Abstract
The stress response in rodents and humans is exquisitely dependent on the environmental context. The interactive element of the environment is typically studied by creating laboratory models of stress-induced plasticity manifested in behavior or the underlying neuroendocrine mediators of the behavior. Here, we discuss three representative sets of studies where the role of the environment in mediating stress sensitivity or stress resilience is considered across varying windows of time. Collectively, these studies testify that environmental variation at an earlier time point modifies the relationship between stressor and stress response at a later stage. The metaplastic effects of the environment on the stress response remain possible across various endpoints, including behavior, neuroendocrine regulation, region-specific neural plasticity, and regulation of receptors. The timescale of such variation spans adulthood, across stages of life history and generational boundaries. Thus, environmental variables are powerful determinants of the observed diversity in stress response. The predominant role of the environment suggests that it is possible to promote stress resilience through purposeful modification of the environment.
Keywords: Amygdala, Behavior, Early-life, Enrichment, Intergenerational, Orexin, Transgenerational
1. Stress, environment and interactions
1.1. Stress and the environment
The concept of stress in biology has a long history (Goldstein and Kopin, 2007). It grew out of two inter-related pre-existing concepts of psychological distress and physiological homeostasis. Thus, the stress response was envisioned as a unitary response to a range of environmental conditions that threatened to move an animal away from its normative physiological state (Selye, 1976). A variety of stressors were conceptualized to result in the same physiological stress response, an idiosyncratic physiological response aimed at restoration of the homeostasis. The environmental variability of stressors was distilled to a qualitatively invariant physiological response. This early concept of stress has undergone two significant modifications. Both of these emphasize a greater role of the environment in the stress response rather than viewing it as an amorphous set of various triggers resulting in the same consequence.
1.2. Variability of stress response according to environmental context
The stress response is not constant; it varies across different types of environmental stressors, with the balance of stress responsiveness regulated by environmentally-sensitive neuromodulator systems, such as orexin/hypocretin (Orx) neuropeptides acting via differential receptor types in pro- and anti-stress neurocircuitries (Yaeger et al., 2020). For example, one could use stressors of the same intensity and frequency and achieve substantially different outcomes if the stress was perceived as controllable versus non-controllable. Animals previously exposed to uncontrollable stress develop learned helplessness (Maier and Seligman, 1976), showing a maladaptive passivity when subsequently tested in an environment where escape from an aversive stimulus is possible. Animals given control over the stressor by allowing a response to terminate the stressor do not show learned helplessness while yoked controls with the same amount of stressor exposure develop passivity (Seligman and Beagley, 1975). Thus, the psychological element of control, by itself, can alter the nature of stress response. Psychological context can create a variable stress response. The similarly poignant nature of environmental context is captured in human studies that look at adverse health effects of long-term stress in the backdrop of socioeconomic gradients. People at lower ends of the socioeconomic gradient often show grater stress-induced damage to the health (Lynch et al., 1996; Steenland et al., 2002; Adler et al., 1993). The connection between socioeconomic status and stress is apparent in the Whitehall study where an inverse relationship is observed between social status and cardiac risk (Marmot et al., 1978). Furthermore, this relationship remains consistent when long-term health outcomes of erstwhile civil servants are studied three decades after retirement in relation to the stratification of socioeconomic status while they were in employment (Marmot et al., 1991). Due to these and similar studies, the concept of stress has slowly been modified to include a more defining role of environmental context in determining the response variability.
1.3. The shaping of the stress response by previous exposure to stressful environment
A critical addition in the vocabulary of the stress response, in this context, is allostasis and allostatic load, again providing a renewed emphasis on the role of the environment in shaping the stress response (McEwen, 2000; Sterling and Eyer, 1988). In contrast to a homeostatic view that emphasizes the defense of a pre-defined physiological set point, the allostasis encompasses plasticity in the set point itself, which depends on the historical environment of the individual animal. Interactions between stress and hippocampus provide an easy exemplar to understand this effect (McEwen, 1999). Adrenal glucocorticoids secreted during stress bind to their receptors within the hippocampus after crossing the blood-brain barrier. Engagement of hippocampal glucocorticoid receptors then provides negative feedback to the hypothalamic components that control the stress endocrine response. This negative feedback loop, in addition to feedback regulation at the level of pituitary and hypothalamus, ensures that the level of circulating glucocorticoids is kept in a homeostatically narrow range. Indeed, there are environmental contexts when a high-stress response is warranted, for example, during the periods of migration and terminal reproduction (Robertson and Wexler, 1957). Similar examples can be found when animals need to adapt to high population density or risk of predation (Dantzer et al., 2013). Several reciprocal feedback loops, operating at multiple levels of the organization, work together to change the requisite set point and exert an allostatic control on the glucocorticoid levels. For example, chronic or traumatic stress causes long-term plasticity within the basolateral amygdala and facilitates future responsivity of the stress endocrine axis to future stressors (Vyas et al., 2002). Thus, allostasis is a metaplastic effort to bring homeostatic machinery in line with the incipient environment. Allostasis describes the idea that the same stressor, for example, the same change in population density, can create two very different stress responses depending on the previous environmental exposure of the individual animals. The historical environment therefore leaves a trace to influence the future stress response.
1.4. Environment dependence of the stress response
These examples demonstrate that the stress is intricately linked to the environmental determinants of the response. The stress as a concept gains meaning only in the context of its interaction with the environmental variability, providing an excellent example of the interaction between a biological system and an environment where the system is embedded. A popular way this interaction has been studied is to create laboratory models of stress-induced plasticity. Typically, mice or rats are exposed to stressors, and then the resultant changes in brain structure, neurochemistry, and behavior are studied. In this review, we will discuss three representative classes of such animal models. Each of these models focuses on distinct modifying roles of the environment in stress sensitivity or stress resilience. In other words, these models show that preceding environmental variability uniquely changes how the subsequent stressors are processed. We will first discuss environment-dependent plasticity in the brain orexin receptors where the modifying environment and the stress response co-occur in adulthood. We will then discuss circumstances where the environmental variability during critical early periods of life causes later changes in the stress response. Lastly, we will discuss instances where pre-conception stress in the parents causes changes in the stress response of the offspring.
2. Neuroendocrine signaling and its relation to gene expression
2.1. Role of neural and endocrine signaling to the promotion of gene expression
Physiological stress is mediated through activation of the hypothalamic-pituitary-adrenal (HPA) axis and involves the discharge of catecholamines, serotonin, and other neurotransmitters (Hanley and Van de Kar, 2003; Pacak et al., 1995; Mora et al., 2012). Glucocorticoids released during a stressful event directly regulate gene expression through the binding of cytosolic receptors that migrate to neuronal (and other cell types) nuclei where activation or suppression of gene transcription takes place (Srinivasan et al., 2013a). Stressors that are chronic (Bergström et al., 2007), occur with high intensity (Török et al., 2019), and/or take place early in development (Provençal and Binder, 2015) alter transcriptional regulation in neurons, producing behaviorally divergent traits.
Chronic and unpredictable stress may lead to psychopathologies, including disruptions in learning (Conrad, 2010), fear (Ressler, 2010), arousal (Sanford et al., 2014), motivation (Sinha and Jastreboff, 2013), and decision-making (Starcke and Brand, 2012) neurocircuits. While the hormonal cascade associated with the HPA axis promotes peripheral and central changes to meet the demands of sympathetic response, several specific brain areas undergo molecular, cellular, and signaling modifications that influence and sometimes dramatically alter, stable neural states. For example, infralimbic (IL) and prelimbic (PrL) subregions of the medial prefrontal cortex project to and affect amygdalar function in an opposing way, with the activity of IL promoting extinction of fear and PrL enhancing fear expression (Sierra-Mercado et al., 2011). During chronic stress, glucocorticoid receptors (GR) are downregulated in parvalbumin (Pv) interneurons of the IL, which leads to reduced excitability of pyramidal projection neurons targeting amygdalar subregions (McKlveen et al., 2016). These examples show that stress-related neurocircuits are plastic, suggesting behavioral adaptations to stressful events require regulatory changes in proteins, like receptors and neuromodulators, that ultimately define phenotype expression.
Products of transcriptional regulation, namely neuropeptides and their corresponding receptors, control stress responses and phenotype formation. Therefore, understanding relationships between these proteins and stress resilience or vulnerability may lead to the classification of psychopathology biomarkers (Walker et al., 2017) and reveal targets for therapeutic intervention (Griebel and Holsboer, 2012). In the following sections, we briefly discuss neuropeptide signaling during stress, with a focus on the orexin/hypocretin system because of the critical role this neuromodulator plays in learning (Sears et al., 2013), arousal (Berridge et al., 2010), motivation (Tsujino and Sakurai, 2013), and decision-making (Karimi et al., 2019; Smith et al., 2014), all of which are essential processes disrupted during stress-induced psychopathologies.
2.2. Interconnectivity of stress signaling circuits
The stress response is created through a complex interdependent circuitry that includes reciprocal connections between limbic structures like the prefrontal cortex (PFC), extended amygdala, hippocampus, and reward elements, including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Hare et al., 2019; Tovote et al., 2015; Kim et al., 2016; Tye, 2012). The connections between these specialized sub-regions, such as the pro-stress pathway from the prelimbic region (PrL) to the anterior basolateral amygdala, often make use of glutamatergic (Glu) pyramidal projection neurons and are regulated by GABAergic, dopaminergic, or orexinergic modulation (Hare et al., 2019; Tovote et al., 2015; Staton et al., 2018; Summers et al., 2020). The GABA dominated local and projection circuitry in the central amygdala, bed nucleus of the stria terminalis (BNST), and NAc are modified by corticotropin-releasing factor (CRF), neuropeptide Y (NPY), neuropeptide S (NPS), and vasopressin (AVP) (Smith et al., 2014; Gilpin, 2012; Albeck et al., 1997). Phenotypes related to stress-induced psychopathology are heavily dependent on the activity and balance of these neuropeptides at specific areas within the stress circuit. These neuromodulators act through specialized receptor subtypes, although there remains some overlap in signal transduction pathways. Together, these neuromodulators produce the phenotypes interpreted as susceptible or resilient to stressful stimuli. For example, CRF and AVP have been demonstrated to be anxiogenic (An and Tai, 2014; Appenrodt et al., 1998; Carpenter et al., 2009a; Takahashi, 2001; Zorrilla and Koob, 2004) and promote susceptibility (Keck et al., 2008; Smith et al., 2016a), while NPY and NPS tend to be anxiolytic (Gilpin, 2012; Ionescu et al., 2012) and promote resilience (Smith et al., 2014; Cohen et al., 2012). However, the molecular signaling pathways for these peptides often share intracellular mechanisms, such that CRF receptors can share activation of phospholipase C (PLC) pathways (Ronan and Summers, 2011) with AVP and NPS (Guerrini et al., 2010), share stimulation of cAMP signaling with NPS and share inhibition of cAMP signaling with NPY. Which means neuropeptides that typically have opposite effects (Gilpin, 2012) sometimes produce identical effects (Tschenett et al., 2003). Contradictory effects are likely dependent on specific cell types (e.g., excitatory or inhibitory) upon which the receptors are expressed, as is the case with opposite actions of two orexin receptors (Yaeger et al., 2020). The final integrated signaling also plays a vital role in activating transcription factors to modify gene expression and promote methylation and acetylation, which alter epigenetic regulation via modification of histone tails or CpG sites and thereby affect the output of behavioral responses characteristic of specific phenotypes.
Similar to the previous examples, distributed CRF outputs also interact directly, indirectly, and reciprocally with CRF receptor-containing Orx neurons in the lateral, dorsomedial and perifornical hypothalamus (LH-DMH/PeF) and in other limbic brain regions, such as the vBNST, dBNST, CeA, PVN, lateral septum (LS) (Achua et al., 2014; Ronan et al., 2016; Sakurai et al., 2005; Yoshida et al., 2006; Slater et al., 2016) that regulate stress, anxious, and depressive behavior. The CRF-positive neurons in the PVN and CeA express Orx receptors (Achua et al., 2014; Ronan et al., 2016); such that microinjection of OrxA into the PVN produces large increases in plasma corticosterone levels (Samson et al., 2002). Within the LH-DMH/PeF, Orx perikarya self-interact, and stress stimulates increased dendritic branching to promote these connections (Grafe et al., 2018). Direct CRF innervation of Orx-expressing neurons yields depolarization via CRF1 receptors and is blocked by CRF1 antagonists or knockout (Winsky-Sommerer et al., 2005; Winsky-Sommerer et al., 2004). This direct CRF1 connection also promotes stress-induced activation of orexinergic outputs that return activation in a positive feedback manner (Winsky-Sommerer et al., 2004). Surprisingly, although CRF1 receptors stimulate activity of LH-DMH/PeF Orx neurons, they only express CRF2 receptors (Slater et al., 2016; Winsky-Sommerer et al., 2004). Thus, Orx regulatory roles in stress-related behavior, and the behavioral phenotypes that result, are derived in conjunction with CRF systems.
2.3. The role of Orx1 receptors in affective behavioral phenotypes
The hypothalamic OrxA and OrxB neuropeptides are cleaved in equal proportions from the same prepro-orexin molecule. These neuropeptides induce cellular effects through interaction with two receptor subtypes (Orx1 and Orx2). Importantly, OrxA demonstrates a high affinity for both receptor subtypes, while OrxB binds Orx2 equally well as OrxA but shows a 10–100x lower affinity for Orx1 (Sakurai et al., 1998; Ammoun et al., 2003). As such, OrxB shows a slight binding preference for Orx2 over Orx1. This binding bias suggests a mechanism for higher Orx2 activation during Orx release; however, regional differences in receptor mRNA and protein abundance (Trivedi et al., 1998; Cluderay et al., 2002) predicts that the receptor that dominates Orx’s role in circuit dynamics may be stimulus and behavior dependent.
The orexin system plays a role in stress responses outside of its essential functions in sleep regulation (Sakurai et al., 2010) and feeding behavior (Sakurai, 2014). Optogenetic stimulation or activation using designer receptors exclusively activated by designer drugs (DREADD) of Orx-producing neurons reduces social interaction (Heydendael et al., 2014) and impairs memory (Eacret et al., 2019). Intracerebroventricular (icv) infusions of OrxA in rodents enhance locomotion (Samson et al., 2010; Matsuzaki et al., 2002), increases time in the dark side of the light/dark box and the closed arms of the elevated plus-maze (Suzuki et al., 2005), and reinstates previously extinguished nicotine-seeking behavior (Plaza-Zabala et al., 2010). In humans, orexin levels are elevated in patients with depression, anxiety disorders, and stress related to alcohol withdrawal (Lu et al., 2017; Ozsoy et al., 2017; von der Goltz et al., 2011). These examples suggest that orexin system activity may exacerbate stress-induced reactions and that they are related to psychopathological states. It is important to note that the influence of these neuromodulators on stress-related behavior is dependent on regionally specific effects in their neurocircuitry.
During social defeat, Orx neuropeptides are reduced in reward (VTA) and decision-making areas (mPFC) of rat brains (Nocjar et al., 2012). Pharmacological inhibition or knockdown of Orx receptors in the reward-associated area of the ventral pallidum (VP) enhances depressive behavior in the forced swim and sucrose preference test in rats (Ji et al., 2019), suggesting activity of endogenous Orx here promotes resilient behavior. However, OrxA and OrxB administered in the paraventricular thalamus (PVT) of rats enhances anxiety in the elevated plus-maze (Samson et al., 2010; Heydendael et al., 2011). A similar effect is observed when Orx neuropeptides are directed into the CeA of hamsters (Avolio et al., 2011) or the BNST of rats (Lungwitz et al., 2012). Together these examples highlight the importance of regional specificity of Orx signaling.
As an example of region-specific effects of the orexin system, our work has previously assessed the Orx1 function in the basolateral amygdala (BLA), an area associated with emotional interpretation, learning, and reward, and its contribution to stress-induced phenotype formation. After ten days of social defeat, Orx1 gene expression in the BLA is negatively correlated with the social preference score in mice, with higher resilience being associated with lower Orx1 expression (Arendt et al., 2014). This finding led to the prediction that Orx1 activity in the BLA may influence stress-induced phenotype formation (Summers et al., 2020; Yaeger et al., 2020). To test this idea, we used a social stress paradigm developed by our lab, called the Stress Alternatives Model (SAM), that introduces test mice to a social aggressor, allowing them to diverge over the course of four days into two distinct behavioral phenotypes, Escape/Resilient or Stay/Vulnerable (Smith et al., 2014; Summers et al., 2020; Blanchard et al., 2013; Robertson et al., 2015). Phenotype commitment occurs at the end of the second day of interaction, allowing us to use days 3 and 4 to test behavioral alterations as a result of pharmacological manipulation. When introducing a single treatment of an Orx1 antagonist (SB-674042) into the BLA of vulnerable mice, behavioral adaptation took place with approximately 65 % of the animals shifting to the resilient phenotype (Yaeger et al., 2020)(Fig. 1), similar to the effect of the CRF1 receptor antagonist antalarmin (Yaeger et al., 2019; Smith et al., 2016b; Carpenter et al., 2009b; Summers et al., 2017). This suggests Orx1 activity within the BLA modulates the development of resilient or susceptible phenotypes.
Fig. 1.

Example of stress signaling interference to promote behavioral flexibility and therapeutic outcomes. Inhibition of Orx1 receptors in the BLA enhances anti-stress behaviors, including social learning (second from right) and escape (far right), while reducing pro-stress behaviors, like contextual (second from left) and cued (far left) freezing to fear conditioning.
Using the SAM, we are uniquely able to test a component of fear learning, where freezing (conditioned response, CR) is measured after pairing a tone (conditioned stimulus, CS) with social aggression (unconditioned stimulus, US). In our paradigm, inhibition of intra-BLA Orx1 reduced both contextual and cued fear learning responses (Fig. 1) (Yaeger et al., 2019, 2020). This result is consistent with traditional shock-induced fear conditioning procedures demonstrating that antagonism of Orx1 (SB-334867) in the BLA, but not in the dorsal hippocampus or IL of the mPFC, promotes fear extinction (Flores et al., 2014). These results characterize the role of intra-BLA Orx1 in fear learning. Further, we observed alternative learning strategies being evoked through these receptors.
After four days of SAM exposure, mice were introduced to the Open Field (OF) Test, where we predicted animals treated with the Orx1 antagonist would exhibit reduced anxious behavioral responses. Curiously, our results showed that inhibition of Orx1 produced divergent behavioral responses in the OF with resilient animals preferring the sides of apparatus and vulnerable mice occupying the anxiogenic center region more regularly (Yaeger et al., 2019). Upon closer inspection, we identified a positive relationship between the amount of time these animals spent in the center of the SAM and the amount of time spent in the center of the OF test. Our interpretation of the results is that intra-BLA Orx1 inhibition enhances a form of social learning (Fig. 1), where animals accustomed to Escape behavior (Resilient) will follow the walls of the SAM to find the exit and avoid social confrontation by leaving the SAM arena. In contrast, mice that choose to Stay (Vulnerable) in the SAM arena may avoid social encounters with the aggressor if they evade the walls of the arena, where the aggressive mouse patrols. With Orx1 inhibition, these behavioral responses in the SAM are transferred to the non-social context of the OF test (Yaeger et al., 2019). Collectively, our results expose a regulatory role of intra-BLA Orx1 in stress-induced phenotype formation through the mediation of learning mechanisms dependent on both fear and the environmental context.
While Orx1 receptor signaling likely plays an important role in the modification of genetically and epigenetically defined phenotypes, it is important to note that the effects of Orx2 receptors must always be taken into consideration commensurately (Yaeger et al., 2020). Here we would like to make a case for opposing actions for Orx1 and Orx2 receptor functions (Yaeger et al., 2020). Reduced behavioral despair in the forced swim test (FST) and tail suspension test (TST) is observed in Orx1 null mice, whereas Orx2 null mice show the opposite effect (Scott et al., 2011). Moreover, we have demonstrated that ten days of social stress increases intra-BLA Orx2 gene expression in resilient mice while decreasing it in susceptible animals (Arendt et al., 2014), suggesting Orx2 activation may have a rehabilitating effect on stress-induced behavioral responses (Yaeger et al., 2020). In fact, we have shown that after stress-induced phenotype formation, icv Orx2 stimulation can reverse maladaptive behavioral reactions in susceptible mice (Staton et al., 2018). Further, intra-BLA knockdown studies by our lab have illustrated that short-hairpin knockdown of Orx2 (Orx2-shRNA) promotes susceptibility in non-stressed mice (Arendt et al., 2014), but Orx1-shRNA in mice exposed to the SAM demonstrate resilience (Yaeger et al., 2019). Together these examples reveal a potential contrasting role of Orx1 and Orx2 signaling, particularly in BLA (Yaeger et al., 2020), where learning, fear, arousal, motivation, and decision-making circuits intersect.
3. Effects of early-life environment
Adult behavior often exhibits considerable variability, even when genetic variability is experimentally constrained. What causes behavioral diversity even when animals with similar genetic backgrounds are studied? A part of the variability, undoubtedly, arises from stochastic differences in the neuroendocrine development of the expression of the behavior. Nevertheless, these random differences do not explain the whole gamut of the behavioral variability because the direction of the observed differences is typically systematic. For example, a genetic mutation in a component of the NMDA receptor causes reduced learning and memory if animals are raised in laboratory cages but not if they live in enriched cages (Rampon et al., 2000). Or, animals of the same inbred strain show high anxiety if they have been exposed to weeks of chronic stress (Koe et al., 2016). In each of these cases, the variability of the behavior arises from the variability of the environmental context for the individual animals.
3.1. The early life environment causes later behavioral variability
The extent to which the environment affects behavior depends on the life stage of the individual. Specifically, experience in early life has a disproportionate influence on the behaviors expressed later during adulthood. Some of these influences encompass environment-expectant traits, an idea that some aspects of the environment are so predictive that their absence in a small minority of cases causes behavioral development to run askew. For example, the proper development of sensory cortices depends on the availability of sensory information from the environment during critical windows of development (Fregnac and Imbert, 1978). Experimentally depriving visual information disrupts the development of the primary visual cortex in kittens. Yet, a vast majority of kittens in nature receive the visual stimulus, and thus, the visual system in this restricted example is environment-expectant. Yet, the role of early environment in shaping the adult brain or behavior often extends beyond environment-expectant behaviors and into the realm of environment-dependent plasticity. Maternal care in rats provides a case in point. Female pups born to high-nursing mothers themselves show a high-nursing phenotype in adulthood, just as expected from a genetically heritable behavior (Bagot and Meaney, 2010). Yet, cross-fostering studies show that the difference between high and low-nursing mothers is caused by the nursing environment rather than genetic differences (Francis et al., 1999). Female pups entrain their maternal behavior to their experience of the maternal environment. Such coupling of past environment and future behavior allows animals to optimize the quantity of offspring vis-a-vis quality of maternal care as a function of environmental variability. A similar argument can be made to explain why higher population density in the prenatal life leads to faster postnatal growth later (Dantzer et al., 2013), to plausibly adjust the life history stage to the incipient environment. Thus, the early environment creates phenotypic plasticity in later life through environment-dependent processes.
The presence of the mother and the quality of maternal interactions are important environmental variables that can have sustained effects on behavioral development. In several bird species, the mother can change many factors of her egg composition like the size of the egg, hormones, nutrients, and many other components, including the sex of the embryo with respect to the changes in the environment (Badyaev and Oh, 2008). Depending on the availability of food, quality of their mating partners, their maternal effects can vary, and this variation has a major impact on the developmental phenotypic plasticity of their offspring that persist across various generations even if the conditions have changed later on (Lindström, 1999; Mousseau and Fox, 1998).
Apart from playing a critical part in the environment of offspring post-delivery, females in numerous vertebrates have an enormous impact on the growth and breeding performance of their offspring depending on their own early life experience (Kan et al., 2016). During prenatal in utero life, poor maternal nutrition can predispose offspring to metabolic disorders later in life. Such predisposition has been observed both in longitudinal studies of humans, e.g., those born during Dutch famine in the Second World War, and in preclinical animal models. Developing a fetus is also exquisitely attuned to the hormonal environment of the mother. For example, glucocorticoids secreted during stress can readily cross placental boundaries and affect the life history choices of the offspring. In squirrels, high population density causes a stress response in adults, including pregnant mothers (Dantzer et al., 2013). Glucocorticoids secreted during the stress, independent of social or nutritional consequences of the overcrowding, can initiate rapid growth in the pups. The critical window of maternal influences extends to early postnatal life. Emotional development in human kids relies on behavioral synchrony between the mother and the child (Feldman, 2012; Pratt et al., 2017). This can be readily demonstrated, for example, in still face experiments where emotional withdrawal of the mother evokes emotional stress in the kid. Case-control studies likewise show that reduced maternal care translates to adverse health outcomes and a shorter lifespan in humans (Pesonen and Räikkönen, 2012). The role of the postnatal maternal environment has been extensively modelled in laboratory rats and mice. Aversive conditioning in pups with or without maternal presence provides a fascinating example of behavioral variability brought about by parental care (Moriceau and Sullivan, 2006a). Rat pups exhibit aversive conditioning to neutral sensory stimuli paired previously with footshock, similar to the learning observed in a similar scenario in adult animals. Yet, this classical conditioning only occurs during the maternal absence and reverts to an atypical attraction to conditioned stimulus if the mother is present during the pairing. The switch between typical and atypical learning, in this case, is mediated through adrenal glucocorticoid acting within the amygdala of the pups, a response that is ablated during maternal presence (Moriceau et al., 2006). Thus, in short, the mother represents an important early-life environmental factor for the later behavioral variance.
3.2. Role of stress and stress hormones
The role of glucocorticoids in mediating maternal influences reinforces a role for these steroid hormones in mediating long-term and environment-dependent behavioral variability in the stress response. Acute stressors typically lead to a biphasic neuroendocrine response. A part of this response is mediated by neurons of autonomic nerves initiating rapid recruitment of the sympathetic system and causing the release of epinephrine from adrenal glands. This short-term component of the response is succeeded by a chain of hormone-mediated events culminating in adrenal glucocorticoid release. These events are mediated by corticotropin-releasing hormones secreted by hypothalamic neurons and leading to adrenocorticotropin release from the anterior pituitary, which eventually binds to its adrenal receptors and causes a surge of glucocorticoids (Herman and Cullinan, 1997; Herman et al., 2003). This endocrine chain is usually referred to by its acronym HPA-axis or hypothalamus-pituitary-adrenal axis.
Central control of the HPA-axis has been intensely studied and plays an important part in metaplastic changes in stress responsivity (Herman and Cullinan, 1997; Herman et al., 2003). Hippocampus was the first brain region where glucocorticoid receptors were discovered (McEwen, 2019). Activation of these receptors leads to inhibition of the HPA axis (Sapolsky et al., 1991; Herman et al., 1989). For example, surgical removal of the hippocampus increases transcription of CRH in the hypothalamus (Herman et al., 1989; Sapolsky et al., 1984). The inhibiting role of the hippocampus provides an important physiological node where the early environment can act to modulate the adult stress response. In keeping with this possibility, lower maternal care causes a sustained reduction in hippocampal glucocorticoid receptors through epigenetic mechanisms, leading to the reduced responsivity of the hippocampus to stress hormones (Weaver et al., 2004). This leads to lesser inhibition on the HPA axis and increased magnitude of stress endocrine response during adulthood. Hippocampus is also involved in cognitive function like learning and the formation of long-term memories. Thus, it is no surprise that early-life stress influences these cognitive functions in parallel to deficits in regulation of endocrine response. Early life stress causes deficits in spatial memory in middle age when tested in both Morris water maze and object recognition tasks (Brunson et al., 2005a; Huot et al., 2002; Brunson et al., 2005b). Basal synaptic transmission and synaptic plasticity are likewise compromised in adult rats stressed in early life, reminiscent of electrophysiological deficits often observed in adult interventions that reduce cognitive performance. Similarly, neurogenesis within hippocampal dentate gyrus is substantially reduced and pruning of mossy fiber terminals at CA3 sub-filed is compromised. This non-exhaustive list suggests that early life stress results in a deficit in hippocampal structure and function that persist in adulthood, with accompanying facilitation of stress responsiveness and predisposing to lower mnemonic functions. Hippocampal formation can be broadly divided into its dorsal and ventral aspects, both with distinct features of stress sensitivity (Pinto et al., 2015). Ventral hippocampus plays an unique role in defensive responses to the stress, including anxiogenesis and retrieval of traumatic memories (Ritov et al., 2014). Dorsal hippocampus, on the other hand, is involved in spatial memory and its modulation in relation to stress (Hawley et al., 2012).
3.3. Role of the basolateral amygdala
Another important node in the behavioral effects of early life stress is the basolateral amygdala. This brain structure is required for the generation and maintenance of fear or conditioned aversion. Stress, specifically chronic immobilization stress, in adulthood causes long-term plasticity within the basolateral amygdala by creating dendritic expansion of principal neurons within this structure (Vyas et al., 2002; Vyas et al., 2003). Such dendritic hypertrophy is known to be dependent on adrenal glucocorticoids because exogenous corticosterone recapitulates structural plasticity, and competition of glucocorticoid receptors prevents it (Mitra et al., 2009a; Mitra and Sapolsky, 2008; Mitra and Sapolsky, 2010a). Amygdalar hypertrophy represents a structural footprint of the stress in the brain and serves to change the behavior long after the original stress episode is over. The most studied behavioral change in this respect is anxiogenesis. Treatments that promote dendritic hypertrophy in the basolateral amygdala cause anxiogenesis and those that block dendritic growth prevent stress-induced anxiogenesis (Mitra and Sapolsky, 2010b). Moreover, the amount of stress hormones after a stressful episode shows a positive association with amygdalar hypertrophy that later develops (Mitra et al., 2009b; Mitra, 2019; Hegde et al., 2017). The relationship between neuronal hypertrophy and anxiogenesis is environment-dependent. Stressors in adults result in anxiety along with amygdalar expansion, if animals are placed in the standard laboratory housing conditions,. Both of these endpoints are blocked if the same strain of animals, exposed to the same stressors, are instead housed in an enriched environment (Koe et al., 2016). Thus, the environment has a principal role in the capacity of stressors to cause neuronal and behavioral change. This role extends to sensitivity to the subsequent stressors whereby living in enriched housing blunts the capacity of subsequent acute stressors to cause the release of adrenal glucocorticoids. Experimental observations suggest that the same framework can be extended to understand the effects of early life experience on later emotional behaviors. Pre-weaning maternal separation causes anxiogenesis and increases the dendritic length of basolateral amygdala neurons when measured after pubescence. These observations again support the narrative that quality and quantity of maternal experience during development creates a permanent metaplasticity in behavior and in brain regions involved in the behavioral regulation.
An early example of environment-induced resilience can be found in the work of Robert Tryon, who selectively bred generations of rats for their cognitive performance in a maze, resulting in artificially selected lines at the extremes of the performance (Tryon, 1940). When these lines were later exposed to a living environment rich in sensory stimulation, the behavioral variability between two lines was obliterated with higher cognitive performance in both groups. Observations like this have led to a large body of research in modern neuroscience where standard laboratory housing environment for rodents is compared with similar animals living in an enriched environment consisting of larger cages, more complex social organization and provision of sensory stimuli. There is a significant diversity of enrichment models whereby some paradigms provide the additional possibility of voluntary exercise, or the protocols differ in terms of duration and age and social composition (Brown et al., 2003; Kempermann et al., 1997; Komitova et al., 2005). Across these various paradigms, environmental enrichment is generally thought to create resilience to stress (Fig. 2). For example, rats and mice living in an enriched environment show improved performance in memory tasks and reduced anxiety post-stress (Koe et al., 2016; Pacteau et al., 1989; Ashokan et al., 2018). These observations have led to experiments addressing the ability of the enriched environment to reverse or prevent the effects of stress both in early life as well as adult-life. Enrichment during the peripubertal stage renormalizes the facilitation of stress endocrine axis brought about by earlier maternal separation. Similarly, the provision of enrichment later in life rescues the effects of maternal separation as well as chronic restraint stress on anxiety and dendritic expansion within the basolateral amygdala. These examples further buttress the role of the environment in sculpting not only brain plasticity and behavior but also the neuroendocrine response of an individual’s stress-susceptibility and resilience through the lifespan.
Fig. 2.
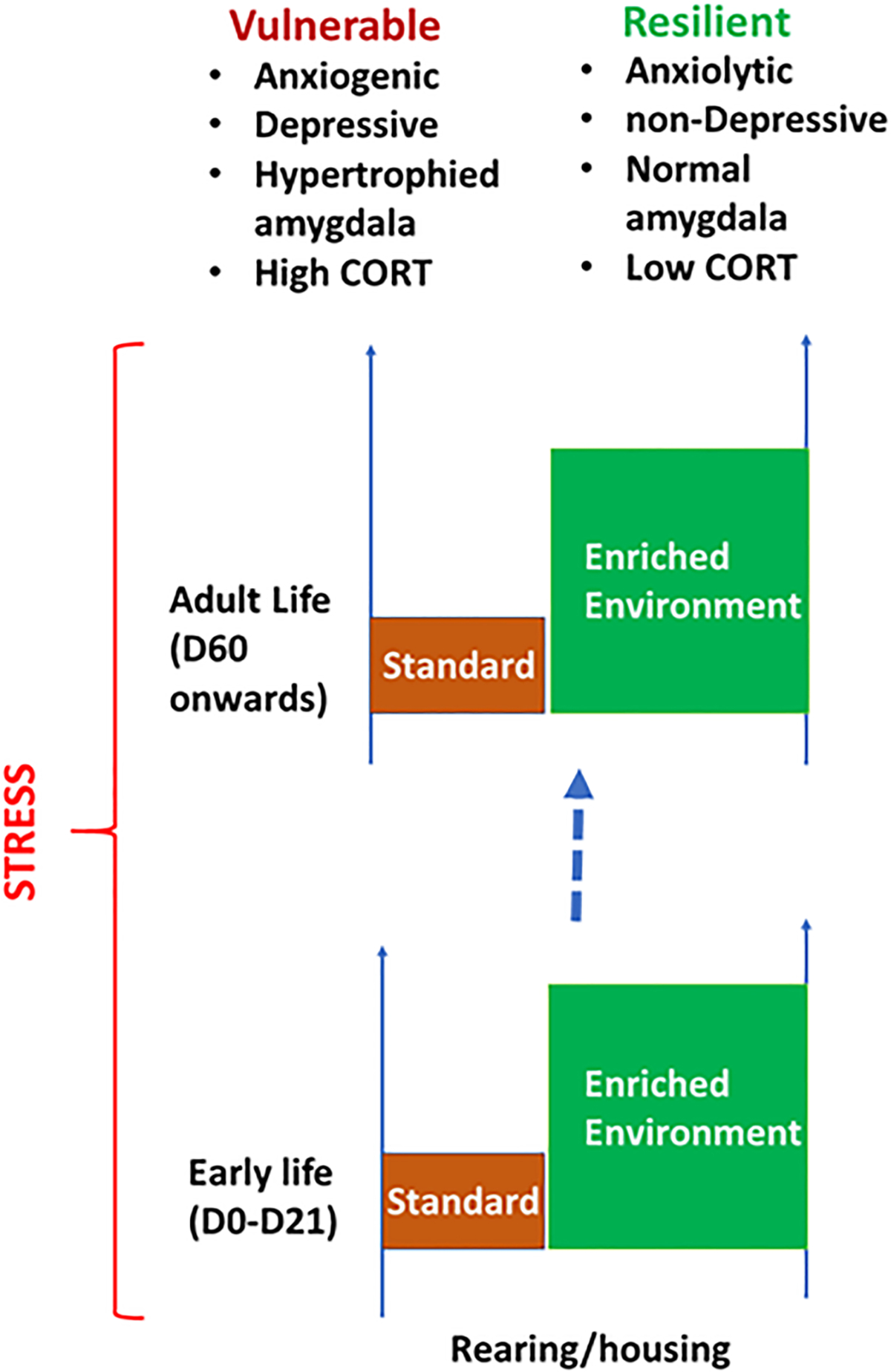
Environment influences stress-induced behavior, neuroplasticity of amygdala, and stress-sensitivity. Animals raised in standard housing are more vulnerable to stress than those raised in enriched environment, during early life or during adulthood.
Stress-induced plasticity in the hippocampus and amygdala likely interact at multiple levels. For example, hippocampus based memory tasks and its modulation by stress and fearful context is influenced by amygdala activation through stress hormone glucocorticoid and its receptors (Roozendaal et al., 2003). At the level of synaptic plasticity, the basolateral amygdala modulates long-term hippocampal potentiation under stressful conditions, specifically enhancing certain memory traces heavily laden with fear or stressful emotion (Bergado et al., 2011).
4. Intergenerational effects of parental stress
It has now become apparent that stress affects not just the individual but holds the capacity to have long-term latent effects on future generations. Much of the intergenerational research to-date has focussed on maternal effects, with multiple studies showing that perturbations to the maternal environment in utero negatively impact the normal neurodevelopment of children via fetal reprogramming (Bronson and Bale, 2016; Bronson et al., 2017). Human epidemiological and animal studies collectively indicate that stressful experiences during pregnancy and the perinatal period affect the endocrine function and brain development, increases stress vulnerability, and risks the development of psychological disorders in subsequent generations (Babenko et al., 2015; Jiang et al., 2019; Zannas and West, 2014). Due to prior comprehensive coverage of maternal stress effects on offspring health (Franke et al., 2020; Frasch et al., 2018), the following section will primarily review the relatively small but growing body of evidence of a role for paternal stress to influence the offspring’s behavioral health and endocrine function.
Human studies of intergenerational and transgenerational effects of stress are instructive. Yet, they need to be complemented by animal models because of the ability to create and compare experimental groups. Animal models also allow precise control over parental exposure and the quantity or quality of parental interactions. These factors, in addition to genetic control over the homogeneity of the study subjects, make animal research an interesting addition to the study of the effects of the stress that span generations.
4.1. Maternal Separation and inter-generational effects of stress during early life
The maternal separation paradigm is a widely studied model of early life adversity (Tractenberg et al., 2016). The core deleterious effects are related to increased anxiety (Huot et al., 2002), impaired fear memory processing (Lukkes et al., 2009; Stevenson et al., 2009; Zoicas and Neumann, 2016), and depression-related behaviors (Lee et al., 2007), which are closely associated with HPA axis dysregulation (Enthoven et al., 2008; Loizzo et al., 2010; Zajdel et al., 2019). However, it suffers from a lack of consistency in its behavioral effects, partly due to variations in the methodological procedures, and there are likely secondary effects due to significant changes in the dam’s behavior (Alves et al., 2019). Interestingly, the initial evidence regarding this controversial paradigm is that it imposes no intergenerational ill-effects on offspring behavioral phenotypes (Weiss et al., 2011). However, in a unique adaptation of the maternal separation paradigm, Mansuy and colleagues then combined early-life maternal separation with maternal unpredictable stress (MSUS) and reported the development of reduced anxiety-like behavior but increased depressive-like behavior in the offspring of MSUS males (Gapp et al., 2014). The intergenerational effects were associated with changes to sperm miRNAs implying that early-life stress triggers long-term physiological adaptations of the male reproductive tract, further strengthened by reproducing the MSUS intergenerational effects in IVF mice after microinjecting the upregulated miRNAs into fertilized oocytes. More recently, the same group also described a potential role for sperm-borne long non-coding RNAs (Gapp et al., 2018). It is still unknown if this MSUS paradigm changes sperm DNA methylation, which has been described to occur in the brain (Murgatroyd, 2014) - specifically for arginine-vasopressin in the hypothalamic paraventricular nucleus (Murgatroyd, 2014). It is unclear if other stress-relevant genes in the hypothalamus also sustain methylation changes, nor has there been validation that these epigenetic modifications are inherited by the offspring.
4.2. Social instability in early life and its inter-generational effects
Adverse childhood experiences are associated with physical and mental health disturbances during adolescence up until early adulthood (Oh et al., 2018). Affected individuals are also at greater risk for chronic cardiovascular and metabolic health conditions later in life (Maunder et al., 2019; McLeod et al., 2019; Robson et al., 2020; Suglia et al., 2018), as well as mental illness and suicide ideation (Evans et al., 2020; Iob et al., 2019; Stansfeld et al., 2017; Turner et al., 2019). However, it is worth noting that this relationship is multifactorial and complex, and there is evidence of significant gene-environment interactions (Lehto et al., 2019). There is a possibility to explore the potential intergenerational implications by investigating experimental paradigms of childhood adversity using transgenic rodent strains modeling genetic susceptibilities to psychiatric illness.
One potential paradigm worthy of further exploration is early life social instability (Koolhaas et al., 2017) that causes anxiety-related behavioral modifications (Caruso et al., 2018; Hodges et al., 2017; Hodges et al., 2018; Pittet et al., 2017). In addition, these animals also show signs of a dysregulated physiological stress response (Hodges et al., 2019) that is attributed to abnormal cellular activation of the oxytocin neurons in the paraventricular nucleus of the hypothalamus, and generally of cortical and hippocampal neurons under social contexts. There are several reasons why these social instability paradigms would make for intriguing intergenerational studies. As mentioned, adolescent social instability results in abnormal oxytocin neurotransmission, a process that is regulated by the paternally imprinted genes, Peg3 and Magel2 (Broad et al., 2009). Separately, the epigenetic regulation of oxytocin receptor expression is hypothesized to govern social behaviors, thereby making it an ideal gene mediator for the intergenerational shifts in behavior. However, this remains controversial in the absence of robust evidence and consistent replication from human and animal studies (Maud et al., 2018). Disruption of oxytocinergic signaling by manipulating the oxytocin receptor (OXTR) expression in mice causes HPA axis dysregulation and pro-anxiety behaviors (Amico et al., 2008; Mantella et al., 2004). Research into the consequences of impoverished early life parental care in prairie voles (taking advantage of their natural biparental caring behavior) reported hypermethylation of the OXTR and reduced OXT density in the brains of offspring (Perkeybile et al., 2019). That latter finding could be highly relevant to humans since there are four homologous CpG sites within the promoter region of the human OXTR gene. Those hypermethylation deficiencies have been separately linked to the atypical development of the left orbitofrontal cortex in children with childhood maltreatment, resulting in distorted attachment formation (Fujisawa et al., 2019) and poorer theory of mind ability in toddlers (MacKinnon et al., 2019).
While not recognized as an imprinted gene, the estrogen receptor-α, and more specifically methylation of the α−1B promotor, has been reported to regulate the intergenerational variation in the quality of maternal care behavior (e.g., pup licking and grooming) (Champagne et al., 2006). Mechanistically, that differential methylation of this promoter region regulates the extent of signal transducer and activator of transcription (STAT)5b binding. Higher levels of Stat5b along with and greater ER-α receptor expression are observed in the medial preoptic area (MPOA) of mice that had experienced high levels of licking/grooming during their first week of life, compared to the offspring of low LG dams. Interestingly, oxytocin receptor density was previously found to be subject to estrogenic regulation (de Kloet et al., 1986). While OXTR density was found to be altered in the ventromedial nucleus of the hypothalamus, the nucleus accumbens and the central amygdala, its expression in the MPOA remains to be confirmed. However, it is possible that estrogen-mediated regulation of the imprinted OXTR gene could be a key mechanism in transgenerational inheritance.
4.3. Pre-conception chronic unpredictable mild stress and transgenerational inheritance of stress-relevant phenotypes
Gestational stress imposed through maternal chronic mild stress during pregnancy is well-documented to results in HPA axis dysregulation and depressive-like behaviors in adult offspring (Fatima et al., 2017). In contrast, it is only more recently shown that chronic variable stress exposure of the paternal lineage preconception did not produce behavioral deficits in the F1 offspring (Rodgers et al., 2013). However, both male and female offspring had blunted serum corticosterone responses to acute restraint stress, which is consistent with the general suppression of HPA axis activity associated with PTSD-like symptoms in humans. Rodgers and colleagues also identified nine miRNAs that were up-regulated in the sperm of stressed breeders that, when microinjected into zygotes, recapitulated the HPA-axis dysregulation in the resulting offspring (Rodgers et al., 2015). The presence of HPA-axis dysregulation is strongly associated with increased vulnerability to mental illness (see review by (Du and Pang, 2015). Therefore, given the deficiency in their stress-induced HPA axis response, and despite them not manifesting behavioral impairments, it would be interesting for future studies to examine how the F1 offspring themselves would respond and adapt to repeated exposures to stress (both mild and severe).
4.4. Chronic social defeat and transgenerational inheritance of stress-relevant phenotypes
Chronic social defeat is a well-established rodent model of posttraumatic stress disorder (Patel et al., 2019; Schoner et al., 2017), with the occurrence of impaired neural plasticity, aberrant white matter connectivity, and neuroinflammation believed to underlie the long-term behavioral abnormalities (Bonnefil et al., 2019; Pfau and Russo, 2016; Qiao et al., 2016). Dietz and colleagues reported that males that experienced social defeat preconception went on to sire offspring with heightened anxiety and depressive-like behaviors, even in the absence of a stressor (Dietz et al., 2011). However, those intergenerational effects were not reproduced by in vitro fertilization (IVF), leading the authors to argue for a minor contribution of epigenetic mechanisms and instead speculate that the offspring phenotypes arise from the females adjusting their maternal investment depending on their mate (Curley et al., 2011). These adaptations could result in further epigenetic variations in the offspring that directly affect their behavior and stress response later in life (Fish et al., 2004). Another speculation for this difference is that there are innate differences in the impact of social compared to asocial stress (Cunningham et al., 2019), thereby accounting for the subtle differences noted across the variety of paternal stress models, but this requires further investigation. Other potential reasons for the discrepancy are that there could be unaccounted bias for sperm selection under IVF conditions (Lee et al., 2002), and IVF is known to alter the epigenome of the fetus, including DNA methylation (Melamed et al., 2015). More comprehensive detailing of how chronic social defeat stress alters germline methylation and sperm ncRNA content is required to further our understanding of the mode of transgenerational transmission in this paternal stress model. There has been one report of social defeat stress-inducing gonadal atrophy and reducing semen quality (Wang et al., 2017), which were ameliorated by pharmacological antagonism of the vasopressin V1b receptor, but there have been no reported of subfertility impacting the paternal studies to-date. This needs to be independently verified but could be an intriguing consideration for post-trauma reproduction plans.
4.5. Paternal glucocorticoid treatment
While the above models of psychosocial stress demonstrate that paternal environments are intergenerational modifiers of offspring stress-relevant phenotypes, they do not directly address that paternal physiological adaptation to stress that drives those effects. We began to address this by adapting a mouse model of metabolic syndrome (Karatsoreos et al., 2010) to model the chronic escalation of HPA axis activity (Short et al., 2016). Limiting the protocol to four weeks of low-dose corticosterone (CORT)-treatment in drinking water avoided secondary metabolic effects such as increased adiposity and abnormal body weight gain. In the absence of behavioral deficits in the CORT-treated animals (F0 generation), we verified the presence of marked indicators of HPA axis adaption, primarily adrenal involution and reduced GR gene expression. We also verified that sperm density and motility were unaffected, but a closer examination of gonadal atrophy was not conducted. We found that adult F1 males were particularly vulnerable to paternal CORT-treatment and developed heightened anxiety in the absence of additional stressors. In contrast, juvenile F1 females displayed impaired extinction of auditory conditioned fear memory and a subtle spatial memory deficit in adulthood (Yeshurun et al., 2017). We hypothesize that the former indicates an early-life vulnerability for developing anxiety-related psychopathologies, which would be consistent with the extended literature (Rapee et al., 2019; Ganella and Kim, 2014). Importantly, we provided evidence of a transgenerational effect of paternal CORT-treatment with both sexes of the F2 generation, also displaying modified anxiety- and depression-related behaviors, although the directionality of those changes was different to that observed in the F1 generation and we have yet to resolve the potential reasons for that.
Our work expanded upon prior studies that reported significant transgenerational effects following exposure of the male germline to synthetic glucocorticoids. Some of these examined antenatal exposure and will be discussed in the next section, as these are technically maternal treatments. There have been only a few intergenerational studies that have treated adult male mice with the GR agonist, dexamethasone, and with different outcomes. A 5-day DEX treatment resulted in persistent sperm DNA hypermethylation (Petropoulos et al., 2014), but a separate study reported no alteration to sperm transcriptome (Bonisch et al., 2016). The former study also reported demethylation of the gene regulatory regions of steroid receptors in the F1 offspring hippocampus but also did not examine the offspring for any potential changes in behavior or stress response. Bonisch and colleagues observed no increased susceptibility to metabolic syndromes associated with access to a high-fat diet, which was in contrast to metabolic reprogramming in a paternal restraint stress model (Wu et al., 2016). While most experimental protocols employ a single period of corticosterone treatment, a cyclical approach to administering low-dose corticosterone could more accurately model the typical mood cycles of patients with depression. This is reported to cause behavioral sensitization and development of depressive-like behaviors in rats (Lebedeva et al., 2017), but the transgenerational implications for offspring in this model have yet to be investigated.
Despite the subtle differences in offspring phenotypes, the epigenetic mechanisms implicated in the intergenerational effects of paternal CORT-treatment are not dissimilar to the psychosocial stress models (Gapp et al., 2014; Rodgers et al., 2013). Chronic CORT-treatment also alters the sperm content of sncRNAs, mainly microRNAs but also tRNAs and piwiRNAs (Short et al., 2016). Further evidence of the consistency across the distinct paternal stress models is that several dysregulated miRNAs are common to the different models (Pang et al., 2017). Future research could perhaps focus on these common targets to identify when the effects of having altered paternal miRNAs can be observed post-fertilization during early embryonic development.
4.5.1. Intergenerational transmission of fearful olfactory cues
Readers should be aware of the controversial transgenerational model of paternal traumatic stress involving the preconceptional cued fear conditioning of male breeder mice paired to the aromatic ketone, acetophenone (Dias and Ressler, 2014). In the studies of Dias and Ressler (2014), they reported that male breeders conditioned to this specific odorant subsequently sired F1 offspring that naturally displayed an aversion to this same odorant, despite themselves having never been exposed or primed to acetophenone. This behavioral sensitivity also manifested in the F2 generation, thereby demonstrating a transgenerational inheritance of olfactory fear memory. Having found CpG hypomethylation of the Olfr151 gene in the F0 and F1 males, supporting evidence from IVF and cross-fostering studies strengthened the argument for inheritance via the paternal gametes. More recently, it was reported that sperm RNAs also had the capacity to convey this olfactory sensitization, although it only affected the female F1 offspring (Aoued et al., 2020). While this highly innovative and eye-opening finding initially drew considerable attention within the field (Welberg, 2014; Szyf, 2014), it is also worth noting that this transgenerational model has not been independently validated. Furthermore, counter-commentary articles have discussed this work (Francis, 2014), arguing for the minuscule statistical probability in the uniformity of experimental success through the series of studies.
4.5.2. Maternal glucocorticoid treatment
While we refer readers to the abundant literature on maternal health influences on offspring health outcomes, the use of glucocorticoids during pregnancy for women at risk for pre-term delivery exposes the fetus to elevated steroid levels, with potential consequences for fetal programming and offspring health (Moisiadis and Matthews, 2014a; Moisiadis and Matthews, 2014b). There are also well-established sex--specific responses to glucocorticoid surges in the placenta (Wieczorek et al., 2019; Braun et al., 2018; Lee et al., 2017), partly due to GR-mediated sex-specific responses of the infant (Togher et al., 2018). Here, we will outline the most recent evidence of transgenerational effects transmitted through males born under such conditions.
Studies of pregnant guinea pigs treated with clinically relevant doses of glucocorticoids have revealed transgenerational changes in metabolism-related gene expression in the paraventricular nucleus of the hypothalamus (Moisiadis et al., 2017). This was complemented by increased novelty-induced corticosterone responses in three generations of females via the paternal lineage. Subsequent studies of the prefrontal cortex (Constantinof et al., 2019a; Constantinof et al., 2019b) in this same model confirmed the presence of wide-spread alterations of gene expression and DNA methylation across three generations of females. Further work is required to determine if stress-relevant genes regulating HPA axis activity are also affected and if stress-relevant behavioral responses are modified across the generations accordingly.
Early work by Jonathan Seckl’s group demonstrated that prenatal overexposure to dexamethasone led to paternal-mediated transgenerational effects by consistently reducing rat pup birth weights across two generations (Drake et al., 2005). However, the altered expression of paternally imprinted gene candidates (e.g., insulin-like growth factor 2, igf2) that were proposed to mediate those early growth effects differed between the F2 and F3 generations (Drake et al., 2011). That implied that sperm-borne DNA methylation changes could not fully account for the observed transgenerational effects. More recent evidence further revealed that histone modifications and alterations to the sperm small RNA content are also unlikely to modulate the transgenerational effects observed in this model (Cartier et al., 2018). Given the intergenerational discrepancies, it is more likely that different epigenetic modifications moderate each generation’s phenotype despite sharing a common ancestral exposure. Interestingly, the female reproductive system appears particularly sensitive to glucocorticoid exposure during pregnancy, given that maternal preconceptional dexamethasone exposure does not impact the metabolic health of rat F1 offspring, albeit a slight augmentation of post-feeding blood glucose concentrations (Natividade da Silva et al., 2019). Stress-induced hyperglycemia is an important physiological stress response, and stress is a significant risk factor for diabetes. Thus, future studies could explore if these offspring are more susceptible to stress-induced metabolic syndromes (including HPA axis function) and if any behavioral deficits would develop as a result. There is precedence for the latter outcome, as it has been reported to occur in non-human primates (de Vries et al., 2007).
It would also be interesting to investigate if low-dose chronic CORT-treatment of adult female rodents prior to mating could also transgenerationally modify offspring behavior. Recent studies indicate that this is a feasible approach, at least in the C57Bl/6 N strain (Berger et al., 2019), which is more susceptible to chronic CORT-treatment compared to C57Bl/6 J mice (Sturm et al., 2015). However, female fertility and subsequent maternal care parameters would first have to be validated before questions regarding offspring health can be addressed.
5. Conclusion
Stress is a very productive research field within neuroendocrinology. For example, there are currently more than 40,000 research articles and in excess of 5000 review articles within PubMed that contain stress in their title and study either brain or hormones or both. Such volume of information requires some sort of introspective model within which to classify the observations and make coherent interpretations. There are several such models available. A partial list will contain models like allostasis, metaplasticity, multiple-hit models, or dose-response relationships (McEwen, 2000; Schmidt et al., 2013; Karst et al., 2010; Currier et al., 2009; Sapolsky, 2015; Schilling et al., 2013; Goldstein and McEwen, 2002; Ashokan et al., 2016). Another productive approach is to look for endophenotypes of stress-related human disorders and model these in the laboratory animals. A partial list of such a phenotype-based framework will contain efforts to model post-traumatic stress disorder, anxiety, or age-related memory decline (McEwen, 1999; Mitra and Sapolsky, 2010b; Cohen et al., 2006; Flandreau and Toth, 2018; Adamec et al., 2012; Lau et al., 2016). An alternative way of organizing the study of stress is to interpret stress biology as a series of nested levels of biological organization. Thus, stress affects neuroendocrinology at the level of molecular plasticity, for example, through transcriptomic changes brought about by occupied glucocorticoid receptors or epigenetic modifications of brain-derived growth factors. These changes create an emergent modulation of the neuronal structure through structural reconfiguration. Such structural changes induce an effect on reciprocally-connected circuits that control endocrine response or behavioral reactivity to subsequent stressors. All of these introspective models have been used with success, be it from perspectives of conceptual entities, known disorders, or biological organization. In this review, we approach stress biology from a different but complementary structure.
We take a temporal approach by first looking at stress plasticity at relatively shorter time windows after stress exposure. Then we look at how stress during one phase of life history can cause sustained changes in neurobiology and behavior through the subsequent stages of life. Subsequently, we integrate work that expands the temporal window further by showing that stress exposure in one generation can transcend the Weismann barrier and cause phenotypic changes in the subsequent generations. The relationship between a stressor and its resultant response is modified by the environmental history of the individual animals. In other words, the conditions of an earlier environment cause plastic changes in the behavior and associated neuroendocrine mediators later. The ‘later,’ or the time difference between the environmental modifier and the subsequent change, can encompass the adult phase of an individual animal or constitute effects of particularly sensitive critical phases of early development, or even reflect maternal or paternal effects on the offspring. Examples discussed until now shows an environmental modification of the stress response at these varying time-profiles.
Physiological and psychological stressors are potent environmental triggers for behavioral adaptation, promoting the emergence of phenotypic responses characteristic of resilient or vulnerable subsets of a population. Physiological stress is mediated through activation of the hypothalamic-pituitary-adrenal (HPA) axis and involves the discharge of catecholamines, serotonin, and other neurotransmitters (Hanley and Van de Kar, 2003; Pacak et al., 1995; Mora et al., 2012). Glucocorticoids released during a stressful event directly regulate gene expression through the binding of cytosolic receptors that migrate to neuronal (and other cell types) nuclei where activation or suppression of gene transcription takes place (Srinivasan et al., 2013b). Stressors that are chronic (Bergström et al., 2007), occur with great intensity (Török et al., 2019), and/or take place early in development (Provençal and Binder, 2015) alter transcriptional regulation in neurons, producing behaviorally divergent traits. Epigenetic regulation of genes tied to stress neurocircuitries, like corticotropin-releasing factor (CRF) and vasopressin (AVP), is linked to stress-related phenotype expression (Zannas and West, 2014). Furthermore, it is plausible based on the available evidence that genetic predisposition to stress-related phenotype formation arises from specific variations of glucocorticoid- and mineralocorticoid-associated proteins (i.e., receptors and chaperone proteins), as well as CRF and its receptor (CRF1) in vulnerable populations (Ebner and Singewald, 2017). Orexin signaling within the basolateral amygdala buttresses the role of variation in the expression of signaling molecules in mediation behavioral inter-individual variation. Thus, changes in the social experience, in the form of social defeat, raises social withdrawal when measured later in time. Yet, not all animals show a comparable modification of social behavior through the previous defeat. The variation in this instance seems to be regulated by variation in expression of Orx1 (orexin receptor 1). Animals with more potent expression of Orx1 within amygdala show greater suppression of social preference. Such modifying role of Orx1 signaling appears to be causally linked to the behavioral adaptation because experimental inhibition of Orx1 signaling induces emotional resilience. Thus, historical experience in adulthood can modify stress susceptibility or stress resilience in an individual animal. Together, these ideas support the likelihood of gene-related modifications as influencers of neural signaling during stable stress states, with the variation in these factors contributing to phenotype development and psychopathologies (Summers et al., 2020).
The effects of early life provide another example of the phenotypic plasticity in the stress response. Phenotypic plasticity refers to the capacity of similar genotypes to give rise to different phenotypes as a function of environmental variability (Pigliucci et al., 2006). Such capacity allows individuals to use environmental cues in order to change the developmental trajectory and create a better fit between phenotypes and future environments (Garland and Kelly, 2006). In this context, the early environment is particularly important because, for many animals, early life represents a critical or sensitive period, a window of time when a change in environment has an outsized effect on later behavioral adaptation (Tau and Peterson, 2010). In many animals, the most crucial essence of the early environment is mediated by maternal interactions. This can be seen in the studies showing that variation in maternal care can induce variation in endocrine response of the offspring to the stressors presented in later adulthood (Liu et al., 1997), mediated by epigenetic regulation of glucocorticoid receptors in the hippocampus (Suderman et al., 2012). Studies of classical conditioning in rat pups are another example of how early life maternal environment can induce behavioral variability. In the absence of the mother, rat pups displayed odor avoidance when exposed to odor-shock conditioning along with amygdala activation. The same conditioning initiates a preference for the odor in the presence of the mother without amygdala activation. The variation in behavior due to maternal presence is regulated by modulating the corticosterone, which in turn helps in moderation of amygdala activity (Moriceau and Sullivan, 2006a; Moriceau and Sullivan, 2006b). Deprivation of maternal care, even for short periods of time, causes a sustained change in the response when offspring are exposed to the stressors during adulthood. In general, maternal deprivation potentiates later stress response. This is mediated, in part, by the long-lasting structural plasticity of principal neurons within the basolateral amygdala. Maternal deprivation causes dendritic expansion of neurons in this brain region. Such dendritic hypertrophy is important for greater anxiety after the stress. This is shown by a reduction in anxiety when dendritic complexity is experimentally reduced through gene therapy, and by increase in anxiety through action of glucocorticoids on these neurons. These examples support the idea that the road to behavior in adult life passes through the experiences in early life, as well as influence of immediate environment on behavior. This is true with respect to sensitivity to stress and it is equally true when resilience to the stress is considered. Together these influences explain often-observed robust inter-individual variability.
While environment during adulthood and a more distant environment in early-life have been long accepted to be modifiers of individual phenotypes, it is only relatively recently that the role of environment has been shown to exert change in the stress reactivity of the next filial generation. As discussed earlier, the maternal environment in early life can have a large influence on stress-related phenotypes during adulthood. The effect of the mother as the environment extends back to prenatal life, whereby stress during pregnancy reprograms the brain, physiology, and behavior of the offspring. Yet, the window of the parental environmental effects can include the pre-conception environment as well. In the context of animal models, this can be seen in both pre-conception chronic unpredictable mild stress and social defeat stress. Parental treatment with glucocorticoids captures some but not all features of such transgenerational behavioral adaptation. Thus, stress affects not just the individual but hold the capacity to have long-term latent effects on future generations.
There are several human examples of transgenerational transmission where the health status of adults is linked to ancestral history of ill-health. For example, the Swedish famine studies elegantly demonstrated a relationship between ancestral food supply during the prepubescent phase and the lifespan of their grandchildren (Bygren et al., 2014; Kaati et al., 2002; Pembrey et al., 2006). In a similar manner, parental exposure to traumatic or chronic stress can lead to intergenerational adaptations of the stress response, as observed in the mental and physical health of children whose parents suffer from conflict-related post-traumatic stress disorder (PTSD) (Dashorst et al., 2019; Pierce et al., 2019). Cohort studies of Holocaust survivors have revealed the complex relationship between parental trauma and the emergence of psychological issues in their adult children (Yehuda et al., 2008; Yehuda et al., 2001; Yehuda et al., 1998). Besides the observed behavioral pathologies, parental Holocaust exposure is associated with cortisol dysregulation (suppression of basal concentrations) and metabolic disorders in adult children (Flory et al., 2011; Yehuda et al., 2000; Yehuda et al., 2007). The underlying molecular pathology is attributed to altered DNA methylation patterns of key stress-regulatory genes, namely GR and FKBP5 (Yehuda et al., 2016; Yehuda et al., 2014). Studies of other conflict-exposed groups have reported similar correlations of paternal PTSD with an increased incidence of psychological disturbances in their children (Rosenheck, 1986; Vaage et al., 2011; Zerach and Solomon, 2016). However, it remains to be seen if there are also consistencies in the intergenerational physiological disturbances (e. g., HPA axis dysregulation) and epigenetic modifications (e.g., DNA methylation). Human epidemiological and animal studies collectively indicate that stressful experiences during pregnancy and the perinatal period affect the endocrine function and brain development, increases stress vulnerability, and risks the development of psychological disorders in subsequent generations (Babenko et al., 2015; Jiang et al., 2019; Zannas and West, 2014).
The varied examples discussed until now demonstrate that the environment is indeed the driving force for the inter-individual variability in stress vulnerability or stress resilience. The environmental influence becomes evident at shorter time windows of behavior within the adulthood of individuals, across stages of life history from early life to adult behavior, and across the generational boundaries. Such environment dependency has two sides. While adverse events in the environment can create long-term facilitation of stress vulnerability, it also follows that a change in the environment in the reverse direction can also create stress resilience. For example, exposure to sensorily rich environments in early life or in adulthood can moderate the effects of altering stress and renormalize the behavioral adaptation. Vulnerabilities caused by the environment can be rescued by the environment. As we further understand the modes and mechanisms of the interactions between the environment and stress-related phenotypes, it will slowly lead us to also understand the mechanisms of resilience and purposeful construction of an environment where individuals can operate in the stress-free milieu.
Acknowledgments
TYP is supported by a project grant from the National Health and Medical Research Council of Australia (APP1184465). JDWY and CHS were supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH104485, and a CBBRe Research Enhancement Pilot grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs or the United States Government. RM is supported by the Ministry of Education, Singapore (RG 144/17).
References
- Achua JK, et al. , 2014. Cross-talk between orexin/hypocretin and corticotropin releasing factor systems: a combined retrograde tracer immunohistochemical study. Soc. Neurosci. Abstr. 40, 78.06. [Google Scholar]
- Adamec R, et al. , 2012. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol. Behav. 105 (3), 628–638. [DOI] [PubMed] [Google Scholar]
- Adler NE, et al. , 1993. Socioeconomic inequalities in health: no easy solution. Jama 269 (24), 3140–3145. [PubMed] [Google Scholar]
- Albeck DS, et al. , 1997. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J. Neurosci. 17 (12), 4895–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves RL, et al. , 2019. Maternal separation effects on mother rodents’ behaviour: a systematic review. Neurosci. Biobehav. Rev. [DOI] [PubMed] [Google Scholar]
- Amico JA, et al. , 2008. Oxytocin knockout mice: a model for studying stress-related and ingestive behaviours. Prog. Brain Res. 170, 53–64. [DOI] [PubMed] [Google Scholar]
- Ammoun S, et al. , 2003. Distinct recognition of OX1 and OX2Receptors by orexin peptides. J. Pharmacol. Exp. Ther. 305 (2), 507–514. [DOI] [PubMed] [Google Scholar]
- An XL, Tai FD, 2014. AVP and Glu systems interact to regulate levels of anxiety in BALB/cJ mice. Dongwuxue Yanjiu 35 (4), 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoued HS, et al. , 2020. Proximate causes and consequences of intergenerational influences of salient sensory experience. Genes Brain Behav. 19 (4), e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenrodt E, Schnabel R, Schwarzberg H, 1998. Vasopressin administration modulates anxiety-related behavior in rats. Physiol. Behav. 64 (4), 543–547. [DOI] [PubMed] [Google Scholar]
- Arendt DH, et al. , 2014. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashokan A, et al. , 2018. Housing environment influences stress-related hippocampal substrates and depression-like behavior. Brain Res. 1683, 78–85. [DOI] [PubMed] [Google Scholar]
- Ashokan A, Sivasubramanian M, Mitra R, 2016. Seeding stress resilience through inoculation. Neural Plast. 2016, 4928081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio E, et al. , 2011. Amygdalar orexinergic–GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav. Brain Res. 218 (2), 288–295. [DOI] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GA, 2015. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 48, 70–91. [DOI] [PubMed] [Google Scholar]
- Badyaev AV, Oh KP, 2008. Environmental induction and phenotypic retention of adaptive maternal effects. BMC Evol. Biol. 8 (1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Meaney MJ, 2010. Epigenetics and the biological basis of gene×environment interactions. J. Am. Acad. Child Adolesc. Psychiatry 49 (8), 752–771. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Lucas M, Richter-Levin G, 2011. Emotional tagging—a simple hypothesis in a complex reality. Prog. Neurobiol. 94 (1), 64–76. [DOI] [PubMed] [Google Scholar]
- Berger S, et al. , 2019. Effect of chronic corticosterone treatment on depression-like behavior and sociability in female and male C57BL/6N mice. Cells 8 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A, et al. , 2007. Molecular pathways associated with stress resilience and drug resistance in the chronic mild stress rat model of depression—a gene expression study. J. Mol. Neurosci. 33 (2), 201–215. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM, 2010. Hypocretin/orexin in arousal and stress. Brain Res. 1314, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ, 2013. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci. Biobehav. Rev. 37 (8), 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonisch C, et al. , 2016. Dexamethasone treatment alters insulin, leptin, and adiponectin levels in male mice as observed in DIO but does not lead to alterations of metabolic phenotypes in the offspring. Mamm. Genome 27 (1–2), 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefil V, et al. , 2019. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun F, et al. , 2018. Sex-specific and lasting effects of a single course of antenatal betamethasone treatment on human placental 11beta-HSD2. Placenta 69, 9–19. [DOI] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB, 2009. Increased apoptosis during neonatal brain development underlies the adult behavioral deficits seen in mice lacking a functional paternally expressed gene 3 (Peg3). Dev. Neurobiol 69 (5), 314–325. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL, 2016. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41 (1), 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Chan JC, Bale TL, 2017. Sex-specific neurodevelopmental programming by placental insulin receptors on stress reactivity and sensorimotor gating. Biol. Psychiatry 82 (2), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, et al. , 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17 (10), 2042–2046. [DOI] [PubMed] [Google Scholar]
- Brunson KL, et al. , 2005a. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 25 (41), 9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, et al. , 2005b. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 25 (41), 9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygren LO, et al. , 2014. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, et al. , 2009a. Corticotropin releasing factor influences aggression and monoamines: modulation of attacks and retreats. Neuroscience 158 (2), 412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, et al. , 2009b. Corticotropin releasing factor influences aggression and monoamines: modulation of attacks and retreats. Neuroscience 158 (2), 412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier J, et al. , 2018. Investigation into the role of the germline epigenome in the transmission of glucocorticoid-programmed effects across generations. Genome Biol. 19 (1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso MJ, et al. , 2018. Adolescent social stress increases anxiety-like behavior and ethanol consumption in adult male and female C57BL/6J mice. Sci. Rep. 8 (1), 10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, et al. , 2006. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147 (6), 2909–2915. [DOI] [PubMed] [Google Scholar]
- Cluderay J, Harrison D, Hervieu G, 2002. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul. Pept. 104 (1), 131–144. [DOI] [PubMed] [Google Scholar]
- Cohen H, et al. , 2012. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 37 (2), 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, et al. , 2006. The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann. N. Y. Acad. Sci. 1071, 335–350. [DOI] [PubMed] [Google Scholar]
- Conrad CD, 2010. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 34 (5), 742–755. [DOI] [PubMed] [Google Scholar]
- Constantinof A, et al. , 2019a. Prenatal glucocorticoid exposure results in changes in gene transcription and DNA methylation in the female juvenile guinea pig hippocampus across three generations. Sci. Rep. 9 (1), 18211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinof A, et al. , 2019b. Antenatal glucocorticoid exposure results in sex-specific and transgenerational changes in prefrontal cortex gene transcription that relate to behavioural outcomes. Sci. Rep. 9 (1), 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AM, Walker DM, Nestler EJ, 2019. Paternal transgenerational epigenetic mechanisms mediating stress phenotypes of offspring. Eur. J. Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, Champagne FA, 2011. Epigenetics and the origins of paternal effects. Horm. Behav. 59 (3), 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JM, Jobe-Shields LE, Phipps S, 2009. Stressful life events and posttraumatic stress symptoms in children with cancer. J. Traumatic Stress 22 (1), 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer B, et al. , 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340 (6137), 1215–1217. [DOI] [PubMed] [Google Scholar]
- Dashorst P, et al. , 2019. Intergenerational consequences of the Holocaust on offspring mental health: a systematic review of associated factors and mechanisms. Eur. J. Psychotraumatol. 10 (1), 1654065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, et al. , 1986. Estradiol modulates density of putative’ oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology 44 (4), 415–421. [DOI] [PubMed] [Google Scholar]
- de Vries A, et al. , 2007. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J. Clin. Invest. 117 (4), 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ, 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17 (1), 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, et al. , 2011. Paternal transmission of stress-induced pathologies. Biol. Psychiatry 70 (5), 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR, 2005. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288 (1), R34–8. [DOI] [PubMed] [Google Scholar]
- Drake AJ, et al. , 2011. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics 6 (11), 1334–1343. [DOI] [PubMed] [Google Scholar]
- Du X, Pang TY, 2015. Is dysregulation of the HPA-Axis a core pathophysiology mediating Co-morbid depression in neurodegenerative diseases? Front. Psychiatry 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacret D, et al. , 2019. Orexin signaling during social defeat stress influences subsequent social interaction behaviour and recognition memory. Behav. Brain Res. 356, 444–452. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N, 2017. Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr. Opin. Behav. Sci. 14, 54–64. [Google Scholar]
- Enthoven L, et al. , 2008. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology 149 (12), 6366–6377. [DOI] [PubMed] [Google Scholar]
- Evans EA, et al. , 2020. Childhood adversity and mental health comorbidity in men and women with opioid use disorders. Addict. Behav. 102, 106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima M, Srivastav S, Mondal AC, 2017. Prenatal stress and depression associated neuronal development in neonates. Int. J. Dev. Neurosci. 60, 1–7. [DOI] [PubMed] [Google Scholar]
- Feldman R, 2012. Bio-behavioral synchrony: a model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting 12 (2–3), 154–164. [Google Scholar]
- Fish EW, et al. , 2004. Epigenetic programming of stress responses through variations in maternal care. Ann. N. Y. Acad. Sci. 1036, 167–180. [DOI] [PubMed] [Google Scholar]
- Flandreau EI, Toth M, 2018. Animal models of PTSD: a critical review. Curr. Top. Behav. Neurosci. 38, 47–68. [DOI] [PubMed] [Google Scholar]
- Flores Á, et al. , 2014. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology 39 (12), 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory JD, Bierer LM, Yehuda R, 2011. Maternal exposure to the holocaust and health complaints in offspring. Dis. Markers 30 (2–3), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G, 2014. Too much success for recent groundbreaking epigenetic experiments. Genetics 198 (2), 449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, et al. , 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286 (5442), 1155–1158. [DOI] [PubMed] [Google Scholar]
- Franke K, et al. , 2020. Effects of maternal stress and nutrient restriction during gestation on offspring neuroanatomy in humans. Neurosci. Biobehav. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch MG, et al. , 2018. Non-invasive biomarkers of fetal brain development reflecting prenatal stress: an integrative multi-scale multi-species perspective on data collection and analysis. Neurosci. Biobehav. Rev. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M, 1978. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J. Physiol. 278, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa TX, et al. , 2019. Oxytocin receptor DNA methylation and alterations of brain volumes in maltreated children. Neuropsychopharmacology 44 (12), 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Kim JH, 2014. Developmental rodent models of fear and anxiety: from neurobiology to pharmacology. Br. J. Pharmacol. 171 (20), 4556–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, et al. , 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17 (5), 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, et al. , 2018. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr., Kelly SA, 2006. Phenotypic plasticity and experimental evolution. J. Exp. Biol. 209 (Pt 12), 2344–2361. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, 2012. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol 46 (4), 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, 2007. Evolution of concepts of stress. Stress 10 (2), 109–120. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, McEwen B, 2002. Allostasis, homeostats, and the nature of stress. Stress 5 (1), 55–58. [DOI] [PubMed] [Google Scholar]
- Grafe LA, et al. , 2018. Reduced orexin system function contributes to resilience to repeated social stress. eNeuro p. ENEURO. 0273–17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holsboer F, 2012. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat. Rev. Drug Discov. 11 (6), 462–478. [DOI] [PubMed] [Google Scholar]
- Guerrini R, et al. , 2010. Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med. Res. Rev 30 (5), 751–777. [DOI] [PubMed] [Google Scholar]
- Hanley NS, Van de Kar L, 2003. Serotonin and the neuroendocrine regulation of the hypothalamic-pituitary-adrenal axis in health and disease. Vitam. Horm. 66, 190–257. [DOI] [PubMed] [Google Scholar]
- Hare BD, et al. , 2019. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun. 10 (1), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DF, et al. , 2012. Differential response of hippocampal subregions to stress and learning. PLoS One 7 (12), e53126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde A, Soh Yee P, Mitra R, 2017. Dendritic architecture of principal basolateral amygdala neurons changes congruently with endocrine response to stress. Int. J. Environ. Res. Public Health 14 (7), 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, 1997. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20 (2), 78–84. [DOI] [PubMed] [Google Scholar]
- Herman JP, et al. , 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 24 (3), 151–180. [DOI] [PubMed] [Google Scholar]
- Herman JP, et al. , 1989. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J. Neurosci. 9 (9), 3072–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, et al. , 2014. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol. Behav. 130, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, et al. , 2011. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152 (12), 4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges TE, et al. , 2017. Social instability stress in adolescent male rats reduces social interaction and social recognition performance and increases oxytocin receptor binding. Neuroscience 359, 172–182. [DOI] [PubMed] [Google Scholar]
- Hodges TE, Baumbach JL, McCormick CM, 2018. Predictors of social instability stress effects on social interaction and anxiety in adolescent male rats. Dev. Psychobiol. 60 (6), 651–663. [DOI] [PubMed] [Google Scholar]
- Hodges TE, et al. , 2019. Effects of oxytocin receptor antagonism on social function and corticosterone release after adolescent social instability in male rats. Horm. Behav. 116, 104579. [DOI] [PubMed] [Google Scholar]
- Huot RL, et al. , 2002. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 950 (1–2), 52–63. [DOI] [PubMed] [Google Scholar]
- Iob E, Lacey R, Steptoe A, 2019. The long-term association of adverse childhood experiences with C-reactive protein and hair cortisol: cumulative risk versus dimensions of adversity. Brain Behav. Immun. [DOI] [PubMed] [Google Scholar]
- Ionescu IA, et al. , 2012. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 37 (6), 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji MJ, et al. , 2019. Orexin prevents depressive-like behavior by promoting stress resilience. Mol. Psychiatry 24 (2), 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, et al. , 2019. Epigenetic modifications in stress response genes associated with childhood trauma. Front. Psychiatry 10, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, Edvinsson S, 2002. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 10 (11), 682–688. [DOI] [PubMed] [Google Scholar]
- Kan JM, Callaghan BL, Richardson R, 2016. A Mother’s Past Can Predict Her Offspring’s Future: Previous Maternal Separation Leads to the Early Emergence of Adult-like Fear Behavior in Subsequent Male Infant Rat Offspring. [DOI] [PubMed]
- Karatsoreos IN, et al. , 2010. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151 (5), 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi S, et al. , 2019. Orexin 1 receptors in the anterior cingulate and orbitofrontal cortex regulate cost and benefit decision-making. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 227–235. [DOI] [PubMed] [Google Scholar]
- Karst H, et al. , 2010. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. 107 (32), 14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, et al. , 2008. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet 147B (7), 1196–1204. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386 (6624), 493. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. , 2016. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19 (12), 1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe AS, Ashokan A, Mitra R, 2016. Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl. Psychiatry 6 (2), e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, et al. , 2005. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur. J. Neurosci. 21 (9), 2397–2405. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, et al. , 2017. Social stress models in rodents: towards enhanced validity. Neurobiol. Stress 6, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, et al. , 2016. Absence of neurogenic response following robust predator-induced stress response. Neuroscience 339, 276–286. [DOI] [PubMed] [Google Scholar]
- Lebedeva KA, Caruncho HJ, Kalynchuk LE, 2017. Cyclical corticosterone administration sensitizes depression-like behavior in rats. Neurosci. Lett. 650, 45–51. [DOI] [PubMed] [Google Scholar]
- Lee JH, et al. , 2007. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci. Res. 58 (1), 32–39. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. , 2002. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development 129 (8), 1807–1817. [DOI] [PubMed] [Google Scholar]
- Lee JY, et al. , 2017. Prenatal exposure to dexamethasone in the mouse induces sex-specific differences in placental gene expression. Dev. Growth Differ. 59 (6), 515–525. [DOI] [PubMed] [Google Scholar]
- Lehto K, et al. , 2019. Childhood adoption and mental health in adulthood: the role of gene-environment correlations and interactions in the UK Biobank. Biol. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Lindström J, 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14 (9), 343–348. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. , 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-Adrenal responses to stress. Science 277 (5332), 1659. [DOI] [PubMed] [Google Scholar]
- Loizzo S, et al. , 2010. Post-natal stress-induced endocrine and metabolic alterations in mice at adulthood involve different pro-opiomelanocortin-derived peptides. Peptides 31 (11), 2123–2129. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. , 2017. Sexually dimorphic changes of hypocretin (Orexin) in depression. EBioMedicine 18, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, et al. , 2009. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 55 (1), 248–256. [DOI] [PubMed] [Google Scholar]
- Lungwitz EA, et al. , 2012. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav. 107 (5), 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW, et al. , 1996. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am. J. Epidemiol. 144 (10), 934–942. [DOI] [PubMed] [Google Scholar]
- MacKinnon AL, et al. , 2019. The interaction between oxytocin receptor gene methylation and maternal behavior on children’s early theory of mind abilities. Dev. Psychopathol. 1–9. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME, 1976. Learned helplessness: theory and evidence. J. Exp. Psychol. Gen. 105 (1), 3. [Google Scholar]
- Mantella RC, et al. , 2004. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287 (6), R1494–504. [DOI] [PubMed] [Google Scholar]
- Marmot MG, et al. , 1978. Employment grade and coronary heart disease in British civil servants. J. Epidemiol. Commun. Health (1978) 32 (4), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, et al. , 1991. Health inequalities among British civil servants: the Whitehall II study. Lancet 337 (8754), 1387–1393. [DOI] [PubMed] [Google Scholar]
- Matsuzaki I, et al. , 2002. Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul. Pept. 104 (1), 119–123. [DOI] [PubMed] [Google Scholar]
- Maud C, et al. , 2018. The role of oxytocin receptor gene (OXTR) DNA methylation (DNAm) in human social and emotional functioning: a systematic narrative review. BMC Psychiatry 18 (1), 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunder RG, et al. , 2019. The prevalence and clinical correlates of adverse childhood experiences in a cross-sectional study of primary care patients with cardiometabolic disease or risk factors. BMC Cardiovasc. Disord. 19 (1), 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2000. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22 (2), 108. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1999. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22 (1), 105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2019. Hormones and behavior and the integration of brain-body science. Horm. Behav. 119, 104619. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, et al. , 2016. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry 80 (10), 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod GF, et al. , 2019. Childhood predictors of adult adiposity: findings from a longitudinal study. N. Z. Med. J. 132 (1507), 11–21. [PubMed] [Google Scholar]
- Melamed N, et al. , 2015. Comparison of genome-wide and gene-specific DNA methylation between ART and naturally conceived pregnancies. Epigenetics 10 (6), 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, 2019. Neuronal plasticity in the amygdala following predator odor exposure. Front. Behav. Neurosci. 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM, 2008. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. 105 (14), 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM, 2010a. Expression of chimeric estrogen-glucocorticoid-receptor in the amygdala reduces anxiety. Brain Res. 1342, 33–38. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM, 2010b. Gene therapy in rodent amygdala against fear disorders. Expert Opin. Biol. Ther. 10 (9), 1289–1303. [DOI] [PubMed] [Google Scholar]
- Mitra R, Ferguson D, Sapolsky RM, 2009a. Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol. Psychiatry 66 (7), 686–690. [DOI] [PubMed] [Google Scholar]
- Mitra R, Adamec R, Sapolsky R, 2009b. Resilience against predator stress and dendritic morphology of amygdala neurons. Behav. Brain Res. 205 (2), 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisiadis VG, Matthews SG, 2014a. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 10 (7), 403–411. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, Matthews SG, 2014b. Glucocorticoids and fetal programming part 1: outcomes. Nat. Rev. Endocrinol. 10 (7), 391–402. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, et al. , 2017. Prenatal glucocorticoid exposure modifies endocrine function and behaviour for 3 generations following maternal and paternal transmission. Sci. Rep. 7 (1), 11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, et al. , 2012. Stress, neurotransmitters, corticosterone and body–brain integration. Brain Res. 1476, 71–85. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM, 2006a. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 9 (8), 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM, 2006b. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 9 (8), 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, et al. , 2006. Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 26 (25), 6737–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW, 1998. Maternal Effects as Adaptations. Oxford University Press. [Google Scholar]
- Murgatroyd C, 2014. Epigenetic programming of neuroendocrine systems during early life. Exp. Physiol. 99 (1), 62–65. [DOI] [PubMed] [Google Scholar]
- Natividade da Silva F, et al. , 2019. Impact of glucocorticoid treatment before pregnancy on glucose homeostasis of offspring exposed to glucocorticoid in adult life. Life Sci. 237, 116913. [DOI] [PubMed] [Google Scholar]
- Nocjar C, et al. , 2012. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 218, 138–153. [DOI] [PubMed] [Google Scholar]
- Oh DL, et al. , 2018. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 18 (1), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, et al. , 2017. Relationship between orexin A and childhood maltreatment in female patients with depression and anxiety. Soc. Neurosci. 12 (3), 330–336. [DOI] [PubMed] [Google Scholar]
- Pacak K, et al. , 1995. Effects of various stressors on in vivo norepinephrine release in the hypothalamic paraventricular nucleus and on the pituitary-adrenocortical axis. Ann. N. Y. Acad. Sci. 771 (1), 115–130. [DOI] [PubMed] [Google Scholar]
- Pacteau C, Einon D, Sinden J, 1989. Early rearing environment and dorsal hippocampal ibotenic acid lesions: long-term influences on spatial learning and alternation in the rat. Behav. Brain Res. 34 (1–2), 79–96. [DOI] [PubMed] [Google Scholar]
- Pang TYC, et al. , 2017. Transgenerational paternal transmission of acquired traits: stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr. Opin. Behav. Sci. 14, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, et al. , 2019. Rodent models of social stress and neuronal plasticity: relevance to depressive-like disorders. Behav. Brain Res. 369, 111900. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, et al. , 2006. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14 (2), 159–166. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, et al. , 2019. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A-K, Räikkönen K, 2012. The lifespan consequences of early life stress. Physiol. Behav. 106 (5), 722–727. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Matthews SG, Szyf M, 2014. Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring. Biol. Reprod. 90 (2), 43. [DOI] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ, 2016. Neuroinflammation regulates cognitive impairment in socially defeated mice. Trends Neurosci. 39 (6), 353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, et al. , 2019. Effects of parental mental illness on children’s physical health: systematic review and meta-analysis. Br. J. Psychiatry 1–10. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ, Schlichting CD, 2006. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209 (Pt 12), 2362–2367. [DOI] [PubMed] [Google Scholar]
- Pinto V, et al. , 2015. Differential impact of chronic stress along the hippocampal dorsal-ventral axis. Brain Struct. Funct. 220 (2), 1205–1212. [DOI] [PubMed] [Google Scholar]
- Pittet F, et al. , 2017. Chronic social instability in adult female rats alters social behavior, maternal aggression and offspring development. Dev. Psychobiol. 59 (3), 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, et al. , 2010. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci. 30 (6), 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M, et al. , 2017. Mother-child adrenocortical synchrony; Moderation by dyadic relational behavior. Horm. Behav. 89, 167–175. [DOI] [PubMed] [Google Scholar]
- Provençal N, Binder EB, 2015. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp. Neurol. 268, 10–20. [DOI] [PubMed] [Google Scholar]
- Qiao H, et al. , 2016. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016, 8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, et al. , 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 3 (3), 238–244. [DOI] [PubMed] [Google Scholar]
- Rapee RM, et al. , 2019. Adolescent development and risk for the onset of social-emotional disorders: a review and conceptual model. Behav. Res. Ther. 123, 103501. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, 2010. Amygdala activity, fear, and anxiety: modulation by stress. Biol. Psychiatry 67 (12), 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov G, Ardi Z, Richter-Levin G, 2014. Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front. Behav. Neurosci. 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson O, Wexler BC, 1957. Pituitary degeneration and adrenal tissue hyperplasia in spawning Pacific salmon. Science 125 (3261), 1295–1296. [DOI] [PubMed] [Google Scholar]
- Robertson JM, et al. , 2015. Nuance and behavioral cogency: how the visible burrow system inspired the stress-alternatives model and conceptualization of the continuum of anxiety. Physiol. Behav. 146, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson E, et al. , 2020. The relationship of early-life adversity with adulthood weight and cardiometabolic health status in the 1946 national survey of health and development. Psychosom. Med. 82 (1), 82–89. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, et al. , 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33 (21), 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, et al. , 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. U. S. A. 112 (44), 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan PJ, Summers CH, 2011. Molecular signaling and translational significance of the corticotropin releasing factor system. Prog. Mol. Biol. Transl. Sci. 98, 235–292. [DOI] [PubMed] [Google Scholar]
- Ronan PJ, et al. , 2016. Topographical organization of perifornical orexin neurons; implications for stressw-induced disorders. Soc. Neurosci. Abstr. 42. [Google Scholar]
- Roozendaal B, et al. , 2003. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 100 (3), 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R, 1986. Impact of posttraumatic stress disorder of World War II on the next generation. J. Nerv. Ment. Dis. 174 (6), 319–327. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2014. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 15 (11), 719. [DOI] [PubMed] [Google Scholar]
- Sakurai T, et al. , 2005. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46 (2), 297–308. [DOI] [PubMed] [Google Scholar]
- Sakurai T, et al. , 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92 (4), 573–585. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N, 2010. The orexin system: roles in sleep/wake regulation. Ann. N. Y. Acad. Sci. 1200 (1), 149–161. [DOI] [PubMed] [Google Scholar]
- Samson WK, et al. , 2002. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul. Pept. 104 (1–3), 97–103. [DOI] [PubMed] [Google Scholar]
- Samson WK, et al. , 2010. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiol. 198 (3), 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Suchecki D, Meerlo P, 2014. Stress, arousal, and sleep, in Sleep, Neuronal Plasticity and Brain Function. Springer, pp. 379–410. [Google Scholar]
- Sapolsky RM, 2015. Stress and the brain: individual variability and the inverted-U. Nat. Neurosci. 18 (10), 1344. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Zola-Morgan S, Squire L, 1991. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J. Neurosci. 11 (12), 3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS, 1984. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. 81 (19), 6174–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling TM, et al. , 2013. For whom the bell (curve) tolls: cortisol rapidly affects memory retrieval by an inverted U-shaped dose–response relationship. Psychoneuroendocrinology 38 (9), 1565–1572. [DOI] [PubMed] [Google Scholar]
- Schmidt M, et al. , 2013. Stress-induced metaplasticity: from synapses to behavior. Neuroscience 250, 112–120. [DOI] [PubMed] [Google Scholar]
- Schoner J, et al. , 2017. Post-traumatic stress disorder and beyond: an overview of rodent stress models. J. Cell. Mol. Med. 21 (10), 2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, et al. , 2011. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav. Brain Res. 222 (2), 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RM, et al. , 2013. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc. Natl. Acad. Sci. 110 (50), 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Beagley G, 1975. Learned helplessness in the rat. J. Comp. Physiol. Psychol. 88 (2), 534. [DOI] [PubMed] [Google Scholar]
- Selye H, 1976. Stress without distress. Psychopathology of Human Adaptation. Springer, pp. 137–146. [Google Scholar]
- Short AK, et al. , 2016. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 6 (6), e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ, 2011. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36 (2), 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM, 2013. Stress as a common risk factor for obesity and addiction. Biol. Psychiatry 73 (9), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K, 2016. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur. J. Neurosci. 43 (2), 220–229. [DOI] [PubMed] [Google Scholar]
- Smith JP, et al. , 2014. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Front. Behav. Neurosci. 8 (121), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, et al. , 2016a. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology 63, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, et al. , 2016b. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Shariff M, Bartlett S, 2013a. The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 4 (68). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Shariff M, Bartlett SE, 2013b. The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 4, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld SA, et al. , 2017. Childhood adversity and midlife suicidal ideation. Psychol. Med. 47 (2), 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Brand M, 2012. Decision making under stress: a selective review. Neurosci. Biobehav. Rev. 36 (4), 1228–1248. [DOI] [PubMed] [Google Scholar]
- Staton CD, et al. , 2018. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology 143, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Henley J, Thun M, 2002. All-cause and cause-specific death rates by educational status for two million people in two American cancer society cohorts, 1959–1996. Am. J. Epidemiol. 156 (1), 11–21. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J, 1988. Handbook of life stress, cognition and health. In: Fisher S, Reason J (Eds.), Copyright 1988. John Wiley 8. Sons. [Google Scholar]
- Stevenson CW, et al. , 2009. Early life programming of fear conditioning and extinction in adult male rats. Behav. Brain Res. 205 (2), 505–510. [DOI] [PubMed] [Google Scholar]
- Sturm M, et al. , 2015. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav. 14 (3), 292–300. [DOI] [PubMed] [Google Scholar]
- Suderman M, et al. , 2012. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl. Acad. Sci. 109 (Supplement 2), 17266–17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, et al. , 2018. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation 137 (5), e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, et al. , 2020. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res. 1731, 146085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers TR, et al. , 2017. Learning and CRF-induced indecision during escape and submission in rainbow trout during socially aggressive interactions in the stress-alternatives model. Front. Neurosci. 11, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, et al. , 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 1044 (1), 116–121. [DOI] [PubMed] [Google Scholar]
- Szyf M, 2014. Lamarck revisited: epigenetic inheritance of ancestral odor fear conditioning. Nat. Neurosci. 17 (1), 2–4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, 2001. Role of CRF 1 and CRF 2 receptors in fear and anxiety. Neurosci. Biobehav. Rev. 25 (7–8), 627–636. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS, 2010. Normal development of brain circuits. Neuropsychopharmacology 35 (1), 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togher KL, et al. , 2018. Placental FKBP51 mediates a link between second trimester maternal anxiety and birthweight in female infants. Sci. Rep. 8 (1), 15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török B, et al. , 2019. Modelling posttraumatic stress disorders in animals. Prog. Neuropsychopharmacol. Biol. Psychiatry 90, 117–133. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A, 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16 (6), 317. [DOI] [PubMed] [Google Scholar]
- Tractenberg SG, et al. , 2016. An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 68, 489–503. [DOI] [PubMed] [Google Scholar]
- Trivedi P, et al. , 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438 (1–2), 71–75. [DOI] [PubMed] [Google Scholar]
- Tryon RC, 1940. Genetic differences in mazelearning ability in rats. Yearbook of the National Society for the Study of Education. [Google Scholar]
- Tschenett A, et al. , 2003. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 18 (1), 143–148. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T, 2013. Role of orexin in modulating arousal, feeding, and motivation. Front. Behav. Neurosci. 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, et al. , 2019. Childhood adversity and clinical and psychosocial outcomes in psychosis. Epidemiol. Psychiatr. Sci. 29, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, 2012. Glutamate inputs to the nucleus accumbens: does source matter? Neuron 76 (4), 671–673. [DOI] [PubMed] [Google Scholar]
- Vaage AB, et al. , 2011. Paternal predictors of the mental health of children of Vietnamese refugees. Child Adolesc. Psychiatry Ment. Health 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Goltz C, et al. , 2011. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm. Behav. 60 (5), 644–650. [DOI] [PubMed] [Google Scholar]
- Vyas A, et al. , 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22 (15), 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Chattarji S, 2003. Enhanced anxiety and hypertrophy in basolateral amygdala neurons following chronic stress in rats. Ann. N. Y. Acad. Sci. 985 (1), 554–555. [Google Scholar]
- Walker FR, et al. , 2017. In the search for integrative biomarker of resilience to psychological stress. Neurosci. Biobehav. Rev. 74, 310–320. [DOI] [PubMed] [Google Scholar]
- Wang B, et al. , 2017. The non-peptide vasopressin V1b receptor antagonist, SSR149415, ameliorates spermatogenesis function in a mouse model of chronic social defeat stress. J. Cell. Biochem. 118 (11), 3891–3898. [DOI] [PubMed] [Google Scholar]
- Weaver IC, et al. , 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7 (8), 847–854. [DOI] [PubMed] [Google Scholar]
- Weiss IC, et al. , 2011. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front. Behav. Neurosci. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, 2014. Epigenetics: a lingering smell? Nat. Rev. Neurosci. 15 (1), 1. [DOI] [PubMed] [Google Scholar]
- Wieczorek A, et al. , 2019. Sex-specific regulation of stress-induced fetal glucocorticoid surge by the mouse placenta. Am. J. Physiol. Endocrinol. Metab 317 (1), E109–E120. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L, 2005. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol. Neurobiol. 32 (3), 285–294. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, et al. , 2004. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 24 (50), 11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. , 2016. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell Metab. 23 (4), 735–743. [DOI] [PubMed] [Google Scholar]
- Yaeger JDW, et al. , 2020. Counterbalanced microcircuits for Orx1 and Orx2 regulation of stress reactivity. Med. Drug Discovery 100059, 1–20. [Google Scholar]
- Yaeger JDW, et al. , 2019. Stress responses are bidirectionally regulated through amygdalar orexin 1 and 2 receptors. Society for Neuroscience Abstracts. Chicago, IL. [Google Scholar]
- Yehuda R, et al. , 2008. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J. Psychiatr. Res. 42 (13), 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Bierer LM, 2001. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J. Psychiatr. Res. 35 (5), 261–270. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. , 1998. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am. J. Psychiatry 155 (9), 1163–1171. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. , 2000. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am. J. Psychiatry 157 (8), 1252–1259. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. , 2007. Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. Am. J. Psychiatry 164 (1), 163–166. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. , 2016. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol. Psychiatry 80 (5), 372–380. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. , 2014. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 171 (8), 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun S, et al. , 2017. Elevated paternal glucocorticoid exposure modifies memory retention in female offspring. Psychoneuroendocrinology 83, 9–18. [DOI] [PubMed] [Google Scholar]
- Yoshida K, et al. , 2006. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 494 (5), 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel J, et al. , 2019. Acute maternal separation potentiates the gene expression and corticosterone response induced by inflammation. Brain Behav. Immun. 77, 141–149. [DOI] [PubMed] [Google Scholar]
- Zannas AS, West AE, 2014. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 264, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerach G, Solomon Z, 2016. Indirect exposure to captivity details is not related to posttraumatic stress symptoms among the spouses and offspring of former prisoners of war. J. Trauma. Stress 29 (6), 530–536. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Neumann ID, 2016. Maternal separation facilitates extinction of social fear in adult male mice. Behav. Brain Res. 297, 323–328. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF, 2004. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin. Investig. Drugs 13 (7), 799–828. [DOI] [PubMed] [Google Scholar]


