Abstract
Introduction
Many patients with brain cancer experience cognitive problems. In this narrative review, we comprehensively evaluated empirical studies on various intervention approaches for cognitive problems in these patients.
Methods
Intervention studies that reported effects on cognitive functioning (either objectively tested or subjectively reported) in adult patients with primary and/or secondary brain tumours were identified through online searches in PubMed (MEDLINE) and Web of Science up to 13 March 2019.
Results
Of the 364 identified records, 10 pharmacological (including five randomised placebo‐controlled trials), 10 cognitive rehabilitation (including five [pilot] RCTs) and two multiple‐group exercise studies matched the inclusion criteria. Seventeen of 22 studies had final sample sizes smaller than 40. Several cognitive rehabilitation studies and some pharmacological approaches (donepezil and memantine) showed (at least partial) benefits for cognitive problems in adults with brain cancer. The effects of other pharmacological and exercise interventions were inconclusive and/or preliminary.
Conclusion
Overall, drawing firm conclusions is complicated due to various methodological shortcomings, including the absence of a (placebo) control group and small sample sizes. Promising effects have been reported for cognitive rehabilitation and some pharmacological approaches. Suggestions for more thorough research with respect to the various approaches are provided.
Keywords: brain neoplasms, cognition, exercise therapy, neoplasm metastasis, pharmacology, rehabilitation
1. INTRODUCTION
Although primary brain tumours are relatively uncommon, representing only 1.6% of all cancers (Bray et al., 2018), they carry with them significant disability and mortality. Glioblastoma, usually diagnosed in the sixth or seventh decade of life, is the most aggressive glioma in adults (Ostrom et al., 2018), with a median survival of one and a half years (Gilbert et al., 2014; Stupp et al., 2017). Patients affected by lower‐grade glioma, which usually present in early‐ to mid‐adulthood, experience more favourable median survival time (DeAngelis, 2001; Ellis, Stieber, & Austin, 2003). Meningioma, generally benign slow‐growing tumours arising from the meninges covering the brain, also has/have a more favourable prognosis (Linsler, Keller, Urbschat, Ketter, & Oertel, 2016) and is/are most common in adults older than 65 years (Dolecek et al., 2015; Ostrom et al., 2018). The current standard of treatment for glioma consists of surgery, radiation and/or chemotherapy, whereas for benign meningioma, surgical resection alone is preferred.
Secondary brain tumours are metastases from cancers elsewhere in the body. Ten to thirty‐five percent of adult cancer patients develop brain metastases during the course of their disease (Arvold et al., 2016). Whole brain radiation therapy (WBRT) is the most commonly used treatment for patients with brain metastases, yet the use of stereotactic radiosurgery has increased in order to spare healthy brain tissue (Arvold et al., 2016). Patients treated for brain metastases have a median survival time of <6 months (Arvold et al., 2016). However, for subgroups of patients median survival time exceeds 15 months (Sperduto et al., 2012).
Patients with primary and secondary brain tumours frequently present with multiple symptoms including headache, seizures, and language and motor impairments (Chandana, Movva, Arora, & Singh, 2008; Patchell, 2003). Additionally, due to the tumour and its treatment, many patients suffer from cognitive deficits during the course of their disease, mainly in the domains of attention, processing speed, memory and executive function (Brown et al., 2018; Edelstein, Richard, & Bernstein, 2017; Lidstone et al., 2003; Meskal, Gehring, Rutten, & Sitskoorn, 2016; Mukand, Blackinton, Crincoli, Lee, & Santos, 2001; Taphoorn & Klein, 2004; Tucha, Smely, Preier, & Lange, 2000). Previous studies in brain tumour and other neurological patients groups report a discrepancy between objectively measured cognitive ability and self‐reported cognitive concerns and that the latter tends to be more associated with anxiety, depression and fatigue (Gehring, Taphoorn, Sitskoorn, & Aaronson, 2015; Hall, Isaac, & Harris, 2009; Kinsinger, Lattie, & Mohr, 2010; McDowell et al., 2019; Pranckeviciene, Deltuva, Tamasauskas, & Bunevicius, 2017).
Although cognitive deficits in patients with primary brain tumours are usually milder and more diffuse from those secondary to stroke (Anderson, Damasio, & Tranel, 1990), cognitive problems can have substantial impact on patients’ lives, particularly as many patients are affected by this diagnosis at a relatively young age when they are often active in their work, family and social life. As patients live longer with possible cognitive problems due to improved treatments (Claus & Black, 2006; Linsler et al., 2016; McKinney, 2004), prevention and treatment of these problems among patients with a brain tumour are important.
Different approaches can be employed when targeting cognitive problems in patients with brain cancer, including pharmacological and exercise interventions, as well as cognitive rehabilitation, including cognitive strategy training and cognitive retraining. In cognitive strategy training, patients are taught strategies to compensate for their cognitive problems to help them adapt and function within their environment, whereas cognitive retraining aims to restore affected cognitive functions by extensive practice over time.
Several previous reviews have been published on interventions for cognitive impairments in patients with brain tumours; however, these reviews are limited in depth (e.g., Day et al., 2016), scope (e.g., Bergo et al., 2016; Day et al., 2014) or focus (e.g., Ali et al., 2018). The aim of the current narrative review was to comprehensively report on the breadth of intervention approaches studied, including pharmacological, cognitive rehabilitation and exercise, for improvement of cognitive function (objectively measured cognitive performance and/or self‐reported cognitive concerns) in patients with brain cancer.
2. METHODS
2.1. Inclusion and exclusion criteria
This narrative review included peer‐reviewed, English language papers on intervention studies that were clearly described and reported effects on cognitive problems (i.e., cognitive functioning and/or cognitive concerns). In the sections below, we refer to cognitive impairment when cognitive function has been assessed by neuropsychological testing and to cognitive concerns in case of self‐report. The term cognitive problems is used when we do not distinguish between cognitive concerns and cognitive impairment.
Particularly for cognitive rehabilitation approaches, we also included studies that incorporated questions on the use of cognitive strategies to evaluate rehabilitation effects. Searches were limited to adult patients with primary and/or secondary brain tumours regardless of treatment history. Studies on functional outcomes of inpatient rehabilitation with no clear description of the cognitive component of the intervention, as well as case reports, study protocols and abstract publications, were excluded. See Table 1 for an overview of the inclusion and exclusion criteria per study characteristic (i.e., design, publication, patient population, intervention and outcome measures).
Table 1.
Study inclusion and exclusion criteria
| Study characteristics | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Design |
|
|
| Publication |
|
|
| Patient population |
|
|
| Intervention |
|
|
| Outcome measures (either primary or secondary) |
|
|
Abbreviation: R(PC)T, randomised (placebo‐controlled) trial.
2.2. Search strategy and study selection
Systematic literature searches were conducted in PubMed (MEDLINE) and Web of Science up to 13 March 2019 (see Appendix for the full search strategy). Additional articles were identified through cross‐references.
Articles were initially screened based on title and abstract. If articles appeared eligible or if eligibility was unclear, the full‐text articles were independently screened by the first (PL) and last author (KG). Potential differences were resolved in scheduled meetings. A flow diagram according to PRISMA guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009) depicts the article selection process.
3. RESULTS
3.1. Characteristics of included studies
The literature search yielded a total of 364 records, of which 22 matched the inclusion criteria (see Figure 1): 10 pharmacological interventions (five randomised placebo‐controlled trials (RPCTs), four single‐group studies and one randomised trial), 10 cognitive rehabilitation approaches (five randomised controlled trials (RCTs), four single‐group studies, one two‐group study) and two exercise interventions (one two‐group study and one three‐group study) (Tables 2 and 3).
Figure 1.
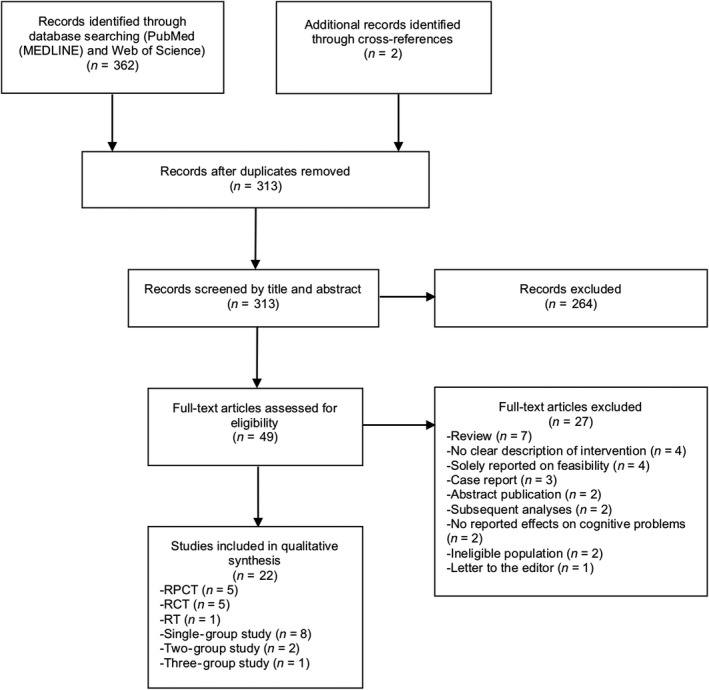
PRISMA flow diagram of article selection. R(PC)T, randomised (placebo‐controlled) trial
Table 2.
Pharmacological studies in brain tumour patients
| Pharmacological approaches | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donepezil | (Dex)MPHa | (Ar)MODb | MPHa + MODb | MEc | Herbsd | |||||
| Shaw (2006) | Rapp (2015) | Correa (2016) | Meyers (1998) | Butler (2007)a | Boele (2013) | Page (2015)b | Gehring (2012) | Brown (2013) | Attia (2012)d | |
| Study type | ||||||||||
| Pilot study | X | X | ||||||||
| Single‐group pre–post‐study | X | X | X | X | ||||||
| Randomised trial (RT) | X | |||||||||
| RPCT | X | X | X | X | X | |||||
| Sample size | ||||||||||
| Recruited number of BT patients | 35 | 198 | 24 | 30 | 68 | 37 | 54 | 34 | 508 | 34 |
| Number of BT patients with complete datae | 24 | 146 | 15 | 26 | 55 | 30 | 39 | 24 | 149 | 19 |
| Sample size of each R(PC)T groupf | 72/74 | 29/26 | 16/14 | 19/20 | 19/5g | 71/78 | ||||
| Demographicsh, i | ||||||||||
| Age (mean or median, rounded)f | 45i | 56/54h | 59 i | 40 h | 52/60h | 48 h | 59/58h | 43/54 h | 60/59h | 47h |
| Sex, male (n)f | 13i | 43/49h | 10i | 20h | 20/17h | 14h | 12/13h | 8/5h | 115/107h | 11h |
| Country | US | US | US | US | US | NL | US | US | US | US |
| Patient population | ||||||||||
| Glioma | X | |||||||||
| Mixed primary brain tumours | X | X | X | X | ||||||
| Primary and metastatic brain tumours | X | X | X | |||||||
| Brain metastases | X | |||||||||
| Not reported | X | |||||||||
| Patients selected based on presence of | ||||||||||
| Cognitive impairmentj | X | X | ||||||||
| Cognitive complaintsk | X | |||||||||
| None | X | X | X | X | X | X | X | |||
| Timing of intervention | ||||||||||
| During RTX | X | X | X | |||||||
| ≥6 months after TX (surgery, RTX or CTX) | X | X | X | X | ||||||
| Cross‐sectional/years after diagnosis | X | X | X | |||||||
| Relevant cognitive outcome(s) | ||||||||||
| Self‐perceived cognitive functioning | X | X | ||||||||
| Tested cognitive functioning | X | X | X | X | X | X | X | X | X | X |
| Reports on longer‐term follow‐up | ||||||||||
| None | X | X | X | X | X | X | ||||
| <6 months | X | X | X | X | ||||||
| Beneficial effects on cognitive outcome | ||||||||||
| No beneficial effects | X | X | X | |||||||
| Improvements possibly due to other effects | X | X | X | X | X | |||||
| (At least partial) beneficial effects | X | X | ||||||||
Abbreviations: BT, brain tumour; CTX, chemotherapy; ME, memantine; MOD, modafinil; MPH, methylphenidate; NL, The Netherlands; NR, not reported; R(PC)T, randomised (placebo‐controlled) trial; RTX, radiotherapy; TX, treatment; US, United States.
The italic values indicate mean age.
(Dex)methylphenidate.
(Ar)modafinil.
Memantine
Ginkgo biloba.
Final number of patients with complete data on the first post‐intervention cognitive assessment.
First number represents the number of patients in the intervention group, whereas the second number represents the number of patients in the control group if applicable.
First number represents the number of patients in the MPH group.
Demographics reported for recruited patients.
Demographics reported for the patients with complete data on the first post‐intervention cognitive assessment.
As determined by neuropsychological testing.
As self‐reported on a questionnaire.
Table 3.
Cognitive rehabilitation and exercise studies in brain tumour patients
| Cognitive rehabilitation | Exercise | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strategy training | Retraining | Combination | ||||||||||
| Locke (2008) | Hassler (2010) | Miotto (2013) | Miotto (2014) | Richard (2019) | Yang (2014) | Maschio (2015) | Gehring (2015) | Zucchella (2013) | Van der Linden (2018) | Han (2015) | Colledge (2018) | |
| Study type | ||||||||||||
| Pilot study | X | X | X | X | X | |||||||
| Single‐group pre–post‐study | X | X | X | X | ||||||||
| Two‐group pre–post‐study | X | X | ||||||||||
| Three‐group pre–post‐study | X | |||||||||||
| RCT | X | X | X | X | X | |||||||
| Sample size | ||||||||||||
| Recruited number of BT patients | 19 | NR | NR | NR | 25 | NR | 16 | 140 | 62 | 15 | NR | 25 |
| Number of BT patients with complete dataa | 14 | 11 | 21 | 9 | 20 | 38 | 12 | 135 | 53 | 13 | 29 | 16 |
| Sample size of each RCT groupb | 8/6 | 10/6/4c | 19/19 | 66/69 | 25/28 | |||||||
| Demographicsd, e | ||||||||||||
| Age (mean or median, rounded) b | 47/60d | 50e | 42 e | 39 e | 48d | 48/53 e | 49 d | 42/44 d | 59/53 e | 52 e | 48 e | 59 e |
| Sex, male (n) b | 7/4d | 7e | 12e | NR | 15d | 9/10e | 9d | 41/40d | 14/13e | 8e | 12e | 8e |
| Country | US | AU | BR | BR | CA | KO | IT | NL | IT | NL | KO | SW |
| Patient population | ||||||||||||
| Glioma | X | X | X | |||||||||
| Meningioma | X | |||||||||||
| Mixed primary brain tumours | X | X | X | X | X | |||||||
| Primary and metastatic brain tumours | X | X | X | |||||||||
| Patients selected based on presence of | ||||||||||||
| Cognitive impairmentf | X | X | X | X | X | X | ||||||
| Cognitive complaintsg | X | X | ||||||||||
| None | X | X | X | X | X | X | ||||||
| Timing of intervention | ||||||||||||
| ≤2 weeks after surgery | X | |||||||||||
| During RTX | X | |||||||||||
| After TX (surgery, RTX or CTX) | X | X | X | X | ||||||||
| Cross‐sectional/years after diagnosis | X | X | X | X | X | X | ||||||
| Relevant outcome(s) | ||||||||||||
| Self‐perceived cognitive functioning | X | X | ||||||||||
| Tested cognitive functioning | X | X | X | X | X | X | X | X | X | X | ||
| Self‐report of strategy use | X | X | X | X | ||||||||
| Imaging | X | X | ||||||||||
| Reports on longer‐term follow‐up | ||||||||||||
| None | X | X | X | X | X | X | X | |||||
| <6 months | X | X | ||||||||||
| ≥6 months to 1 year | X | X | X | |||||||||
| Beneficial effects on cognitive outcome | ||||||||||||
| Improvements possibly due to other effects | X | X | X | X | ||||||||
| (At least partial) beneficial effects | X | X | X | X | X | X | X | X | ||||
Abbreviations: AU, Austria; BR, Brazil; BT, brain tumour; CA, Canada; CTX, chemotherapy; IT, Italy; KO, Korea; NL, The Netherlands; NR, not reported; RCT, randomised controlled trial; RTX, radiotherapy; SW, Switzerland; TX, treatment; US, United States.
The italic values indicate mean age.
Final number of patients with complete data on the first post‐intervention cognitive assessment.
First number represents the number of patients in the intervention group, whereas the second number represents the number of patients in the control group if applicable.
The first number represents the number of patients in cognitive strategy training, and the second number and third number represent the number of patients in the psychoeducation (active control) group and passive (wait‐list) control group, respectively.
Demographics reported for the recruited patients.
Demographics reported for the patients with complete data on the first post‐intervention cognitive assessment.
As determined by neuropsychological testing.
As self‐reported on a questionnaire.
Recruited sample sizes of all included intervention studies ranged from 19 to 508 patients. Four cognitive rehabilitation studies and one exercise intervention study did not report recruited sample size. The number of patients with complete data on the first post‐intervention cognitive assessment ranged from 9 to 149, and these are the numbers we will use in our description of the studies below unless otherwise stated. The majority of the studies were conducted in the United States (n = 10), followed by the Netherlands (n = 3), and included samples with mixed primary brain tumours, followed by primary and metastatic brain tumours and other more homogeneous brain tumour groups. One pharmacological intervention study did not specify the type of brain tumour (Attia et al., 2012). The presence of cognitive impairment and/or cognitive concerns was a baseline inclusion criterion in nine of the 22 included studies. Sixteen studies reported intervention outcomes with respect to objectively tested cognitive function, four studies reported on both objectively tested cognitive function and self‐reported cognitive concerns, and two studies included solely self‐reported strategy as an outcome measure (see Tables 2 and 3 for a more detailed overview of the study characteristics).
3.2. Pharmacologic approaches
Of the 10 studies reviewed here (Table 2), five evaluated the effects of stimulant‐like agents ((dex)methylphenidate, (ar)modafinil), followed by three evaluating an acetylcholinesterase inhibitor (donepezil), one investigating an N‐methyl‐d‐aspartate (NMDA)‐receptor antagonist (memantine) and one examining a herb extract (ginkgo biloba).
Meyers, Weitzner, Valentine, and Levin (1998) were the first to evaluate the effects of a psychostimulant (i.e., methylphenidate) in the treatment of cognitive deficits among patients with brain cancer. Thirty patients with glioma, who scored below normative value (≥1 SD) on at least one neuropsychological test, were re‐assessed during increasing dosages of methylphenidate (i.e., 10, 20 and/or 30 mg doses twice daily). Significant improvements were found for 26 patients on tests of verbal memory, visual‐motor function, psychomotor speed, executive function, and motor speed and dexterity at the 10‐mg twice daily dose of methylphenidate. The majority of patients (78%) on this dose also reported increased energy and improved concentration and mood. As a control group was absent in this study, as was also the case in other studies reviewed here, there was no control for the possibility of improved test scores due to repeated neuropsychological testing (i.e., practice effects).
More recent attempts including RPCTs, controlling for placebo and/or practice effects, could not demonstrate beneficial effects of comparable agents (i.e., dexmethylphenidate and (ar)modafinil) on tests of cognitive functioning (or fatigue) in patients with brain cancer (Boele et al., 2013; Butler et al., 2007; Page et al., 2015). In these three RPCTs (samples ranging from 30 to 55 patients), participants were not selected on the basis of cognitive problems, as fatigue was the primary outcome. One randomised trial evaluated the cognitive effects of methylphenidate versus modafinil in a small sample (n = 24) of primary brain tumour patients with self‐reported cognitive decline or fatigue (Gehring et al., 2012). Improvements on tests of processing speed and executive function in both groups were larger for those with greater baseline deficits. Slow accrual of patients was reported by Boele et al. (2013), Butler et al. (2007) and Gehring et al. (2012).
More favourable and consistent findings have been documented for the use of donepezil, an acetylcholinesterase inhibitor, in patients with brain cancer (Correa, Kryza‐Lacombe, Baser, Beal, & DeAngelis, 2016; Rapp et al., 2015; Shaw et al., 2006). In both a single‐group study (n = 24) (Shaw et al., 2006) and a subsequent RPCT (n = 146) (Rapp et al., 2015), neither employing cognitive eligibility criteria, among previously irradiated patients with primary and secondary brain tumours, significant improvements were found on tests of attention/concentration, memory (Shaw et al., 2006), and motor speed and dexterity (Rapp et al., 2015) following 24 weeks of donepezil. In the RPCT, no significant improvements were found for the primary outcome (cognitive composite test score) (Rapp et al., 2015), but benefits of donepezil were more profound for patients with greater pre‐treatment cognitive deficits. A more recent uncontrolled pilot study, that selected patients on the basis of cognitive impairment, also documented positive effects of donepezil in 15 primary brain tumour patients (Correa et al., 2016). However, the study was closed due to slow accrual.
A large RPCT evaluated the potential neuroprotective effects of memantine (Brown et al., 2013), an NMDA‐receptor antagonist, involved in learning and memory (Stahl, 2013). In total, 508 patients with brain metastases were randomly assigned to receive either placebo or memantine for 24 weeks during WBRT. After attrition due to death, withdrawal of consent or non‐compliance with cognitive testing (of which the reasons were unspecified or not reported), 149 patients completed the cognitive assessment at 24 weeks. Following treatment, patients in the memantine arm showed a non‐significant trend of less decline in the primary endpoint of a test of delayed verbal recall as compared to the placebo group. The memantine group showed significantly longer time to cognitive decline, and had reduced decline rates, in executive function, delayed recognition and processing speed as compared to patients who received placebo.
A single‐group study by Attia et al. (2012) among an undefined group of 19 irradiated brain tumour patients on gingko biloba demonstrated improvements on tests of executive function, attention and non‐verbal memory after 24 weeks of treatment. The authors suggested that practice effects are unlikely to account for these improvements, as the majority of the outcome measures did not improve after discontinuation of the extract.
3.3. Cognitive rehabilitation
Ten studies incorporated cognitive rehabilitation approaches in patients with brain cancer (Table 3). Most of these studies investigated strategy training, followed by studies combining both strategy training and retraining, or evaluated retraining alone.
3.3.1. Cognitive strategy training
In a pilot RCT, Locke et al. (2008) examined the feasibility of a combined cognitive strategy and problem‐solving intervention (six sessions each) provided concurrently with radiotherapy in 14 primary brain tumour patients with mild‐to‐moderate cognitive deficits over a period of 2 weeks. Accrual was slow and most patients did not return for neuropsychological assessment 3 months post‐intervention, leaving the effects of the intervention on cognitive performance unevaluated. At 3 months of follow‐up, seven out of eight participants who received the intervention reported using the taught compensatory strategies at least once a week and the same proportion evaluated the intervention as helpful.
Hassler et al. (2010) investigated the effects of 10 weekly group sessions of holistic mnemonic training among 11 patients with high‐grade glioma in a single‐group pre–post‐study. After training, mean group scores on a verbal memory test improved significantly. Patients demonstrated great variability in their individual performances, with worsening, improvement and stabilisation over time. All patients reported that they were satisfied with the programme and expressed interest in participation in a refresher course.
Examination of the neural correlates (i.e., BOLD activation) associated with a semantic strategy training session in patients with primary brain tumours has been done with pre‐ and post‐training functional MRI (fMRI) scans (Miotto et al., 2014,2013). Miotto et al. (2013) scanned 21 patients with distinct pre‐frontal cortex lesions due to resection of various primary brain tumours during different word list encoding conditions (unrelated, related‐non‐structured and related‐structured words) and a control condition, of which the presentation order was randomised. After scanning, all patients underwent a 30‐min semantic organisation training during which they were taught to apply semantic organisational learning and memory strategies to different word lists. Participants then again underwent the fMRI scanning and were instructed to apply the learned strategies to novel words. Following the training, participants demonstrated significant improvements in memory and their use of semantic strategies during the learning trials. Moreover, increased activation in the pre‐frontal cortical (executive) network was identified, although exact location hereof varied according to lesion location. In their subsequent study (Miotto et al., 2014), they employed the same fMRI paradigm in nine patients who had undergone resection of left hemisphere low‐grade glioma and 15 healthy controls. Post‐training, both groups improved in the use of semantic strategies and memory performance for the related‐non‐structured words condition. Moreover, patients showed increased activation in the right inferior frontal gyrus during encoding for this condition following strategy training, which the authors attributed to post‐training compensatory recruitment of contralateral homologous areas.
A recent pilot RCT (Richard et al., 2019) evaluated the efficacy of Goal Management Training (GMT), an intervention combining both mindfulness and strategy training for improvement of executive functioning, among patients with primary brain tumours self‐identifying with cognitive concerns. Patients were randomly assigned to GMT, an active control group receiving education and activities to promote brain health, or a wait‐list control group. Patients in the intervention and active control group received eight weekly individual sessions and homework assignments. Executive function improved non‐significantly from pre‐ to post‐training for the GMT group (n = 10) but did significantly at 4 months of follow‐up. There were no significant changes for the active (n = 6) and wait‐list control group (n = 4). Both the GMT group and the active control group improved in processing speed (non‐trained function) post‐training, with maintenance at 4 months of follow‐up only for the GMT group. All groups improved at 4 months of follow‐up in a memory composite score. Both the GMT and the active control group reported fewer cognitive concerns post‐training and at follow‐up, and both groups reported using the learned strategies at least two to three times a week, which decreased slightly at 4 months of follow‐up for both groups.
3.3.2. Cognitive retraining
The two cognitive retraining studies in adults with brain tumours both used computer programmes (Maschio, Dinapoli, Fabi, Giannarelli, & Cantelmi, 2015; Yang, Chun, & Son, 2014). In a pilot study, Maschio et al. (2015) evaluated the effects of 10 weekly 1‐hr sessions of cognitive training (RehabTr) in 16 patients with primary and secondary brain tumours who presented with tumour‐related epilepsy and cognitive deficits. Tests with available parallel forms were used. Accrual was slow. Significant improvements were found among the 12 patients with complete data on tests of attention, memory and verbal fluency following the programme, which remained stable until 6 months of follow‐up.
Yang et al. (2014) evaluated the effectiveness of 4 weeks of VR training in primary and metastatic brain tumour patients. Thirty‐eight patients, presenting with cognitive impairments, were randomly assigned to the intervention group (VR training and computer‐assisted retraining, n = 19) or the control group (computer‐assisted retraining only, n = 19). The VR training included five different individually tailored exercises in which patients had to move, grab, hit or catch objects; the computer‐assisted retraining consisted of several attention and memory exercises. The intervention group showed greater improvements on tests of visual and auditory attention, verbal and visual memory, and visual‐motor coordination as compared to the control group.
3.3.3. Combined approaches of strategy training and retraining
Gehring et al. (2009) conducted an RCT in patients with lower‐grade glioma evaluating a programme combined of strategy training and computerised retraining. A total of 135 patients with complete follow‐up data reporting both cognitive concerns and demonstrating cognitive deficits were randomised to either the wait‐list control group (n = 69), receiving care as usual, or the intervention group (n = 66). The intervention consisted of six weekly 2‐hr home‐based sessions during which patients received individual compensatory strategy training and practiced with a computerised attention retraining game together with weekly homework assignments. The intervention group showed subjective cognitive benefits immediately post‐intervention as compared to the control group, but not at 6 months of follow‐up. At 6 months of follow‐up, the intervention group also performed significantly better on tests of attention and verbal memory, and self‐reported mental fatigue, as compared to the control group. Older participants benefited less from the cognitive rehabilitation programme (Gehring, Aaronson, Gundy, Taphoorn, & Sitskoorn, 2011) and evaluated its homework assignments as more burdensome than younger participants (Gehring, Aaronson, Taphoorn, & Sitskoorn, 2011).
Van der Linden, Sitskoorn, Rutten, and Gehring (2018) converted the programme into an iPad‐based application, “ReMIND,” available in both Dutch and English. In a pilot study on the feasibility of this intervention in the clinical setting, 13 patients with low‐grade glioma or meningioma followed the 10‐week programme 3 months after surgery. There was no control group, and recruitment of participants was challenging. On average, the 13 participants completed 71% of the iPad‐based strategy training and 76% of the attention retraining. Overall, eight participants indicated that they applied the taught strategies in daily life and six participants reported that the impact of their cognitive problems had positively changed. Twelve patients found an iPad application an appropriate mode of delivery of cognitive rehabilitation, whereas all participants indicated that they would recommend the application to other brain tumour patients.
One RCT conducted by Zucchella et al. (2013) investigated whether a combined cognitive intervention early (i.e., within 2 weeks) after tumour resection improves neuropsychological test performance in a mixed group of primary brain tumour patients. Patients with demonstrated cognitive deficits were allocated to the intervention or control group. The intervention group received 16 1‐hr individual sessions of therapist‐guided cognitive retraining and strategy training in a period of 4 weeks. Immediately following the intervention, the intervention group (n = 25) showed significantly better performance on tests of visual attention and verbal memory as compared to the control group (n = 28). The study did not include a long‐term follow up assessment to evaluate the potential protective effects of the early intervention.
3.4. Exercise approaches
Two studies among patients with brain cancer evaluated cognitive effects of an exercise intervention (Colledge et al., 2018; Han, Chun, Kim, & Kim, 2015), yet both studies compared the outcomes of brain tumour patients to those of (sub)acute stroke patients after the same physical training. Han et al. (2015) evaluated the effects of early conventional rehabilitation (among others, physical therapy, aerobic exercises, occupational therapy) on functional improvement among 29 primary and metastatic brain tumour patients (mean time since surgery or biopsy 25.5 days) and 26 stroke patients (mean time since stroke onset 28.1 days) and found that both groups improved in Mini‐Mental State Examination scores following 4 weeks of rehabilitation.
In a more recent exploratory study, Colledge et al. (2018) compared the effects of 12 weeks of home‐based individualised moderate aerobic exercise on several outcomes including psychological function, verbal learning and sleep in 15 survivors of aneurysmal subarachnoid haemorrhage with its effects in control groups of 16 meningioma patients and of 17 healthy participants. They documented improvement in the one cognitive test (verbal learning) that was included for all three groups.
4. DISCUSSION
4.1. Strengths and limitations of the included studies and main findings
This review provides an overview of empirical studies on interventions for cognitive problems in adults with brain cancer. Drawing firm conclusions on the effects of the different approaches is complicated by methodological shortcomings, of which the absence of a non‐intervention or placebo control group is the most prominent. Twelve out of 22 intervention studies did not incorporate such a control group and were therefore not able to preclude non‐specific effects, such as spontaneous recovery (especially during early intervention), regression to the mean, practice and/or placebo effects. Reducing the possibility of such effects is of great importance, as improvements in performance on cognitive tests due to repeated testing can be mistakenly attributed to the intervention. In the absence of a control group, alternate/parallel neuropsychological test forms may be used to have some control over practice effects, which was only the case in five out of the 12 studies in which a non‐intervention or placebo control group was absent (i.e., three cognitive rehabilitation (Maschio et al., 2015; Miotto et al., 2014; Miotto et al., 2013) and two pharmacological studies (Correa et al., 2016; Gehring et al., 2012)).
Another limitation is that 17 out of 22 studies had final sample sizes smaller than 40, which likely resulted in underpowered statistical testing and/or basic statistical testing procedures. Only two cognitive rehabilitation studies and three pharmacological studies included samples of reasonable size. In most studies, if reported, accrual of patients was often challenging, yet attrition from recruitment to first post‐intervention assessment, especially for cognitive rehabilitation studies, was relatively low.
Overall, the few somewhat larger, better‐designed cognitive rehabilitation studies (i.e., retraining (Yang et al., 2014) and a combination of strategy training and retraining (Gehring et al., 2009; Zucchella et al., 2013)) and pharmacological approaches (i.e., donepezil (Rapp et al., 2015) and memantine (Brown et al., 2013)) showed (at least partially) promising effects in the management of cognitive problems (especially for objective cognitive function) in patients with brain cancer. Evidence for the use of other pharmacological (i.e., (dex)methylphenidate, (ar)modafinil and gingko biloba) and exercise interventions remains inconclusive and/or preliminary.
4.2. Considerations with respect to the designs of studies
The majority of studies examined the effect of an intervention that was carried out several months after tumour treatment or even years after initial diagnosis when patients start to experience cognitive problems. Patients with more favourable prognosis might be more motivated to participate in and may benefit more from late intervention. A few studies investigated interventions employed within the first weeks after surgery (Han et al., 2015; Zucchella et al., 2013) or during radiotherapy (Brown et al., 2013; Butler et al., 2007; Locke et al., 2008; Page et al., 2015). Early intervention may prevent worsening of cognitive deficits over time or after adjuvant treatments, whether it concerns strengthening intact cognitive functions and use of compensatory strategies, or, in case of pharmacological interventions, protecting and/or changing the cellular milieu. To determine whether early intervention is able to prevent or delay (further) cognitive decline, studies need long‐term follow‐up assessments. However, only two out of the five aforementioned early intervention studies reported long‐term follow‐up findings (Butler et al., 2007; Locke et al., 2008).
Screening of the presence of cognitive problems, as confirmed with neuropsychological tests or self‐reports, was done by all six cognitive rehabilitation studies that specifically aimed at ameliorating (objectively tested) cognitive deficits, except for one (Hassler et al., 2010). Of the six pharmacological studies that specifically aimed for amelioration of cognitive problems, three studies did not employ eligibility criteria with respect to cognitive problems, which might have resulted in substantial numbers of patients without the target symptom being included and who are less likely to benefit from the intervention. In fact, in some studies larger treatment effects have been reported in patients who had greater cognitive impairments at baseline (Gehring et al., 2012; Rapp et al., 2015). Neither of the two exercise studies defined an aim for amelioration of cognitive problems nor included cognitive selection criteria. Experiencing cognitive concerns to some extent might be crucial in the motivation of patients to adhere to (additional) medication regimes or to participate in interventions for which active participation is required. Of the studies that used eligibility criteria with respect to cognitive problems (either concerns or deficits), the vast majority selected these patients on the basis of low performance on neuropsychological test(s) rather than self‐reported cognitive concerns on questionnaires. It should be noted that for studies evaluating preventative effects, it is not necessary to select patients with cognitive deficits or concerns, as prevention may of course be of interest to both patients with and without cognitive problems. It is important for research in this area to identify more robust predictive risk factors associated with the development of these adverse outcomes so that study enrolment of vulnerable patients can be enriched and power can be achieved in the prevention setting.
With respect to outcome assessment, there has been a lack of uniform tests and measures across studies. Furthermore, neuropsychological tests and self‐report questionnaires are not always able to capture the effects of an intervention, especially after cognitive rehabilitation. For instance, the application of taught strategies following cognitive strategy training might not always be measurable with objective neuropsychological tests. Here, self‐report of daily cognitive function or strategy use may be more suitable, although this, as discussed above, may not relate to objectively tested cognitive performance and is often subject to fluctuations in mood or fatigue. Neuroimaging techniques, in particular fMRI, might assist in understanding the neural processes underlying potential behavioural effects of and differences between intervention approaches. Only two studies used this technique and did so with respect to a cognitive strategy training in patients with a brain tumour and demonstrated compensatory activation in pre‐frontal areas after 30‐min semantic strategy training (Miotto et al., 2014,2013). However, the significance of such compensatory brain activity for patients’ daily life functioning needs to be investigated.
Furthermore, the majority of the studies reviewed included heterogeneous samples of primary and/or secondary brain tumours. Findings from these studies might lack generalisability, as differences in types and grades of brain tumours and (histories of) treatments thereof can result in different patterns and severity of cognitive problems, but can also impact the brain's reorganisational processes in different ways. Differences in response to interventions for cognitive deficits, and maintenance of potential intervention effects, might therefore be expected between different brain tumour samples.
4.3. Implications for practice and research
With respect to the use of cognitive interventions in clinical practice, it is important to note that despite high levels of patients’ care needs, the actual use of supportive services (such as cognitive rehabilitation) in patients with brain tumours is relatively low, which might be the result of several patient factors, such as the inability to recognise symptoms or understanding the treatability thereof, altering expectations or minimising losses experienced following diagnosis, and the preference for self‐management (Langbecker, Ekberg, & Yates, 2017). Especially in patients with brain tumours, cognitive impairments including lack of self‐awareness might further limit their ability to recognise their needs. On the other hand, clinicians often have a limited knowledge of the benefits of such services, especially for patients with poor prognosis.
More thorough research is still needed with respect to the pharmacological and cognitive rehabilitation approaches in patients with brain tumours. While the cognitive benefits of exercise training have been described in other populations (Gomez‐Pinilla & Hillman, 2013; Riggs et al., 2017; Sofi et al., 2011; van Uffelen, Chin, Hopman‐Rock, & van Mechelen, 2008), future studies might further investigate the cognitive effects in patients with brain cancer, as an exercise approach among patients with various types of brain tumours has already be shown to be feasible (Baima, Omer, Varlotto, & Yunus, 2017; Capozzi, Boldt, Easaw, Bultz, & Culos‐Reed, 2016; Cormie, Nowak, Chambers, Galvao, & Newton, 2015; Gehring et al., 2018; Hansen, Sogaard, Minet, & Jarden, 2018).
Based on the aforementioned methodological shortcomings, some common themes should be considered in future research in this area. First, inclusion of a non‐intervention or placebo control group allows for a more reliable interpretation of treatment effects. In addition, larger and/or more homogeneous samples are needed for proper statistical testing and may enable subgroup analyses and/or increase generalisability. At the same time, studies should anticipate a long recruitment period and/or collaborate in multicenter studies. Dependent on the timing of the intervention and the intervention goal (amelioration or prevention respectively), patients either must, or do not have to, be screened for the presence of cognitive problems, and longer‐term follow‐ups may be very informative. Outcome measures at the level of objective testing and/or self‐report of cognitive function and strategy utilisation and/or fMRI should be selected carefully, dependent on, as noted above, the aim and nature of the intervention. If neuropsychological testing is relevant, the core set of sensitive tests, as recommended by the International Cognition and Cancer Task Force, may be suitable (Wefel, Vardy, Ahles, & Schagen, 2011), and practice effects should be taken into account. In addition to mean group comparisons, analyses of individual responses and predictors thereof may further help in our understanding of the type of patients who benefit most (and least) and in adapting interventions to yield optimal benefit for larger proportions of patients. As the ultimate goal is to implement effective interventions in clinical and/or daily practice, designing and investigating interventions for such purpose, as well as disseminating positive findings from intervention studies, may help to better embed interventions in the clinical care of these patients.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENT
None.
APPENDIX 1.
Search strategy
(neuropsycholog* [ti] OR cognit* [tiab] OR neurocognit* [ti] OR attention* [ti] OR memory [tiab] OR “brain injury” [ti] OR neurobehavior* [ti] OR neurobehaviour* [ti] OR “problem solving” [ti]) AND (remediation [ti] OR intervention* [ti] OR training [ti] OR retraining [ti] OR telerehabilitation [ti] OR rehabilitation [ti] OR efficacy [ti] OR improv* [ti] OR effect* [ti] OR protect* [ti] OR neuroprotect* [ti] OR prevent* [ti] OR alleviat* [ti] OR ameliorat* [ti] OR restor* [ti] OR exercise [ti] OR “physical activity” [ti] OR “physical training” [ti] OR TMS [ti] OR stimulation [ti] OR mindfulness [ti] OR meditat* [ti] OR relax* [ti] OR yoga [ti] OR neurofeedback [ti] OR biofeedback [ti] OR methylphenidate [ti] OR modafinil [ti] OR armodafinil [ti] OR donepezil [ti] OR ginkgo biloba [ti] OR hyperbaric oxygen [ti] OR erythropoietin [ti] OR epoetin alpha [ti] OR darbepoetin [ti] OR alpha‐tocopherol [ti] OR vitamin E [ti] OR naltrexone [ti] OR indomethacin [ti] OR memantine [ti] OR paroxetine [ti] OR selective serotonin reuptake inhibitor [tiab] OR SSRI [tiab] OR stimulant* [tiab] OR psychostimulant* [tiab] OR NSAID* [tiab] OR anti‐inflammatory [tiab]) AND (glioma* [ti] OR glioblastoma* [ti] OR astrocytoma* [ti] OR oligodendroglioma* [ti] OR meningioma* [ti] OR ependymoma* [ti] OR ((brain [ti] OR cerebral [ti] OR cranial [ti] OR “central nervous system” [ti] OR glial [ti] OR nonglial [ti]) AND (tumor [ti] OR tumour [ti] OR tumors [ti] OR tumours [ti] OR neoplasm* [ti] OR metastasis [ti] OR metastases [ti] OR cancer [ti] OR radiation [ti] OR irradiation [ti] OR radiotherap* [ti]))) AND patients [tiab] NOT (childhood [tiab] OR children [tiab] OR pediatric [tiab] OR paediatric [tiab])
van Lonkhuizen PJC, Klaver KM, Wefel JS, Sitskoorn MM, Schagen SB, Gehring K. Interventions for cognitive problems in adults with brain cancer: A narrative review. Eur J Cancer Care. 2019;28:e13088. 10.1111/ecc.13088
Funding information
KG is funded by ZonMw, a Dutch national organisation for Health Research and Development (project numbers 842003006, 842003007, 842003008 and 842003009). JSW is consultant to AbbVie, AngioChem, Bayer, Juno, Magnolia Tejas, Novocure, Vanquish Oncology and member of advisory boards of AbbVie, Bayer, Blueprint Medicines and Magnolia Neurosciences.
REFERENCES
- Ali, F. S. , Hussain, M. R. , Gutierrez, C. , Demireva, P. , Ballester, L. Y. , Zhu, J. J. , … Esquenazi, Y. (2018). Cognitive disability in adult patients with brain tumors. Cancer Treatment Reviews, 65, 33–40. 10.1016/j.ctrv.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Anderson, S. W. , Damasio, H. , & Tranel, D. (1990). Neuropsychological impairments associated with lesions caused by tumor or stroke. Archives of Neurology, 47(4), 397–405. [DOI] [PubMed] [Google Scholar]
- Arvold, N. D. , Lee, E. Q. , Mehta, M. P. , Margolin, K. , Alexander, B. M. , Lin, N. U. , … Wen, P. Y. (2016). Updates in the management of brain metastases. Neuro‐Oncology, 18(8), 1043–1065. 10.1093/neuonc/now127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia, A. , Rapp, S. R. , Case, L. D. , D'Agostino, R. , Lesser, G. , Naughton, M. , … Shaw, E. G. (2012). Phase II study of Ginkgo biloba in irradiated brain tumor patients: Effect on cognitive function, quality of life, and mood. Journal of Neuro‐Oncology, 109(2), 357–363. 10.1007/s11060-012-0901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima, J. , Omer, Z. B. , Varlotto, J. , & Yunus, S. (2017). Compliance and safety of a novel home exercise program for patients with high‐grade brain tumors, a prospective observational study. Supportive Care in Cancer, 25(9), 2809–2814. 10.1007/s00520-017-3695-7 [DOI] [PubMed] [Google Scholar]
- Bergo, E. , Lombardi, G. , Pambuku, A. , Della Puppa, A. , Bellu, L. , D'Avella, D. , & Zagonel, V. (2016). Cognitive rehabilitation in patients with gliomas and other brain tumors: State of the art. Biomed Research International, 2016, 3041824. 10.1155/2016/3041824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele, F. W. , Douw, L. , de Groot, M. , van Thuijl, H. F. , Cleijne, W. , Heimans, J. J. , … Klein, M. (2013). The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: A multicenter randomized controlled trial. Neuro‐Oncology, 15(10), 1420–1428. 10.1093/neuonc/not102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, F. , Ferlay, J. , Soerjomataram, I. , Siegel, R. L. , Torre, L. A. , & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Brown, P. D. , Ahluwalia, M. S. , Khan, O. H. , Asher, A. L. , Wefel, J. S. , & Gondi, V. (2018). Whole‐brain radiotherapy for brain metastases: Evolution or revolution? Journal of Clinical Oncology, 36(5), 483–491. 10.1200/JCO.2017.75.9589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P. D. , Pugh, S. , Laack, N. N. , Wefel, J. S. , Khuntia, D. , Meyers, C. , … Radiation Therapy Oncology Group (2013). Memantine for the prevention of cognitive dysfunction in patients receiving whole‐brain radiotherapy: A randomized, double‐blind, placebo‐controlled trial. Neuro‐Oncology, 15(10), 1429–1437. 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, J. M. Jr , Case, L. D. , Atkins, J. , Frizzell, B. , Sanders, G. , Griffin, P. , … Shaw, E. G. (2007). A phase III, double‐blind, placebo‐controlled prospective randomized clinical trial of d‐threo‐methylphenidate HCl in brain tumor patients receiving radiation therapy. International Journal of Radiation Oncology, 69(5), 1496–1501. 10.1016/j.ijrobp.2007.05.076 [DOI] [PubMed] [Google Scholar]
- Capozzi, L. C. , Boldt, K. R. , Easaw, J. , Bultz, B. , & Culos‐Reed, S. N. (2016). Evaluating a 12‐week exercise program for brain cancer patients. Psycho‐Oncology, 25(3), 354–358. 10.1002/pon.3842 [DOI] [PubMed] [Google Scholar]
- Chandana, S. R. , Movva, S. , Arora, M. , & Singh, T. (2008). Primary brain tumors in adults. American Family Physician, 77(10), 1423–1430. [PubMed] [Google Scholar]
- Claus, E. B. , & Black, P. M. (2006). Survival rates and patterns of care for patients diagnosed with supratentorial low‐grade gliomas: Data from the SEER program, 1973–2001. Cancer, 106(6), 1358–1363. 10.1002/cncr.21733 [DOI] [PubMed] [Google Scholar]
- Colledge, F. , Brand, S. , Puhse, U. , Holsboer‐Trachsler, E. , Zimmerer, S. , Schleith, R. , & Gerber, M. (2018). A twelve‐week moderate exercise programme improved symptoms of depression, insomnia, and verbal learning in post‐aneurysmal subarachnoid haemorrhage patients: A comparison with meningioma patients and healthy controls. Neuropsychobiology, 76(2), 59–71. 10.1159/000486903 [DOI] [PubMed] [Google Scholar]
- Cormie, P. , Nowak, A. K. , Chambers, S. K. , Galvao, D. A. , & Newton, R. U. (2015). The potential role of exercise in neuro‐oncology. Frontiers in Oncology, 5, 85. 10.3389/fonc.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa, D. D. , Kryza‐Lacombe, M. , Baser, R. E. , Beal, K. , & DeAngelis, L. M. (2016). Cognitive effects of donepezil therapy in patients with brain tumors: A pilot study. Journal of Neuro‐Oncology, 127(2), 313–319. 10.1007/s11060-015-2035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, J. , Gillespie, D. C. , Rooney, A. G. , Bulbeck, H. J. , Zienius, K. , Boele, F. , & Grant, R. (2016). Neurocognitive deficits and neurocognitive rehabilitation in adult brain tumors. Current Treatment Options in Neurology, 18(5), 22. 10.1007/s11940-016-0406-5 [DOI] [PubMed] [Google Scholar]
- Day, J. , Zienius, K. , Gehring, K. , Grosshans, D. , Taphoorn, M. , Grant, R. , … Brown, P. D. (2014). Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation. The Cochrane Database of Systematic Reviews, (12), CD011335. 10.1002/14651858.CD011335.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis, L. M. (2001). Brain tumors. New England Journal of Medicine, 344(2), 114–123. 10.1056/NEJM200101113440207 [DOI] [PubMed] [Google Scholar]
- Dolecek, T. A. , Dressler, E. V. , Thakkar, J. P. , Liu, M. , Al‐Qaisi, A. , & Villano, J. L. (2015). Epidemiology of meningiomas post‐Public Law 107–206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer, 121(14), 2400–2410. 10.1002/cncr.29379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein, K. , Richard, N. M. , & Bernstein, L. J. (2017). Neurocognitive impact of cranial radiation in adults with cancer: An update of recent findings. Current Opinion in Supportive and Palliative Care, 11(1), 32–37. 10.1097/SPC.0000000000000255 [DOI] [PubMed] [Google Scholar]
- Ellis, T. L. , Stieber, V. W. , & Austin, R. C. (2003). Oligodendroglioma. Current Treatment Options in Oncology, 4(6), 479–490. [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Aaronson, N. K. , Gundy, C. M. , Taphoorn, M. J. , & Sitskoorn, M. M. (2011). Predictors of neuropsychological improvement following cognitive rehabilitation in patients with gliomas. Journal of the International Neuropsychological Society, 17(2), 256–266. 10.1017/S1355617710001530 [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Aaronson, N. , Taphoorn, M. , & Sitskoorn, M. (2011). A description of a cognitive rehabilitation programme evaluated in brain tumour patients with mild to moderate cognitive deficits. Clinical Rehabilitation, 25(8), 675–692. 10.1177/0269215510395791 [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Kloek, C. J. , Aaronson, N. K. , Janssen, K. W. , Jones, L. W. , Sitskoorn, M. M. , & Stuiver, M. M. (2018). Feasibility of a home‐based exercise intervention with remote guidance for patients with stable grade II and III gliomas: A pilot randomized controlled trial. Clinical Rehabilitation, 32(3), 352–366. 10.1177/0269215517728326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, K. , Patwardhan, S. Y. , Collins, R. , Groves, M. D. , Etzel, C. J. , Meyers, C. A. , & Wefel, J. S. (2012). A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. Journal of Neuro‐Oncology, 107(1), 165–174. 10.1007/s11060-011-0723-1 [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Sitskoorn, M. M. , Gundy, C. M. , Sikkes, S. A. , Klein, M. , Postma, T. J. , … Aaronson, N. K. (2009). Cognitive rehabilitation in patients with gliomas: A randomized, controlled trial. Journal of Clinical Oncology, 27(22), 3712–3722. 10.1200/JCO.2008.20.5765 [DOI] [PubMed] [Google Scholar]
- Gehring, K. , Taphoorn, M. J. , Sitskoorn, M. M. , & Aaronson, N. K. (2015). Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neuro‐Oncology Practice, 2(1), 20–31. 10.1093/nop/npu035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, M. R. , Dignam, J. J. , Armstrong, T. S. , Wefel, J. S. , Blumenthal, D. T. , Vogelbaum, M. A. , … Mehta, M. P. (2014). A randomized trial of bevacizumab for newly diagnosed glioblastoma. New England Journal of Medicine, 370(8), 699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pinilla, F. , & Hillman, C. (2013). The influence of exercise on cognitive abilities. Comprehensive Physiology, 3(1), 403–428. 10.1002/cphy.c110063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, K. E. , Isaac, C. L. , & Harris, P. (2009). Memory complaints in epilepsy: An accurate reflection of memory impairment or an indicator of poor adjustment? A review of the literature. Clinical Psychology Review, 29(4), 354–367. 10.1016/j.cpr.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Han, E. Y. , Chun, M. H. , Kim, B. R. , & Kim, H. J. (2015). Functional improvement after 4‐week rehabilitation therapy and effects of attention deficit in brain tumor patients: Comparison with subacute stroke patients. Annals of Rehabilitation Medicine, 39(4), 560–569. 10.5535/arm.2015.39.4.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, A. , Sogaard, K. , Minet, L. R. , & Jarden, J. O. (2018). A 12‐week interdisciplinary rehabilitation trial in patients with gliomas – A feasibility study. Disability and Rehabilitation, 40(12), 1379–1385. 10.1080/09638288.2017.1295472 [DOI] [PubMed] [Google Scholar]
- Hassler, M. R. , Elandt, K. , Preusser, M. , Lehrner, J. , Binder, P. , Dieckmann, K. , … Marosi, C. (2010). Neurocognitive training in patients with high‐grade glioma: A pilot study. Journal of Neuro‐Oncology, 97(1), 109–115. 10.1007/s11060-009-0006-2 [DOI] [PubMed] [Google Scholar]
- Kinsinger, S. W. , Lattie, E. , & Mohr, D. C. (2010). Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology, 24(5), 573–580. 10.1037/a0019222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbecker, D. , Ekberg, S. , & Yates, P. (2017). Don't need help, don't want help, can't get help: How patients with brain tumors account for not using rehabilitation, psychosocial and community services. Patient Education and Counseling, 100(9), 1744–1750. 10.1016/j.pec.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Lidstone, V. , Butters, E. , Seed, P. T. , Sinnott, C. , Beynon, T. , & Richards, M. (2003). Symptoms and concerns amongst cancer outpatients: Identifying the need for specialist palliative care. Palliative Medicine, 17(7), 588–595. [DOI] [PubMed] [Google Scholar]
- Linsler, S. , Keller, C. , Urbschat, S. , Ketter, R. , & Oertel, J. (2016). Prognosis of meningiomas in the early 1970s and today. Clinical Neurology and Neurosurgery, 149, 98–103. 10.1016/j.clineuro.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Locke, D. E. , Cerhan, J. H. , Wu, W. , Malec, J. F. , Clark, M. M. , Rummans, T. A. , & Brown, P. D. (2008). Cognitive rehabilitation and problem‐solving to improve quality of life of patients with primary brain tumors: A pilot study. Journal of Supportive Oncology, 6(8), 383–391. [PubMed] [Google Scholar]
- Maschio, M. , Dinapoli, L. , Fabi, A. , Giannarelli, D. , & Cantelmi, T. (2015). Cognitive rehabilitation training in patients with brain tumor‐related epilepsy and cognitive deficits: A pilot study. Journal of Neuro‐Oncology, 125(2), 419–426. 10.1007/s11060-015-1933-8 [DOI] [PubMed] [Google Scholar]
- McDowell, L. J. , Ringash, J. , Xu, W. , Chan, B. , Lu, L. , Waldron, J. , … Bernstein, L. J. (2019). A cross sectional study in cognitive and neurobehavioral impairment in long‐term nasopharyngeal cancer survivors treated with intensity‐modulated radiotherapy. Radiotherapy and Oncology, 131, 179–185. 10.1016/j.radonc.2018.09.012 [DOI] [PubMed] [Google Scholar]
- McKinney, P. A. (2004). Brain tumours: Incidence, survival, and aetiology. Journal of Neurology, Neurosurgery, and Psychiatry, 75 (Suppl 2), ii12–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskal, I. , Gehring, K. , Rutten, G. J. , & Sitskoorn, M. M. (2016). Cognitive functioning in meningioma patients: A systematic review. Journal of Neuro‐Oncology, 128(2), 195–205. 10.1007/s11060-016-2115-z10.1007/s11060-016-2115-z [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, C. A. , Weitzner, M. A. , Valentine, A. D. , & Levin, V. A. (1998). Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. Journal of Clinical Oncology, 16(7), 2522–2527. 10.1200/jco.1998.16.7.2522 [DOI] [PubMed] [Google Scholar]
- Miotto, E. C. , Balardin, J. B. , Vieira, G. , Sato, J. R. , Martin Mda, G. , Scaff, M. , … Junior, E. A. (2014). Right inferior frontal gyrus activation is associated with memory improvement in patients with left frontal low‐grade glioma resection. PLoS ONE, 9(8), e105987. 10.1371/journal.pone.0105987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto, E. C. , Savage, C. R. , Evans, J. J. , Wilson, B. A. , Martin, M. G. , Balardin, J. B. , … Amaro Junior, E. (2013). Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clinical Neurology and Neurosurgery, 115(3), 309–316. 10.1016/j.clineuro.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukand, J. A. , Blackinton, D. D. , Crincoli, M. G. , Lee, J. J. , & Santos, B. B. (2001). Incidence of neurologic deficits and rehabilitation of patients with brain tumors. American Journal of Physical Medicine & Rehabilitation, 80(5), 346–350. 10.1097/00002060-200105000-00005 [DOI] [PubMed] [Google Scholar]
- Ostrom, Q. T. , Gittleman, H. , Truitt, G. , Boscia, A. , Kruchko, C. , & Barnholtz‐Sloan, J. S. (2018). CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro‐Oncology, 20(Suppl_4), iv1‐iv86. 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, B. R. , Shaw, E. G. , Lu, L. , Bryant, D. , Grisell, D. , Lesser, G. J. , … Chan, M. D. (2015). Phase II double‐blind placebo‐controlled randomized study of armodafinil for brain radiation‐induced fatigue. Neuro‐Oncology, 17(10), 1393–1401. 10.1093/neuonc/nov084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchell, R. A. (2003). The management of brain metastases. Cancer Treatment Reviews, 29(6), 533–540. [DOI] [PubMed] [Google Scholar]
- Pranckeviciene, A. , Deltuva, V. P. , Tamasauskas, A. , & Bunevicius, A. (2017). Association between psychological distress, subjective cognitive complaints and objective neuropsychological functioning in brain tumor patients. Clinical Neurology and Neurosurgery, 163, 18–23. 10.1016/j.clineuro.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Rapp, S. R. , Case, L. D. , Peiffer, A. , Naughton, M. M. , Chan, M. D. , Stieber, V. W. , … Shaw, E. G. (2015). Donepezil for irradiated brain tumor survivors: A phase III randomized placebo‐controlled clinical trial. Journal of Clinical Oncology, 33(15), 1653–1659. 10.1200/JCO.2014.58.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, N. M. , Bernstein, L. J. , Mason, W. P. , Laperriere, N. , Maurice, C. , Millar, B.‐A. , … Edelstein, K. (2019). Cognitive rehabilitation for executive dysfunction in brain tumor patients: A pilot randomized controlled trial. Journal of Neuro‐Oncology, 142, 565–575. 10.1007/s11060-019-03130-1 [DOI] [PubMed] [Google Scholar]
- Riggs, L. , Piscione, J. , Laughlin, S. , Cunningham, T. , Timmons, B. W. , Courneya, K. S. , … Mabbott, D. J. (2017). Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro‐Oncology, 19(3), 440–450. 10.1093/neuonc/now177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, E. G. , Rosdhal, R. , D'Agostino, R. B. Jr , Lovato, J. , Naughton, M. J. , Robbins, M. E. , & Rapp, S. R. (2006). Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology, 24(9), 1415–1420. 10.1200/JCO.2005.03.3001 [DOI] [PubMed] [Google Scholar]
- Sofi, F. , Valecchi, D. , Bacci, D. , Abbate, R. , Gensini, G. F. , Casini, A. , & Macchi, C. (2011). Physical activity and risk of cognitive decline: A meta‐analysis of prospective studies. Journal of Internal Medicine, 269(1), 107–117. 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Sperduto, P. W. , Kased, N. , Roberge, D. , Xu, Z. , Shanley, R. , Luo, X. , … Mehta, M. (2012). Summary report on the graded prognostic assessment: An accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. Journal of Clinical Oncology, 30(4), 419–425. 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, S. M. ( 2013). Stahl's essential psychopharmacology: Neuroscientific basis and practical applications( 4th ed.). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Stupp, R. , Taillibert, S. , Kanner, A. , Read, W. , Steinberg, D. , Lhermitte, B. , … Ram, Z. (2017). Effect of tumor‐treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. Journal of the American Medical Association, 318(23), 2306–2316. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taphoorn, M. J. , & Klein, M. (2004). Cognitive deficits in adult patients with brain tumours. The Lancet Neurology, 3(3), 159–168. 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- Tucha, O. , Smely, C. , Preier, M. , & Lange, K. W. (2000). Cognitive deficits before treatment among patients with brain tumors. Neurosurgery, 47(2), 324–333. 10.1097/00006123-200008000-00011 [DOI] [PubMed] [Google Scholar]
- Van der Linden, S. D. , Sitskoorn, M. M. , Rutten, G. M. , & Gehring, K. (2018). Feasibility of the evidence‐based cognitive telerehabilitation program Remind for patients with primary brain tumors. Journal of Neuro‐Oncology, 137, 523–532. 10.1007/s11060-017-2738-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen, J. G. , Chin, A. P. M. J. , Hopman‐Rock, M. , & van Mechelen, W. (2008). The effects of exercise on cognition in older adults with and without cognitive decline: A systematic review. Clinical Journal of Sport Medicine, 18(6), 486–500. 10.1097/JSM.0b013e3181845f0b [DOI] [PubMed] [Google Scholar]
- Wefel, J. S. , Vardy, J. , Ahles, T. , & Schagen, S. B. (2011). International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology, 12(7), 703–708. 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- Yang, S. , Chun, M. H. , & Son, Y. R. (2014). Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Annals of Rehabilitation Medicine, 38(6), 726–733. 10.5535/arm.2014.38.6.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchella, C. , Capone, A. , Codella, V. , De Nunzio, A. M. , Vecchione, C. , Sandrini, G. , … Bartolo, M. (2013). Cognitive rehabilitation for early post‐surgery inpatients affected by primary brain tumor: A randomized, controlled trial. Journal of Neuro‐Oncology, 114(1), 93–100. 10.1007/s11060-013-1153-z [DOI] [PubMed] [Google Scholar]


