Abstract
High hydrostatic pressure is a new food preservation technology known for its capacity to inactivate spoilage and pathogenic microorganisms. That inactivation is usually assessed by the number of colonies growing on solid media after treatment. Under normal conditions the method does not permit recovery of damaged cells and may underestimate the number of cells that will remain viable and grow after a few days in high-pressure-processed foodstuffs. This study investigated the damage inflicted on Listeria monocytogenes cells treated by high pressure for 10 min at 400 MPa in pH 5.6 citrate buffer. Under these conditions, no cell growth occurred after 48 h on plate count agar. Scanning electron microscopy, light scattering by flow cytometry, and cell volume measurements were compared to evaluate the morphological changes in cells after pressurization. All these methods revealed that cellular morphology was not really affected. Esterase activity, as assessed either by enzymatic activity assays or by carboxy fluorescein diacetate fluorescence monitored by flow cytometry, was dramatically lowered, but not totally obliterated, under the effects of treatment. The measurement of propidium iodide uptake followed by flow cytometry demonstrated that membrane integrity was preserved in a small part of the population, although the membrane potential measured by analytical methods or evaluated by oxonol uptake was reduced from −86 to −5 mV. These results showed that such combined methods as fluorescent dyes monitored by flow cytometry and physiological activity measurements provide valuable indications of cellular viability.
In recent years, high-pressure technology has increasingly found new applications in biological systems. High-pressure treatment for the preservation of foodstuffs has been proposed as a viable alternative to conventional heat treatment. The main advantage of these processes is that they preserve the characteristics of the initial product (vitamins and flavor). Microorganisms show variable resistance to high pressure according to genus, although as a rule the reduction of microbial populations is directly linked to the hydrostatic pressure applied (19, 24, 31). Typically, the effectiveness of high pressure on microbial destruction is estimated from the reduction of the baseline population. The number of surviving microorganisms is determined by plating the bacterial suspension on solid media before and after treatment (19, 25). It is widely recognized that classic culture techniques may underestimate the numbers of truly viable bacteria, especially when cells have been damaged by physical treatments. Among the methods used for viable cell determination in such cases, fluorescent staining and detection by microscopy or flow cytometry are the most widely used. A wide range of fluorescent dyes are available which are aimed at specific cellular targets, like DNA, enzyme activities, internal pH, or the cytoplasmic membrane (29). Cell viability can be assessed using fluorescein derivatives, like carboxy fluorescein diacetate (cFDA) or carboxy-fluorescein succinimidyl ester. Membrane integrity and activity have also been described as good indicators of viability; these properties can be studied with fluorescent markers, like propidium iodide (PI) or oxonols. The advantages of using flow cytometry in microscopy lie in the possibility of analyzing cells according to a multiplicity of parameters. Most cytometers feature lateral and forward light scatterers and two or more fluorescent detectors. Therefore, cellular size, cytoplasm content, and physiological state can be simultaneously and swiftly documented and/or recorded for any given bacterial population. This technique has demonstrated its potential as a means to count viable cell (18) or to assess the physiological state of damaged cells (30). The purpose of this study was to characterize the morphological and physiological states of cells when high-pressure treatment was applied to microbial populations to induce a maximal reduction of 8 log10, as measured by plate count. Cellular morphology was studied by estimating the cellular volume measured by light-scattering flow cytometry and by the Rottenberg method (22). These results were compared with scanning electron microscopy (SEM) images. Flow cytometry was also used and compared with analytical methods to assess the membrane integrity, membrane potential, and enzymatic activities of Listeria monocytogenes cells treated with high pressure.
MATERIALS AND METHODS
Bacterial culture preparation.
Listeria monocytogenes Scott A (CIP 103575) was obtained from the Collection of the Pasteur Culture Collection (Paris, France). It was stored at −30°C in cryobeads (A.E.S., Combourg, France). The bacterial culture was obtained by inoculation of a cryobead in brain heart infusion, (Biokar, Beauvais, France) and incubation at 37°C for 24 h. This first culture was used to prepare a subculture inoculated with 0.1% (vol/vol) of the first culture in fresh brain heart infusion and incubated for 18 h at 37°C.
Suspension medium preparation and high-pressure treatment.
The pressurized samples were composed of 1 ml of this subculture diluted in 9 ml of citrate buffer (pH 5.6) (0.2 M Na2HPO4, 0.1 M C6H8O7; Merck, Darmstadt, Germany). Each sample was placed in a sterile mini-stomacher bag (A.E.S.). The bags were sealed and placed in a hydrostatic pressure vessel (3 liters) in a high pressure apparatus (Alstom, Nantes, France). High-pressure levels were generated using water pressurized by a hydrostatic pump. L. monocytogenes cells were pressurized from 200 to 400 MPa at 20°C for 10 min. For dead-cell control, identically prepared samples were heat treated (i.e., autoclaved) for 15 min at 121°C.
Survivor cell count.
The number of cells was determined before and after treatment by plating 0.1 ml of treated samples twice on plate count agar (PCA) (Biokar) and leaving it to incubate for 48 h at 37°C. For low viable cell counts, 10 ml of cell suspension was divided between two plates and poured with PCA to lower the detection threshold to 0.1 cells · ml−1. The reduction in population was computed as the difference in cell count before and after pressurization.
Flow cytometry.
One milliliter of each cell suspension was resuspended in phosphate-buffered saline after centrifugation (12,000 × g, 5 min) and diluted to obtain nearly 106 cells · ml−1 before staining. Cells were then stained with either 30 μl of cFDA (1 mmol · liter−1), 10 μl of PI (1 mg · ml−1; Sigma Chemical Co., St. Louis, Mo.), or 10 μl of DiBac4(3) [bis-(1,3-dibutylbarbituric acid)] trimethine oxonol, 10 μmol · liter−1. Molecular Probes, Eugene, Oreg.]. Before flow cytometric analysis, the cell suspensions were incubated in the dark for 15 min at 37°C for cFDA and DiBac4(3) or 2 min at 4°C for PI. As a DiBac4(3) staining control, cells were treated with gramicidin (5 or 50 μmol · liter−1, 10 min; Sigma).
A FACScan flow cytometer (Becton Dickinson Immunocytochemistry Systems, San Jose, Calif.) was used for single-cell light scattering and fluorescence intensity measurements. Samples were illuminated with an argon ion laser (488 nm), and fluorescence emission at 500 to 560 nm for cFDA and DiBac4(3) (FL1 detector) and at 650 nm for PI (FL3 detector) and forward light scatters were recorded simultaneously. The data collected were processed with Lysis II software. Flow cytometric detectors were calibrated with flow count fluorospheres (Becton Dickinson).
Protein concentrations in dense cell suspensions.
Cellular suspensions of L. monocytogenes were centrifuged for 30 min at 13,000 × g. Pellets were resuspended in 2 to 10 ml of the resulting supernatant in order to obtain dense cell suspensions with an average protein concentration of 1 to 10 mg · ml−1. The dense cell suspensions were used to measure the cell volumes. The protein content of these suspensions was determined by the Lowry method (11); with bovine serum albumin as the standard.
Intracellular volume measurement.
The intracellular volume was measured with radiolabeled probes according to Rottenberg's method (22). One hundred microliters (37 kBq) of a stock solution of [carboxyl-14C]dextran (370 kBq of [14C]dextran ml−1 dissolved in distilled water) and 20 μl (74 kBq) of 3H2O (7.4 MBq g− 1) were added to 2 ml of dense cell suspension (4 mg of protein · ml−1), mixed, and incubated for 10 min with gentle shaking. Three 300-μl samples were then layered in tubes containing 100 μl of perchloric acid (1 mol · liter−1) and 300 μl of silicone oil 508V70 (density [d] = 1.03), as previously described (15). The cells were then centrifuged at 13,000 × g for 15 min so that they passed through the oil layer and were packed in the bottom of the tube. Radioactivity was then measured in the supernatant on top of the oil and in the packed cells. Scintillation vials containing the double-labeled samples were counted on a Betamatic liquid scintillation counter from Kontron Instruments (Montigny-le-Bretonneux, France) with a manually optimized 14C, 3H double-labeling program.
Membrane potential measurement.
Membrane potentials were measured in cell suspensions (4 mg of cell protein · ml−1). Tetra[3H]phenylphosphonium bromide ([3H]TPP+) was used as a radioactive probe for membrane potential measurements as previously described (1). One hundred microliters (185 kBq) of a stock solution of [3H]TPP+ (1.4 TBq mmol−1, ethanolic solution) was added to 2 ml of dense-cell suspension, along with 100 μl of unlabeled TTP+ stock solution (final concentration, 10 mmol · liter−1) to prevent nonspecific binding of the radioactive probe to cells. The incubation procedure, supernatant and packed-cell harvesting, and radioactivity counts were performed as previously described for internal volume measurements (22). Cells (10 mg of protein · ml−1) were first harvested and washed once in 120 mmol of Tris-HCl buffer, pH 8.0, liter−1. EDTA-KOH, pH 7.0, was then added to a final concentration of 1 mmol · liter−1, and incubation was continued for 2 min under gentle agitation. Cells were then centrifuged at 10,000 × g for 10 min at room temperature. The cell pack was washed by resuspension and centrifugation in 5 mmol of Tris liter−1–5 mmol of 4-morpholineethanesulfonic acid liter−2–HCl buffer (pH 7.0) and then centrifuged again and resuspended in citrate buffer.
Determination of esterasic activity by spectrocolorimetry.
Sixty milliliters of L. monocytogenes cell suspension was centrifuged at 12,000 × g for 10 min at 4°C. The pellet was resuspended in 500 ml of phosphate buffer (0.1 mol · liter−1, pH 7.0). Two hundred milliliters of that suspension was added to 1 ml of para-nitrophenyl butyric acid. After 1 h at 30°C, the samples were centrifuged at 12,000 × g for 10 min at 4°C. The optical density of the samples was measured at 405 nm. The esterasic activity was estimated with a stock solution of para-nitrophenol and was converted to millimoles of para-nitrophenol produced per milligram of proteins per hour of incubation time.
SEM.
L. monocytogenes cell suspensions were fixed for at least 4 h in a 1.25% (vol/vol) glutaraldehyde solution in 0.1 mol liter of sodium cacodylate buffer liter−1. Aliquots containing approximately 106 cells were filtered through a 25-mm diameter, 0.25-μm porosity Anodisc (Whatman, Maidstone, United Kingdom). The filters were then rinsed five times for 10 min in the sodium cacodylate solution. Postfixation was performed for at least 4 h in a 1% OsO4 sodium cacodylate buffer. Filters were then rinsed five times in ultrapure water. The samples underwent further dehydration in a graded ethanol series (50, 70, 95, 100, and 100%). Soaking in isopentyl acetate was performed before critical-point drying in CO2 medium with an EMSCOPE CPD 750 apparatus as previously described (6). Filters were then attached to large SEM stubs with double-stick tape and coated with gold-palladium by cathodic spreading in a Polaron E5100 coater. Sample observation was done and microphotographs were taken on a JEOL JSM35CF scanning electron microscope operating at 15 kV.
RESULTS
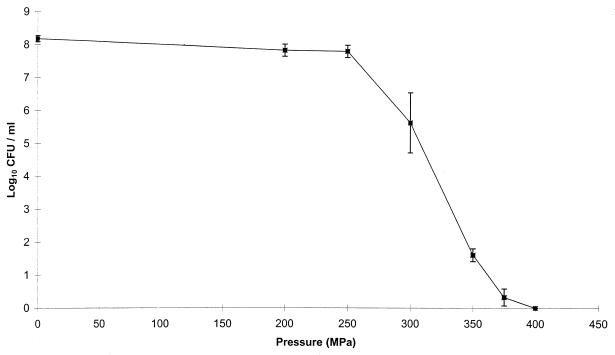
Inactivation of L. monocytogenes by high pressure at pH 5.6 was determined by microbial counts (Fig. 1). No significant inactivation was recorded below 300 MPa; the number of survivors then decreased dramatically when pressure increased from 300 to 400 MPa, when no colony could be detected in the entire treated medium after normal incubation and inactivation was considered complete. Raffalli et al. demonstrated that bacterial cells inactivated by such treatments were able to recover some growth potential after repair time (21). To characterize the deteriorations endured by inactivated L. monocytogenes cells, cells treated at 400 MPa (10 min) in pH 5,6 citrate buffer were checked for several physiological characteristics and compared with viable (nontreated) cells or dead (heat-treated) cells.
FIG. 1.
Effects of high-hydrostatic-pressure treatment on inactivation of L. monocytogenes in citrate buffer (pH 5.6). After pressurization (200 to 400 MPa for 10 min at 20°C), the survival of microorganisms was measured on PCA.
Measurement of cellular morphology.
Cellular morphology, especially cellular volume, was expected to be altered by high-pressure treatment. Three different methods were used in this study to assess changes in cellular morphology before and after treatment.
(i) Flow cytometry.
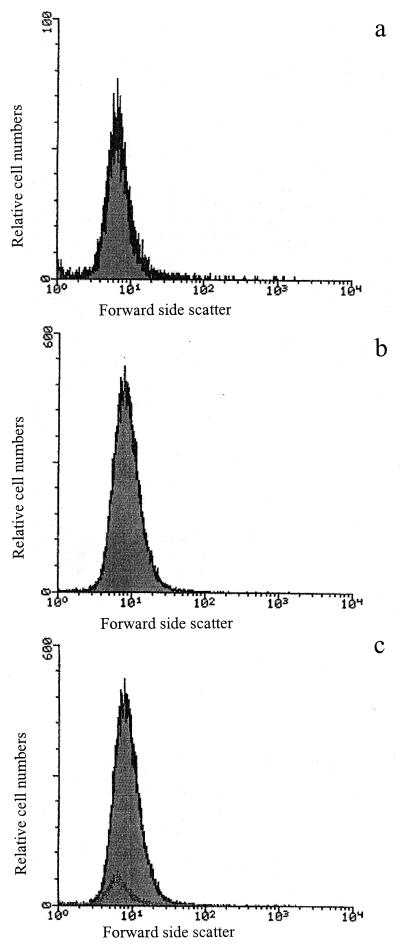
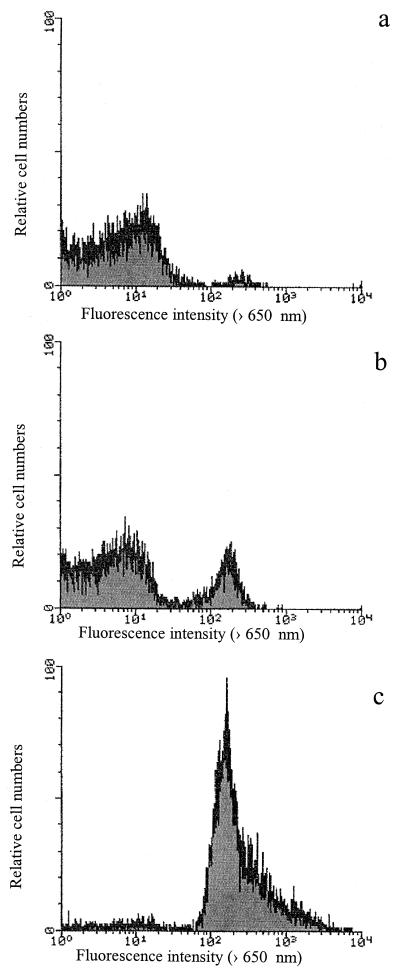
Flow cytometry was used to assess the modifications in bacterial size that could be correlated to the scattergrams of the intensity of forward-angle light scattering. Figure 2 shows the scattergrams recorded for the initial, inactivated, and heat-treated populations. The distributions of the populations according to forward scattered light diffusion were similar for the three conditions, suggesting that no major cellular size modifications occurred after high pressure and heat treatments.
FIG. 2.
Scattergrams of forward-angle light intensity recorded for untreated (a), pressure-treated (400 MPa, 10 min) (b), and heat-treated (121°C, 15 min) (c) cell populations.
(ii) Analytical methods.
The cellular volume modifications induced by the pressure treatment are as follows: no treatment, 2.70 ± 0.08 μl · mg of protein−1; 275 MPa, 2.05 ± 0.15 μl · mg of protein−1; 325 MPa, 2.48 ± 0.52 μl · mg of protein−1; and 400 MPa, 4.71 ± 0.02 μl mg of protein−1 (values are means ± standard deviations). The internal water volume of L. monocytogenes cell suspensions remained constant after high pressure treatment. Nevertheless, with the highest pressure treatment (400 MPa) we noticed a remarkable increase in cellular water volume, probably due to nonspecific absorption of radiolabeled probes by the cellular remains.
(iii) SEM observations.
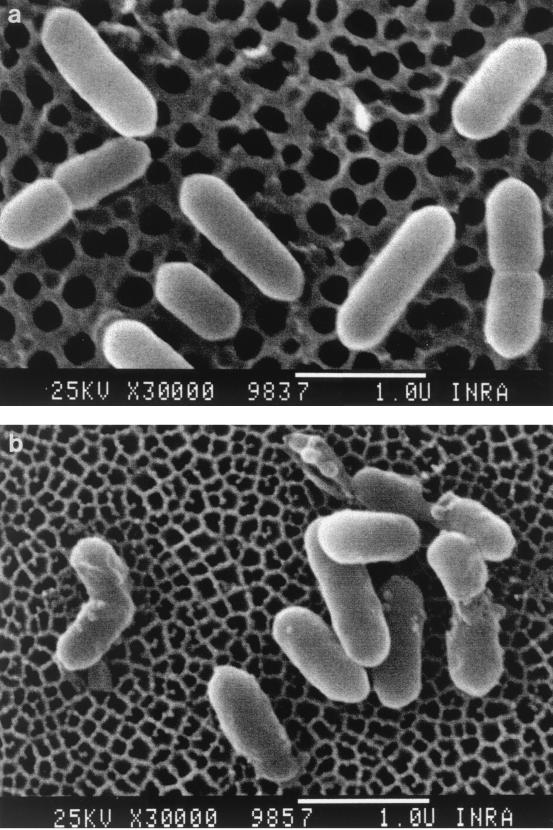
The cells of L. monocytogenes suspension were observed before and after treatment (400 MPa in citrate buffer [pH 5.6] for 10 min at 20°C). An average cellular size of 0.7 μm in length and 0.4 μm in diameter was commonly measured; some cells were still dividing. Before treatment, the cell surface was smooth (Fig. 3a). After pressure treatment, bud scars appeared on the surfaces of the cells. The number of buds was broadly proportional to the treatment pressure. In addition, as shown in Fig. 3b, the 400-MPa treatment could cause some cell disruptions. Nevertheless, the whole population seemed to keep its morphological characteristics.
FIG. 3.
Effects of high-hydrostatic-pressure treatment on L. monocytogenes cells. Scanning electron micrographs show untreated (a) and pressure-treated (400 MPa, 10 min) (b) cells in citrate buffer (pH 5.6). Bars, 1 μm. Magnification, ×30,000.
Physiological characteristics.
Viability is usually assessed by the ability of cells to grow and make colonies on suitable media. However, it has been shown that nonculturable cells can be considered viable on the basis of active metabolism, enzyme activities, or membrane integrity (2). Fluorescent dyes and flow cytometry detection were used in combination with analytical methods to characterize the viability of pressurized cells.
(i) Determination of esterasic activity by spectrocolorimetry.
The aim of determining esterasic activity by spectrocolorimetry was to identify a cellular parameter that could be correlated to cellular viability. Esterase activity is an indicator of metabolic activity, and comparing this activity before and after pressurization can help to assess the physiological state of pressurized cells.
In heat-treated cells 95.20% ± 0.9% of esterase activity was lost in comparison with the untreated population, whereas in pressurized cells (400 MPa) it decreased only by 69.05% ± 3.09%. However, because the enzymatic activity was measured in the supernatant, it was not possible to see whether esterase activity was lowered in the entire population after high-pressure treatment or if it was the mean value of maximal activity in viable cells and no activity in dead cells. For that reason, we elected to use flow cytometry with cFDA to obtain more information on the distribution of esterase activity among the cellular population.
(ii) Flow cytometry.
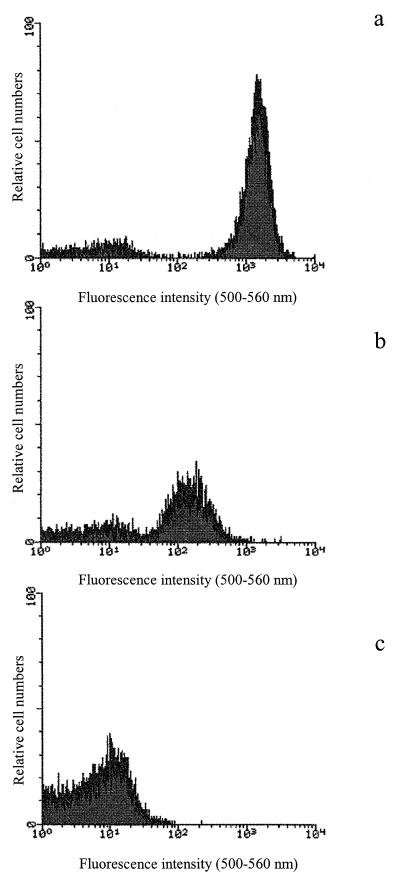
Fluorescein esters, like cFDA, are charged by intact cells and cleaved by intracellular esterases into fluorescent products. Figure 4 shows the cFDA staining of untreated, high-pressure-treated, and heat-treated cells. In the case of untreated cells, esterase activity led to intracellular cleavage of cFDA, as shown by the results of fluorescence emission in Fig. 4a. In contrast, only background fluorescence was recorded in heat treated cells after cFDA staining. High-pressure-treated cells exhibited lower fluorescence intensity than untreated cells, suggesting that some persistent esterase activity could be detected. Therefore, no differences could be detected among the population.
FIG. 4.
Fluorescence intensities of cFDA-stained untreated (a), pressure-treated (400 MPa, 10 min) (b), and heat-treated (121°C, 15 min) (c) cells.
(iii) Membrane integrity.
The bacterial membrane is assumed to be the primary target for several inactivation treatments applied in the food industry. PI is a stain commonly used for determination of membrane integrity. This dye is mutagenic, positively charged, and almost membrane-impermeative. It can enter cells mainly via the damaged membranes of injured or dead cells, and it can intercalate into DNA or RNA, resulting in red fluorescence emission. Figure 5 shows the fluorescence intensities of untreated, heat-treated, and high-pressure-treated cells after PI staining. Background fluorescence was recorded in intact cells (untreated cells), whereas heat-treated cells exhibited high levels of fluorescence intensity. The population of high-pressure-treated cells appeared to be divided into two parts: cells showing a fluorescence intensity matching that of heat-treated cells and cells with a lower fluorescence intensity. These results suggest that a small part of the high-pressure-treated population kept its membrane integrity.
FIG. 5.
Fluorescence intensities of PI-stained untreated (a), pressure-treated (400 MPa, 10 min) (b), and heat-treated (121°C, 15 min) (c) cells.
(iv) Membrane potential. (a) Flow cytometry.
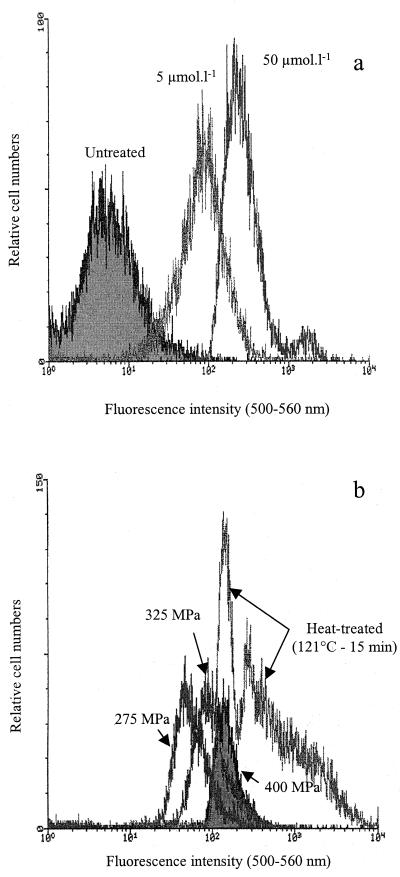
The negatively charged oxonol dyes, e.g., DiBac4(3), undergo potential-dependent distributions between the cytoplasmic membrane and the extracellular medium. DiBac4(3) enters depolarized cells, where it binds to lipid-rich intracellular components. Its fluorescence is enhanced upon accumulation. The efficacy of DiBac4(3) in showing membrane potential modifications was assessed using gramicidin, a depolarizing agent. Listeria cells were exposed to variable gramicidin concentrations before DiBac4(3) staining. Membrane depolarization, enhanced by gramicidin, leads to DiBac4(3) accumulation on the cell membrane, resulting in increased fluorescence intensity (Fig. 6a). Cells treated at 275, 325, and 400 MPa were stained by DiBac4(3) and analyzed by flow cytometry (Fig. 6b). The fluorescence of these populations increased with the intensity of treatment, suggesting that the cell membrane is submitted to depolarization after high-pressure treatment.
FIG. 6.
Fluorescence intensity recorded after staining with DiBac4(3). (a) L. monocytogenes populations left untreated or treated by gramicidin (5 μmol · liter−1 for 15 min) or (50 μmol · liter−1 for 15 min); (b) L. monocytogenes populations treated by high pressure (275, 325, or 400 MPa; 10 min) or heat-treated (121°C, 15 min).
(b) Analytical method.
To confirm these observations, the membrane potential of high-pressure-treated cells was measured by an analytical method using radioactive probes. The membrane potential of untreated cells in citrate buffer was −86 ± 11 mV. It decreased as the intensity of treatment increased to reach values of −41 ± 1, −34 ± 8, and −5 ± 2 mV in the populations treated at 275, 325, and 400 MPa, respectively. These results confirm those obtained by flow cytometry and the effect of high-pressure treatment on membrane depolarization.
DISCUSSION
High-pressure treatments have been proposed as an alternative to classic heat treatment to preserve foodstuffs. The development of high-hydrostatic-pressure technology depends primarily on its capacity to eradicate microorganisms in foodstuffs. In microbiology, the effectiveness of preservative treatments is usually assessed by the difference in bacterial cell counts before and after treatment. This method was also used for the inactivation of L. monocytogenes by high-pressure treatment (17, 20, 31). Nevertheless, it has been widely accepted that classic culture techniques may underestimate the number of truly viable bacterial cells. The aim of this study was to characterize the morphological and physiological states of the population after maximal inactivation (8 log10) using viability indicators other than the classic plating method. Flow cytometry was chosen for its capacity of multiparameter analysis and the possibility of using several fluorescent viability markers that are not commonly used in microscopy. This approach has been commonly used in several studies to assess the viability of microbial cells after starvation or exposure to antibiotics or disinfectant (7, 10, 14). In most cases, the viability of the cells was determined by using fluorescent dyes with different targets in the cells. In our study, the effectiveness of flow cytometric multiparameter analysis in characterizing the physiological state of cells after pressurization was validated by other analytical methods whenever possible.
The morphological state of high-pressure treated L. monocytogenes was studied using three methods. Flow cytometry forward-scatter measurement had already been used for bacterium size determination (4). Analysis of the histograms recorded for initial, pressurized, and heat-treated populations showed that pressure treatment did not induce any greater modification of cellular size. Measurements of intracellular volume (22) confirmed cellular size preservation except under the highest pressure treatment (400 MPa), which led to a notable increase in intracellular volume, probably due to nonspecific absorption of radiolabeled probes by the cellular remains. SEM revealed that pressure treatment induced the occurence of bud scars on the surfaces of the cells. These observations suggest that the cellular wall or membrane could be targets of high-pressure treatment. In addition, SEM examination showed that the 400-MPa treatment could induce some cell disruptions. The same was observed with Salmonella in other studies (13, 28). Whatever the method used, these results show that even if the pressure treatment leads to total inactivation of the population, individual cells retain their morphological characteristics. Since maximal inactivation should not be based on morphological damage alone, we investigated the physiological deterioration that could explain cellular inactivation.
Some authors have shown that L. monocytogenes intracellular enzymes could be affected by high-pressure treatments (26). The electrophoretic mobility of those enzymes was modified after treatment corresponding to some variations of their conformational forms. In some instances, the activity of those enzymes appeared to decrease as pressure increased.
Studying esterase properties revealed that cellular activity was modified after treatment. Spectrocolorimetric assessment of this activity showed a mean 69.05% decrease in activity in the overall population, thus revealing that esterase activity was altered by high-pressure treatment, although it has yet to be determined whether that decrease in esterase activity was the compound result of differently affected viable and dead cells or if each individual cell suffered the same reduction of its esterase activity. Intracellular cleavage of the fluorogenic substrate cFDA into fluorescein is also an indication of cellular esterase activity (27). It has been used successfully with flow cytometric monitoring for viability counts of L. monocytogenes cells (18) or for viability assessment of nonculturable cells from aquatic environments (32). In our experiment, the fluorescence intensity recorded with pressurized cells after cFDA staining was between the maximum intensity of unpressurized cells and the minimum intensity of dead cells. These results are in agreement with the esterase activity assays and suggest that the reduction of esterase activity involves the whole population. However, the activity recorded by cFDA staining corresponds to overall activity and can mask heterogeneity in enzyme sensitivity to pressure. Indeed, individual activities of enzymes with the same metabolic function could be differently affected by high-pressure treatments (26). The efflux of cFDA from cells could also explain the decrease in fluorescence intensity. It was described in an earlier study for cells with damaged membranes (3), and this study has shown that high-pressure-treated cells do exhibit membrane injuries.
Examining membrane integrity was a supplemental means to characterize the physiological status of inactivated cells. It has been suggested previously that the cell membrane could be a target for high pressure by disorganization among membrane phopholipids (12). Staining with the nucleic acid probe PI has been widely used for membrane integrity determination, either alone or combined with other dyes (9, 10, 16). It is also being used as a viability indicator with the LIVE/DEAD BackLight viability kit (Molecular Probes) in combination with SYTO9, another nucleic acid dye which stains all cells regardless of their membrane integrity. Our experiments with PI show that after high-pressure treatment, bacterial cells were divided into two different populations with regard to membrane integrity. A small proportion of cells did not take up PI, suggesting that their membranes were not seriously damaged. Another part of the cell population appeared to have been stained by PI, like dead cells. Examining the cells that were still detectable on solid media after low-pressure treatment revealed that they were not stained by PI (data not shown). Using PI staining followed by flow cytometry revealed that membrane integrity was not homogeneous across the cellular population. Although high-hydrostatic-pressure treatments are considered to be isostatic (i.e., equal pressure at every point of the treatment vessel), this study showed that cellular damage is not equally withstood by all the cells, suggesting that more resistant or less damaged cells are present in the pressurized cell population. Furthermore, membrane potential results raise the same questions. The analytical techniques applied to the overall population revealed a decrease in membrane potential. Therefore, the flow cytometry study with an oxonol indicates that even if the membrane potential of most pressurized cells is similar to that of depolarized or dead cells, some of the pressurized cells do not share these characteristics. These cells have a membrane potential halfway between those of untreated and pressurized cells. This intermediate physiological state could be reversible if the residual metabolic activity permitted it.
In conclusion, this study made it possible to investigate various cellular targets for high-pressure treatment on bacterial cells. It has shown that pressurization does not significantly affect cellular volume. Nevertheless, some physical damage was inflicted, as reflected by the occurrence of buds on the cell surface, and membrane integrity was lost in the greater part of the cell population. This study also investigated modifications of the physiological characteristics of cells. Most of the cells suffered a decrease in metabolic activity and membrane potential. The effects of high pressure on cell components such as nucleic acids (5) or ribosomes (23) have been studied, but our data represent the first observations of the effects of high pressure on bacterial cell physiology.
Using flow cytometry in combination with analytical methods constitutes an original approach to investigating the physiological state of damaged cells. We showed that the fluorescent dyes used in this study ensured clear discrimination between dead and living cells. However, in most cases, pressurized cells did not behave like dead or live cells, and it was necessary to supplement flow cytometry results with quantitative physiological parameters to determine the physiological state of the damaged cell population. Accumulation of such parameters has been used previously to assess the viability of bacterial cells after the action of disinfecting agents (8). Using all of these methods, we have shown that even if the population appears totally inactivated by treatment and no culture growth is recorded, variable degrees of injury can be inflicted on the cells by high pressure treatment. This heterogeneity of the treated cell population suggests that reversible damage may occur and cellular repair under favorable conditions should not be ruled out. Those conditions are currently being investigated in our laboratory and will be a determining factor in further high-pressure applications in the food preservation industry.
ACKNOWLEDGMENTS
This work was supported by grants from the INRA (AIP Listeria No. 97/P00180) and from the Région des Pays de la Loire.
English proofreading and rewriting were done by Philip Rousseau-Cunningham.
REFERENCES
- 1.Bakker E P, Rottenberg H, Caplan S R. An estimation of light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta. 1976;440:557–572. doi: 10.1016/0005-2728(76)90042-6. [DOI] [PubMed] [Google Scholar]
- 2.Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–137. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- 3.Breeuwer P, Abee T. Assessment of viability of microorganisms employing fluorescent techniques. Int J Food Microbiol. 2000;55:193–200. doi: 10.1016/s0168-1605(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 4.Davey H M, Davey C L, Kell D B. On the determination of the size of microbial cells using flow cytometry. In: Lloyd D, editor. Flow cytometry in microbiology. New York, N.Y: Springer-Verlag; 1993. pp. 49–65. [Google Scholar]
- 5.Heden C G, Lindhal T, Toplin I. The stability of deoxyribonucleic acid solutions under high pressure. Acta Chem Scand. 1964;18:1150–1158. [Google Scholar]
- 6.Kobs M. A procedure for preparing microscopic organisms for SEM. Eur Microsc Anal. 1995;35:25. [Google Scholar]
- 7.Langsrud S, Sundheim G. Flow cytometry for rapid assessment of viability after exposure to a quaternary ammonium compound. J Appl Bacteriol. 1996;81:411–418. doi: 10.1111/j.1365-2672.1996.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 8.Lisle J T, Pyle B H, Feters G A. The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia coli O157:H7. Lett Appl Microbiol. 1999;29:42–47. doi: 10.1046/j.1365-2672.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Amoros R, Castel S, Comas J, Vives-Rego J. Assessment of E. coli and Salmonella viability and starvation by confocal laser microscopy and flow cytometry using rhodamine 123, DiBAC4(3), propidium iodide, and CTC. Lett Appl Microbiol. 1997;15:125–132. doi: 10.1002/(sici)1097-0320(19971201)29:4<298::aid-cyto6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Amoros R, Comas J, Vives-Rego J. Flow cytometric assessment of Escherichia coli and Salmonella typhimurium starvation-survival in seawater using rhodamine 123, propidium iodide, and oxonol. Appl Microbiol Biotechnol. 1995;61:2521–2526. doi: 10.1128/aem.61.7.2521-2526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Macdonald A G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Phil Trans R Soc Lond. 1984;B304:47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- 13.Mackey B M, Forestière K, Isaacs N S, Stenning R, Brooker B. The effect of high hydrostatic pressure on Salmonella thompson and Listeria monocytogenes examined by electron microscopy. Lett Appl Microbiol. 1994;19:429–432. [Google Scholar]
- 14.Magarinos B, Romalde J L, Cid A, Toranzo A E. Viability of starved Pasteurella piscicida in seawater monitored by flow cytometry and the effect of antibiotics on its resuscitation. Lett Appl Microbiol. 1997;24:122–126. [Google Scholar]
- 15.Miguelez E, Gilmour D J. Regulation of cell volume in the salt tolerant bacterium Halomonas elongata. Lett Appl Bacteriol. 1994;19:363–365. [Google Scholar]
- 16.Miller J S, Quarles J M. Flow cytometric identification of microorganisms by dual staining with FITC and PI. Cytometry. 1990;11:667–675. doi: 10.1002/cyto.990110603. [DOI] [PubMed] [Google Scholar]
- 17.Murano E A, Murano P S, Brennan R E, Shenoy K, Moreira R G. Application of high hydrostatic pressure to eliminate Listeria monocytogenes from fresh pork sausage. J Food Prot. 1999;62:480–483. doi: 10.4315/0362-028x-62.5.480. [DOI] [PubMed] [Google Scholar]
- 18.Nexmann Jacobsen C, Rasmussen J, Jakobsen M. Viability staining and flow cytometric detection of Listeria monocytogenes. J Microbiol Methods. 1997;28:35–43. [Google Scholar]
- 19.Patterson M F, Quinn M, Simpson R, Gilmour A. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J Food Prot. 1995;58:524–529. doi: 10.4315/0362-028X-58.5.524. [DOI] [PubMed] [Google Scholar]
- 20.Prestamo G, Sanz P D, Fonberg-Broczek M, Arroyo G. High pressure response of fruit jams contaminated with Listeria monocytogenes. Lett Appl Microbiol. 1999;28:313–316. doi: 10.1046/j.1365-2672.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 21.Raffalli J, Rosec J P, Carlez A, Dumay E, Richard N, Cheftel J C. Stress et inactivation par haute pression de Listeria innocua introduites dans une crème laitière. Sci Aliments. 1994;14:349–358. [Google Scholar]
- 22.Rottenberg H. The measurement of membrane potential and DpH in cells, organelles and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 23.Schultz E, Ludmann H-D, Jaenicke R. High pressure equilibrium studies on the dissociation-association of Escherichia coli ribosomes. FEMS Microbiol Lett. 1976;64:40–43. doi: 10.1016/0014-5793(76)80243-8. [DOI] [PubMed] [Google Scholar]
- 24.Shigehisa T, Ohmori T, Saito A, Taji S, Hayashi R. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat and meat products. Int J Food Microbiol. 1991;12:207–216. doi: 10.1016/0168-1605(91)90071-v. [DOI] [PubMed] [Google Scholar]
- 25.Simpson R K, Gilmour A. The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J Appl Microbiol. 1997;83:181–188. doi: 10.1046/j.1365-2672.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Simpson R K, Gilmour A. The effect of high hydrostatic pressure on the activity of intracellular enzymes of Listeria monocytogenes. Lett Appl Microbiol. 1997;25:48–53. doi: 10.1046/j.1472-765x.1997.00168.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugata K, Ohnishi T, Matsumoto K. Rapid counting method of living cells by fluorescent enzyme substrates. Biomed Mater Eng. 1991;1:115–125. [PubMed] [Google Scholar]
- 28.Tholozan J L, Ritz M, Jugiau F, Federighi M, Tissier J P. Physiological effects of high hydrostatic pressure treatments on Listeria monocytogenes and Salmonella typhimurium. J Appl Microbiol. 1999;88:202–212. doi: 10.1046/j.1365-2672.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 29.Ueckert J, Breeuwer P, Abee T, Stephens P. Flow cytometry applications in physiological study and detection of food-borne microorganisms. Int J Food Microbiol. 1995;28:317–326. doi: 10.1016/0168-1605(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 30.Ueckert J E, Nebe von-Caron G, Bos A P, Ter Steeg P F. Flow cytometric analysis of Lactobacillus plantarum to monitor lag times, cell division and injury. Lett Appl Microbiol. 1997;25:295–299. doi: 10.1046/j.1472-765x.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 31.Winckel E, Ritz M, Pilet M F, Mescle J F, Federighi M. Effets de traitements par hautes pressions hydrostatiques sur Salmonella typhimurium, Listeria monocytogenes et Enterococcus faecalis. Microbiol Aliments Nutr. 1997;15:131–138. [Google Scholar]
- 32.Yamaguchi N, Nasu M. Flow cytometric analysis of bacterial respiratory and enzymatic activity in the natural aquatic environment. J Appl Microbiol. 1997;83:43–52. [Google Scholar]