Abstract
Although techniques such as subepithelial connective tissue graft are now widely used for root coverage procedures, free gingival graft (FGG) continues to be a common mucogingival procedure used to increase keratinized tissue dimensions. However, the palatal donor site heals with secondary intention and requires a longer healing time causing more discomfort and pain to the patient. A healthy 22-year-old female patient presented with Miller's class II recession in relation to the tooth 31 with high frenal attachment and a shallow vestibule, which was treated using FGG, and the donor site was bandaged with advanced-platelet-rich fibrin (A-PRF). This report evaluates the healing of the donor site over a 12-month period and assesses the root coverage as well as the postoperative discomfort after the harvesting of graft. In terms of healing, the use of A-PRF membrane as a palatal bandage appears to accelerate healing at the donor site, thereby reducing postoperative complications.
Keywords: Donor site, free gingival graft, gingival recession, healing, pain, platelet-rich fibrin
Introduction
The free gingival graft (FGG) remains to be a common root coverage procedure in areas of inadequate attached gingiva. However, post harvesting of the graft, the donor site is subjected to discomfort and pain due to healing by secondary intention.[1] In recent reports and findings, platelet-rich fibrin (PRF) has been highly recommended as a palatal bandage to protect the donor sites for FGG. Balaram et al.[2] reported that PRF has favorable healing and tissue regeneration properties by sustained release of growth factors such as platelet-derived growth factor, transforming growth factor-beta, and insulin-like growth factor 1. This is generally seen in between 1 and 4 weeks, thus stimulating the environment for wound healing by inducing angiogenesis and osteogenesis.[1] Conventional PRF-based matrices such as leukocyte and PRF, due to excessive centrifugal forces, show compromises in the protein structure. Studies have shown higher qualitative outcome in terms of growth factor release by reduction in centrifugation speed and increase in time leading to the formation of advanced-PRF (A-PRF).[3] This report states the usage of A-PRF as a palatal bandage following FGG for a single tooth recession.
Case Report
A 22-year-old healthy female patient reported to our oral health center with the chief complaint of sensitivity and unesthetic appearance in her lower front teeth region. Upon intraoral examination, Miller's class II gingival recession or Recession Type 1 (RT1) as per the classification by Cairo et al.,[4] was seen in relation to tooth 31. There was a loss of clinical attachment up to 5mm and the probing pocket depth was 1mm with inadequate width of attached gingiva [Figure 1].
Figure 1.
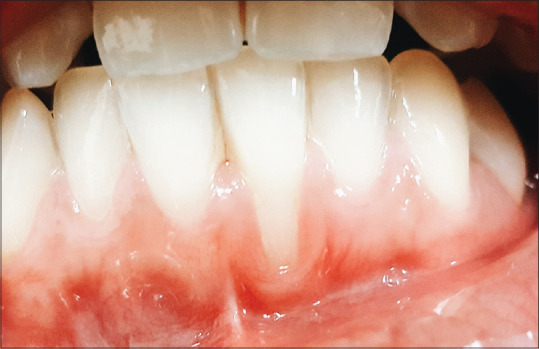
Pre-operative photograph of #24
After obtaining informed and written consent from the patient, the decision was made for root coverage using FGG technique accompanied with vestibuloplasty in relation to tooth 31. The placement of A-PRF at the palatal donor site in relation to tooth 24 and 25 was concurrently considered.
Case management
Platelet-rich fibrin preparation
Ten milliliters of venous blood were drawn into a tube without anticoagulant from the medial cubital vein (VACUETTE; PRF Process™, Nice, France). A-PRF was prepared following manufacturer's instructions.[5]
Surgical procedure
The patient was advised a pre-procedural mouthrinse with 15 ml of 0.12% Chlorhexidine (Periogard®, Colgate, Malaysia) post scaling. Local anesthesia using 2% of Lidocaine containing 1:100,000 epinephrine was administered in relation to 24 and 25. Vestibulopasty incision was performed in relation to teeth 32–42 using a no. 15 BP-blade (Swann-Morton®, England) after which the recipient site was de-epithelialized. The dimension of the site measured 4cm × 3cm, which was recorded using an aluminum foil. It was later transferred to the left palatal mucosa in relation to 24 and 25 tooth region for FGG to be harvested [Figure 2].
Figure 2.
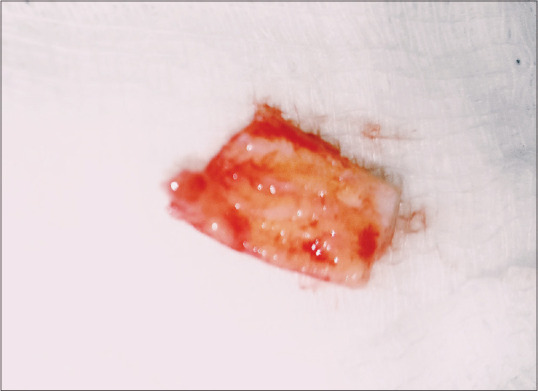
Graft measured to be 4 by 3cm
The donor site was irrigated with saline, and the A-PRF prepared earlier was placed as a fibrin banadage [Figure 3], which was then carefully protected by a eugenol-free periodontal dressing, (Coe-Pak™, GC, Australasia) followed by placement of a surgical stent. The graft was then positioned and stabilized by placing two modified sling sutures together with three interupted sutures using 4–0 nonresorbable silk sutures [Figure 4]. It was then covered with a layer of aluminum foil and Coe-Pak™.
Figure 3.
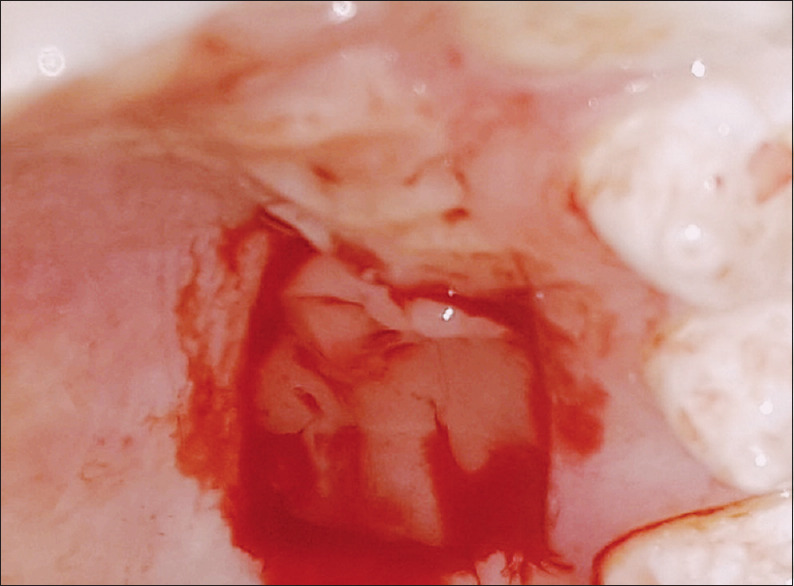
Platelet-rich fibin placed at donor site
Figure 4.
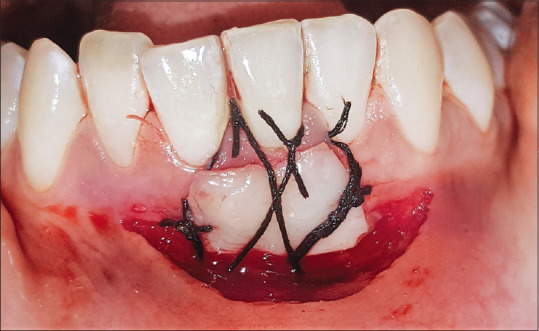
Free gingival graft was stabilized at the recipient site with 2 modified sling sutures
For pain assessment, the 101-numerical rating scale (NRS-10) was used. The patient's comfort level was recorded using the four-point verbal rating scale (VRS-4). The patient was advised to record her readings every hour for the first 8-hours after surgery followed by three times a day on the 2nd, 3rd, 4th, and the 7th day.[6,7]
Clinical outcomes
During the 1st week of postoperative follow-up, the patient reported postoperative pain at 1 h postsurgery, with an NRS-10 score of 50 and severe discomfort with a VRS-4 score. However, the pain and comfort scores showed to be at constant throughout the remaining 7 h postsurgery, with a NRS-10 value of 30 and VRS-4 at moderate discomfort [Table 1]. On the 2nd and 3rd day, the patient showed no changes during the morning and the afternoon [Table 2] that continued until the 4th postoperative day [(Table 3]. However, on the 7th day, patient scores improved drastically [Table 4]. Clinically, after 1 week of surgery, the healing of the palatal site was uneventful with moderate inflammation. During the second week, the donor site at the palatal region showed improved re-epithelization with marked reduction in inflammation [Figure 5].
Table 1.
Patient’s Numerical Rating Scale - 10 and Verbal Rating Scale - 4 score day 1 after surgery
| Day 1 (h) | NRS-10 | VRS-4 |
|---|---|---|
| 1st | 50 | Severe discomfort |
| 2nd | 30 | Moderate discomfort |
| 3rd | 30 | Moderate discomfort |
| 4th | 30 | Moderate discomfort |
| 5th | 30 | Moderate discomfort |
| 6th | 30 | Moderate discomfort |
| 7th | 30 | Moderate discomfort |
| 8th | 30 | Moderate discomfort |
NRS-10: Numerical Rating Scale-10, VRS-4: Verbal Rating Scale
Table 2.
Patient’s Numerical Rating Scale - 10 and Verbal Rating Scale - 4 score day 2 and 3 after surgery
| Day 2 and 3 | NRS-10 | VRS-4 |
|---|---|---|
| Morning | 20 | Moderate discomfort |
| Afternoon | 20 | Moderate discomfort |
| Night | 30 | Moderate discomfort |
NRS-10: Numerical Rating Scale-10, VRS-4: Verbal Rating Scale
Table 3.
Patient’s Numerical Rating Scale - 10 and Verbal Rating Scale - 4 score day 4,5 and 6 after surgery
| Day 4,5 and 6 | NRS-10 | VRS-4 |
|---|---|---|
| Morning | 20 | Mild discomfort |
| Afternoon | 20 | Mild discomfort |
| Night | 20 | Mild discomfort |
NRS-10: Numerical Rating Scale-10, VRS-4: Verbal Rating Scale
Table 4.
Patient’s Numerical Rating Scale - 10 and Verbal Rating Scale - 4 score day 7 after surgery
| Day 7 | NRS-10 | VRS-4 |
|---|---|---|
| Morning | 10 | No discomfort |
| Afternoon | 10 | No discomfort |
| Night | 10 | No discomfort |
NRS-10: Numerical Rating Scale-10, VRS-4: Verbal Rating Scale
Figure 5.
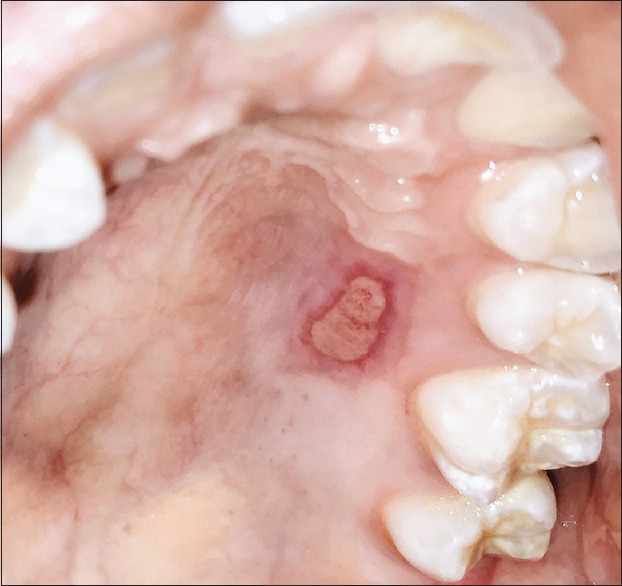
Palatal donor site 2 weeks postsurgery
Months later, the color and texture of the donor site appeared similar to the surrounding palatal tissues [Figure 6], similarly, the recipient site showed improved healing in terms of consistency and contour of the gingival margin [Figure 7]. One-year postoperative evaluation revealed that the recipient and the donor site showed no signs of inflammation [Figures 8 and 9].
Figure 6.
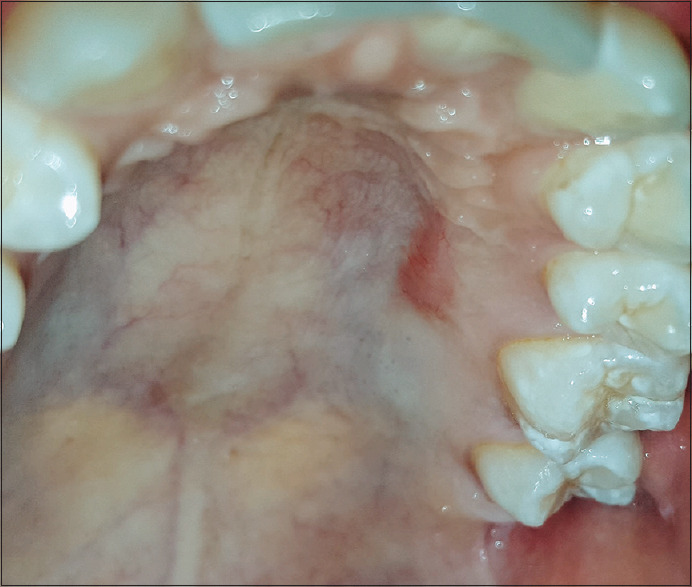
Palatal donor site 6 months postsurgery
Figure 7.
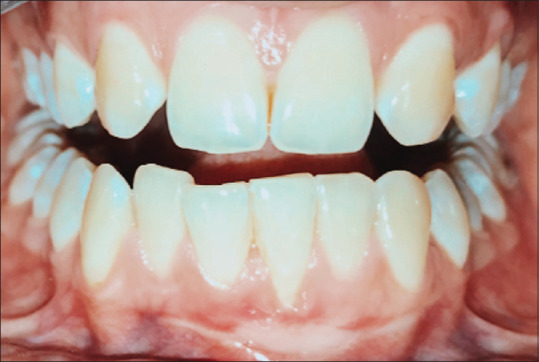
Recipient site after 6 months of surgery
Figure 8.
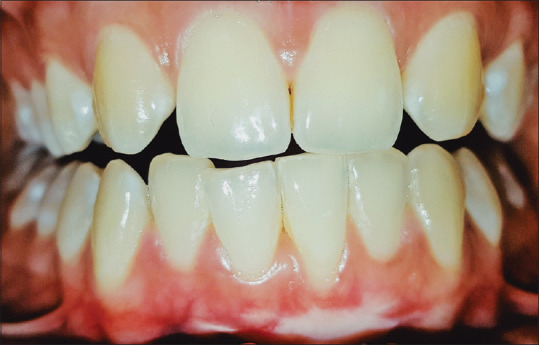
One year follow up showing an increase in width of attachment and clinical attachment loss
Figure 9.
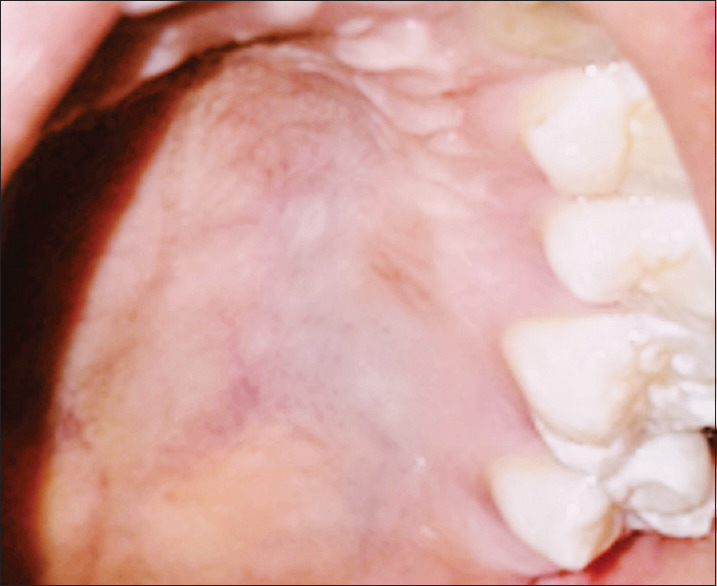
One year follow up of the palatal donor site with reduced redness
Discussion
The use of FGG, in this case, was most favorable as it provides root coverage in cases of narrow recessions (<3 mm) with increase in the width of attached gingiva.[8] At the palatal donor site, A-PRF was used as a palatal bandage along with a periodontal dressing as it protects the wound and accelerates the healing mechanism through angiogenesis, immunity, epithelial proliferation, and release of various inflammatory cytokines such as interleukin-1 (IL-1β), IL-6, and tumor necrosis factor-α, This could have resulted in less postoperative discomfort.[1]
The structure of A-PRF allows remodeling of fibrin in a more resistant connective tissue.[8] Our report showed a significant difference in the pain scores by the first week itself. Also, clinically, there was a total merge in the color and texture of the palatal tissues within 6 months to 1 year, similar to previous studies that stated a considerable shorter healing time which resulted in less postoperative discomfort and morbidity in the patients.[9,10]
Although PRF application improves the clinical parameters and decreases discomfort and pain in the early periods of healing, there are certain disadvantages of using PRF. Hence, we used A-PRF, as it has an increased number of leukocytes within the PRF matrix scaffolds.[11] Another key benefit of A-PRF is that it is a low centrifugation speed (1500 rpm for 14 min) concept that results in enhanced growth factor release entrapped in the fibrin clot.[12]
The key to the successful management of this case was the accelerated healing process induced by A-PRF as shown by the favorable pain scores of the patient. Not suturing the A-PRF membrane also aided in complete usage of the membrane, without sloughing. However, extensive trials and systematic reviews will be needed to confirm the long-term benefits of usage of A-PRF as a palatal bandage.
Summary
This case has some new information as it demonstrates a relatively new technique for accelerating healing at the donor site by usage of A-PRF membrane, a third-generation product as a palatal bandage to protect the palatal donor site. The advantage is that it is relatively easier to procure and an economic replacement for any other type of membranes. However, the major limitation of this case was the absence of a control site to compare the healing of the donor site after conventional harvesting of the graft.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bahammam MA. Effect of platelet-rich fibrin palatal bandage on pain scores and wound healing after free gingival graft: A randomized controlled clinical trial. Clin Oral Investig. 2018;22:3179–88. doi: 10.1007/s00784-018-2397-y. [DOI] [PubMed] [Google Scholar]
- 2.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16:284–93. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto-Peñaloza D, Peñarrocha-Diago M, Cervera-Ballester J, Peñarrocha-Diago M, Tarazona-Alvarez B, Peñarrocha-Oltra D. Pain and quality of life after endodontic surgery with or without advanced platelet-rich fibrin membrane application: A randomized clinical trial. Clin Oral Investig. 2020;24:1727–38. doi: 10.1007/s00784-019-03033-5. [DOI] [PubMed] [Google Scholar]
- 4.Cairo F, Nieri M, Cincinelli S, Mervelt J, Pagliaro U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J Clin Periodontol. 2011;38:661–6. doi: 10.1111/j.1600-051X.2011.01732.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 6.Houde RW. Methods for measuring clinical pain in humans. Acta Anaesthesiol Scand Suppl. 1982;74:25–9. doi: 10.1111/j.1399-6576.1982.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldman HM, Cohen DW. 5th ed. St. Louis: C.V. Mosby Co; 1973. Periodontal Therapy; pp. 715–8. [Google Scholar]
- 8.Hartshorne J, Gluckman H. A Comprehensive Clinical Review of Platelet-Rich Fibrin (PRF) and its Role in Promoting Tissue Healing and Regeneration: Part 2. Implant Practice. 2019. [Last accessed on 2020 Apr 24]. Available from: http://www.implantpractice.com .
- 9.Aravindaksha SP, Batra P, Sood V, Kumar A, Gupta G. Use of platele-rich fibrin membrane as a palatal bandage. Clin Adv Periodontics. 2014;4:246–50. [Google Scholar]
- 10.Femminella B, Iaconi MC, Di Tullio M, Romano L, Sinjari B, D’Arcangelo C, et al. Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: A randomized clinical trial. J Periodontol. 2016;87:103–13. doi: 10.1902/jop.2015.150198. [DOI] [PubMed] [Google Scholar]
- 11.Bahammam MA. Effect of platelet-rich fibrin palatal bandage on pain scores and wound healing after free gingival graft: A randomized controlled clinical trial. Clin Oral Investig. 2018;22:3179–88. doi: 10.1007/s00784-018-2397-y. [DOI] [PubMed] [Google Scholar]
- 12.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]


