Abstract
Background:
Practitioners and policy makers need evidence to facilitate the selection of effective prevention interventions that can address the ongoing opioid overdose epidemic in the United States.
Methods:
We conducted a systematic review of publications reporting on rigorous evaluations of systems-level interventions to address provider and patient/public behavior and prevent prescription and illicit opioid overdose. A total of 251 studies were reviewed. Interventions studied included 1) state legislation and regulation, 2) prescription drug monitoring programs (PDMPs), 3) insurance strategies, 4) clinical guideline implementation, 5) provider education, 6) health system interventions, 7) naloxone education and distribution, 8) safe storage and disposal, 9) public education, 10) community coalitions, and 11) interventions employing public safety and public health collaborations.
Results:
The quality of evidence supporting selected interventions was low to moderate. Interventions with the strongest evidence include PDMP and pain clinic legislation, insurance strategies, motivational interviewing in clinical settings, feedback to providers on opioid prescribing behavior, intensive school and family-based programs, and patient education in the clinical setting.
Conclusions:
Although evidence is growing, further high-quality research is needed. Investigators should aim to identify strategies that can prevent overdose, as well as influence public, patient, and provider behavior. Identifying which strategies are most effective at addressing prescription compared to illicit opioid misuse and overdose could be fruitful, as well as investigating synergistic effects and unintended consequences.
Keywords: Opioid, Overdose, Prevention, Policy, Prescribing
1. Introduction
The burden associated with the opioid crisis in the U.S. continues to grow, necessitating up-to-date evidence to guide selection of prevention and response strategies. In 2017 there were 47,600 opioid overdose deaths; 17,029 involved prescription opioids (including natural and semisynthetic opioids and methadone), 15,482 involved heroin, and 28,466 involved synthetic opioids (other than methadone) (Scholl et al., 2018). Although opioid prescribing has been declining since 2012, there were still over 191 million opioid prescriptions dispensed by retail pharmacies in 2017, a rate of 58.5 per 100 persons, with 17% of the population filling at least one prescription for an opioid (CDC, 2018). Over 11 million people were estimated to misuse prescription pain relievers in 2017, an estimated 886,000 reported use of heroin, and 2.1 million people were estimated to have an opioid use disorder (SAMHSA, 2019). The total cost of the opioid overdose epidemic, including costs associated with the use and misuse of prescription and illicit opioids in 2015, was estimated at over $500 billion (Council of Economic Advisors, 2017).
While the epidemic’s roots began with prescription opioids, a second wave of the epidemic became apparent with greater involvement of heroin in overdose deaths starting in 2010, and in 2013, the U.S. entered a third wave after the introduction of illicitly manufactured fentanyl (IMF) (Seth et al., 2018b). Although the greatest overdose burden is currently linked to synthetic opioids, a majority of recent users of heroin report starting their opioid misuse trajectories with prescription opioids (Compton et al., 2016). It remains vital that prevention strategies continue to target both prescription and illicit opioid misuse.
State and local public health departments, policymakers, and health systems play a critical role in prevention and response. To assist and inform state prevention efforts, in 2014 Haegerich and colleagues published a systematic review of what was known about what works to prevent prescription drug overdose with a focus on opioids (Haegerich et al., 2014). Promising strategies identified in the review included PDMPs, insurer strategies, pain clinic legislation, clinical guidelines, and naloxone distribution; the overall quality of evidence was deemed to be low. The findings directly informed strategies targeted for implementation in state programs supported by the U.S. Centers for Disease Control and Prevention (CDC) (https://www.cdc.gov/drugoverdose/states/index.html).
Since publication of that review, evaluation research on opioid overdose prevention interventions has rapidly evolved. Significant funding has been deployed to support prevention efforts. There is a pressing need for an update on the evidence to inform the prioritization of effective primary and secondary prevention strategies. The current review provides a recent synthesis on the effectiveness of prevention strategies that address prescription and illicit opioid overdose.
2. Method
2.1. Data sources and searches
We conducted a systematic search of electronic databases including CINAHL, Medline, PsychInfo, and Scopus with the assistance of a research librarian to identify English language abstracts of studies published January 2013-May 2018 for inclusion in the review. Search terms included those associated with opioids (e.g., analgesics, opioid, opiate, painkiller, pain reliever, heroin), key outcomes of interest (e.g., overdose, death, abuse, misuse), and intervention strategies (e.g., prescribing, guideline, legislation, education, naloxone) (see Supplementary Appendix: Search Strategy).
The authors searched the reference lists of identified publications to find studies not detected in the search; when potentially relevant publications were identified, the authors screened abstracts to determine appropriateness for inclusion. Authors nominated publications for inclusion that were otherwise known based on experience (N = 23); such studies could have been published after the end date of the electronic abstract search up until the review activities were completed near the end of 2018 to allow for the most up-to-date review as practical.
2.2. Selection criteria
Selection criteria included consideration of publications including studies that were part of the previous review (Haegerich et al., 2014), evaluated interventions intended to prevent opioid overdose, conducted in the United States, printed in English language, and with rigorous designs such as experimental or quasi-experimental designs (e.g., RCT, time series, pre-post, or pre-post with comparison). Non-comparative studies were included or retained from the original systematic review (Haegerich et al., 2014) if they represented a novel intervention with promise but with a small number of studies with rigorous designs evaluating them to inform effectiveness (e.g., naloxone distribution). Some studies included in the previous review are not included within this review if they were non-US studies or focused solely on drugs other than opioids (e.g., benzodiazepines).
Included interventions were determined to have systems-level implementation feasibility through state and local health agencies or collaborations with health systems. Evaluations must have included reports of desired outcomes, including provider behavior (e.g., opioid prescribing, guideline-concordant care), patient behavior (e.g., use of multiple providers or pharmacies, opioid misuse), and health outcomes (e.g., overdose from prescription or illicit opioids). The term “patient” was used to reflect findings relevant to the general public. When publications of educational interventions assessed knowledge only, the study was included, and the outcome was coded as “behavior”.
Consistent with our prior systematic review, we excluded biochemical and animal studies; case reports; and evaluations of abuse-deterrent formulations, opioid use disorder treatment (e.g., methadone, buprenorphine, or naltrexone), and compulsory drug treatment/drug courts. Medications for opioid use disorder have been demonstrated to save lives (NASEM, 2019). Evaluations of public health approaches intended to enhance linkage to opioid use disorder treatment, including medications for treating opioid use disorder, were included when they were retrieved by the literature search, although the search strategy was not engineered to fully capture all relevant reports in this area. For a more in-depth review of models of care for medication-assisted treatment for opioid use disorder, see Korthuis et al. (2017). Evaluations of strategies to address other opioid-related harms (e.g., HIV/HCV) that also aim to prevent overdose (e.g., comprehensive syringe services programs; see Des Jarlais et al., 2015), were not specifically searched for inclusion in the review. Drug consumption/safe injection facilities (e.g., see Andresen and Boyd, 2010) which are available internationally but not Federally sanctioned in the United States were excluded. Interventions implemented in community settings were included; interventions focused within correctional settings (e.g., enhancing access to medications for opioid use disorder in jails or prisons) were excluded.
2.3. Data extraction and synthesis
The database search identified a total of 12,558 unique publications (Fig. 1). After screening titles, 1246 publications that clearly did not meet screening criteria were removed. Of the remaining 11,312 publications, we retained 156 publications for inclusion in the review based on abstract review; an additional 23 publications were nominated by study authors based on reference list searches and general awareness of reports (N = 179 total new publications). When questions arose about inclusion criteria, selection was determined after discussion and agreement among authors. The 179 new publications combined with 72 publications retained from the original systematic review resulted in a total of 251 publications included in the review.
Fig. 1.
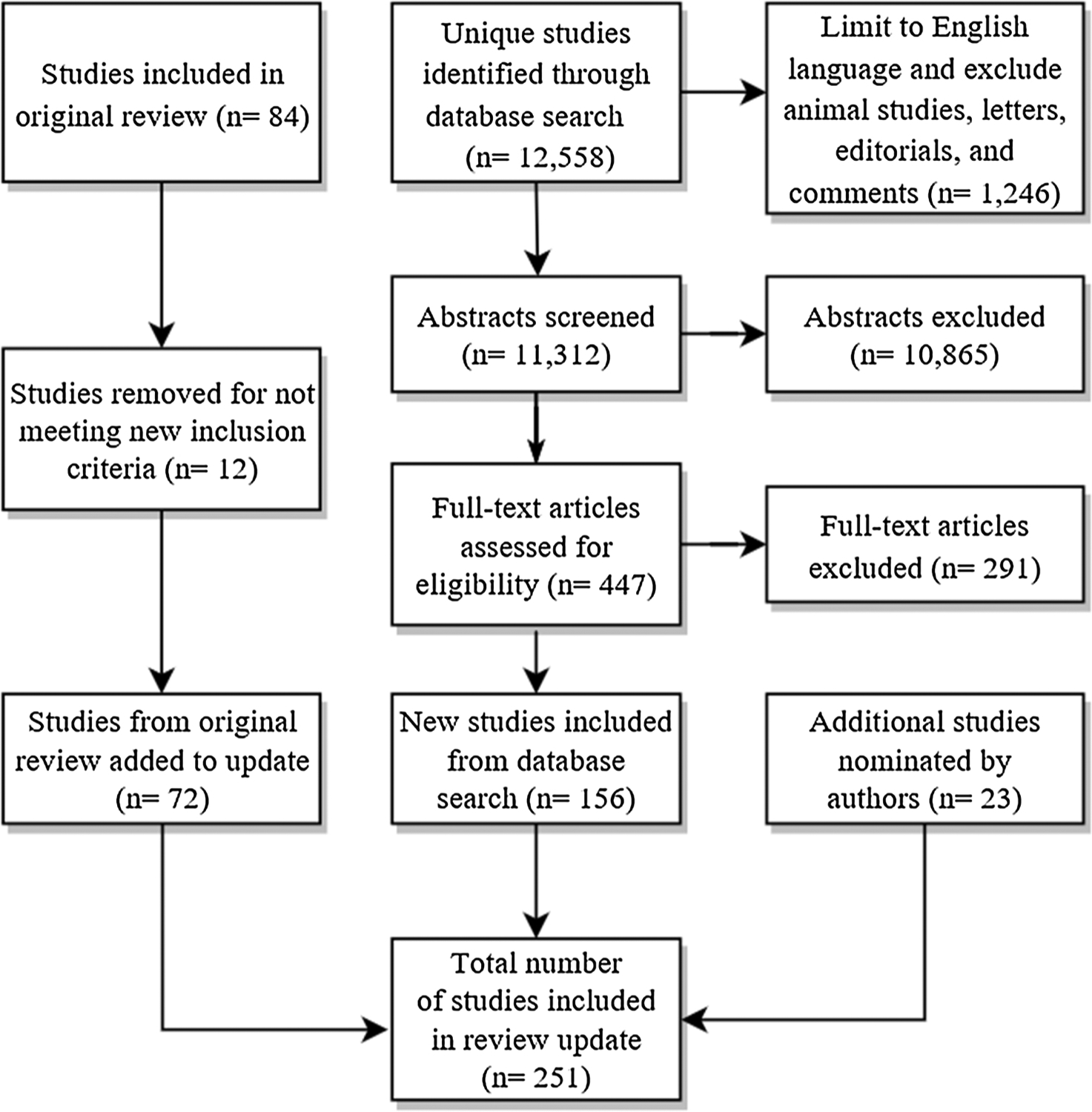
Data sources and searches: Articles included in review.
The categories of interventions identified include: 1) state prescribing legislation or regulation, 2) prescription drug monitoring programs (PDMPs), 3) insurance and pharmacy benefit management strategies, 4) clinical guideline implementation, 5) provider education, 6) clinical health system interventions, 7) naloxone education and distribution, 8) safe storage and disposal, 9) public education, 10) community coalitions, and 11) interventions employing public safety and public health collaboration. Interventions covered in the current review differ slightly from the previous review (Haegerich et al., 2014) given the evolving nature of the research in this area and the addition of innovative approaches evaluated in the field. Some intervention categories remained similar in scope (e.g., PDMPs), some categories were further separated for clarity (e.g., guideline implementation, provider education, and health system interventions are now distinguished categories), and other categories were newly added (e.g., public safety and public health collaboration).
The authors independently reviewed articles within assigned intervention categories, documenting the study design and outcomes examined (see Supplementary Tables 1–11). The study design algorithm from the CDC Community Guide was used to categorize study design (Zaza et al., 2000). When questions arose about study design or outcomes, one author consulted a second author for discussion and validation until agreement was reached. Given the variation in interventions, study designs, and outcomes assessed, it was not practical to synthesize the results through meta-analysis. We developed a narrative synthesis for each intervention area, summarizing results from studies included in the previous review along with new studies to provide a holistic picture of the available evidence, focusing on the studies with the greatest scientific rigor in the narrative review (e.g., use of randomized trials, time series analysis, and comparison groups).
Authors assigned evidence quality ratings for each intervention category, including very low, low, moderate, or high, according to methods used in the previous review (see Haegerich et al., 2014) and inspired by the GRADE approach (Balshem et al., 2011). In brief, intervention categories comprised of primarily observational studies would receive an initial assignment of low evidence quality, while categories comprised of primarily randomized controlled trials would receive an initial assignment of high evidence quality. Ratings could be modified downward based on factors such as study limitations, inconsistency of results, and indirectness of evidence; ratings could be modified upward based on factors such as large magnitude of effect and dose response. When quality of evidence is low, there is low confidence that the effect is a true effect, and further research is likely to change judgments of effectiveness. When quality of evidence is high, there is confidence that the true effect is close to what evaluations have estimated. Studies were considered to offer indirect evidence when a health outcome (e.g., opioid overdose) was not assessed.
3. Results
In the summaries below, we review findings from studies of the highest design quality in each intervention area, given the large number of studies identified and the greater confidence in findings from studies with higher internal validity. A detailed description of all individual studies, designs, outcomes, and findings for each intervention can be found in the tables in the Supplemental Appendix. As can be seen in Fig. 2, there is significant variation in the number of studies addressing each intervention type, with the greatest number of studies examining effectiveness of naloxone distribution, clinical guidelines, and health system interventions, and the lowest number of studies examining effectiveness of public education, coalitions, and public safety/public health partnerships. However, the proportion of high quality studies [(including RCT and time series (with or without concurrent controls)] compared to lower quality studies (all other studies) also varies across intervention type. Interventions with the greatest overall number of high quality studies include clinical health systems, PDMPs, and guideline implementation. Interventions with the greatest proportion of high quality studies includes public education, state legislation, and PDMPs; interventions with the lowest proportion of high quality studies include public safety/public health partnerships, provider education, safe storage and disposal, and naloxone distribution (Fig. 3). Overall, the greatest proportion of studies examine provider behavior, followed by patient behavior, then health outcomes (Fig. 4). Attention to outcome type in the studies also varies by intervention type. The highest number of studies focusing on health outcomes such as overdose includes those evaluating naloxone distribution, PDMPs, and state legislation; the number of studies focusing on provider outcomes such as prescribing is highest for guideline implementation, provider education, and naloxone distribution. Finally, naloxone distribution, clinical health system interventions, and safe storage and disposal are the interventions with the highest number of studies examining patient outcomes and behaviors.
Fig. 2.
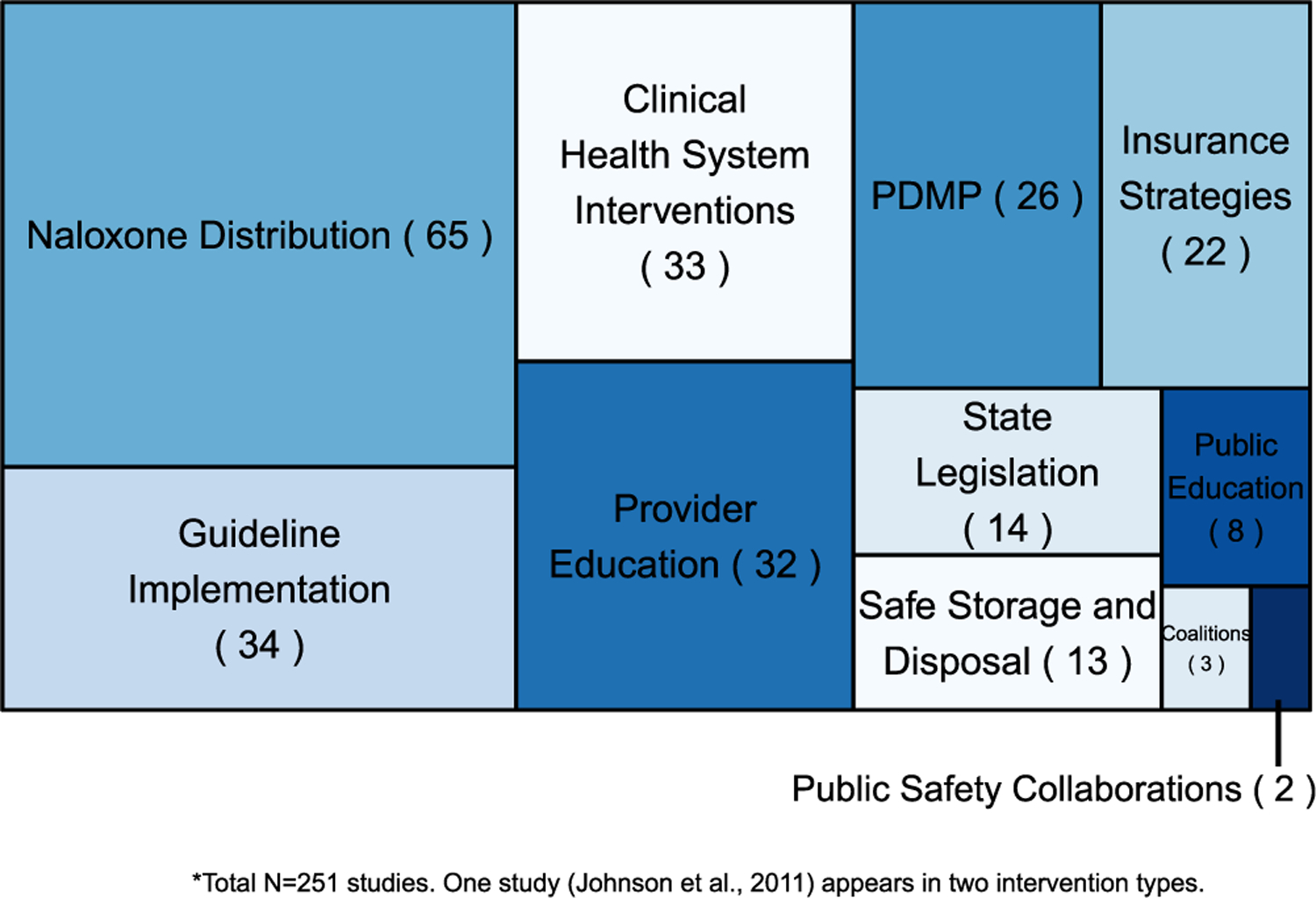
Proportion of studies by intervention type.
*Total N = 251 studies. One study (Johnson et al., 2011) appears in two intervention types.
Fig. 3.
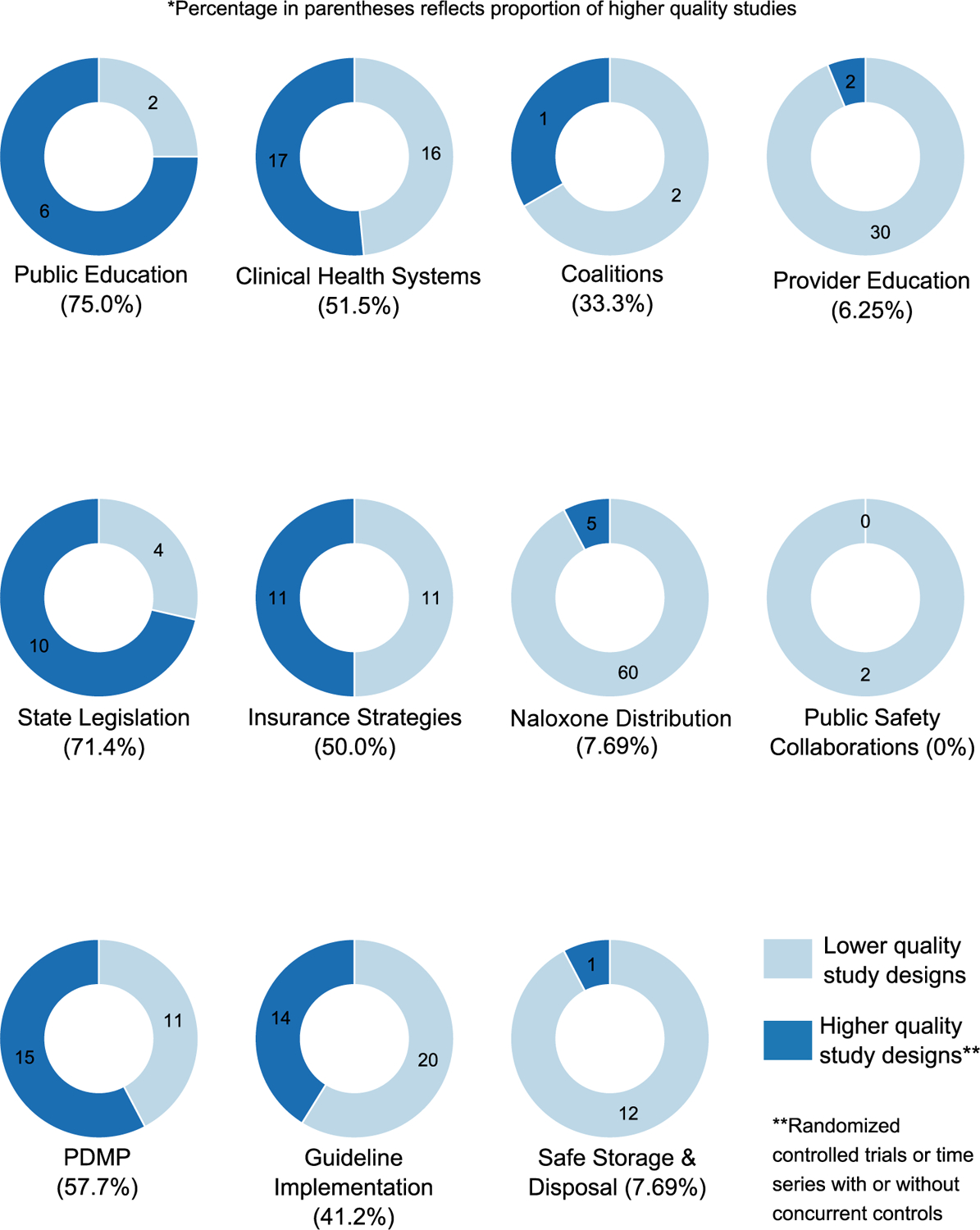
Proportion of higher and lower quality studies by intervention type.
Fig. 4.
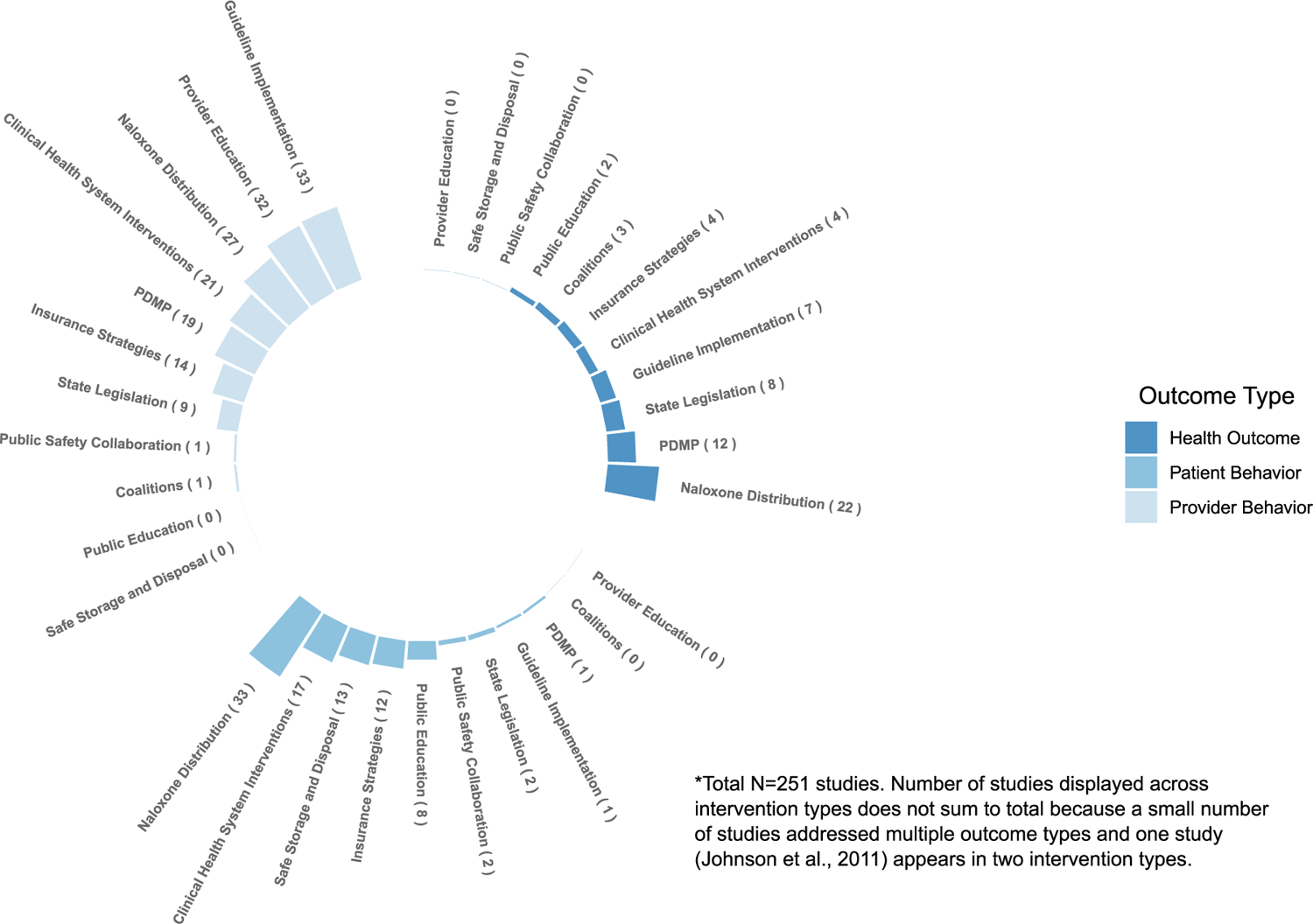
Number of studies by outcome and intervention type.
3.1. Opioid-relevant state policy (legislation/regulation)
State policy (e.g., legislation and regulations) intended to prevent opioid overdose were examined in 14 studies (see Table 1, Supplementary Appendix), including policies addressing pain clinics (often in combination with PDMPs), immunity from prosecution, physical examinations, and comprehensive regulation. Because multiple policies are often implemented simultaneously within and between states, there are significant challenges in assessing the unique impact of specific state policies. The most rigorous studies employing time series designs with concurrent comparison states have evaluated pain clinic legislation; particularly, in Florida, and in combination with implementation of Prescription Drug Monitoring Programs (PDMPs). Among the most rigorous studies in Florida, two found a significant decrease in opioid volume and morphine milligram equivalent (MME) prescribed, especially among higher-risk prescribers (Chang et al. (2016); Rutkow et al. (2015)). In a study comparing opioid overdose mortality rates in Florida to North Carolina, a state without pain clinic regulations, Kennedy-Hendricks et al. (2016) estimated that Florida’s legislation saved 1029 lives in the 34 months following implementation in the state. Other, less rigorous studies in Texas have also found decreases in the amounts of opioids prescribed after implementation of pain clinic regulation, although in one study decreases were detected only in the short-term (Lyapustina et al. (2016); Raji et al. (2017)).
Some have raised concerns that regulating pain clinics could result in a shift in patients receiving opioids from clinics to obtaining opioids on the illicit market, and thus increasing overdoses from illicit opioids. In a time series analysis (without comparison), Johnson et al. (2014) reported a 27% decrease in the prescription opioid overdose death rate after policy changes in Florida. There was a concurrent increase in the heroin overdose death rate, but this increase was relatively small (from 0.3 to 0.6 per 100,000) and it is unclear whether this increase was due to state policy changes or due to changes in the illicit drug supply and secular drug use patterns during the study period. A national study conducted by Dowell et al. (2016) found that the combined implementation of pain clinic policies and mandatory PDMP review was associated with a significant 8% decrease in opioid prescribing and 12% decrease in prescription opioid overdose deaths (pain clinic policies alone did not significantly reduce these rates); however, no significant increase was detected for heroin overdose rates.
There is very limited evidence of the impact of Good Samaritan laws – laws providing varying degrees of legal protection for individuals engaged in seeking help during an overdose event. A cross sectional analysis of overdose mortality in all states showed that Good Samaritan laws were associated with a significantly lower (15%) incidence of overdose mortality (McClellan et al., 2018) while a non-comparative study reported an increased willingness to call 911 among people using opioids once the law was understood (Banta-Green et al., 2011).
We assigned the overall quality of evidence as low for state policies overall given the limitations of the study designs. However, evidence supporting the combination of pain clinic and PDMP legislation could be upgraded to moderate given enhanced rigor of study designs and consistency in findings.
3.2. State prescription drug monitoring programs (PDMPs)
State prescription drug monitoring programs (PDMPs) were evaluated as the primary intervention in 26 studies (Tables 1 and 2, Supplementary Appendix). Eight studies evaluated PDMPs in combination with other state policies [Al Achkar et al., 2018; Chang et al., 2016; Dowell et al., 2016; Johnson et al., 2014; Meara et al., 2016; Penm et al., 2017; Rutkow et al., 2015; Surratt et al., 2014]). In the studies evaluating PDMPs as the primary intervention, designs were structured to either test their overall impact once established, or to assess impact of specific PDMP characteristics.
Because PDMPs have evolved significantly since their inception, the most recent analyses have focused on PDMP characteristics that are intended to increase their utilization (e.g., mandatory registration and/or use), and the degree to which such characteristics influence prescribing and health outcomes. In the most rigorous studies, compared to states without mandatory use, PDMPs with mandatory use demonstrated significant decreases in the days’ supply of opioids prescribed (Yarbrough, 2018), as well as deaths involving prescription opioids when combined with pain clinic regulation (Dowell et al. (2016). More broadly, stronger PDMP states, such as those that required mandatory use, monitored more than schedule II drugs, and updated more frequently (e.g., daily), demonstrated greater reductions in overdose deaths involving prescription opioids (Pardo, 2016).
However, findings of impact on prescribing and health outcomes are mixed in the most rigorous studies examining the impact of the presence of PDMPs alone. We identified 10 studies that implemented time series analyses examining PDMP states with concurrent comparison states. Of the six studies that examined impacts of presence of a PDMP on prescribing behavior, four found significant decreases in prescribing, as evidenced by prescriptions for schedule II opioids (Bao et al., 2016; Simeone and Holland, 2006), oxycodone shipments, (Reisman et al., 2009), and opioid volume among Medicare enrollees (Moyo et al., 2017); however, two studies were unable to detect significant differences in average MME dispensed in states with PDMPs compared to those without (Brady et al., 2014; Paulozzi et al., 2011). Of the three studies that examined impact on overdose, two found no significant changes or differences in drug or opioid overdose mortality (Nam et al., 2017; Paulozzi et al., 2011). Yet, one found significantly lower opioid-related death rates in states with a PDMP compared to those without, particularly when the PDMP was more robust in terms of number of drug schedules monitored, mandated use, and update frequency (Patrick et al., 2016); estimating there could have been 600 fewer opioid overdose deaths in 2016 if Missouri adopted a PDMP and other states enhanced their programs. In two studies examining treatment admissions in PDMP states compared to non-PDMP states, one study found a significant decrease in PDMP states (Simeone and Holland, 2006) while the other did not (Reifler et al., 2012).
Findings are likely mixed due to the complexities involved in isolating the impact of PDMPs within the context of a worsening epidemic, a rapidly shifting intervention landscape, varying PDMP characteristics across states, and lack of information about specific implementation strategies (e.g., provider education on use). Yet, we assigned the level of evidence as moderate, particularly when examining impact on prescribing behavior, when taking into consideration both the study designs employed and the consensus of findings. Additional rigorous examinations of the impact of PDMPs on distal health outcomes are necessary, including more fine-tuned examination of PDMP characteristics that could influence effectiveness (e.g.. mandatory use and registration, delegate access, reporting frequency, and inclusion of risk scores to identify high-risk patients). Studies are also needed to examine actual provider PDMP use, beyond overall policy implementation.
3.3. Insurance strategies
Insurance interventions, such as those that identify high-risk opioid prescribing, lock in patients to specific providers or pharmacies (i.e., patient review and restriction), or require prior approval of medications before reimbursement, were evaluated in 22 studies (Table 3, Supplementary Appendix). Approximately half of the studies utilized a more rigorous time series design (N = 10), and one employed a randomized controlled trial design.
Three time series studies evaluated lock-in programs within Medicaid populations. All reported positive changes in prescribing behavior, including significant decreases in polypharmacy, increases in number of patients filling opioids only from assigned prescribers, and decreases in quantities of opioids prescribed or filled (Blake et al., 1999; Mitchell, 2009; Skinner et al., 2016). Mitchell (2009) reported a significant decrease in emergency department visits as well (the other more rigorous studies did not examine health outcomes). However, less methodologically rigorous studies of lock-in programs reported an unintended consequence: a significant increase in non-Medicaid reimbursed opioid prescriptions (e.g., cash payment) after program implementation (Naumann et al., 2018; Roberts et al., 2016).
Three time series studies focused on prior authorization (PA) policies in the Medicaid population. Morden et al. (2008) found that only strict PA policies – such as those implementing “fail first” protocols and requiring medical documentation for opioid use for pain – were associated with a significant decrease (34%) in oxycodone use. Another study found that PA policies for extended release/long acting (ER/LA) opioids significantly decreased prescriptions for those types of medications, including high-dose prescriptions (Oregon State University (OSU, 2012). Such prescribing outcomes could be considered to be positive impacts. However, a lower quality study found that a decline in ER/LA opioid prescribing after prior authorization coincided with a significant increase in prescriptions for short acting opioids illustrating a displacement effect; further, no differences in ED visits and hospitalizations were seen, questioning whether changes in prescribing translate to changes in health outcomes (Keast et al., 2018). In evaluating a PA policy for buprenorphine used in medication treatment for opioid use disorder to limit dose and prescription length, Clark et al. (2014) noted a decrease from 16.5% to 4.1% in doses exceeding 24 mg per day (the maximum recommended therapeutic dose), accompanied by a temporary increase in relapse for some patients. Hence, while prior authorization for prescription opioids might be helpful in terms of changing prescribing behavior, with uncertain impacts on health outcomes, prior authorization for treatments for opioid use disorder have the potential for harm by facilitating resumption of drug use.
Pertaining to drug utilization review, two methodologically rigorous studies using time series and randomized trial designs evaluated retrospective drug reviews of patients by employing letters mailed to providers that prescribed to high-risk patients. Both studies illustrated significant decreases in the number of controlled substances filled, with one illustrating greater reductions when letters provided more patient-specific information (Daubresse et al., 2014; Gonzalez and Kolbasovsky, 2012). Similarly, Zarowitz et al. (2005) reported a decrease in both the number of prescriptions per member and in polypharmacy events after the implementation of a pharmacist-led provider education intervention based on the results of drug-utilization review. Unfortunately, no data are available from rigorous studies about impacts of drug utilization review on patient health outcomes.
In an evaluation of a comprehensive commercial payer opioid utilization policy that included patient-provider agreements, risk assessments, lock-in programs, PA policy, a mail-order ban, and quantity limits, there were significant decreases in both the short-acting opioid prescription rate (−6.1%) and the LA opioid prescription rate (−9.1%) (Garcia et al., 2016). Similarly, Garcia et al. (2014) evaluated a comprehensive Medicaid initiative aimed at reducing LA opioid prescribing and found a significant, 17.8% reduction in the number of members utilizing LA opioids.
Reviewed studies indicate decreases in prescribing are possible through requiring prior authorization for opioids for pain management, restricting the number of providers and pharmacies that can prescribe and dispense to a patient when patients are identified as high-risk, and reviewing medication use with patient-specific provider feedback; comprehensive policies hold particular promise. We assigned an evidence strength of moderate for prescribing behaviors, but more research is needed to evaluate the impact on health outcomes such as overdose, as well as improvements in patient pain and function, such as through encouraging use of non-opioid strategies. Concern has been raised that insurance policies motivated by clinical guidelines but implemented inflexibly or without adequate consideration of patient context could result in patient harm; thus, further evaluation of insurance strategies are warranted to assess for intended as well as unintended consequences (Dowell et al., 2019; Kroenke et al., 2019). Additional research on prior authorization policies focused on medications used for treatment of opioid use disorder could inform how such policies might positively or negatively affect treatment access, retention, relapse, diversion, overdose, and other outcomes.
3.4. Clinical guideline implementation
National, state, local, and professional society clinical practice guideline implementation was evaluated in 34 studies (Table 4, Supplementary Appendix). Evaluated guidelines focused primarily on opioid prescribing, such as limiting the dosage or duration of prescriptions, using PDMPs and urine drug testing, starting with short-acting opioids, and avoiding co-prescribing of opioids and benzodiazepines. Strategies for implementation included limited efforts of distribution (e.g., paper/ electronic dissemination of recommendations, small group training), as well as intensive efforts such as integration of recommendations within the electronic health record (EHR) that provides clinical decision support at the point of care, and structured educational visits to offer tailored training in evidence-based practice (i.e., academic detailing). Several studies (n = 10) examined impact of opioid prescribing guidelines on provider behavior using before/after designs, with only a minority of studies (n = 7) examining patient health outcomes. State guidelines were evaluated using more rigorous time series designs (n = 7). Randomized trials (n = 5) were employed primarily to test different ways of presenting guideline information and improving knowledge, without “no treatment” controls.
At a national level, one rigorous interrupted time series analysis of the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain illustrated significant declines in the overall opioid prescribing rate after guideline release, beyond existing declining trends, including decreases in the rate of high-dosage opioid prescriptions and overlapping opioid and benzodiazepine prescriptions (Bohnert et al., 2018). Time series analyses of the Washington State opioid dosing guideline have illustrated declines in number and amount of opioid prescriptions, as well as fewer patients newly initiating opioids becoming long-term users in a worker’s compensation population (Franklin et al., 2012; Garg et al., 2013). Significant changes in prescribing were also detected among the Washington state Medicaid population, with significant decreases in high MME dosages and use of LA opioids overall, and for dispensing after an ED visit specifically, including among patients with prior risky opioid use (Sullivan et al., 2016; Sun et al., 2017). Similarly, reductions in opioid prescriptions dispensed through EDs were seen after Ohio emergency physician opioid prescribing guidelines were released, compared to pre-guideline trends; impact on patient health outcomes were not assessed (Weiner et al., 2017). When patient health outcomes have been assessed, findings are mixed. In the Washington state worker’s compensation population after guideline implementation, one study illustrated a decrease in number of unintentional deaths related to prescription opioid use (Franklin et al., 2012) and another study found no declines in ED visits for poisoning or adverse effects involving opioids (Fulton-Kehoe et al., 2013). Yet, this was during a time of rapid increases in ED visits nationally.
Guideline impact can be influenced by implementation strategies. For example, communication strategies that provide recommendations within the clinical context (i.e., present information in narrative format rather than summary format) can enhance knowledge improvements (Kilaru et al., 2014; Meisel et al., 2016). To achieve behavior change, however, additional efforts may be required. In rigorous randomized controlled trials, investigators have found that education, monitoring, and feedback to providers on guideline implementation for management of chronic pain are sometimes not sufficient to facilitate use of recommended practices or improvements in patient outcomes (Corson et al., 2011; McCracken et al., 2012). However, pairing guidelines with more intensive efforts, such as academic detailing, systems consultation, and coordinated care plans illustrates particular promise for changing prescribing behavior (e.g., decrease in high opioid dosages) and influencing patient outcomes, such as decreased overdose mortality (Paone et al., 2015; Quanbeck et al., 2018; Von Korff et al., 2016).
We determine the overall quality of evidence to be low given the limitations of designs in many studies (e.g., pre-post only without control), mixed findings on behavioral outcomes, and the limited number of studies examining patient health outcomes. However, there were several studies with strong designs, such as time series designs, that illustrated positive impacts on prescribing behaviors. More intensive implementation strategies, such as making recommendations available at the point of care and using academic detailing and coordinated care plans implemented in tandem with guideline dissemination, hold particular promise for changing prescribing behavior and decreasing overdose mortality. Similar to considerations for insurance strategies, further research is needed to identify impacts on patient health outcomes including both intended and unintended consequences, given concerns about harms associated with implementation of recommended practices without adequate consideration of patient characteristics (Dowell et al., 2019; Kroenke et al., 2019).
3.5. Provider education
Provider education was evaluated in 32 studies (Table 5, Supplementary Appendix). The predominance of studies (n = 24) evaluated provider education on opioid prescribing for acute or chronic pain; four studies included education on opioid use disorder and prescribing medication for opioid use disorder treatment, and six included education on overdose and prescribing or dispensing naloxone. Some educational programs focused on students or residents, while others focused on educating experienced providers, with some targeted to providers based on a history of high risk prescribing (e.g., referral by the medical board). In some cases, providers were educated about their prescribing history specifically, in comparison to other providers. Educational strategies included in-person presentations and workshops, web-based training, presentation combined with direct application in the clinical setting (e.g., in combination with structured clinical examination), and telehealth strategies with case-based learning (e.g., project ECHO). Most studies were descriptive, with only 2 studies using randomized trial designs and 9 studies using comparison groups. Most studies evaluated the impact of education on knowledge improvements, self-efficacy, and self-reported behavioral intentions or changes in practice; only one third of studies evaluated direct changes in prescribing behavior or other objective changes in provider behavior (e.g., PDMP registration, use of risk mitigation strategies), and none evaluated impacts on patient health outcomes.
In one randomized trial, Michael et al. (2018) offered emergency department physicians feedback on how their prescribing behaviors compared to their peers, finding that those in the intervention group who had initially underestimated their own prescribing had a significantly larger decrease in prescribing than physicians in the control group. In another randomized trial, residents who received web-based training on guideline recommendations had a greater increase in knowledge and self-rated competence of patient management than residents who only received the guideline document (Sullivan et al., 2010). Other findings from before and after studies with comparison groups revealed that provider education generally is effective in increasing provider knowledge and self-efficacy, intention to change, and self-reported change in prescribing behavior (e.g., Alford et al., 2016; Cardarelli et al., 2018; Zisblatt et al., 2017). One study found no differences in knowledge between in-person versus online training format (Berland et al., 2017), with another finding that online training out-performed document dissemination (Kim et al., 2016). In terms of objective measures of provider behavior, Ury et al. (2002) illustrated improvements in recommended prescribing practices after education on palliative care and pain management. Telehealth education, namely within the Project ECHO model, increased provider knowledge and self-efficacy for opioid prescribing for pain, with some evidence for change in prescribing behavior, referrals, and use of risk mitigation strategies (Anderson et al., 2017). Mixed findings have been found after naloxone training for providers, with some programs showing no differences in knowledge (Jacobson et al., 2018), and others showing increases in naloxone prescribing rates (Taylor et al., 2018).
We assigned an overall evidence quality of low given the limitations of the study designs (e.g., primarily before-after studies without true control), mixed findings on objectively measured provider behavior outcomes, and the lack of studies examining patient health outcomes. Provider education that goes beyond curriculum presentation with additional supports (e.g., provider feedback or tools) could increase education effectiveness. Distance education through telehealth can increase knowledge and practice changes for providers who have difficulty accessing in-person education opportunities. More rigorous research is needed to identify if education that focuses on obtaining waivers for prescribing buprenorphine for opioid use disorder has the potential to increase capacity for treatment, in particular.
3.6. Clinical health system interventions
Clinical health system interventions were evaluated in 33 studies (Table 6, Supplementary Appendix). A wide variety of interventions are included in this category, including: integrated pharmacist services and multidisciplinary team approaches to manage pain; brief motivational interviewing to reduce opioid misuse or overdose risk; patient education on pain management, opioid risks, and overdose; EHR clinical decision support and opioid quantity defaults; audit and feedback of providers on opioid prescribing; and system policies concerning opioid dosage and risk mitigation strategies. Compared to other categories of interventions evaluated in this review that are most directly relevant to clinical care (e.g., guideline implementation, provider education), evaluations of health systems incorporated more rigorous study designs, including randomized trials (n = 14) and time series analyses (n = 3); however, descriptive and before/after studies were also numerous. Studies primarily assessed impacts on provider and patient behavior; three studies examined patient health outcomes.
Among randomized or nonrandomized trials, multidisciplinary team approaches have been shown to reduce opioid prescribing after intervention (Neven et al., 2016). Patient education about opioid risks and overdose can increase patient knowledge and behavioral intentions (Dunn et al., 2017; McCarthy et al., 2015). Mehl-Madrona et al. (2016) demonstrated that patients educated on nonpharmacologic pain treatment who remain engaged in support sessions significantly reduced or discontinued opioid use, with reductions in pain and improvement in quality of life.
Compared to patients receiving usual care, patients engaged in brief interventions by clinical providers, including motivational interviewing about their opioid misuse, report less misuse after intervention (Bernstein et al., 2005; Bohnert et al., 2016; Gelberg et al., 2015; Gryczynski et al., 2015). Mobile psychosocial interventions for increasing adherence to medications for opioid use disorder treatment also shows promise (Guarino et al., 2016). However, findings are mixed when more objective measures are assessed (e.g., through toxicology testing), and in some cases, significant differences in outcomes have not been detected between intervention and control groups (Ondersma et al., 2014; Saitz et al., 2014) or between patients receiving in-person consultation versus computer administration (Schwartz et al., 2014). In one study, motivational interviewing unintentionally reduced the likelihood of receipt of substance use disorder treatment (Kim et al., 2016). Yet optimistically, in a pilot randomized controlled trial, Coffin et al. (2017) demonstrated that individuals with opioid use disorder and overdose history who engaged in motivational interviewing including follow-up counseling in a public health naloxone education and distribution program experienced a significant decrease in overdose events compared to individuals engaged in overdose education as usual.
Providing decision support at the point of care, such as via the EHR, and providing feedback to providers on their prescribing was found to decrease opioid prescribing after implementation (Gugelmann et al., 2013; Lin et al., 2017) as well as to decrease ED visits by patients (Ringwalt et al., 2015). System policies such as opioid dosing limits in combination with provider education have resulted in reductions in opioid prescribing and dosage, with no significant differences in pain or quality of life among patients after policy initiation (Weimer et al., 2016).
We judge the overall quality of evidence to be moderate. Study designs included randomized trials and time series analyses, and studies have provided overall consistent findings of improvements in provider and patient behavior, and in some cases patient health outcomes. Randomized studies did include small sample sizes, however, and other important limitations such as high attrition. Findings suggest that educating patients can improve knowledge and intentions, but interventions such as motivational interviewing that engage patients in understanding discrepancies in behavior, resolving ambivalence, and exploring motivations and plans for change hold particular promise in reducing risky behavior and affecting health outcomes among those at highest risk for overdose. Multidisciplinary interventions and those that engage providers on recommended clinical practices at the point of care with feedback on prescribing behavior have the potential to change not only provider behavior but patient health outcomes as well.
3.7. Naloxone education and distribution
A total of 65 studies examined naloxone education and distribution (Table 7, Supplementary Appendix). The venue for naloxone education and distribution varied and included naloxone co-prescribing with opioids, first responder training, pharmacy-, health system-, and community-based naloxone distribution, and bystander engagement.
Patient behaviors, such as acceptance of a naloxone kit, were the outcomes of interest for the majority of studies (n = 33). Fewer studies examined provider behavior (n = 27) or health outcomes such as number of reversals (n = 20). Most studies within this section used low-quality study designs, such as non-comparison (n = 34) or before-and-after (n = 18); however, three randomized controlled trials evaluated impacts on patient behavior and health outcomes.
The preponderance of studies employed descriptive techniques that illustrated characteristics of naloxone programs, policies, and distribution sites; the types of participants receiving services; the number of participants trained; or amount of naloxone distributed. In prospective cohort and randomized trials, patients receiving naloxone training and kits exhibited significantly greater knowledge of overdose symptoms and when naloxone was indicated after training (Green et al., 2008; Huhn et al., 2018). Reported use of distributed naloxone kits in reversing an overdose has also been documented (e.g., Papp and Schrock, 2017; Spelman et al., 2017; Walley et al., 2013b).
At the community level, targeted messaging to increase public support has been found to be most effective in increasing support for naloxone policies when it includes both factual information and a sympathetic narrative (Bachhuber et al., 2015). A time series analysis with concurrent controls identified that overdose death rates were significantly reduced in communities with opioid education and naloxone distribution (OEND) programs compared to communities without these programs (Walley et al., 2013a). Further, a time series analysis of data from a parent randomized control study found that individuals involved in syringe services programs with naloxone education and distribution reported fewer days of heroin use compared to baseline use (Kidorf et al., 2013).
In the clinical environment, a retrospective cohort study found that providers that had at least one academic detailing session were more likely to prescribe naloxone to their patients (Bounthavong et al., 2017). However, another retrospective cohort study showed no significant difference in naloxone administration rates between patients who received naloxone education and receipt than those that did not (Doe-Simkins et al., 2014). In a nonrandomized intervention study, Coffin et al. (2016) documented a decrease in opioid-related ED visits after providers and clinic staff were trained in naloxone prescribing, with a focus on indications for prescribing, language to use with patients, formulations, payer coverage, and naloxone use. However, in a randomized trial, Banta-Green et al. (2011) conducted overdose education, brief counseling, and naloxone prescription for patients at elevated risk for an overdose after an ED visit and found that overdose events did not significantly differ between intervention and control participants.
We determined the quality of evidence to be low given study designs, despite the preponderance of evidence of naloxone as a vital clinical tool and consensus of the large volume of findings. Studies with more robust methodological designs could identify the most effective training and delivery modalities and for which populations. Which other components may be necessary for effective harm reduction (e.g. wrap-around care for infectious diseases, such as comprehensive syringe services programs and linkage to treatment after an overdose) should be explored.
3.8. Safe storage and disposal
Safe storage and disposal interventions were evaluated in 13 studies (Table 8, Supplementary Appendix). Studies were predominantly non-comparative (N = 6) or before-after designs (n = 3) focusing on patient behaviors. Two cross sectional studies and one before-after study focused on wide-reaching media campaigns that included safe storage and disposal components, examining target population reach, attitudes, and self-reported behaviors. Non-comparative descriptive studies primarily reported on amounts and proportions of discarded drugs across different geographic areas. Findings generally showed small percentages of controlled substances, including opioids, disposed relative to disposal of other types of medications.
In a before-after study with a concurrent comparison group, Hasak et al. (2018) educated surgery patients on the safe disposal of opioids with an educational brochure and found a doubling of the disposal rate (11%–22%) after brochure distribution. De La Cruz et al. (2017) evaluated a patient educational program in a cancer outpatient palliative care clinic that uses educational material focused on proper prescription opioid use, storage, and disposal in a non-randomized trial. After receiving the intervention, patients had significantly greater knowledge of proper disposal methods (76% vs. 28%) and were significantly less likely to practice unsafe use of prescription opioids (18% vs. 25%) compared to the control group. In the only RCT identified, a program promoting pharmacy-based drug disposal after dental surgery was evaluated. Compared to the control group, patients who received the brief behavioral intervention were 22% more likely to report disposal or intent to dispose of unused prescription opioids; however, the sample size was small and results were not statistically significant (Maughan et al., 2016).
Safe storage and disposal interventions could play an important a role in limiting the availability of prescription opioids for diversion, but we judge the quality of the available evidence as low. Safe storage and disposal education through both multimedia campaigns and patient-targeted material can increase participant knowledge, but little is known about how that translates to health outcomes, such as reductions in misuse or overdose. Rigorous studies evaluating the impact of disposal boxes and take-back events on health outcomes are needed. Most studies have limited sample sizes with before-after designs that focus on patient behaviors. Other study limitations include the lack of baseline data and comparison groups, unassessed health outcomes, and other events occurring simultaneously that could be responsible for effects. Although findings are encouraging, they mainly highlight the need for rigorous evaluations examining health outcomes.
3.9. Public education
Public education interventions were evaluated in 8 studies (Table 9, Supplementary Appendix; with one study also reviewed for safe storage and disposal). Given the small number of studies and variety of content and modalities used, findings are reviewed from all studies, regardless of methods employed. Studies included a mix of before/after designs (n = 2) and small randomized trials (n = 6), with a predominant focus on patient behavior outcomes. In the clinical setting, Chakravarthy et al. (2018) illustrated that video-based education on safe opioid use upon discharge in the ED can improve patients’ recall about safe opioid use and disposal. Similarly, Guarino et al. (2018) documented in a randomized trial that a web-based behavioral intervention for patients with chronic pain and a history of opioid misuse reduced aberrant drug-related behavior and ED visits compared to patients receiving treatment as usual. Pre-operative education on safe opioid use was demonstrated in a randomized trial to encourage discontinuation of opioid use, and lower opioid consumption six weeks and three months after surgery (Syed et al., 2018).
Klisch et al. (2013) examined the effectiveness of forensic science games that address drug misuse for integration in the school science curricula; compared to pretest, student attitudes were more negative toward drug abuse after playing the games. In a small study, Fang and Schinke (2013) demonstrated that an online substance abuse prevention program for mothers and daughters that focused on relationships and resilience reduced intentions and self-report of substance misuse. The most rigorous evidence comes from evaluations of universal school and family-based preventive interventions focused on developing youth competencies (Crowley et al., 2014; Spoth et al., 2013). Such programs focus on nurturing skills that support children and dealing with peer pressure for parents, and self-management, social skills, and drug resistance skills for children, and have evidenced significant reductions in prescription opioid misuse. Finally, a before/after evaluation of the “Use Only As Directed” statewide media campaign in Utah that included messages about safe use of prescription pain medications and proper disposal through TV/radio spots, posters, advertising, news releases, and information cards found that survey respondents were less likely to share medications and take medications not prescribed to them after the campaign. Implementation was accompanied by a 14% one-year reduction in unintentional opioid-related drug overdose deaths (Johnson et al., 2011).
Overall, education efforts have some, yet limited, promise for increasing knowledge and awareness of opioid-related harms and decreasing substance misuse, with the most rigorous evidence coming from intensive school and family-based interventions for youth and randomized trials in the clinical setting focused on patient use of opioids; impact on health outcomes was not assessed. Overall quality of evidence for this heterogeneous group of interventions is judged as moderate, given the strength of study designs, and assessment of misuse (in the case of school and family-based interventions). Media campaigns to prevent opioid overdose have promise, yet require further rigorous evaluation, particularly given lack of evidence for changes in health outcomes, and mixed findings from previous evaluations of campaigns focused on illicit drugs beyond opioids, not included in the current review due to their expanded scope (Allara et al., 2015).
3.10. Community coalitions
Interventions involving coalitions formed to coordinate opioid overdose prevention efforts across a state were evaluated in 3 studies (Table 10, Supplementary Appendix). All three studies evaluated Project Lazarus in North Carolina. Project Lazarus includes coordination among county health departments, chronic pain initiatives, and substance abuse task forces on overdose prevention. Albert et al. (2011) and Brason et al. (2013) reported reductions in overdose mortality, but impacts of Project Lazarus independent of other state- or local-level interventions could not be determined. In a time series design with concurrent comparison groups, Alexandridis et al. (2017) documented in the longer-term, programs for patients with pain were associated with lower mortality rates, and naloxone policies and expansion of medication-assisted treatment was associated with lower ED visits (but the effect of treatment stabilized and rates began to increase again). Diversion control was associated with counter-intuitive increases in ED visits.
Interventions involving coalitions could play an important role in the coordination of opioid overdose prevention efforts but we judged the quality of the evidence to be very low with more research needed. More rigorous evaluations are needed to analyze the independent effects of such coalitions on health outcomes of participants.
3.11. Public Safety/Public health collaboration
Interventions involving collaborations between public safety and public health in the community were assessed in 2 before-after studies evaluating policy-led linkage to treatment, both of which are reviewed here (Table 11, Supplementary Appendix). Medications for opioid use disorder treatment is recognized as the most-effective treatment for opioid use disorder (SAMHSA, 2018). However, medication-based treatment is underused and linkage to treatment could help improve treatment access and health outcomes for individuals recently treated for an overdose.
The first study, a training program on opioid overdose prevention and naloxone administration for law enforcement officers with a referral to treatment component resulted in approximately 20% of patients seeking treatment after an overdose (Dahlem et al., 2017). Officers were also significantly more knowledgeable about naloxone and drug overdose reversal after the intervention. The second study, the addiction treatment referral program led by the Gloucester Massachusetts police department was successful at accommodating 75% of participants who sought a referral (Schiff et al., 2017). Although the difference was not statistically significant, program participants were more likely to report drug abstinence at the mean follow-up time of 6.7 months compared to individuals who did not participate (40.9% v. 26.0%). Although these two studies lack rigorous designs and large sample sizes, the results show some promise.
Overall, we assess that the quality of evidence supporting public safety and public health collaborations to prevent opioid overdose is very low. More rigorous evaluations of public safety-led linkage to treatment interventions in the community are needed to determine their effectiveness in improving access to medication-based treat.ment and long-term health outcomes. Additional studies could address the effectiveness of strategic use of surveillance data to inform public safety and public health partnership efforts. For example, collaborative efforts such as the RxStat model established in New York City that aim to share health and public safety data among stakeholders to target and tailor programs, practices, and policies to address opioid misuse and overdose may hold promise (Heller et al., 2014). Further, efforts to improve the provision of medication-based treatment during incarceration and enhance linkage to treatment and ongoing supports upon release (beyond the scope of this review) have demonstrated positive impacts on overdose, but could benefit from further evaluation (Green et al., 2018).
4. Discussion
There has been a substantial increase in the number of studies examining overdose prevention interventions in recent years. Due to the use of lower quality research designs, however, the increase in volume has not significantly improved the level of evidence across a range of interventions. The following strategies have moderate quality of evidence: (1) PDMP and pain clinic legislation; (2) insurance strategies such as lock-in programs, drug utilization review, and prior authorization; (3) clinical health system interventions such as motivational interviewing for those at risk for overdose and interventions that provide recommendations to providers at the point of care with feedback on opioid prescribing behavior; and (4) public education, including intensive school and family-based programs and patient education in the clinical setting. Although promising, all other strategies were found to be supported by low quality evidence, either because of lower quality study designs, mixed findings across studies, and/or a lack of evidence showing impact on health outcomes such as overdose.
States and health systems are trying aggressively to address the epidemic through multi-component interventions and strategies. Federal agencies, such as CDC, are providing information on the evidence for interventions, working with states to implement interventions, and conducting evaluations. For example, through its Opioid Prevention in States initiative, CDC has supported state programs aimed at maximizing PDMPs, implementing health system and insurer interventions, and evaluating state policies and interventions. Continued expansion of these initiatives, such as through Overdose Data to Action (OD2A), is expected to further address the epidemic.
Additional research is critical to inform the selection of strategies that impact overdose. Beyond studies with more rigorous, prospective, experimental research designs, it is important to determine which strategies might be more or less effective in addressing different aspects of the opioid crisis – including those likely to impact prescription opioids versus illicit opioids such as heroin and IMF. Distinct prevention strategies may be required in areas burdened by overdoses involving prescription opioids (e.g., guideline implementation, provider education, clinical health systems interventions, and insurance interventions) versus those burdened by the introduction of IMF in the drug supply (e.g., collaborations between public safety and public health, expansion of naloxone for overdose reversal; Seth et al., 2018a). Understanding how different policies interact and influence outcomes in synergistic or antagonistic ways should be a central area of focus in future research; it is important to understand both intended and unintended consequences. Finally, additional research could also shed light on the utility of science-based community prevention capacity building systems, such as Communities that Care (CTC), to prevent further escalation of the crisis among children and adolescents. The CTC model was evaluated by several group RCTs that examined adolescent behavior, such as drug use, after the intervention (Brown et al., 2014; Oesterle et al., 2015; Rhew et al., 2016). However, none of these studies evaluated the effect of CTC on outcomes specifically related to opioid use or overdose.
This review is subject to important limitations. The review included a systematic search of the available evidence, yet we generated high-level assessments to synthesize the overall quality of the body of evidence for each intervention category and did not systematically rate individual study quality. The review did not fully identify grey literature (e.g., non-indexed, independent publications by the federal government or nongovernmental organizations). It is possible that relevant articles were not identified. While some interventions addressing linkage to treatment for opioid use disorder were captured within this review, the review did not fully capture models of care; other sources may allow for a greater understanding of the effectiveness of such strategies. Finally, the review itself is subject to publication bias as studies that found no significant effects might not have been published.
5. Conclusion
The opioid crisis continues to exact a significant, health, economic, and social toll on communities across the United States. Assessing the availability and strength of the evidence to support state and health system overdose prevention interventions can help facilitate the adoption of the policies, programs, and practices most likely to produce positive health outcomes. Several interventions identified in our review, such as PDMPs and pain clinic policies, insurer strategies, and select clinical and community-based interventions, had moderate quality evidence and are ripe for broader implementation. Other strategies such as innovative public safety and public health collaborations potentially show promise, but need further study. The evidence available, while currently low to moderate quality, is sufficient to catalyze primary and secondary prevention strategies in states. Such prevention strategies, in combination with expanding access to and delivery of evidence-based treatment for opioid use disorder, in particular treatment of opioid use disorder with medications, may hold promise for turning around the overdose epidemic.
Supplementary Material
Role of funding source
Authors employed by the Centers for Disease Control and Prevention conducted this work as a part of federal employment. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Competing Interest
No conflicts declared.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2019.107563.
References
- Al Achkar M, Grannis S, Revere D, MacKie P, Howard M, Gupta S, 2018. The effects of state rules on opioid prescribing in Indiana. BMC Health Serv. Res 18 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert S, Brason FW 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B, 2011. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 12 (Suppl 2), S77–85. [DOI] [PubMed] [Google Scholar]
- Alexandridis AA, McCort A, Ringwalt CL, Sachdeva N, Sanford C, Marshall SW, Mack K, Dasgupta N, 2017. A statewide evaluation of seven strategies to reduce opioid overdose in North Carolina. Inj. Prev 24 (1), 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford DP, Carney BL, Brett B, Parish SJ, Jackson AH, 2016. Improving residents’ safe opioid prescribing for chronic pain using an objective structured clinical examination. J. Grad. Med. Educ 8 (3), 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allara E, Ferri M, Bo A, Gasparrini A, Faggiano F, 2015. Are mass-media campaigns effective in preventing drug use? A Cochrane systematic review and meta-analysis. BMJ Open 5 (9), e007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Zlateva I, Davis B, Bifulco L, Giannotti T, Coman E, Spegman D, 2017. Improving pain care with project ECHO in community health centers. Pain Med. 18 (10), 1882–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MA, Boyd N, 2010. A cost-benefit and cost-effectiveness analysis of Vancouver’s supervised injection facility. Int. J. Drug Policy 21 (1), 70–76. [DOI] [PubMed] [Google Scholar]
- Bachhuber MA, McGinty EE, Kennedy-Hendricks A, Niederdeppe J, Barry CL, 2015. Messaging to Increase Public Support for Naloxone Distribution Policies in the United States: Results from a Randomized Survey Experiment. PLoS One 10 (7), e0130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH, 2011. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol 64 (4), 401–406. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, K. PC, Coffin PO, Schoeppe JA, 2011. Washington’s 911 Good Samaritan Drug Overdose Law: Initial Evaluation Results. Alcohol & Drug Abuse Institute, University of Washington. [Google Scholar]
- Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, Schackman BR, 2016. Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health Aff. (Millwood) 35 (6), 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland N, Lugassy D, Fox AD, Tofighi B, Hanley K, 2017. A comparative analysis of online vs in-person opioid overdose prevention training for first year medical students as an adjunct to first responder training using cardiopulmonary resuscitation. Ann. Emerg. Med 70 (4), S71–S72. [Google Scholar]
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R, 2005. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 77 (1), 49–59. [DOI] [PubMed] [Google Scholar]
- Blake SG, F. JF, Hunter TS, Rappaport H, Holt G, Medon PJ, 1999. The Effect of the Louisiana Medicaid Lock-in on Prescription Drug Utilization and Expenditures. Drug Benefit Trends, CDC, Atlanta 72. [Google Scholar]
- Bohnert AS, Bonar EE, Cunningham R, Greenwald MK, Thomas L, Chermack S, Blow FC, Walton M, 2016. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug Alcohol Depend. 163, 40–47. [DOI] [PubMed] [Google Scholar]
- Bohnert ASB, Guy GP Jr., Losby JL, 2018. Opioid prescribing in the United States before and after the centers for disease control and prevention’s 2016 opioid guideline. Ann. Intern. Med 169 (6), 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounthavong M, Harvey MA, Wells DL, Popish SJ, Himstreet J, Oliva EM, Kay CL, Lau MK, Randeria-Noor PP, Phillips AG, Christopher MLD, 2017. Trends in naloxone prescriptions prescribed after implementation of a National Academic Detailing Service in the Veterans Health Administration: a preliminary analysis. J. Am. Pharm. Assoc 57 (2S), S68–S72 (2003). [DOI] [PubMed] [Google Scholar]
- Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G, 2014. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 129 (2), 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brason FW 2nd, Roe C, Dasgupta N, 2013. Project Lazarus: an innovative community response to prescription drug overdose. N. C. Med. J 74 (3), 259–261. [PubMed] [Google Scholar]
- Brown EC, Hawkins JD, Rhew IC, Shapiro VB, Abbott RD, Oesterle S, Arthur MW, Briney JS, Catalano RF, 2014. Prevention system mediation of communities that care effects on youth outcomes. Prev. Sci 15 (5), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli R, Elder W, Weatherford S, Roper KL, King D, Workman C, Stewart K, Kim C, Betz W, 2018. An examination of the perceived impact of a continuing interprofessional education experience on opiate prescribing practices. J. Interprof. Care 32 (5), 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Annual Surveillance Report of Drug-related Risks and Outcomes — United States. Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Opioid Overdose: State Information. Available at https://www.cdc.gov/drugoverdose/states/index.html. [Google Scholar]
- Chakravarthy B, Somasundaram S, Mogi J, Burns R, Hoonpongsimanont W, Wiechmann W, Lotfipour S, 2018. Randomized pilot trial measuring knowledge acquisition of opioid education in emergency department patients using a novel media platform. Subst. Abus 39 (1), 27–31. [DOI] [PubMed] [Google Scholar]
- Chang HY, Lyapustina T, Rutkow L, Daubresse M, Richey M, Faul M, Stuart EA, Alexander GC, 2016. Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: a comparative interrupted time series analysis. Drug Alcohol Depend. 165, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Baxter JD, Barton BA, Aweh G, O’Connell E, Fisher WH, 2014. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence. Health Serv. Res 49 (6), 1964–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Behar E, Rowe C, Santos GM, Coffa D, Bald M, Vittinghoff E, 2016. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann. Intern. Med 165 (4), 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Matheson T, Behar E, Rowe C, Rubin T, Silvis J, Vittinghoff E, 2017. Behavioral intervention to reduce opioid overdose among high-risk persons with opioid use disorder: A pilot randomized controlled trial. PLoS One 12 (10), e0183354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374 (2), 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson K, Doak MN, Denneson L, Crutchfield M, Soleck G, Dickinson KC, Gerrity MS, Dobscha SK, 2011. Primary care clinician adherence to guidelines for the management of chronic musculoskeletal pain: results from the study of the effectiveness of a collaborative approach to pain. Pain Med. 12 (10), 1490–1501. [DOI] [PubMed] [Google Scholar]
- Crowley DM, Jones DE, Coffman DL, Greenberg MT, 2014. Can we build an efficient response to the prescription drug abuse epidemic? Assessing the cost effectiveness of universal prevention in the PROSPER trial. Prev. Med 62, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem CHG, King L, Anderson G, Marr A, Waddell JE, Scalera M, 2017. Beyond rescue: implementation and evaluation of revised naloxone training for law enforcement officers. Public Health Nurs. 34 (6), 516–521. [DOI] [PubMed] [Google Scholar]
- Daubresse M, Gleason PP, Peng Y, Shah ND, Ritter ST, Alexander GC, 2014. Impact of a drug utilization review program on high-risk use of prescription controlled substances. Pharmacoepidemiol. Drug Saf 23 (4), 419–427. [DOI] [PubMed] [Google Scholar]
- de la Cruz M, Reddy A, Balankari V, Epner M, Frisbee-Hume S, Wu J, Liu D, Yennuraialingam S, Cantu H, Williams J, Bruera E, 2017. The impact of an educational program on patient practices for safe use, storage, and disposal of opioids at a comprehensive Cancer center. Oncologist 22 (1), 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D, 2015. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas - United States, 2013. MMWR Morb. Mortal. Wkly. Rep 64 (48), 1337–1341. [DOI] [PubMed] [Google Scholar]
- Doe-Simkins M, Quinn E, Xuan Z, Sorensen-Alawad A, Hackman H, Ozonoff A, Walley AY, 2014. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health 14, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich T, Chou R, 2019. No shortcuts to safer opioid prescribing. N. Engl. J. Med 380 (24), 2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Zhang K, Noonan RK, Hockenberry JM, 2016. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff. 35 (10), 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Yepez-Laubach C, Nuzzo PA, Fingerhood M, Kelly A, Berman S, Bigelow GE, 2017. Randomized controlled trial of a computerized opioid overdose education intervention. Drug Alcohol Depend. 173 (Suppl 1), S39–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Schinke SP, 2013. Two-year outcomes of a randomized, family-based substance use prevention trial for Asian American adolescent girls. Psychol. Addict. Behav 27 (3), 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D, 2012. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am. J. Ind. Med 55 (4), 325–331. [DOI] [PubMed] [Google Scholar]
- Fulton-Kehoe D, Garg RK, Turner JA, Bauer AM, Sullivan MD, Wickizer TM, Franklin GM, 2013. Opioid poisonings and opioid adverse effects in workers in Washington state. Am. J. Ind. Med 56 (12), 1452–1462. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Dodek AB, Kowalski T, Fallon J, Lee SH, Iademarco MF, Auerbach J, Bohm MK, 2016. Declines in opioid prescribing after a private insurer policy change - Massachusetts, 2011–2015. MMWR Morb. Mortal. Wkly. Rep 65 (41), 1125–1131. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P, 2014. Implementation of an opioid management initiative by a state Medicaid program. J. Manag. Care Spec. Pharm 20 (5), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RK, Fulton-Kehoe D, Turner JA, Bauer AM, Wickizer T, Sullivan MD, Franklin GM, 2013. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J. Pain 14 (12), 1620–1628. [DOI] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Afifi AA, Leake BD, Arangua L, Vahidi M, Singleton K, Yacenda-Murphy J, Shoptaw S, Fleming MF, Baumeister SE, 2015. Project QUIT (Quit using Drugs Intervention Trial): a randomized controlled trial of a primary care-based multi-component brief intervention to reduce risky drug use. Addiction 110 (11), 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Kolbasovsky A, 2012. Impact of a managed controlled-opioid prescription monitoring program on care coordination. Am. J. Manag. Care 18, 512–516. [PubMed] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BDL, Alexander-Scott N, Boss R, Rich JD, 2018. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry 75 (4), 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Heimer R, Grau LE, 2008. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction 103 (6), 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, Ondersma SJ, O’Grady KE, Schwartz RP, 2015. A randomized trial of computerized vs. Inperson brief intervention for illicit drug use in primary care: outcomes through 12 months. J. Subst. Abuse Treat 50, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino H, Acosta M, Marsch LA, Xie H, Aponte-Melendez Y, 2016. A mixed-methods evaluation of the feasibility, acceptability, and preliminary efficacy of a mobile intervention for methadone maintenance clients. Psychol. Addict. Behav 30 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino H, Fong C, Marsch LA, Acosta MC, Syckes C, Moore SK, Cruciani RA, Portenoy RK, Turk DC, Rosenblum A, 2018. Web-based cognitive behavior therapy for chronic pain patients with aberrant drug-related behavior: outcomes from a randomized controlled trial. Pain Med. 19 (12), 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugelmann H, Shofer FS, Meisel ZF, Perrone J, 2013. Multidisciplinary intervention decreases the use of opioid medication discharge packs from 2 urban EDs. Am. J. Emerg. Med 31 (9), 1343–1348. [DOI] [PubMed] [Google Scholar]
- Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM, 2014. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 145, 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasak JM, Roth Bettlach CL, Santosa KB, Larson EL, Stroud J, Mackinnon SE, 2018. Empowering post-surgical patients to improve opioid disposal: a before and after quality improvement study. J. Am. Coll. Surg 226 (3), 235–240 e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D, Bradley O’Brien D, Harocopos A, Hreno J, Lerner J, McCoy EB, Nolan M, Phillips LP, Tuazon E, Parker C, Kunins H, Paone D, 2014. RxStat: Technical Assistance Manual. 2014. New York City.. [Google Scholar]
- Huhn AS, Garcia-Romeu AP, Dunn KE, 2018. Opioid overdose education for individuals prescribed opioids for pain management: randomized comparison of two computer-based interventions. Front. Psychiatry 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AN, Bratberg JP, Monk M, Ferrentino J, 2018. Retention of student pharmacists’ knowledge and skills regarding overdose management with naloxone. Subst. Abus 39 (2), 193–198. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Porucznik CA, Anderson JW, Rolfs RT, 2011. State-level strategies for reducing prescription drug overdose deaths: utah’s prescription safety program. Pain Med. 12 (Suppl 2), S66–72. [DOI] [PubMed] [Google Scholar]
- Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B, 2014. Decline in drug overdose deaths after state policy changes - Florida, 2010–2012. MMWR Morb. Mortal. Wkly. Rep 63 (26), 569–574. [PMC free article] [PubMed] [Google Scholar]
- Keast SL, Kim H, Deyo RA, Middleton L, McConnell KJ, Zhang K, Ahmed SM, Nesser N, Hartung DM, 2018. Effects of a prior authorization policy for extended-release/long-acting opioids on utilization and outcomes in a state Medicaid program. Addiction. 113 (9), 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, Richey M, McGinty EE, Stuart EA, Barry CL, Webster DW, 2016. Opioid overdose deaths and Florida’s crackdown on pill mills. Am. J. Public Health 106 (2), 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, King VL, Peirce J, Kolodner K, Brooner RK, 2013. An observation of lower rates of drug use over time in community syringe exchangers. Am. J. Addict 22 (3), 271–276. [DOI] [PubMed] [Google Scholar]
- Kilaru AS, Perrone J, Auriemma CL, Shofer FS, Barg FK, Meisel ZF, 2014. Evidence-based narratives to improve recall of opioid prescribing guidelines: a randomized experiment. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine 21 (3), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Heverling H, Cordeiro M, Vasquez V, Stolbach A, 2016. Internet training resulted in improved trainee performance in a simulated opioid-poisoned patient as measured by checklist. J. Med. Toxicol 12 (3), 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch Y, Bowling KG, Miller LM, Ramos MA, 2013. The impact of science education games on prescription drug abuse attitudes among teens: a case study. J. Drug Educ 43 (3), 255–275. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, Grusing S, Devine B, Chou R, 2017. Primary care-based models for the treatment of opioid use disorder: a scoping review. Ann. Intern. Med 166 (4), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, Haake KJ, Hanling S, Hooten WM, Kertesz SG, Kravitz RL, Krebs EE, Stanos SP, Sullivan M, 2019. Challenges with implementing the centers for disease control and prevention opioid guideline: a consensus panel report. Pain Med. 20 (4), 724–735. [DOI] [PubMed] [Google Scholar]
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA, 2017. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain 158 (5), 833–839. [DOI] [PubMed] [Google Scholar]
- Lyapustina T, Rutkow L, Chang H-Y, Daubresse M, Ramji AF, Faul M, Stuart EA, Alexander GC, 2016. Effect of a “pill mill” law on opioid prescribing and utilization: the case of Texas. Drug Alcohol Depend. 159, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carrasco LR, Rhodes KV, 2016. Unused opioid analgesics and drug disposal following outpatient dental surgery: a randomized controlled trial. Drug Alcohol Depend. 168, 328–334. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Wolf MS, McConnell R, Sears J, Chevrier A, Ahlstrom E, Engel KG, Cameron KA, Adams JG, Courtney DM, 2015. Improving patient knowledge and safe use of opioids: a randomized controlled trial. Acad. Emerg. Med 22 (3), 331–339. [DOI] [PubMed] [Google Scholar]
- McClellan C, Lambdin BH, Ali MM, Mutter R, Davis CS, Wheeler E, Pemberton M, Kral AH, 2018. Opioid-overdose laws association with opioid use and overdose mortality. Addict. Behav 86, 90–95. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Boichat C, Eccleston C, 2012. Training for general practitioners in opioid prescribing for chronic pain based on practice guidelines: a randomized pilot and feasibility trial. J. Pain 13 (1), 32–40. [DOI] [PubMed] [Google Scholar]
- Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ, Morden NE, 2016. State legal restrictions and prescription-opioid use among disabled adults. N. Engl. J. Med 375 (1), 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl-Madrona L, Mainguy B, Plummer J, 2016. Integration of complementary and alternative medicine therapies into primary-care pain management for opiate reduction in a rural setting. J. Altern. Complement. Med 22 (8), 621–626. [DOI] [PubMed] [Google Scholar]
- Meisel ZF, Metlay JP, Sinnenberg L, Kilaru AS, Grossestreuer A, Barg FK, Shofer FS, Rhodes KV, Perrone J, 2016. A Randomized Trial Testing the Effect of Narrative Vignettes Versus Guideline Summaries on Provider Response to a Professional Organization Clinical Policy for Safe Opioid Prescribing. Ann. Emerg. Med 68 (6), 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SS, Babu KM, Androski C, Reznek MA, Kline JA, 2018. Effect of a Data-driven Intervention on Opioid Prescribing Intensity Among Emergency Department Providers: A Randomized Controlled Trial. Acad. Emerg. Med 25 (5), 482–493. [DOI] [PubMed] [Google Scholar]
- Mitchell L, 2009. Lock-in program promotes appropriate use of resource. J. Okla. State Med. Assoc 102. [PubMed] [Google Scholar]
- Morden NE, Zerzan JT, Rue TC, Heagerty PJ, Roughead EE, Soumerai SB, Ross-Degnan D, Sullivan SD, 2008. Medicaid prior authorization and controlled-release oxycodone. Med. Care 46 (6), 573–580. [DOI] [PubMed] [Google Scholar]
- Moyo P, Simoni-Wastila L, Griffin BA, Onukwugha E, Harrington D, Alexander GC, Palumbo F, 2017. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US States. Addiction 112 (10), 1784–1796. [DOI] [PubMed] [Google Scholar]
- Nam YH, Shea DG, Shi Y, Moran JR, 2017. State prescription drug monitoring programs and fatal drug overdoses. Am. J. Manag. Care 23 (5), 297–303. [PubMed] [Google Scholar]
- National Academies of Sciences Engineering, and Medicine, 2019. Medications for opioid use disorder save lives. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Naumann RB, Marshall SW, Lund JL, Gottfredson NC, Ringwalt CL, Skinner AC, 2018. Evaluating short- and long-term impacts of a Medicaid “lock-in” program on opioid and benzodiazepine prescriptions dispensed to beneficiaries. Drug Alcohol Depend. 182, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven D, Paulozzi L, Howell D, McPherson S, Murphy SM, Grohs B, Marsh L, Lederhos C, Roll J, 2016. A Randomized Controlled Trial of a Citywide Emergency Department Care Coordination Program to Reduce Prescription Opioid Related Emergency Department Visits. J. Emerg. Med 51 (5), 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle S, Hawkins JD, Kuklinski MR, Fagan AA, Fleming C, Rhew IC, Brown EC, Abbott RD, Catalano RF, 2015. Effects of Communities That Care on Males’ and Females’ Drug Use and Delinquency 9 Years After Baseline in a Community-Randomized Trial. Am. J. Community Psychol 56 (3–4), 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N, 2014. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: a randomized trial. J. Subst. Abuse Treat 46 (1), 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oregon State University (OSU), 2012. Drug Use Evaluation: Long-Acting Opioids (LAO). Oregon State University, Salem, OR. [Google Scholar]
- Paone D, Tuazon E, Kattan J, Nolan ML, O’Brien DB, Dowell D, Farley TA, Kunins HV, 2015. Decrease in rate of opioid analgesic overdose deaths - Staten Island, New York City, 2011–2013. MMWR Morb. Mortal. Wkly. Rep 64 (18), 491–494. [PMC free article] [PubMed] [Google Scholar]
- Papp J, Schrock J, 2017. 1 EMF take-home naloxone rescue kits following heroin overdose in the emergency department to prevent opioid overdose-related repeat emergency department visits, hospitalization, and death: a pilot study. Ann. Emerg. Med 70 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, 2016. Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction 112 (10), 1773–1783. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Fry CE, Jones TF, Buntin MB, 2016. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff. (Millwood) 35 (7), 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Desai HA, 2011. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 12 (5), 747–754. [DOI] [PubMed] [Google Scholar]
- Penm J, MacKinnon NJ, Boone JM, Ciaccia A, McNamee C, Winstanley EL, 2017. Strategies and policies to address the opioid epidemic: a case study of Ohio. J. Am. Pharm. Assoc 57 (2), S148–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanbeck A, Brown RT, Zgierska AE, Jacobson N, Robinson JM, Johnson RA, Deyo BM, Madden L, Tuan W-J, Alagoz E, 2018. A randomized matched-pairs study of feasibility, acceptability, and effectiveness of systems consultation: a novel implementation strategy for adopting clinical guidelines for Opioid prescribing in primary care. Implement. Sci 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji MA, Kuo Y-F, Chen N-W, Hasan H, Wilkes DM, Goodwin JS, 2017. Impact of Laws Regulating Pain Clinics on Opioid Prescribing and Opioid-Related Toxicity Among Texas Medicare Part D Beneficiaries. J. Pharm. Technol 33 (2), 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifler LM, Droz D, Bailey JE, Schnoll SH, Fant R, Dart RC, Bucher Bartelson B, 2012. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 13 (3), 434–442. [DOI] [PubMed] [Google Scholar]
- Reisman RM, S. PJ, Atherly AJ, Flowers CR, 2009. Prescription opioid usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst. Abus. Res. Treat 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhew IC, Hawkins JD, Murray DM, Fagan AA, Oesterle S, Abbott RD, Catalano RF, 2016. Evaluation of community-level effects of communities that care on adolescent drug use and delinquency using a repeated cross-sectional design. Prev. Sci 17 (2), 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwalt C, Shanahan M, Wodarski S, Jones J, Schaffer D, Fusaro A, Paulozzi L, Garrettson M, Ford M, 2015. A randomized controlled trial of an emergency department intervention for patients with chronic noncancer pain. J. Emerg. Med 49 (6), 974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Farley JF, Holmes GM, Oramasionwu CU, Ringwalt C, Sleath B, Skinner AC, 2016. Controlled Substance Lock-In Programs: Examining An Unintended Consequence Of A Prescription Drug Abuse Policy. Health Aff. 35 (10), 1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC, 2015. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern. Med 175 (10), 1642–1649. [DOI] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH, 2014. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA 312 (5), 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration), 2018. TIP 63: Medications for Opioid Use Disorder. https://store.samhsa.gov/system/files/sma18-5063fulldoc.pdf. [PubMed]
- Substance Abuse and Mental Health Services Administration, 2018. Key Substance Use and Mental Health Indicators in the United States: Results From the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Schiff DM, Drainoni ML, Weinstein ZM, Chan L, Bair-Merritt M, Rosenbloom D, 2017. A police-led addiction treatment referral program in Gloucester, MA: implementation and participants’ experiences. J. Subst. Abuse Treat 82, 41–47. [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2018. Drug and opioid-involved overdose deaths - United States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep 67 (5152), 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, Ondersma SJ, O’Grady KE, 2014. Computerized versus in-person brief intervention for drug misuse: a randomized clinical trial. Addiction 109 (7), 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, Haegerich TM, 2018a. Quantifying the epidemic of prescription opioid overdose deaths. Am. J. Public Health 108 (4), 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018b. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep 67 (12), 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Holland L, 2006. An Evaluation of Prescription Drug Monitoring Programs. US Department of Justice, Office of Justice Programs, Washington, DC. [Google Scholar]
- Skinner AC, Ringwalt C, Naumann RB, Roberts AW, Moss LA, Sachdeva N, Weaver MA, Farley J, 2016. Reducing opioid misuse: evaluation of a medicaid controlled substance lock-in program. J. Pain 17 (11), 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman JF, Peglow S, Schwartz AR, Burgo-Black L, McNamara K, Becker WC, 2017. Group visits for overdose education and naloxone distribution in primary care: a pilot quality improvement initiative. Pain Med. 18 (12), 2325–2330. [DOI] [PubMed] [Google Scholar]
- Spoth R, Trudeau L, Shin C, Ralston E, Redmond C, Greenberg M, Feinberg M, 2013. Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am. J. Public Health 103 (4), 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Bauer AM, Fulton-Kehoe D, Garg RK, Turner JA, Wickizer T, Franklin GM, 2016. Trends in opioid dosing among washington state medicaid patients before and after opioid dosing guideline implementation. J. Pain 17 (5), 561–568. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Gaster B, Russo J, Bowlby L, Rocco N, Sinex N, Livovich J, Jasti H, Arnold R, 2010. Randomized trial of web-based training about opioid therapy for chronic pain. Clin. J. Pain 26 (6), 512–517. [DOI] [PubMed] [Google Scholar]
- Sun BC, Lupulescu-Mann N, Charlesworth CJ, Kim H, Hartung DM, Deyo RA, John McConnell K, Griffey RT, 2017. Impact of hospital “Best practice” mandates on prescription opioid dispensing after an emergency department visit. Acad. Emerg. Med 24 (8), 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt HL, O’Grady C, Kurtz SP, Stivers Y, Cicero TJ, Dart RC, Chen M, 2014. Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiol. Drug Saf 23 (3), 314–320. [DOI] [PubMed] [Google Scholar]
- Syed UAM, Aleem AW, Wowkanech C, Weekes D, Freedman M, Tjoumakaris F, Abboud JA, Austin LS, 2018. Neer Award 2018: the effect of preoperative education on opioid consumption in patients undergoing arthroscopic rotator cuff repair: a prospective, randomized clinical trial. J. Shoulder Elbow Surg 27 (6), 962–967. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Rapoport AB, Rowley CF, Mukamal KJ, Stead W, 2018. An opioid overdose curriculum for medical residents: impact on naloxone prescribing, knowledge, and attitudes. Subst. Abus 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Council of Economic Advisers, 2017. The Underestimated Cost of the Opioid Crisis.
- Ury WA, Rahn M, Tolentino V, Pignotti MG, Yoon J, McKegney P, Sulmasy DP, 2002. Can a pain management and palliative care curriculum improve the opioid prescribing practices of medical residents? J. Gen. Intern. Med 17 (8), 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Dublin S, Walker RL, Parchman M, Shortreed SM, Hansen RN, Saunders K, 2016. The impact of opioid risk reduction initiatives on high-dose opioid prescribing for patients on chronic opioid therapy. J. Pain 17 (1), 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013b. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346, f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013a. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346, f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer MB, Hartung DM, Ahmed S, Nicolaidis C, 2016. A chronic opioid therapy dose reduction policy in primary care. Subst. Abus 37 (1), 141–147. [DOI] [PubMed] [Google Scholar]
- Weiner SG, Baker O, Poon SJ, Rodgers AF, Garner C, Nelson LS, Schuur JD, 2017. The effect of opioid prescribing guidelines on prescriptions by emergency physicians in Ohio. Ann. Emerg. Med 70 (6), 799–808 e791. [DOI] [PubMed] [Google Scholar]
- Yarbrough CR, 2018. Prescription drug monitoring programs produce a limited impact on painkiller prescribing in medicare part d. Health Serv. Res 53 (2), 671–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarowitz BJ, Stebelsky LA, Muma BK, Romain TM, Peterson EL, 2005. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy 25 (11), 1636–1645. [DOI] [PubMed] [Google Scholar]
- Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kulis VG, Teutsch SM, Pappaioanou M, 2000. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Task Force on Community Preventive Services. American journal of preventive medicine 18 (1 Suppl), 44–74. [DOI] [PubMed] [Google Scholar]
- Zisblatt L, Hayes SM, Lazure P, Hardesty I, White JL, Alford DP, 2017. Safe and competent opioid prescribing education: increasing dissemination with a train-the-trainer program. Subst. Abus 38 (2), 168–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


