Abstract
Objective: To determine the frequency of specific and non-specific inhibitors in haemophilia A patients.
Study design: This is a cross-sectional study.
Patients and methods: A total of 150 male haemophilia A patients were included in this cross-sectional study at the National Institute of Blood Diseases and Bone Marrow Transplant (NIBD), Karachi, Pakistan, from September 2019 to January 2022.
Results: Among 150 patients included in this study, 23 (15.3%) had an inhibitor and 127 (84.6%) did not. All patients had specific inhibitors against Factor VIII (FVIII). Non-specific inhibitors were not identified in our population. Among the patients in the inhibitor group, there were 13 (56.5%) in the severe (<1%) category. There were 10 (43.5%) patients in the moderate (1-5%) category. There were no patients in the mild category. The median inhibitor level was 15.4 Bethesda unit (BU).
Conclusion: The development of inhibitors has not been identified as a major problem in our population. However, it is noteworthy that only 15.3% of patients with haemophilia A developed inhibitors in this data set. They were essentially treated with plasma and its products.
Keywords: fviii, non specific inhibitors, plasma, factor viii inhibitors, factor viii, s: haemophilia complications
Introduction
Haemophilia A affects one in 5000 men. It is an X-linked hereditary disorder characterized by bleeding due to the lack of clotting factor VIII (FVIII). FVIII is a cofactor, which is responsible in the instigation of factor X via factor IXa. Classification of point mutations in the human FVIII gene has been rather challenging owing to its huge size and its increasingly complex design [1].
Subjects are noted to present themselves to the hospital with bleeding gums, epistaxis, hemarthrosis, and hematoma [2]. It is challenging to control incidents of bleeding among these patients. Literature shows that replacing FVIII is a reliable treatment option to halt bleeding [3]. However, the development of neutralizing antibodies against the administered exogenous FVIII, which are known as inhibitors, has been a longstanding complication [4].
Inhibitors are classified as specific and non-specific inhibitors (lupus). They are antibodies that are responsible for neutralizing factors that result in clotting. FVIII inhibitor results from alloantibodies among subjects with haemophilia A who have been given exogenous FVIII [5]. The development of antibodies is multifaceted, linking both non-genetic risk factors as well as endogenous genetic factors.
FVIII mutation has been researched widely. Scientists have demonstrated an increasing interest in the effect of polymorphisms when studying immune-regulatory genes. Approximately 20-30% of severe haemophilia A cases and 5-15% of moderate to mild haemophilia A cases tend to acquire inhibitors [4,5]. It has been indicated in research that huge deletions, impede mutations of codon and inversions 22 were correlated with bigger odds to make the inhibitor as opposed to missense mutations and minor deletions (5% vs 35% ). Exposure to certain commodities in the early part of life escalates the probability of its development [6].
Scientific progress has led to the manufacturing of plasma-derived, virally attenuated, coagulation factor products. There are recombinant FIX and FVIII distillates available as well. All these developments have resulted in the elimination of complications from severe bleeding, for instance, the danger of transmission of infections and haemophilic arthropathy. Literature has shown that one of the challenging complications of haemophilia therapy is the development of inhibitory antibodies [7]. The consequence of the development of inhibitors among haemophiliac patients is a very problematic hemostasis, particularly post elective surgery and through acute episodes of bleeding, consequently raising mortality and morbidity [7].
It is estimated that about 80% of the haemophiliacs are in developing countries, where receiving appropriate treatment is possible only for a few patients [4]. In Pakistan, it is noted that haemophilia management and treatment are inadequate. Due to the increased costs and unavailability of factor concentrates for use in our clinical setting, fresh frozen plasma (FFP) is frequently utilized as the therapeutic modality [4].
This study aims to determine the frequency of specific and non-specific inhibitors among haemophilia A patients.
Materials and methods
A total of 150 haemophilia A male patients were included in this cross-sectional single-centre study at the National Institute of Blood Diseases and Bone Marrow Transplant (NIBD), Karachi, Pakistan, from September 2019 to January 2022. Patients who had bleeding disorders other than haemophilia A were excluded. Approval of the Institutional Review Board/Ethics Committee, Haematology, NIBD, Karachi, Pakistan, was taken prior to conducting the study (approval number NIBD/RD-199/10-2019). Informed consent was obtained from the parents of patients, following complete disclosure about the risks and benefits of the study. Strict International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) good clinical practise guidelines were followed.
Thorough history comprising bleeding episodes, the start of signs and symptoms, treatment particulars, kind of factor concentrates, and length was noted in a questionnaire. Venous blood samples were gathered in tubes enclosing 0.109 M (3.2%) trisodium citrate in a proportion of nine parts blood to one part anticoagulant, which was then centrifuged immediately at 1200 G for 15 minutes. FVII inhibitor was assessed by utilizing activated partial thromboplastin time (APTT)-based method. Normal pooled plasma and subjects' plasma together in a 50:50 mix were incubated for a period of 120 minutes, at a temperature of 37°C [8]. A quantitative assay was done and Bethesda unit (BU) was noted for patients who had positive inhibitor screening. BU is expressed as the quantity of inhibitor that would eventually neutralize half of one unit of added FVIII in 120 minutes at a temperature of 37°C. FVIII inhibitors are noted to be essentially time-dependent and are labelled as low titre when they are fewer than 5 BU detected, though high titre implies that more than 5 BU [9]. In case a test sample contained no inhibitor, the FVIII activity in the test sample mixture was expected to be equivalent to the control, and the residual FVIII activity was expected to be 100%. If the residual FVIII activity in a sample was between 80-100%, it was considered that sample contained no inhibitor.
Statistical analysis
Data analysis was carried out on IBM SPSS Statistics for Windows, Version 23.0 (Released 2015; IBM Corp., Armonk, New York, United States). Descriptive analysis, i.e., frequency and percentage were computed for categorical variables of gender, family history, and factors, whereas mean and standard deviation were predictable for quantitative variables of age, age of diagnosis in years and inhibitor in BU. However, Chi-square was applied for categorical variables, i.e., specific and non-specific inhibitors.
Results
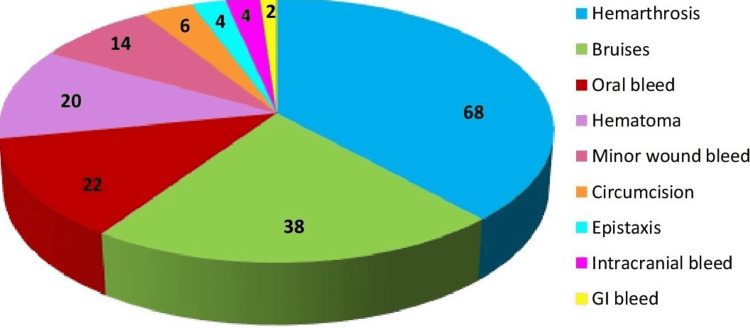
A total of 150 patients were included in this study. All of them (100%) were males. The median age at diagnosis was 0.8 years. Family history for haemophilia A was present in 60 (40%) patients. There was no family history of inhibitors in our patients. The frequency of bleeding type in our patients is shown in Figure 1. There were nine (6%) mild, 58 (38.7%) moderate, and 83 ( 55.3%) severe haemophilia A patients. There were 23 (15.3%) patients who had the inhibitor and 127 (84.6%) who did not. Table 1 shows the demographic characteristics of our patients.
Table 1. Demographic characteristics of patients in the study.
| Table 1: Demographic Characteristics (N=150) | ||
| Age (Median (IQR)) | ||
| Years | 15 (15.5) | |
| Age of diagnosis (Median (IQR)) | ||
| Years | 0.8 (1.5) | |
| Gender (%) | ||
| Male | 150 (100) | |
| Family history of haemophilia A (%) | ||
| Positive | 60 (40) | |
| Factor (%) | ||
| Mild | 9 (6) | |
| Moderate | 58 (38.7) | |
| Severe | 83 (55.3) | |
| Inhibitor (%) | ||
| Specific | 23 (15.3) | |
| Non-specific | 0 | |
| Inhibitor Titre (Median(IQR)) | ||
| Bethesda unit | 15.4 (31.2) | |
Figure 1. Frequency of bleeding in haemophilia A patients in the study.
After stratifying the data in Inhibitor positive patients, the data showed that there were 13 (56.5%) patients in the severe (<1%) category, 10 (43.5%) patients in the moderate (1-5%) category, and no patients in the mild category. All patients had FVIII specific inhibitor and non-specific inhibitor (lupus antibody) was not identified in any patient. The results are shown in Table 2.
Table 2. Association of inhibitor with severity of haemophilia A.
Note: P-value > 0.05 found to be insignificant
| Specific Inhibitor (BU) | P-value | ||
| Severity | Positive | Negative | 0.407 |
| Mild | - | 9 (7.1%) | |
| Moderate | 10 (43.5%) | 48 (37.8%) | |
| Severe | 13 (56.5%) | 70 (55.1%) | |
Discussion
It is noteworthy that only 15.3% of the haemophilia A patients had developed inhibitors in this data set. They were essentially treated with plasma and its products. Haemophilia has a treatment cost that is excessive and, hence, its management is a burden for patients dwelling in developing countries. Preceding research regarding the range of congenital bleeding disorders has demonstrated that haemophilia A is found among the population of Pakistan [10]. Haemophilia is managed by using factor replacement therapy (FRT), which is primarily composed of FFP. Access to FFP is more conceivable for the Pakistani population. The challenges faced by patients in the developing world vary from a dearth of access to the suitable treatment and incorrect diagnosis owing to inadequate comprehension and lack of resources, resulting in an increased risk of morbidities among these subjects. Inhibitors are detected when a subject with haemophilia experiences an immune reaction towards clotting factor distillates. Allo-antibodies are present in about 20-30% of the haemophilia A subjects and are prevalent among those with severe disease as compared to moderate or mild categories [11]. Our study also found inhibitor development to be in a higher proportion among the severe category, that is 56.5%, as opposed to moderate and mild types and these results are close to a prior meta-analysis [12]. Further, results from Borhany et al. [4] have shown comparable results to our study.
Many factors are responsible for inhibitor development. For instance, the nature of gene defect, ethnicity, the severity of haemophilia A, intensive factor exposure when surgery takes place, on-demand treatment regimens, or prophylactic treatments. Also, literature shows that it matters what source of FVIII is used in the replacement therapy as it might have an influence on the development of the inhibitor [13]. However, Gouw et al. [14] had come across contradictory evidence in their study. They demonstrated that there was no significant distinction in the risk of emerging inhibitors among subjects who were getting FVIII. A literature review conducted by Iorio et al. showed that it was rather impossible to disprove or prove the theory that the use of rFVIII products is superior to that of pdFVIII and vice versa, in preventing the risk of developing inhibitors [15].
A Spanish research study demonstrated the cumulative incidence of inhibitors at different ages. According to the study, at three years of age, the incidence of inhibitors in patients of haemophilia A who had been treated with clotting factors was noted to be 41% before the age of six months, 29% between the age of six and 12 months, and 12% after one year [16]. A comparable inclination was detected in a study conducted in the Netherlands [17].
Clinically important FVIII inhibitors are typically detected when there is a lack of response to the replacement therapy. According to Borhany et al., about 15% of cases among a total of 21 haemophilia A cases were recognized with the help of inhibitors presence [4]. However, eight cases had a lower BU inhibitor titre and it was observed among 13 patients that there was a high BU. In that study, there was no family history of inhibitors [4]. Our study too did not show any family history of inhibitors in our patients. Literature has noted that the inhibitor formation pathogenesis and the cause that few inhibitors vanish is rather inadequately comprehended [18]. It has been noted that there is evidence suggesting that the development of inhibitory antibodies is a rather multifaceted activity linking together both genetic and non-genetic factors [19].
Limited resources and being a single-centre study were the major limitations of our study. Thus, further studies are needed in the future so that the multifaceted nature of the causes involved in inhibitor formation among the subjects may become more clear.
Conclusions
The development of inhibitors has not been identified as a major problem in our population. However, it is noteworthy that only 15.3% of patients with haemophilia A developed inhibitors in this data set. Inhibitors are classified as specific and non-specific inhibitors, and non-specific inhibitors were not identified in our population.
Hemophilia A is mostly managed by FFP due to limited access to recombinant FVIII in developing countries. Our study population was essentially treated with plasma and its products.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Review Board/Ethics Committee, Haematology, National Institute of Blood Diseases and Bone Marrow Transplantation, Karachi, Pakistan issued approval NIBD/RD-199/10-2019
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.The molecular genetic analysis of hemophilia A: a directed search strategy for the detection of point mutations in the human factor VIII gene. Pattinson J, Millar DS, McVey J, et al. 1990;76:2242–2248. [PubMed] [Google Scholar]
- 2.Hemophilia treatment in historical perspective: a review of medical and social developments. Rosendaal FR, Smit C, Briët E. https://pubmed.ncbi.nlm.nih.gov/1903310/ Ann Hematol. 1991;62:5–15. doi: 10.1007/BF01714977. [DOI] [PubMed] [Google Scholar]
- 3.Development of inhibitors in patients with haemophilia from India. Ghosh K, Shetty S, Kulkarni B, et al. Haemophilia. 2001;7:273–278. doi: 10.1046/j.1365-2516.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Frequency of factor VIII (FVIII) inhibitor in haemophilia A. Borhany M, Kumari M, Shamsi T, Naz A, Farzana T. https://pubmed.ncbi.nlm.nih.gov/22538032/ J Coll Physicians Surg Pak. 2012;22:289–293. [PubMed] [Google Scholar]
- 5.Frequency and levels of factor eight inhibitors in known haemophiliacs. Amir M, Hassan ZU, Aziz OBA, Ali N, Ahmad SQ. https://www.pafmj.org/index.php/PAFMJ/article/view/17 PAFMJ. 2018;68:712–716. [Google Scholar]
- 6.Haemophilia A: mutation type determines risk of inhibitor formation. Schwaab R, Brackmann HH, Meyer C, et al. https://pubmed.ncbi.nlm.nih.gov/8772209/ Thromb Haemost. 1995;74:1402–1406. [PubMed] [Google Scholar]
- 7.Inhibitors in haemophilia: clinical aspects. Dimichele D, Rivard G, Hay C, Antunes S. Haemophilia. 2004;10 Suppl 4:140–145. doi: 10.1111/j.1365-2516.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 8.Proceedings: a more uniform measurement of factor VIII inhibitors. Kasper C, Aledort L, Aronson D, et al. https://pubmed.ncbi.nlm.nih.gov/1198543/ Thromb Diath Haemorrh. 1975;34:612. [PubMed] [Google Scholar]
- 9.Laffan MA, Manning R. Dacie and Lewis Practical Haematology (Twelfth Edition) Amsterdam: Elsevier; 2017. Investigation of haemostasis; pp. 366–409. [Google Scholar]
- 10.Clinical audit of inherited bleeding disorders in a developing country. Sajid R, Khalid S, Mazari N, Azhar WB, Khurshid M. https://www.ijpmonline.org/text.asp?2010/53/1/50/59183. Indian J Pathol Microbiol. 2010;53:50–53. doi: 10.4103/0377-4929.59183. [DOI] [PubMed] [Google Scholar]
- 11.Why do inhibitors develop? Principles of and factors influencing the risk for inhibitor development in haemophilia. Astermark J. Haemophilia. 2006;12 Suppl 3:52–60. doi: 10.1111/j.1365-2516.2006.01261.x. [DOI] [PubMed] [Google Scholar]
- 12.Incidence of inhibitors in haemophilia A patients—a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Scharrer I, Bray GL, Neutzling O. Haemophilia. 1999;5:145–154. doi: 10.1046/j.1365-2516.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- 13.Incidence of factor IX inhibitor development in severe haemophilia B patients treated with only one brand of high purity plasma-derived factor IX concentrate. Parquet A, Laurian Y, Rothschild C, et al. Thrombosis and haemostasis. 1999;82:1247–1249. [PubMed] [Google Scholar]
- 14.Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Gouw SC, van der Bom JG, Auerswald G, Ettinghausen CE, Tedgård U, van den Berg HM. Blood. 2007;109:4693–4697. doi: 10.1182/blood-2006-11-056317. [DOI] [PubMed] [Google Scholar]
- 15.Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma‐derived or recombinant factor VIII concentrates: a systematic review. Iorio A, Halimeh S, Holzhauer S, et al. Journal of Thrombosis and Haemostasis. 2010;8:1256–1265. doi: 10.1111/j.1538-7836.2010.03823.x. [DOI] [PubMed] [Google Scholar]
- 16.Incidence of factor VIII inhibitors in severe haemophilia: the importance of patient age. Lorenzo JI, López A, Altisent C, Aznar JA. Br J Haematol. 2001;113:600–603. doi: 10.1046/j.1365-2141.2001.02828.x. [DOI] [PubMed] [Google Scholar]
- 17.Age at first treatment and immune tolerance to factor VIII in severe hemophilia. van der Bom JG, Mauser-Bunschoten EP, Fischer K, van den Berg HM. https://pubmed.ncbi.nlm.nih.gov/12624630/ Thromb Haemost. 2003;89:475–479. [PubMed] [Google Scholar]
- 18.Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. N Engl J Med. 1993;328:453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 19.Understanding inhibitor development in haemophilia A: towards clinical prediction and prevention strategies. Coppola A, Santoro C, Tagliaferri A, Franchini M, DI Minno G. Haemophilia. 2010;16:13–19. doi: 10.1111/j.1365-2516.2009.02175.x. [DOI] [PubMed] [Google Scholar]