Abstract
When Campylobacter jejuni NCTC 11351 was grown microaerobically in rich medium at 39°C, entry into stationary phase was followed by a rapid decline in viable numbers to leave a residual population of 1% of the maximum number or less. Loss of viability was preceded by sublethal injury, which was seen as a loss of the ability to grow on media containing 0.1% sodium deoxycholate or 1% sodium chloride. Resistance of cells to mild heat stress (50°C) or aeration was greatest in exponential phase and declined during early stationary phase. These results show that C. jejuni does not mount the normal phenotypic stationary-phase response which results in enhanced stress resistance. This conclusion is consistent with the absence of rpoS homologues in the recently reported genome sequence of this species and their probable absence from strain NCTC 11351. During prolonged incubation of C. jejuni NCTC 11351 in stationary phase, an unusual pattern of decreasing and increasing heat resistance was observed that coincided with fluctuations in the viable count. During stationary phase of Campylobacter coli UA585, nonmotile variants and those with impaired ability to form coccoid cells were isolated at high frequency. Taken together, these observations suggest that stationary-phase cultures of campylobacters are dynamic populations and that this may be a strategy to promote survival in at least some strains. Investigation of two spontaneously arising variants (NM3 and SC4) of C. coli UA585 showed that a reduced ability to form coccoid cells did not affect survival under nongrowth conditions.
Members of the genus Campylobacter are the major cause of bacterial gastroenteritis in the developed world, and in many countries the incidence of infection continues to increase (1). In the United Kingdom, for example, cases of Campylobacter infection have been increasing annually for the last 10 years and the number of cases of gastroenteritis attributed to Campylobacter is now more than triple that associated with Salmonella (http://www.phls.co.uk). In the United States it is estimated that Campylobacter strains cause more than two million cases of diarrhea annually (43). Although the symptoms can be severe, the illness is generally self-limiting and uncomplicated. However, serious sequelae, including acute neuromuscular paralysis due to Guillain-Barré syndrome and Miller-Fisher syndrome, can affect one in a thousand patients (29).
Campylobacter jejuni and Campylobacter coli are commensals of many domesticated animals and birds. Consequently, food, especially poultry, is considered to be the main vehicle of transmission. Although the ability of campylobacters to survive in food and in the environment is cardinal to their infective and contamination cycles, we know little of the mechanisms which influence the persistence of these pathogens outside the host. Campylobacter survival is, however, influenced by two important factors. First, the organisms are thermophilic and have a minimum growth temperature of 30°C (42). Consequently, when cells of the organism are excreted into the environment or introduced into food, they are unable to grow. Second, although there have been some reports which have demonstrated the aerobic growth of certain strains of C. jejuni and C. coli (18), they are generally considered to be microaerophilic; that is, they are unable to grow in, or tolerate, the normal atmospheric concentration of oxygen, and they grow best in atmospheres containing around 5% oxygen (24).
As Campylobacter cultures age or are exposed to stress conditions, morphological changes occur within cells. During exponential growth, vibrioid or bacillary forms predominate, whereas coccoid cells are formed as the culture ages or is exposed to stress (30, 37). A similar reduction in cell size also occurs in organisms such as Vibrio vulnificus and Helicobacter pylori and has been associated with transformation to the viable but nonculturable state (34). Other evidence suggests that coccoid cells are not formed by an active differentiation process but represent a degenerate form of cell which may still retain metabolic activity for a period prior to death (3, 13, 26).
When pure cultures of many bacterial species are grown in standard laboratory media, the organisms grow exponentially until conditions no longer support rapid growth, and the cells then enter stationary phase. In the majority of bacterial species characterized to date, entry into the stationary phase, or starvation, is accompanied by profound structural and physiological changes that result in increased resistance to heat shock, oxidative, osmotic, and acid stresses (19, 21, 33, 46). In Escherichia coli, for example, the expression of 30 or more genes is induced in response to entry into stationary phase or starvation, a process that is regulated by the stationary-phase sigma factor RpoS (23).
The aim of this study was to evaluate the responses of C. jejuni and C. coli to stationary phase in order to determine whether or not mechanisms for stationary-phase adaptation existed. In addition, since coccoid cells are formed during prolonged exposure to stationary phase, we also sought to elucidate the role that this cell type played within the population. In this context, two variants of C. coli UA585 which had reduced rates of coccoid cell production were also studied.
MATERIALS AND METHODS
Bacterial strains.
C. jejuni NCTC 11351 (type strain) was obtained from the National Collection of Type Cultures (Colindale, United Kingdom). C. coli UA585, originally isolated from a diarrheic pig, was a generous gift from D. E. Taylor (University of Alberta, Edmonton, Canada). All strains were stored at −70°C in Microbank vials (Pro-Lab Diagnostics, Neston, Cheshire, United Kingdom).
Growth of organisms.
Campylobacters were grown in BBFBP, consisting of brucella broth (Difco, East Moseley, United Kingdom) containing one vial of FBP campylobacter growth supplement (Oxoid, Basingstoke, United Kingdom) per 500 ml. Cultures were prepared as follows. A frozen bead was inoculated into 50 ml of fresh BBFBP contained in a 100-ml flask. This was then incubated at 39°C on a shaking platform at 150 rpm for 24 h under microaerobic conditions (5% [vol/vol] oxygen, 10% [vol/vol] carbon dioxide, and 85% [vol/vol] nitrogen) maintained using a variable-atmosphere incubator (VAIN) (Don Whitely, Otley, United Kingdom). The cells were then diluted 1:100 into 50 ml of fresh BBFBP and grown for 24 h to stationary phase before being used as an inoculum for batch culture studies. Experimental cultures were produced by inoculating 100 ml of fresh BBFBP with 200 μl of 24-h stationary-phase culture (see above) and incubating at 39°C with shaking in the VAIN cabinet. Cultures were incubated for 6 or 24 h to produce exponential- or stationary-phase cells, respectively.
Viable counts.
Serial 10-fold dilutions were prepared in maximum-recovery diluent (Oxoid), and 20-μl volumes were spread onto fresh BMHA plates, comprising Mueller-Hinton agar (MHA) (Oxoid) containing 5% defibrinated sheep's blood (TCS, Basingstoke, United Kingdom) and one vial of FBP campylobacter supplement per 500 ml. The number of CFU was assessed after plates had been incubated at 39°C in the VAIN for a minimum of 48 h.
Measurement of sublethal injury.
Sublethally injured cells were defined as those unable to tolerate 0.1% sodium deoxycholate (DOC) or 1% sodium chloride in the growth medium (15). The proportion of sublethally injured cells in the population was thus estimated by comparing plate counts on BMHA and MHA containing 0.1% (wt/vol) DOC (MHAD) or 1% (wt/vol) sodium chloride (MHAN). Sensitivity to bile salts and sodium chloride in gram-negative bacteria is generally attributed to disturbance of membrane structure and/or function (16, 25, 31). Preliminary experiments established that recovery on the restrictive media was 100% for uninjured cells.
Heat resistance.
C. jejuni 11351 cultures were grown to the desired growth phase in BBFBP at 39°C in the VAIN. Samples (1 ml) were aseptically transferred to sterile glass freeze-drying vials (Fisher, Loughborough, United Kingdom), sealed immediately in a flame, and immersed in a water bath set at 50°C. The temperature was monitored using a mercury-in-glass thermometer calibrated to British Standard BS1704. At intervals a vial was removed, cooled on ice, and then broken, and viable counts were determined on BMHA.
Resistance to aeration.
Cultures at the required growth phase were diluted 1:20 in 100 ml of prewarmed phosphate-buffered saline (PBS) (Oxoid) in a 250-ml flask to give cell concentrations of approximately 106 CFU ml−1 for exponential-phase cells and 107 CFU ml−1 for stationary-phase cultures. The flasks were incubated in air at 37°C in a shaking water bath set at 90 rpm. Samples were removed at intervals for the determination of viable counts by plating onto BMHA.
Estimation of coccoid cell numbers by microscopy.
Samples (2 ml) collected from cultures or cell suspensions were fixed by the addition of formaldehyde to a final concentration of 3.7% (vol/vol). The proportions of vibrioid and coccoid cells were estimated by microscopy of cells immobilized on an agar slide. A clean microscope slide was dipped into molten 1% agar and allowed to air dry, and agar was removed from the underside. Two orientation markers were spotted onto a coverslip (50 by 22 mm; Merck) using a permanent marker pen, and a small volume (approximately 1 μl) of well-mixed cell suspension was placed between them. The coverslip was then turned upside down and gently lowered onto the agar. The slide was viewed by phase-contrast microscopy using a 100× oil immersion lens on a Nikon Microphot SA microscope. Twenty fields were counted for each preparation, and results were expressed as the percentage of each cell type.
Isolation of C. coli UA585 variants with altered motility.
C. coli UA585 was inoculated into Mueller-Hinton broth (Oxoid) and incubated, with shaking, at 42°C for 72 h. Dilutions of the resulting culture were plated onto MHA or MHAD. Growth of surviving cells on MHAD revealed the presence of equal numbers of motile (large, swarming morphology) and nonmotile (pinpoint morphology) colonies. Motility of these variants was assessed as described previously (8). Colonies of representative motile and nonmotile variants were picked, purified by restreaking, and designated C. coli SC4 and C. coli NM3, respectively.
RESULTS
Occurrence of sublethal injury in stationary-phase cells of C. jejuni
Viable counts of C. jejuni NCTC 11351 (type strain) were monitored on BMHA, MHAD, or MHAN during growth and as cells entered the stationary phase (Fig. 1). Immediately after inoculation with stationary-phase cells, the number of colonies recorded on medium containing 1% NaCl was 100-fold less than that recorded on either BMHA or MHAD, demonstrating the existence of sublethally injured cells in the inoculum. After cells had emerged from a short lag phase, the counts became similar on all media, indicating that sublethal injury was absent in growing cells.
FIG. 1.
Sublethal injury and loss of viability in C. jejuni NCTC 11351 following entry into stationary phase. Cells were grown in shaken culture under microaerobic conditions in BBFBP at 39°C. Viable counts were determined on BMHA (○), MHAD (●), and MHAN (▾). The experiment was repeated four times, and results of a single experiment are shown.
After maximum cell density had been achieved at about 20 h, viable numbers estimated on MHA remained roughly constant for about 5 h and then began to decline. However, during the interval before total viable numbers decreased, cells became sensitive first to NaCl and then to DOC, as evidenced by the decrease in counts on MHAN and MHAD, respectively. Increased sensitivity to bile salts or sodium chloride in gram-negative organisms is often taken to indicate loss of integrity or impairment of homeostatic functions associated with the outer and cytoplasmic membranes, respectively (16, 25). This suggests that membrane damage in the stationary-phase population preceded loss of viability. After about 50 h of incubation, counts on all three media were broadly similar again, indicating that the population remaining after the initial decline in numbers was not sublethally injured.
The degree of inhibition of sublethally injured cells by selective agents depends on the severity of injury and concentration of the selective agent (25). It appears that 1% NaCl was more inhibitory to injured cells than 0.1% DOC, because loss of resistance to NaCl preceded loss of resistance to DOC during stationary phase and because injured cells in the inoculum were sensitive to NaCl but not DOC.
Sensitivity of stationary-phase populations of C. jejuni to stress.
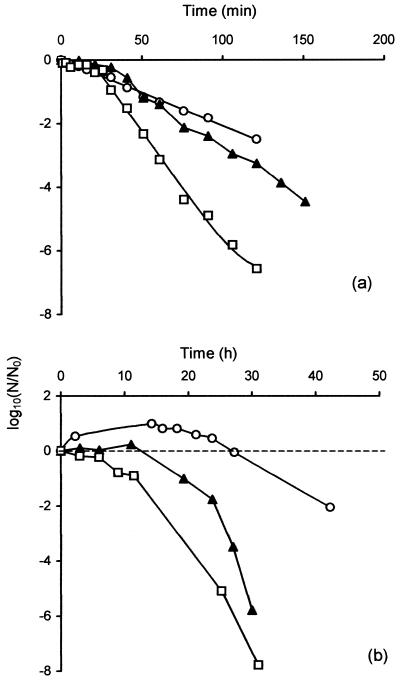
A residual population of cells, representing about 1% of the maximum stationary-phase population, remained viable for an extended period (Fig. 1). It was of interest to determine whether this population, of cells that had just entered stationary phase, displayed the increased resistance to stress characteristic of the stationary-phase response of other bacteria. Samples taken during exponential growth (6 h), during early stationary phase (24 h), or after 48 h (residual population) were heated at 50°C or aerated in PBS at 37°C. Resistance to heating at 50°C was greatest in exponential-phase cells and least in the residual population in late stationary phase (48 h). Cells from early stationary phase were of intermediate heat resistance (Fig. 2a).
FIG. 2.
Effect of growth phase on resistance of C. jejuni NCTC 11351 to heat and aeration. Cells were grown in BBFBP to exponential phase (○), early stationary phase (24 h) (▴), and late stationary phase (48 h) (□). Samples were heated to 50°C (a) or diluted 1:20 in prewarmed PBS prior to aeration at 37°C and 90 rpm (b). The experiment was repeated three times, and results from a single experiment are shown.
A similar order of resistance was seen in aerated cells (Fig. 2b). In all cases there was a delay before viable numbers decreased, with this delay being shortest in the more sensitive late-stationary-phase cells. Viable counts of exponential-phase cells increased slightly before subsequently decreasing. Although the absolute level of resistance to heat and aeration varied somewhat between experiments, the same relative order of resistance in exponential-, early-stationary-, and late-stationary-phase cultures was confirmed in each of three independent experiments.
Heat resistance of C. jejuni cells during extended stationary phase.
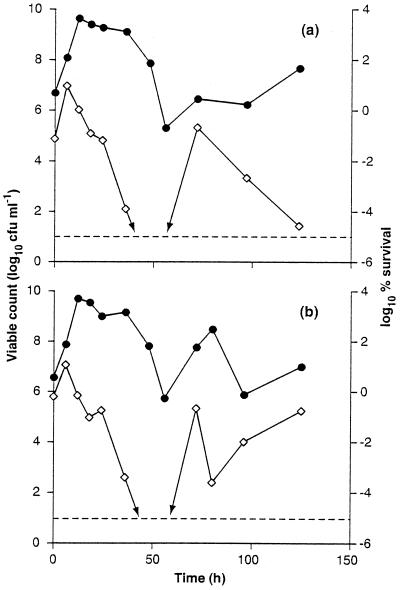
To obtain more detailed information about changes in resistance during the growth cycle of C. jejuni, heat resistance was monitored during extended incubation in stationary phase. Samples were removed at intervals and subjected to a standard heat challenge of 50°C for 75 min. Data from two representative experiments are presented in Fig. 3. Heat resistance initially increased as cells from the inoculum entered exponential growth, but once the culture entered stationary phase, total viable numbers declined and there was a progressive decrease in heat resistance. After about 50 h of incubation, survival after the heat treatment had decreased to below the limits of detection (100 CFU/ml). Unexpectedly, heat resistance increased after this, and survivors were once more detected following the heat challenge to the 72-h sample. After 72 h there were further fluctuations in total viable cell numbers and heat resistance. In both experiments the reappearance of cells surviving heat treatment coincided with an increase in cell numbers which probably reflected cryptic bacterial growth in the population. The heat resistance data are expressed as percent survival to correct for changes in the total viable population. The results thus reflect changes in resistance and are not simply due to changes in the initial (preheat) numbers of cells present. Nevertheless, the failure to recover cells after a heat challenge to the 72-h cultures is undoubtedly due to low inherent heat resistance combined with low initial cell numbers in these samples.
FIG. 3.
Changes in viable numbers and heat resistance of C. jejuni NCTC 11351 during extended incubation in stationary phase. Cells were grown to stationary phase in BBFBP under microaerobic conditions at 39°C. Samples were removed at intervals for the determination of viable counts (●) and survival after a heat challenge at 50°C for 75 min (⋄). Results from two representative experiments are shown. Data in panel a are from a single determination at each time interval, and those in panel b are the means of duplicates.
Role of coccoid cells in the persistence of C. coli during stationary phase.
The survival of C. coli strain UA585 during stationary phase was also studied. The general pattern of survival was very similar to that seen in C. jejuni; i.e., following entry into the stationary phase, there was a period of about 5 h when viable numbers remained more or less constant, followed by a decline to leave a residual population that persisted for at least a further 200 h (data not shown).
During these experiments, it was noted that cells taken from cultures 72 h old or older gave rise to two different colony morphology phenotypes, in approximately equal numbers, when plated on medium containing 0.1% DOC. Both a flat, spreading colony variant, reminiscent of the wild type, and a small, domed colony type were apparent. After isolation, the colony variants could be maintained as stable cultures. A representative of the flat, spreading colony variant, designated SC4, and one of the small, domed colony type, designated NM3, were chosen for further study.
Preliminary microscopic observation of SC4 and NM3 revealed a number of distinctive phenotypic characteristics compared to those of the wild type (UA585). First, although strain SC4 was motile, NM3 was not. Second, when cells of SC4 and NM3 were taken from aged cultures, there appeared to be fewer cocci present than were seen in cultures of UA585 of the same age. The occurrence of similar pinpoint and spreading colonies in strains of C. jejuni has previously been associated with phase variation in the expression of flagella but not with changes in the ability to form cocci (8).
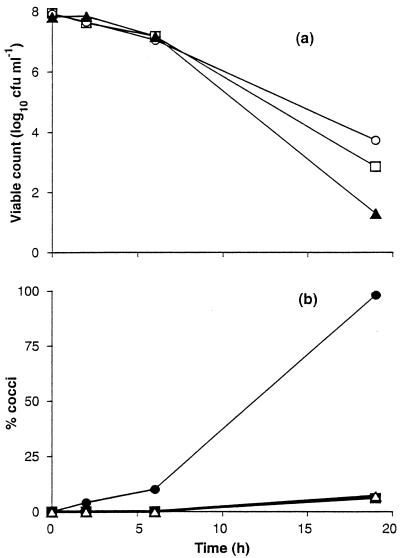
To confirm the altered ability of NM3 and SC4 to form coccoid cells, exponentially grown cells were introduced into PBS and then incubated aerobically with shaking, a condition which has previously been shown to induce the formation of cocci (20, 26, 41). Over the period of incubation (20 h), survival of the wild-type UA585 and the two variants SC4 and NM3 was found to be similar (Fig. 4). However, the variants gave rise to significantly fewer cocci that the wild-type C. coli UA585 (Fig. 4). For example, at the end of the incubation period, cocci accounted for 98% of the C. coli UA585 cell population, while the populations of NM3 and SC4 contained only 6 and 7% cocci, respectively.
FIG. 4.
Coccoid cell formation and survival in C. coli exposed to air. Stationary-phase cells grown in BBFBP were diluted 1:20 in PBS and aerated at 37°C at 90 rpm. Viability (a) was determined at intervals for C. coli UA585 (○), C. coli NM3 (□), and C. coli SC4 (▴). The formation of coccoid cells (b) was determined at the same time for C. coli UA585 (●), C. coli NM3 (▪), and C. coli SC4 (▵). The curves show the data from a single experiment.
To determine whether the diminished rate of formation of cocci seen with NM3 and SC4 was due to a specific response to the aerobic conditions used or to a more general deficiency in the ability of cells to form cocci, cultures of UA585, NM3, and SC4 were shaken at 39°C in BBFBP under microaerobic conditions, and the ability to form cocci was assessed (Table 1). Again, the variants SC4 and NM3 gave rise to fewer cocci. For example, after 126 h of incubation, cocci represented 97% of the UA585 cell population but only 16 and 19% the populations of SC4 and NM3, respectively. Thus, it would appear that the relative inability of NM3 and SC4 to form cocci is caused by a general defect in the mechanism that governs coccoid cell formation.
TABLE 1.
Percentages of coccoid cells within cultures of C. coli UA585, SC4, and NM3 incubated in BBFBP at 39°C under microaerobic conditions for extended periods of time
| Incubation time (h) | % of coccoid cells of C. coli:
|
||
|---|---|---|---|
| UA585 | SC4 | NM3 | |
| 24 | 7 | 1 | 1 |
| 48 | 27 | 4 | 7 |
| 126 | 97 | 16 | 19 |
| 267 | 99 | 17 | 22 |
DISCUSSION
It has been shown for several species of bacteria that entry into the stationary phase is accompanied by structural and physiological changes that result in the increased resistance of the cell to a variety of inimical conditions (21, 33, 46). However, despite the significance of campylobacters as food-borne pathogens, we know little of the response of these organisms to aging and starvation and of their adaptation to stationary phase. This is perhaps surprising, since these conditions are especially relevant to their survival in food and the environment. In E. coli, other members of the Enterobacteriaceae, Pseudomonas aeruginosa, and Vibrio cholerae, the central regulator for many stationary-phase-induced changes is the sigma factor RpoS (11, 17, 48), which, accordingly, is critical for the survival of the bacterial cell in stationary phase and during exposure to unfavorable conditions (14, 19, 23, 28, 45). However, in Legionella pneumophila an rpoS gene homologue has been identified, but it appears to have a function different from that in E. coli (12).
To study the process of aging and stationary-phase adaptation in C. jejuni, we initially assessed sublethal injury in stationary-phase and aged cultures of C. jejuni by recovering cells on media containing either 0.1% DOC or 1% NaCl. In gram-negative enteric organisms and pseudomonads, resistance to lipophilic dyes, bile salts, and many lipophilic antibiotics is due to their restricted penetration through the outer membrane combined with active efflux through multidrug transporter systems (32). Resistance to elevated levels of salt depends on the passive barrier properties of the cytoplasmic membrane to sodium ions combined with the action of a battery of osmoregulatory transport mechanisms (2, 7, 47). Although there is little information available specifically for campylobacters, it is likely that increased sensitivity to DOC and sodium chloride reflects perturbation of the barrier properties of outer and cytoplasmic membranes, respectively, and/or interference with active homeostatic mechanisms. Consequently, it is likely that disruption to both the inner and outer membrane structures or functions occurs upon entry into stationary phase and upon aging. The appearance of such sublethally injured cells is inconsistent with the paradigm of increased resistance of stationary-phase cells, which has been observed for many other gram-negative bacteria.
Since cells taken from the stationary phase of growth were also found to be more sensitive to mild heat stress and aeration under nongrowing conditions than exponential-phase cells, it is unlikely that these resistance mechanisms are regulated in a stationary-phase-dependent manner. Analysis of the C. jejuni NCTC 11168 genome sequence (36) indicates that RpoS is absent from this organism. While the genome sequence of strain NCTC 11351 has not yet been determined, it is very likely that RpoS is also absent from this strain, since we have been unable to isolate the rpoS gene from this strain using two alternative techniques. In hybridization-based assays a DNA probe containing a fragment of the E. coli rpoS gene (27) failed to hybridize to any homologous DNA in the genome of NCTC 11351 (S. F. Park, unpublished data). A second technique used in an attempt to isolate an rpoS gene from this Campylobacter strain was complementation of an rpoS-deficient strain of E. coli, a technique which has been used to isolate rpoS from other organisms (40). However, when a plasmid library of strain NCTC 11351 chromosomal DNA (38) was used in an attempt to complement an rpoS mutant of E. coli, no complementing plasmids were identified. The absence of an RpoS homologue is entirely consistent with the failure of C. jejuni NCTC 11351 to induce stress resistance in the stationary phase.
The survival of campylobacters during exposure to heat stress and aeration may involve aspects of the heat shock and oxidative stress responses, respectively. While superoxide dismutase is known to be essential for the survival of campylobacters during exposure to air (39), little is known of the mechanisms which regulate the expression of this enzyme. Again, while C. jejuni is known to elicit a heat shock response (22), the regulatory mechanisms governing this response have not yet been studied in detail. However, there are potentially three alternative regulatory systems controlling the induction of the heat shock response in C. jejuni, the RacRS regulon (4) and orthologues of HrcA and HspR (35). In view of the increased sensitivity of stationary-phase cells compared with exponentially grown cells, it seems unlikely that aspects of the heat shock response and oxidative stress response in C. jejuni are upregulated in a stationary-phase-dependent manner by an alternative regulator of the stationary-phase response.
The lack of a stationary-phase response in C. jejuni was demonstrated here in a single strain, NCTC 11351. Although the results are consistent with the absence of an rpoS homologue in this strain, it is possible that other strains may behave differently given the genetic plasticity of this species (36). Recently, Cappelier et al. (6) reported an increase in heat resistance in C. jejuni strain 79 as cells entered starvation in a surface water microcosm. The reason for the difference between their results and ours may perhaps be due to strain differences or differences in methodology. In our work, the method of assessing viability after a heat challenge was colony formation, whereas the method of Cappelier et al. (6) was based on loss of metabolic activity in single cells. Alternatively, the increase in heat resistance shown by Cappelier et al. may be due to an rpoS-independent mechanism.
When the resistance of cultures of C. jejuni to heat was monitored during more prolonged periods of aging, an unusual fluctuating profile of heat resistance was observed, in which resistance decreased progressively soon after cells entered stationary phase and then increased again. In all repeats of this experiment, the restoration of heat resistance coincided with a point in the survival curve where total viable numbers increased. It thus appears likely that the appearance of heat resistance is associated with the emergence of a new population due to the regrowth of bacterial cells in the stationary-phase culture. At present it is not clear whether these cells have the same genotype and phenotype as the original inoculum or whether they represent a subpopulation of cells with a competitive advantage in stationary phase, similar to GASP (growth advantage in stationery phase) mutants of E. coli (10, 49, 50, 51) or phenotypic variants of Mycobacterium smegmatis (44).
When cells from cultures of C. coli UA585 which had been incubated for 72 h were plated onto MHAD, equal numbers of motile (large, swarming morphology) and nonmotile (pinpoint morphology) colonies emerged. Intriguingly, strains derived from both of these colony types showed a reduced ability to form cocci compared to the wild-type strain that had been used as an inoculum for the culture. The appearance of these variant cell types, with different phenotypic characteristics, following prolonged incubation is consistent with the fact that aged stationary-phase cultures of campylobacters are dynamic populations of variant cell types. Indeed since 10 of 10 survivors tested at this stage had this phenotype, it is likely that these variants had largely replaced the original population.
The availability of variants compromised in the ability to form cocci also allowed the role of this cell type in stationary-phase survival of Campylobacter to be investigated. Although NM3 and SC4 produced significantly fewer cocci, their survival rates were similar to that of the parental strain under the conditions tested. Thus, the formation of cocci did not enhance the ability of C. coli to persist in nongrowth environments. The onset of sublethal injury as cells enter stationary phase would be consistent with the early stages of a degenerative process in a fraction of the population leading to the production of coccoid cells. This view is consistent with the findings of Hazeleger et al. (13) that transition to the coccoid stage is not an active process, implying that coccoid cells are unlikely to represent differentiated resistant forms produced in response to stress. Moreover, the maintenance of long-term viability in starved cells of C. jejuni was recently shown to be associated with vibrioid cells in the population rather than cocci, which is also consistent with the conclusions drawn here (5, 9).
Generally, it is assumed that bacteria isolated from the stationary phase are more resistant to environmental stresses and toxic agents than cells in the exponential phase of growth and that this is a programmed adaptation mediated by alternative sigma factors, including RpoS in gram-negative bacteria. The results presented here argue that stationary-phase cultures of C. jejuni do not enter an RpoS-mediated resistant state as has been observed for a number of other gram-negative bacteria. Instead, the fluctuations in heat resistance in stationary-phase cultures of C. jejuni and the emergence of variants of C. coli with altered phenotypes that apparently replace the original population support the hypothesis that stationary-phase cultures of C. jejuni and C. coli are dynamic populations of cells and that this may be an alternative mechanism for promoting continued survival.
ACKNOWLEDGMENT
We are grateful to the Food Standards Agency/Ministry of Agriculture Fisheries and Food, London, United Kingdom, for financial support of this work.
REFERENCES
- 1.Blaser M J. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl 2):S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 2.Booth I R. The regulation of intracellular pH in bacteria. In: Chadwick D J, Cardew G, editors. Bacterial responses to pH. Chichester, United Kingdom: John Wiley and Sons; 1999. pp. 19–37. [Google Scholar]
- 3.Boucher S N, Slater E R, Chamberlain A H, Adams M R. Production and viability of coccoid forms of Campylobacter jejuni. J Appl Bacteriol. 1994;77:303–307. doi: 10.1111/j.1365-2672.1994.tb03078.x. [DOI] [PubMed] [Google Scholar]
- 4.Bras A, Chatterjee M S, Wren B W, Newell D G, Ketley J M. A novel Campylobacter jejuni two-component regulatory system important for the temperature-dependent growth and colonization. J Bacteriol. 1999;181:3298–3302. doi: 10.1128/jb.181.10.3298-3302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappelier J M, Magras C, Jouve J L, Federighi M. Recovery of viable but non-culturable Campylobacter jejuni cells in two animal models. Food Microbiol. 1999;16:375–383. [Google Scholar]
- 6.Cappelier J M, Rossero A, Federighi M. Demonstration of a protein synthesis in starved Campylobacter jejuni cells. Int J Food Microbiol. 2000;55:63–67. doi: 10.1016/s0168-1605(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 7.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1210–1223. [Google Scholar]
- 8.Diker K S, Hascelik G, Akan M. Reversible expression of flagella in Campylobacter spp. FEMS Microbiol Lett. 1992;99:261–264. doi: 10.1016/0378-1097(92)90037-o. [DOI] [PubMed] [Google Scholar]
- 9.Federighi M, Tholozan J L, Cappelier J M, Tissier J P, Jouve J L. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998;15:539–550. [Google Scholar]
- 10.Finkel S E, Kolter R. Evolution of microbial diversity during prolonged starvation. Proc Natl Acad Sci USA. 1999;96:4023–4027. doi: 10.1073/pnas.96.7.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita M, Tanaka K, Takahashi H, Amemura A. Transcription of the principal sigma-factor genes, rpoD and rpoS, in Pseudomonas aeruginosa is controlled according to the growth phase. Mol Microbiol. 1994;13:1071–1077. doi: 10.1111/j.1365-2958.1994.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 12.Hales L M, Shuman H A. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol. 1999;181:4879–4889. doi: 10.1128/jb.181.16.4879-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazeleger W C, Janse J D, Koenraad P M, Beumer R R, Rombouts F M, Abee T. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl Environ Microbiol. 1995;61:2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey T J, Cruickshank J G. Antibiotic and deoxycholate resistance in Campylobacter jejuni following freezing or heating. J Appl Bacteriol. 1985;59:65–71. doi: 10.1111/j.1365-2672.1985.tb01777.x. [DOI] [PubMed] [Google Scholar]
- 16.Hurst A. Bacterial injury: a review. Can J Microbiol. 1977;23:936–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- 17.Iriarte M, Stainier I, Cornelis G R. The rpoS gene from Yersinia enterocolitica and its influence on expression of virulence factors. Infect Immun. 1995;63:1840–1847. doi: 10.1128/iai.63.5.1840-1847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D M, Sutcliffe E M, Rios R, Fox A J, Curry A. Campylobacter jejuni adapts to aerobic metabolism in the environment. J Med Microbiol. 1993;38:145–150. doi: 10.1099/00222615-38-2-145. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen F, Bally M, Chapon-Herve V, Michel G, Lazdunski A, Williams P, Stewart G S. RpoS-dependent stress tolerance in Pseudomonas aeruginosa. Microbiology. 1999;145:835–844. doi: 10.1099/13500872-145-4-835. [DOI] [PubMed] [Google Scholar]
- 20.Karmali M A, Allen A K, Fleming P C. Differentiation of catalase-positive campylobacters with special reference to morphology. Int J Syst Bacteriol. 1981;3:64–71. [Google Scholar]
- 21.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 22.Konkel E M, Kim B J, Klena J D, Young C R, Ziprin R. Characterization of the thermal stress response of Campylobacter jejuni. Infect Immun. 1998;66:3666–3672. doi: 10.1128/iai.66.8.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewen P C, Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 24.Luechtefeld N W, Reller L B, Blaser M J, Wang W L. Comparison of atmospheres of incubation for primary isolation of Campylobacter fetus subsp. jejuni from animal specimens: 5% oxygen versus candle jar. J Clin Microbiol. 1982;15:53–57. doi: 10.1128/jcm.15.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackey B M. Injured bacteria. In: Lund B M, Baird-Parker A, Gould R, editors. The microbiological safety and quality of food. Gaithersburg, Md: Aspen Publishers Inc.; 2000. pp. 315–341. [Google Scholar]
- 26.Moran A P, Upton M E. A comparative study of the rod and coccoid forms of Campylobacter jejuni ATCC 29428. J Appl Bacteriol. 1986;60:103–110. doi: 10.1111/j.1365-2672.1986.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 27.Mulder N J, Powles R E, Zappe H, Steyn L M. The Mycobacterium tuberculosis mysB gene product is a functional equivalent of the Escherichia coli sigma factor, KatF. Gene. 1999;240:361–370. doi: 10.1016/s0378-1119(99)00430-8. [DOI] [PubMed] [Google Scholar]
- 28.Munro P M, Flatau G N, Clement R L, Gauthier M J. Influence of the RpoS (KatF) sigma factor on maintenance of viability and culturability of Escherichia coli and Salmonella typhimurium in seawater. Appl Environ Microbiol. 1995;61:1853–1858. doi: 10.1128/aem.61.5.1853-1858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachamkin I, Allos B M, Ho T. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng L K, Sherburne R, Taylor D E, Stiles M E. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985;164:338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido H. Microdermatology: cell surface in the interaction of microbes with the external world. J Bacteriol. 1999;181:4–8. doi: 10.1128/jb.181.1.4-8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nystrom T, Flardh K, Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990;172:7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Publishing Corporation; 1993. pp. 239–272. [Google Scholar]
- 35.Park S F. Environmental regulatory genes. In: Nachamkin I, Blaser M J, editors. Campylobacter. Washington, D.C.: American Society for Microbiology; 2000. pp. 423–440. [Google Scholar]
- 36.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the foodborne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 37.Pead P J. Electron microscopy of Campylobacter jejuni. J Med Microbiol. 1979;12:383–385. doi: 10.1099/00222615-12-3-383. [DOI] [PubMed] [Google Scholar]
- 38.Purdy D, Park S F. Cloning, nucleotide-sequence and characterisation of a gene encoding superoxide-dismutase from Campylobacter jejuni and Campylobacter coli. Microbiology. 1994;140:1203–1208. doi: 10.1099/13500872-140-5-1203. [DOI] [PubMed] [Google Scholar]
- 39.Purdy D, Cathraw S, Dickinson J H, Newell D G, Park S F. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in campylobacter survival and colonization. Appl Environ Microbiol. 1999;65:2540–2546. doi: 10.1128/aem.65.6.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-Gonzalez M, Molin S. Cloning, sequencing, and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J Bacteriol. 1998;180:3421–3431. doi: 10.1128/jb.180.13.3421-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skirrow B M, Benjamin J. ‘1001 Campylobacters’: cultural characteristics of intestinal campylobacters from man and animals. J Hyg Camb. 1979;85:427–442. doi: 10.1017/s0022172400063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skirrow B M, Blaser M J. Clinical and epidemiological considerations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 3–8. [Google Scholar]
- 44.Smeulders J M, Keer J, Speight R A, Williams H D. Adaptation of Mycobacterium smegmatis to stationary phase. J Bacteriol. 1999;181:270–283. doi: 10.1128/jb.181.1.270-283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh S J, SiloSuh L, Woods D E, Hassett D J, West S E H, Ohman D E. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trainor V C, Udy R K, Bremer P J, Cook G M. Survival of Streptococcus pyogenes under stress and starvation. FEMS Microbiol Lett. 1999;176:421–428. doi: 10.1111/j.1574-6968.1999.tb13692.x. [DOI] [PubMed] [Google Scholar]
- 47.Weerkamp A, Geerts W, Vogels G D. Conditional killing effect of Staphylococcin 1580 and repair of sublethal injury in Staphylococcus aureus. Antimicrob Agents Chemother. 1977;12:314–321. doi: 10.1128/aac.12.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yildiz F H, Schoolnik G K. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;180:773–784. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zambrano M M, Siegele D A, Almiron M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]
- 50.Zinser E R, Kolter R. Mutations enhancing amino acid catabolism confer a growth advantage in stationary phase. J Bacteriol. 1999;181:5800–5807. doi: 10.1128/jb.181.18.5800-5807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinser E R, Kolter R. Prolonged stationary-phase incubation selects for lrp mutations in Escherichia coli K-12. J Bacteriol. 2000;182:4361–4365. doi: 10.1128/jb.182.15.4361-4365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]