Abstract
Polyclonal antibodies against the bacteriocin propionicin PLG-1 were produced in rabbits at high titer (256,000 to 512,000, as determined by indirect enzyme-linked immunosorbent assay [ELISA]). Anti-PLG-1 antiserum neutralized the antimicrobial activity of PLG-1 preparations in a well diffusion assay. Cross-reacting protein was detected using an indirect ELISA of the culture supernatant from a fed-batch fermentation of the producer strain Propionibacterium thoenii P127 within the first 24 h of incubation, but bacteriocin activity was not detected in the same culture until 217 h of incubation. Culture supernatants from 156 strains of classical dairy propionibacteria were tested by indirect ELISA at 5 and 12 days of incubation for production of cross-reacting protein and by well diffusion assay for bacteriocin activity. Cross-reacting protein was detected in 52 strains: all of the tested strains of P. thoenii, most of the strains of Propionibacterium jensenii, and a minority of the Propionibacterium acidipropionici and Propionibacterium freudenreichii strains. Of these 52 strains, only 4 strains of P. thoenii showed bacteriocin activity in a well diffusion assay. Eight bacteriocin-negative mutants of strain P127 were negative in both ELISA and well diffusion assays. Western blot analysis showed that three protein bands bound anti-PLG-1 antibodies in culture supernatants: a 9.1-kDa band that is assumed to be the PLG-1 monomer and 16.2- and 27.5-kDa bands that may be precursors, multimers, or complexes of PLG-1.
The best characterized bacteriocin from the dairy propionibacteria is propionicin PLG-1, which is produced by Propionibacterium thoenii P127 (14). This bacteriocin is moderately heat-stable, sensitive to proteolytic enzymes, and stable at pH 3 to 9 (15). It contains 99 amino acid residues, has a molecular mass of 9,328 Da, and seems unrelated to other bacteriocins from lactic acid bacteria based on a comparison of its N-terminal amino acid sequence to others in the SWISS-PROT data bank (21). Propionicin PLG-1 is rapidly bactericidal against other dairy propionibacteria and lactic acid bacteria (14, 16). Methods for PLG-1 production and purification have been optimized (10, 21).
The use of immunological methods in bacteriocin research has been limited. Recent attempts to produce bacteriocin-specific antibodies have had varied results (1, 3, 4, 12, 17, 18). The relatively low molecular mass (<5,000 Da) of many bacteriocins makes them poorly or nonimmunogenic (22); conjugation of small bacteriocins to carrier proteins can improve their immunogenicity. Immunological techniques based on immunoblotting and enzyme-linked immunosorbent assays (ELISA) can be useful in investigating details of bacteriocin production, structure, and function (17, 18). Production of bacteriocin-specific antibodies has enabled development of sensitive immunoassays for nisin (7, 22) and pediocins (1).
The objective of this work was to produce polyclonal antibodies against propionicin PLG-1 to enable immunoassay development. These assays were used for detection of PLG-1 and other cross-reacting proteins, which might be novel bacteriocins or other forms of PLG-1.
MATERIALS AND METHODS
Microorganisms and media.
All cultures were from the Iowa State University Department of Food Science and Human Nutrition culture collection. P. thoenii P127 was the PLG-1 producer strain. Bacteriocin-negative mutants of P127 obtained by nitrosoguanidine mutagenesis have been described previously (15). All propionibacteria were propagated in sodium lactate broth (NLB) at 32°C (14). Indicator strain Lactobacillus delbrueckii ATCC 4797 and other lactic acid bacteria were propagated in lactobacilli MRS broth (Difco Laboratories, Detroit, Mich.) as previously described (10). Other bacterial strains were propagated in tryptic soy broth (Difco) statically at 37°C. Working cultures were stored on the appropriate agar medium with 1.5% Bacto agar (Difco) added. Long-term storage was at −80°C in the appropriate medium with 20% glycerol added (10). Viable counts of propionibacteria were obtained on sodium lactate agar (NLA) after 5-day anaerobic incubation at 32°C (14).
Cultures tested for antibody cross-reactivity were grown statically at the appropriate temperature. Supernatant samples taken after 5 or 12 days of incubation were filter sterilized and stored at −20°C until required. The numbers of strains of each organism tested for cross-reactivity were as follows: P. thoenii, 8; Propionibacterium jensenii, 26; Propionibacterium acidipropionici, 19; Propionibacterium freudenreichii subsp. shermanii, 79; P. freudenreichii subsp. freudenreichii, 14; unclassified Propionibacterium strains, 10; bacteriocin-negative (bac−) mutants of P. thoenii P127, 8; Lactobacillus bulgaricus, 3; L. delbrueckii, 1; Lactobacillus casei, 1; Streptococcus cremoris, 1; Streptococcus diacetylactis, 1; Streptococcus lactis, 1; Streptococcus thermophilus, 1; Bacillus cereus, 1; Escherichia coli, 1; Pseudomonas aeruginosa, 1; and Staphylococcus aureus, 1.
Bacteriocin production and purification.
Strain P127 was grown in 14-day fed-batch fermentations in the Iowa State University Fermentation Facility. The cells were removed by centrifugation (8,000 × g; 30 min), and propionicin PLG-1 was harvested from the cell-free supernatant and purified using ammonium sulfate precipitation, ion exchange chromatography, and reverse-phase high-performance liquid chromatography as described previously (21). Purification was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of aliquots of the sample, followed by silver staining (21). The gel was overlaid with the indicator organism L. delbrueckii ATCC 4797 to confirm that the stained bands had biological activity, as described by Bhunia and Johnson (2). Purified PLG-1 batches were combined and stored at −80°C until required for immunizations.
Bacteriocin assay.
Bacteriocin activity was determined in an agar well diffusion assay (10, 21) using L. delbrueckii ATCC 4797 as an indicator organism. To test the ability of the polyclonal antiserum to neutralize bacteriocin activity, serial dilutions of bacteriocin were mixed with an equal volume of undiluted antiserum in each well prior to adding the overlay. Preimmune serum and sterile deionized water were mixed with bacteriocin in the control wells. All tests were run in duplicate.
Immunization and serum harvest.
All procedures were conducted at the Iowa State University Cell and Hybridoma Facility. Three New Zealand white rabbits were immunized both subcutaneously and intramuscularly with 1 ml of antigen solution containing approximately 200 μg of PLG-1, using either Freund's complete adjuvant for the initial immunization or Freund's incomplete adjuvant for subsequent booster immunizations at 4-week intervals. After titers had reached acceptable levels, one rabbit was chosen for antibody production and maintained for production bleeds (a 30-day cycle of immunization followed by three 50-ml bleeds at 10-day intervals). All blood samples were refrigerated for 24 h and centrifuged (450 × g; 10 min) at room temperature. The serum was then stored at −20°C.
Indirect ELISA.
An indirect ELISA based on the methods of Engvall and Perlmann (6) and similar to that described by Fan and Chu (8) was used for antigen detection. Antigen-containing samples (100 μl) were dispensed into duplicate wells of COSTAR polystyrene microtiter plates (Corning, Inc., Corning, N.Y.), which were then sealed in plastic bags and incubated at 4°C overnight. Wells were washed twice with a solution consisting of phosphate buffered saline (PBS), 1.9 mM NaH2PO4, 8.1 mM Na2HPO4, 154 mM NaCl (pH 7.4) and 0.05% (vol/vol) Tween 20 (Sigma, St. Louis, Mo.) (PBST), blocked with blocking buffer (PBST + 2% [wt/vol] casein) for 30 min at 37°C, and washed twice with PBST.
Primary antibody (100 μl of rabbit serum diluted 1:20,000 in PBS) was then added to the wells and incubated for 120 min at 37°C. Wells were washed three times with PBST before the secondary antibody (100 μl of goat anti-rabbit immunoglobulin G [IgG]-horseradish peroxidase conjugate [Sigma]; 1:5,000 dilution in PBS) was added. After 90 min of incubation at 37°C, wells were washed three times with PBST, and then o-phenylenediamine (Sigma) substrate solution at a concentration of 0.66 mg/ml plus 0.012% (vol/vol) H2O2 was added, and plates were incubated at room temperature for 10 min in the dark.
The reaction was stopped by addition of 0.5 M sulfuric acid (Fisher) and the absorbance (492 nm) of each well was read with a Bio Kinetics EL 340 microtiter plate reader (Bio Tek Instruments, Inc., Winooski, Vt.). Average absorbance due to nonspecific binding of secondary antibody was subtracted from the absorbance values of all samples. Purified PLG-1 solutions at concentrations of 12.5 and 6.25 μg/ml in PBS were used as standards. To allow comparison of values across different plates, all values were normalized to a standard value of 0.500 for the 6.25 μg of PLG-1 per ml control. Concentrations of PLG-1 greater than 0.08 μg/ml were detected.
The titer of the rabbit antiserum was determined by testing serum diluted in PBS against purified PLG-1 (2 μg/ml in PBS) using indirect ELISA. Titer was defined as the reciprocal of the highest dilution with an absorbance at least twice the average absorbance for all dilutions of preimmune serum. Rabbit IgG at concentrations greater than 0.23 ng/ml was detected.
SDS-PAGE and Western blot.
Gel electrophoresis was performed using the buffer system described by Laemmli (13) in 10 to 20% gradient polyacrylamide gels (mini-PROTEAN II ready gels; Bio-Rad Laboratories, Hercules, Calif.). Proteins were separated at 180 V for 45 min by using the Bio-Rad mini-PROTEAN II electrophoresis apparatus. Gels were fixed in 30% ethanol-10% glacial acetic acid and silver-stained according to the manufacturer's instructions (Bio-Rad).
For Western blotting (5), the separated proteins were transferred to a polyvinyl difluoride membrane (Immobilon-P; Millipore Corp., Bedford, Mass.) by using a Bio-Rad Trans-Blot electrotransfer apparatus according to the manufacturer's instructions. After blocking with Tris-buffered saline (TBS) (20 mM Tris base, 0.5 M NaCl [pH 7.5]) plus 5% (wt/vol) nonfat dry milk at room temperature for 1 h, the membrane was incubated, first with primary antibody (rabbit antiserum diluted 1:2,000 in TBS plus 1% nonfat dry milk plus 0.3% Tween 20) and then with secondary antibody (goat anti-rabbit IgG horseradish peroxidase conjugate diluted 1:2,000 in the same buffer). Each incubation was at room temperature for 1 h with gentle agitation. Bound antibodies were visualized with diaminobenzidine (Sigma).
Colony blot.
The protocol for the colony blot assay was modified from a procedure described by Noel et al. (20). Organisms were grown on NLA for 5 days at 32°C. A moist nitrocellulose membrane disk cut to fit the petri dish was pressed gently onto the colonies, and the dish was incubated for 45 min at room temperature. The disk was then removed and reacted first with primary antibody (rabbit antiserum diluted 1:2,000 in TTBS [Tris-buffered saline plus 0.05% Tween 20] plus 1.0% gelatin) for 2 h at room temperature and then with secondary antibody (goat anti-rabbit IgG horseradish peroxidase conjugate diluted 1:2,000 in TTBS plus 1.0% gelatin) for 90 min at room temperature. Antibodies bound to colonies were visualized with diaminobenzidine.
RESULTS AND DISCUSSION
Antibody production.
The titers of polyclonal antibodies against propionicin PLG-1 were at 256,000 to 512,000 in all three rabbits after the first booster immunization (34 days), and they remained at this level throughout the antiserum production cycle (152 days). Propionicin was large enough (9.3 kDa) to be immunogenic itself; conjugation with a carrier protein was not needed. Antiserum from a single rabbit was used for all of the experiments reported here.
Neutralization of bacteriocin activity.
Undiluted antiserum mixed with an equal volume of a PLG-1 preparation reduced bacteriocin activity by 98 to 100% in the well diffusion assay; preimmune serum had no effect on activity (Table 1). Partially purified bacteriocin samples contained much more total protein than did the purified propionicin; this may have interfered with antibody binding to PLG-1. These results suggested that specific antibodies against PLG-1 were present in the antiserum and that these antibodies interacted with the active bacteriocin molecules.
TABLE 1.
Inhibition of the bacteriocin activity of propionicin PLG-1 preparations by anti-PLG antiserum as measured in a well diffusion assay
| PLG-1 preparationa | Protein (μg/ml) | Activity units per milliliter when mixed with
|
Activity reduction by antiserum (%) | ||
|---|---|---|---|---|---|
| Water | Preimmune serum | Antiserum | |||
| A, partially purified | 8,800 | 1,280 | 1,280 | 20 | 98.4 |
| B, partially purified | 6,640 | 10,240 | 10,240 | 160 | 98.4 |
| C, purified | 50 | 100 | 100 | 0 | 100 |
Preparations A and B were obtained from two different large-scale fermentations and consisted of proteins precipitated with 75% saturated ammonium sulfate (21). Preparation C was a pooled sample from several fermentations, consisting of propionicin PLG-1 purified by sequential ammonium sulfate precipitation, ion-exchange chromatography, and reverse-phase high-performance liquid chromatography (21).
Propionicin PLG-1 detection in fermentation supernatant.
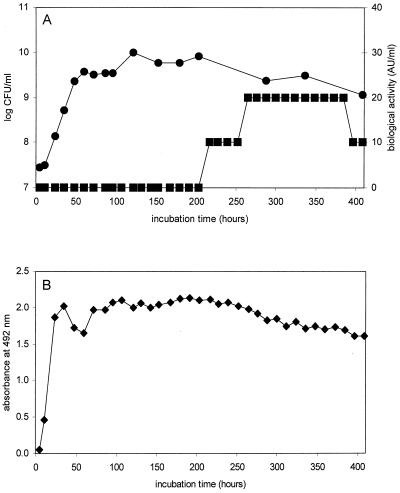
A fed-batch fermentation of P. thoenii P127 in NLB with a feeding of 0.6% sodium lactate every 12 h was monitored for viable counts, PLG-1 content (indirect ELISA), and bacteriocin activity (well diffusion assay). Viable counts and bacteriocin activity levels were similar to those previously reported by Paik and Glatz (21) (Fig. 1A). Cell numbers plateaued at approximately 60 h of incubation, but bacteriocin activity was not detected in the supernatant until 217 h, a pattern typical of secondary metabolite synthesis (15). However, supernatant samples from as early as 5 h of incubation gave a response in the indirect ELISA (Fig. 1B). The response was high and relatively constant for the duration of the fermentation. All components of NLB as well as 0.1 and 1.0% (wt/vol) solutions of propionic and acetic acid (typical concentrations produced by propionibacteria in fermentation) produced no response in the ELISA.
FIG. 1.
Growth and bacteriocin production by P. thoenii P127 in fed-batch fermentation. (A) ●, viable counts (log CFU/ml); ■, bacteriocin activity in well diffusion assay (activity units/ml). (B) ⧫, absorbance at 492 nm of supernatant samples that reacted with anti-PLG-1 antiserum in the indirect ELISA.
The difference in response pattern between the ELISA and the well diffusion assay suggested that anti-PLG-1 antibodies recognized peptides or proteins synthesized during logarithmic growth with no antimicrobial activity or with activity below the assay's detection limit. These might be precursors of the bacteriocin, complexes of bacteriocin with other molecules, or other cross-reacting proteins.
Immunoblotting of PLG-1 and fermentation supernatant.
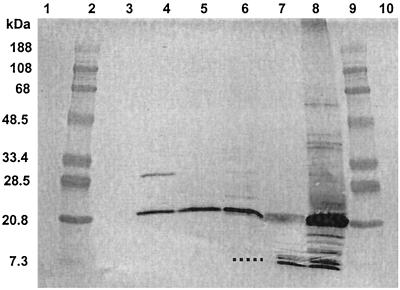
To determine what components in the fermentation supernatant reacted with anti-PLG-1 antibodies, the proteins in supernatants from two different fermentations as well as purified and partially purified preparations of PLG-1 were separated by SDS-PAGE and probed in a Western blot procedure using anti-PLG-1 antiserum (Fig. 2).
FIG. 2.
Western blot analysis of preparations of propionicin PLG-1 and fermentation supernatants probed with anti-PLG-1 antiserum. The lanes show the following: lanes 1, 3, and 10, empty; lanes 2 and 9, prestained molecular weight standards; lanes 4, 5, and 6, fermentation supernatants from 72- and 312-h (fermentation 1) and 206-h (fermentation 2) incubation, respectively; lane 7, purified PLG-1; and lane 8, partially purified PLG-1. The dotted line represents the position of a faint band that was not visible in the scanned image.
Two bands appeared in the purified PLG-1 sample: a doublet band with an apparent molecular mass of approximately 9.1 kDa (closely matching the previously reported [21] molecular mass of PLG-1 [9,328 Da]), and a diffuse band with a molecular mass of approximately 16.2 kDa. Others have noted that the hydrophobic nature of some bacteriocins can cause inconsistent protein migration in SDS-PAGE separations (19). It is possible that hydrophobic interactions may have caused spontaneous formation of PLG-1 dimers during purification, concentration, or storage; these dimers may be represented by the diffuse band.
Partially purified PLG-1, which contained proteins concentrated from a 336-h fermentation supernatant by ammonium sulfate precipitation, contained several protein bands that reacted with anti-PLG-1 antibodies. The two most intense bands had approximate molecular masses of 9.1 and 16.2 kDa, and less intense bands had approximate molecular masses of 10.7, 12.8, 40.5, and 72.1 kDa. It is possible that PLG-1 associated with either other small proteins or larger proteins or that it formed multimers during precipitation as has been suggested previously (15).
The fermentation supernatant samples all contained an intense 16.2-kDa band in the Western blot; this could represent a PLG-1 dimer, as seen in the purified bacteriocin preparation. The earliest fermentation sample (72 h of incubation in fermentation 1) also contained a reactive band at 27.5 kDa, but this band was absent in a later sample (312 h) from the same fermentation. It is possible that this larger band represented a PLG-1 trimer or an association with other proteins. A 206-h supernatant sample from fermentation 2 also contained faint bands at 27.5 and 9.2 kDa (the latter was visible to the eye but was not visible in the scanned image). Both the 206-h and 312-h fermentation samples contained measurable bacteriocin activity, but the 72-h sample did not.
Bacteriocins of other gram-positive bacteria have been reported to be synthesized as biologically inactive precursor peptides (prepeptides) that are later enzymatically modified into the active bacteriocin form (propeptide) by removal of a peptide leader sequence (11). Bhunia (1) observed cross-reaction of anti-pediocin monoclonal antibodies with larger proteins in supernatants of Pediococcus acidilactici cultures and assumed that these proteins were prepediocin molecules, because they were biologically inactive and disappeared with culture aging.
It is possible that PLG-1 may appear first during culture growth as a multimer; the active monomer may be released only after prolonged incubation. Upon concentration and storage, multimers may reform. The biological activity of the multimeric forms is yet to be determined precisely but seems low.
Alternatively, the 16.2- and 27.5-kDa proteins may be precursor forms of PLG-1 that contain a leader sequence. As the prepeptide is enzymatically cleaved, the smaller, biologically active PLG-1 molecule is released. The 16.2-kDa precursor may be copurified with PLG-1 and thus appear in purified bacteriocin preparations. Elution of reactive bands from gels followed by treatment with stronger dissociating agents and/or protein sequencing could help to clarify whether larger molecular weight forms are precursors or multimers.
No bands appeared in Western blots when samples were probed with preimmune serum or with secondary antibody, which suggests that the bands were not caused by nonspecific binding or by cross-reaction with normal rabbit or goat antibodies.
Detection of cross-reactive proteins from other bacteria.
A total of 156 strains of propionibacteria and representative cultures of other gram-positive and gram-negative bacteria were tested for bacteriocin activity in the well diffusion assay and for production of proteins that reacted with anti-PLG-1 antibodies in the indirect ELISA. Culture supernatants harvested after 5 and 12 days of incubation were used.
Only four strains (all strains of P. thoenii), P15, P20, P126, and P127, produced zones of inhibition in the well diffusion assay. Both the 5- and 12-day supernatants of strain P126 were active; this strain produces the bacteriocin jenseniin G (9). Only the 12-day supernatants of the other strains were active. Bacteriocin activity is typically seen at 12 days for P127, the PLG-1 producer strain (15). This was the first time that strains P15 and P20 were seen to produce bacteriocin-like activity. Supernatants of bac− mutants of P127 and of all other bacteria tested did not produce zones of inhibition.
A supernatant was considered to be reactive with anti-PLG-1 antiserum in the indirect ELISA if its absorbance was at least 10-fold the average nonspecific absorbance value of 0.010. Using this criterion, supernatants from 52 strains of propionibacteria were considered to be reactive at one or both of the testing times (Table 2).
TABLE 2.
Number of propionibacteria strains whose supernatants reacted with anti-PLG-1 antiserum in the indirect ELISA
| Species | Strains tested (n) | Reactive strains (n) |
|---|---|---|
| P. thoenii | 8 | 8 |
| P. jensenii | 26 | 19 |
| P. acidipropionici | 19 | 2 |
| P. freudenreichii subsp. shermanii | 79 | 16 |
| P. freudenreichii subsp. freudenreichii | 14 | 4 |
| Unclassified | 10 | 3 |
| Total | 156 | 52 |
The species of propionibacteria varied widely in the number of strains that were reactive in the indirect ELISA and in the strength of that reaction. All of the strains of P. thoenii were reactive, as were the majority of strains of P. jensenii. Reactions for P. thoenii strains were moderate, with absorbance values ranging from 0.16 to 1.63; the highest absorbance value seen with strain P127 was 0.88. The most intense reactions (absorbance values >2.00) were observed with supernatants from 11 P. jensenii strains. In contrast, most strains of P. acidipropionici and P. freudenreichii were nonreactive, and many of those identified as reactive had weak responses (absorbance values <0.20). No reactions were observed for the eight bac− mutants of P. thoenii P127 and for the other bacterial strains tested. The only exception was the 5-day supernatant from P. aeruginosa, whose absorbance value (0.13) was considered a marginally positive response.
These results suggest that protein(s) immunologically similar to propionicin PLG-1 are unique to the propionibacteria and perhaps to certain species of propionibacteria. It may be possible to use anti-PLG-1 antiserum in a simple screening test to tentatively identify P. thoenii and P. jensenii strains.
Intensity of response in the ELISA did not correlate with bacteriocin activity. The absorbance values of several strains with no detectable bacteriocin activity were much higher than those of the four strains that were biologically active. It is likely that the bacteriocin activity of strain P126 was due to the presence of jenseniin G, a different bacteriocin, but the ELISA response of this strain was very similar to that of strain P127. The nature of the cross-reacting material in supernatants that gave a positive response in the ELISA was studied further by Western blot analysis.
Western blot analysis of reactive supernatants.
The same 5- or 12-day supernatants from strains that had moderate to strong ELISA reactions also were subjected to SDS-PAGE and probed with anti-PLG-1 antibodies using the Western blot procedure. Some supernatants from ELISA-negative strains, including the bac− mutants of strain P127, also were tested, as was the marginally reactive supernatant from P. aeruginosa. Results are summarized in Table 3.
TABLE 3.
Strains producing 16.2 and 27.5-kDa proteins that reacted with anti-PLG-1 serum, as identified by Western blot analysis
| Organism | Strains (n)
|
|||
|---|---|---|---|---|
| Tested | 16.2-kDa band only | 27.5-kDa band only | 16.2- and 27.5-kDa bands | |
| P. thoenii | 8 | 6 | 0 | 2 |
| P. jensenii | 19 | 1 | 11 | 6 |
| P. acidipropionici | 1 | 1 | 0 | 0 |
| P. freudenreichii subsp. shermanii | 7 | 2 | 0 | 3 |
| P. freudenreichii subsp. freudenreichii | 2 | 1 | 0 | 0 |
| Unclassified propionibacteria | 3 | 0 | 1 | 2 |
| P. thoenii P127 bac− | 5 | 0 | 2 | 0 |
| P. aeruginosa | 1 | 0 | 0 | 0 |
None of the supernatants, even those with biological activity in the well diffusion assay, contained a reactive 9.1-kDa band, which is hypothesized to be the biologically active monomer form of PLG-1. A reactive 16.2-kDa band and/or a 27.5-kDa band, similar in size to reactive bands previously seen in P127 supernatant, were seen in most supernatants. The distribution of these bands varied among species. The 16.2-kDa band was seen in all P. thoenii strains and most of the tested strains of P. acidipropionici and P. freudenreichii; some of these strains also contained the 27.5-kDa band. In contrast, all but one P. jensenii strain contained the 27.5-kDa band, and six of these strains also contained the 16.2-kDa band. Just one strain contained only the 16.2-kDa band. The unclassified strains either contained both bands or only the 27.5-kDa band.
No reactive proteins were seen in supernatants from three ELISA-positive strains of P. freudenreichii, an ELISA-negative strain of P. jensenii tested as a negative control, P. aeruginosa, and three of five bac− mutants of P127. The other two bac− mutants contained very faint 27.5-kDa bands.
These results suggest that many strains of propionibacteria produce proteins similar to the proteins produced by strain P127 that bind anti-PLG-1 antibodies. If these are inactive precursors or multimers of the bacteriocin, strains without bacteriocin activity must lack the mechanism required to release the active form of the bacteriocin. The failure to find a reactive 9.1-kDa band, hypothesized to be the active form of PLG-1, in biologically active supernatants, particularly in strain P127, is puzzling. The protein might be present at low concentration, below the detection limit of the Western blot assay, or it may readily form complexes with other proteins that remain intact in SDS-PAGE. The absence or low intensity of reactive protein bands in bac− mutants of P127 supports the hypothesis that these bands represent precursor or multimer forms of PLG-1.
Colony blot analysis.
To test whether anti-PLG-1 antiserum could be used in a colony blot procedure as a relatively rapid, simple test for production of bacteriocin or cross-reacting protein, several colonies each of strain P127, two bac− mutants of P127, two ELISA-negative strains of P. freudenreichii and P. acidipropionici, and a strain of B. cereus were examined using the colony blot procedure after 5 days of growth on NLA.
All colonies of P. thoenii P127 gave very dark reactions, which indicated that high levels of reactive proteins were present. No reactions were seen with the colonies of P. freudenreichii, P. acidipropionici, and B. cereus. All of the colonies of one bac− mutant gave dark reactions, whereas the colonies of the other bac− mutant gave very faint reactions. Perhaps the bac− mutants produced levels of cross-reactive proteins that were undetectable in culture supernatants by the ELISA or Western blot procedures but were concentrated at the colony surface on solid medium and thus were visible in the colony blot. Alternatively, growth on solid medium may favor production of these proteins.
After colony growth and transfer of proteins to nitrocellulose membranes for probing with antiserum, the L. delbrueckii indicator organism was overlaid onto the plates to detect bacteriocin activity in the colonies. No inhibition of the indicator organism was seen for any colony. This was expected for all colonies except those of strain P127. It is possible that a sufficient quantity of active PLG-1 had not yet accumulated in the P127 colonies to give a zone of inhibition (the assay was done after 5 days of incubation, when biological activity typically only starts to appear) or that the blotting procedure removed much of the active form from the plate.
The colony blot assay was able to detect cross-reactive proteins; like the ELISA, it did not distinguish which or how many proteins were reactive. Several colonies could be grown on each plate and probed simultaneously on the same membrane. No specialized equipment was necessary to complete the assay. This procedure may be useful as a qualitative test to screen propionibacteria for bacteriocin analog production.
In conclusion, propionicin PLG-1 is antigenic in rabbits. Polyvalent anti-PLG-1 antiserum neutralizes biological activity of PLG-1 in the well diffusion assay and reacts not only with the bacteriocin itself but also with precursors, multimers, and/or complexes of the bacteriocin in indirect ELISA, Western blot, and colony blot procedures. Many strains of propionibacteria, especially P. thoenii and P. jensenii strains, produce cross-reacting proteins that do not seem to have antimicrobial activity. The nature of these proteins and their function(s) in the propionibacteria must still be elucidated.
ACKNOWLEDGMENTS
This research was supported by research grant US-2614-95C from BARD, the United States-Israel Binational Agricultural Research and Development Fund. Project 3475 is supported by Hatch Act and State of Iowa funds.
We are deeply indebted to Joan Cunnick, Department of Microbiology, Iowa State University, for her advice and for the use of her laboratory facilities to carry out much of this work.
Footnotes
Journal paper No. J-18987 of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, Project 3475.
REFERENCES
- 1.Bhunia A K. Monoclonal antibody-based enzyme immunoassay for pediocins of Pediococcus acidilactici. Appl Environ Microbiol. 1994;60:2692–2696. doi: 10.1128/aem.60.8.2692-2696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhunia A K, Johnson M G. A modified method to directly detect in SDS-PAGE the bacteriocin of Pediococcus acidilactici. Lett Appl Microbiol. 1992;15:5–7. [Google Scholar]
- 3.Bhunia A K, Johnson M G. Monoclonal antibody-colony immunoblot method specific for isolation of Pediococcus acidilactici from foods and correlation with pediocin (bacteriocin) production. Appl Environ Microbiol. 1992;58:2315–2320. doi: 10.1128/aem.58.7.2315-2320.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhunia A K, Johnson M C, Ray B, Belden E L. Antigenic property of pediocin AcH produced by Pediococcus acidilactici H. J Appl Bacteriol. 1990;69:211–215. doi: 10.1111/j.1365-2672.1990.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnette W N. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 6.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA): quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–879. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 7.Falahee M B, Adams M R, Dale J W, Morris B A. An immunoassay for nisin. Int J Food Sci Technol. 1990;25:590–595. [Google Scholar]
- 8.Fan T S L, Chu F. Indirect enzyme-linked immunosorbent assay for detection of aflatoxin B1 in corn and peanut butter. J Food Prot. 1984;47:263–266. doi: 10.4315/0362-028X-47.4.263. [DOI] [PubMed] [Google Scholar]
- 9.Grinstead D A, Barefoot S F. Jenseniin G, a heat-stable bacteriocin produced by Propionibacterium jensenii P126. Appl Environ Microbiol. 1992;58:215–220. doi: 10.1128/aem.58.1.215-220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh H-Y, Paik H-D, Glatz B A. Improvement of detection and production of propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii. J Food Prot. 1996;59:734–738. doi: 10.4315/0362-028X-59.7.734. [DOI] [PubMed] [Google Scholar]
- 11.Jack R W, Tagg J, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joerger M C, Klaenhammer T R. Cloning, expression, and sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. J Bacteriol. 1990;172:6339–6347. doi: 10.1128/jb.172.11.6339-6347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lyon W J, Glatz B A. Partial purification and characterization of a bacteriocin produced by Propionibacterium thoenii. Appl Environ Microbiol. 1991;57:701–706. doi: 10.1128/aem.57.3.701-706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon W J, Glatz B A. Isolation and purification of propionicin PLG-1, a bacteriocin produced by a strain of Propionibacterium thoenii. Appl Environ Microbiol. 1993;59:83–88. doi: 10.1128/aem.59.1.83-88.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyon W J, Sethi J K, Glatz B A. Inhibition of psychrotrophic organisms by propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii. J Dairy Sci. 1993;76:1506–1513. doi: 10.3168/jds.S0022-0302(93)77482-2. [DOI] [PubMed] [Google Scholar]
- 17.Martinez J M, Martinez M I, Suarez A M, Herranz C, Casaus P, Cinta L M, Rodriguez J M, Hernandez P E. Generation of polyclonal antibodies of predetermined specificity against pediocin PA-1. Appl Environ Microbiol. 1998;64:4536–4545. doi: 10.1128/aem.64.11.4536-4545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez M I, Rodriguez J M, Suarez A, Martinez J M, Azcona J I, Hernandez P E. Generation of polyclonal antibodies against a chemically synthesized N-terminal fragment of the bacteriocin pediocin PA-1. Lett Appl Microbiol. 1997;24:488–492. doi: 10.1046/j.1472-765x.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 19.Nettles C G, Barefoot S. Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid bacteria. J Food Prot. 1993;56:338–356. doi: 10.4315/0362-028X-56.4.338. [DOI] [PubMed] [Google Scholar]
- 20.Noel T, Nicolas J, Boulo V, Mialhe E, Roch P. Development of a colony-blot ELISA assay using monoclonal antibodies to identify Vibrio P1 responsible for “brown ring disease” in the clam Tapes philippinarum. Aquaculture. 1996;146:171–178. [Google Scholar]
- 21.Paik H-D, Glatz B A. Purification and partial amino acid sequence of propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii P127. Lait. 1995;75:367–377. [Google Scholar]
- 22.Suarez A M, Rodriguez J, Hernandez P, Azcona-Olivera J. Generation of polyclonal antibodies against nisin: immunization strategies and immunoassay development. Appl Environ Microbiol. 1996;62:2117–2121. doi: 10.1128/aem.62.6.2117-2121.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]