Abstract
Objective
Follow‐up after breast cancer can be divided into surveillance and aftercare. It remains unclear how follow‐up can ideally be organised from the perspective of health care professionals (HCPs). The aim of this study was to gain insight in the organisation of follow‐up in seven Dutch teaching hospitals and to identify best practices and opportunities for improvement of breast cancer (all stages) follow‐up as proposed by HCPs.
Methods
Semi‐structured in‐depth group interviews were performed, one in each of the participating hospitals, with in total 16 HCPs and 2 patient advocates. To describe the organisation of follow‐up, transcripts were analysed using a deductive approach. Best practices and opportunities were derived using an inductive approach.
Results
Variation was found in the organisation of aftercare, especially in timing, frequency, and disciplines of involved HCPs. Less variation was observed for surveillance, which was guided by the national guideline. Best practices focused on case management and adequate collaboration between HCPs of different disciplines. Mentioned opportunities were improving the structured monitoring of patients' needs and a comprehensive guideline for organisation and content of aftercare.
Conclusions
Variation in follow‐up existed between hospitals. Shared decision‐making (SDM) about surveillance is desirable to ensure that surveillance matches the patient needs, preferences, and personal risk for recurrences.
Keywords: aftercare, breast cancer, care pathways, follow‐up, personalised care, survivorship care
1. INTRODUCTION
In the Netherlands, almost 15,000 women are diagnosed with breast cancer every year. The average survival rate after 5 years has increased by 10% in the last 30 years to 88% for patients diagnosed in the period from 2011 to 2017 (Integraal Kankercentrum Nederland [IKNL], n.d.). The increased incidence and survival rate results in an increasing prevalence, stretching resources for follow‐up (Chopra & Chopra, 2014; Lafranconi et al., 2017).
Follow‐up can be subdivided into surveillance and aftercare. In the Dutch national guideline for general practitioners (GPs), the term surveillance is defined as “the regularly scheduled medical examination for early detection of recurrent breast cancer or secondary primary tumours” (De Bock et al., 2016). According to the national guideline, surveillance is equal for each curatively treated breast cancer patient (all stages): an annual mammogram and physical examination for at least 5 years following treatment (NABON, 2012). Aftercare focuses on “limiting the burden of disease, rehabilitation and signalling, guiding and treating (late) consequences of (the treatment of) cancer” (De Bock et al., 2016). Although a national guideline for aftercare exists, the number and timing of pre‐scheduled consultations is not specified. In the Netherlands, HCPs receive no formal training regarding follow‐up care.
Follow‐up has changed over time. The traditional idea of monitoring cancer recurrence has broadened to a concern for recovery and well‐being that includes comprehensive management of psychosocial and physical effects, promotion of healthy lifestyles, and care coordination of the various health care professionals (HCPs) involved (Sisler et al., 2016). Besides, there is a growing demand for personalised care planning within cancer follow‐up (Chopra & Chopra, 2014; van Hezewijk et al., 2014; Zorginstituut Nederland [ZINL], 2016). Following the current national guideline, a personalised aftercare plan should be composed containing information about the received treatment, the risk for potential late effects, the risk for recurrences, and a personalised schedule for surveillance (NABON, 2012). The content of the personalised schedule is not specified. Personalisation of follow‐up (e.g., of the frequency and timing of imaging and aftercare consultations) can be supported through the process of shared decision‐making (SDM) in which decisions are made in a collaborative way (Elwyn et al., 2017).
To our knowledge, no studies are available about the ideal organisation of follow‐up, from the perspectives of HCPs. Therefore, the aim of this study is to gain insight into the organisation of follow‐up in seven teaching hospitals in the Netherlands and to identify best practices and opportunities for improvement of follow‐up after breast cancer (all stages) as proposed by HCPs.
2. METHODS
2.1. Study design and setting
We used an explorative design, including semi‐structured in‐depth group interviews with multiple HCPs involved in breast cancer care. This study took place in seven teaching hospitals, which together form the Santeon hospital group. The Santeon hospitals are large teaching hospitals spread over the Netherlands with dedicated breast centres, treating about 11% of all Dutch breast cancer patients.
2.2. Respondents
In each of the hospitals, the leading medical specialists of the breast cancer teams (mainly surgical oncologists) were asked to identify two to three HCPs who could best explain clinical practice regarding breast cancer follow‐up as representatives for their hospital. The appointed HCPs were then invited for a group interview. All of the invited HCPs accepted the invitation. Patient advocates from the Dutch Breast Cancer Society (BVN) were present at two of the interviews. Table 1 displays the interviewed respondents and their discipline per hospital.
TABLE 1.
Respondents
| Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4 | Hospital 5 | Hospital 6 | Hospital 7 | ||
|---|---|---|---|---|---|---|---|---|
| Surgical oncologist | X | X | X | X | 4 | |||
| NP surgery department | X | X | X | X | X | 5 | ||
| NP oncology department | X | X | 2 | |||||
| Breast cancer nurse | X | X | X | X | 4 | |||
| General physician | X | 1 | ||||||
| Patient advocate BVN | X | X | 2 | |||||
| Total respondents | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 18 |
Abbreviations: BVN, Dutch Breast Cancer Society; NP, nurse practitioner.
2.3. Procedures
Before the interviews took place, the HCPs were asked to send the following information (if available), which served as input for the interviews: (1) the transmural care pathway (i.e., a care pathway defined by cooperating intra‐ and extra‐mural health care organisations); (2) the institutional follow‐up care pathway (i.e., a care pathway defined for a specific hospital); (3) an example of an aftercare plan for patients; and (4) outcomes on patient‐reported experience measures (PREMs) and patient‐reported outcome measures (PROMs).
The HCPs in each hospital were interviewed together during a group interview to ensure that the participants could complement and correct each other. During the interviews, participants were actively asked to add to the information given by other participants. After the interviews, the summary notes were sent to all participants for correction/additions.
The interviews lasted about 1 h each and were conducted at the hospital location by two researchers (JA and JvH), both trained in conducting interviews. All interviews were audio‐recorded—with prior permission of respondents—and transcribed verbatim.
This study was no subject to approval by an ethical committee. All participants were informed about the aims and procedures of the study before the group interview and gave oral consent for audio‐recording of the interview and processing and reporting of the data for scientific publication.
2.4. Interview scheme
The interviews focused on the following topics: (1) roles of different HCPs within the process of follow‐up, (2) frequency of scheduled care moments, (3) referrals to other HCPs for aftercare, (4) tools used in follow‐up, (5) personalisation of follow‐up, and (6) SDM regarding follow‐up, (7) “best practices” and (8) “opportunities” for improvement of follow‐up. The topics were selected based on a literature review, the authors' clinical expertise, and the information needs of the Dutch Breast Cancer Patient Association.
To identify best practices, respondents were asked to indicate what makes them proud regarding follow‐up care for breast cancer patients in their hospital. Furthermore, respondents were asked to identify opportunities for the improvement of follow‐up.
2.5. Data analysis
Two researchers (JA and JvH) coded all transcripts independently. The coders read and reread all transcripts to familiarise themselves with the content. Then, relevant fragments were selected and categorised in one of the main themes, either related to the organisation of follow‐up (topics 1 to 6 in the interview scheme), best practices or opportunities for improvement of follow‐up. These three main themes were defined a priori based on the research questions of this study.
Fragments related to the organisation of follow‐up were analysed using deductive analysis, meaning that fragments were coded based on a pre‐defined set of topics, in this case the six first topics in the interview scheme. Fragments related to best practices and opportunities for improvement of follow‐up were analysed using an inductive approach. Relevant themes were derived from patterns found in the data.
All data were analysed on hospital level. This means that quotes of individual respondents in each hospital were gathered and labelled with a hospital number. Analysing data on hospital level allowed comparison of clinical practice (organisation) and perceived best practices and opportunities between hospitals in order to translate these findings to relevant topics for the future of breast cancer follow‐up.
The coders (JA and JvH) discussed their individual findings several times, and any differences in coding were solved based on consensus. Remaining inconsistencies in coding were discussed with a third coder (CD) until consensus was reached.
3. RESULTS
3.1. Organisation and variation in follow‐up
The results mainly represent the follow‐up trajectories in the surgery department, with the exception of Hospital 2, in which the surgery trajectory is integrated with the follow‐up trajectory in the oncology department.
3.2. Roles in follow‐up
3.2.1. Aftercare and surveillance
Figure 1 displays the HCPs involved in follow‐up and the structural care moments for aftercare and/or surveillance. Wide variation was found between the hospitals regarding the HCPs involved in aftercare. Regarding surveillance, less variation was found. In most hospitals, surveillance was conducted by the surgical oncologist (SO) or the nurse practitioner (NP) of the surgery department. Depending on the received treatment, patients visited the medical oncologist or the radiotherapist during the follow‐up phase, often in a separate aftercare trajectory. For example, HCPs indicated that guidance of anti‐hormonal therapy was mostly performed by NPs of the oncology department or a medical oncologist. Generally, the case manager (i.e., a HCP that is a contact person for the patient and that oversees the whole care process) was a HCP with a nursing background such as a breast cancer nurse (BCN) or a NP of the surgery or oncology department.
FIGURE 1.
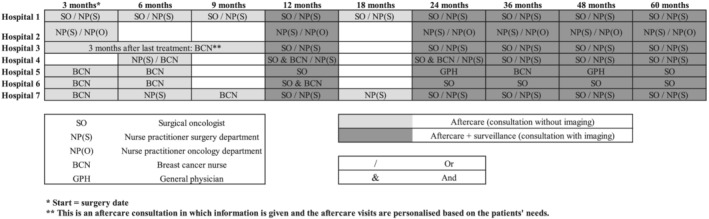
Scheduled care moments for follow‐up and roles of health care professional per hospital. Note: This figure only represents the follow‐up trajectories in the surgery department of the hospitals. Hospital 2 is the only hospital in which this trajectory is integrated with the follow‐up trajectory in the oncology department
3.2.2. Involvement of the general practitioner
In most hospitals (4), HCPs indicated that the GP was not actively involved in the follow‐up for patients with breast cancer. However, HCPs actively informed GPs about the patient's aftercare and surveillance. Furthermore, the GP was consulted by HCPs in case patients suffered from complaints or distress unrelated to the experienced breast cancer (treatment). HCPs had differing opinions on the extent to which the GP should be actively involved in breast cancer follow‐up:
I always tell patients: if there is something wrong, call us. Because the GP is an extra link in the chain and they refer back to us anyway. (NP surgery department, H3)
The group of patients who do not receive additional hormonal treatment, but experience long‐term effects from chemotherapy, may be missing something. Perhaps the GP could play an active role in that group. (NP oncology department, H6)
3.3. Frequency scheduled care moments
The moments at which aftercare takes place during the first and second year after surgery vary (Figure 1).
Two of the seven hospitals have pre‐scheduled consultations for aftercare every 3 months in the first year, every 6 months in the second year, and after that annual consultations until 5 years after treatment. In the other hospitals, aftercare was provided at a lower frequency. In most cases, the aftercare schedule is defined by the team of HCPs. This schedule is often based on health care providers' perspectives on the frequency and content of aftercare. These perspectives range from leaving the responsibility to the patients to contact their HCP when they need care to providing a structured program with regularly planned care moments to assure that the patient receives adequate care:
We have agreed that we will leave it up to the patient to plan more aftercare moments. (NP surgery department, H4)
It is not suggested in the guideline to provide aftercare so often, but we do it frequently. Especially to provide extra psychosocial care in the first year. (NP surgery department, H7)
The scheduled care moments (i.e., pre‐scheduled consultations) for surveillance were similar in all hospitals and correspond to the national guideline (an annual mammogram and physical examination for at least 5 years following treatment).
3.4. Referrals
Respondents mentioned that patients are referred to other (specialised) HCPs based on their needs and preferences in the follow‐up phase. For example, patients with physical complaints were referred to (oncological) physiotherapists and lymphedema therapists. For (psycho‐)social problems related to the disease or treatment, patients were mostly referred to social workers or medical psychologists within the hospital. In case of more general (psycho‐)social complaints, a referral was made to external psychologists or general practice‐based nurse specialists.
Patient referral to rehabilitation and re‐integration (i.e., care that can help patients to regain abilities needed for daily life after their illness) mainly took place in case of multiple physical and psychosocial complaints in the first years after treatment.
3.5. Tools
3.5.1. Follow‐up care pathway and aftercare plan
In most hospitals (6), a follow‐up care pathway was available for HCPs (either digitally or on paper). However, none of the respondents referred to actively using these described care pathways during follow‐up care. In two hospitals, HCPs indicated that they do not consult the care pathway on a daily basis, but that they are familiar with the content. In three hospitals, respondents mentioned that the care pathway for follow‐up was in revision. In one hospital, the care pathway was recently transformed into a flowchart and integrated into the electronic medical record (EMR).
A personalised aftercare plan as recommended by the Dutch guideline was provided to patients in only one hospital. This plan was developed within a regional collaboration with other regional hospitals and aimed to provide insight into different treatments the patients had undergone, the possible late effects of these treatments, and to inform patients about aftercare and surveillance. In three hospitals, an aftercare plan was under development at the time of the interview.
3.5.2. Other tools
In most hospitals, one or more tools were used for patients with breast cancer within the follow‐up phase (Table 2). In one hospital, no tools were used yet, but the HPCs were looking for tools to use. The most often mentioned tool was the distress thermometer (DT), which is a short self‐report measure to assess distress in the emotional, practical, physical, spiritual, and social functioning domains (Roth et al., 1998; Tuinman et al., 2008). In three hospitals, respondents indicated that they made use of PREMs outcomes.
TABLE 2.
Instruments used in the follow‐up phase
|
Tools to screen for (psychosocial) problems
|
|
Tools for self‐management
|
|
Tools for prediction of risks for recurrence
|
|
Tools to evaluate and improve care
|
Abbreviation: PREM, patient‐reported experience measure.
3.6. Personalisation
According to the respondents, aftercare was more personalised than surveillance because it was in particular organised according to the needs of patients. Personalisation of aftercare mostly took place in an non‐structured way based upon the patients' needs and wishes.
Surveillance was mainly offered according to the Dutch general guideline (an annual mammogram and physical examination for at least 5 years following treatment) and not considered as care that is personalised. Yet, HCPs indicated that in some cases, surveillance deviated from the guideline based on patient or treatment‐related factors, which can be seen as informal personalisation. In two hospitals (hospitals 4 and 7), respondents indicated a desire for personalisation of surveillance based on tumour‐ and patient characteristics:
“Now you assume a common denominator and then you can monitor certain patients more or less closely” (SO, H4).
3.7. Shared decision‐making
In none of the hospitals, structured SDM was practiced when making decisions about the planning of aftercare or surveillance. Nevertheless, in three hospitals, HCPs mentioned that decisions about the organisation of the aftercare and surveillance take place after discussion with the patient. In two hospitals (hospitals 1 and 2), HCPs expressed a wish for SDM regarding follow‐up to tailor care to the patients' needs and preferences:
Our ambition is to look at the needs of the patient from an SDM perspective. We want to use the guideline to see what is required and offer extra support based on needs. (NP oncology department, H2)
3.8. Best practices and opportunities for improvement of breast cancer follow‐up
An overview of mentioned best practices and opportunities is provided in Table 3.
TABLE 3.
Best practices and opportunities for improvement
| Best practices |
|
| Opportunities for improvement |
|
Abbreviations: EMR, electronic medical record; HCP, health care professional; PROM, patient‐reported outcome measure; SDM, shared decision‐making.
3.9. Best practices
In five hospitals, respondents valued their case management in the follow‐up phase. HCPs had the impression that patients valued the flexibility, accessibility and continuity of such a case manager.
“Case management is a priority for us and therefore something we are proud of, as it offers a low threshold for care and continuity for the patient” (NP surgery department, H1).
In six hospitals, the collaboration of HCPs (e.g., surgical oncologists, medical oncologists, NPs, and BCNs) in the follow‐up phase was also named as a best practice since this collaboration provides the opportunity to deliver efficient and adequate care:
“I am proud of our team, which collaborates and is very approachable to each other” (SO, H4).
Other best practices such as sufficient possibilities for referral and close contact with primary care providers (PCPs), personalised aftercare visits, and an integrated follow‐up trajectory were mentioned less often and are displayed in Table 3.
3.10. Opportunities for improvement of follow‐up
The HCPs described some opportunities for improvement. Most mentioned was the exploration of the use of PROMs for monitoring patient's needs in follow‐up. Described obstacles for this implementation were as follows: the burden of the current extensive PROMs inquiry, required resources for implementation, low uptake by patients, different IT systems in the participating hospitals, and lack of knowledge on how to use the outcomes in clinical practice: “We are going to implement PROMs. The question is whether we should do it with this large amount of questions” (SO, H3).
A second opportunity was to develop a (nation‐wide) guideline about the ideal organisation and content of aftercare. Examples of what this guidelines should include according to the respondents were as follows: (1) organisational aspects (e.g., a specific starting point for aftercare), (2) a description of the role of the case manager during follow‐up, (3) possibilities for follow‐up outside of hospital, and (4) an oversight of referral options for specific patients' needs (e.g., sexuality, fatigue, or neuropathy):
“Currently, we are very busy defining the potential content of aftercare” (BCN, H5).
Other opportunities such as better collaboration between HCPs, integration of follow‐up trajectories, SDM regarding personalised follow‐up and risk‐based surveillance were mentioned less often and are displayed in Table 3.
4. DISCUSSION
The results of this study showed substantial variation in the organisation of aftercare, but less variation in surveillance. Best practices mainly focused on case management and adequate collaboration between HCPs. Opportunities for improvement were seen in improving the structured monitoring of patients' needs and a more comprehensive guideline for the organisation and content of aftercare.
HCPs in this study indicated perspectives on the frequency of aftercare widely ranging from leaving the responsibility for organisation of aftercare with the patients as much as possible to providing a structured program with planned care moments, to make sure that all patients receive sufficient aftercare. Additionally, HCPs involved in aftercare varied considerably in disciplines and coordination of care, possibly due to a lack of standard protocols as seen in other studies (Neuman et al., 2017; Tucholka et al., 2018). One way to streamline care and to reduce inefficiency is to appoint a case manager that has an overview of the care process and acts as a contact person for patients. In our study, we found that many hospitals started with case management and reported to be proud of it and that the concept can be improved in the future.
Opinions on involving primary care providers (PCPs), such as GPs and general practice‐based nurse practitioners in aftercare and surveillance strongly differed. On one hand, involving PCPs may create future opportunities in terms of reducing the HCPs workload and improvement of meeting the wishes and needs of patients and reducing distress levels combined with a higher satisfaction of care (e.g., by providing aftercare closer to home in a familiar environment) (Lai et al., 2019). On the other hand, barriers still exist regarding communication and coordination between PCPs and secondary HCPs and especially doubts regarding the level of expertise of PCPs on breast cancer specific aftercare (de Ligt et al., 2019).
Personalisation of surveillance hardly takes place and surveillance is one‐size‐fits‐all. Studies have reported that for some patients, the frequency of surveillance stated in the guideline may be appropriate, while for a proportion of patients, less intensive surveillance would be sufficient (Witteveen et al., 2020). Moreover, more intensive surveillance is not more effective in terms of timeliness of recurrence detection or survival as opposed to less intensive surveillance (Høeg et al., 2019; Lafranconi et al., 2017; Moschetti et al., 2016). The INFLUENCE‐nomogram—a prediction tool for the risk for locoregional recurrences (LRRs)—can be used to personalise surveillance for breast cancer survivors (Witteveen et al., 2015). The INFLUENCE‐nomogram was used by two hospitals to inform patients about their risk for LRRs, but not yet for personalisation of surveillance.
Furthermore, SDM about surveillance is currently not common practice. De Ligt et al. (2019) defined decisions about surveillance for recurrent or secondary breast cancer as preference‐sensitive, meaning that decisions need to be based on the best available (medical) evidence and that these should reflect patient needs and values. SDM is advised as an optimal decision‐making process for this type of decisions (Elwyn et al., 2017). A tool to guide SDM regarding personalised surveillance integrating relevant outcome information (e.g., personal risks of LRR and second primary tumours or relevant PROs) can therefore be helpful. The effectiveness of (a tool for) SDM supported by outcome information about surveillance should be assessed in future research.
Although aftercare was described as highly personalised care, it was mainly personalised in an non‐structured way. HCPs indicated the need for tools to personalise aftercare according to the patients' needs. A patient decision aid (PtDA), such as the Aftercare Patient Decision Aid, developed by Klaassen et al. (2018), could be used for personalisation of aftercare. Respondents in our study indicated that the use of PROMs to monitor and meet needs of patients could be an opportunity for improvement. At the same time, they mentioned barriers for the implementation and use of PROMs such as the extensiveness of the current questionnaires, required resources for implementation, low uptake by patients, a variety of IT systems for the collection of PROMs, and a lack of options to show and communicate outcomes and give patients feedback when they visit the hospital. Van Egdom et al. (2019) and Riis et al. (2019) reported similar opportunities and barriers, which calls for the development of a standard set of patient‐ and HCP‐friendly PROMs to support implementation in clinical practice. The use of PROMs for personalisation of aftercare should be addressed in future research.
Little reference was made to the opportunity for distant care and telemedicine in follow‐up in the interviews (e.g., the use of telemedicine, EMRs or patient portals in follow‐up). However, these interviews were performed just before the COVID‐19 pandemic. Meanwhile, COVID‐19 gave rise to the use of these types of technologies in follow‐up and may cause a long‐lasting shift in care delivery in follow‐up.
5. STRENGTHS AND LIMITATIONS
To our knowledge, this study is the first study to assess the similarities and differences in the organisation of follow‐up and to identify best practices and opportunities for improvement of follow‐up for patients with breast cancer as proposed by HCPs. A strength of this study is the use of semi‐structured group interviews, which enabled the HCPs to complement each other and give a comprehensive overview about the actual follow‐up practices.
Even though this study focused on the perspective of HCPs on follow‐up, effort was made to take into account patients' views. The Dutch Breast Cancer Association (BVN) provided input for the study design and patient representatives were present at two interviews. A considerable amount of information about the needs and preferences of patients in breast cancer follow‐up is already present in literature (Berendsen et al., 2016; Brandenbarg et al., 2017; Feiten et al., 2016; Kwast et al., 2013; Lubberding et al., 2015; Onuma et al., 2019; Tucholka et al., 2018; van Hezewijk et al., 2011).
The presence of the patient representatives at two of the interviews may have influenced the information that was disclosed by HCPs. HCPs might have been more prone to mention more patient‐related best practices and opportunities for improvement. However, there were no signs that their presence has influenced the completeness of the data.
A limitation of this study is that the study is conducted within one nation, the Netherlands, including only teaching hospitals. This may influence generalisability to other types of institutions (e.g., academic hospitals) and institutions in other countries. Nevertheless, this study generated valuable insights, because even in comparable contexts (teaching hospitals) follow‐up varied considerably.
The number of HCPs that were interviewed for this study is another limitation of this study. Even though HCPs were carefully selected to be representative, the sample of 16 HCPs in seven hospitals remains relatively small. However, we feel that these respondents provided sufficient information to answer the research question.
In addition, interviewing the HCPs in each hospital in a group interview instead of in separate interviews could potentially create a bias because one HCP could overrule the other HCP(s). We managed this bias by actively asking all participants for additions/corrections during and after the interviews.
6. CONCLUSION
Variation is present in the organisation of aftercare. Case management and collaboration between HCPs of different disciplines is seen as a best practice. HCPs particularly see opportunities for improving the monitoring of patients' needs and a more comprehensive guideline for aftercare. Surveillance was organised according to the guideline and hardly personalised. SDM about surveillance is currently not practiced but is desirable to ensure that surveillance matches the patients' needs, preferences, and personal risk for recurrences.
Recommendations for practice:
Appoint a case manager for each patient, including during the follow‐up trajectory;
Explore collaboration between hospitals and PCPs during follow‐up;
Improve use of PROMs to personalise aftercare;
Personalise surveillance based on SDM and personal risk for recurrences.
CONFLICT OF INTEREST
Jet W. Ankersmid, Jolanda C. van Hoeve, Luc J.A. Strobbe, Yvonne E. A. van Riet, Cornelia F. van Uden‐Kraan, Sabine Siesling, and Constance H. C. Drossaert certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non‐financial interest in the subject matter or materials discussed in this manuscript.
INFORMED CONSENT
Participants in this study were health care professionals (HCPs), who were informed about the aim and method of the study. By participating in the interviews, HCPs gave informed consent for their participation. During the interviews, the researchers asked for consent for recording the interviews and for the processing and publishing of the data resulting from the interviews. This way of obtaining informed consent for interviews with HCPs is common practice in the Netherlands.
ACKNOWLEDGEMENTS
We would like to thank the health care professionals that have participated in this research for their meaningful contributions. Also we would like to thank Mirjam Velting (Dutch Breast Cancer Patient Association) for her help and input in the earlier phases of this research.
This work was supported by ZonMW as part of the “Experiment Uitkomstindicatoren” (projectnr. 516007001), which is carried out by Santeon.
APPENDIX A.
The Santeon Value‐Based Healthcare (VBHC) Breast Cancer Group (collaborators) are: Y.E.A. van Riet and J.M. Bode‐Meulepas, Catharina Hospital, Eindhoven, The Netherlands; L.J.A. Strobbe, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands; A.E. Dassen, Medisch Spectrum Twente, Enschede, The Netherlands; A.F.T. Olieman, Martini Hospital, Groningen, The Netherlands; H.H.G. Witjes, OLVG, Amsterdam, The Netherlands; R. Koelemij, St. Antonius Hospital, Utrecht, The Netherlands; C.M.E. Contant, Maasstad Hospital, Rotterdam, The Netherlands.
Ankersmid, J. W. , van Hoeve, J. C. , Strobbe, L. J. A. , van Riet, Y. E. A. , van Uden‐Kraan, C. F. , Siesling, S. , Drossaert, C. H. C. , & the Santeon VBHC Breast Cancer Group (2021). Follow‐up after breast cancer: Variations, best practices, and opportunities for improvement according to health care professionals. European Journal of Cancer Care, 30(6), e13505. 10.1111/ecc.13505
Funding information ZonMW, Grant/Award Number: 516007001
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Berendsen, A. J. , Roorda, C. , Jansen, L. , & de Bock, G. H. (2016). Patients' beliefs about the aims of breast cancer follow‐up: A qualitative study. Maturitas, 91, 140–144. 10.1016/j.maturitas.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Brandenbarg, D. , Berendsen, A. J. , & de Bock, G. H. (2017). Patients' expectations and preferences regarding cancer follow‐up care. Maturitas, 105, 58–63. 10.1016/j.maturitas.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Chopra, I. , & Chopra, A. (2014). Follow‐up care for breast cancer survivors: Improving patient outcomes. Patient Related Outcome Measures, 7, 71–85. 10.2147/PROM.S49586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock, G. , Bronsgeest, M. , Corsten, M. , Hinloopen, R. , Korver, J. , De Meij, M. , Verstappen V, van der Weele GM, Wittenberg, J. (2016). NHG‐Standaard Borstkanker.
- de Ligt, K. M. , van Egdom, L. S. E. , Koppert, L. B. , Siesling, S. , & van Til, J. A. (2019). Opportunities for personalised follow‐up care among patients with breast cancer: A scoping review to identify preference‐sensitive decisions. European Journal of Cancer Care, 28(3), e13092. 10.1111/ecc.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, G. , Durand, M. A. , Song, J. , Aarts, J. , Barr, P. J. , Berger, Z. , Cochran, N. , Frosch, D. , Galasiński, D. , Gulbrandsen, P. , Han, P. K. J. , Härter, M. , Kinnersley, P. , Lloyd, A. , Mishra, M. , Perestelo‐Perez, L. , Scholl, I. , Tomori, K. , Trevena, L. , … Van Der Weijden, T. (2017). A three‐talk model for shared decision making: Multistage consultation process. BMJ (Online), 359, 1–7. 10.1136/bmj.j4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiten, S. , Dünnebacke, J. , Friesenhahn, V. , Heymanns, J. , Köppler, H. , Meister, R. , Thomalla, J. , van Roye, C. , Wey, D. , & Weide, R. (2016). Follow‐up reality for breast cancer patients—Standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Und Frauenheilkunde, 76(05), 557–563. 10.1055/s-0042-106210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høeg, B. L. , Bidstrup, P. E. , Karlsen, R. V. , Friberg, A. S. , Albieri, V. , Dalton, S. O. , Saltbæk, L. , Andersen, K. K. , Horsboel, T. A. , & Johansen, C. (2019). Follow‐up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database of Systematic Reviews, 2019(11). 10.1002/14651858.CD012425.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integraal Kankercentrum Nederland (IKNL) . (n.d.). NKR cijfers. https://www.iknl.nl/nkr-cijfers
- Klaassen, L. A. , Dirksen, C. D. , Boersma, L. J. , Hoving, C. , Portz, M. J. G. , Mertens, P. M. G. , Janssen‐Engelen, I. L. E. , Lenssen, A. M. M. R. N. , Marais, M. B. C. B. , Starren‐Goessens, C. M. J. , Kurris, F. , Degenaar, A. , & van Lierop, K. (2018). A novel patient decision aid for aftercare in breast cancer patients: A promising tool to reduce costs by individualizing aftercare. Breast, 41, 144–150. 10.1016/j.breast.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Kwast, A. B. G. , Drossaert, C. H. C. , Siesling, S. , Klaase, J. , de Vries, H. , & IJzerman, M. (2013). Breast cancer follow‐up: From the perspective of health professionals and patients. European Journal of Cancer Care, 22(6), 754–764. 10.1111/ecc.12094 [DOI] [PubMed] [Google Scholar]
- Lafranconi, A. , Pylkkänen, L. , Deandrea, S. , Bramesfeld, A. , Lerda, D. , Neamțiu, L. , Saz‐Parkinson, Z. , Posso, M. , Rigau, D. , Sola, I. , Alonso‐Coello, P. , & Martinez‐Zapata, M. J. (2017). Intensive follow‐up for women with breast cancer: Review of clinical, economic and patient's preference domains through evidence to decision framework. Health and Quality of Life Outcomes, 15(1), 206. 10.1186/s12955-017-0779-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, X. B. , Ching, S. S. Y. , Wong, F. K. Y. , Leung, C. W. Y. , Lee, L. H. , Wong, J. S. Y. , & Lo, Y. F. (2019). A nurse‐led care program for breast cancer patients in a chemotherapy day center. Cancer Nursing, 42(1), 20–34. 10.1097/NCC.0000000000000539 [DOI] [PubMed] [Google Scholar]
- Lubberding, S. , van Uden‐Kraan, C. F. , Te Velde, E. A. , Cuijpers, P. , Leemans, C. R. , & Verdonck‐de Leeuw, I. M. (2015). Improving access to supportive cancer care through an eHealth application: A qualitative needs assessment among cancer survivors. Journal of Clinical Nursing, 24(9–10), 1367–1379. 10.1111/jocn.12753 [DOI] [PubMed] [Google Scholar]
- Moschetti, I. , Cinquini, M. , Lambertini, M. , Levaggi, A. , & Liberati, A. (2016). Follow‐up strategies for women treated for early breast cancer. Cochrane Database of Systematic Reviews, 2016(5). 10.1002/14651858.CD001768.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NABON . (2012). Breast cancer—Dutch guideline, version 2.0.
- Neuman, H. B. , Schumacher, J. R. , Schneider, D. F. , Winslow, E. R. , Busch, R. A. , Tucholka, J. L. , Smith, M. A. , & Greenberg, C. C. (2017). Variation in the types of providers participating in breast cancer follow‐up care: A SEER‐Medicare analysis. Annals of Surgical Oncology, 24(3), 683–691. 10.1245/s10434-016-5611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma, A. E. , Palmer Kelly, E. , Chakedis, J. , Paredes, A. Z. , Tsilimigras, D. I. , Wiemann, B. , Johnson, M. , Merath, K. , Akgul, O. , Cloyd, J. , & Pawlik, T. M. (2019). Patient preferences on the use of technology in cancer surveillance after curative surgery: A cross‐sectional analysis. Surgery (United States)., 165, 782–788. 10.1016/j.surg.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis, C. L. , Bechmann, T. , Jensen, P. T. , Coulter, A. , & Steffensen, K. D. (2019). Are patient‐reported outcomes useful in post‐treatment follow‐up care for women with early breast cancer? A scoping review. Patient Related Outcome Measures, 10, 117–127. 10.2147/prom.s195296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, A. J. , Kornblith, A. B. , Batel‐Copel, L. , Peabody, E. , Scher, H. I. , & Holland, J. C. (1998). Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer, 82, 1904–1908. [DOI] [PubMed] [Google Scholar]
- Sisler, J. , Chaput, G. , Sussman, J. , & Ozokwelu, E. (2016). Follow‐up after treatment for breast cancer: Practical guide to survivorship care for family physicians. Canadian Family Physician Medecin de Famille Canadien, 62(10), 805–811. http://www.ncbi.nlm.nih.gov/pubmed/27737976 [PMC free article] [PubMed] [Google Scholar]
- Tucholka, J. L. , Jacobson, N. , Steffens, N. M. , Schumacher, J. R. , Tevaarwerk, A. J. , Anderson, B. , Wilke, L. G. , Greenberg, C. C. , & Neuman, H. B. (2018). Breast cancer survivor's perspectives on the role different providers play in follow‐up care. Supportive Care in Cancer, 26(6), 2015–2022. 10.1007/s00520-018-4042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinman, M. A. , Gazendam‐Donofrio, S. M. , & Hoekstra‐Weebers, J. E. (2008). Screening and referral for psychosocial distress in oncologic practice. Cancer, 113(4), 870–878. 10.1002/cncr.23622 [DOI] [PubMed] [Google Scholar]
- van Egdom, L. S. E. , Oemrawsingh, A. , Verweij, L. M. , Lingsma, H. F. , Koppert, L. B. , Verhoef, C. , Klazinga, N. S. , & Hazelzet, J. A. (2019). Implementing patient‐reported outcome measures in clinical breast cancer care: A systematic review. Value in Health, 22(10), 1197–1226. 10.1016/j.jval.2019.04.1927 [DOI] [PubMed] [Google Scholar]
- van Hezewijk, M. , Ranke, G. M. C. , van Nes, J. G. H. , Stiggelbout, A. M. , de Bock, G. H. , & van de Velde, C. J. H. (2011). Patients' needs and preferences in routine follow‐up for early breast cancer; an evaluation of the changing role of the nurse practitioner. European Journal of Surgical Oncology (EJSO), 37(9), 765–773. 10.1016/j.ejso.2011.06.007 [DOI] [PubMed] [Google Scholar]
- van Hezewijk, M. , Smit, D. J. F. , Bastiaannet, E. , Scholten, A. N. , Ranke, G. M. C. , Kroep, J. R. , Marijnen, C. A. M. , & van de Velde, C. J. H. (2014). Feasibility of tailored follow‐up for patients with early breast cancer. The Breast, 23(6), 852–858. 10.1016/j.breast.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Witteveen, A. , Munck, L. , Groothuis‐Oudshoorn, C. G. M. , Sonke, G. S. , Poortmans, P. M. , Boersma, L. J. , Smidt, M. L. , Vliegen, I. M. H. , IJzerman, M. J. , & Siesling, S. (2020). Evaluating the age‐based recommendations for long‐term follow‐up in breast cancer. The Oncologist, 25(9), e1330–e1338. 10.1634/theoncologist.2019-0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen, A. , Vliegen, I. M. H. , Sonke, G. S. , Klaase, J. M. , IJzerman, M. J. , & Siesling, S. (2015). Personalisation of breast cancer follow‐up: A time‐dependent prognostic nomogram for the estimation of annual risk of locoregional recurrence in early breast cancer patients. Breast Cancer Research and Treatment, 152(3), 627–636. 10.1007/s10549-015-3490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorginstituut Nederland (ZINL) . (2016). Verbetersignalement Zinnige nacontrole bij vrouwen behandeld voor borstkanker [in Dutch]. https://www.zorginstituutnederland.nl/werkagenda/publicaties/rapport/2016/10/31/zinnige‐zorg‐verbetersignalement‐zinnige‐nacontrole‐bij‐vrouwen‐behandeld‐voor‐borstkanker
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


