Abstract
Objective
We aim to describe treatment patterns and overall survival (OS) among a Portuguese cohort of patients with small cell lung cancer (SCLC).
Methods
This study utilised a database held by IPO‐Porto, Portugal's largest oncology hospital. Adult patients diagnosed with SCLC at IPO‐Porto between January 2012 and June 2017, with follow‐up to December 2017, were included. Patients were stratified into subgroups with limited disease (LD) or extensive disease (ED). Treatment analyses were performed from 2015 onwards.
Results
Overall, 227 patients diagnosed with SCLC (37 LD; 190 ED) were analysed. Median OS (interquartile range [IQR]) was 15.0 months (3.8–39.3) for LD‐SCLC and 5.0 months (1.7–10.3) for ED‐SCLC. Among 19 patients diagnosed with LD‐SCLC from 2015 onwards, 12 (63.2%) received initial treatment with systemic anticancer therapy (SACT) ± radiotherapy; 6 (31.6%) received best supportive care (BSC). Among 89 patients with ED‐SCLC, 57 (68.5%) received SACT ± palliative radiotherapy; 28 (31.5%) received BSC. For patients receiving platinum doublet chemotherapy (±radiotherapy), median OS (IQR) was not reached for LD‐SCLC and 5.4 months (2.3–10.9) for ED‐SCLC.
Conclusion
This real‐world data analysis from a large Portuguese oncology hospital demonstrates a high disease burden for patients diagnosed with SCLC, particularly those with ED, and highlights a need for more effective therapies.
Keywords: chemotherapy, I‐O Optimise, radiotherapy, real‐world, small cell lung cancer, survival
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related death, both in Portugal and worldwide (GLOBOCAN, 2020; Sung et al., 2021). Small cell lung cancer (SCLC) is an aggressive high‐grade neuroendocrine tumour that makes up 13%–15% of all lung cancers globally (Sabari et al., 2017). SCLC is typically characterised by rapid growth and early metastatic spread and, as a consequence, is associated with limited treatment options and poor prognosis (Fruh et al., 2013; Sabari et al., 2017).
Since prognosis of SCLC is heavily dependent on tumour stage, the most recent European Society for Medical Oncology (ESMO) management guidelines for SCLC, last updated in 2013, include first‐line treatment options based on the tumour, node, metastasis (TNM) staging system (Fruh et al., 2013). Surgery with adjuvant chemotherapy is recommended as a potentially curative approach for small resectable tumours with no or minimal lymph node involvement and no distant metastases (T1‐T2, N0‐N1, M0); concomitant chemoradiotherapy as a potentially curative option for non‐metastatic disease involving lymph nodes (T1‐T4, N2‐N3, M0) or disease involving solitary, but not confirmed, metastases (T1‐T4, N1‐N3, M1a/b); and chemotherapy alone as palliative therapy in the case of multiple or confirmed metastases (Fruh et al., 2013). Regardless of stage, prophylactic cranial irradiation is recommended when there is a positive response to treatment or stable disease.
In the past 2–3 years, the management of lung cancer, including SCLC, has been transformed by approvals of immune checkpoint inhibitors. In the United States, several inhibitors targeting the programmed death‐1 (PD‐1)/PD‐1 ligand 1 (PD‐L1) pathway are now recommended for first‐line use in combination with chemotherapy for extensive disease SCLC (ED‐SCLC) or as monotherapy for subsequent post‐relapse disease (National Comprehensive Cancer Network®, 2021). In Europe, the PD‐L1 inhibitors atezolizumab and durvalumab have recently been approved for first‐line use in combination with standard chemotherapy in patients with ED‐SCLC (AstraZeneca, 2020; Roche, 2019). At the time of writing, no other immune checkpoint inhibitors are approved in Europe for the first‐line treatment of SCLC, and none are approved for second or later lines of therapy.
In order to fully evaluate the benefits of anticancer therapies, there is a need to assess effectiveness in real‐life clinical practice. Moreover, in the context of assessing the impact of newer treatments, for example, immune checkpoint inhibitors, it is important to understand treatment patterns, patient outcomes and disease burden in the period before these treatments became available. Real‐world databases represent valuable evidence sources as they can provide information to rapidly evaluate the impact of new treatment options and practical insights that complement clinical trial data. I‐O Optimise, a multinational, observational research initiative, has been designed to utilise existing real‐world data sources to provide valuable insights on the evolving treatment landscape for thoracic malignancies, including SCLC, across several countries in Europe (Ekman et al., 2019). The database held by the IPO‐Porto hospital is one of the data sources in this collaborative initiative. In this analysis, we describe the treatment patterns and survival outcomes among patients diagnosed with SCLC at IPO‐Porto between 2012 and 2017.
2. PATIENTS AND METHODS
2.1. Database and study overview
IPO‐Porto, a single‐site oncology hospital treating 15%–20% of all cancer patients in Portugal, holds a research database capturing data on all cancer types, as previously described (Soares et al., 2020), In this retrospective observational cohort study, adult patients (≥18 years old) were eligible if they received a new diagnosis of lung cancer (International Statistical Classification of Diseases and Related Health Problems, 10th Revision [ICD‐10] code C33 or C34) with SCLC morphology (International Classification of Diseases for Oncology, 3rd version [ICD‐0‐3] codes 80413, 80423, 80433, 80443, and 80453) between 1 January 2012 and 30 June 2017. Patients were excluded from the study if they had missing data on age or sex; had a concomitant primary tumour within 5 years before, or 1.5 years after, their SCLC diagnosis (except in the case of concomitant non‐metastatic non‐melanoma skin cancer [ICD‐10 codes C44 and C4A] and in situ or benign neoplasms); had received treatment for SCLC prior to admission to IPO‐Porto; and/or had no lung multidisciplinary or medical oncology consultation at IPO‐Porto.
Data extraction was performed using the Vision database (developed by IPO‐Porto), a system that relies on constant integration of selected outcomes and baseline and treatment variables. Data on systemic anticancer therapy (SACT) were only available from 1 January 2015. As such, treatment analyses were performed from that date through to end of the study. Patients were followed from their initial diagnosis until end of follow‐up (31 December 2017), death or loss to follow‐up, whichever occurred first.
The study was conducted in accordance with International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Epidemiology Practices and ethical principles originating from the Declaration of Helsinki. All relevant Portuguese laws and regulatory requirements were followed. The study protocol was approved by the Ethics Committee of the IPO‐Porto. Due to the retrospective, observational nature of the study, all patient data were anonymised. Patients were not contacted or directly impacted by study participation in any way; thus, obtaining informed consent was not applicable.
2.2. Data analysis
Baseline patient and clinical characteristics are reported using descriptive statistics. For descriptions of treatment patterns, ‘initial treatment’, including the categories of ‘surgery’, ‘radiotherapy’ and ‘SACT’, was defined as the first treatment received within 3 months of SCLC diagnosis, associated with any other treatment received within a time period following first treatment (see Table 1). Data outputs utilising these definitions were validated by clinicians at IPO‐Porto who were actively involved in the study. Overall survival (OS) was calculated using either (1) the time from date of diagnosis to date of death from any cause during the study period (for all patients and relevant subgroups diagnosed from 2012 onwards and for subgroups diagnosed from 2015 onwards receiving radiotherapy only or best supportive care [BSC]) or (2) the time from start of initial treatment to date of death from any cause during the study period (for subgroups diagnosed from 2015 onwards receiving SACT). The Kaplan–Meier method was used to estimate OS (medians and landmark probabilities) with the associated interquartile range (IQR) or 95% confidence interval (CI) reported.
TABLE 1.
Definitions of initial treatment
| Initial treatment category | Definitions |
|---|---|
| A. Surgery | Surgery for SCLC at any time point (regardless of any other treatment received) |
| B. Radiotherapy alone | Radiotherapy + no SACT nor surgery within 90 days (3 months) after radiotherapy |
| C. SACT + radiotherapy | Sum of C1, C2, C3 and C4 |
| C1. Radiotherapy followed by SACT | Radiotherapy + identification of SACT within 90 days (3 months) after 1st radiotherapy regimen + no surgery |
| C2. SACT followed by radiotherapy | SACT + identification of radiotherapy within 42 days (6 weeks) after start of last SACT cycle + no surgery |
| C3. Induction chemoradiotherapy | SACT + radiotherapy within less than 54 days (9 weeks/~3 cycles) of SACT initiation + no surgery |
| C4. Non‐induction chemoradiotherapy | SACT + radiotherapy starting at least 54 days (9 weeks/~3 cycles) after SACT initiation but before the end of the start of the last SACT cycle + no surgery |
| D. SACT alone | SACT + no radiotherapy nor surgery within 150 days (5 months) of 1st SACT regimen start |
| E. Not treated | No SACT, surgery or radiotherapy identified over entire follow‐up period |
Note: Initial treatment was defined as the first treatment received within 3 months of diagnosis, associated with any other treatment received within a time period following first treatment as described above.
Abbreviations: SACT, systemic anticancer therapy; SCLC, small cell lung cancer.
For the current analyses, eligible patients were classified according to the American Joint Committee on Cancer (AJCC) 7th Edition TNM staging system (Edge et al., 2010) and stratified into subgroups according to (1) the Veterans Administration Lung Study Group (VALSG) stage categorising patients as having limited disease SCLC (LD‐SCLC) or ED‐SCLC, and (2) when treatment data were available, the initial treatment received (SACT plus radiotherapy vs. SACT alone vs. radiotherapy alone vs. no SACT and no radiotherapy [i.e. BSC]). LD‐SCLC was defined as disease confined to the ipsilateral hemithorax, which could be safely encompassed within a tolerable radiation field (T any, N any, M0 except T3–T4 due to multiple lung nodules that do not fit in a tolerable radiation field). ED‐SCLC was defined as disease extending beyond the ipsilateral hemithorax, which could include malignant pleural or pericardial effusion or haematogenous metastases (T any, N any, M1a/b/c or T3–T4, N any, M0 due to multiple lung nodules that do not fit in a tolerable radiation field). VALSG staging was assessed by the medical team through a review of medical records.
3. RESULTS
3.1. Population characteristics
A total of 230 patients were diagnosed with SCLC at IPO‐Porto between January 2012 and June 2017. Since only one patient underwent surgical resection after SCLC diagnosis, analysis of ‘surgery’ as an initial treatment was not possible, and this patient was excluded. In addition, two other patients had missing TNM staging data and were also excluded. Therefore, the reported analyses focused on the cohort of 227 patients who did not undergo surgery as initial treatment and who had known TNM staging. Among these patients, median age was 65 years (range = 43–87), 190 (83.7%) were male, and 73.1% were diagnosed with TNM stage IV disease (Table 2). Thirty‐seven patients (16.3%) had LD‐SCLC, and 190 (83.7%) had ED‐SCLC. Among patients with ED‐SCLC, the most commonly reported distant metastases were bone (41.6%), liver (39.5%), lymph node (15.3%) and brain/CNS (11.6%).
TABLE 2.
Patient populations and characteristics at diagnosis
| Variable | Full analysis population a (2012–2017) | Sub‐cohort with treatment data b (2015–2017) | ||||
|---|---|---|---|---|---|---|
| All patients (N = 227) | LD‐SCLC (n = 37) | ED‐SCLC (n = 190) | All patients (N = 108) | LD‐SCLC (n = 19) | ED‐SCLC (n = 89) | |
| Age, years | ||||||
| Median (range) | 65 (43–87) | 70 (43–86) | 64 (43–87) | 66 (46–87) | 72 (46–81) | 66 (47–87) |
| Age category, n (%) | ||||||
| <65 years | 111 (48.9) | 15 (40.5) | 96 (50.5) | 49 (45.4) | M | 41 (46.1) |
| 65 to <75 years | 69 (30.4) | 7 (18.9) | 62 (32.6) | 34 (31.5) | M | 30 (33.7) |
| ≥75 years | 47 (20.7) | 15 (40.5) | 32 (16.8) | 25 (23.1) | 7 (36.8) | 18 (20.2) |
| Sex, n (%) | ||||||
| Male | 190 (83.7) | 29 (78.4) | 161 (84.7) | 96 (88.9) | 15 (78.9) | 81 (91.0) |
| TNM stage, n (%) | ||||||
| I‐II | 13 (5.7) | 13 (35.1) | 0 | 8 (7.4) | 8 (42.1) | 0 |
| IIIA | 24 (10.6) | 24 (64.9) | 0 | 11 (10.2) | 11 (57.9) | 0 |
| IIIB | 24 (10.6) | 0 | 24 (12.6) | 6 (5.6) | 0 | 6 (6.7) |
| IV | 166 (73.1) | 0 | 166 (87.4) | 83 (76.9) | 0 | 83 (93.3) |
| Site of metastasis, n (%) c | ||||||
| Bone | 79 (34.8) | 0 | 79 (41.6) | 40 (37.0) | 0 | 40 (44.9) |
| Brain/CNS | 22 (9.7) | 0 | 22 (11.6) | 12 (11.1) | 0 | 12 (13.5) |
| Liver | 75 (33.0) | 0 | 75 (39.5) | 39 (36.1) | 0 | 39 (43.8) |
| Lymph node | 29 (12.8) | 0 | 29 (15.3) | 17 (15.7) | 0 | 17 (19.1) |
Note: Data are masked if there were five or fewer patients (or to prevent calculation of masked categories).
Abbreviations: CNS, central nervous system; ED, extensive disease; M, masked; LD, limited disease; SCLC, small cell lung cancer; TNM, tumour, node, metastasis; VALSG, Veterans Administration Lung Study Group.
All eligible patients diagnosed with SCLC between January 2012 and June 2017 who did not undergo surgery as initial treatment for SCLC and who had available TNM and VALSG staging at diagnosis.
Treatment data were only available from January 2015 onwards; includes all eligible patients diagnosed with SCLC between January 2015 and June 2017 who did not undergo surgery as initial treatment for SCLC and who had available TNM and VALSG staging at diagnosis.
Patients could have more than one site of metastasis.
One hundred and eight patients were diagnosed with SCLC between January 2015 and June 2017, time for which treatment data were available; population characteristics of these patients were generally comparable to the overall analysis cohort (Table 2).
3.2. Treatment patterns (from 2015 onwards)
Thirteen of 19 patients (68.4%) diagnosed with LD‐SCLC and 61 of 89 patients (68.5%) diagnosed with ED‐SCLC between January 2015 and June 2017 received active initial treatment (i.e. SACT and/or radiotherapy); the remaining patients (representing 31.6% and 31.5% of LD‐SCLC and ED‐SCLC patients, respectively) received BSC only. Median time between diagnosis and initial treatment was 39 days for all treated patients with LD‐SCLC and 24 days for all treated patients with ED‐SCLC.
Among the 13 treated patients with LD‐SCLC, 23.1% received chemoradiotherapy, 30.8% received chemotherapy followed by radiotherapy and 30.8% received chemotherapy alone (Figure 1). Among the 61 treated patients with ED‐SCLC, 63.9% received chemotherapy alone and 29.5% received chemotherapy with palliative radiotherapy. The remaining treated patients with ED‐SCLC received palliative radiotherapy alone.
FIGURE 1.
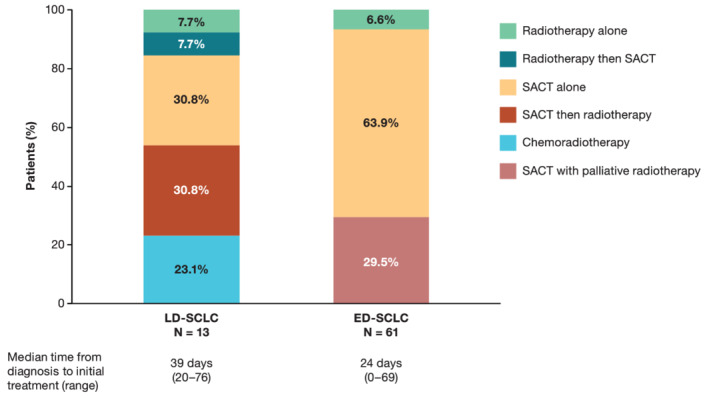
Initial treatment for subgroups based on VALSG stage in treated patients diagnosed with SCLC between 2015 and 2017. Abbreviations: ED, extensive disease; LD, limited disease; SACT, systemic anticancer therapy; SCLC, small cell lung cancer
Of the patients receiving SACT (alone or with radiotherapy) as initial treatment, all with LD‐SCLC and 98.2% of those ED‐SCLC received platinum doublet chemotherapy with carboplatin and/or cisplatin plus etoposide. Table 3 presents baseline characteristics for patients with LD‐SCLC and ED‐SCLC who received platinum doublet chemotherapy as initial treatment.
TABLE 3.
Population characteristics for patients receiving initial treatment with platinum doublet chemotherapy
| Variable | Sub‐cohort with treatment data a (2015–2017) receiving platinum doublet chemotherapy | |
|---|---|---|
| LD‐SCLC (n = 12) | ED‐SCLC (n = 56) | |
| Age, years | ||
| Median (range) | 65 (46–79) | 63 (47–87) |
| Sex, n (%) | ||
| Male | 11 (91.7) | 53 (94.6) |
| Site of metastasis, n (%) b | ||
| Bone | 0 | 28 (50.0) |
| Brain/CNS | 0 | 8 (14.3) |
| Liver | 0 | 20 (35.7) |
| Lymph node | 0 | 10 (17.9) |
Note: Data on age distribution and TNM staging are not included due to small patient numbers and the requirement for data masking if there are five or fewer patients in a specific category (or to prevent calculation of masked categories).
Abbreviations: CNS, central nervous system; ED, extensive disease; LD, limited disease; SCLC, small cell lung cancer; TNM, tumour, nodes, metastasis; VALSG, Veterans Administration Lung Study Group.
All eligible patients diagnosed with SCLC between January 2015 and June 2017 who did not undergo surgery as initial treatment for SCLC and who had available TNM and VALSG staging at diagnosis.
Patients could have more than one site of metastasis.
Among the patients with LD‐SCLC or ED‐SCLC receiving any initial treatment (n = 74), 14.9% received a second line of therapy (15.4% of treated patients with LD‐SCLC and 14.8% of treated patients with ED‐SCLC). The most common second‐line therapies were carboplatin plus etoposide and topotecan alone.
3.3. Survival outcomes
Median OS (IQR) from diagnosis for all 227 patients diagnosed with SCLC between January 2012 and June 2017 was 5.9 months (1.9–12.6), with 1‐ and 2‐year OS probabilities (95% CI) of 27% (22–34) and 11% (7–16), respectively. Median OS (IQR) in all patients with LD‐SCLC was 15.0 months (3.8–39.3) and in all patients with ED‐SCLC was 5.0 months (1.7–10.3) (Figure 2).
FIGURE 2.
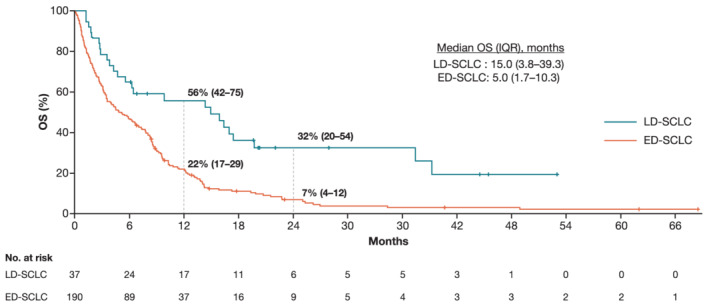
Overall survival from diagnosis for subgroups based on VALSG stage in all patients with SCLC between 2012 and 2017. Abbreviations: CI, confidence interval; ED, extensive disease; IQR, interquartile range; LD, limited disease; OS, overall survival; SCLC, small cell lung cancer
Among 68 patients receiving SACT with platinum doublet chemotherapy (alone or with radiotherapy) as initial treatment, median OS (IQR) from start of therapy was not reached (14.7–not evaluable) for the 12 patients with LD‐SCLC and 5.4 months (2.3–10.9) for the 56 patients with ED‐SCLC. Respective 1‐ and 2‐year OS probabilities (95% CIs) were 83% (65–100) and 52% (25–100) and 21% (11–37) and 5% (1–29).
Among the seven patients with LD‐SCLC not receiving platinum doublet chemotherapy, who received either radiotherapy alone (n = 1) or BSC (n = 6), median OS (IQR) from diagnosis was 2.8 months (1.2–6.4) with a 1‐year OS probability (95% CI) of 14% (2–88). In the 32 patients with ED‐SCLC who did not receive platinum doublet chemotherapy and who received either radiotherapy alone (n = 4) or BSC (n = 28), median OS (IQR) from diagnosis was 1.0 month (0.6–2.7); no patients were alive or uncensored 12 months after diagnosis.
4. DISCUSSION
Of the patients presenting with SCLC at IPO‐Porto, most were diagnosed with metastatic disease and only around one‐quarter were estimated to be alive one year after diagnosis, regardless of treatment. Approximately one‐third of all patients with SCLC at IPO‐Porto remained untreated (i.e. received BSC only). Assessing the generalisability of this finding is challenging as most real‐world SCLC analyses focus on treated populations (i.e. untreated patients are excluded prior to the analysis). In a Dutch population‐based study of 368 patients diagnosed with LD‐SCLC between 1997 and 2004, 41.6% received BSC only (Janssen‐Heijnen et al., 2014) which is a higher proportion than reported here (31.6%). However, the study population differed from ours as it was restricted to patients aged ≥75 years; moreover, their definition of ‘BSC’ included palliative radiotherapy. In another analysis of 792 patients diagnosed with ED‐SCLC between 2008 and 2014 at six teaching hospitals in the Netherlands, 28% received BSC only (Cramer‐van der Welle et al., 2020) which is similar to the proportion reported here (31.5%). Although our analysis was not designed to determine reasons for non‐treatment of patients with SCLC, it might be related to patient age, their relative frailty, and/or presence of aggressive disease. Despite small sample sizes it is noteworthy that patients in our study who received platinum doublet chemotherapy had a lower median age than the respective full populations (see Tables 2 and 3) suggesting patient age may have influenced the decision to administer chemotherapy. Of note, in the aforementioned Dutch population‐based study, increasing patient age was significantly associated with greater receipt of BSC in patients with LD‐SCLC (Janssen‐Heijnen et al., 2014). Likewise, in the Dutch teaching hospitals analysis, BSC‐treated patients with ED‐SCLC tended to be older, have worse performance status and have more co‐morbidities compared with those receiving active initial treatment (Cramer‐van der Welle et al., 2020).
Among the treated patients with LD‐SCLC, most initially received SACT followed by radiotherapy or SACT alone, rather than receiving concomitant chemoradiotherapy as recommended in the ESMO guidelines (Fruh et al., 2013). The low use of concomitant chemoradiotherapy (only 23% of treated patients with LD‐SCLC) was mainly related to patient‐specific clinical factors such as tumour size or the number and/or type of co‐morbidities. Treatment selection for treated patients with ED‐SCLC at IPO‐Porto was generally consistent with ESMO guidelines (Fruh et al., 2013) with almost all patients (93.4%) receiving SACT. Of these, around two‐thirds received SACT alone and around one‐third received SACT with radiotherapy administered either before or after SACT as palliation for bone or brain/CNS metastases. Most SACT‐treated patients with ED‐SCLC received platinum doublet chemotherapy with carboplatin and/or cisplatin plus etoposide, which is consistent with other real‐world studies of ED‐SCLC populations (Al Farsi et al., 2017; Cramer‐van der Welle et al., 2020).
Published real‐world survival data for SCLC populations are relatively limited and are often reported regardless of the stage of disease or treatment administered (Povsic et al., 2019). However, some studies have reported survival outcomes separately for LD‐ and ED‐SCLC populations. For example, in a Swedish retrospective, single‐centre cohort study, OS medians for 544 patients diagnosed with LD‐ or ED‐SCLC between 2008 and 2016 and receiving first‐line platinum doublet chemotherapy were 24.2 and 7.0 months, respectively (Tendler et al., 2020). In a Canadian retrospective chart review, OS medians for 229 patients diagnosed with LD‐ or ED‐SCLC between 2011 and 2014, of whom 96% had received cisplatin or carboplatin plus etoposide, were 21.7 and 8.9 months, respectively (Al Farsi et al., 2017). In two independent Dutch studies, median OS for > 1,600 patients with LD‐SCLC receiving chemotherapy plus thoracic radiotherapy was 21 months (Damhuis et al., 2018) and for 568 patients with ED‐SCLC mostly receiving cisplatin/carboplatin plus etoposide was 7.4 months (Cramer‐van der Welle et al., 2020). Finally, in the prospective German TLK cohort study, median OS for 314 patients with ED‐SCLC treated with first‐line platinum‐based chemotherapy ranged from 9.3 to 12.2 months depending on the regimen used (Steffens et al., 2019). In our analysis, median OS among patients with LD‐SCLC receiving platinum doublet chemotherapy was not reached. While this may appear to prevent comparison with the other real‐world studies, the 2‐year OS probability for this patient subgroup was 52%, suggesting that the median OS would fall close to this landmark and would be relatively aligned with the 21 to 24 months median range reported elsewhere (Al Farsi et al., 2017; Damhuis et al., 2018; Tendler et al., 2020). Among patients with ED‐SCLC receiving platinum doublet chemotherapy, the median OS in our analysis (5.4 months) was lower than the 7 to 12 month median range reported in other real‐world studies (Al Farsi et al., 2017; Steffens et al., 2019; Tendler et al., 2020; Cramer‐van der Welle et al., 2020). This may relate to differences in population characteristics (particularly in the context of performance status or co‐morbidities, which were not available for our analysis) and/or the use and type of second or later lines of therapy. In our study, only 15% of treated patients with ED‐SCLC received second‐line therapy; in contrast, other ED‐SCLC real‐world studies reported between 26% and 50% of treated patients receiving second‐line therapy (Steffens et al., 2019; Tendler et al., 2020; Cramer‐van der Welle et al., 2020).
As with any single‐site study, our analysis is limited to the population of patients referred to a single hospital at the time of SCLC diagnosis and findings may not be representative of overall clinical practice in the rest of the country or across Europe. The current analysis is also limited by the relatively low number of patients with treatment information and by missing data on certain baseline characteristics of interest, such as smoking history, performance status and/or co‐morbidities.
In conclusion, the results of our analysis of patients diagnosed with SCLC at IPO‐Porto add to the growing literature showing that treatment options for patients with SCLC in Europe have been suboptimal and associated survival outcomes poor. These collective data highlight the importance of rapidly implementing more effective treatment options, such as immune checkpoint inhibitor‐based regimens, into the treatment paradigm for SCLC patient populations.
CONFLICT OF INTERESTS
M. Soares, L. Antunes, P. Redondo, M. Borges and M.J. Bento are employees of IPO‐Porto. M. Soares, L. Antunes, P. Redondo and M.J. Bento report no further conflicts of interest. M. Borges received personal fees and non‐financial support from Roche and non‐financial support from Janssen outside the submitted work. F.R. Gonçalves reports no conflicts of interest. F. Grimson was an employee of IQVIA at the time of the reported analysis. R. Hermans is a current employee of IQVIA. C. Chaib, A. Juarez‐Garcia, M.J. Daumont and J.R. Penrod are employees of Bristol Myers Squibb. C. Chaib and J.R. Penrod report stock ownership in Bristol Myers Squibb. L. Lacoin was contracted (paid) as consultant by Bristol Myers Squibb to support the I‐O Optimise initiative and is an employee of Epi‐Fit.
ACKNOWLEDGMENTS
Professional writing and editorial assistance were provided by Richard Daniel, PhD, of Parexel, funded by Bristol Myers Squibb. This work was supported by Bristol Myers Squibb. IQVIA received funding from Bristol Myers Squibb to coordinate the data analyses presented in this manuscript. IPO‐Porto received funding from IQVIA to perform the analyses planned in the study protocol. All named authors were involved in the study design; in the analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Soares, M. , Antunes, L. , Redondo, P. , Borges, M. , Grimson, F. , Hermans, R. , Chaib, C. , Lacoin, L. , Juarez‐Garcia, A. , Daumont, M. J. , Penrod, J. R. , Bento, M. J. , & Gonçalves, F. R. (2021). Small cell lung cancer treatment and survival in Portugal: A retrospective analysis from the I‐O Optimise initiative. European Journal of Cancer Care, 30(6), e13496. 10.1111/ecc.13496
Funding information Bristol Myers Squibb
DATA AVAILABILITY STATEMENT
No data sharing is planned. Patient level data cannot to be shared due to regulatory and confidentiality reasons. Aggregated results from the study are presented in this manuscript.
REFERENCES
- Al Farsi, A. , Swaminath, A. , & Ellis, P. (2017). Patterns of relapse in small cell lung cancer (SCLC): A retrospective analysis of outcomes from a single Canadian center. Journal of Thoracic Oncology, 12(1S), S727–S728. 10.1016/j.jtho.2016.11.962 [DOI] [Google Scholar]
- AstraZeneca . (2020). Imfinzi approved in the EU for the treatment of extensive‐stage small cell lung cancer.
- Cramer‐van der Welle, C. M. , Schramel, F. M. N. H. , van Leeuwen, A. S. , Groen, H. J. M. , van de Garde, E. M. W. , & Santeon SCLC Study Group . (2020). Real‐world treatment patterns and outcomes of patients with extensive disease small cell lung cancer. European Journal of Cancer Care, 29(5), e13250. 10.1111/ecc.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damhuis, R. , Widder, J. , & Senan, S. (2018). Population‐based results of chemoradiotherapy for limited stage small cell lung cancer in the Netherlands. Clinical Oncology (Royal College of Radiologists), 30(1), 17–22. 10.1016/j.clon.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Edge, S. B. , Byrd, D. R. , Compton, C. C. , Fritz, A. G. , Greene, F. L. , & Trotti, A. (2010). AJCC Cancer Staging Manual (7th ed.). New York, NY: Springer. [Google Scholar]
- Ekman, S. , Griesinger, F. , Baas, P. , Chao, D. , Chouaid, C. , O'Donnell, J. C. , Penrod, J. R. , Daumont, M. , Lacoin, L. , McKenney, A. , Khovratovich, M. , Munro, R. E. J. , Durand‐Zaleski, I. , & Johnsen, S. P. (2019). I‐O Optimise: A novel multinational real‐world research platform in thoracic malignancies. Future Oncology, 15(14), 1551–1563. 10.2217/fon-2019-0025 [DOI] [PubMed] [Google Scholar]
- Früh, M. , De Ruysscher, D. , Popat, S. , Crinò, L. , Peters, S. , Felip, E. , & on behalf of the ESMO Guidelines Working Group . (2013). Small‐cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 24(S6), vi99–105. 10.1093/annonc/mdt178 [DOI] [PubMed] [Google Scholar]
- GLOBOCAN . (2020). Global Cancer Observatory 2020—Portugal Fact Sheet. International Agency for Research on Cancer. Available from https://gco.iarc.fr/ [Google Scholar]
- Janssen‐Heijnen, M. L. G. , Maas, H. A. A. M. , Koning, C. C. E. , van der Bruggen‐Bogaarts, B. A. H. A. , Groen, H. J. M. , & Wymenga, A. N. M. (2014). Tolerance and benefits of treatment for elderly patients with limited small‐cell lung cancer. Journal of Geriatric Oncology, 5(1), 71–77. 10.1016/j.jgo.2013.07.008 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network® . (2021). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Small Cell Lung Cancer ‐ Version 2.2021.
- Povsic, M. , Enstone, A. , Wyn, R. , Kornalska, K. , Penrod, J. R. , & Yuan, Y. (2019). Real‐world effectiveness and tolerability of small‐cell lung cancer (SCLC) treatments: A systematic literature review (SLR). PLoS ONE, 14(7), e0219622. 10.1371/journal.pone.0219622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche . (2019). European Commission approves Roche's Tecentriq in combination with chemotherapy for the initial treatment of people with extensive‐stage small cell lung cancer.
- Sabari, J. K. , Lok, B. H. , Laird, J. H. , Poirier, J. T. , & Rudin, C. M. (2017). Unravelling the biology of SCLC: Implications for therapy. Nature Reviews. Clinical Oncology, 14(9), 549–561. 10.1038/nrclinonc.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, M. , Antunes, L. , Redondo, P. , Borges, M. , Hermans, R. , Patel, D. , Grimson, F. , Munro, R. , Chaib, C. , Lacoin, L. , Daumont, M. , Penrod, J. R. , O'Donnell, J. C. , Bento, M. J. , & Gonçalves, F. R. (2020). Real‐world treatment patterns and survival outcomes for advanced non‐small cell lung cancer in the pre‐immunotherapy era in Portugal: A retrospective analysis from the I‐O Optimise initiative. BMC Pulmonary Medicine, 20, 240. 10.1186/s12890-020-01270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens, C.‐C. , Elender, C. , Hutzschenreuter, U. , Dille, S. , Binninger, A. , Spring, L. , Jänicke, M. , Marschner, N. , & TLK‐Group (Tumour Registry Lung Cancer) . (2019). Treatment and outcome of 432 patients with extensive‐stage small cell lung cancer in first, second and third line—Results from the prospective German TLK cohort study. Lung Cancer, 130, 216–225. 10.1016/j.lungcan.2019.02.026 [DOI] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tendler, S. , Zhan, Y. , Pettersson, A. , Lewensohn, R. , Viktorsson, K. , Fang, F. , & de Petris, L. (2020). Treatment patterns and survival outcomes for small‐cell lung cancer patients—A Swedish single center cohort study. Acta Oncologica, 59(4), 388–394. 10.1080/0284186X.2019.1711165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data sharing is planned. Patient level data cannot to be shared due to regulatory and confidentiality reasons. Aggregated results from the study are presented in this manuscript.


