polymicrobial solution was difficult and took several attempts until the solution was able to remain viable. This is due to the global pH of the system causing dominance of one bacterium over the other.
A major weakness of this pilot study is the potential for damaging the teeth during specimen preparation and desiccation during EtOH sterilization. Although every effort was made to preserve the integrity of the samples, several suffered fractures during preparation and sterilization. These samples, which were significant in number, had to be eliminated from data analysis. Any unidentified microcracks may confound the model and the analysis. These cracks are additional avenues down which the bacterial can infiltrate to cause demineralization in unintended areas. Special care was taken to eliminate this trauma and allow for more accurate data analysis. Additional repetition may result in statistical differences between groups where trends were beginning to emerge in this study. Likewise, the fluoride content of the individual teeth and substrates of those teeth would be beneficial to know to see if a correlation exists between fluoride levels and the ability to resist demineralization of exposed dentin.
Conclusions
It was shown within the limitations of this pilot study that CuI doped adhesives did reduce the amount of demineralization evident after breakdown of the adhesive layer and subsequent colonization of the microbes known to cause dental caries. The caries model itself showed the potential to effectively investigate how antimicrobial adhesive systems are able to control bacterial proliferation at the vulnerable tooth/restoration interface once the bond has broken down. Much was learned in this study that will hopefully be carried forth in future studies to increase sample size and better pin down statistical certainties.
Financial support and sponsorship
This study was funded through an NIH STTR grant, #1R41DE026085-01.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Rep. 2007;122:657–63. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadowsky S. An overview of treatment considerations for esthetic restorations: A review of the literature. J Prosth Dent. 2006;96:433–42. doi: 10.1016/j.prosdent.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Varughese R, Andrews P, Sigal M, Azarpazhooh A. An assessment of direct restorative material use in posterior teeth by American and Canadian pediatric dentists: I. Material choice. Pediatr Dent. 2016;38:502–8. [PubMed] [Google Scholar]
- 4.Khalichi P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004;25:5467–72. doi: 10.1016/j.biomaterials.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Samuel S, Li S, Mukherjee I, Guo Y, Patel A, Baran G, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Ferracane J. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Moraschini V, Fai CK, Alto RM, Dos Santos GO. Amalgam and resin composite longevity of posterior restorations: A systematic review and meta-analysis. J Dent. 2015;43:1043–50. doi: 10.1016/j.jdent.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Weir M, Zhang K, Wu E, Xu S, Zhou X, et al. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent Mater. 2012;28:853–62. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo M, Cheng L, Weir M, Hsia RC, Rodrigues L, Xu H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2012;101B:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamouda M. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26:143–51. doi: 10.7555/JBR.26.20120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira-Cenci T, Cenci MS, Fedorowicz Z, Marchesan MA. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst Rev. 2009;(3):CD007819. doi: 10.1002/14651858.CD007819.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Sarrett D. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials–Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Xie D, Weng Y, Guo X, Zhao J, Gregory R, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Fan C, Chu L, Rawls H, Norling B, Cardenas H, Whang K. Development of an antimicrobial resin – A pilot study. Dent Mater. 2011;27:322–8. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 18.Murray P, Windsor L, Smyth T, Hafez A, Cox C. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Bio Med. 2002;13:509–20. doi: 10.1177/154411130201300607. [DOI] [PubMed] [Google Scholar]
- 19.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Project 2-95. Int Dent J. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 20.Weston JF. Predictable nanohybrid composite systems and techniques for anterior and posterior direct restorations. Compend Contin Educ Dent. 2013;34(Spec No 5):8–12. [PubMed] [Google Scholar]
- 21.Mousavinasab S, Atai M, Salehi N, Salehi A. Effect of shade and light curing mode on the degree of conversion of silorane-based and methacrylate-based resin composites. J Dent Biomater. 2016;3:299–305. [PMC free article] [PubMed] [Google Scholar]
- 22.Shah P, Stansbury J. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater. 2014;30:586–93. doi: 10.1016/j.dental.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–6. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsujimoto A, Barkmeier WW, Takamizawa T, Watanabe H, Johnson WW, Latta MA, et al. Comparison between universal adhesives and two-step self-etch adhesives in terms of dentin bond fatigue durability in self-etch mode. Eur J Oral Sci. 2017;125:215–22. doi: 10.1111/eos.12346. [DOI] [PubMed] [Google Scholar]
- 25.Amsler F, Peutzfeldt A, Lussi A, Flury S. Long-term bond strength of self-etch adhesives to normal and artificially eroded dentin: Effect of relative humidity and saliva contamination. J Adhes Dent. 2017;19:169–76. doi: 10.3290/j.jad.a38101. [DOI] [PubMed] [Google Scholar]
- 26.Sai K, Shimamura Y, Takamizawa T, Tsujimoto A, Imai A, Endo H, et al. Influence of degradation conditions on dentin bonding durability of three universal adhesives. J Dent. 2016;54:56–61. doi: 10.1016/j.jdent.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Makishi P, André C, Ayres A, Martins A, Giannini M. Effect of storage time on bond strength and nanoleakage expression of universal adhesives bonded to dentin and etched enamel. J Oper Dent. 2016;41:305–17. doi: 10.2341/15-163-L. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz MA, Luque I, Hass V, Reis A, Loguercio AD, Bombarda NH. Immediate bonding properties of universal adhesives to dentine. J Dent. 2013;41:404–11. doi: 10.1016/j.jdent.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–6. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 30.Burke FJ, Lucarotti PS. The ultimate guide to restoration longevity in England and Wales. Part 4: Resin composite restorations: Time to next intervention and to extraction of the restored tooth. Br Dent J. 2018;224:945–56. doi: 10.1038/sj.bdj.2018.443. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS) June 17-19, 2004, Oregon Health and Science University, Portland, Oregon. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Ferracane J. Resin composite – State of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Demarco F, Corrêa M, Cenci M, Moraes R, Opdam N. Longevity of posterior composite restorations: Not only a matter of materials. Dent Mater. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS. Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res. 2014;93:1243–9. doi: 10.1177/0022034514550039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadeghinejad L, Cvitkovitch D, Siqueira W, Merritt J, Santerre J, Finer Y. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent Mater. 2017;33:175–90. doi: 10.1016/j.dental.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26:571–8. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 38.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 39.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 40.Zhang N, Ma Y, Weir M, Xu H, Bai Y, Melo M. Current insights into the modulation of oral bacterial degradation of dental polymeric restorative materials. Materials (Basel) 2017;10:507. doi: 10.3390/ma10050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith DJ, Taubman MA. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–73. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vacca Smith AM, Scott-Anne KM, Whelehan MT, Berkowitz RJ, Feng C, Bowen WH. Salivary glucosyltransferase B as a possible marker for caries activity. Caries Res. 2007;41:445–50. doi: 10.1159/000107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita Y, Bowen W, Burne R, Kuramitsu H. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–17. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K, Melo M, Cheng L, Weir M, Bai Y, Xu H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melo M, Cheng L, Zhang K, Weir M, Rodrigues L, Xu H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatini C, Mennito AS, Wolf BJ, Pashley DH, Renné WG. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J Dent. 2015;43:546–55. doi: 10.1016/j.jdent.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renné W, Lindner A, Mennito A, Agee K, Pashley D, Willett D, et al. Antibacterial properties of copper iodide-doped glass ionomer-based materials and effect of copper iodide nanoparticles on collagen degradation. Clin Oral Invest. 2016;21:369–79. doi: 10.1007/s00784-016-1799-y. [DOI] [PubMed] [Google Scholar]
- 48.Alghanem A, Fernandes G, Visser M, Dziak R, Renné W, Sabatini C. Biocompatibility and bond degradation of poly-acrylic acid coated copper iodide-adhesives. Dent Mater. 2017;33:336–47. doi: 10.1016/j.dental.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutiérrez MF, Malaquias P, Hass V, Matos TP, Lourenço L, Reis A, et al. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J Dent. 2017;61:12–20. doi: 10.1016/j.jdent.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Thomas RZ, Ruben JL, ten Bosch JJ, Huysmans MC. Effect of ethylene oxide sterilization on enamel and dentin demineralization in vitro. J Dent. 2007;35:547–51. doi: 10.1016/j.jdent.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Ferracane JL. Models of caries formation around dental composite restorations. J Dent Res. 2017;96:364–71. doi: 10.1177/0022034516683395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutiérrez MF, Malaquias P, Matos TP, Szesz A, Souza S, Bermudez J, et al. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent Mater. 2017;33:309–20. doi: 10.1016/j.dental.2016.12.011. [DOI] [PubMed] [Google Scholar]


