1. INTRODUCTION
The investigation of biological processes involved in cardiovascular diseases and the study of new cardioprotective strategies and therapeutic targets in human specimens are challenging to date. This is partly due to the almost absent availability of control cardiac samples caused by stringent ethical guidelines of the interested country and the continual development of semi‐invasive percutaneous procedures, which make it difficult to perform and process the cardiac biopsy for experiments on viable myocytes.
Real and attractive alternatives may exist, such as the creation of pathological models with immortalised ventricular human myocytes and the direct reprogramming of human endogenous cells into cardiomyocytes using induced pluripotent stem cell technology, but these methods also include limitations. 1
The obstacles to developing an appropriate cell model to study a given pathology are not limited to the cardiovascular field. Several studies, including mitochondrial disorders and neurodegenerative diseases, 2 , 3 , 4 have adopted alternative and more feasible experimental models, such as skin‐biopsy‐derived fibroblasts, to perform functional analyses on a pathological phenotype.
In our previous publication 5 to which this short letter refers, we showed a strong and positive correlation between the mitochondrial permeability transition pore (mPTP) opening measured in fibroblasts from ST‐segment elevation myocardial infarction (STEMI) patients and reperfusion injury (RI) evaluated by cardiac magnetic resonance imaging of the same patients. Moreover, we found significant intersubject variability in mPTP opening, and the patients with hyperresponsive mPTP opening also had greater reperfusion damage. 5 This was an interesting finding considering the fact that, to the best of our knowledge, no data on mPTP and RI in humans are available to date. However, the question of how mPTP measured in fibroblasts reflects the reperfusion damage of a given patient, as well as how mPTP function (or other biological readouts) can be assessed directly in patients affected by cardiac ischaemia–reperfusion (I/R), remains unanswered.
mPTP is a multiprotein complex with channel function. 6 , 7 , 8 , 9 , 10 , 11 It is of great interest in the cardiovascular field because it is a key step in cell death during I/R episodes (i.e., in MI) in its “open state” and is considered one of the main culprits of RI. For these reasons, it constitutes an important target for cardioprotection, as already shown in cells and animal models. 8 , 12 , 13
Here, in an effort to explain our findings mentioned above, we provide an update on the differences that may occur between cardiomyocytes and fibroblasts in the analysis of mPTP activity in ischaemic patients. Indeed, with this pilot study, we detected negligible differences in mPTP function between both cell lines. This study is part of a larger project aimed to provide genetic and functional information on the mPTP in the RI directly in humans and refers to our previous publication.
2. PATIENTS AND METHODS
2.1. Study population
Simultaneous evaluation of the mPTP in CaRdiOmyocytes and FibroblasTs in patients undergoing cardiac surgery (CROFT) was a single‐centre, investigator‐driven, prospective study conducted at the cardiac surgery unit of Maria Cecilia Hospital of Cotignola, Ravenna, Italy. The trial was performed according to the Declaration of Helsinki and approved by the local ethics committee (Comitato Etico Area Vasta Emilia Romagna). Patients with a surgical indication for cardiac revascularisation or heart valve surgery, >18 years old, and with documented coronary artery disease upon coronary artery angiography were enrolled. The exclusion criteria were as follows: refused informed consent, contraindication to statin therapy, known haemorrhagic disease, known disease of the mitochondria, neoplasia treated <5 years ago, chemotherapy <5 years ago, life expectancy <1 year, suspected neoplasia, use of oral contraceptives, pregnancy or breast feeding. All enrolled patients underwent a skin biopsy to obtain a fibroblast culture and myocardial biopsy to obtain atrial cardiomyocyte cultures. As a basic research pilot study, sample size calculation was not feasible. 14 Considering similar studies, we enrolled 20 patients in whom the twofold biopsy (skin fibroblasts and atrial cardiomyocytes) was performed.
2.2. Skin biopsy and fibroblast extraction protocol
Patients undergoing coronary artery bypass graft needed the packaging of one or more saphenous or autologous mammary bypasses. Thus, a skin biopsy was performed at the site of the autologous saphenous bypass or at the level of the thoracic skin incision. The skin tissue was collected with a clamp and introduced into a sterile container prefilled with an adequate quantity of preservative solution. Fibroblasts were isolated from the epidermis, dermis and hypodermis. After the surgical intervention, the cutaneous biopsy tissue was stored overnight in HBSS containing 3% penicillin–streptomycin (PS) and 1% amphotericin B. After being washed and minced into smaller pieces, the tissues were arranged in 25‐cm2 flasks covered by a thin layer of FBS. Then, for the following 10 days, every day, 500 µl of DMEM with 50% FBS was added to the culture. After approximately 15 days, fibroblasts were removed from the biopsy samples and trypsinised and amplified for experiments.
2.3. Myocardial biopsy and cardiomyocyte extraction protocol
A single tissue sample was collected at the level of the auricula where the tobacco pouch was made for venous cannulation. The tissue was collected with a clamp and introduced into a sterile container prefilled with an adequate quantity of preservative solution. Cardiomyocytes were isolated from the myocardium. The procedure was performed in accordance with standard procedures widely described in the literature. 15 Briefly, within 20 min after myocardial biopsy, atrial appendages were digested using a two‐step protocol involving first 30 min of protease XXIV followed by 60 min of collagenase II digestion in a buffer with low calcium at 37°C and continuously oxygenated. The digestion product was filtered with a 300‐μm nylon mesh and carefully centrifuged at 100 g for 5 min. Cells were counted and seeded on 24‐mm glass coverslips, and a laminin coating was applied on these coverslips to promote cell attachment. All experiments, from sample collection to cell attachment on the coverslips, were performed within 6 h.
2.4. Evaluation of mPTP opening
As mPTP opening leads to the loss of the proton gradient across mitochondrial membranes, it can be measured with tetramethylrhodamine methyl ester (TMRM), a chemical, cationic and cell‐permeable dye that accumulates in the mitochondrial matrix. The opening of the mPTP triggers the efflux of TMRM from the organelle in a short time, resulting in a progressive decrease in fluorescence intensity (Figure 1A). This change in fluorescence, in addition to the basal TMRM intensity loaded by the cell at resting conditions, can be monitored and recorded with confocal microscopy to obtain kinetics (Figure 1A). These data were analysed considering the slope (excel function) of the kinetics from the time of the stimulus administration until the end of the live imaging. In detail, cells were loaded with 20 nM TMRM for 30 min at 37°C; once on the microscope, TMRM basal intensity was first acquired at resting conditions for 30 s. Then, mPTP opening was stimulated by Ca2+ (10 nM ionomycin)‐ and oxidative stress (500 μM H2O2)‐dependent conditions in living cardiomyocytes and fibroblasts (Figure 1A). TMRM assay was sensitive to mPTP‐dependent stimuli as its fluorescence decreased after their addition, but also to Cyclosporin A (CsA), as the cells pretreated with the mPTP inhibitor and then stimulated with Ca2+ overload and pro‐oxidants did not experience TMRM fluorescence decrease in the same extend of the first case (Figure 1A). Imaging was performed with a Nikon Eclipse Ti confocal microscope with a 40 ×/0.60 SPlanFluor objective. On average, 15 cardiomyocytes and 40 fibroblasts per patient were evaluated.
FIGURE 1.
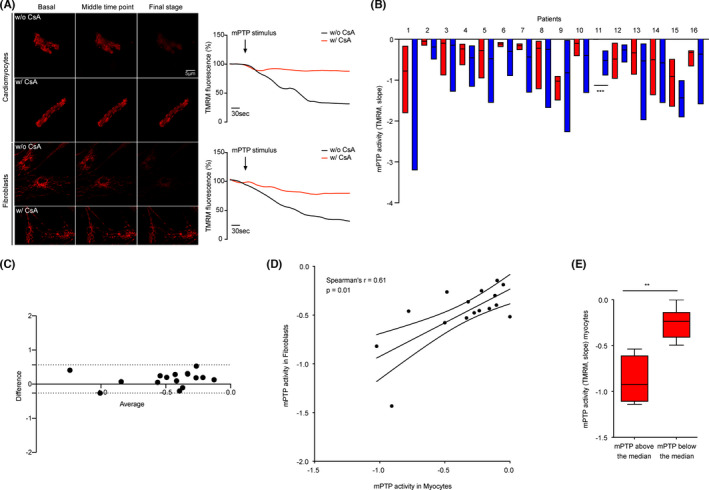
Main findings from the CROFT clinical study. (A) Set‐up of the experiments in which TMRM probe is shown to be sensitive to mPTP stimuli and CsA pretreatment in the evaluation of mPTP opening. In the upper panel, images and kinetics of mPTP opening in cardiomyocytes with (w/) and without (w/o) CsA; in the bottom panel, images and kinetics of mPTP opening in fibroblasts in the same conditions. (B) mPTP activity measured with the TMRM probe in both myocytes (red) and fibroblasts (blue) from the same patient. (C) Bland–Altman test: bias =0.15; 95% limits of agreement =dotted lines; Y axis =difference between measures; X axis =average of mPTP measures. (D) Graph showing the correlation (Spearman's r index) between mPTP measured in both myocytes (X axis) and fibroblasts (Y axis) for each patient in the CROFT study. (E) Stratification of mPTP function measured in myocytes (Y axis) according to the median fibroblast mPTP value
2.5. Statistical analysis
The Shapiro–Wilk test was used to check data for normality. The results are shown as the median ± range. Statistical significance was investigated by the Mann–Whitney test for two‐group comparisons and by the Kruskal–Wallis test for comparisons of more than two groups. A Bland–Altman plot was used to analyse the agreement between two different measures. A correlation analysis was performed by applying the Spearman rank test.
3. RESULTS
3.1. mPTP opening varies among patients
The CROFT study initially involved 20 patients undergoing cardiac surgery. In these samples, we performed mPTP functional analysis by analysing the mitochondrial membrane potential under Ca2+‐ and oxidative stress‐dependent conditions, as previously described and shown in Figure 1A. Indeed, it was impossible to perform the more direct calcein–cobalt assay in myocytes due to the toxicity of the CoCl2 reagent needed for the test or longer protocols due to the short viability of cells after extraction. Samples from 4 out of 20 enrolled patients were used to set experimental conditions; thus, they were excluded from the study. In the remaining 16 patients (Table 1), by measuring mPTP opening in both fibroblasts and myocytes, we reported a significant intersubject variability of the channel (p < 0.0001 for myocytes and p < 0.0001 for fibroblasts) (Figure 1B). This finding confirmed the results obtained in 5 and supported the hypothesis that this functional difference may have a pathological implication.
TABLE 1.
Table containing statistics and information on the 16 patients enrolled in the CROFT study
| (A) Variable | Summary statistics | Patient population N = 16 |
|---|---|---|
| Age (years) | Mean ± SD; Median (min‐max) | 64.8 ± 13.1; 67(33–83) |
| Male sex | N (%) | 12 (75%) |
| Body mass index (kg/m2) | Mean ± SD; Median (min‐max) | 27.8 ± 5.6; 26.2 (19.6–39.5) |
| Extracardiac arteriopathy | N (%) | 1 (6%) |
| Sinus rhythm | N (%) | 15 (88%) |
| Urgent indication | N (%) | 5 (29%) |
| Aetiology: | ||
| Mitral/Aortic valve disease | N (%) | 9 (53%) |
| Coronary artery disease | N (%) | 5 (29%) |
| Valvular and ascending aorta | N (%) | 2 (12%) |
| Valvular and coronary disease | N (%) | 1 (6%) |
| Family history of cardiovascular disease | N (%) | 6 (35%) |
| Hypertension | N (%) | 12 (71%) |
| Diabetes mellitus | N (%) | 4 (23%) |
| Smoker | N (%) | 3 (18%) |
| COPD | N (%) | 1 (6%) |
| Renal failure | N (%) | 2 (12%) |
| Cerebrovascular accident | N (%) | 2 (12%) |
| Leukocyte | Mean ± SD; Median (min‐max) | 6.3 × 109 ± 2; 6.3 × 109 (3.1–10.4) |
| Creatinine (mg/dl) | Mean ± SD; Median (min‐max) | 1.01 ± 0.33; 0.89 (0.65–1.77) |
| C‐Reactive protein (mg/dl) | Mean ± SD; Median (min‐max) | 0.3 ± 0.2; 0.3 (0–0.7) |
| Hemoglobin (g/dl) | Mean ± SD; Median (min‐max) | 13.4 ± 1.9; 13.3 (10.5–16.5) |
| NYHA III ‐ IV | N (%) | 2 (12%) |
| Ejection fraction (%) | Mean ± SD; Median (min‐max) | 56.8 ± 10.4; 60 (35–75) |
| euro SCORE I | Mean ± SD; Median (min‐max) | 4.8 ± 3.5; 5 (0–11) |
| euro SCORE II | Mean ± SD; Median (min‐max) | 6.2 ± 7.1; 3.98 (0.88–24.9) |
| Logistic EuroSCORE | Mean ± SD; Median (min‐max) | 3.1 ± 3.9; 1.98 (0.56–15.81) |
3.2. mPTP opening in fibroblasts correlates with myocytes
Although the values obtained from patient ID11 differ significantly between myocytes and fibroblasts and considering that different cellular types can have different sensitivities to either Ca2+‐ or ROS‐dependent stimuli, overall, the analysis of the agreement between both measures confirms that they are interchangeable. Indeed, the Bland–Altman test (Figure 1C) yielded a bias = 0.15 and a narrow range of 95% limits of agreement (dotted lines), and all values fell within that limit, suggesting that the differences between mPTP opening in myocytes and fibroblasts are close to 0. In addition, the recorded values showed a positive correlation (Spearman's r = 0.61, p = 0.01) (Figure 1D). Overall, as shown in Figure 1E, patients with lower mPTP activity recorded in fibroblasts (mPTP of fibroblasts below the median value) also had lower mPTP activity in myocytes than other patients. Taken together, these data highlighted fibroblasts from skin biopsies as a feasible model for obtaining crucial information on mPTP function in cardiovascular diseases where cardiac biopsies cannot be collected (i.e., MI).
4. DISCUSSION
Obtaining and processing heart samples for experimental research are challenging, even considering the continual development of semi‐invasive percutaneous procedures in the treatment of I/R‐based pathologies. In vitro models to study the molecular mechanisms of human cardiac diseases exist, but they include several limitations. Moreover, clinical studies involving both basic and translational research lines are still scarce. 16 Thus, it is necessary to explore additional routes to investigate the molecular pathways behind the phenotype, as was done in the past by several research groups in other fields. The most widespread, already used for mitochondrial disorders or neurodegenerative pathologies where human specimens are rare or impossible to be taken, is skin biopsy‐derived fibroblasts. 2 , 3 , 4
In line with this previous literature and in understanding how the function of mPTP activity measured in fibroblasts from STEMI patients correlates with RI, we analysed the activity of mPTP both in fibroblasts and myocytes in ischaemic patients undergoing cardiac surgery. We believe this addendum may provide at least one more explanation in support of those findings.
Overall, in the CROFT study, we first confirmed our previous results about the significant intersubject variability of mPTP opening, which further supports the hypothesis that this functional difference may have a pathological implication in the clinical outcome of those patients (such as RI). Second, we detected negligible differences in terms of mPTP activity between fibroblasts and cardiomyocytes recorded in patients undergoing cardiac surgery. Indeed, the trend of variation in mPTP activity between fibroblasts and cardiomyocytes among patients was positively and significantly correlated. The fact that ID11 assumes statistically significant values between cell lines may on the one hand confirm the fact that different cell types sense stimuli in different ways; on the other hand, it further validates our data output; indeed, although the cohort is small, only one sample differed from the overall trend.
Nevertheless, the comparative analyses represented by the Bland–Altman test and the correlation analysis provide a link between fibroblasts and cardiomyocytes in terms of mPTP opening under certain pathological conditions.
5. LIMITATIONS OF THE STUDY
We are aware that our study contains some limitations. The small population size is affected by restrictions due to ethical guidelines. Further studies should be conducted to broaden the findings of this pilot study. Another limitation is the evaluation of mPTP activity using only one assay. Unfortunately, the cardiac tissues allowed were small, and explanted myocytes were viable only for a few hours after cardiac surgery, in agreement with the literature in the field. Thus, longer experimental protocols were not applicable, and the more direct assay (calcein–cobalt technique) for analysing mPTP was excluded due to the toxicity of the CoCl2 reagent.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
P.P. is grateful to Camilla degli Scrovegni for continuous support. The Signal Transduction Laboratory (www.unife.it/labs/signaltransduction) was supported by the Italian Association for Cancer Research (AIRC: IG‐23670 to P.P. and IG‐19803 to C.G.), A‐ROSE, Progetti di Rilevante Interesse Nazionale (PRIN2017E5 L5P3 to P.P. and PRIN20177E9EPY to C.G.), the Italian Ministry of Health (GR‐2013‐02356747 to C.G.), the European Research Council (ERC; 853057‐InflaPML to C.G.) and local funds from the University of Ferrara to P.P. and C.G. G.M. was supported by the Italian Ministry of Health (GR‐2019‐12369862). M.R.W. was supported by the Polish National Science Centre (Grants: UMO‐2014/15/B/NZ1/00490 and UMO‐2018/29/B/NZ1/00589). Open Access Funding provided by Universita degli Studi di Ferrara within the CRUI‐CARE Agreement. [Correction added on 17 May 2022, after first online publication: CRUI funding statement has been added.]
Morciano G, Pedriali G, Mikus E, et al. Similarities between fibroblasts and cardiomyocytes in the study of the permeability transition pore. Eur J Clin Invest. 2022;52:e13764. doi: 10.1111/eci.13764
Contributor Information
Giampaolo Morciano, Email: mrcgpl@unife.it.
Gianluca Campo, Email: cmpglc@unife.it.
Paolo Pinton, Email: paolo.pinton@unife.it.
REFERENCES
- 1. Knollmann BC. Induced pluripotent stem cell‐derived cardiomyocytes: boutique science or valuable arrhythmia model? Circ Res. 2013;112(6):969‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakrabarty B, Sharma MC, Gulati S, Sarkar C. Skin biopsy for diagnosis of ullrich congenital muscular dystrophy: an observational study. J Child Neurol. 2017;32(14):1099‐1103. [DOI] [PubMed] [Google Scholar]
- 3. Jimenez‐Mallebrera C, Maioli MA, Kim J, et al. A comparative analysis of collagen VI production in muscle, skin and fibroblasts from 14 Ullrich congenital muscular dystrophy patients with dominant and recessive COL6A mutations. Neuromuscul Disord. 2006;16(9–10):571‐582. [DOI] [PubMed] [Google Scholar]
- 4. Mak SK, Tewari D, Tetrud JW, Langston JW, Schüle B. Mitochondrial dysfunction in skin fibroblasts from a Parkinson’s disease patient with an alpha‐synuclein triplication. J Parkinsons Dis. 2011;1(2):175‐183. [DOI] [PubMed] [Google Scholar]
- 5. Morciano G, Pedriali G, Bonora M, et al. A naturally occurring mutation in ATP synthase subunit c is associated with increased damage following hypoxia/reoxygenation in STEMI patients. Cell Rep. 2021;35(2):108983. [DOI] [PubMed] [Google Scholar]
- 6. Pinke G, Zhou L, Sazanov LA. Cryo‐EM structure of the entire mammalian F‐type ATP synthase. Nat Struct Mol Biol. 2020;27(11):1077‐1085. [DOI] [PubMed] [Google Scholar]
- 7. Morciano G, Naumova N, Koprowski P, et al. The mitochondrial permeability transition pore: an evolving concept critical for cell life and death. Biol Rev Camb Philos Soc. 2021;96(6):2489‐2521. [DOI] [PubMed] [Google Scholar]
- 8. Hausenloy DJ, Schulz R, Girao H, et al. Mitochondrial ion channels as targets for cardioprotection. J Cell Mol Med. 2020;24(13):7102‐7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amodeo GF, Krilyuk N, Pavlov EV. Formation of High‐Conductive C subunit channels upon interaction with cyclophilin D. Int J Mol Sci. 2021;22(20):11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campo G, Pavasini R, Morciano G, et al. Clinical benefit of drugs targeting mitochondrial function as an adjunct to reperfusion in ST‐segment elevation myocardial infarction: A meta‐analysis of randomized clinical trials. Int J Cardiol. 2017;1(244):59‐66. [DOI] [PubMed] [Google Scholar]
- 11. Bonora M, Giorgi C, Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol. 2021; 10.1038/s41580-021-00433-y [DOI] [PubMed] [Google Scholar]
- 12. Nesci S. The mitochondrial permeability transition pore in cell death: A promising drug binding bioarchitecture. Med Res Rev. 2020;40(2):811‐817. [DOI] [PubMed] [Google Scholar]
- 13. Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res. 2020;126(2):280‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307‐312. [DOI] [PubMed] [Google Scholar]
- 15. Bird SD, Doevendans PA, van Rooijen MA, et al. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58(2):423‐434. [DOI] [PubMed] [Google Scholar]
- 16. Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289(1):H237‐242. [DOI] [PubMed] [Google Scholar]


