Abstract
Objective
This study aimed to assess the genotoxicity and cytotoxicity of Sealer Plus BC (SBC), AH Plus (AHP) and MTA Fillapex (MTF).
Methods
Human periodontal ligament dental stem cells (hPDLSCs) from third molars were isolated and cultured in a clonogenic medium. Cells were maintained in an incubator, and cell growth was monitored daily. hPDLSCs were characterised under flow cytometry and stem cell surface markers. The tested groups were a control group, SBC, AHP and MTF. Each sealer was prepared according to the manufacturer's instructions and placed in a clonogenic medium to produce a conditioned media. Conditioned media were then diluted to 10% to be placed in contact with culture cells in cell viability assay afterwards. The cells were harvested and plated into 96 wells culture plates. Genotoxicity was assessed by evaluation of micronucleus formation and cytotoxicity by MTT-based assay. All experiments were performed in triplicate. Data normality was verified by the Kolmogorov-Smirnov test. Statistical analysis for genotoxicity was performed with Kruskal-Wallis and Dunn's tests and two-way ANOVA for cytotoxicity, both with a significance level of 5%.
Results
Cells expressed typical levels of mesenchymal stem cell surface markers. No differences in the number of micronuclei were observed among all groups (P>0.05). In all periods analysed (24, 48, and 72 h), the sealers presented statistically different results for cell viability (P<0.05), with SBC presenting the lowest cytotoxicity, followed by the control group, MTF, and AHP.
Conclusion
All sealers presented low genotoxicity, and Sealer Plus BC presented the lowest cytotoxicity.
Keywords: Biocompatibility, cytotoxicity, endodontic sealers, genotoxicity
HIGHLIGHTS
Cytotoxicity and genotoxicity tests are important markers for the clinical use of materials;
Sealer Plus BC had the lowest cytotoxicity;
All tested sealers had low genotoxicity.
INTRODUCTION
Calcium silicate-based sealers have been presenting promising results regarding physicochemical (1, 2) and antibacterial (3) properties. Moreover, its main characteristics are related to its biocompatibility (2, 4, 5) and bioactivity, which is the capacity to chemically bond to the dentinal walls, forming an apatite-like structure that could favour the sealing ability within the root dentine (6).
With the introduction of these materials, new formulations have been presented. For example, Sealer Plus BC (MK Life, Porto Alegre, Brazil) is a ready-to-use sealer composed of calcium disilicate, calcium trisilicate, zirconium oxide and calcium hydroxide (7). When compared to AH Plus (Dentsply De Trey Gmbh, Konstanz, Germany), it presents higher solubility, pH and calcium ion release and less flow and radiopacity (7).
Since one of the main purposes of root canal treatments is the healing of the periapical tissues, it is necessary that the materials used inside the root canal favour this repair or at least does not promote any additional harm to these tissues (8). Cytotoxicity and genotoxicity are well-established methods used to verify these implications. Therefore, these parameters should be verified prior to the employment of the materials in clinical practice. While there are many studies on the cytotoxicity of bioceramic sealers, there are only a small number of studies that investigated the genotoxicity of these sealers (4, 9-11).
Specifically, on Sealer Plus BC, there is no information about its genotoxicity and only a few studies about its cytotoxicity (12-14). Thus, this study aimed to assess the genotoxicity and cytotoxicity of the Sealer Plus BC and compare it to the gold standard, AH Plus, and to a salicylate resin-based sealer, MTA Fillapex (Angelus Dental Solutions, Londrina, Brazil), containing mineral trioxide aggregate (MTA) on its composition. Therefore, the null hypotheses of the study are: (i) there are no differences among the cytotoxicity presented by the tested sealers; (ii) there are no differences in the genotoxicity among the tested sealers.
MATERIALS AND METHODS
This research was approved by the University of São Paulo ethics committee (CAAE: 40392214.5.0000.0075).
hPDLSC Cell Line (hPDLSCs) selection and growth
In this study, human periodontal ligament dental stem cells (hPDLSCs) from third molars were isolated for the tests. hPDLSCs between the 3rd and 6th passages were cultured in a clonogenic medium composed of alpha-minimum essential medium (a- MEM; Gibco, Grand Island, NY, US) supplemented with 10% fetal bovine Serum [MSC FBS; Mesenchymal Stem Cell-qualified Fetal Bovine Serum; (Gibco)], 100 µM ascorbic acid (Sigma-Aldrich, St. Louis, Missouri, US), 2 mM de L-glutamine (Gibco), penicillin (100 U/mL; Gibco), and streptomycin (100 mg/mL; Gibco). Cells were maintained in an incubator at 37°C in a humid atmosphere containing 5% CO2 and 95% air humidity. Cell growth was monitored daily under a phase-contrast microscope. The cell culture medium was changed every 2 or 3 days depending on the cell metabolism, and a subculture was made whenever necessary. Finally, the cells were harvested and plated into 96 wells culture plates for the experiments.
Human periodontal ligament dental stem cells (hPDLSCs) characterisation
In the second passage (P2), the cells were analysed by flow cytometry to confirm their stem cell nature. Briefly, aliquots of the cells (1×105 cells) were washed and resuspended in phosphate-buffered saline (PBS) containing saturating concentrations (1:200) of the following panel of primary antibodies, conjugated with allophycocyanin (APC), fluorescein (FITC), or phycoerythrin (PE), against human surface molecules. Cells were classified on a flow cytometer (FACS Calibur, BD Biosciences), and 50,000 events were analysed using FlowJo software version 9.6.2 (Tree Star, Ashland, OR, USA).
Preparation of conditioned culture media
All sealers were used according to the manufacturer's instructions for all tests. The compositions of all evaluated sealers, according to the manufacturer, are shown in Table 1.
TABLE 1.
Chemical composition of the tested sealers
| Endodontic sealer | Chemical composition |
|---|---|
| Sealer Plus BC (MK Life) | Calcium disilicate, calcium trisilicate, zirconium oxide, calcium hydroxide, propylene glycol |
| AH Plus (Dentsply) | Paste A: Bisphenol-A epoxy resin, bisphenol-F epoxy resin, calcium tungstate, zirconium oxide, silica, iron oxide pigments; Paste B: Dibenzyldiamine, aminoadamantane, tricyclodecane-diamine, calcium tungstate, zirconium oxide, silica, silicon oil; |
| MTA Fillapex (Angelus) | Base paste: Salicylate resin, natural resin, calcium tungstate, nanoparticulated silica, pigments; Catalyst paste: Diluting resin, mineral trioxide aggregate, nanoparticulated silica, pigments |
The conditioned media (e.g. culture medium containing substances leached from the sealers) were obtained as recommended by the American Society for Testing Material (ASTM, 1992). Each sealer was placed on the bottom of 50 mL Falcon tubes filled with a clonogenic medium (0.2 g/mL). Conditioning was carried out for 24 h at 37°C. After this period, each conditioned medium was collected, centrifuged at 300x g for 30 s to remove fragments of the sealers and then filtered through 0.2 µm syringe filters to sterilise the samples. These conditioned media were then diluted to a 1:10 (10%) extract, according to the described in a previous study (15), in a fresh clonogenic culture medium to be further placed in contact with cultured cells.
Experimental groups
Four groups were created as follows:
Control: hPDLSCs grown in a fresh clonogenic medium;
Sealer Plus BC (SBC): hPDLSCs grown in medium conditioned with Sealer Plus BC;
AH Plus (AHP): hPDLSCs grown in medium conditioned with AH Plus;
MTA Fillapex (MTF): hPDLSCs grown in medium conditioned with MTA Fillapex.
Genotoxicity
A genotoxicity test was performed according to the methodology previously described (4). Cells were seeded (3×103 cells well-1) on a glass coverslip placed on the bottom of 35-mm cell culture plates. These cells were incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2. Then, the culture medium was replaced by the diluted conditioned medium and incubated for another 24hs. After this period, the conditioned medium was discarded, and the cells were washed twice with PBS. Next, cells were fixed with a 1.5% formaldehyde solution at room temperature for 20 minutes. The formaldehyde solution was discarded and replaced by a cold 100% methanol solution (-20°C), and the cells were left at room temperature for 20 min. The methanol solution was discarded, and the cells were washed three times with PBS. Next, Hoechst solution (Sigma, St Louis, MO, USA) was placed on the top of the cells and incubated for 15min at room temperature. Cells were then rinsed five times with PBS. The glass coverslips with the cells were visualized and photographed in a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The percentage of micronuclei was determined by the number of cells with micronucleus in 100 cells observed in five predetermined microscopic fields (at the four corners and in the centre of the coverslip) with a magnification of 400x. All experimental groups were tested in triplicate.
Cytotoxicity analysis
Cytotoxicity evaluation was performed according to ISO 10993-5 specifications (International Organization for Standardization 2009).
Cell viability assay
For the experiments, cells were plated (1×104 cells/well) in 96-well culture plates and maintained in a humified chamber at 37°C. The culture medium was replaced by the either conditioned medium (experimental groups) or the fresh medium (control group) 24 hours later. The cultures were incubated in a humified chamber at 37°C for 24, 48 and 72 h. After 48 h, half of the medium in each well was exchanged for fresh medium to simulate the solubility of endodontic sealers into periapical tissues. The cells were submitted to the MTT reduction assay to evaluate cytotoxicity, and the concentration was determined by absorbance at a 562 nm filter. In addition, three isolated experiments were performed.
Statistical analysis
Normality analysis of the data was evaluated with the Kolmogorov-Smirnov test. Next, genotoxicity data were statistically analysed by the Kruskal-Wallis test, followed by Dunn's test for multiple comparisons. Finally, cytotoxicity data were statistically analysed by Two-Way Analysis of Variance (ANOVA) followed by Bonferroni post-hoc test using BioEstat® 5.0 software and GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). The results were expressed as the mean and standard deviation of the mean. The significance level was established at 5% (P<0.05).
RESULTS
Cell characterization by the immunoprofile of the surface molecules
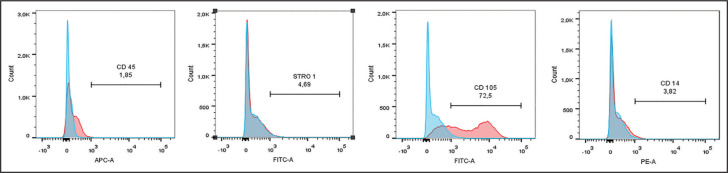
The hLPSCs expressed the typical levels of MSCs associated surface markers (Fig. 1). The cultures expressed positivity for CD105 and STRO-1, whereas the haematopoietic cell markers (CD45, CD14) were minimally expressed. The percentage of positive cells for the CD105 was 72.5%, and STRO-1 was 4.69% for the hLPSCs population. The percentage of cells for CD14 was 3.82%, and CD45 was 1.85% for the hLPSCs population.
Figure 1.
Flow cytometry analysis showed that human ligament periodontal stem cells (hPDLSCs) express mesenchymal stem cell surface markers. Positive for CD105, STRO-1 and lower expression of CD14 and CD45
APC-A: Panel of primary antibodies, conjugated with allophycocyanin, FITC: Fluorescein, PE: Phycoerythrin
Genotoxicity test
Figure 2 illustrates the genotoxicity analysis of all experimental groups. No statistical differences in the number of micronuclei were observed among all groups (P>0.05).
Figure 2.
Photographs of optical fluorescence images showing micronuclei formation in all experimental groups. (a) Control group showing no micronucleus formation. (b) Cells after exposure to AHP with one micronucleus at the arrow. (c) Cells after exposure to MTF showing two micronuclei formation at the arrows. (d) Cells after exposure to SBC showing one micronucleus formation at the arrow. (e) Graphical representation for the mean micronuclei formation of the different groups. Bars represent the standard deviation. No statistical differences were found (P>0.05)
Cytotoxicity test
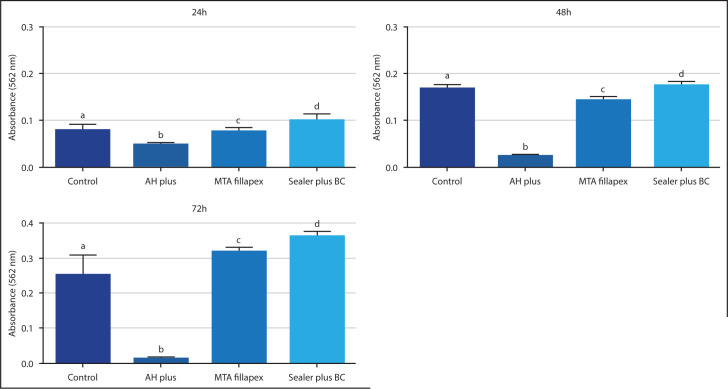
The cytotoxicity results of the control and experimental groups are shown in Figure 3. In 24 h, 48 h, and 72 h of culture, absorbance results from the endodontic sealers were significantly different between them (P<0.05) and also when compared to the control group (P<0.05). The BCS presented the highest absorbance in all periods analyzed, followed by MTF, control groups and AH Plus with the lowest (P<0.05).
Figure 3.
MTT assay results in 24, 48, and 72 h. Different letters indicate intergroup significant absorbance differences
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
DISCUSSION
When in contact with cells of the periapical tissues, soluble chemicals derived from endodontic materials may lead to inflammation or necrosis (16), which could interfere with the periapical healing process (17-19). Therefore, it is possible to infer that in the case of extrusion or higher solubility of the endodontic sealer, a greater contact of its chemical substances with the periapical tissues occurs, making it necessary to verify its biological impact before clinical applications.
The selection of human periodontal ligament dental stem cells (hPDLSCs) for the tests is corroborated due to their direct involvement with the endodontic sealer at the root end (20) and their role in tissue repair, remodelling and regenerative processes (21). Thereby, these cells better simulate the clinical environment in vitro.
In addition, to mimic in vivo conditions where the ligament stem cells are in contact with substances leached or dissolved from root canal filling materials into apical fluids, the sealers were placed indirectly in contact with the stem cell cultures by applying conditioned culture media. This dilution and media exchange are appropriate since, in periapical tissue, the cell numbers are higher than the number of cells in a culture well. At the same time, blood and lymphatic vessels are present in living tissue, diluting the substances.
In this study, SBC, a calcium silicate-based sealer, was tested and compared to a control group, AHP, an epoxy resin-based sealer, and MTF, a salicylate resin-based sealer. As a result, it has been verified that this sealer presents appropriate physicochemical properties such as setting time, pH, calcium ion release, flow and radiopacity, except for its solubility, which is higher than those established by the International Organization for Standardization (ISO) 6876:2012 (7).
Genotoxicity tests are performed to verify the influence of the tested material on the cell's genetic material, which may influence its integrity (4, 22). Mutagenicity can be assessed in vitro using the Ames test, cytogenetics, or micronucleus. The present research evaluated the genotoxicity of the substances leached from the endodontic sealers through the micronuclei formation assay (MNT assay). This test is a reliable method to evaluate the carcinogen (genotoxic) effect of chemicals, being the test recommended by the OECD guideline for testing chemicals (23). The MNT assay is based on the loss of entire chromosomes or their fragments during cell mitosis, which are not reinstated by the nucleus after cell division and therefore are transformed into smaller nuclei or micronuclei (22). These in vitro assays do not consider the complexity of a living organism or the clinical presentation of the apical region. So, it is also mandatory to determine the biocompatibility of a material within an in vivo setting in future studies (24). Regarding genotoxicity, the null hypothesis was accepted.
This study is the first to assess Sealer Plus BC genotoxicity. Therefore, a direct comparison of results is not possible. However, a low genotoxicity was verified for this sealer, which is in accordance with the results presented by calcium silicate-based sealers in general (4, 9-11, 22, 25).
In relation to the results presented by the MTF group, although not presenting a statistical difference, MTF had the highest genotoxicity compared to the other sealers, which must be considered during clinical practice. Previous studies also reported similar results (9, 11). This fact can probably be explained due to its salicylate resin composition, causing salicylate precipitation in the cell cytoplasm, and the fragmentation of the cell genetic material (26).
As for the results of the AHP group, the possible explanation for its low genotoxicity relies on the fact that, when diluted, there is a decrease of the resinous compound present in the sealer composition, which allows the sealer to demonstrate a similar behaviour to the control group, as also previously reported in another study (22) that tested AHP in dilutions higher than 1:8 (22), corroborating with the found data. However, it is important to emphasise that the sealer may be initially presented in higher concentrations when extrusion occurs. Therefore, it can potentially present a greater genotoxic effect in contact with the periapical tissues (11).
Regarding cytotoxicity, the null hypothesis was rejected. Cytotoxicity tests are usually performed to assess biologic compatibility by analyzing the survival of cells after exposure to materials at determined experimental periods. In this study, the MTT assay was used since it has long been regarded as the gold standard of cytotoxicity assays as it is highly sensitive and has been miniaturised for use as a high-throughput screening assay. Also, the MTT assay is recommended to evaluate cellular viability and proliferation of cell cultures because they are applicable for adherent or suspended cell lines, are easy to perform, and are comparably economical (4). The formation of needlelike formazan crystals destroys the cell's integrity and thus leads to cell death. This breakdown in cell metabolism leads to a quick interrupting of the reaction of MTT to formazan, being called an end-point determination. Because the crystals are formed intracellularly, MTT-based assay protocols usually include a cell lysis step and a formazan-dissolving step before a spectroscopic measurement can be performed. Despite its advantages of being rapid and simple, the formation of an insoluble product and the necessity to dissolve it exclude this assay from any real-time assays. Researchers have proposed modifications to improve the performance and sensitivity of this assay, but the problem of dissolving solid formazan crystals still exists (27).
In the present study, SBC presented better results than all the other groups, during all experimental times. Our results are in agreement if previous studies (13, 14) that used less-diluted extracts and compared to AHP (13, 14) and MTF (14). Only one previous study (12) that evaluated SBC cytotoxicity and compared it to the same sealers (AHP and MTF) reported different results, suggesting that SBC is more cytotoxic than AHP in the highest dilution (1:50; 1:100; 1:200). Therefore, it is possible to suggest that such disagreement on the results is due to the methodologies adopted.
SBC's lower cytotoxicity can probably be explained due to its bioceramic components, calcium hydroxide, propylene glycol and resin-free composition, favouring an alkaline pH, greater release of Ca2+ ions and hydroxyapatite formation, which could favour the hPDLSCs biological activity (1, 2, 4).
Regarding the results presented by the AHP group, it has been verified that it presented lower cell viability. This result is probably related to the epoxy resin of its composition, mainly due to the amine component, which is a mutagenic substance (22). Furthermore, previous studies that evaluated this sealer's cytotoxicity on hPDLSCs reported similar results (28-31).
In relation to MTF, although it presents MTA in its composition, this sealer is mainly composed of salicylate resin, which could induce cell apoptosis (12), explaining the greater cytotoxicity when compared to SBC and the control group. However, lower cytotoxicity was observed compared to the AHP group, disagreeing with previous studies (12, 16, 31, 32). A possible explanation for these conflicting results could be related to the radiopacifier presented on the sealer. The first version of this sealer contained bismuth oxide, a strong cell death inducer (33), while more recently, it was replaced by calcium tungstate.
CONCLUSION
Within the limitations of this study, it is possible to conclude that Sealer Plus BC presented the lowest cytotoxicity and all the sealers are equally low genotoxic.
Footnotes
Please cite this article as: Barcelos Só B, Martins MD, Reis Só MV, Weissheimer T, Marques MM, Moreira MS. Genotoxicity and Cytotoxicity Comparison of Calcium Silicate-Based and Resin-Based Sealers on Human Periodontal Ligament Stem Cells. Eur Endod J 2022; 7: 129-34
Disclosures
Conflict of interest:
The authors deny any conflict of interest.
Ethics Committee Approval:
This study was approved by The University of São Paulo Ethics Committee (Date: 03/03/2015, Number: CAAE: 40392214.5.0000.0075).
Peer-review:
Externally peer-reviewed.
Financial Disclosure
This study did not receive any financial support.
Authorship contributions
Concept – B.B.S., M.D.M., M.V.R.S., M.M.M., M.S.M.; Design – B.B.S., M.D.M., M.V.R.S., M.M.M., M.S.M.; Supervision – M.D.M., M.M.M., M.S.M.; Funding - M.D.M., M.M.M., M.S.M.; Materials - M.D.M., M.M.M., M.S.M.; Data collection and/or processing – M.D.M., M.M.M., M.S.M.; Analysis and/or interpretation – B.B.S., M.D.M., M.V.R.S., T.W.; Literature search – B.B.S., M.D.M., M.V.R.S., T.W.; Writing – B.B.S., M.D.M.; Critical Review – M.V.R.S., T.W.
References
- 1.Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38(6):842–5. doi: 10.1016/j.joen.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, et al. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37(5):673–7. doi: 10.1016/j.joen.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35(7):1051–5. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Candeiro GTM, Moura-Netto C, D'Almeida-Couto RS, Azambuja-Júnior N, Marques MM, Cai S, et al. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. 2016;49(9):858–64. doi: 10.1111/iej.12523. [DOI] [PubMed] [Google Scholar]
- 5.Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q. Cytotoxicity evaluation of Gutta Flow and Endo Sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):657–61. doi: 10.1016/j.tripleo.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 6.Prati C, Gandolfi MG. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent Mater. 2015;31(4):351–70. doi: 10.1016/j.dental.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mendes AT, Silva PBD, Só BB, Hashizume LN, Vivan RR, Rosa RAD, et al. Evaluation of physicochemical properties of new calcium silicate-based sealer. Braz Dent J. 2018;29(6):536–40. doi: 10.1590/0103-6440201802088. [DOI] [PubMed] [Google Scholar]
- 8.Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Top. 2005;12(1):25–38. [Google Scholar]
- 9.Eldeniz AU, Shehata M, Högg C, Reichl FX. DNA double-strand breaks caused by new and contemporary endodontic sealers. Int Endod J. 2016;49(12):1141–51. doi: 10.1111/iej.12577. [DOI] [PubMed] [Google Scholar]
- 10.Nair AV, Nayak M, Prasada LK, Shetty V, Kumar CNV, Nair RR. Comparative evaluation of cytotoxicity and genotoxicity of two bioceramic sealers on fibroblast cell line: an in vitro study. J Contemp Dent Pract. 2018;19(6):656–61. [PubMed] [Google Scholar]
- 11.Erdogan H, Yildirim S, Cobankara FK. Cytotoxicity and genotoxicity of salicylate-and calcium silicate-based root canal sealers on primer human periodontal ligament fibroblasts. Aust Endod J. 2021;47(3):645–53. doi: 10.1111/aej.12537. [DOI] [PubMed] [Google Scholar]
- 12.Benetti F, de Azevedo Queiroz ÍO, Oliveira PHC, Conti LC, Azuma MM, Oliveira SHP, et al. Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz Oral Res. 2019;33:e042. doi: 10.1590/1807-3107bor-2019.vol33.0042. [DOI] [PubMed] [Google Scholar]
- 13.Zordan-Bronzel CL, Tanomaru-Filho M, Torres FFE, Chávez-Andrade GM, Rodrigues EM, Guerreiro-Tanomaru JM. Physicochemical properties, cytocompatibility and antibiofilm activity of a new calcium silicate sealer. Braz Dent J. 2021;32(4):8–18. doi: 10.1590/0103-6440202103314. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira GC, Pinheiro LS, Nunes JS, de Almeida Mendes R, Schuster CD, Soares RG, et al. Evaluation of the biological and physicochemical properties of calcium silicate-based and epoxy resin-based root canal sealers. J Biomed Mater Res B Appl Biomater. 2022;110(6):1344–53. doi: 10.1002/jbm.b.35004. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcanti BN, Rode SM, Marques MM. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endod J. 2005;38(8):505–9. doi: 10.1111/j.1365-2591.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino P, Nishiyama CK, Modena KC, Santos CF, Sipert CR. In vitro cytotoxicity of white MTA, MTA Fillapex® and Portland cement on human periodontal ligament fibroblasts. Braz Dent J. 2013;24(2):111–6. doi: 10.1590/0103-6440201302115. [DOI] [PubMed] [Google Scholar]
- 17.Aminoshariae A, Kulild JC. The impact of sealer extrusion on endodontic outcome: A systematic review with meta-analysis. Aust Endod J. 2020;46(1):123–9. doi: 10.1111/aej.12370. [DOI] [PubMed] [Google Scholar]
- 18.Murazabal M, Erausquin J, Devoto FH. A study of periapical overfilling root canal treatment in the molar of the rat. Arch Oral Biol. 1966;11(4):373–83. doi: 10.1016/0003-9969(66)90103-8. [DOI] [PubMed] [Google Scholar]
- 19.Ricucci D, Rôças IN, Alves FR, Loghin S, Siqueira JF., Jr Apically Extruded Sealers: Fate and Influence on Treatment Outcome. J Endod. 2016;42(2):243–9. doi: 10.1016/j.joen.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Mielert J, Kleinheinz J, Dammaschke T. Human oral cells' response to different endodontic restorative materials: an in vitro study. Head Face Med. 2014;10:55. doi: 10.1186/s13005-014-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyko GA, Melcher AH, Brunette DM. Formation of new periodontal ligament by periodontal ligament cells implanted in vivo after culture in vitro A preliminary study of transplanted roots in the dog. J Periodontal Res. 1981;16(1):73–88. doi: 10.1111/j.1600-0765.1981.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 22.Bin CV, Valera MC, Camargo SE, Rabelo SB, Silva GO, Balducci I, et al. Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. J Endod. 2012;38(4):495–500. doi: 10.1016/j.joen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Martinho FC, Camargo SEA, Fernandes AMM, Campos MS, Prado RF, Camargo CHR, et al. Comparison of cytotoxicity, genotoxicity and immunological inflammatory biomarker activity of several endodontic sealers against immortalized human pulp cells. Int Endod J. 2018;51(1):41–57. doi: 10.1111/iej.12785. [DOI] [PubMed] [Google Scholar]
- 24.Dos Santos Costa FM, Fernandes MH, Batistuzzo de Medeiros SR. Genotoxicity of root canal sealers: a literature review. Clin Oral Investig. 2020;24(10):3347–62. doi: 10.1007/s00784-020-03478-z. [DOI] [PubMed] [Google Scholar]
- 25.Jafari F, Jafari S, Etesamnia P. Genotoxicity, bioactivity and clinical properties of calcium silicate based sealers: a literature review. Iran Endod J. 2017;12(4):407–13. doi: 10.22037/iej.v12i4.17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdi JG, Alkarrawi MA, Mahdi AJ, Bowen ID, Humam D. Calcium salicylate-mediated apoptosis in human HT-1080 fibrosarcoma cells. Cell Prolif. 2006;39(4):249–60. doi: 10.1111/j.1365-2184.2006.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Präbst K, Engelhardt H, Ringgeler S, Hübner H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. Methods Mol Biol. 2017;1601:1–17. doi: 10.1007/978-1-4939-6960-9_1. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Lozano FJ, López-García S, García-Bernal D, Tomás-Catalá CJ, Santos JM, Llena C, et al. Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int Endod J. 2020;53(9):1216–28. doi: 10.1111/iej.13327. [DOI] [PubMed] [Google Scholar]
- 29.López-García S, Pecci-Lloret MR, Guerrero-Gironés J, Pecci-Lloret MP, Lozano A, Llena C, et al. Comparative cytocompatibility and mineralization potential of Bio-C Sealer and TotalFill BC Sealer. Materials (Basel) 2019;12(19):3087. doi: 10.3390/ma12193087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JK, Kim S, Lee S, Kim HC, Kim E. In vitro comparison of biocompatibility of calcium silicate-based root canal sealers. Materials (Basel) 2019;12(15):2411. doi: 10.3390/ma12152411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod. 2017;43(5):816–22. doi: 10.1016/j.joen.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013;39(2):274–7. doi: 10.1016/j.joen.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Ahamed M, Akhtar MJ, Khan MAM, Alrokayan SA, Alhadlaq HA. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019;216:823–31. doi: 10.1016/j.chemosphere.2018.10.214. [DOI] [PubMed] [Google Scholar]