Abstract
Beer would not exist without microbes. During fermentation, yeast cells convert cereal‐derived sugars into ethanol and CO2. Yeast also produces a wide array of aroma compounds that influence beer taste and aroma. The complex interaction between all these aroma compounds results in each beer having its own distinctive palette. This article contains all protocols needed to brew beer in a standard lab environment and focuses on the use of yeast in beer brewing. More specifically, it provides protocols for yeast propagation, brewing calculations and, of course, beer brewing. At the end, we have also included protocols for analyses that can be performed on the resulting brew, with a focus on yeast‐derived aroma compounds. © 2019 The Authors.
Keywords: beer, brewing, fermentation, Saccharomyces cerevisiae, yeast
INTRODUCTION
Beer brewing is intrinsically a biotechnological process: the conversion of raw materials into beer relies on many different enzymatic reactions and microbial activity. Beer is traditionally made from four key ingredients: malted cereals (barley or other), water, hops, and yeast. Each of these ingredients contributes to the final taste and aroma of beer.
Beer production starts with the malting of barley (or other cereals, such as wheat, sorghum, rye, or oats). The main goal of malting is to activate enzymes within the grain. These enzymes will hydrolyze starch and other compounds within the kernels during mashing (Goldammer, 2008; Kunze, 2004). During malting, barely kernels are soaked in water and periodically aerated, the so‐called steeping and germination phase. During germination, three important groups of enzymes are activated: (i) amylases, (ii) proteases/peptidases, and (iii) beta‐glucanases. Each of these enzymes have an important function during the malting and downstream brewing process: (i) amylases convert starch, present in the barley kernels, into fermentable sugars; (ii) proteases and peptidases break down proteins and release free amino nitrogen (FAN), while (iii) beta‐glucanases degrade the endosperm cell wall, allowing other enzymes access to the endosperm. Next, in the drying and kilning phase, kernels are dried and heated. This stops germination, arrests enzymatic activity within the kernels, reduces spoilage risks, and determines the impact of malt on the final aroma and color of the beer.
The actual brewing process consists of five steps. The main goal is to convert insoluble malt or grain material into a soluble and fermentable extract.
Milling of malted grains (i) and mashing (ii)
In this step, milled grains are mixed with warm water. This mash is kept at specific temperatures and pH to ensure proper enzymatic conversion of starch and proteins. Traditionally, a starting temperature of 45°C is used. At this temperature, proteases are activated and degrade proteins to short peptides and amino acids, that will form the major nitrogen source for yeast during fermentation. The mash is then heated to 62°C‐64°C, at which starch will gelatinize and become accessible to amylases. Beta‐amylases will cleave off maltose from starch molecules. The mash is then heated to 72°C for 15‐25 min, allowing further breakdown of long chain polysaccharides by alpha‐amylases. Finally, the temperature of the mash is raised to 78°C, stopping nearly all enzymatic activity.
Modern, highly modified malts allow mashing in directly at temperatures >60°C since the protein breakdown has already been completed by the maltster.
Filtering/lautering (iii)
During this step, the insoluble fraction (spent grains) is separated from the soluble extract. The remaining extract (wort) is transferred to the boiling vessel.
Boiling (iv)
During boiling, hops and other spices are added. These contribute to bitterness and aroma of the final beer. More specifically, hops contain alpha acids and during boiling, these acids will isomerize into iso‐alpha acids, the major bittering substances in beer.
Bitter hops contain high concentrations of alpha acids (6%‐16%) and are often added at the beginning of the boil. Aroma hops have a high hop oil content (>1%), which contains 200‐250 different compounds that contribute to the characteristic aroma of hops (e.g., myrcene, linalool, and nonenal) (Kunze, 2004). Aroma hops are typically only added towards the end of the boil, or in the dry‐hopping of green beer to reduce the stripping of aroma‐active compounds.
Other major effects of wort boiling include protein denaturation and aggregation, concentration of the wort, stripping of off‐flavors such as dimethyl sulfide (DMS), and sanitization of the wort. The boiled wort is then transferred to a whirlpool to remove the aggregated protein and insoluble hop components (hot trub). Finally, the wort is cooled, aerated, and transferred into the fermentor, where yeast is added.
Fermentation (v)
During fermentation, yeast converts fermentable sugars into CO2 and ethanol. At the same time, hundreds of secondary metabolites that influence the aroma and taste of beer are produced. Variation in these metabolites across different yeast strains is what allows yeast to so uniquely influence beer flavor. Examples of typical yeast‐derived beer flavors are the esters isoamylacetate (banana aroma), ethyl acetate (solvent‐like aroma), and ethyl hexanoate (pineapple aroma). For reviews on the different aroma's produced by yeast, and the biochemical pathways involved, see for example (Dzialo, Park, Steensels, Lievens, & Verstrepen, 2017; Pires, Teixeira, Branyik, & Vicente, 2014)
Primary fermentation is typically completed within 10 days, and the end‐product of this fermentation is called green beer. Afterwards, most of the yeast is removed, and the green beer is transferred to a maturation tank and stored at low temperatures (−1°C to 5°C), for several days (ale beers) or up to a couple of weeks (lager beers). During this maturation, the remaining yeast are still metabolically active and can produce additional CO2 and ethanol as well as reduce off‐flavors such as diacetyl (buttery, rancid aroma).
Many breweries have their own proprietary yeasts for their specific beers. Two main types of yeast are used in brewing: Saccharomyces cerevisiae is a top‐fermenting yeast used to make ales while Saccharomyces pastorianus is a bottom‐fermenting yeast used in lager brewing. The latter is actually a hybrid yeast and combines phenotypes of both its parental species: the high fermentation capacity of S. cerevisiae and the cold tolerance of S. eubayanus (Libkind et al., 2011).
Although most breweries use pure yeast cultures for fermentation, spontaneous or mixed fermentation is still used for some specialty beers. These kinds of fermentations usually involve a mix of different yeast species (and bacteria as well) that appear sequentially over time, giving the beer an added complexity. For example, Brettanomyces yeast species are commonly present during the later stages of Belgian lambic beer fermentation (Bokulich, Bamforth, & Mills, 2012; Steensels et al., 2015). Commonly referred to as Brett by brewers, these yeasts can metabolize longer chain carbohydrates and produce a number of distinctive compounds that provide the characteristic Brett aromas (barnyard, spicy, smoky, metallic etc.).
Since yeast is the driving force behind making beer, this protocol will focus on the yeast side of beer brewing. More specifically, this article describes the different steps in producing your own beer, starting from some basic brewing calculations to yeast propagation and chemical analysis of the end product. We deliberately only included one beer recipe, so you can experiment with how different yeast strains can influence beer aroma. Since the brewing world is full of its own jargon, we have included a glossary of brewing terminology and abbreviations (Table 1). The methods provided in this protocol are designed to have a minimal equipment requirement and is similar to a small‐scale home brewing approach. Far more advanced brewing setups are available but are often costly and vary greatly in the specifics of usage and are, thus, beyond the scope of this protocol unit. Each of the brewing‐specific materials can be purchased from various suppliers, we have listed some of them in Table 2.
Table 1.
Glossary of Brewing Terms
| Brewing term | Explanation |
|---|---|
| °P | Degree Plato; unit for the mass percentage of dissolved solids (mostly carbohydrates) in wort. By definition, wort with 1°P has the same density as a solution of 1 g sucrose in 100 g pure water. |
| 4‐VG | 4‐Vinyl guaiacol; phenolic compound originating from ferulic acid |
| Active dried yeast | Dried yeast in granulated form with a residual water content of approx. 5% and >6 × 109 viable cells per gram. Shelf life of 3 months at room temperature without losing viability or up to 36 months at <10°C with decreasing viability. Common suppliers are Lallemand and Fermentis. |
| Aeration | Dissolving of oxygen into the cold wort before fermentation. Important for the production of unsaturated fatty acids and sterols needed for yeast membrane production. |
| Alpha acids | Class of hop compounds, also known as humulones. Show a low solubility in water but isomerize during the boiling process into water soluble iso‐α‐acids. |
| Attenuation | The percentage of original extract consumed during fermentation. The maximum attenuation is determined by wort composition whereas the actual attenuation is largely determined by the yeast strain. |
| Base malt | Malt with full enzymatic potential, cell walls, and proteins are well degraded (highly modified malt), starch has not been degraded. Usually constitutes the bulk of the grain bill. |
| Brew house yield | Ratio of the extract (kg) in the cast wort and the mass of the malt used (kg) in percent. |
| Cast wort | The wort obtained after the boiling process including the hot trub. |
| Cold crash | Process in which the green beer is chilled to −1°C to 4°C to facilitate precipitation of cold trub. |
| Cold trub (also cold break) | A precipitate of formerly dissolved proteins, polyphenols, and carbohydrates that occurs upon cooling of the boiled wort. |
| Dry hopping | Addition of hops to the fermenting wort or green beer. During dry hopping predominantly aroma oils dissolve into the wort, while almost no bitterness is added. |
| EBC | European Brewing Congress. The abbreviation EBC is also used as the unit for the color of malts and wort. |
| Extract | Total amount of dissolved solids in the brewing water after the mashing process. |
| FAN | Free Amino Nitrogen; sum of all amino acids and peptides present in wort which can be utilized by yeast |
| Final gravity (FG) | Specific gravity of the beer after fermentation is finished. |
| Grain bill | Amount of malt(s) that is needed to brew a beer of desired volume and gravity. |
| Gravity | See specific gravity. |
| Green beer | Beer at the end of the fermentation, before lagering and maturation. |
| Grist | Ground malt. |
| Hop utilization% | Degree to which hop alpha acids are isomerized during the boiling process. Depends on the boiling time and wort gravity. |
| Hot trub (also hot break) | Insoluble material that occurs due to heat denaturation and coagulation of proteins during wort boiling. Also includes the remaining hop material. |
| IBU | International Bittering Unit; 1 IBU = 1 mg/L iso‐alpha acids |
| Iso‐alpha acids | Water soluble isomerization products of alpha acids. cis and trans form possible, both contribute equally to the bitterness of beer. |
| Kilning | Part of the malting process in which the germinated grains are dried and, if necessary, roasted to a desired color. |
| Krausen | Foam layer that occurs on ale beers (and to a lesser extent also on lager beers) during fermentation. Contains yeast cells, protein, and hop resins. |
| Lautering | Process in which the insoluble fraction of a mash (afterward referred to as spent grain) is separated from the liquid fraction (wort). |
| Liquid yeast | Yeast slurry/paste with a high cell density provided in tubes or sachets with 1‐2 × 109 viable cells/ml. Shelf life of 4 months at 4°C. Typical suppliers are White Labs and Wyeast. |
| Malt | Cereals in which the enzymatic potential has been released by a process of germination and subsequent heat drying (kilning). |
| Malt modification | Modification describes the enzymatic breakdown of biopolymers (mostly proteins and pentosanes) during malting. A highly modified malt is typically suitable for direct mash‐in at amylase rest temperatures without a previous protein rest. |
| Maltster | Professional maker of malt from raw cereals by directed germination and kilning processes (see Malt). |
| Mash | Mixture of grist and water, held at specific temperature for the enzymatic breakdown of proteins into FAN and of starch into the fermentable sugars that constitute wort. |
| Maturation | Also referred to as conditioning. Period after the end of fermentation during which the beer is rounding off its flavor profile, e.g., by reduction of diacetyl by yeast. |
| Off‐flavor | Collective term for aroma active compounds which are (usually) undesirable in beer, e.g., diacetyl, DMS, 4‐VG. |
| Original gravity (OG) | Specific gravity of the finished wort before fermentation starts. |
| Pitching rate | Amount of viable cells used to inoculate the wort. Benchmark for ale fermentations: 0.75 million cells/ml/°P and for lager fermentations: 1.5 million cells/ml/°P |
| POF | Phenolic Off Flavor; specific off‐flavor often described as smoky, clove like, sometimes medicinal or burned plastic. Caused by phenolic compounds, most prominently 4‐vinylguaiacol (4‐VG). |
| Saccharomyces cerevisiae | So called “brewer's and baker's” yeast. S. cerevisiae strains are typically used for ales/top‐fermented beers, e.g., Belgian specialty beers, (India) pale ale, and Hefeweizen. |
| Saccharomyces pastorianus | S. cerevisiae × S. eubayanus hybrid used for lager/bottom‐fermented beers. Shows a better cold tolerance than ale yeasts. |
| Sparging | The process of sequential addition of hot water to cast wort from the spent grains during lautering. |
| Specialty malt | Malt used to adjust the body or add flavor to a beer, typically no more than 10% of the grain bill. Usually no enzymatic potential, cell walls, proteins, and starch are well degraded, free sugars are caramelized during kilning and subsequent roasting. |
| Specific gravity (SG) | Ratio of the density of wort and that of pure water at the same temperature (usually 20°C). |
| Spent grains | Solid fraction that remains after the lautering. |
| Steeping | First step of the malting process, in which cereals are soaked in water to start germination. |
| Trub | Collective term for “hot trub” and “cold trub.” |
Table 2.
Suppliers for Brewing Equipment
| Country | Supplier Web site |
|---|---|
| US | https://www.northernbrewer.com |
| UK | https://www.themaltmiller.co.uk |
| Germany | https://www.hobbybrauerversand.de |
| Belgium | https://www.brouwland.be |
Please note that it is beyond the scope of this article to give you a detailed overview of all different beer brewing methods and recipes available. For this, we refer readers to, for example Goldammer, 2008; Snyder, 1997, and to the Internet Resources listed at the end of the article.
Basic Protocol 1. PREPARING 20 L OF 12°P WORT STARTING FROM WHOLE GRAINS
Wort production can be broken down into three essential steps − mashing, lautering, and boiling. Making a wort recipe from scratch allows complete control of this process. This can be important if the experiments in question require specific modifications to wort composition. Here we provide a basic wort recipe starting from whole grains that can easily be scaled or modified as needed.
The recipe is for a generic ale beer with 25 international bittering units (IBU) (see also Support Protocol 1). It is not designed to mimic a specific beer style but rather produce a lightly hopped wort that can be used as a growth medium for fermentation assays. Individual styles are beyond the scope of this method but there are many resources and software available for developing recipes within individual beer style guidelines, see Internet Resources.
This protocol uses a single temperature infusion mash—a compromise between the different thermal optima of malt enzymes and overall efficiency. For yeast propagation and simple fermentation tests, wort prepared according to Alternate Protocol 1 can also be used.
Materials
70% (v/v) ethanol
Pale ale malt
Water
Bittering hops (such as magnum hops 15% alpha acids)
200 ppm zinc stock solution (see recipe)
Roller mill for grinding malt (optional if buying pre‐ground malts)
30‐L stainless steel electric kettle with stainless steel tap for use as mash tun
Thermometer
Brewers paddle or large spoon to stir the mash
30‐L stainless steel pot with tap and false bottom for use as a lauter tun
500‐ml beaker
30‐L stainless steel electric kettle with stainless steel tap for use as boil kettle
Cooling coil with hose tap attachments
Sterile 20‐L canister
Centrifuge
Four sterile 5‐L canisters
Additional reagents and equipment for performing an iodine start test (see Support Protocol 4)
Prepare equipment
-
1
Sanitize all equipment with 70% ethanol and allow the ethanol to evaporate completely before use.
Do not use ethanol with additives such as methyl ethyl ketone (MEK), since this is not suitable for human consumption.
Mashing
-
2
Grind 4.2 kg of pale ale malt using a roller mill set to 0.7 mm.
See Support Protocol 2 for grain bill calculation.
Properly ground malt should have particles small enough to facilitate efficient hydration. It should also still include unshredded husks to create a stable grain bed during the lautering and prevent a stuck mash (see also Troubleshooting).
-
3
Add 12 L of water to the mash tun and pre‐heat to a strike temperature of 71°C
See Support Protocol 3 for the calculation of strike water temperature.
-
4
Gently stir in the grist (ground malt produced in step) ensuring that any clumps of malt are broken up.
-
5
Maintain the temperature of the mash at 65°C for 60 min. During this time, gently stir the mash every 10 min.
The mash temperature can be between 63°C and 72°C. Lower temperatures will result in a highly fermentable wort, whereas higher temperatures will increase the amount of non‐fermentable sugars in the wort and can increase mouthfeel and apparent sweetness in the final beer.
-
6
Perform an iodine starch test to ensure the mash has undergone complete saccharification.
See Support Protocol 4 for the iodine starch test.
-
7
When the iodine starch test is negative (which means the starch is sufficiently digested), heat the mash up to 78°C.
During this step, residual amylases will be deactivated. The wort's viscosity is also decreased at higher temperatures, which helps in the subsequent lautering. Make sure not to heat above 80°C as this would release undigested starch to the wort.
Lautering
-
8
Place the false bottom in the lauter tun, transfer in the mash contents, and allow to settle for 20 min.
Make sure to elevate the lauter tun, so that its contents can drain back into the cleaned electric kettle.
-
9
While the mash is settling, heat up 16 L of water in the cleaned mash tun to 78°C for use in the sparge.
-
10
Slowly drain the mash into a 500‐ml beaker and gently pour this back into the lauter tun.
The first runnings will be cloudy and full of particulate matter.
-
11
Repeat this process until the wort begins to run clear.
-
12
Once the wort begins to run clear, it can be drained directly into the boil kettle.
Start draining with a low flow rate, which can be increased as a stable grain bed settles on the false bottom of the mash tun (see also Troubleshooting). A low flow rate is needed to avoid collapse or creation of channels through the grain bed.
-
13
As the mash drains, additional sparge water can be gently added on top of the mash and continually collected as the mash drains into the boil kettle. As the lautering progresses keep track of the wort gravity (see Basic Protocol 2 or Alternate Protocol 2).
-
14
Continue to add sparge water and lauter until the gravity of the wort is 10% below the desired final gravity (FG) (i.e., 10.8°P for a FG of 12°P).
Note that this percentage can be adapted to match the evaporation rate of your brewing setup in the subsequent boiling step.
Boiling
-
15
Turn on the kettle heating and bring the wort to a boil.
-
16
Add 12 g of bittering hops (15% AA).
See Support Protocol 1 for calculating how much hops need to be added to reach a specific IBU.
-
17
Boil for 60 min.
Cooling
-
18
Attach the sanitized cooling coil to a cold water outlet and place the coil into the brew kettle. Turn on the water and allow to run until the wort has cooled below 25°C.
-
19
Remove the cooling coil and transfer the cooled wort into a 20‐L canister and store overnight at 4°C.
-
20
Decant the wort, leaving as much of the trub behind as possible.
-
21
Ensure the wort is homogeneous and dilute the wort to 12°P with sterile water if required.
Ideally, the final volume should be 20 L but this will depend on the efficiency of the mashing and lautering procedures, which can be influenced by a number of factors (see Troubleshooting).
-
22
Add zinc to a final concentration of 500 ppb (2.5 ml stock solution per liter wort).
If needed, wort can be sterilized by autoclaving for 10 min at 105°C.
-
23
Optional: Centrifuge for 5 min at 1,500‐3,000 × g, 4°C, completely separate any remaining trub.
-
24
Collect the clarified wort in sterile 5 L canisters.
-
25
Store the wort at 4°C until use.
For long‐term storage, the wort can be kept at −20°C (>6 months).
Support Protocol 1. PREDICTING BITTERNESS
Bitterness of beer is measured in international bittering units (IBU), which represent the amount of iso‐alpha acids per liter (quantified as mg/L or ppm). Importantly, the IBU value of a beer may not match its perceived bitterness. The bitterness of a beer can be affected by, among others, the alcohol level, residual sugars or the use of roasted malts.
Estimation of the expected bitterness of a beer can be calculated using the following formula:
| (1) |
where:
-
Alpha acid% = alpha acid content of hops
This is provided by the hop producer. Alpha acid% greatly depends on hop variety, harvest year, and hop storage conditions.
-
Utilization% = hop utilization rate
The hop utilization rate represents the percentage of hop alpha acids that are first solubilized in wort and later isomerized into iso‐alpha acids. This isomerization reaction requires heat and is dependent on the time of hop addition to the boil and the gravity of the wort. In Table 3, utilization% is provided for a range of boiling times and wort gravities (Mosher & Finkel, 1993).
V = volume of cooled wort (L)
Table 3.
Utilization%, Adjusted From Mosher & Finkel (1993)
| Specific gravity | |||||||
|---|---|---|---|---|---|---|---|
| Boiling times | 1.030 | 1.040 | 1.050 | 1.060 | 1.070 | 1.080 | 1.090 |
| Whole hops | |||||||
| 5 min | 5% | 5% | 4% | 4% | 3% | 3% | 3% |
| 15 min | 12% | 12% | 11% | 11% | 11% | 10% | 9% |
| 30 min | 17% | 17% | 16% | 16% | 15% | 15% | 13% |
| 45 min | 21% | 21% | 20% | 19% | 18% | 17% | 16% |
| 60 min | 24% | 23% | 23% | 22% | 21% | 20% | 18% |
| 90 min | 28% | 27% | 26% | 26% | 25% | 23% | 21% |
| Hop pellets | |||||||
| 5 min | 6% | 6% | 5% | 5% | 4% | 4% | 3% |
| 15 min | 15% | 15% | 14% | 14% | 13% | 13% | 11% |
| 30 min | 22% | 21% | 21% | 20% | 19% | 18% | 16% |
| 45 min | 26% | 26% | 25% | 24% | 23% | 22% | 21% |
| 60 min | 29% | 28% | 28% | 27% | 26% | 25% | 23% |
| 90 min | 35% | 34% | 33% | 32% | 31% | 29% | 27% |
Support Protocol 2. DETERMINING THE GRAIN BILL TO ACHIEVE A TARGET EXTRACT AND WORT VOLUME
The grain bill is the amount of malt needed to produce a wort of desired final gravity (FG) and volume. It can be estimated using the following formula
| (2) |
where:
V [L] = the desired volume of cooled wort at 20°C
ρ [kg/L] = the density of the desired wort at 20°C and x°P (Table 4)
°P [% w/w] = the desired wort gravity expressed in degrees Plato
Table 4.
Conversion of °P to SG and density at 20°C
| °P (g/100 g) | SG | ρ (kg/L) | °P (g/100 g) | SG | ρ (kg/L) | °P (g/100 g) | SG | ρ (kg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.00388 | 1.00208 | 7.5 | 1.02976 | 1.02792 | 14 | 1.05684 | 1.05495 |
| 1.5 | 1.00583 | 1.00403 | 8 | 1.03180 | 1.02995 | 14.5 | 1.05898 | 1.05708 |
| 2 | 1.00779 | 1.00598 | 8.5 | 1.03385 | 1.03199 | 15 | 1.06112 | 1.05922 |
| 2.5 | 1.00975 | 1.00794 | 9 | 1.03590 | 1.03404 | 15.5 | 1.06327 | 1.06137 |
| 3 | 1.01172 | 1.00991 | 9.5 | 1.03796 | 1.03610 | 16 | 1.06543 | 1.06352 |
| 3.5 | 1.01370 | 1.01188 | 10 | 1.04003 | 1.03817 | 16.5 | 1.06760 | 1.06568 |
| 4 | 1.01568 | 1.01386 | 10.5 | 1.04211 | 1.04024 | 17 | 1.06977 | 1.06785 |
| 4.5 | 1.01767 | 1.01585 | 11 | 1.04419 | 104232 | 17.5 | 1.07195 | 1.07003 |
| 5 | 1.01967 | 1.01784 | 11.5 | 1.04628 | 1.04440 | 18 | 1.07414 | 1.07222 |
| 5.5 | 1.02167 | 1.01984 | 12 | 1.04838 | 1.04650 | 18.5 | 1.07634 | 1.07441 |
| 6 | 1.02369 | 1.02185 | 12.5 | 1.05048 | 1.04860 | 19 | 1.07855 | 1.07661 |
| 6.5 | 1.02570 | 1.02386 | 13 | 1.05260 | 1.05071 | 19.5 | 1.08076 | 1.07882 |
| 7 | 1.02773 | 1.02589 | 13.5 | 1.05472 | 1.05283 | 20 | 1.08298 | 1.08104 |
The brewhouse yield displays the relation between the mass of the malt used and the mass of the dissolved extract obtained in the wort (Kunze, 2004). It is unique to each brewing setup and recipe and has to be determined empirically. However, a 60% brewhouse yield can be used as a reasonable estimate to calculate the first brew. For more details on brewhouse yield estimation, see Kunze (2004).
For example, for 20 L of a 12°P wort with a brew house yield of 60%, we need the following amount of malt:
Support Protocol 3. CALCULATE REQUIRED STRIKE WATER TEMPERATURE
The required temperature of the strike water can be determined according to (Holle & Klimovitz, 2003):
| (3) |
where:
GW [kg] = the weight of the added malt/grain
WW [kg] = the weight of the added strike water
TM [°C] = wanted final temperature of the mash
TG [°C] = the temperature of the added malt/grain
Support Protocol 4. IODINE TEST FOR SACCHARIFICATION OF MASH
During mashing, complex carbohydrates need to be hydrolyzed into sugars that can be consumed by yeast, such as glucose, maltose, and maltotriose. This protocol describes how to check if the starch has been sufficiently hydrolyzed.
Materials
Mash solution (see Basic Protocol 1)
Lugol's iodine solution (see recipe)
White porcelain bowl
-
1
Add a few drops of the mash solution to a white porcelain bowl.
Avoid any grain particles as these can lead to a false‐positive reaction.
-
2
Add a single drop of Lugol's iodine solution and note the color.
Yellow‐amber red colors indicate complete conversion of starch (Fig. 1C). Blue/black indicates incomplete conversion of starch (Fig. 1A‐B) and the mash should be given an additional 15 min to complete.
Figure 1.

Iodine starch test. (A) Positive starch result, sample from the beginning of a mash. (B) Partial starch conversion mid‐mash. (C) Negative starch result, complete starch conversion from the end of a mash.
Alternate Protocol 1. DRIED MALT EXTRACTRECIPE OF 12°P WORT (1 L)
This recipe is ideal for yeast propagation and simple tests (e.g., characterization of the basic fermentation properties of different yeasts). Dried malt extract (DME) wort offers a very reproducible recipe that can be easily prepared without the specialized equipment required for an all grain‐derived wort (see Basic Protocol 1). DME, or spray malt, is available in a range of colors and comes in hopped and unhopped varieties. Here we use light unhopped DME, but the recipe can be readily adapted using other DME variants. This recipe can also be used as a concentrated stock if lower gravity wort is required (e.g., for yeast propagation).
Materials
Light unhopped dry malt extract [DME: color: 8 European Brewery Convention (EBC)]
Deionied water
Zinc chloride solution at 200 ppm (see recipe)
5‐L bottle
Autoclave
Sterile 500‐ml buckets/bottles to centrifuge wort
Centrifuge
Density meter (e.g., Anton Paar DMA 35)
-
1
In a 5‐L bottle, prepare DME solution by dissolving 549.5 g of light (8 EBC) unhopped DME in water to a total volume of 2 L.
During autoclaving, proteins precipitate and the wort needs to be clarified afterwards. Because of this, twice the volume is prepared to ensure there is sufficient clarified wort harvested in the end.
-
2
Sterilize by autoclaving for 10 min at 105°C.
Do not autoclave at 121°C as this temperature facilitates maillard reactions between carbohydrates and amino‐compounds.
-
3
Cool the wort down overnight to 4°C.
This will assist protein precipitation and improve the clarity of the final wort.
-
4
Gently decant the wort into sterile 500‐ml centrifuge buckets, leaving as much of the trub behind as possible.
-
5
Centrifuge for 5 min at 1,500‐3,000 × g, 4°C. Collect 1 L of the clarified wort.
-
6
Measure gravity of the wort with the density meter and dilute to 12°P using sterile water if required (see Basic Protocol 2 or Alternate Protocol 2).
-
7
Add zinc to a final concentration of 500 ppb (2.5 ml zinc solution per liter wort).
Zinc is required as a co‐factor for a number of enzymes, such as alcohol dehydrogenase (Kunze, 2004), and is commonly added as supplement to wort.
Basic Protocol 2. EXTRACT MEASUREMENT OF WORT USING A HYDROMETER
For brew house calculations, brewers mostly rely on measurements of the wort's or beer's density. Traditionally, wort density is measured using a hydrometer. A hydrometer is a floating body with a thicker bulb for a base, sometimes containing a thermometer, and a thinner upper stem holding a mass percentage (°P) or specific gravity (SG) scale, or both. The value can be determined as the point to which the stem sinks into the fluid. A hydrometer will sink deeper into a sample with lower density and vice versa.
The extract measured before fermentation is called original extract (OE) if measured in °P, or original gravity (OG) if measured as SG. Extract values measured after the fermentation is finished are called residual extract (RE) or FG, respectively.
Here, we give a protocol to determine wort extract using a hydrometer.
Materials
Wort sample (see Basic Protocol or Alternate Protocol 1)
250‐ml glass bottle with screwcap
Cooling bath at 20°C
Thermometer
200‐ml glass cylinder
Hydrometer
-
1
Collect a homogeneous sample (200 ml) of the wort in a 250‐ml glass bottle and bring it to 20°C using a water bath.
Close the bottle to avoid evaporation during the cooling process.
-
2
Make sure the sample is thoroughly mixed and transfer to the glass cylinder.
Avoid foam on top of the wort sample.
-
3
Wet the dry hydrometer by immersing it into the wort sample. Pull it up again and then lower it gently until the expected value is almost reached.
Make sure no gas bubbles adhere to the hydrometer.
-
4
Read the gravity value of the wort as the point where the meniscus touches the stem of the hydrometer.
Hydrometers for brewing are often calibrated to be read at the highest point of the meniscus which forms around the stem, whereas hydrometers for laboratory use are calibrated to be read at the lowest point of the meniscus. Please check the user manual.
Alternate Protocol 2. EXTRACT MEASUREMENT OF WORT USING AN OSCILLATING U‐TUBE DENSITY METER
A faster and easier way of measuring the wort's extract is using a density meter. The working principle of a density meter is based on an oscillating U‐tube made of glass. The U‐tube gets excited to vibrate at its characteristic frequency. Depending on the density of the tested sample this characteristic frequency changes, which allows calculation of the actual density (DMA 35 Portable Density Meter, Instruction Manual, Anton Paar GmbH, Austria, 2009). This calculation is automatically done by density meter devices for beverage use (e.g., Anton Paar DMA 35).
Materials
Warm tap water (40°C)
Demineralized water
100‐ml glass bottle with screwcap
Cooling bath at 20°C
Density meter (e.g., Anton Paar DMA 35)
Plastic syringes
-
1
Transfer a homogeneous sample (∼50 ml) of the wort into a 100‐ml glass bottle and bring the temperature to 20°C.
Close the bottle to avoid evaporation.
-
2
Rinse the density meter's U‐tube with your sample before you take the actual measurement. To this end, aspire the liquid into the U‐tube using the built‐in sample tube and pump lever or inject the sample with a plastic syringe. Then dispose of the liquid by depressing the pump lever.
-
3
Take the actual sample in the same way as for the rinsing step; the device will automatically show the density value or any other derived value by choice (e.g., °P or SG).
-
4
Take additional two readings to ensure consistency.
-
5
Rinse the U‐tube with warm tap water followed by a final rinse with demineralized water.
Basic Protocol 3. YEAST PROPAGATION FROM CRYO CULTURES, AGAR PLATES, OR SLANTS
Because of the tremendous effect of yeast‐produced aroma compounds on the quality and character of the final beer, selecting an appropriate yeast is an important and crucial step during brewing. Nowadays, different brewing yeasts are commercially available. These yeast strains are most often supplied as liquid yeast slurries or as active dried yeast powder. For use of these yeast starter cultures, we refer to the manufacturers protocol. Another option is to start from a yeast strain that is stored at −80°C (see Support Protocol 5). The protocol below describes how to propagate yeast from such a cryo culture to sufficient cell densities to pitch it into 20 L of a 12°P wort. Using the protocol below, a cell density of ∼2 × 108 cells per ml with a viability >95% can usually be obtained. Please check Critical Parameters and Troubleshooting if the cell counts are much lower than expected.
Materials
Yeast strain of choice, as cryo culture or streaked on an agar plate or agar slant
YPD (2% glucose) agar plate
Liquid wort (see Alternate Protocol 1) or YP maltose (see recipe)
Sterile wooden sticks (toothpicks work fine) or inoculation loop
Sterile test tubes
Shaking incubator
Test tube rotator
Sterile Erlenmeyer flasks
-
1
Use the inoculation loop/wooden stick to streak a bit of the frozen material from the cryo culture onto a YPD agar plate and incubate for 2 days at 30°C.
This step can be skipped if starting from agar plate or slant.
For lager yeasts, incubate at 20°C rather than 30°C.
Make sure to not thaw and refreeze the cryo culture, since this will significantly reduce cell viability.
-
2
Use a sterilized inoculation loop/wooden stick to pick up cell material from roughly 15‐30 different colonies from the plate or slant.
Avoid starting with a single colony as this will increase the risk of selecting a colony that may have acquired a deleterious mutation.
-
3
Submerge the inoculation loop/wooden stick in 5 ml liquid medium in a test tube and gently shake yeast cells free.
Use either wort or YP maltose as starter medium.
-
4
Place the tube in a tube rotator and incubate for 24 hr at 18°C‐24°C. The culture is now ready for the next propagation step.
If starting from a commercially available yeast strain, check instructions of yeast supplier for exact temperatures to grow your yeast at.
-
5
Propagation cultures can be serially stepped up in size by a factor 10 until the desired number of viable cells is achieved by inoculating the entirety of the previous starter culture into the next step, e.g., 5 ml → 50 ml → 500 ml. Volumes larger than 5 ml should be grown in Erlenmeyer flasks in a shaking incubator.
Starter wort gravity is advised to be between 7 and 10°P when brewing a beer with an OE of 10 to 14°P.
Note that using medium containing only glucose as the carbon source is not recommended as the yeast will need time to adapt metabolism to utilize the maltose in wort. The pH of the starter wort should be around 5.
Support Protocol 5. STORING ANY YEAST STRAIN AT −80°C BY MAKING A GLYCEROL STOCK
For the long‐term storage of any yeast strain we recommend glycerol cryo stocks stored at −80°C.
Materials
Dried yeast
YPD (2% glucose) agar plates
YPD (2% glucose) liquid medium
50% glycerol (sterile)
Incubator
Inoculation loop/sterile wooden sticks
Test tubes
Test tube rotator
2‐ml Cryo‐vials (Corning, REF 430659)
−80°C freezer
When starting from (commercially available) liquid cultures or dried yeast
-
1
Rehydrate dried yeast following the manufacturers protocol.
-
2
Dilute liquid (rehydrated) yeast culture to ∼2‐3 × 103 cells/ml (see Basic Protocol 4).
-
3
Streak 100 µl of this dilution on a YPD agar plate and incubate for 2 days at 30°C.
For commercially available strains, check the manufacturers protocol for recommended incubation temperatures.
When yeast strains are already streaked on agar plates
-
4
Pick roughly 15‐30 colonies from the agar plate using an inoculation loop or wooden stick.
Avoid starting with a single colony as this will increase the risk of selecting a colony that may have acquired a deleterious mutation.
-
5
Submerge the inoculation loop/wooden stick in 5 ml YPD liquid medium in test tube and gently shake yeast cells free.
-
6
Place the tube in a test tube rotator and incubate at 18°C‐24°C for 24 hr.
If starting from a commercially available yeast strain, check instructions of yeast supplier for exact temperatures to grow your yeast at.
-
7
Transfer 500 µl of the overnight culture into a cryo‐vial.
-
8
Mix gently with the same amount of 50% glycerol (final glycerol concentration 25%).
-
9
Freeze to −80°C until further use.
Basic Protocol 4. ASSESSING YEAST CELL NUMBER AND VIABILITY
Prior to pitching the starter culture, most brewers check cell count and cell viability. One of the most widely used methods in the brewing industry requires a microscope, hemacytometer, and staining with methylene blue or methylene violet (Smart, Chambers, Lambert, Jenkins, & Smart, 1999). All yeast cells take up the dye, but only viable cells can reduce it to a colorless product.
This protocol describes how to determine the total number of cells in a liquid culture, as well as the number of viable cells, using a methylene violet staining protocol. Cell counts and viability measurements can also be automated, for example by using a TC20 Automated Cell Counter (Bio‐Rad).
Materials
Deionized water
Yeast sample
0.9% (w/v) NaCl solution or PBS
0.1 M glycine buffer, pH 10.6 (adjusted with NaOH)
Methylene violet solution (0.1% w/v), filter sterilized
Hemacytometer (Neubauer‐improved type) with cover slips or, alternatively, Kova Glasstic slides (e.g., from FisherScientific)
Lint‐free paper tissues (e.g., Kimwipes)
Pipettes
Brightfield microscope (total magnification of 400×)
Prepare the cell count
-
1
Clean the hemacytometer with water before using it. Use a lint‐free paper tissue to remove excess water.
This step is not required when using Kova Glasstic slides.
-
2
Dilute yeast sample to an appropriate concentration for cell counting.
Try to have around 100 cells or less per microscope field. Usually a 1:100 or a 1:1000 dilution of your original culture works well. Dilute your samples in 0.9% (w/v) NaCl solution or PBS.
If yeast cells are flocculent, make your dilution using EDTA (50 mM, pH 7.0). This will break up the clumps and will allow you to count the individual cells.
-
3
Mix the sample well by pipetting up and down several times.
-
4
Dilute methylene violet stock solution 1/10 using 0.1 M glycine buffer at pH 10.6.
-
5
Add 1 ml of a methylene violet solution to 1 ml of (diluted) yeast culture.
-
6
Mix the sample well by pipetting up and down several times.
Count the yeast cells
-
7
Gently apply 10 µl of the well‐mixed sample between coverslip and hemacytometer. Avoid air bubbles under the coverslip.
The sample will almost immediately be pulled under the coverslip by capillary action.
When using Kova Glasstic slides, simply pipette 6.6 µl (the maximum volume of each chamber) of the well‐mixed sample onto the slides.
-
8
Place the hemacytometer on the microscopy stage.
-
9
Locate yeast cells and focus the microscope.
-
10
Count the cells in squares in the hemacytometer.
If yeast cells are evenly distributed over sample, you can count within the five numbered squares (see Fig. 2).
Be consistent when counting and make sure to establish a counting protocol (e.g., always count cells on upper and right edge, and never on lower and left edge of the squares).
For the Kova Glasstic slides: Count 10 of the small grids.
Figure 2.
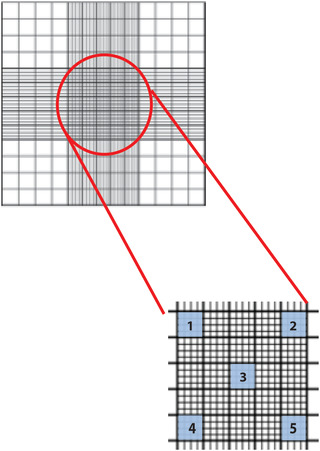
A hemacytometer is used to count the number of cells in a suspension. It basically comprises a chamber, created by a grid of perpendicular lines that are carved into glass (upper panel). The area bound by each line, as well as the volume of each chamber that is created in this way, is known. By counting cells in a specific area of the grid (e.g.,, in the five indicated areas in lower panel), it is possible to calculate the original overall cell concentration in the suspension that was pipetted in the chamber.
-
11
Note down the total number of cells as well as the number of viable cells.
Dead cells stain violet, viable (or at least metabolically active) cells do not have a color. In general, pale violet cells and budding yeast cells are considered as viable cells.
-
12
Determine fraction of viable cells.
-
13
Calculate cell concentration in original culture using Equation 4.
This equation is for a Neubauer‐improved type of hemacytometer.
This calculation assumes you only counted 5 of the in total 25 squares of the counting chamber. Each counted square holds the precise volume of 4 nl. If you counted 5 squares, you have the cell count for a total volume of 20 nl. To determine cell count in 1 ml, multiply by 50,000 (5 × 104). Please note that volume of counting chamber is given for a Neubauer‐improved hemocytometer. If you are using a different type, please check the manufacturer's instructions on how to determine total cell count. For example, for the Kova Glasstic slides, the volume of each small grid is 0.011 µl; so, if you counted 10 small grids, you have determined the total number of cells in 0.11 µl.
When calculating the dilution factor, take into account that the addition of methylene blue also dilutes your cells twofold.(4)
Basic Protocol 5. BATCH FERMENTATION AND BOTTLING OF BEER (12 L)
This protocol describes how to perform batch fermentations and how to bottle the resulting beer.
Materials
Wort (see Basic Protocol 1)
Propagated yeast with sufficiently high cell number (see Basic Protocol 3)
Priming sugar (Sigma)
Sterile 15‐L bottle
Air pump with 0.2 µm filter attached and sterile tubing (e.g., Eheim 400 air pump)
Balance (min. 0.1 g resolution)
Sanitized automatic siphon with bottle filling attachment/bottling wand
Sanitized/aseptic beer bottles (330 ml)
Crown capper (with 26‐mm attachment)
Sanitized crown caps 26 mm
Incubator that can maintain 20°C
Fermentation of the wort
-
1
Transfer 12 L of 12°P wort into a 15‐L sterile bottle and bring to the desired fermentation temperature.
-
2
Count the yeast cell concentration in the starter culture (see Basic Protocol 4).
-
3
Allow the yeast starter to settle and decant as much of the supernatant off as possible.
Alternatively, the starter culture can be centrifuged for 5 min at room temperature, to facilitate this step.
-
4
Pump sterile air into the wort to aerate for 30 to 60 min.
Maintain a sufficiently low flow rate to prevent excessive foaming of the wort.
-
5
Inoculate the wort with 0.75 × 106 cells/ml/°P for ale brewing and 1.5 × 106 cells/ml/°P for lager brewing.
For the given protocol, this sums up to 108 billion cells and 216 billion cells, respectively.
Avoid temperature shocking the yeast by keeping the starter culture in a range of ±2°C of the wort/fermentation temperature.
-
6
Ferment in the incubator at 20°C if using ale yeast or 10°C if using lager yeast.
The exact fermentation temperature depends on your experimental setup.
Maturation of the beer
-
7
Towards the end of the fermentation, measure the gravity daily (see Basic Protocol 2 or Alternate Protocol 2).
Degas samples before measuring gravity (see Support Protocols 6‐7).
-
8
When the gravity no longer decreases, the fermentation can be considered complete.
Alternatively, fermentation progress can be tracked by monitoring weight loss, using a balance. This process will take around 6 days for an ale and 10 days for a lager.
-
9
Once the primary fermentation has finished, ale beer should be matured at a lower temperature (0°C‐4°C) for 3 days and lager beers allowed to warm up to 16°C for 2 days followed by 10 days at 0°C‐4°C.
The 16°C step during maturation of lager beer allows the yeast to reduce the off‐flavor diacetyl (buttery, rancid aroma) to acetoin and, subsequently, 2,3‐butanediol.
During the cold temperature step (also referred to as “cold crash”) yeast cells, as well as cold trub, settle to the bottom of the fermentation vessel, allowing them to be easily separated from the finished beer.
Bottling the beer
-
10
Place the sanitized automatic siphon into the fermenter (15‐L bottle), attach the bottling wand to the end of the siphon tubing, and pump a few times to prime the siphon.
The siphon should not be lowered completely to avoid disturbing the sediment layer at the bottom of the fermenter.
-
11
Transfer 330 ml beer into each beer bottle. Lower the bottling wand to the bottom of the bottle and gently depress to slowly begin filling while being careful of avoid aeration of the beer.
Avoid splashing or excessive air contact of the green beer during steps 10 and 11, as this will lead to oxidation of the final beer.
-
12
Add priming sugar to each bottle.
For a standard pale ale beer, a carbonation level of 0.5 g CO2/100 g beer is often suggested. For fermentation at 20°C, add 2.37 g of pure glucose per bottle of 330 ml.
See the Internet Resources to calculate the amount of sugar needed for other conditions.
-
13
Place a sanitized crown cap on each bottle and crown bottles following the manufacturer's instructions.
-
14
Allow secondary fermentation in the bottles to complete for 14 days at 20°C.
Basic Protocol 6. SMALL‐SCALE FERMENTATIONS AND SAMPLING
Here we describe a setup for small lab‐scale fermentations that can be useful when comparing a large number of yeast strains or brewing variables. Often it is useful to take samples throughout the fermentation process to measure metabolite production or track fermentation kinetics. Here we describe a setup where samples can be taken while avoiding contamination of the beer (see also Fig. 3). Full‐scale industrial fermentations take place under very different physical conditions compared to lab‐scale fermentations. There is a number of ways to mimic the conditions of larger scale fermentations. One method is to simply use stirred fermentations. This protocol is described below.
Figure 3.
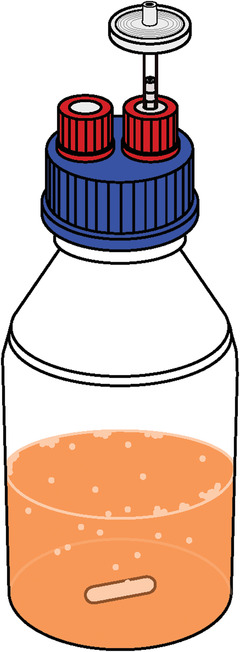
Schematic of small‐scale fermentation setup in bottles. The 2‐µm filters allow gas escape as CO2 is produced during fermentation. The septum allows for sampling without risk of contamination.
Materials
Wort (see Basic Protocol 1)
Saline solution (0.9% w/v NaCl)
70% (v/v) ethanol
Dual port GL45 screw caps (e.g., Duran, cat. no. 1129750)
12‐mm silicone septa (e.g., Duran, cat. no. 292460503)
GL14 screw caps with aperture (e.g., Duran, cat. no. 292270508)
Straight tubing connector for GL14 cap (Duran, cat. no. 292550603)
Silicone tubing (5 mm internal, 8 mm external diameter)
25‐mm PTFE 0.2‐µm filters with female luer lock
4‐mm polypropylene hose barbs with male luer lock
Rubber stopper with airlock
Autoclave
30‐mm magnetic stir bars
Sterile cylinder (250 ml; to transfer wort to bottles)
Sterile conical centrifugation tubes (50 ml)
Centrifuge
250‐ml GL45 glass bottles for fermentations (GL45 thread)
Incubator that can maintain 20°C
Magnetic stir plates (e.g., IKA RT 15)
Balance (min. 0.1 g resolution)
70‐mm, 20‐G needles with luer adapter
Syringes with luer adapter
Preparation of the fermentation vessels
-
1Set up the dual port fermentation caps as follows
- On one port, place a silicone septum and secure it with a GL14 screw cap with aperture.
- To the other port, attach a tubing connector and secure with a GL14 screw cap with aperture.
- Attach 50‐mm silicone tubing to the tubing connector and insert a hose barb into the free end.
-
Attach 0.2‐µm filter to the luer lock of the hose barb.If sampling is not required throughout the experiment, a simple rubber stopper with airlock can be used instead. In this case, the stopper and airlock should be sanitized with 70% ethanol prior to use.
-
2
Autoclave bottles with magnetic stir bar and caps for 20 min at 121°C.
-
3
If wort has been stored at 4°C, bring to the desired fermentation temperature and fill each bottle with 150 ml of 12°P wort.
This protocol can be scaled up to use glass bottles of any size. Do not use more than 80% of the total volume to avoid over foaming. In some cases, with yeast that ferment heavily or produce large krausen even smaller volumes should be used.
Inoculate the ferementations with yeast
-
4
Prepare a 50 ml yeast starter culture as described in Basic Protocol 3 (steps 1 to 5).
-
5
Transfer the yeast starter into a 50‐ml conical centrifuge tube.
-
6
Centrifuge for 3 min at 1,500‐3,000 × g, room temperature.
-
7
Discard the supernatant.
-
8
Resuspend the cell pellet in 5 ml saline solution and take a cell count (see Basic Protocol 4).
-
9
Inoculate each bottle with a pitch rate of 0.75 × 106 cells/ml/°P for ale yeasts and 1.5 × 106 cells/ml/°P for lager yeasts.
The exact pitch rate will depend on your experimental conditions. The given protocol will lead to total pitch rates of 1.35 billion cells for ale yeasts and 2.7 billion cells for lager yeasts.
-
10
Attach caps and place fermentation bottles on magnetic stir plates with stirring at 200 rpm in an incubator set to 20°C.
Monitor and sample the fermentations
-
11
Monitor fermentation progress by regularly measuring weight loss over time on an analytical balance as a measure of CO2 release.
The fermentation is complete when the mass of fermentation bottles stays constant.
-
12
Samples can be taken throughout the experiment by sampling through the septa with needle and syringe.
Sanitize septa with 70% ethanol prior to sampling.
If monitoring fermentation progression by weight loss, be sure to take measurements before and after sampling.
Basic Protocol 7. SPECTROPHOTOMETRIC METHOD TO DETERMINE THE COLOR OF WORT AND BEER
In Europe, the color of a beer is specified in European Brewery Convention (EBC). EBC can be converted to standard reference method (SRM), which is used in the Americas.
| (5) |
EBC can be measured spectrophotometrically at 430 nm wavelength and is defined as:
| (6) |
where:
d = the dilution factor of the wort/beer sample
A430 nm = the measured absorbance at λex = 430 nm.
Materials
Membrane filter (0.45 µm)
Syringe membrane filter
Small plastic vessel to collect filtered samples
10‐mm cuvettes
Spectrophotometer
-
1
Filter 5 ml of wort or degassed beer through a 0.45‐µm membrane filter (see Support Protocol 7 or 8).
-
2
Fill a 10‐mm cuvette with demineralized water, place it in the spectrophotometer, and capture a blank measurement at 430 nm.
-
3
Measure absorbance of wort or beer sample at 430 nm.
Make sure the measured value is within the linear range of the spectrophotometer. In case the measured absorbance is too high, use a dilution of the sample.
-
4
Calculate the EBC value using Equation 6.
Basic Protocol 8. SPECTROPHOTOMETRIC MEASUREMENT OF BITTERNESS
The following protocol describes the procedure to determine the IBU of a sample spectrophotometrically.
Materials
6 M hydrochloric acid
Iso‐octane for UV spectroscopy (e.g., from Sigma‐Aldrich)
50‐ml Erlenmeyer flask with ground glass joint and glass stopper
Orbital shaker
UV spectrophotometer
1‐cm silica cuvettes
-
1
Transfer 10 ml of a degassed beer sample into a 50‐ml Erlenmeyer flask with ground glass joint. (see Support Protocols 6‐7).
-
2
Add 0.5 ml hydrochloric acid and 20 ml iso‐octane to this sample.
-
3
Close the flask with a glass stopper.
-
4
Shake the flasks at 20°C on a rotary shaker.
The shaking frequency and duration have to be optimized for the given equipment by repeated measurement of samples from the iso‐octane layer at an absorption wavelength of 275 nm of until no further increase can be detected.
-
5
Measure pure iso‐octane at 275 nm as a blank.
-
6
Measure the absorbance of the iso‐octane layer of each sample at 275 nm.
-
7Calculate the IBU value as:
(7) where A275 nm = the absorbance of a sample at λex = 275 nm.
Basic Protocol 9. APPARENT ATTENUATION OF A FERMENTATION
The degree of attenuation achieved at the end of a fermentation is an important characteristic of the yeast strain used. In contrast to the wort, which can be considered as a mixture of water and dissolved extract, the final beer also includes alcohol. Therefore, only the apparent attenuation can be determined based on density measurements.
The apparent degree of attenuation (ADA) can be determined as follows:
| (8) |
where:
ADA [%] = apparent degree of attenuation
RE [°P] = residual extract
OE [°P] = original extract
Basic Protocol 10. ESTIMATED ALCOHOL CONTENT OF A BEER
Based on the OG of the wort before fermentation and the FG after fermentation, the alcohol content of a beer can be predicted using:
| (9) |
where:
ABV [% (v/v)] = percentage alcohol by volume
OG [SG] = original gravity
FG [SG] = final gravity
For this calculation gravity values in °P have to be converted to SG using Table 4. Specialized equipment, such as an Anton Paar Alcolyzer, can be used to measure alcohol content of the final beer.
Basic Protocol 11. GC MEASUREMENT OF VOLATILE ESTERS
During fermentation, yeast produces hundreds of secondary metabolites that influence the aroma and taste of beer. Variation in these metabolites across different yeast strains is what allows yeast to so uniquely influence beer flavor. The protocol below describes how to detect several of the most important yeast‐produced flavor compounds, namely ester compounds.
Materials
Internal standard (10× stock solution of 2‐heptanol = 129.8 ppm in EtOH)
External standard (see Table 5 for concentrations and retention times of standards; all compounds can be purchased from Sigma‐Aldrich)
NaCl (e.g., from VWR or Sigma‐Aldrich)
GC vials (Headspace screw neck vials with precision thread, ND18; e.g., VWR International)
Chemical hood
Headspace autosampler (PAL system, CTC analytics)
HS‐GC‐FID‐FPD system (e.g., from Shimadzu)
Chromatography column (e.g., DB‐WAXETR column (length: 30 m, internal diameter: 0.25 mm, layer thickness: 0.5 mm) from Agilent Technologies)
Analysis software (e.g., GC solutions software, Shimadzu)
Table 5.
External Standards for GC Measurements
| Compound | Aroma | Expected retention time (min) | Stock concentration (µg/L) |
|---|---|---|---|
| Ethyl acetate | Fruity, sweet | 3.000 | 100 |
| Ethyl propionate | Fruity, sweet, rum, juicy, grape, pineapple | 4.070 | 2 |
| Propyl acetate | Fruity, solvent, sweet | 4.410 | 1 |
| Ethyl isobutyrate | Fruity, sweet, ethereal, alcoholic, fusel | 4.182 | 0.25 |
| Isobutyl acetate | Fruity, sweet, tropical | 5.315 | 0.5 |
| Ethyl butyrate | Sweet, fruity, pineapple | 5.950 | 2.5 |
| Ethyl 2‐methyl butyrate | Green, apple, sweet, fruity, tropical | 6.380 | 0.5 |
| Ethyl isovalerate | Sweet, fruity, metallic, green, pineapple | 6.800 | 0.5 |
| Isoamyl acetate | Fruity, banana, pear, pine apple | 8.585 | 12.5 |
| Pentyl acetate | Fruity, pear, apple | 10.263 | 0.25 |
| Ethyl hexanoate (caproate) | Sweet, fruity, pineapple, waxy | 12.138 | 1.25 |
| Hexyl acetate | Fruity, green, apple, banana, sweet, apple, pear | 13.440 | 0.25 |
| Ethyl octanoate (caprylate) | Fruity, sweet, wine, pineapple, creamy, fatty, mushroom | 18.600 | 5 |
| Octyl acetate | Floral, green, earthy, mushroom, herbal, waxy | 19.925 | 0.25 |
| Phenetyl acetate | Floral, rose, sweet, honey, tropical, fruity | 30.200 | 3 |
| Ethyl decanoate (caprate) | Sweet, fruity, waxy, apple, grape | 24.816 | 2 |
| Ethyl valerate | Fruity, sweet, apple, pineapple, green, tropical | 9.000 | 1 |
-
1Generate standard curves for external standard compounds (see Table 5) as follows:
-
Prepare a 500× stock solution of each compound in 96% EtOH.Prepare this in volumetric 10‐ml flasks.Make sure to properly close stock solutions to prevent evaporation.
-
Prepare the different standard dilutions by diluting in beer or wort.Prepare this in a volumetric 10‐ml flask.Dilutions used to generate standard curve are: 1:2; 1:3; 1:4; 1:8; 1:9; 1:27; 1:81; 1:162Prepare each dilution in triplicate.
-
-
2
Prepare samples and standards by adding 5 ml of each sample and standard dilution to a GC vial containing 1.75 g NaCl.
Adding NaCl increases the efficiency of extraction of less volatile compounds in the sample.
Make sure to immediately close and cool each vial to minimize evaporation of volatile compounds.
-
3
Dilute the internal standard 10‐fold and add 100 µl of this diluted internal standard to each sample in chemical hood.
Dilute the internal standard in water.
The internal standard allows calculation of the actual concentrations of each compound after analysis.
Final concentration of the internal standard in each sample is 2.44 ppm.
-
4
Place samples in the autosampler.
For external standards, measure the triplicates of each dilution.
-
5Adjust settings on the HS‐GC‐FID as follows:
- Temperature split‐splitless injector: 250°C
- Temperature FID: 250°C
- Temperature injection needle: 105°C
- Flow rate carrier gas (N2): 2.6 ml/min
- Sample heating: 70°C for 25 min
- Oven temperature profile: See Table 6
Table 6.
Temperature Profile Oven for GC Measurements
| Time (min) | Temperature (°C) |
|---|---|
| 0.0 | 50°C |
| 5.0 | Increase by 4°C/min to a final T of 80°C |
| 20.0 | Increase by 5°C/min to a final T of 200°C |
| 44 | 200°C for 3 min |
-
6
Use data acquisition software to acquire chromatograms and interpolate concentrations of esters in samples from standard curve.
Basic Protocol 12. SPECTROPHOTOMETRIC MEASUREMENT OF PHENOLIC OFF‐FLAVORS
During the mashing process, hydroxycinnamic acids such as ferulic acid are released into the wort by hydrolase activity. Decarboxylation of ferulic acid by yeast during fermentation leads to formation of 4‐vinyl guaiacol (4‐VG). 4‐VG has a very distinctive smoky, clove‐like aroma. In most beers, except for particular styles such as Belgian witbier and German hefeweizen, 4‐VG is considered a yeast‐derived off‐flavor; often also referred to as a phenolic off‐flavor (POF).
The protocol below tests the capacity of a yeast strain to convert ferulic acid to 4‐VG (Mertens et al., 2017). The method measures absorbance of ferulic acid before and after fermentation as a measure of ferulic acid consumption, inferring 4‐VG production.
Please note that this protocol only tests the capacity of a yeast strain to produce 4‐VG. Whether 4‐VG will actually be produced during fermentation or perceived in the final beer, depends on many additional factors.
Materials
Yeast strain(s) of interest
POF+ control strain: e.g., SafAle WB‐06 (Fermentis)
POF‐ control strain: e.g., SafLager W34/70 (Fermentis)
YPD (see recipe)
Ferulic acid, 50 g/L (500×) stock concentration in 96% EtOH
96‐well plates (e.g., CellStar 96‐well plates, flat bottom)
Aluminum seals for 96‐well plates (e.g., silverseal aluminum seals, Greiner BioOne)
Incubator at 30°C
Plateshaker (e.g., Heidolph Titramax 1000)
Centrifuge
Plate reader (e.g., Tecan Infinity Pro)
-
1
Inoculate yeast strain of interest, plus a POF+ and a POF‐ strain, in 150 µl YPD, supplemented with 100 mg/L ferulic acid in a standard 96‐well plate.
Test each strain of interest in biological and technical replicate.
Ferulic acid is added to YPD medium after autoclaving.
Include non‐inoculated wells as blanks.
-
2
Seal the plates with aluminum seal.
-
3
Incubate for 5 days on a shaking platform (900 rpm) in an incubator at 30°C.
-
4
Centrifuge the plates for 3 min at 1,500‐3,000 × g, room temperature.
-
5
Transfer 100 µl of the supernatant into a new 96‐well plate.
-
6
Measure absorbance at 325‐nm using a plate reader.
Strains are considered POF+ if the measured amount of ferulic acid is below the 90% confidence interval of the blank.
Support Protocol 6. DEGASSING OF SAMPLES
Every sample taken during or after the fermentation process needs to be degassed prior further analysis due to the CO2 produced during fermentation. This is to avoid false results due to gas bubbles adhering to measuring devices (e.g., hydrometer) or emerging during an actual measurement (e.g., density meter). For all protocols handling fermenting wort or beer, this degassing protocol should be followed.
Materials
Sample to degass
0.5‐L Erlenmeyer flasks with stopper
Plastic funnel
Filter papers (e.g., MACHERY‐NAGEL MN 713 ¼)
-
1
Transfer 150‐200 ml of the sample into a 0.5‐L Erlenmeyer flask.
-
2
Shake the flask gently with the stopper on until no more CO2 escapes from the liquid.
An overpressure might develop within the flask during this step. Release the pressure carefully by lifting the stopper from time to time.
-
3
Pour the sample through the funnel containing a filter paper. Discard the first 20 ml of flow through and collect the rest into the second Erlenmeyer flask.
-
4
Repeat steps 1 and 2 until the sample appears sufficiently degassed.
Support Protocol 7. DEGASSING OF SMALLER VOLUMES
This protocol can be used when smaller volumes (<5 ml) need to be degassed prior to analyses.
Materials
Sample to degass
20‐ml plastic syringe (sterile)
Stopper for plastic syringe
-
1
Aspirate ∼5 ml of your fermenting wort or beer using a (sterile) plastic syringe.
-
2
Close the syringe using a stopper
-
3
Pull the lever of the syringe to create an under pressure within the syringe and wait until no more CO2 degasses from the liquid.
-
4
Release the CO2 carefully from the syringe without losing any sample volume.
-
5
Repeat steps 1 to 4 until the sample appears sufficiently degassed.
REAGENTS AND SOLUTIONS
Lugol's iodine 1% (w/v)
Weigh 0.1 g iodine (I2) and 0.2 g potassium iodide (KI).
Dissolve the solids in 30 ml dH2O.
Store up to 1 year at room temperature
YP maltose
Weigh 10 g yeast extract and 20 g bactopeptone into a 1‐L bottle
Add 900 ml of dH2O
Sterilize by autoclaving for 20 min at 121°C
After autoclaving, add 100 ml of a 20% (w/v) maltose stock solution
Store up to several weeks at room temperature
YPD
Weigh 10 g yeast extract and 20 g bactopeptone in a 1‐L bottle
Add 900 ml of dH2O
Sterilize by autoclaving for 20 min at 121°C
After autoclaving, add 100 ml of a 20% (w/v) d‐glucose stock solution
Store up to several weeks at room temperature
Zinc chloride solution
Prepare a stock solution of zinc chloride at 200 ppm (417 mg/L ZnCl2).
Sterilize by autoclaving for 20 min at 121°C
Store up to several at room temperature
COMMENTARY
Background Information
Humans have been brewing beer and other fermented beverages in one way or another since prehistoric times (Barnett, 1998, 2000, 2003; Hornsey & Royal Society of Chemistry (Great Britain), 2003). Initially, the production of beer was a spontaneous, uncontrolled process that relied on microbes that were haphazardly present, for example on the raw materials or instruments used for brewing. Because of this spontaneous process, fermentation results often varied a lot, with big differences in flavor and aroma between different fermentation rounds. To create more consistency and predictability in the quality of the end product, early brewers started using a small fraction of a previously fermented batch to inoculate a new batch; effectively (but unknowingly) recycling the microbes from the previous fermentation round to the next. This practice is called backslopping and promoted adaptation of microbes to the fermentation environment, a process called domestication. This backslopping, and the concomitant domestication, ultimately resulted in the present‐day yeast strains that are used in current breweries (Fay et al., 2019; Gallone et al., 2018; Gallone et al., 2016; Goncalves et al., 2016; Hornsey & Royal Society of Chemistry (Great Britain), 2003; Liti et al., 2009). The process of backslopping is not used often anymore in modern‐day breweries. Instead, brewers preserve pure yeast strains as frozen stock and only recycle their yeast from one fermentation to another for a couple of batches before starting a new culture from their frozen stock.
It was not until 1680 that yeast was discovered by Antonie van Leeuwenhoek, and even later that Pasteur discovered that fermentation was carried out by yeast cells. Many scientific breakthroughs were made during brewing‐related research. For example, in 1883, Emil Christian Hansen, while working at the Carlsberg Laboratory in Copenhagen, developed a method that allowed the isolation of pure yeast cultures (Barnett & Lichtenthaler, 2001; Hornsey & Royal Society of Chemistry (Great Britain), 2003).
Recently, there is an increasing customer demand for product diversity, both in terms of flavor and alcohol content. Driven by this demand, brewers and researchers are currently investigating the potential of alternative yeast species (both Saccharomyces and non‐Saccharomyces) for creating beers with different, more complex flavor profiles as well as the production of flavorful low/no alcohol beers (Gibson et al., 2017; Steensels & Verstrepen, 2014).
Critical Parameters and Troubleshooting
Stuck mash during lautering
A stuck mash is a common issue during lautering. Follow the tips below to avoid or solve the problem.
Grind the malt in a way that the husks are still intact, and the starch body is broken up but not ground too finely. Avoid a high flour percentage.
Allow the solid to settle down properly before starting the lautering process (∼20‐30 min).
Do not lauter too fast. It helps to fully open the tap of the lauter tun two to three times in the beginning for a short time (∼2 s) to remove bigger particles from underneath the false bottom. Afterwards open the tap just far enough to get a constant flow of 0.5 L/min.
If the flow rate starts to decrease, do not open the tap further to compensate. This will most likely just worsen the problem by contracting the filter bed. Instead, lower the flow rate and carefully cut the filter bed with a long knife in a rhombus like pattern. Don't cut all the way down to the false bottom.
If everything fails and the mash is stuck: Close the tap completely, stir up the filter bed completely, and start all over again.
Yeast cell number is too low after propagation
A number of factors can be checked/adjusted to increase yeast cell number during propagation.
Make sure to use “fresh” yeast as much as possible: Do not use agar plates or slants older than 4 weeks and re‐streak from a cryo stock.
Always pick multiple colonies from an agar plate to avoid starting from cells that may have acquired deleterious mutations.
Aerate larger volumes of propagation medium and stir or shake during incubation to keep the yeast in suspension.
Some yeast strains grow slower than others. In this case extend the incubation time.
Incubate each strain at its optimal temperature.
Low yield of brew
If the yield of the brew was low (less wort or less extract than expected) there a number of factors that can be adjusted to compensate for this.
A finer malt grind can improve the yield. Beware that this can also lead to lautering problems (see above).
Monitor the pH of your mash. Allow the mash to equilibrate and take the first measurement after 10 min. A typical mash pH should be between 5.2 and 5.5, but depends on the malt and water used. If necessary, adjust the pH by titrating with food grade hydrochloric/lactic acid or sodium hydroxide.
Allow the trub to settle as much as possible before removing the wort from it.
If there was more than the expected 20 L of wort then the boil may not have been strong enough. Adjust by boiling more vigorously, increasing the duration or by reducing the sparge water volume.
Moderately modified malts may require multiple mash temperature steps for complete saccharification, using highly modified malts helps to avoid this.
Last resort: Increase the amount of malt in the recipe to compensate the low efficiency.
Fermentation stops unexpectedly early
Typically, beer yeast strains reach an ADA of ∼80%, based on the sugar composition of the wort. Check the following points if the fermentation stops with an unexpectedly low ADA.
Check if the yeast strain is able to ferment maltotriose. Strains unable to ferment this trisaccharide typically yield ADA around 60%.
Streaking a strain on YP maltotriose plates and checking for growth is a quick test to see if strains can use maltotriose.
If propagating yeast from a cryo culture; make sure to aerate both the propagation medium and the wort before fermentation. Insufficient aeration limits cell membrane production can lead to unhealthy yeast.
Increasing fermentation temperature towards the end of the fermentation by 2°C can support the yeast to completely finish fermentation.
A lack of FAN can also lead to a stuck fermentation. Moderately modified malts may require a protease rest while mashing to release enough FAN to the wort.
Off‐flavors are present in final beer
See Table 7.
Table 7.
Off‐Flavors
| Off‐flavor | Aroma | Potential cause | Possible solution |
|---|---|---|---|
| 4‐VG | Cloves, smoky, spicy | Contamination of the fermentation with POF+ yeast |
After fermentation, clean all brewing equipment thoroughly. Check under microscope for potential contamination of starter culture |
| DMS | Cooked corn, cabbage |
Boiling was too short or not thorough enough. Cooling took too long. |
Increase boiling time or vigor. Cool hot wort as fast as possible. Do not cover pot during the boil. |
| Diacetyl | Buttery, rancid | Maturation was not long enough | Increase time and temperature of maturation |
| Trans‐2‐nonenal | Cardboard, stale, oxidized | Oxidation of the finished beer |
Minimize exposure of the finished beer to oxygen, especially while bottling. Keep the finished beer dark and cold. |
| Acetaldehyde | Fruity, green apple, sharp |
Overpitching yeast. Some yeast strains produce more acetaldehyde. Maturation not long enough. |
Increase time of maturation to give yeast sufficient time to reduce acetaldehyde to ethanol. |
| H2S | Rotten egg, foul | FAN content of wort too low |
Increase time of maturation, to allow H2S to evaporate. Can be stripped by bubbling CO2 through the green beer. |
| Acetic acid | Vinegar, sharp, sour | Contamination of wort or beer with bacteria (e.g., acetobacter) in combination with too high oxygen content. | Clean equipment thoroughly. Minimize oxygen exposure of the finished beer. |
Understanding Results
Production of wort and fermentation with any beer yeast strain
The basic protocols produce 20 L of a wort with 12°P. The wort can subsequently be used for two purposes as described here: (i) Batch fermentation of the wort using a single yeast strain with the aim to bottle beer for later use, or (ii) small‐scale fermentation experiments, which allow monitoring the fermentation characteristics of multiple yeast strains at a time. Using the calculations and techniques given in these protocols, up or downscaling of the process is possible. Please check the Critical Parameters and Troubleshooting section if unanticipated results occur.
Measurement of beer/fermentation characteristics
Basic Protocols 8 to 12 enable the reader to determine several standard characteristics of beer (color, bitterness, alcohol content, apparent attenuation, and volatile composition). All of these characteristics are influenced by multiple parameters of the brewing process, e.g., malt composition, fermentation temperature, hop variety, and yeast. Using controlled conditions, a standard wort, and consistent fermentation conditions, these measurements can be used to characterize a given yeast strain. These measurements can be further used, for example, to check if the resulting beer falls within specific style guidelines.
Table 8 lists values for typical color, bitterness, alcohol, and attenuation values for a few common beer styles, which can be used for comparison (Brücklmeier, 2018).
Table 8.
Values for Typical Color, Bitterness, ABV, and Attenuation Values for Some Common Beer Styles (Brücklmeier, 2018)
| Style | Fermentation | Color (EBC) | Bitterness (IBU) | ABV [% (v/v)] | Attenuation [%] |
|---|---|---|---|---|---|
| Bavarian Helles | Lager | 8 to 12 | 16 to 25 | 5.0 to 6.0 | 76 to 82 |
| Bohemian Pilsner | Lager | 5 to 15 | 30 to 45 | 4.0 to 5.5 | 64 to 68 |
| Dubbel | Ale | 30 to 75 | 20 to 35 | 6.0 to 8.0 | ∼79 |
| Pale ale | Ale | 10 to 25 | 20 to 40 | 4.0 to 5.5 | 71 to 80 |
GC‐based analysis
The proposed gas chromatography method explained in Basic Protocol 11 allows to determine concentrations of 17 different yeast related aroma active compounds. If the compounds are present in beer below the detection limit of the used setup, they are often represented as “not detected” (N.D.). Table 9 contains data of these aroma compounds for two “standard” types of beer, as well as the concentration ranges in beer reported for each of these compounds, found in literature (Chemists).
Table 9.
Expected Concentrations of Aroma Active Yeast Metabolitesa
| Compound | Reported concentration range (PPM) | Example standard American ale (mg/L) | Example standard lager (mg/L) |
|---|---|---|---|
| Ethyl acetate | 5‐50 | 21.89 | 17.98 |
| Ethyl propionate | 0.03‐0.2 | 0.06 | 0.05 |
| Propyl acetate | 0.01‐0.012 | 0.03 | 0.02 |
| Ethyl isobutyrate | 0.002‐0.01 | N.D. | N.D. |
| Isobutyl acetate | 0.01‐0.8 | 0.06 | 0.03 |
| Ethyl butyrate | 0.004‐0.4 | 0.20 | 0.11 |
| Ethyl 2‐methyl butyrate | 0.0004‐0.003 | N.D. | N.D. |
| Ethyl isovalerate | 0.0006‐0.007 | N.D. | 0.00 |
| Isoamyl acetate | 0.3‐8 | 0.93 | 1.10 |
| Pentyl acetate | 0.006‐0.015 | 0.01 | 0.01 |
| Ethyl hexanoate (caproate) | 0.008‐1.5 | 0.19 | 0.19 |
| Hexyl acetate | 0.003‐0.015 | 0.01 | N.D. |
| Ethyl octanoate (caprylate) | 0.04‐1.5 | 1.12 | 0.68 |
| Octyl acetate | 0.03‐0.05 | N.D. | N.D. |
| Phenetyl acetate | 0.03‐1.5 | 0.14 | 0.26 |
| Ethyl decanoate (caprate) | 0.01‐1 | 0.28 | 0.53 |
| Ethyl valerate | 0.001‐0.01 | N.D. | N.D. |
aIn column two, the measured concentration ranges for 17 different yeast‐related aroma active compounds in beer are represented (according to Chemists). As an example, the obtained results of a standard, laboratory‐brewed American ale (OE = 13°P, fermented at 20°C with a commercial American ale yeast) and lager beer (OE = 12°P, fermented at 12°C with a standard lager yeast) are reported.
bAbbreviations: N.D., not detected.
Spectrophotometric measurement of phenolic off‐flavors
Basic Protocol 12 enables the reader to test whether a given yeast strain has the capability of transforming the malt derived compound ferulic acid to 4‐VG. In contrast to the protocols before, this experiment does not include the production of a wort but can be performed in standard YPD medium. Production of POF is undesirable for most beer yeast strains and beer styles, with a few exceptions. In some Belgian specialty beers POF is tolerated and for Wit as well as German Hefeweizen, it is even a characteristic of the style.
Time Considerations
An example brewing experiment, following the recipes and protocols given in this article, would look similar to the following:
Streak yeast strain from stock (15 min of work, 2 days incubation).
Prepare wort (1 day initial preparation, 30‐60 min clarification)
Grow yeast strain to sufficiently high densities and pitch into wort: Pre‐growth (1 day) and propagation (1‐2 days)
Fermentations: 6 days for ale beer, 10 days for lager beer.
Maturation: 3 days for ale beer, 12 days for lager beer.
Bottling: 30‐60 min
Secondary fermentation in bottle: 14 days.
Flavor analyses using GC protocol: 53 min/sample.
Acknowledgments
FAT is supported by the 3rd Hydrophobin Chair and ASBC Research Council Grant 2019. KV is supported by a FWO postdoctoral fellowship. Original research in the lab of KV is supported by KU Leuven Program Financing, European Research Council (ERC) Consolidator Grant CoG682009, VIB, European Molecular Biology Organization (EMBO) Young Investigator program, FWO, and VLAIO (Vlaams Agentschap Innoveren en Ondernemen).
Thesseling, F. A. , Bircham, P. W. , Mertens, S. , Voordeckers, K. , & Verstrepen, K. J. (2019). A hands‐on guide to brewing and analyzing beer in the laboratory. Current Protocols in Microbiology, 54, e91. doi: 10.1002/cpmc.91
Literature Cited
- Barnett, J. A. (1998). A history of research on yeasts. 1: Work by chemists and biologists 1789–1850. Yeast, 14(16), 1439–1451. doi: . [DOI] [PubMed] [Google Scholar]
- Barnett, J. A. (2000). A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850‐1880. Yeast, 16(8), 755–771. doi: . [DOI] [PubMed] [Google Scholar]
- Barnett, J. A. (2003). Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology, 149(Pt 3), 557–567. doi: 10.1099/mic.0.26089-0 [DOI] [PubMed] [Google Scholar]
- Barnett, J. A. , & Lichtenthaler, F. W. (2001). A history of research on yeasts 3: Emil Fischer, Eduard Buchner and their contemporaries, 1880–1900. Yeast, 18(4), 363–388. doi: . [DOI] [PubMed] [Google Scholar]
- Bokulich, N. A. , Bamforth, C. W. , & Mills, D. A. (2012). Brewhouse‐resident microbiota are responsible for multi‐stage fermentation of American coolship ale. PLoS One, 7(4), e35507. doi: 10.1371/journal.pone.0035507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brücklmeier, J. (2018). Bier brauen: Grundlagen, Rohstoffe, Brauprozess. Stuttgart, Germany: Verlag Eugen Ulmer. [Google Scholar]
- Chemists, A. S. O. B. , from American Society of Brewing Chemists. Retrieved from http://methods.asbcnet.org/Flavors_Database.aspx
- Dzialo, M. C. , Park, R. , Steensels, J. , Lievens, B. , & Verstrepen, K. J. (2017). Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiology Reviews, 41(Suppl_1), S95–S128. doi: 10.1093/femsre/fux031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, J. C. , Liu, P. , Ong, G. T. , Dunham, M. J. , Cromie, G. A. , Jeffery, E. W. , … Dudley, A. M. (2019). A polyploid admixed origin of beer yeasts derived from European and Asian wine populations. PLoS Biology, 17(3), e3000147. doi: 10.1371/journal.pbio.3000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallone, B. , Mertens, S. , Gordon, J. L. , Maere, S. , Verstrepen, K. J. , & Steensels, J. (2018). Origins, evolution, domestication and diversity of Saccharomyces beer yeasts. Current Opinion in Biotechnology, 49, 148–155. doi: 10.1016/j.copbio.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Gallone, B. , Steensels, J. , Prahl, T. , Soriaga, L. , Saels, V. , Herrera‐Malaver, B. , … Verstrepen, K. J. (2016). Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell, 166(6), 1397–1410.e1316. doi: 10.1016/j.cell.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, B. , Geertman, J. A. , Hittinger, C. T. , Krogerus, K. , Libkind, D. , Louis, E. J. , … Sampaio, J. P. (2017). New yeasts‐new brews: Modern approaches to brewing yeast design and development. FEMS Yeast Research, 17(4). doi: 10.1093/femsyr/fox038. [DOI] [PubMed] [Google Scholar]
- Goldammer, T. (2008). The Brewer's handbook (2nd ed.). Clifton, VA: Apex Publishers. [Google Scholar]
- Goncalves, M. , Pontes, A. , Almeida, P. , Barbosa, R. , Serra, M. , Libkind, D. , … Sampaio, J. P. (2016). Distinct domestication trajectories in top‐fermenting beer yeasts and wine yeasts. Current Biology, 26(20), 2750–2761. doi: 10.1016/j.cub.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Holle, S. R. , & Klimovitz, R. (2003). A Handbook of Basic Brewing Calculations. St. Paul, Minn.: Master Brewers Association of the Americas. [Google Scholar]
- Hornsey, I. S. & Royal Society of Chemistry (Great Britain) . (2003). A History of beer and brewing. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- Kunze, W. (2004). Technology brewing and malting. Berlin, Germany: Versuchs‐ und Lehranstalt für Brauerei. [Google Scholar]
- Libkind, D. , Hittinger, C. T. , Valerio, E. , Goncalves, C. , Dover, J. , Johnston, M. , … Sampaio, J. P. (2011). Microbe domestication and the identification of the wild genetic stock of lager‐brewing yeast. Proceedings of the National Academy of Sciences of the United States of America, 108(35), 14539–14544. doi: 10.1073/pnas.1105430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti, G. , Carter, D. M. , Moses, A. M. , Warringer, J. , Parts, L. , James, S. A. , … Louis, E. J. (2009). Population genomics of domestic and wild yeasts. Nature, 458(7236), 337–341. doi: 10.1038/nature07743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, S. , Steensels, J. , Gallone, B. , Souffriau, B. , Malcorps, P. , & Verstrepen, K. J. (2017). Rapid screening method for phenolic off‐flavor (POF) production in yeast. Journal of the American Society of Brewing Chemists, 75(4), 15. doi: 10.1094/ASBCJ-2017-4142-01. [DOI] [Google Scholar]
- Mosher, R. , & Finkel, C. (1993). The Brewer's companion: A Source‐Book for the Small‐Scale Brewer. Seattle, WA: Alephenalia Publications. [Google Scholar]
- Pires, E. J. , Teixeira, J. A. , Branyik, T. , & Vicente, A. A. (2014). Yeast: The soul of beer's aroma—A review of flavour‐active esters and higher alcohols produced by the brewing yeast. Applied Microbiology and Biotechnology, 98(5), 1937–1949. doi: 10.1007/s00253-013-5470-0. [DOI] [PubMed] [Google Scholar]
- Smart, K. A. , Chambers, K. M. , Lambert, I. , Jenkins, C. , & Smart, C. A. (1999). Use of methylene violet staining procedures to determine yeast viability and vitality. Journal of the American Society of Brewing Chemists, 57(1), 18–23. doi: 10.1094/ASBCJ-57-0018. [DOI] [Google Scholar]
- Snyder, S. (1997). The Brewmaster's Bible: The Gold Standard for Home Brewers. New York, NY: William Morrow Cookbooks. [Google Scholar]
- Steensels, J. , Daenen, L. , Malcorps, P. , Derdelinckx, G. , Verachtert, H. , & Verstrepen, K. J. (2015). Brettanomyces yeasts–From spoilage organisms to valuable contributors to industrial fermentations. International Journal of Food Microbiology, 206, 24–38. doi: 10.1016/j.ijfoodmicro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Steensels, J. , & Verstrepen, K. J. (2014). Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annual Review of Microbiology, 68, 61–80. doi: 10.1146/annurev-micro-091213-113025. [DOI] [PubMed] [Google Scholar]
Internet Resources
Brew recipe developer software, free of charge.
BeerSmith, commercial Web site.
Web sites with recipes for different beer types
This database gives an overview of the different aroma compounds in beer including their concentration range in beer.


