Abstract
In the vertebrate brain, neural stem cells (NSCs) generate both neuronal and glial cells throughout life. However, their neuro‐ and gliogenic capacity changes as a function of the developmental context. Despite the growing body of evidence on the variety of intrinsic and extrinsic factors regulating NSC physiology, their precise cellular and molecular actions are not fully determined. Our review focuses on thyroid hormone (TH), a vital component for both development and adult brain function that regulates NSC biology at all stages. First, we review comparative data to analyse how TH modulates neuro‐ and gliogenesis during vertebrate brain development. Second, as the mammalian brain is the most studied, we highlight the molecular mechanisms underlying TH action in this context. Lastly, we explore how the interplay between TH signalling and cell metabolism governs both neurodevelopmental and adult neurogenesis. We conclude that, together, TH and cellular metabolism regulate optimal brain formation, maturation and function from early foetal life to adult in vertebrate species.
Keywords: cell fate, development, evo‐devo, metabolism, mitochondria, neural stem cell, thyroid hormone
1. INTRODUCTION
The development of the vertebrate central nervous system (CNS) is a complex process that starts early in embryogenesis, and continues throughout adult life. It involves many coordinated cellular events including cell proliferation, cell fate decision, cell survival and cell death, migration, differentiation and maturation of both neuronal and glial cells. Both cell types are derived from neural stem cells (NSCs) populating the pseudostratified neuro‐epithelium along the rostro‐caudal axis of the CNS primordium.1 They first divide symmetrically to enlarge the NSC pool while transforming into radial glia (RG), giving rise to the ventricular zone (VZ). They gradually start to divide asymmetrically, generating post‐mitotic neuroblasts. Only after generating the bulk of neurons that make up the brain, RG become gliogenic, establishing the astrocyte and oligodendrocyte populations.2, 3, 4 The entire process produces a fixed number of cells ultimately determining brain size.
The adult brain retains some of its neuro‐ and gliogenic potential, although considerable differences exist among vertebrates.5 While neurogenic regions containing RG‐like NSCs are widespread in the anamniote brain, sustaining neuro‐ and gliogenesis throughout life, amniotic species and mammals in particular only retain a few distinct telencephalic regions that support de novo generation of neural cells. This physiological trait is directly related to the rather limited capacity for self‐repair in the mammalian brain, as compared to the high regenerative potential seen for instance in fish and amphibians.5, 6 NSCs in the adult mammalian brain are predominantly found in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus, and are generally in a quiescent state.7 The default state of the adult SVZ niche is gliogenic,8 but several external and internal stimuli can induce specific cellular programs favouring a neurogenic environment. Elucidating which factors are involved, and how they interact with the SVZ niche in the developing, adult and ageing brain, will enhance our understanding of its underlying physiology, and how NSC homeostasis changes during the course of ageing. Moreover, unravelling the mechanisms underlying the regenerative potential, as well as NSC fate choice in pathophysiological conditions, is the key to understanding how NSCs can induce repair and regeneration in the adult brain.9
One of the most intriguing factors regulating NSC behaviour throughout vertebrate life is thyroid hormone (TH). An adequate TH supply is crucial for brain development and function throughout life in all vertebrates, including humans.10 This vital role is well demonstrated in human cases of severe hypothyroidism during critical phases of neurodevelopment, resulting in an aberrant structure and function of brain regions such as the cerebellum, hippocampus, and neocortex.11, 12 However, while most studies have focussed on examining these adverse neurological outcomes following TH insufficiency in late pregnancy (reviewed in13), it remains debated whether THs are equally involved in embryonic development.14 We do however know that maternal hypothyroidism in rodents and in humans during stages prior to thyroid gland activation and hypothalamus‐pituitary‐thyroid (HPT) axis maturation, can irreversibly damage the brain, leading to distinctive psychomotor and cognitive deficits in the progeny.15, 16 For instance, epidemiological data have shown that maternal hypothyroidism during the first trimester of pregnancy alters white/grey matter ratios, and is associated with a lower offspring general IQ and a decrease in verbal and motor performances during childhood.17 Moreover, recent experimental findings also showed that TH is a crucial regulator of NSC fate determination in the two main neurogenic niches in mammals.18, 19, 20, 21 The question arises whether TH action converges on common pathways in embryonic neural progenitors.
Cell‐specific TH action is tightly controlled by a complex set of evolutionary conserved proteins (eg, TH‐transporters (THTs), deiodinases (DIOs), receptors), enabling coordination of tissue development, growth, maintenance and repair in vertebrates, from teleosts to mammals.22, 23 Triiodothyronine (T3), the main biologically active form of THs, binds to TH receptors (TRs), positively or negatively regulating specific gene expression. Cellular uptake and efflux of THs occurs via transmembrane THTs. Monocarboxylate transporter 8 (MCT8) is a highly specific THT in the CNS, while MCT10 and organic anion transporting polypeptide 1C1 (OATP1C1) transport THs along with other substrates.24 They have orthologues in all vertebrates showing similar transport characteristics.25, 26, 27 Inside the cell, DIOs (in)activate THs.28 DIO2 activates tetraiodothyronine (T4) into T3, while DIO3 inactivates T4 into reverse T3, and T3 into diiodothyronine (T2), and both are highly expressed in the brain. DIO1 catalyses all reactions but has a much lower affinity for THs,29 and, while tissue‐specific mRNA expression has been detected, no DIO1 activity has been reported in the vertebrate brain so far.28, 30, 31
In addition, THs are major modulators of cell metabolism, by affecting genomic and non‐genomic pathways regulating mitochondrial activity.32, 33 NSC determination and differentiation also implicate metabolic shifts. Proliferating cells mainly use glycolysis associated with a low mitochondrial activity to generate energy, whereas differentiated cells primarily rely on oxidative phosphorylation (OXPHOS).34, 35 Moreover, NSC fate choice is associated with specific changes in metabolic status and mitochondrial morphology.36, 37 Increasing evidence shows that a complex interplay between TH signalling and cell metabolism is involved in cell commitment and differentiation.20
In this review, we focus on TH‐dependent molecular and metabolic mechanisms governing NSC physiology in the developing and adult brain. First, we provide a vertebrate wide view on how TH locally influences NSC fate in the embryonic/foetal brain. Second, we elaborate on the role of THs in NSC fate in the adult mammalian brain, and specifically focus on an emerging concept that TH, as a key regulator of cellular metabolism, can mediate NSC fate decisions via mitochondrial pathways.
2. EVIDENCE FOR thyroid hormone REGULATING EMBRYONIC NSC PHYSIOLOGY
2.1. In non‐mammalian vertebrates
Work on several non‐mammalian vertebrates has contributed to understanding TH action in the developing brain. Besides the essential requirement of THs for development and metamorphic events, common mechanisms regulate TH uptake and metabolism across vertebrates. Moreover, experimental TH insufficiency or excess affects brain structure and function similarly in mammals and humans, indicating that TH action during neurodevelopment is conserved throughout evolution.38 Furthermore, non‐mammalian embryos (eg, those of birds, reptiles, amphibians and teleost fish) develop in a readily accessible extra‐uterine environment, during which THs are derived from a maternal stock in the egg yolk. This fact allows manipulation of TH homeostasis from early stages onwards, independent of maternal physiology.39, 40 Here, we focus on the role of THs on embryonic neural progenitors in the most commonly used animal models in thyroid stem cell research.
2.1.1. Zebrafish
The zebrafish (Danio rerio), a teleost, became a popular model thanks to its rapid and transparent early development, and the recent emergence of a wide variety of genetic techniques allowing the generation of mutants.41 As in other vertebrates, the zebrafish CNS consists of neurons, oligodendrocytes and other glial cells, but so far mature astrocytes have not been identified. Moreover, neuro‐ and oligodendrogenesis continue to occur at a relatively high rate throughout many regions of the adult zebrafish brain.42, 43 The majority of neuronal and oligodendroglial precursor cells (NPCs and OPCs respectively) are generated between 24 and 72 hours post‐fertilization (hpf) (Figure 1A), and arise from stem cells in the VZ throughout the CNS.42 A typical model used to investigate this process is the motor neuron progenitor (pMN) domain of the ventral spinal cord, where olig2‐expressing progenitors generate motor neurons and OPCs in two successive waves under the influence of Hedgehog signalling.44, 45, 46 The neuronal vs glial output is thereby under strict control of the evolutionary conserved Notch signalling pathway. High Notch signalling maintains the self‐renewal state of neural progenitors and is necessary for OPC production, while decreased Notch signalling causes premature neurogenesis and the formation of primary motor neurons at the expense of OPCs and secondary motor neurons.46, 47, 48, 49
Figure 1.
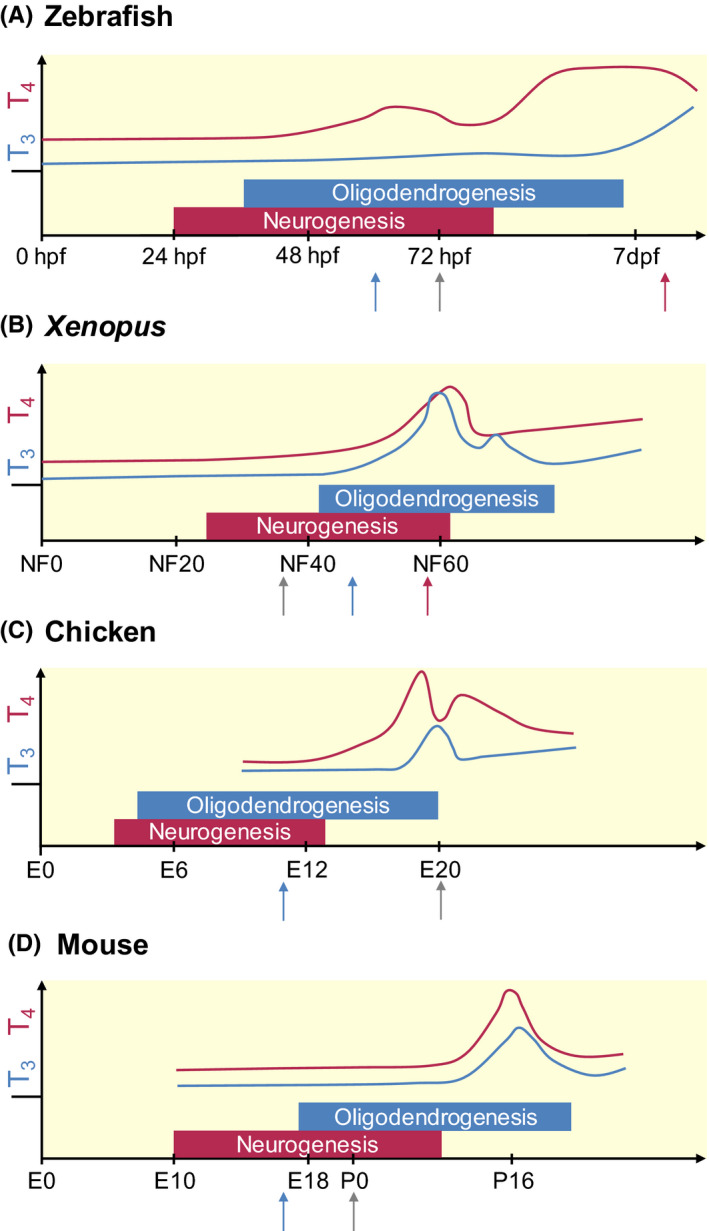
Schematic representation of TH levels, and the timing of neurogenesis and oligodendrogenesis during the development of 4 classical vertebrate models. The x‐axis represents the relative time scale of development for each species, the y‐axis the relative concentrations of serum T4 (red line) and T3 (blue line) based on available data. The moment of hatching/birth is depicted by the grey arrow. Metamorphosis in fish (A) and amphibians (B) (red arrow) is characterized by a high TH peak. In contrast, the TH peak in chicken (C), a precocial bird, occurs at hatching, while a sharp TH peak is observed more than 2 weeks after birth in mice (D), a typical altricial species. For these, and possibly all vertebrates, the period of neurogenesis starts prior to that of oligodendrogenesis, although both phases overlap to some extent. The timing and regulation of these processes relies for a major part on maternal TH supply, prior to endogenous TH production by the foetal thyroid gland (tetrapods) or the thyroid follicles (fish) (blue arrow). While circulating TH levels are low in embryonic stages and generally reflect a high T4/T3 ratio, local regulators of T3 availability are expressed during these stages in all vertebrates, enabling temporal and cell‐specific control of TH action. dpf, days post fertilization; E, embryonic day; hpf, hours post fertilization; NF, Nieuwkoop‐Faber stage; P, postnatal day. T3: triiodothyronine, T4: tetraiodothyronine
During the 3‐day period of embryogenesis, maternal THs are derived from the egg yolk prior to the activation of the HPT axis around 60‐72 hpf.50 In the last decade, several mutant fish for regulators of TH availability enabled studying the consequences of altered TH signalling.41 Blocking TH uptake by either mct8 knockdown (KD) or knockout (KO) strongly impairs zebrafish organ development, with a profound impact on the CNS.51, 52, 53 In humans, inactivating mutations in the MCT8 gene are responsible for the Allan‐Herndon‐Dudley Syndrome (AHDS), an X‐linked psychomotor retardation syndrome.54, 55 In zebrafish, a transcriptome analysis at 25 hpf following KO of mct8 showed dysregulation of the Wnt/Notch signalling pathways.56 This downregulated the expression of her2, an important target of Notch and a homologue of the well‐known TH‐responsive Hairless gene in mammals. Her2 is normally required to maintain neural progenitor proliferation and to postpone neuronal differentiation during the neurogenic wave, while stimulating glial differentiation later in development, supporting a time‐dependent balance between neuro‐ and gliogenesis.57 Downregulation of Notch signalling also increased the expression of the cell cycle inhibitor genes pax6a and neurod6a, resulting in a premature depletion of the NSC population.56 Correspondingly, the neural progenitor pool was smaller in the 48 hpf mct8 mutant zebrafish brain.58 This precocious cell cycle exiting of neuroblasts could explain the decrease or lack of certain neuronal cell types in the mid‐ and hindbrain of mct8 morphants. In addition, medial spinal cord neurons were absent, while more ventral motor neurons were observed, and only one type of dorsal neurons developed.52 This imbalance indicates incorrect neuronal cell specification because of impaired TH uptake. Furthermore, impaired neuronal differentiation and/or altered cell survival could have contributed to, and possibly aggravated the phenotype, resulting in different local outcomes.52, 53
Detailed cell counts in juvenile or adult mct8 mutant zebrafish could indicate the extent of neuron and OPC production limitation in early stages. While no data were gathered on OPC numbers, decreased expression of mbp (a well‐established TH‐target gene involved in OPC differentiation) and p0 (a marker of myelinating oligodendrocytes)51 did suggest a failure of OPCs to differentiate into mature myelinating oligodendrocytes,59 a process normally favoured by THs.60 Consequently, the CNS of mct8 KO zebrafish is in a hypomyelinated state.51, 59 Another surprising observation was the increased number of neural crest‐derived Schwann cells in the peripheral nervous system (PNS),51 suggesting that THs could affect cell fate and differentiation differentially throughout the nervous system. The authors suggested that Mct8 deficiency induces a hyperthyroid state in PNS cells, thereby enhancing Schwann cell maturation,59 but direct evidence is missing.
While less attention was paid to effects on neuro‐ and oligodendrogenesis in other zebrafish TH regulator mutants, KD of dio3b (the most important inactivating deiodinase in zebrafish61) also increased neural crest cell proliferation, migration and apoptosis, and thraa KD increased apoptosis, suggesting they are also involved in different cellular processes.62 This is not surprising given that TR isoforms are expressed in the zebrafish CNS from 24 hpf onwards.63, 64 Activation of distinct thraa‐regulated gene sets, sometimes in combination with other transcription factors such as retinoic acid,62 as well as the unliganded aporeceptor all play an important role in early zebrafish development.38, 61 Another, less thoroughly explored action might occur via T4 binding to the membrane‐situated integrin αVβ3 receptor.65 Similarly, the spatio‐temporal expression patterns of other regulators of TH availability throughout the developing zebrafish CNS41 indicate they might also contribute to determine local neurogenesis and gliogenesis.
These findings suggest that the availability of maternal THs is one of the major factors involved in (a) maintaining normal physiology of the neural progenitor pool, and (b) the neuronal vs oligodendrocyte balance in the early developing zebrafish brain. It is worth mentioning that these actions largely precede endogenous TH production by the thyroid follicles (Figure 1A). How exactly these actions take place at the molecular level awaits a more in‐depth transcriptomic and proteomic profiling.
2.1.2. Xenopus laevis
Xenopus laevis is another frequently used model to investigate the role of THs in early neurogenesis, though most studies focused on metamorphosis.66, 67 However, earlier stages have been included to examine whether THs also impact NSC biology underlying neural circuit development. As in zebrafish, TH signalling is already present prior to thyroid gland activation68 (Figure 1B). Both mRNAs encoding TRα and TRβ isoforms are detected in oocytes and embryos,69, 70, 71 as are dio1, dio2 and dio3 mRNAs from the neurula stage onwards, enabling control of TH action in a tissue‐dependent manner.72
Neuro‐ and oligodendrogenesis in the developing tadpole brain relies on widely conserved regulatory mechanisms and multiple genes controlling cell proliferation and differentiation, from pre‐metamorphic stages onwards73, 74 (Figure 1B). The potential neurogenic action of TH was analysed in the midbrain‐derived tectum opticum (TeO) and the forebrain‐derived neuroretina. Administration of THs during a 72‐hour period, either to the water or by local injection in the ventricles, or knocking down the TH‐inactivating dio3 during pre‐metamorphosis (Nieuwkoop‐Faber stage (NF) 46‐49), increased expression of the proliferative genes pcna and mcm5. Consequently, increased NSC proliferation because of shortening of the cell cycle led to larger sizes of the TeO and retina, consistent with an increased number of neurons.75 This action is likely TRα‐dependent, as it is the only TR isoform detected in neurogenic areas of the embryonic tadpole brain.67 Following expansion of the progenitor pool, THs induce a switch from a proliferative to a differentiation state, thereby orchestrating the timing of consecutive cellular processes necessary to establish a properly functioning neural circuit.75
Adding THs to the aquarium water prior to NF44 did not affect cell proliferation,68, 69 indicating that TH action is spatio‐temporally controlled by local cellular regulators of TH availability, thus preventing precocious neurogenesis.76 Accordingly, Dio3 was found to be strongly expressed in the dorsal ciliary margin zone of the eye, inhibiting the stimulatory effect of T3 on retinal precursor cell proliferation, and notably also in rods and the shortwavelength‐sensitive cones, thus limiting precocious TH‐dependent responses in specific retinal cell types.77, 78 Overexpression of a mutant TRβ ineffective for gene repression strongly impaired eye development and disrupted expression of several important genes.70 This suggests that unliganded TR action could be the mode of action for correct eye development at first. Other regulators of TH availability such as mct8 and oatp1c1 are also expressed from early stages onwards,27 and are probably involved in these processes as well.
It was not investigated whether the generation of OPCs in the TeO was also affected by altered TH signalling,75 although oligodendrocytes have been detected from NF41‐42.79 However, disrupting TH signalling during these early stages by exposing Xenopus tadpoles to a mixture of environmental chemicals found in human amniotic fluid impaired the neuron vs oligodendrocyte ratio at the expense of neuroblasts.69 Accordingly, the expression of the pluripotency genes (eg, sox2, bdnf, tub2b and mcep2) was reduced, as was that of the neuronal marker dcx and the myelination marker mbp,69 suggesting that oligodendrocyte differentiation and maturation could be affected too. Altogether, these results suggest that elevated TH signalling favours progenitor cell commitment towards a neuronal fate in the pre‐metamorphic tadpole brain. So far, the molecular programs that underlie these cell fate changes remain largely enigmatic. Early perturbations in TH signalling might produce irreversible long‐term defects in brain structure and function, ultimately hampering locomotor and other behaviours, as for instance corroborated by the lower distance travelled by Xenopus larvae exposed to the mixture of TH disruptors.69
2.1.3. Chicken
In birds, maternal THs stored in the egg yolk are taken up in the amniotic circulation via several THTs,80 stimulating correct brain development prior to mid‐embryogenesis when the thyroid gland starts producing THs81 (Figure 1C). For instance, already at day 1 (E1) of the 21‐day embryonic development, neural tube closure is responsive to T3 signalling in the chicken (Gallus gallus) embryo,82 and regulators of TH availability are present throughout the E4 and E8 brain.28, 83 The TeO of the chicken brain is particularly interesting to study the role of THs in the process of neuro‐ and gliogenesis, since it displays several features of cortical development and shares a similar cyto‐architecture, such as the occurrence of layers, expression of the TH‐responsive gene RELN (encoding reelin) and an inside‐out developmental gradient.84 Furthermore, both neurons and glial cells arise from a common progenitor during embryonic TeO development, giving rise to a structure that is essentially mature prior to hatching at E20.85, 86, 87 KD of MCT8 in TeO neural progenitors as early as E3 reduced intracellular TH signalling, decreased NSC proliferation, and caused a premature depletion of the progenitor cell pool, resulting in cellular hypoplasia at later stages. Consequently, glutamatergic and GABAergic neuronal populations were smaller, possibly disrupting the TeO visual processing function.88 Since cell production has ceased by then, it is almost certain that the loss of cells is irreversible. Thus, these data indicate that MCT8‐dependent, cellular uptake of THs in the chicken embryo is not only required to maintain neural progenitor proliferation, but also to induce a precisely timed switch from proliferation to differentiation.88 A similar loss of cells was observed in the MCT8‐deficient retina, which was linked to reduced proliferation of retinal precursor cells. More strikingly, cell fate was also altered in MCT8‐deficient cones, with increased commitment towards more UV/blue cones at the expense of green/red cones.89 Whether or not MCT8 KD in the TeO or retina affected OPC generation remains to be investigated, as are the underlying signalling pathways. Furthermore, as elaborated on earlier, the role of other regulators of TH availability besides MCT8 are worthwhile to explore in this context too. Most of them are dynamically expressed throughout various embryonic CNS regions, such as the pallium,90, 91 cerebellum,92, 93 and spinal cord.26 Because TH signalling is vital for the development of each of these brain regions,93 one may reasonably expect other THTs, DIOs and TRs to be involved in local neurogenesis and gliogenesis as well.
2.2. In mammals
Most evidence for a role of THs in embryonic neural progenitors has been obtained from mammalian models, especially eutherian species, because of the easy genetic engineering as well as their genetic, anatomical and physiological similarity to humans. In these species, maternal THs are transferred to the embryo via the placenta, and are implicated in neurodevelopment from early stages onwards13, 94, 95 (Figure 1D). For instance, expression of Thra, Dio2 and Mct8 mRNAs in the VZ of the embryonic rat neocortex suggests that THs act on neural progenitors prior to the activation of the thyroid gland. Furthermore, maternal hypothyroidism during neocorticogenesis delayed mitotic divisions, and shortened cell cycle length, reducing the progenitor pool.96 These effects echo observations in non‐mammalian vertebrates following MCT8 deficiency, causing altered TH activity in neural progenitors.51, 88 The adverse effects of maternal TH deficiency on progenitor cell pool size ultimately resulted in cellular hypoplasia in the telencephalon, which at least partially contributed to the learning and memory impairment observed when these rats reached adulthood.96, 97
Excess T3, mimicking a state of hyperthyroidism, also reduces the progenitor cell pool size. Although it may seem contradictory, this reflects the current idea that tilting the TH balance towards either end outside of the normal TH range negatively affects early brain development.13, 17 T4 administration increased Hes expression, hampered neuronal differentiation and likely altered cell specification in the foetal rat cortex,98 while Hes inhibition stimulated pro‐neural gene expression in NSCs, thereby accelerating neurogenesis.99 Similarly, exposing neural cultures derived from the E16 rat cortex to 3 nM T3 also promoted generation of oligodendrocytes at the expense of neurons.100 In contrast, somewhat unexpectedly, adding T3 to mouse cell cultures derived from the embryonic telencephalon resulted in neurogenic effects through TRα1 while inhibiting gliogenesis.97 Similarly, hyperthyroidism stimulated in vivo dopaminergic neuronal differentiation through increased TRα‐induced Otx2 expression in the ventral mouse midbrain.57 Either these observations could be a consequence of in vitro vs in vivo conditions, or they could imply that regulators of TH availability are able to confine TH action to certain CNS regions, resulting in different spatio‐temporal responses. It is plausible that TH acts as a neurogenic signal in one brain area, while acting as a gliogenic signal elsewhere and/or at another time point. A difference in regional or cellular TH responsiveness could for instance explain why hypothyroid perinatal mice displayed increased 5′‐bromo‐2′‐deoxyuridine (BrdU)‐labelling in the olfactory bulb (OB) and cerebral cortex, but decreased labelling in the SVZ and neocortex, while no change was observed in the hypothalamus.16, 101 The importance of local TH regulators is well demonstrated in Mct8/Oatp1c1 double KO mice with their complex phenotype characterized by profound hypomyelination, delayed cerebellar development and reduced γ‐aminobutyric acid (GABA)ergic cell differentiation, because of a strongly impaired TH uptake.102 Whether neuro‐ and oligodendrogenesis were affected per se remains unanswered and could be relevant for future studies in mouse models harbouring mutations in one or several genes encoding other TH regulators.103
In addition to its stimulatory effect on cell proliferation, T3 also induces a switch to neuronal differentiation in the developing brain,97, 104 and later stimulates OPC differentiation into mature oligodendrocytes,60 indicating that specific generic programs are initiated in a precisely timed manner during development. For example, COUP‐TF1 is an important transcription factor regulating the timing of T3‐stimulated gene expression required for normal corticogenesis.105 In fact, THs influence transcription of numerous genes to guide these complex processes in a coordinated manner.106, 107, 108
Another interesting question is whether TH deficiency during embryogenesis has long‐term effects on the biology of adult NSC pools, notably by mechanisms such as epigenetic modulation that alter the gene response repertoire over the course of time.109, 110 This hypothesis fits with the “developmental origins of health and disease” (DOHaD) paradigm, stating that many non‐communicable diseases originate in early development.111 While defects might be less evident at early stages of development, they could render the brain more susceptible to pathological processes later in life, and could for instance affect the potency of adult NSCs to respond to brain damage caused by acute insults or neurodegenerative diseases. In rats, propylthiouracil (PTU)‐induced hypothyroidism during gestation and perinatal development reduced cell proliferation and survival in the adult hippocampus, a well‐established neurogenic niche (see part 3, Thyroid hormones regulate NSC cell fate in the adult mammalian brain), leading to smaller brains.104 Furthermore, prenatal PTU exposure led to the occurrence of heterotopias, resulting in abnormal brain function in later life.112, 113 These data indicate that embryonic NSCs are sensitive to altered TH signalling from early stages onwards with potentially adverse long‐term effects in the adult brain, even if the period of TH perturbation was only transient.113
As far as we are aware, little is known regarding the role of THs in embryonic NSCs in non‐human primates and humans. However, especially with regard to the gyrencephalic neocortex, the situation is deemed far more complex in the sense that a higher NSC output is required to allow for its disproportionate expansion relative to the rest of the brain.114, 115 Additional progenitor cell types have been identified in the developing neocortex, among which intermediate progenitors serve to amplify cell production.116 THs could have played a crucial role guiding these local changes in the growth of the human neocortex throughout the course of evolution.117 One group proposed that T4 could act on the integrin αVβ3 receptor, thereby stimulating basal progenitor proliferation, with consequent exponential expansion of the progenitor cell pool.118
Lastly, magnetic resonance imaging‐based studies in humans have revealed structural alterations and hypomyelination as key features following maternal hypothyroidism,119, 120 indicating problems with the generation of NPCs and OPCs, and/or the differentiation of these progenitors into mature neurons and oligodendrocytes. Moreover, a large cohort study recently showed that both mild hypo‐ and hyperthyroidism in the first trimester of prenatal brain development altered grey/white matter ratios in the progeny, suggesting that lineage specification was altered in embryonic development.17 Other neurological disorders including attention‐deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and schizophrenia, characterized by abnormal neuronal numbers and networks, are also suspected to have a developmental aetiology of which suboptimal TH levels might be one of the underlying causes.14, 121, 122, 123, 124, 125, 126, 127 Lastly, a histological analysis of the brain of an MCT8‐deficient foetus showed loss of some neuronal subtypes, and a hypomyelinated state, suggesting similar problems in NSC biology and specification caused by insufficient TH uptake during early development.128 To elucidate the associated pathophysiological mechanisms, Vatine and co‐workers recently generated a “disease in a dish” model using induced pluripotent stem cells (iPSCs) obtained from healthy individuals and MCT8‐deficient human patients.129 They showed that the lack of a functional MCT8 in neural cells significantly reduced TH transport, but without affecting proliferation, neuronal differentiation and TH‐dependent neuronal maturation. However, by allowing MCT8‐deficient iPSCs to differentiate into brain endothelial cells, the same authors showed that the loss of MCT8‐dependent TH transport across the in vitro BBB impaired proper neural differentiation. Hence, they concluded that the cause of the human AHDS phenotype is primarily the result of impaired TH uptake at the BBB, rather than an incapacity of MCT8‐deficient neural cells to generate mature neurons.129 This implies the BBB itself can be considered as an additional regulator in controlling TH‐induced NSC differentiation. Applying cell type‐specific approaches, both in vitro and in vivo, could provide an all‐encompassing understanding of the local role of THs, in addition to the current approaches that predominantly rely on systemic hypo‐ and hyperthyroidism.
Taken together, data derived from non‐mammalian as well as mammalian species have provided substantial evidence that (a) tightly regulated TH levels are essential for embryonic brain development prior to foetal thyroid gland activation, (b) THs regulate the proliferative potential of neural progenitors and commitment to neuronal and glial precursors and (c) THs are most likely able to alter NSC fate within a temporally and spatially refined manner, ultimately affecting the final cyto‐architecture and function of presumably every brain region.
3. THYROID HORMONES REGULATE NSC FATE IN THE ADULT MAMMALIAN BRAIN
As described above (see part 2), the role of THs has been largely studied during neurodevelopment in most vertebrate species. However, many studies highlight that THs continue to regulate NSC behaviour in the young and ageing brain, especially in mammals.130, 131 In non‐mammalian vertebrates (eg, zebrafish and amphibians), a role in CNS regeneration has been investigated, suggesting that TH acts on adult brain function.67, 132, 133 However, a direct action of THs on NSCs has not been demonstrated in these models yet. Here, we focus on the critical influence of TH in the regulation of NSC fate and its functional implication on neurocognitive outcome in mammals.
3.1. Heterogeneous populations of NSCs
In the adult rodent brain, NSCs are almost exclusively located in two main germinal brain regions: the SVZ, lining the lateral ventricles and the SGZ of the dentate gyrus of the hippocampus. Both sources of NSCs differentially sustain lifelong neurogenesis and oligodendrogenesis. However, unless specifically exposed to pro‐oligodendrogenic molecules, NSCs derived from the SGZ seem to exclusively produce new neurons134, 135 that subsequently migrate to the granule cell layer of the dentate gyrus where they give rise to glutamatergic granule cells.136 Under homeostatic conditions, adult NSCs derived from the SVZ generate mainly neuroblasts via highly proliferative progenitors. Then, mature neuroblasts migrate towards the OB where they differentiate into different types of interneurons.137 A few NSCs are also able to generate glial cells, both astrocytes and oligodendrocytes.137 However, the oligodendrogenic potential of SVZ‐NSCs strongly increases in response to a pharmacologically demyelinating insult.19, 138, 139 SVZ‐derived OPCs migrate towards the corpus callosum, the striatum and the cerebral cortex within close range of the lateral ventricle, where they differentiate into mature myelinating oligodendrocytes.137
It is still unknown whether all NSCs in different SVZ microdomains are able to either generate both neurons and glia, or if distinct neurogenic and gliogenic NSCs exist. Recent evidence shows that adult NSC functions are heterogeneous and depend primarily on their regional identity acquired during development. Within the adult SVZ, it is well‐established that NSCs reside in regionally distinct microdomains, thus determining the type of neurons generated in the OB.140 Adult NSCs that retain RG hallmarks2, 141 are generated during early embryonic development, then entering in a quiescent state until being activated in the adult.142 Hence, the adult neurogenic niche is constituted of a mosaic of NSCs regionally located in distinct microdomains that reflect their developmental identities.143, 144 Given the crucial role of THs during (a) neurodevelopment and (b) adult NSC behaviour (see below), it will be interesting to investigate whether THs regulate embryonic SVZ‐NSC specification, thereby determining NSC long‐term behaviour, especially the neurogenic and gliogenic potential. Notably, which SVZ microdomain(s) is (are) specifically targeted by TH signalling to generate either neuroblasts or SVZ‐OPCs remains unexplored. Moreover, impaired NSC behaviour caused by disrupted TH signalling during early development could render SVZ‐NSCs unable to respond to a brain insult in the adult, thus limiting NSC plasticity in response to injury.
3.2. TH action on NSC behaviour
In the murine SVZ, adult onset hypothyroidism decreases NSC and progenitor proliferation by blocking cell cycle progression.145 An exogenous TH‐pulse is sufficient to restore cell proliferation within the adult SVZ.145 In the rodent SGZ, the consequences of hypothyroidism on cell proliferation and survival are more obscure. In some studies, hypothyroidism in adult rats decreased progenitor survival without affecting cell proliferation.146, 147 Again, TH treatment was sufficient to restore progenitor survival and neuronal differentiation.146 In contrast, other studies showed that progenitor proliferation decreased without any effect on cell survival.148 These inconsistent data may originate either from distinct experimental approaches used to induce adult hypothyroidism (eg, goitrogens or thyroidectomy), or because of labelling tissues with BrdU.149 Recently generated transgenic mice, expressing specific markers of progenitor cells at different stages of lineage development, provided more compelling evidence that adult hypothyroidism decreases the number of immature and post‐mitotic neuroblasts without affecting the proliferation of early non‐committed progenitors within the SGZ.150, 151
In the adult SVZ, a hypothyroid state is also associated with lower numbers of both early committed neuronal progenitors and mature neuroblasts,20 thus decreasing migrating neuroblasts along the rostral migratory stream (RMS).18, 145 Similarly, low TH levels reduce the generation of immature hippocampal neurons in the adult SGZ.146, 147, 148 Taken together, these results show that TH acts as a neurogenic factor both in the SVZ and in the SGZ by promoting NSC commitment towards a neuronal fate.18, 19, 20, 130
In contrast, a TH‐free environment promotes the generation of new OPCs derived from SVZ‐NSCs under physiological and pathological conditions.19, 20 Following a demyelinating insult, these newly generated SVZ‐OPCs generate mature myelinating oligodendrocytes in the corpus callosum.19 Compared to remyelination accomplished by resident parenchymal OPCs, myelin formed by oligodendrocytes derived from the SVZ has a normal thickness, and these newly generated OPCs provide functional remyelination, restoring normal conduction speed.19, 139, 152 Correspondingly, a one‐month TH‐free window enhanced the regenerative capacity by triggering oligodendrogenesis from SVZ‐NSCs.19
Thus, TH availability determines opposing effects of TH‐dependent NPC and OPC commitment in the adult SVZ. Interestingly, the fate of newly generated neural cells changes with ageing: neurogenesis declines153, 154 whereas oligodendrogenesis is preserved.155, 156 Moreover, low circulating THs are a typical feature of normal ageing.157 Thus, declining circulating TH levels as a function of ageing could preserve generation of oligodendroglial cells while decreasing neuron production. Taken together, these results identify TH signalling as a master pathway, tightly regulating the balance between neurogenesis and oligodendrogenesis in the young, adult and ageing brain.
3.3. Regulation of TH availability in the adult NSC niche
TH entry into the brain is facilitated by THTs at the BBB and the blood‐cerebrospinal fluid barrier (BCSFB). Since the SVZ is located adjacent to the ventricular walls, T4 is most likely supplied via the cerebrospinal fluid (CSF), through binding of T4 to transthyretin (TTR), a TH distributing protein synthesized by the choroid plexus epithelial cells.158, 159, 160, 161, 162 TTR null mice have lower TH levels in the brain, associated with reduced apoptosis of NSCs and progenitors within the adult SVZ.159, 162 Interestingly, the level of apoptosis of NSCs/progenitors in TTR null mice was similar to wild type mice rendered hypothyroid.162 So far, it is unknown whether TTR influences SVZ‐NSC fate. Following entry into the brain, local TH availability is tightly regulated, allowing precise temporal and cell‐specific control of TH action.29, 163, 164 Fine‐tuning of cell‐specific TH availability is particularly important in complex target cell populations such as the NSC niche, as it could affect cell fate choices during different stages of neurogenesis and oligodendrogenesis.
In the adult mouse SVZ, only the TRα1 isoform is detected.18, 131, 145 Interestingly, TRα1 is not detected by immunohistochemistry in SVZ‐NSCs but starts being expressed in early committed neuronal progenitors and strongly persists in mature neuroblasts.18 In contrast, TRα1 is not detected in SVZ‐OPCs, showing that TRα1 expression is mainly associated with neuronal cells19 (Figure 2). Accordingly, TRα1 overexpression induces the generation of new migrating neuroblasts18 whereas shRNA directed against TRα1 mRNA increases expression of NSC/progenitor (Sox2, Musachi) and oligodendroglial (Ng2) markers, suggesting that the pool of NSC/progenitors and OPCs is enlarged.18 Moreover, DIO3, the T3‐inactivating enzyme, is strongly expressed in SVZ‐OPCs, showing that glial fate determination requires a TH‐free environment (Figure 2). Accordingly, shRNA directed against Dio3 increases the number of SVZ‐OPCs.19 Thus, OPCs are protected from the neuralizing effects of T3, by the combination of the absence of TRα1 and the expression of DIO3. So far, the potential role of DIO2 and THTs in these cell types has not been investigated. Few TH target genes have been identified underlying the control of adult SVZ‐NSC fate. In presence of T3, TRα1 downregulates two cell cycle genes (c‐Myc and Ccnd1) within the adult mouse SVZ145, 165 and acts as a neurogenic switch by repressing (i) Sox2 in progenitors, a gatekeeper of NSC identity18 and (ii) Egfr, a well‐established gliogenic factor19 (Figure 2).
Figure 2.
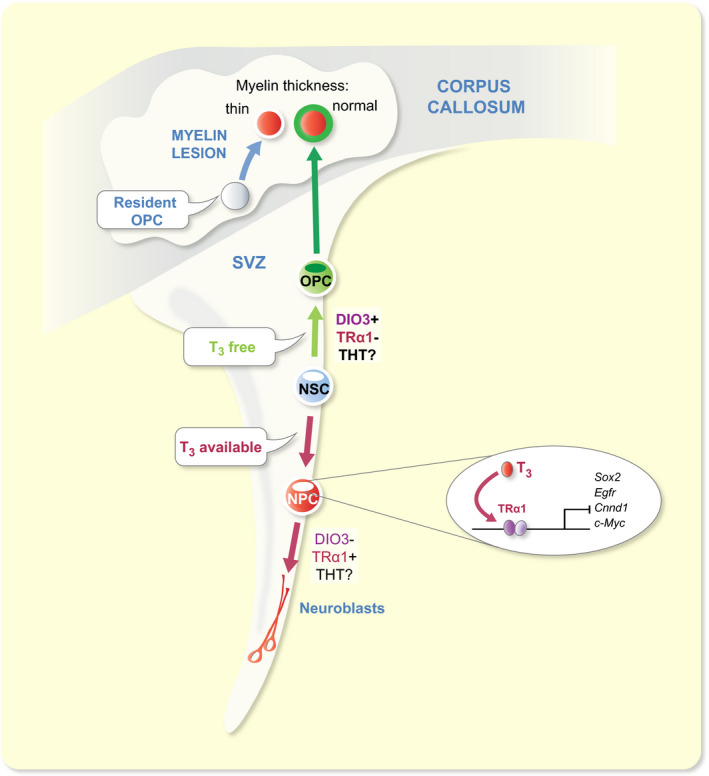
TH signalling regulates cell fate decision within the SVZ niche of the adult mouse. T3 drives NSC commitment preferentially towards a neuronal fate. T3, through its nuclear receptor TRα1, downregulates many target genes, thus promoting de novo generation of neuroblasts. In contrast, determination of glial cell fate depends on a T3‐free window. SVZ‐OPCs are protected from the effects of T3 by (i) the expression of DIO3 and (ii) the absence of TRα1. Later, T3 is necessary to induce differentiation and maturation of oligodendrocytes capable to functionally repair a myelin lesion in the corpus callosum. Compared to SVZ‐OPCs (complete remyelination), resident parenchymal OPCs (thin, insufficient remyelination) are less efficient for functional brain repair
In the adult mouse SGZ, TRα1 is also mainly detected in the neuronal lineage, especially in post‐mitotic hippocampal progenitors, but not in non‐committed proliferating progenitors.21, 130, 150 This suggests that, similar to the adult SVZ, TRα1 promotes neuronal fate in the adult SGZ, but at later steps of neuronal lineage progression. In a mutant mouse line that overexpresses TRα1 (the unliganded TRα1 aporeceptor is overexpressed in TRα2−/− mice), neurogenesis is decreased, showing that unliganded TRα aporeceptor overexpression recapitulates the effects induced by a lack of T3 in hippocampal neurogenesis.146, 147, 148 Accordingly, exogenous T3 treatment rescued the decrease in neurogenesis.166 In contrast, both progenitor survival and immature hippocampal neurons are increased in TRα null mice (TRα1−/− ), without effect on early progenitor proliferation.166 Thus, like in the adult SVZ, T3 through TRα1 enhances neuronal differentiation. To further elucidate how TH availability is locally controlled in the adult SGZ, studies on the expression and function of both DIOs and THTs are needed.
3.4. Functional significance of TH‐dependent adult neurogenesis
Adult neurogenesis is involved in several cognitive functions, notably memory and learning,167, 168 olfactory discrimination,169 and social and reproductive behaviour.170 In rodents, a decline in neurogenesis impairs olfactory function, thus influencing olfactory‐dependent/driven behaviour.171 One might therefore expect that decreased SVZ‐neurogenesis caused by hypothyroidism affects olfactory function. Hypothyroidism reduces mature olfactory receptor neurons172 and induces anosmia (loss of smell) in adult mice.173 Interestingly, TH signalling involving TRβ is also required in the olfactory epithelium of Pacific salmons to discriminate their natal stream during the seaward migration.174 However, the links between neurogenesis underlying TH and olfactory function are not established yet in this model.
In humans, neurocognitive deficits as well as neurodegenerative diseases are also associated with alterations in adult neurogenesis, especially within the hippocampus.175, 176, 177 Moreover, neuropsychiatric manifestations ranging from anxiety, depression and dementia to schizophrenia are linked to adult hypothyroidism178, 179, 180, 181 or developmental hypothyroidism.121 Again, further studies are needed to explore whether TH‐dependent neurogenesis is altered in neurological diseases to provide a mechanistic basis for the epidemiological observations. Several research groups have demonstrated that the generation of new neuronal cells occurs throughout adult life.182, 183, 184 However, more recent papers show contradicting results. One study shows that neurogenesis in the dentate gyrus is limited to the first year of life,185 whereas two others demonstrate that hippocampal adult neurogenesis persists throughout ageing.186, 187 These discrepancies probably relate to different methods used (post‐mortem brain samples or fresh tissues from biopsy). The small sample sizes available for human analysis are another limiting factor to draw unequivocal conclusions.
3.5. Emerging areas in research on adult NSC and THs
One key question is how does ageing alter the balance between neurogenesis and oligodendrogenesis? Ageing decreases NSC activity and the neuroblast population size in both the SVZ and the SGZ.153, 156, 188 Furthermore, several studies have shown that fate of newly generated cells changes with ageing: neurogenesis declines whereas oligodendrogenesis seems to be preserved within the SVZ.189, 190 Apparently, myelin loss and disruption with ageing are not caused by OPC depletion since numbers of OPCs appear stable.191, 192 The higher level of oligodendrogenesis in the ageing brain could be crucial for maintenance of brain functions, also in humans. Although the generation of new SVZ‐derived neuroblasts is still debated in humans,185, 186 oligodendroglial cells are detected throughout adult life, both in the young human SVZ under physiological189 and pathological138 conditions, and in the ageing SVZ.156, 193 Given the well‐established role of TH on NSC fate, it will be interesting to analyse how the slightly lower TH levels and/or TSH levels associated with healthy longevity157, 194 might preserve the oligodendrogenic capacity of the neurogenic niche in the ageing brain. Interestingly, Egfr mRNA levels increased throughout adulthood in the human SVZ.156 Moreover, Egfr, a key gliogenic factor,195 is downregulated by T3 through TRα1 in the young adult SVZ.19 Thus, upregulation of Egfr during ageing, combined with a decreasing TH environment may be a key molecular mechanism modulating neuronal and glial fate determination in the ageing SVZ. Further research should address this point, notably in the context of myelin maintenance in ageing brains and degenerative diseases.
In this context, it is worth noting that the contribution of TH signalling to the thickness of the myelin sheath remains unclear in the adult brain. Two types of OPCs are potential candidates to participate in myelin repair in the adult mammalian brain: (i) the pre‐existing resident parenchymal OPCs (pOPCs)196 and (ii) newly‐generated OPCs from NSCs within the SVZ.138 However, pOPCs repair myelin poorly, producing thin myelin.139, 152 Recent research demonstrates that SVZ‐OPCs are key targets for enhancing endogenous myelin repair.19, 139, 152 After a demyelinating insult, SVZ‐OPCs restore myelin of normal sheath thickness, whereas pOPCs do not. In particular, a TH‐free environment enhances generation of SVZ‐OPCs, but not pOPCs, thereby promoting functional remyelination.19 Understanding how THs differentially regulate SVZ‐OPCs vs resident OPCs could shed new light on how a key endocrine signal specifically targets SVZ‐OPCs, a promising source of remyelinating cells for demyelinating diseases.
Despite a wealth of data describing how the perinatal and adult SVZ generate distinct neuronal populations from spatially segregated microdomains (see above TH action on NSC behaviour), it remains largely unknown whether there is a regionalized origin of fully remyelinating oligodendrocytes within the adult SVZ. It is well‐established that morphogen signalling pathways determine the regionalized origin of neuronal cells. For example, Sonic Hedgehog (Shh) is expressed specifically in the ventral SVZ and contributes to driving NSC fate towards specific ventral neuronal cell types.197 In contrast, canonical Wnt signalling determines dorsalization of the adult SVZ.198 Azim et al. (2014)199 showed that Wnt/β‐catenin signalling promotes NSC determination towards a glial fate in the adult SVZ. However, the precise location of the remyelinating oligodendrocytes capable of producing a myelin sheath of normal thickness remains unknown. An interesting hypothesis is that TH availability is differentially regulated across the SVZ, creating a dorso‐ventral gradient, thus affecting NSC fate differentially in the dorsal vs latero‐ventral SVZ.
4. IMPLICATIONS OF CELL METABOLISM ON NEUROGENESIS AND TH‐induced CELL FATE DETERMINATION
Besides changes in terms of gene and protein regulation, stem cell activation and commitment also involve profound metabolic modifications. These internal changes, intrinsically linked to each other, allow differentiating cells to acquire their functional and morphological specificities. Such metabolic changes are crucial for NSC fate commitment and differentiation.200 THs are major modulators of cell metabolism, and TH‐induced mitochondrial responses are directly implicated in stem cell differentiation.32, 33, 201 Here, we will discuss how changes in cell metabolism influence NSC commitment and differentiation, and how TH signalling impacts cell metabolism during neurogenesis.
4.1. Mitochondrial activity regulates NSC proliferation and fate decisions
Stem cell niches such as the SVZ generally experience lower oxygen tensions compared to surrounding tissues, rendering them hypoxic.202 Hypoxic conditions favour both stem cell proliferation and multipotency.202, 203, 204 NSCs and progenitors principally rely on a high glycolytic metabolism and a low mitochondrial respiration, as opposed to mature differentiated cells, which primarily produce their energy using OXPHOS.204, 205, 206 The hypoxia inducible factor 1 α (HIF1α), a key factor in glycolysis activation and OXPHOS inhibition, is only stable in low intracellular oxygen conditions, and its degradation allows differentiating cells to shift from a glycolytic to an OXPHOS‐based metabolism.34, 207 Interestingly, HIF1α directly induces Dio3 expression,208, 209 and thus could be involved in regulating the TH‐free environment required for both undetermined cells and OPC commitment, as discussed earlier.
Mitochondrial activation, which allows the glycolytic to OXPHOS‐based metabolism shift, is necessary for NSC and progenitor differentiation. Most enzymes involved in glycolysis decrease during neuronal differentiation of human iPSCs, and genes associated with mitochondrial respiration simultaneously increase.34 Similar observations were made in cells isolated from the adult mouse SVZ210 and SGZ.211 Moreover, perturbations of mitochondrial activity strongly alter neuronal differentiation during both embryonic and adult neurogenesis, confirming that increased mitochondrial respiration is necessary for this process.207, 212, 213 Inversely, stimulating mitochondrial respiration helped to restore normal adult‐born hippocampal neuron maturation rates in a mouse model of Alzheimer's disease.214
Changes in cell respiration also influence NSC proliferation and fate choice. For instance, culturing foetal and postnatal human SVZ progenitor cells in hypoxia (5% of O2, physiological oxygen tension in the nervous system) strongly increases proliferation, whereas 20% of O2 (ambient oxygen level) is more favourable for cell differentiation.36 This result confirms that proliferative and differentiating cells require distinct oxygenation levels. Moreover, hypoxic conditions favour progenitor cells to commit to OPCs, while exposing OPCs to 20% of O2 stimulates differentiation, as seen in cultured human SVZ precursors36 and in primary cultures of rat oligodendroglial progenitors.215 The inhibition of HIF1α signalling in human iPSCs promotes neuronal rather than glial differentiation, confirming that low mitochondrial activity is required during glial commitment.37 Inhibiting mitochondrial respiration in adult mice SVZ progenitors similarly impairs their commitment towards a neuronal phenotype.20, 216 Thus, NSCs differentiating into glia maintain a high glycolytic activity, whereas neuronal commitment requires an early increase in OXPHOS.
The modulating effects of THs on NSC mitochondrial metabolism are not well understood yet, especially during embryonic neurogenesis. In contrast, in adult rodents, it was demonstrated that NSC fate is modulated by TH‐mediated metabolic changes in a cell lineage‐specific manner. Using a mitochondrial membrane potential dye, Gothié et al (2017) showed that hypothyroidism decreased mitochondrial activity in neuroblasts in vivo, whereas mitochondrial respiration remained low in SVZ‐derived OPCs both in euthyroid and hypothyroid adult mice.20 Inhibiting mitochondrial activity in vitro using Oligomycin significantly reduced the proportion of SVZ‐derived NSCs committed to neuroblasts, confirming the importance of TH‐induced mitochondrial activation for neuronal differentiation20 (Figure 3).
Figure 3.
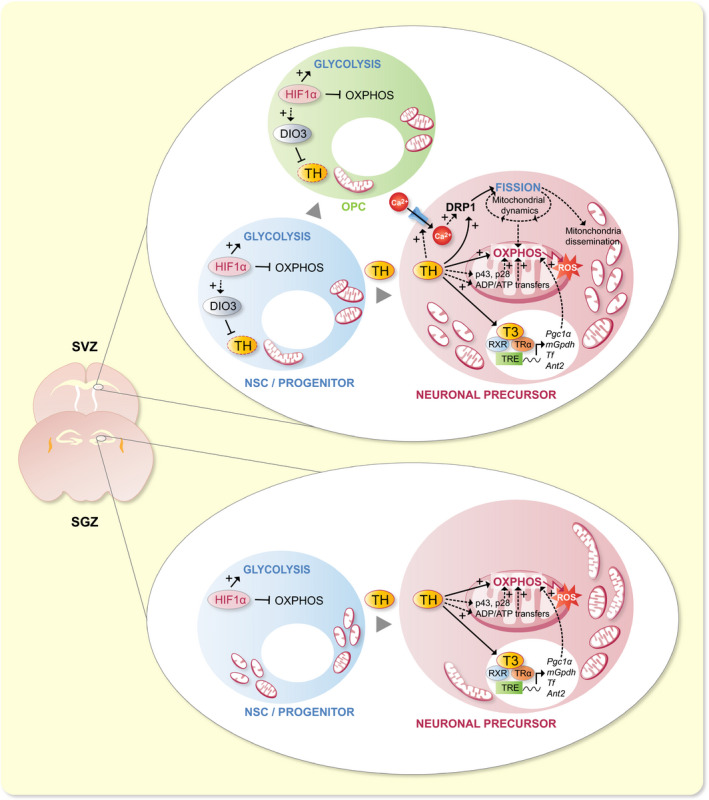
TH signalling activates mitochondrial metabolism during NSC commitment to neuronal precursor cells in rodents. In the adult murine SVZ, HIF1α activates OXPHOS and inhibits glycolysis in NSCs. TH signalling activates OXPHOS and drives NSC commitment towards a neuronal precursor cell (NPC) fate. The metabolism of cells committing to an oligodendroglial fate (OPC) maintains predominantly based on glycolysis. HIF1α may participate in the activation of Dio3.208, 209 THs also activate DRP1, which induces mitochondrial fission in NPCs. The same process might occur in the embryonic SVZ, where mitochondria fragment during NPC commitment. Processes involved may include the activation of Ca2+ entry in the cell, interaction with mitochondria‐specific TH receptors (p43 and p28), activation of ADP/ATP transfer between mitochondria and the cytoplasm, and the activation of TH‐target gene expression. In the adult SGZ, NSC commitment and differentiation is also associated with an increase in OXPHOS. In contrast to what is observed in SVZ cells, mitochondria appear as fragmented in NSCs, and elongated in NPCs in the SGZ. Here too, TH signalling is needed for NSC commitment to NPC and OXPHOS activation, through processes poorly explored to date in the SGZ
4.2. Mitochondrial dynamics are involved in the regulation of NSC differentiation
Mitochondrial dynamics (involving mitogenesis, mitochondrial fusion and fission, intracellular trafficking and mitophagy) is adjusted appropriately in response to changing energetic needs.217 Accordingly, the metabolic shift associated with NSC differentiation implies important changes in mitochondrial morphology: increased mitochondrial activity involves high mitochondrial dynamics. Mitogenesis and fusion increase mitochondrial volume. Fission is implicated in segregation of damaged mitochondria before their destruction (mitophagy), and is essential for mitochondrial trafficking and distribution in the cell and its extensions.218, 219 Interestingly, defects in mitochondrial dynamics are associated with various neurodegenerative disorders, including Alzheimer's disease,220, 221, 222 Parkinson's disease,223 Huntington's disease224, 225 and multiple sclerosis,226, 227 suggesting that impaired dynamics could be detrimental for brain cell physiology.
In the embryonic SVZ of mice, mitochondria appear elongated in NSCs and progenitors, fragmented in cells committing towards a neuronal fate, and elongated again in mature neurons.212 These morphological changes are important to increase OXPHOS and ROS production during neuronal differentiation.212 In the adult SVZ, the main inducer of mitochondrial fission, dynamin‐related protein 1 (DRP1), is acutely activated during neuronal commitment.20 Although morphological changes in mitochondria have not been formally studied in the adult SVZ, this result suggests that mitochondria are fragmented during early neuronal differentiation, similar to the situation in the embryonic SVZ. In the adult SGZ, however, mitochondria appear small and fragmented in NSCs, and progressively elongate during neuronal differentiation.207 The fact that normal cell migration requires mitochondrial fragmentation228, 229 could explain the differences observed between the SVZ and the SGZ. SVZ neuroblasts migrate further than those of the SGZ do, and an increase in mitochondrial fission may be necessary for migration through the RMS towards the OBs. Accordingly, DRP1 inhibition prevents the migration of SVZ‐derived neuroblasts, and hinders their differentiation,216 bolstering this hypothesis.
THs amplify the activation of DRP1 during NSC neuronal commitment in the adult SVZ20 (Figure 3). However, the molecular mechanisms underlying DRP1 activation during neuronal commitment are still unknown. Given that the calcium‐dependent phosphatase calcineurin favours DRP1 activation,230, 231 and that THs can non‐genomically activate Ca2+‐ATPases,232, 233 an interesting hypothesis to test is whether a TH‐mediated increase in intracellular calcium leads to DRP1 activation. Besides, it has been hypothesized that the Doublecortin (DCX) protein, which drives microtubules polymerization specifically in neuroblasts, could be activated by Ca2+‐dependent signalling.234 This assumption is based on a strong sequence homology with a Ca2+/calmodulin dependent kinase in both mice and humans, and remains to be verified. Altogether, these data suggest that TH‐induced Ca2+ intake could play a major role in neuronal determination of progenitor cells by promoting DCX activation and microtubule formation, together with mitochondrial fission and dissemination along microtubules, which also depend on calcium.235
THs also directly induce the expression of Pgc1α (peroxisome proliferator‐activated receptor gamma coactivator 1α), a master regulator of mitochondrial biogenesis.236 PGC1α activation indirectly induces the expression of mitochondrial transcription factor A (TFAM), that in turn activates the expression of many genes required for mitochondrial activity.237 The impact of THs on both TFAM and PGC1α has mostly been studied in the postnatal rat brain. Pups born from hypothyroid dams show severely decreased expression levels for both proteins in the cerebellum, as well as significantly lower mitochondrial protein expression.238 However, the functional impact of TH‐mediated induction of PGC1α and TFAM has not been explored specifically in the context of neurogenesis so far.
4.3. Implication of reactive oxygen species in NSC fate decisions and differentiation
Reactive oxygen species (ROS) generated as side products of mitochondrial activity can trigger stem cell differentiation.239, 240, 241, 242 Experimental evidence indicates that increased ROS production is involved in NSC and progenitor differentiation in both the developing and adult brain, as shown in rodents and in human cells.205, 212, 243, 244, 245, 246, 247 NSC differentiation in the adult SVZ also requires TH‐dependent activation of mitochondrial metabolism and ROS production in cells committing towards a neuronal fate.20 In turn, ROS production is impacted by mitochondrial dynamics. Khacho et al212 showed that mitochondrial fragmentation observed during embryonic NSC commitment is a key trigger for increasing mitochondrial ROS production. ROS signalling activates nuclear respiratory factor 2 (NRF2), which in turn activates genes involved in inhibiting proliferation (Notch), and activating differentiation (Isl1, Nkx2.1, Lhx5 and Sim1). Interestingly, NADPH oxidase 2 (NOX2)‐derived ROS production appears necessary for cell proliferation in adult mouse activated NSCs.248 Similar effects are observed in newt, where inhibiting ROS production by NOX reduced NSC proliferation, whereas inhibiting mitochondrial function did not.249 These results reveal a distinct impact of mitochondrial ROS vs ROS produced by NOXs on cell proliferation and differentiation.
ROS production, by modifying a cell's oxidative state, also impacts NSC fate choice. For instance, hindering NOX2‐derived ROS production in cultured adult mouse SVZ‐NSCs increased the number of neurospheres composed exclusively of glial cells by about 30% compared to wild‐type cultures.248 Accordingly, in vitro exposure to the superoxide indicator hydroethydine showed that SVZ‐derived NSCs committed to a neuronal fate produce more ROS than those differentiating towards an oligodendroglial fate.20 In contrast, an increase in intracellular ROS following glutathion synthetase inhibition induced embryonic cortical progenitor cells to generate astrocytes, at the expense of neurons.250 This process implicates an induction of the protein deacetylase SIRT1, a pivotal regulator of cell metabolism and mitochondrial biogenesis,251, 252 which is also involved in maintaining the undifferentiated state of NSCs and progenitors.253, 254 Furthermore, SIRT1 can promote neuronal differentiation in embryonic progenitors by interacting with the N‐CoR corepressor,255 involved in TH‐target gene inhibition on positively regulated genes. In the adult SVZ, SIRT1 is expressed by a majority of cells engaged in the neuronal differentiation pathway, and only in a minority of cells engaged in glial differentiation,20 suggesting that its involvement in adult NSC commitment mirrors the embryonic conditions. Lastly, acute T3 exposure also increased ROS production in the neuronal lineage of the adult mouse SVZ, but not in the glial lineage.20 The cellular oxidative state could therefore play an important role in NSC fate choice, with a crucial, fine‐tuned control of glycolytic/mitochondrial metabolism, ROS production and ROS detoxification.
4.4. Implications of iron metabolism in NSC fate decisions and differentiation
TH‐dependent regulation of mitochondrial activity also involves iron metabolism. In particular, prolyl hydroxylase (PHD) enzymes use iron to catalyse HIF1α inactivation,256, 257 thus in turn potentially modifying Dio3 expression (Figure 4). Moreover, the mitochondrial electron transport chain depends on iron‐sulphur clusters.258 In the blood stream, iron (Fe3 +) is mainly bound by the glycoprotein transferrin (Tf). THs can directly induce Tf transcription, as shown in human hepatocellular carcinoma cells.259 Furthermore, hypothyroidism decreases Tf expression in the brain of postnatal mice,260 suggesting that THs also regulate Tf expression in vivo. From capillary vessels, Tf can cross the BCSFB261, 262 and the BBB,263 but the mechanisms involved are not clear.264 Iron‐loaded Tf can also interact with specific cell surface receptors (TfR, Transferrin receptor). Then, the complex is internalized by endocytosis and Fe3+ is reduced to Fe2+ and released in the cytoplasm.
Figure 4.
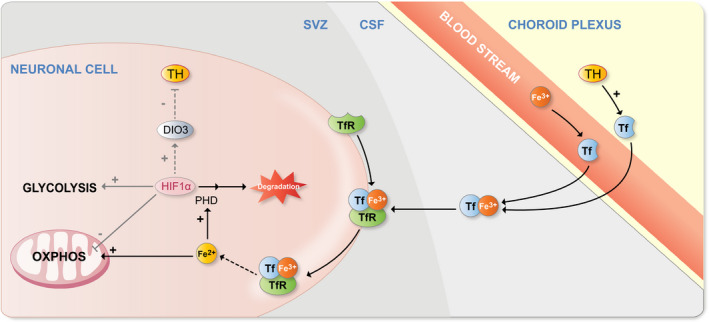
Implication of iron in regulating the metabolic switch during neurogenesis. THs activate transferrin (Tf) transcription in choroid plexus cells. Tf is transferred to the cerebrospinal fluid (CSF) from the choroid plexus cells themselves, as well as from the blood stream, carrying iron in its Fe3+ form. The Tf/ Fe3+ complex binds to the cell‐surface Tf receptor (TfR), facilitating their internalization. Fe3+ is then reduced to Fe2+ in the cytoplasm. Prolyl hydroxylases (PHDs) need both oxygen and Fe2+ to be activated and to provoke HIF1α degradation. Fe2+‐Sulphur clusters are necessary for mitochondrial electron transport chain functioning in mitochondria, making iron necessary for OXPHOS activation. HIF1α degradation may be involved in preventing DIO3 activation, as shown in many cell types (eg, hepatocytes and cardiomyocytes). TfR expression in neuronal precursors has not been demonstrated yet, but has been shown in neurons, while absent from oligodendrocytes. Therefore, TfR expression could be initiated early during neuronal cell commitment
Tf can also be directly synthesized by oligodendrocytes265, 266 and choroid plexus cells in rodents.265, 267 While Tf expression and secretion by the choroid plexus presumably favours iron transport to the CSF,268 its role in oligodendrocytes remains unclear. In fact, they do not secrete the glycoprotein,269, 270 and, unlike adult neurons271, 272 or OPCs in rat neonates,273 they do not express TfR.274, 275, 276 However, iron could enter oligodendrocytes through other receptors.276 It has been suggested that Tf allows intracellular iron storage.277 Marziali et al.260 studied the influence of THs and Tf on oligodendrocyte differentiation and maturation, showing that both hyperthyroidism and intracerebral injection of apo‐Tf (Tf that does not bind iron) decrease OPC numbers, and increase mature oligodendrocytes in the corpus callosum in postnatal mice. Inversely, hypothyroidism during embryonic development (i) increased OPC numbers in postnatal mice, a phenotype reversed following an apo‐Tf injection, and (ii) reduced the number of mature oligodendrocytes.260 These results are in line with studies showing that short‐term hypothyroidism favours NSC determination towards OPCs in the adult mouse SVZ.19, 20 They also reveal that TH can influence iron metabolism, through Tf induction, thus modulating NSC fate. This could constitute one of the TH‐triggered pathways allowing mitochondrial activation during neurogenesis, and it would be particularly interesting to study the interplay between THs and iron metabolism in this context.
4.5. Future research on metabolism and NSCs
It would be interesting to investigate other factors involved in TH‐mediated regulation of mitochondria in the context of neurogenesis and NSC fate determination. For instance, TH activation of adenine nucleotide translocators (ANTs), transporters of ATP and ADP between cytoplasm and mitochondria,278, 279 and that of the mitochondrial glycerol 3P dehydrogenase (mGPDH), an activator of mitochondrial electron transfer directly activated by THs.278, 280 Additionally, T3 can interact with 2 truncated mitochondrial TRα isoforms, p43 and p28.281, 282, 283 Even though we are currently not aware how T3 binds to p28 to affect mitochondrial function, p43 itself strongly in vitro stimulates mitochondrial biogenesis and activity in presence of T3.281, 284, 285, 286, 287
4.5.1. Notch and Wnt signalling pathways
Modulation of signalling pathways such as the Notch pathway could also be involved in mitochondrial changes during NSC commitment and differentiation. Activation of Notch signalling provokes the inhibition of mitochondrial fragmentation in vitro via the activation of Akt and MFN1/2.288, 289 As mentioned above, the Notch pathway maintains the NSC pool in the adult SVZ290, 291, 292 and is also required for OPC generation in the embryo.293 Moreover, Notch signalling responds to THs.98, 294 Thus, in the adult, Notch‐induced inhibition of fission might be involved in the reduced activation of DRP1 in SVZ‐OPCs.20 Furthermore, activation of Wnt/β‐catenin in tumour cells promotes aerobic glycolysis,295, 296 and modulates mitochondrial dynamics by decreasing DRP1 interaction with mitochondria and increasing expression of mitofusin, a key player of mitochondrial dynamics.297 Since a crosstalk between THs and Wnt is well‐demonstrated in many stem cell types in several organisms,298, 299, 300 Wnt could be a key TH‐target pathway playing a role in mitochondria dependent NSC fate decision.
4.5.2. Lipid metabolism
Glycolytic metabolism favours the production of lipids, essential for cell proliferation, in NSCs and progenitors. During cell commitment to neuronal differentiation, lipid biosynthesis decreases following the expression of Spot14.301 T3 can directly induce Spot14 expression,302, 303 and could thus be a key element in lipid biosynthesis decrease during cell differentiation. Hence, we can hypothesize that DIO3 in OPCs19 may prevent a T3‐mediated inhibition of lipid production during commitment to the glial lineage.
Myelinating oligodendrocytes also produce an important quantity of lipids to generate myelin. Besides, oligodendrocytes use large amounts of lactate,304, 305 and several studies suggest that oligodendrocyte processes contain less mitochondria than neuronal projections.306, 307, 308 Thus, metabolic differences between lineages initiated during early cell commitment may persist throughout cell maturation, possibly under the influence of TH signalling.
5. CONCLUSION
THs play a central role in governing neurogenesis and gliogenesis in the embryonic, postnatal and adult brain. We have reviewed the evidence that TH components (eg, transporters, carrier proteins, deiodinases and receptors) participate in fine‐tuning of cell type‐specific regulation of intracellular TH levels. These cellular and molecular TH‐dependent mechanisms are largely conserved through vertebrate evolution. In particular, TH signalling favours NSC commitment towards a neuronal fate in adult rodent neurogenic niches, while SVZ‐OPC determination requires TH absence. Cell metabolic state also affects NSC proliferation, fate choice and differentiation. Increasing evidence shows that TH signalling triggers fate‐specific metabolic changes, involving modifications in mitochondrial activity, mitochondrial dynamics and/or ROS production. More research is needed to explore whether the lower circulating TH levels observed during ageing could be involved in the onset of certain neurodegenerative diseases, known to be associated with important mitochondrial defects. Thus, investigating the role of TH signalling in neuro‐ and gliogenesis during development as well as in the adult and ageing brain will be critical to fully grasp the underlying cellular and metabolic mechanisms. Each of these basic research areas can generate new findings that could provide a solid foundation for novel therapeutic purposes for neurodegenerative diseases.
CONFLICT OF INTEREST
All the authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the EU H2020 contract Thyrage (Grant n°666869), the french ANR grant OLGA and the CNRS.
Gothié J‐D, Vancamp P, Demeneix B, Remaud S. Thyroid hormone regulation of neural stem cell fate: From development to ageing. Acta Physiol. 2020;228:e13316. 10.1111/apha.13316
REFERENCES
- 1. Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777‐788. [DOI] [PubMed] [Google Scholar]
- 2. Kriegstein A, Alvarez‐Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014;15(4):351‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Tilborg E, de Theije CGM, van Hal M, et al. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia. 2018;66(2):221‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alunni A, Bally‐Cuif L. A comparative view of regenerative neurogenesis in vertebrates. Development. 2016;143(5):741‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonfanti L. The (real) neurogenic/gliogenic potential of the postnatal and adult brain parenchyma. ISRN Neurosci. 2013;2013:354136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morizur L, Chicheportiche A, Gauthier LR, Daynac M, Boussin FD, Mouthon M‐A. Distinct molecular signatures of quiescent and activated adult neural stem cells reveal specific interactions with their microenvironment. Stem Cell Reports. 2018;11(2):565‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Capilla‐Gonzalez V, Lavell E, Quiñones‐Hinojosa A, Guerrero‐Cazares H. Regulation of subventricular zone‐derived cells migration in the adult brain. Adv Exp Med Biol. 2015;853:1‐21. [DOI] [PubMed] [Google Scholar]
- 9. Sun D. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen Res. 2014;9(7):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3(3):249‐259. [DOI] [PubMed] [Google Scholar]
- 11. Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20(6):784‐794. [DOI] [PubMed] [Google Scholar]
- 12. Zimmermann MB. The role of iodine in human growth and development. Semin Cell Dev Biol. 2011;22(6):645‐652. [DOI] [PubMed] [Google Scholar]
- 13. Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson W, Russell G, Baragwanath G, Matthews J, Vaidya B, Thompson‐Coon JO. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: A systematic review and meta‐analysis. Clin Endocrinol (Oxf). 2018;88(4):575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Escobar GM, Obregó MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18(2):225‐248. [DOI] [PubMed] [Google Scholar]
- 16. Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16(10):809‐818. [DOI] [PubMed] [Google Scholar]
- 17. Korevaar TIM, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population‐based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 18. López‐Juárez A, Remaud S, Hassani Z, et al. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10(5):531‐543. [DOI] [PubMed] [Google Scholar]
- 19. Remaud S, Ortiz FC, Perret‐Jeanneret M, et al. Transient hypothyroidism favors oligodendrocyte generation providing functional remyelination in the adult mouse brain. Elife. 2017;6:pii: e29996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gothié JD, Sébillot A, Luongo C, et al. Adult neural stem cell fate is determined by thyroid hormone activation of mitochondrial metabolism. Mol Metab. 2017;6(11):1551‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fanibunda SE, Desouza LA, Kapoor R, Vaidya RA, Vaidya VA. Thyroid hormone regulation of adult neurogenesis. Vitam Horm. 2018;106:211‐251. [DOI] [PubMed] [Google Scholar]
- 22. Bianco AC. Minireview: cracking the metabolic code for thyroid hormone signaling. Endocrinology. 2011;152(9):3306‐3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gothié J‐D, Demeneix B, Remaud S. Comparative approaches to understanding thyroid hormone regulation of neurogenesis. Mol Cell Endocrinol. 2017;459:104‐115. [DOI] [PubMed] [Google Scholar]
- 24. Kinne A, Schüein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011;4(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arjona FJ, de Vrieze E, Visser TJ, Flik G, Klaren PH. Identification and functional characterization of zebrafish solute carrier Slc16a2 (Mct8) as a thyroid hormone membrane transporter. Endocrinology. 2011;152(12):5065‐5073. [DOI] [PubMed] [Google Scholar]
- 26. Bourgeois NM, Van Herck SL, Vancamp P, et al. Characterization of chicken thyroid hormone transporters. Endocrinology. 2016;157(6):2560‐2574. [DOI] [PubMed] [Google Scholar]
- 27. Mughal BB, Leemans M, Lima de Souza EC, et al. Functional characterization of xenopus thyroid hormone transporters mct8 and oatp1c1. Endocrinology. 2017;158(8):2694‐2705. [DOI] [PubMed] [Google Scholar]
- 28. Van Herck SLJ, Geysens S, Delbaere J, Tylzanowski P, Darras VM. Expression profile and thyroid hormone responsiveness of transporters and deiodinases in early embryonic chicken brain development. Mol Cell Endocrinol. 2012;349(2):289‐297. [DOI] [PubMed] [Google Scholar]
- 29. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571‐2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bárez‐López S, Montero‐Pedrazuela A, Bosch‐García D, Venero C, Guadaño‐Ferraz A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology. 2017;84:51‐60. [DOI] [PubMed] [Google Scholar]
- 31. Bates JM, St. Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140(2):844‐851. [DOI] [PubMed] [Google Scholar]
- 32. Yehuda‐Shnaidman E, Kalderon B, Bar‐Tana J. Thyroid hormone, thyromimetics, and metabolic efficiency. Endocr Rev. 2014;35(1):35‐58. [DOI] [PubMed] [Google Scholar]
- 33. Lanni A, Moreno M, Goglia F. Mitochondrial actions of thyroid hormone. Compr Physiol. 2016;6(4):1591‐1607. [DOI] [PubMed] [Google Scholar]
- 34. Zheng X, Boyer L, Jin M, et al. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. 2016;5:pii: e13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito K, Suda T. Metabolic requirements for the maintenance of self‐renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pistollato F, Chen H‐L, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci. 2007;35(3):424‐435. [DOI] [PubMed] [Google Scholar]
- 37. Xie Y, Zhang J, Lin Y, et al. Defining the role of oxygen tension in human neural progenitor fate. Stem Cell Reports. 2014;3(5):743‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darras VM, Houbrechts AM, van Herck SL. Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor‐mediated impact on vertebrate development. Biochim Biophys Acta. 2015;1849(2):130‐141. [DOI] [PubMed] [Google Scholar]
- 39. Darras VM, Van Herck SL, Heijlen M, De Groef B. Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res. 2011;2011:402320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Herck SLJ, Geysens S, Bald E, et al. Maternal transfer of methimazole and effects on thyroid hormone availability in embryonic tissues. J Endocrinol. 2013;218(1):105‐115. [DOI] [PubMed] [Google Scholar]
- 41. Vancamp P, Houbrechts AM, Darras VM. Insights from zebrafish deficiency models to understand the impact of local thyroid hormone regulator action on early development. Gen Comp Endocrinol. 2019;279:45–52. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt R, Sträle U, Scholpp S. Neurogenesis in zebrafish ‐ from embryo to adult. Neural Dev. 2013;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindsey BW, Hall ZJ, Heuzé A, Joly J‐S, Tropepe V, Kaslin J. The role of neuro‐epithelial‐like and radial‐glial stem and progenitor cells in development, plasticity, and repair. Prog Neurogibol. 2018;170:99‐114. [DOI] [PubMed] [Google Scholar]
- 44. Ravanelli AM, Appel B. Motor neurons and oligodendrocytes arise from distinct cell lineages by progenitor recruitment. Genes Dev. 2015;29(23):2504‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131(23):5959‐5969. [DOI] [PubMed] [Google Scholar]
- 46. Park HC, Shin J, Roberts RK, Appel B. An olig2 reporter gene marks oligodendrocyte precursors in the postembryonic spinal cord of zebrafish. Dev Dyn. 2007;236(12):3402‐3407. [DOI] [PubMed] [Google Scholar]
- 47. Park HC, Appel B. Delta‐Notch signaling regulates oligodendrocyte specification. Development. 2003;130(16):3747‐3755. [DOI] [PubMed] [Google Scholar]
- 48. Park HC, Boyce J, Shin J, Appel B. Oligodendrocyte specification in zebrafish requires notch‐regulated cyclin‐dependent kinase inhibitor function. J Neurosci. 2005;25(29):6836‐6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim H, Shin J, Kim S, Poling J, Park HC, Appel B. Notch‐regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev Dyn. 2008;237(8):2081‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porazzi P, Calebiro D, Benato F, Tiso N, Persani L. Thyroid gland development and function in the zebrafish model. Mol Cell Endocrinol. 2009;312(1‐2):14‐23. [DOI] [PubMed] [Google Scholar]
- 51. Zada D, Tovin A, Lerer‐Goldshtein T, Vatine GD, Appelbaum L. Altered behavioral performance and live imaging of circuit‐specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet. 2014;10(9):e1004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campinho MA, Saraiva J, Florindo C, Power DM. Maternal thyroid hormones are essential for neural development in zebrafish. Mol Endocrinol. 2014;28(7):1136‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Vrieze E, van deWiel SM, Zethof J, Flik G, Klaren PH, Arjona FJ. Knockdown of monocarboxylate transporter 8 (mct8) disturbs brain development and locomotion in zebrafish. Endocrinology. 2014;155(6):2320‐2330. [DOI] [PubMed] [Google Scholar]
- 54. Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan‐Herndon‐Dudley syndrome. Best Pract Res Clin Endocrinol Metab. 2007;21(2):307‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dumitrescu AM, Liao X‐H, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74(1):168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silva N, Louro B, Trindade M, Power DM, Campinho MA. Transcriptomics reveal an integrative role for maternal thyroid hormones during zebrafish embryogenesis. Sci Rep. 2017;7(1):16657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng Y‐C, Chiang M‐C, Shih H‐Y, et al. The transcription factor hairy/E(spl)‐related 2 induces proliferation of neural progenitors and regulates neurogenesis and gliogenesis. Dev Biol. 2015;397(1):116‐128. [DOI] [PubMed] [Google Scholar]
- 58. Vatine GD, Zada D, Lerer‐Goldshtein T, et al. Zebrafish as a model for monocarboxyl transporter 8‐deficiency. J Biol Chem. 2013;288(1):169‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zada D, Tovin A, Lerer‐Goldshtein T, Appelbaum L. Pharmacological treatment and BBB‐targeted genetic therapy for MCT8‐dependent hypomyelination in zebrafish. Dis Model Mech. 2016;9(11):1339‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee JY, Petratos S. Thyroid hormone signaling in oligodendrocytes: from extracellular transport to intracellular signal. Mol Neurobiol. 2016;53(9):6568‐6583. [DOI] [PubMed] [Google Scholar]
- 61. Heijlen M, Houbrechts AM, Bagci E, et al. Knockdown of type 3 iodothyronine deiodinase severely perturbs both embryonic and early larval development in zebrafish. Endocrinology. 2014;155(4):1547‐1559. [DOI] [PubMed] [Google Scholar]
- 62. Bohnsack BL, Kahana A. Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development. Dev Biol. 2013;373(2):300‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marelli F, Carra S, Agostini M, et al. Patterns of thyroid hormone receptor expression in zebrafish and generation of a novel model of resistance to thyroid hormone action. Mol Cell Endocrinol. 2016;424:102‐117. [DOI] [PubMed] [Google Scholar]
- 64. Takayama S, Hostick U, Haendel M, Eisen J, Darimont B. An F‐domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. Gen Comp Endocrinol. 2008;155(1):176‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yonkers MA, Ribera AB. Molecular components underlying nongenomic thyroid hormone signaling in embryonic zebrafish neurons. Neural Dev. 2009;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246(1‐2):10‐20. [DOI] [PubMed] [Google Scholar]
- 67. Denver RJ, Hu F, Scanlan TS, Furlow JD. Thyroid hormone receptor subtype specificity for hormone‐dependent neurogenesis in Xenopus laevis. Dev Biol. 2009;326(1):155‐168. [DOI] [PubMed] [Google Scholar]
- 68. Schlosser G, Koyano‐Nakagawa N, Kintner C. Thyroid hormone promotes neurogenesis in the Xenopus spinal cord. Dev Dyn. 2002;225(4):485‐498. [DOI] [PubMed] [Google Scholar]
- 69. Fini JB, Mughal BB, LeMével S, et al. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci Rep. 2017;7:43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Havis E, LeMevel S, Dubois GM, et al. Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J. 2006;25(20):4943‐4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oofusa K, Tooi O, Kashiwagi A, et al. Expression of thyroid hormone receptor betaA gene assayed by transgenic Xenopus laevis carrying its promoter sequences. Mol Cell Endocrinol. 2001;181(1‐2):97‐110. [DOI] [PubMed] [Google Scholar]
- 72. Morvan‐Dubois G, Demeneix BA, Sachs LM. Xenopus laevis as a model for studying thyroid hormone signalling: from development to metamorphosis. Mol Cell Endocrinol. 2008;293(1‐2):71‐79. [DOI] [PubMed] [Google Scholar]
- 73. Lau M, Li J, Cline HT. In Vivo Analysis of the Neurovascular Niche in the Developing Xenopus Brain. eNeuro. 2017;4(4:pii: ENEURO.0030-17.2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bestman JE, Lee‐Osbourne J, Cline HT. In vivo time‐lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles. J Comp Neurol. 2012;520(2):401‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thompson CK, Cline HT. Thyroid hormone acts locally to increase neurogenesis, neuronal differentiation, and dendritic arbor elaboration in the tadpole visual system. J Neurosci. 2016;36(40):10356‐10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Préau L, Le Blay K, Saint Paul E, Morvan‐Dubois G, Demeneix BA. Differential thyroid hormone sensitivity of fast cycling progenitors in the neurogenic niches of tadpoles and juvenile frogs. Mol Cell Endocrinol. 2016;420:138‐151. [DOI] [PubMed] [Google Scholar]
- 77. Le Blay K, Préau L, Morvan‐Dubois G, Demeneix B. Expression of the inactivating deiodinase, Deiodinase 3, in the pre‐metamorphic tadpole retina. PLoS ONE. 2018;13(4):e0195374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marsh‐Armstrong N, Huang H, Remo BF, Liu TT, Brown DD. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron. 1999;24(4):871‐878. [DOI] [PubMed] [Google Scholar]
- 79. Kaya F, Mannioui A, Chesneau A, et al. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J Neurosci. 2012;32(37):12885‐12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Too HC, Shibata M, Yayota M, Darras VM, Iwasawa A. Expression of thyroid hormone regulator genes in the yolk sac membrane of the developing chicken embryo. J Reprod Dev. 2017;63(5):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Darras VM. The Role of Maternal Thyroid Hormones in Avian Embryonic Development. Front Endocrinol (Lausanne). 2019;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Flamant F, Samarut J. Involvement of thyroid hormone and its alpha receptor in avian neurulation. Dev Biol. 1998;197(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 83. Forrest D, Hallböök F, Persson H, Vennström B. Distinct functions for thyroid hormone receptors alpha and beta in brain development indicated by differential expression of receptor genes. EMBO J. 1991;10(2):269‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nomura T, Hattori M, Osumi N. Reelin, radial fibers and cortical evolution: insights from comparative analysis of the mammalian and avian telencephalon. Dev Growth Differ. 2009;51(3):287‐297. [DOI] [PubMed] [Google Scholar]
- 85. Galileo DS, Gray GE, Owens GC, Majors J, Sanes JR. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell type‐specific antibodies. Proc Natl Acad Sci U S A. 1990;87(1):458‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gray GE, Sanes JR. Lineage of radial glia in the chicken optic tectum. Development. 1992;114(1):271‐283. [DOI] [PubMed] [Google Scholar]
- 87. Lever M, Brand‐Saberi B, Theiss C. Neurogenesis, gliogenesis and the developing chicken optic tectum: an immunohistochemical and ultrastructural analysis. Brain Struct Funct. 2014;219(3):1009‐1024. [DOI] [PubMed] [Google Scholar]
- 88. Vancamp P, Deprez MA, Remmerie M, Darras VM. Deficiency of the thyroid hormone transporter monocarboxylate transporter 8 in neural progenitors impairs cellular processes crucial for early corticogenesis. J Neurosci. 2017;37(48):11616‐11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vancamp P, Bourgeois NM, Houbrechts AM, Darras VM. Knockdown of the thyroid hormone transporter MCT8 in chicken retinal precursor cells hampers early retinal development and results in a shift towards more UV/blue cones at the expense of green/red cones. Exp Eye Res. 2018;178:135‐147. [DOI] [PubMed] [Google Scholar]
- 90. Geysens S, Ferran JL, VanHerck SL, Tylzanowski P, Puelles L, Darras VM. Dynamic mRNA distribution pattern of thyroid hormone transporters and deiodinases during early embryonic chicken brain development. Neuroscience. 2012;221:69‐85. [DOI] [PubMed] [Google Scholar]
- 91. Van Herck SLJ, Geysens S, Delbaere J, Darras VM. Regulators of thyroid hormone availability and action in embryonic chicken brain development. Gen Comp Endocrinol. 2013;190:96‐104. [DOI] [PubMed] [Google Scholar]
- 92. Delbaere J, Van Herck SLJ, Bourgeois NMA, et al. Mosaic Expression of Thyroid Hormone Regulatory Genes Defines Cell Type‐Specific Dependency in the Developing Chicken Cerebellum. Cerebellum. 2016;15(6):710‐725. [DOI] [PubMed] [Google Scholar]
- 93. Vancamp P, Darras VM. Dissecting the role of regulators of thyroid hormone availability in early brain development: Merits and potential of the chicken embryo model. Mol Cell Endocrinol. 2017;459:71‐78. [DOI] [PubMed] [Google Scholar]
- 94. Ausó E, Lavado‐Autric R, Cuevas E, et al. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037‐4047. [DOI] [PubMed] [Google Scholar]
- 95. Lavado‐Autric R, Ausó E, García‐Velasco JV, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111(7):1073‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mohan V, Sinha RA, Pathak A, et al. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol. 2012;237(2):477‐488. [DOI] [PubMed] [Google Scholar]
- 97. Chen C, Zhou Z, Zhong M, et al. Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRα1. Stem Cells Dev. 2012;21(14):2667‐2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bansal R, You S‐H, Herzig CTA, Zoeller RT. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs). Brain Res Dev Brain Res. 2005;156(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 99. Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008;50(Suppl 1):S97‐103. [DOI] [PubMed] [Google Scholar]
- 100. Johe KK, Hazel TG, Muller T, Dugich‐Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10(24):3129‐3140. [DOI] [PubMed] [Google Scholar]
- 101. Hadj‐Sahraoui N, Seugnet I, Ghorbel MT, Demeneix B. Hypothyroidism prolongs mitotic activity in the post‐natal mouse brain. Neurosci Lett. 2000;280(2):79‐82. [DOI] [PubMed] [Google Scholar]
- 102. Mayerl S, Müller J, Bauer R, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124(5):1987‐1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Richard S, Flamant F. Regulation of T3 availability in the developing brain: the mouse genetics contribution. Front Endocrinol (Lausanne). 2018;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gilbert ME, Goodman JH, Gomez J, Johnstone AF, Ramos RL. Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology. 2017;59:9‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Teng X, Liu YY, Teng W, Brent GA. COUP‐TF1 modulates thyroid hormone action in an embryonic stem‐cell model of cortical pyramidal neuronal differentiation. Thyroid. 2018;28(5):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chatonnet F, Flamant F, Morte B. A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta. 2015;1849(2):122‐129. [DOI] [PubMed] [Google Scholar]
- 107. Bernal J. Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol. 2017;232(2):R83‐R97. [DOI] [PubMed] [Google Scholar]
- 108. Gil‐Ibáñez P, García‐García F, Dopazo J, Bernal J, Morte B. Global transcriptome analysis of primary cerebrocortical cells: identification of genes regulated by triiodothyronine in specific cell types. Cereb Cortex. 2017;27(1):706‐717. [DOI] [PubMed] [Google Scholar]
- 109. Mi D, Li Z, Lim L, et al. Early emergence of cortical interneuron diversity in the mouse embryo. Science. 2018;360(6384):81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Telley L, Jabaudon D. A mixed model of neuronal diversity. Nature. 2018;555(7697):452‐454. [DOI] [PubMed] [Google Scholar]
- 111. Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27(2):248‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. O'Shaughnessy KL, Kosian PA, Ford JL, Oshiro WM, Degitz SJ, Gilbert ME. Developmental thyroid hormone insufficiency induces a cortical brain malformation and learning impairments: a cross‐fostering study. Toxicol Sci. 2018;163(1):101‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. O'Shaughnessy KL, Thomas SE, Spring SR, Ford JL, Ford RL, Gilbert ME. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Sci Rep. 2019;9(1):4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dehay C, Kennedy H, Kosik KS. The outer subventricular zone and primate‐specific cortical complexification. Neuron. 2015;85(4):683‐694. [DOI] [PubMed] [Google Scholar]
- 115. Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141(11):2182‐2194. [DOI] [PubMed] [Google Scholar]
- 116. Martínez‐Cerdeño V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152‐i161. [DOI] [PubMed] [Google Scholar]
- 117. Stenzel D, Huttner WB. Role of maternal thyroid hormones in the developing neocortex and during human evolution. Front Neuroanat. 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stenzel D, Wilsch‐Bräuninger M, Wong FK, Heuer H, Huttner WB. Integrin αvβ3 and thyroid hormones promote expansion of progenitors in embryonic neocortex. Development. 2014;141(4):795‐806. [DOI] [PubMed] [Google Scholar]
- 119. Lischinsky JE, Skocic J, Clairman H, Rovet J. Preliminary findings show maternal hypothyroidism may contribute to abnormal cortical morphology in offspring. Front Endocrinol (Lausanne). 2016;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33(4):842‐852. [DOI] [PubMed] [Google Scholar]
- 121. Gyllenberg D, Sourander A, Surcel H‐M, Hinkka‐Yli‐Salomäki S, McKeague IW, Brown AS. Hypothyroxinemia during gestation and offspring schizophrenia in a national birth cohort. Biol Psychiatry. 2016;79(12):962‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Modesto T, Tiemeier H, Peeters RP, et al. Maternal mild thyroid hormone insufficiency in early pregnancy and attention‐deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. 2015;169(9):838‐845. [DOI] [PubMed] [Google Scholar]
- 123. Salazar P, Cisternas P, Martinez M, Inestrosa NC. Hypothyroidism and cognitive disorders during development and adulthood: implications in the central nervous system. Mol Neurobiol. 2018;56(4):2952‐2963. [DOI] [PubMed] [Google Scholar]
- 124. Berbel P, Navarro D, Romá GC. An evo‐devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism. Front Endocrinol (Lausanne). 2014;5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121(11):1365‐1374. [DOI] [PubMed] [Google Scholar]
- 126. Korevaar T, Tiemeier H, Peeters RP. Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf). 2018;89(2):129‐138. [DOI] [PubMed] [Google Scholar]
- 127. Andersen SL, Andersen S, Vestergaard P, Olsen J. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish nationwide case‐cohort study. Thyroid. 2018;28(4):537‐546. [DOI] [PubMed] [Google Scholar]
- 128. López‐Espíndola D, Morales‐Bastos C, Grijota‐Martínez C, et al. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab. 2014;99(12):E2799‐E2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vatine GD, Al‐Ahmad A, Barriga BK, et al. Modeling psychomotor retardation using iPSCs from MCT8‐deficient patients indicates a prominent role for the blood‐brain barrier. Cell Stem Cell. 2017;20(6):831‐843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kapoor R, Fanibunda SE, Desouza LA, Guha SK, Vaidya VA. Perspectives on thyroid hormone action in adult neurogenesis. J Neurochem. 2015;133(5):599‐616. [DOI] [PubMed] [Google Scholar]
- 131. Remaud S, Gothié J‐D, Morvan‐Dubois G, Demeneix BA. Thyroid hormone signaling and adult neurogenesis in mammals. Front Endocrinol (Lausanne). 2014;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bhumika S, Lemmens K, Vancamp P, Moons L, Darras VM. Decreased thyroid hormone signaling accelerates the reinnervation of the optic tectum following optic nerve crush in adult zebrafish. Mol Cell Neurosci. 2015;68:92‐102. [DOI] [PubMed] [Google Scholar]
- 133. Endo T, Yoshino J, Kado K, Tochinai S. Brain regeneration in anuran amphibians. Dev Growth Differ. 2007;49(2):121‐129. [DOI] [PubMed] [Google Scholar]
- 134. Braun SM, Pilz GA, Machado RA, et al. Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Rep. 2015;11(11):1679‐1685. [DOI] [PubMed] [Google Scholar]
- 135. Rolando C, Erni A, Grison A, et al. Multipotency of adult hippocampal NSCs in ivo is restricted by Drosha/NFIB. Cell Stem Cell. 2016;19(5):653‐662. [DOI] [PubMed] [Google Scholar]
- 136. Seri B, Herrera DG, Gritti A, et al. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(Suppl 1):i103‐i111. [DOI] [PubMed] [Google Scholar]
- 137. Menn B, Garcia‐Verdugo JM, Yaschine C, Gonzalez‐Perez O, Rowitch D, Alvarez‐Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907‐7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Nait‐Oumesmar B, Picard‐Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104(11):4694‐4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xing YL, Röth PT, Stratton JA, et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014;34(42):14128‐14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Merkle FT, Mirzadeh Z, Alvarez‐Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381‐384. [DOI] [PubMed] [Google Scholar]
- 141. Götz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and Genome‐wide expression analysis. Glia. 2015;63(8):1452‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fuentealba LC, Rompani SB, Parraguez JI, et al. Embryonic origin of postnatal neural stem cells. Cell. 2015;161(7):1644‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez‐Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17(2):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chaker Z, Codega P, Doetsch F. A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip Rev Dev Biol. 2016;5(6):640‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lemkine GF, Raj A, Alfama G, et al. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 2005;19(7):863‐865. [DOI] [PubMed] [Google Scholar]
- 146. Ambrogini P, Cuppini R, Ferri P, et al. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81(4):244‐253. [DOI] [PubMed] [Google Scholar]
- 147. Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29(3):414‐426. [DOI] [PubMed] [Google Scholar]
- 148. Montero‐Pedrazuela A, Venero C, Lavado‐Autric R, et al. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive‐like behavior. Mol Psychiatry. 2006;11(4):361‐371. [DOI] [PubMed] [Google Scholar]
- 149. Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53(1):198‐214. [DOI] [PubMed] [Google Scholar]
- 150. Kapoor R, Desouza LA, Nanavaty IN, Kernie SG, Vaidya VA. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J Neuroendocrinol. 2012;24(9):1259‐1271. [DOI] [PubMed] [Google Scholar]
- 151. Sánchez‐Huerta K, García‐Martínez Y, Vergara P, Segovia J, Pacheco‐Rosado J. Thyroid hormones are essential to preserve non‐proliferative cells of adult neurogenesis of the dentate gyrus. Mol Cell Neurosci. 2016;76:1‐10. [DOI] [PubMed] [Google Scholar]
- 152. Brousse B, Magalon K, Durbec P, Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open. 2015;4(8):980‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kuhn HG, Dickinson‐Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age‐related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bouab M, Paliouras GN, Aumont A, Forest‐Berard K, Fernandes KJ. Aging of the subventricular zone neural stem cell niche: evidence for quiescence‐associated changes between early and mid‐adulthood. Neuroscience. 2011;173:135‐149. [DOI] [PubMed] [Google Scholar]
- 155. Capilla‐Gonzalez V, Herranz‐Péez V, Garcí‐Verdugo JM. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front Cell Neurosci. 2015;9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Weissleder C, Fung SJ, Wong MW, et al. Decline in proliferation and immature neuron markers in the human subependymal zone during aging: relationship to EGF‐ and FGF‐related transcripts. Front Aging Neurosci. 2016;8:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591‐2599. [DOI] [PubMed] [Google Scholar]
- 158. Schreiber G, Aldred AR, Jaworowski AN, Nilsson CH, Achen MG, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol. 1990;258(Pt 2):R338‐R345. [DOI] [PubMed] [Google Scholar]
- 159. Richardson SJ, Wijayagunaratne RC, D'Souza DG, Darras VM, VanHerck SL. Transport of thyroid hormones via the choroid plexus into the brain: the roles of transthyretin and thyroid hormone transmembrane transporters. Front Neurosci. 2015;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Alshehri B, D'Souza DG, Lee JY, Petratos S, Richardson SJ. The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol. 2015;27(5):303‐323. [DOI] [PubMed] [Google Scholar]
- 161. Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95‐122. [DOI] [PubMed] [Google Scholar]
- 162. Richardson SJ, Lemkine GF, Alfama G, Hassani Z, Demeneix BA. Cell division and apoptosis in the adult neural stem cell niche are differentially affected in transthyretin null mice. Neurosci Lett. 2007;421(3):234‐238. [DOI] [PubMed] [Google Scholar]
- 163. Visser TJ. Thyroid hormone transporters and resistance. Endocr Dev. 2013;24:1‐10. [DOI] [PubMed] [Google Scholar]
- 164. Schweizer U, Körle J. Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta. 2013;1830(7):3965‐3973. [DOI] [PubMed] [Google Scholar]
- 165. Hassani Z, Francois JC, Alfama G, et al. A hybrid CMV‐H1 construct improves efficiency of PEI‐delivered shRNA in the mouse brain. Nucleic Acids Res. 2007;35(9):e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kapoor R, van Hogerlinden M, Wallis K, et al. Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J. 2010;24(12):4793‐4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bruel‐Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18(2):93‐114. [DOI] [PubMed] [Google Scholar]
- 168. Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167(4):897‐914. [DOI] [PubMed] [Google Scholar]
- 169. Arenkiel BR. Adult neurogenesis supports short‐term olfactory memory. J Neurophysiol. 2010;103(6):2935‐2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Migaud M, Butruille L, Duittoz A, Pillon D, Batailler M. Adult neurogenesis and reproductive functions in mammals. Theriogenology. 2016;86(1):313‐323. [DOI] [PubMed] [Google Scholar]
- 171. Kageyama R, Imayoshi I, Sakamoto M. The role of neurogenesis in olfaction‐dependent behaviors. Behav Brain Res. 2012;227(2):459‐463. [DOI] [PubMed] [Google Scholar]
- 172. Paternostro MA, Meisami E. Lack of thyroid hormones but not their excess affects the maturation of olfactory receptor neurons: a quantitative morphologic study in the postnatal rat. Int J Dev Neurosci. 1991;9(5):439‐452. [DOI] [PubMed] [Google Scholar]
- 173. Beard MD, Mackay‐Sim A. Loss of sense of smell in adult, hypothyroid mice. Brain Res. 1987;433(2):181‐189. [DOI] [PubMed] [Google Scholar]
- 174. Kudo H, Eto A, Abe T, Mochida K. Detection and localization of the thyroid hormone receptor beta mRNA in the immature olfactory receptor neurons of chum salmon. Heliyon. 2018;4(8):e00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270‐276. [DOI] [PubMed] [Google Scholar]
- 176. Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63(3):155‐164. [DOI] [PubMed] [Google Scholar]
- 177. Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression‐like behaviors. Neuropsychopharmacology. 2015;40(10):2368‐2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(6):925‐935. [DOI] [PubMed] [Google Scholar]
- 179. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158(13):1413‐1418. [DOI] [PubMed] [Google Scholar]
- 180. Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26(1):45‐60. [DOI] [PubMed] [Google Scholar]
- 181. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. A reappraisal using objective psychological measurement. Arch Gen Psychiatry. 1969;20(1):48‐63. [DOI] [PubMed] [Google Scholar]
- 182. Eriksson PS, Perfilieva E, Björk‐Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313‐1317. [DOI] [PubMed] [Google Scholar]
- 183. Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42(7):621‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sorrells SF, Paredes MF, Cebrian‐Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589‐599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Moreno‐Jiménez EP, Flor‐García M, Terreros‐Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25(4):554‐560. [DOI] [PubMed] [Google Scholar]
- 188. Hamilton LK, Joppé SE, Cochard LM, Fernandes KJ. Aging and neurogenesis in the adult forebrain: what we have learned and where we should go from here. Eur J Neurosci. 2013;37(12):1978‐1986. [DOI] [PubMed] [Google Scholar]
- 189. Bergmann O, Liebl J, Bernard S, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74(4):634‐639. [DOI] [PubMed] [Google Scholar]
- 190. Capilla‐Gonzalez V, Cebrian‐Silla A, Guerrero‐Cazares H, Garcia‐Verdugo JM, Quiñones‐Hinojosa A. Age‐related changes in astrocytic and ependymal cells of the subventricular zone. Glia. 2014;62(5):790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sim FJ, Zhao C, Penderis J, Franklin RJ. The age‐related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Capilla‐Gonzalez V, Cebrian‐Silla A, Guerrero‐Cazares H, Garcia‐Verdugo JM, Quiñones‐Hinojosa A. The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front Cell Neurosci. 2013;7:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072‐1083. [DOI] [PubMed] [Google Scholar]
- 194. Rozing MP, Houwing‐Duistermaat JJ, Slagboom PE, et al. Familial longevity is associated with decreased thyroid function. J Clin Endocrinol Metab. 2010;95(11):4979‐4984. [DOI] [PubMed] [Google Scholar]
- 195. Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3(3):209‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Dawson MR, Polito A, Levine JM, Reynolds R. NG2‐expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24(2):476‐488. [DOI] [PubMed] [Google Scholar]
- 197. Ihrie RA, Shah JK, Harwell CC, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71(2):250‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ortega F, Gascón S, Masserdotti G, et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15(6):602‐613. [DOI] [PubMed] [Google Scholar]
- 199. Azim K, Fischer B, Hurtado‐Chong A, et al. Persistent Wnt/βcatenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells. 2014;32(5):1301‐1312. [DOI] [PubMed] [Google Scholar]
- 200. Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurogibol. 2011;93(2):182‐203. [DOI] [PubMed] [Google Scholar]
- 201. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mohyeldin A, Garzó‐Muvdi T, Quiñnes‐Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150‐161. [DOI] [PubMed] [Google Scholar]
- 203. Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298‐310. [DOI] [PubMed] [Google Scholar]
- 204. Shyh‐Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140(12):2535‐2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Wanet A, Arnould T, Najimi M, Renard P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015;24(17):1957‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Beckervordersandforth R, Ebert B, Schäffner I, et al. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93(3):560‐573.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia‐inducible factor induces local thyroid hormone inactivation during hypoxic‐ischemic disease in rats. J Clin Invest. 2008;118(3):975‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ciavardelli D, Bellomo M, Crescimanno C, Vella V. Type 3 deiodinase: role in cancer growth, stemness, and metabolism. Front Endocrinol (Lausanne). 2014;5:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Llorens‐Bobadilla E, Zhao S, Baser A, Saiz‐Castro G, Zwadlo K, Martin‐Villalba A. Single‐cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17(3):329‐340. [DOI] [PubMed] [Google Scholar]
- 211. Shin J, Berg DA, Zhu Y, et al. Single‐cell RNA‐seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17(3):360‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Khacho M, Clark A, Svoboda DS, et al. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell. 2016;19(2):232‐247. [DOI] [PubMed] [Google Scholar]
- 213. Calingasan NY, Ho DJ, Wille EJ, et al. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153(4):986‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Richetin K, Moulis M, Millet A, et al. Amplifying mitochondrial function rescues adult neurogenesis in a mouse model of Alzheimer's disease. Neurobiol Dis. 2017;102:113‐124. [DOI] [PubMed] [Google Scholar]
- 215. Janowska J, Ziemka‐Nalecz M, Sypecka J. The differentiation of rat oligodendroglial cells is highly influenced by the oxygen tension. In vitro model mimicking physiologically normoxic conditions. Int J Mol Sci. 2018;19(2):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kim HJ, Shaker MR, Cho B, et al. Dynamin‐related protein 1 controls the migration and neuronal differentiation of subventricular zone‐derived neural progenitor cells. Sci Rep. 2015;5:15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120(Pt 5):838‐848. [DOI] [PubMed] [Google Scholar]
- 218. Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105‐117. [DOI] [PubMed] [Google Scholar]
- 219. Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872‐884. [DOI] [PubMed] [Google Scholar]
- 220. Silva DF, Selfridge JE, Lu J, et al. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet. 2013;22(19):3931‐3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20(13):2495‐2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet. 2011;20(23):4515‐4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Bose A, Beal MF. Mitochondrial dysfunction in Parkinson's disease. J Neurochem. 2016;139(Suppl 1):216‐231. [DOI] [PubMed] [Google Scholar]
- 224. Bossy‐Wetzel E, Petrilli A, Knott AB. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31(12):609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Song W, Chen J, Petrilli A, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin‐related protein‐1 and increases its enzymatic activity. Nat Med. 2011;17(3):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bando Y, Nomura T, Bochimoto H, et al. Abnormal morphology of myelin and axon pathology in murine models of multiple sclerosis. Neurochem Int. 2015;81:16‐27. [DOI] [PubMed] [Google Scholar]
- 227. Campbell GR, Worrall JT, Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Mult Scler. 2014;20(14):1806‐1813. [DOI] [PubMed] [Google Scholar]
- 228. da Silva AF, Mariotti FR, Máximo V, Campello S. Mitochondria dynamism: of shape, transport and cell migration. Cell Mol Life Sci. 2014;71(12):2313‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203(13):2879‐2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Cereghetti GM, Stangherlin A, DeBrito OM, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105(41):15803‐15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Mylotte KM, Cody V, Davis PJ, Davis FB, Blas SD, Schoenl M. Milrinone and thyroid hormone stimulate myocardial membrane Ca2+‐ATPase activity and share structural homologies. Proc Natl Acad Sci U S A. 1985;82(23):7974‐7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Davis PJ, Davis FB, Lawrence WD. Thyroid hormone regulation of membrane Ca2(+)‐ATPase activity. Endocr Res. 1989;15(4):651‐682. [DOI] [PubMed] [Google Scholar]
- 234. Sossey‐Alaoui K, Hartung AJ, Guerrini R, et al. Human doublecortin (DCX) and the homologous gene in mouse encode a putative Ca2+‐dependent signaling protein which is mutated in human X‐linked neuronal migration defects. Hum Mol Genet. 1998;7(8):1327‐1332. [DOI] [PubMed] [Google Scholar]
- 235. Liu X, Hajnóczky G. Ca2+‐dependent regulation of mitochondrial dynamics by the Miro‐Milton complex. Int J Biochem Cell Biol. 2009;41(10):1972‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wulf A, Harneit A, Kröger M, Kebenko M, Wetzel MG, Weitzel JM. T3‐mediated expression of PGC‐1alpha via a far upstream located thyroid hormone response element. Mol Cell Endocrinol. 2008;287(1‐2):90‐95. [DOI] [PubMed] [Google Scholar]
- 237. Picca A, Lezza AM. Regulation of mitochondrial biogenesis through TFAM‐mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67‐75. [DOI] [PubMed] [Google Scholar]
- 238. Sinha RA, Pathak A, Mohan V, et al. Evidence of a bigenomic regulation of mitochondrial gene expression by thyroid hormone during rat brain development. Biochem Biophys Res Commun. 2010;397(3):548‐552. [DOI] [PubMed] [Google Scholar]
- 239. Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325‐339. [DOI] [PubMed] [Google Scholar]
- 240. Chen C, Liu Y, Liu R, et al. TSC‐mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205(10):2397‐2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Renault VM, Rafalski VA, Morgan AA, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Estrada JC, Torres Y, Benguria A, et al. Human mesenchymal stem cell‐replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rharass T, Lemcke H, Lantow M, Kuznetsov SA, Weiss DG, Panáková D. Ca2+‐mediated mitochondrial reactive oxygen species metabolism augments Wnt/βcatenin pathway activation to facilitate cell differentiation. J Biol Chem. 2014;289(40):27937‐27951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Walton NM, Shin R, Tajinda K, et al. Adult neurogenesis transiently generates oxidative stress. PLoS One. 2012;7(4):e35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141(22):4206‐4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cho YM, Kwon S, Pak YK, et al. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348(4):1472‐1478. [DOI] [PubMed] [Google Scholar]
- 247. Saretzki G, Walter T, Atkinson S, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26(2):455‐464. [DOI] [PubMed] [Google Scholar]
- 248. le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self‐renewal and neurogenesis in a PI3K/Akt‐dependant manner. Cell Stem Cell. 2011;8(1):59‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Hameed LS, Berg DA, Belnoue L, Jensen LD, Cao Y, Simon A. Environmental changes in oxygen tension reveal ROS‐dependent neurogenesis and regeneration in the adult newt brain. Elife. 2015;4:e08422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Prozorovski T, Schulze‐Topphoff U, Glumm R, et al. Sirt1 contributes critically to the redox‐dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385‐394. [DOI] [PubMed] [Google Scholar]
- 251. Lagouge M, Argmann C, Gerhart‐Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1alpha. Cell. 2006;127(6):1109‐1122. [DOI] [PubMed] [Google Scholar]
- 252. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC‐1{alpha}. J Biol Chem. 2005;280(16):16456‐16460. [DOI] [PubMed] [Google Scholar]
- 253. Saharan S, Jhaveri DJ, Bartlett PF. SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J Neurosci Res. 2013;91(5):642‐659. [DOI] [PubMed] [Google Scholar]
- 254. Rafalski VA, Ho PP, Brett JO, et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat Cell Biol. 2013;15(6):614‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Hisahara S, Chiba S, Matsumoto H, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105(40):15599‐15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393‐402. [DOI] [PubMed] [Google Scholar]
- 257. Nandal A, Ruiz JC, Subramanian P, et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011;14(5):647‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Stiban J, So M, Kaguni LS. Iron‐sulfur clusters in mitochondrial metabolism: multifaceted roles of a simple cofactor. Biochemistry (Mosc). 2016;81(10):1066‐1080. [DOI] [PubMed] [Google Scholar]
- 259. Lin KH, Lee HY, Shih CH, et al. Plasma protein regulation by thyroid hormone. J Endocrinol. 2003;179(3):367‐377. [DOI] [PubMed] [Google Scholar]
- 260. Marziali LN, Garcia CI, Pasquini JM. Transferrin and thyroid hormone converge in the control of myelinogenesis. Exp Neurol. 2015;265:129‐141. [DOI] [PubMed] [Google Scholar]
- 261. Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992;592(1‐2):8‐16. [DOI] [PubMed] [Google Scholar]
- 262. Hoshi K, Matsumoto Y, Ito H, et al. A unique glycan‐isoform of transferrin in cerebrospinal fluid: a potential diagnostic marker for neurological diseases. Biochim Biophys Acta Gen Subj. 2017;1861(10):2473‐2478. [DOI] [PubMed] [Google Scholar]
- 263. Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood‐brain barrier. J Neurochem. 2001;76(5):1597‐1600. [DOI] [PubMed] [Google Scholar]
- 264. Leitner DF, Connor JR. Functional roles of transferrin in the brain. Biochim Biophys Acta. 2012;1820(3):393‐402. [DOI] [PubMed] [Google Scholar]
- 265. Connor JR, Benkovic SA. Iron regulation in the brain: histochemical, biochemical, and molecular considerations. Ann Neurol. 1992;32(Suppl):S51‐S61. [DOI] [PubMed] [Google Scholar]
- 266. de los Monteros AE, Sawaya BE, Guillou F, Zakin MM, DeVellis J, Schaeffer E. Brain‐specific expression of the human transferrin gene. Similar elements govern transcription in oligodendrocytes and in a neuronal cell line. J Biol Chem. 1994;269(39):24504‐24510. [PubMed] [Google Scholar]
- 267. Aldred AR, Dickson PW, Marley PD, Schreiber G. Distribution of transferrin synthesis in brain and other tissues in the rat. J Biol Chem. 1987;262(11):5293‐5297. [PubMed] [Google Scholar]
- 268. Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. 1999;56(2):113‐122. [DOI] [PubMed] [Google Scholar]
- 269. Møllgård K, Dziegielewska KM, Saunders NR, Zakut H, Soreq H. Synthesis and localization of plasma proteins in the developing human brain. Integrity of the fetal blood‐brain barrier to endogenous proteins of hepatic origin. Dev Biol. 1988;128(1):207‐221. [DOI] [PubMed] [Google Scholar]
- 270. de Arriba Zerpa, GA , Saleh MC, Fernández PM, et al. Alternative splicing prevents transferrin secretion during differentiation of a human oligodendrocyte cell line. J Neurosci Res. 2000;61(4):388‐395. [DOI] [PubMed] [Google Scholar]
- 271. Connor JR, Menzies SL. Cellular management of iron in the brain. J Neurol Sci. 1995;134(Suppl):33‐44. [DOI] [PubMed] [Google Scholar]
- 272. Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol. 1996;375(4):675‐692. [DOI] [PubMed] [Google Scholar]
- 273. Pérez MJ, Fernandez N, Pasquini JM. Oligodendrocyte differentiation and signaling after transferrin internalization: a mechanism of action. Exp Neurol. 2013;248:262‐274. [DOI] [PubMed] [Google Scholar]
- 274. Han J, Day JR, Connor JR, Beard JL. Gene expression of transferrin and transferrin receptor in brains of control vs iron‐deficient rats. Nutr Neurosci. 2003;6(1):1‐10. [PubMed] [Google Scholar]
- 275. Moos T, Nielsen TR, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. 2007;103(5):1730‐1740. [DOI] [PubMed] [Google Scholar]
- 276. Todorich B, Zhang X, Slagle‐Webb B, Seaman WE, Connor JR. Tim‐2 is the receptor for H‐ferritin on oligodendrocytes. J Neurochem. 2008;107(6):1495‐1505. [DOI] [PubMed] [Google Scholar]
- 277. Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467‐478. [DOI] [PubMed] [Google Scholar]
- 278. Dümmler K, Müller S, Seitz HJ. Regulation of adenine nucleotide translocase and glycerol 3‐phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem J. 1996;317(Pt 3):913‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Schönfeld P, Wiêckowski MR, Wojtczak L. Thyroid hormone‐induced expression of the ADP/ATP carrier and its effect on fatty acid‐induced uncoupling of oxidative phosphorylation. FEBS Lett. 1997;416(1):19‐22. [DOI] [PubMed] [Google Scholar]
- 280. Weitzel JM, Kutz S, Radtke C, Grott S, Seitz HJ. Hormonal regulation of multiple promoters of the rat mitochondrial glycerol‐3‐phosphate dehydrogenase gene: identification of a complex hormone‐response element in the ubiquitous promoter B. Eur J Biochem. 2001;268(14):4095‐4103. [DOI] [PubMed] [Google Scholar]
- 281. Wrutniak C, Cassar‐Malek I, Marchal S, et al. A 43‐kDa protein related to c‐Erb A alpha 1 is located in the mitochondrial matrix of rat liver. J Biol Chem. 1995;270(27):16347‐16354. [DOI] [PubMed] [Google Scholar]
- 282. Wrutniak‐Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. J Mol Endocrinol. 2001;26(1):67‐77. [DOI] [PubMed] [Google Scholar]
- 283. Pessemesse L, Lepourry L, Bouton K, et al. p28, a truncated form of TRα1 regulates mitochondrial physiology. FEBS Lett. 2014;588(21):4037‐4043. [DOI] [PubMed] [Google Scholar]
- 284. Casas F, Rochard P, Rodier A, et al. A variant form of the nuclear triiodothyronine receptor c‐ErbAalpha1 plays a direct role in regulation of mitochondrial RNA synthesis. Mol Cell Biol. 1999;19(12):7913‐7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Rochard P, Rodier A, Casas F, et al. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J Biol Chem. 2000;275(4):2733‐2744. [DOI] [PubMed] [Google Scholar]
- 286. Grandemange S, Seyer P, Carazo A, et al. Stimulation of mitochondrial activity by p43 overexpression induces human dermal fibroblast transformation. Cancer Res. 2005;65(10):4282‐4291. [DOI] [PubMed] [Google Scholar]
- 287. Seyer P, Grandemange S, Rochard P, et al. P43‐dependent mitochondrial activity regulates myoblast differentiation and slow myosin isoform expression by control of Calcineurin expression. Exp Cell Res. 2011;317(14):2059‐2071. [DOI] [PubMed] [Google Scholar]
- 288. Perumalsamy LR, Nagala M, Sarin A. Notch‐activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci U S A. 2010;107(15):6882‐6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24(12):761‐770. [DOI] [PubMed] [Google Scholar]
- 290. Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30(9):3489‐3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278(25):23196‐23203. [DOI] [PubMed] [Google Scholar]
- 292. Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self‐renewal. Nature. 2010;467(7313):323‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Genoud S, Lappe‐Siefke C, Goebbels S, et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158(4):709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Sirakov M, Boussouar A, Kress E, et al. The thyroid hormone nuclear receptor TRα1 controls the Notch signaling pathway and cell fate in murine intestine. Development. 2015;142(16):2764‐2774. [DOI] [PubMed] [Google Scholar]
- 295. Sherwood V, Chaurasiya SK, Ekström EJ, et al. WNT5A‐mediated βcatenin‐independent signalling is a novel regulator of cancer cell metabolism. Carcinogenesis. 2014;35(4):784‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Pate KT, Stringari C, Sprowl‐Tanio S, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33(13):1454‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Brown K, Yang P, Salvador D, et al. WNT/βcatenin signaling regulates mitochondrial activity to alter the oncogenic potential of melanoma in a PTEN‐dependent manner. Oncogene. 2017;36(22):3119‐3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Catalano V, Dentice M, Ambrosio R, et al. Activated thyroid hormone promotes differentiation and chemotherapeutic sensitization of colorectal cancer stem cells by regulating Wnt and BMP4 signaling. Cancer Res. 2016;76(5):1237‐1244. [DOI] [PubMed] [Google Scholar]
- 299. Fernández‐Pernas P, Fafián‐Labora J, Lesende‐Rodriguez I, et al. 3, 3′, 5‐triiodo‐l‐thyronine Increases in vitro chondrogenesis of mesenchymal stem cells from human umbilical cord stroma through SRC2. J Cell Biochem. 2016;117(9):2097‐2108. [DOI] [PubMed] [Google Scholar]
- 300. Skah S, Uchuya‐Castillo J, Sirakov M, Plateroti M. The thyroid hormone nuclear receptors and the Wnt/βcatenin pathway: an intriguing liaison. Dev Biol. 2017;422(2):71‐82. [DOI] [PubMed] [Google Scholar]
- 301. Knobloch M, Braun SM, Zurkirchen L, et al. Metabolic control of adult neural stem cell activity by Fasn‐dependent lipogenesis. Nature. 2013;493(7431):226‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Seelig S, Liaw C, Towle HC, Oppenheimer JH. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A. 1981;78(8):4733‐4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Cunningham BA, Moncur JT, Huntington JT, Kinlaw WB. “Spot 14” protein: a metabolic integrator in normal and neoplastic cells. Thyroid. 1998;8(9):815‐825. [DOI] [PubMed] [Google Scholar]
- 304. Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31(2):538‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Sánchez‐Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36(3):321‐329. [DOI] [PubMed] [Google Scholar]
- 306. Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26(26):7035‐7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Macaskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20(2):102‐112. [DOI] [PubMed] [Google Scholar]
- 308. Rinholm JE, Vervaeke K, Tadross MR, et al. Movement and structure of mitochondria in oligodendrocytes and their myelin sheaths. Glia. 2016;64(5):810‐825. [DOI] [PubMed] [Google Scholar]


