Abstract
Dimethyl fumarate (DMF) is a fumaric acid esters derivate approved for plaque psoriasis as first‐line systemic therapy. It has been available in Italy since 2017 and an increasing number of patients are treated with this drug. To evaluate DMF effectiveness, side effects and drug survival in a dermatological real‐life setting. We performed a retrospective multi‐center study in five dermatologic clinics in Emilia‐Romagna, Northern Italy, which included all consecutive patients affected by moderate–severe psoriasis treated with DMF. We assessed effectiveness (in terms of PASI50 and PASI75 in an intention to treat observation) and safety (occurrence of side effects) of DMF and their association with demographic and disease characteristics, mean daily dose taken and treatment discontinuation. We included 103 patients, 78 (75.72%) had at least one comorbidity including 19 (18.44%) with a history of cancer; the mean treatment duration was 23.61 ± 17.99 weeks (min 4, max 130) and the mean daily dose was 262.13 ± 190.94 mg. Twenty‐four patients (23.30%) reached PASI75 at week 12, while a further 18 patients (17.47%) reached it at week 26. Side effects occurred in 63 patients (61.16%), the most frequent were diarrhea, epigastric discomfort, nausea, and flushing. Sixteen patients (15.53%) showed an alteration of laboratory tests. In some cases side effects were transitory, while in 53 patients (51.45%) they led to cessation of therapy. The median daily dose showed a direct association with PASI50 achievement and an indirect association with treatment discontinuation. Our study shows the peculiarities of DMF in a real‐world setting: effectiveness is often reached after 12 weeks of treatment and side effects could limit the continuation of the therapy but, at the same time, DMF has no major contraindications and, due to the wide range of dosage, it can allow both to manage side effects and to personalize the prescription for each patient.
Keywords: dimethyl fumarate, psoriasis, Skilarence, therapy
1. INTRODUCTION
Systemic therapy for psoriasis is based on a wide range of treatment options, including traditional and biologic therapies. In the expanding setting of the molecules usable to date, since 2017 a “traditional” systemic drug, namely dimethyl fumarate (DMF), has been available in Italy. It is a small molecule, which belongs to fumaric acid esters (FAEs), a group of derivates of fumaric acid with anti‐inflammatory and immunomodulating effects. 1 The first fumarate (Fumaderm) has a long history: in 1994 it was approved for severe psoriasis in Germany, and then for moderate psoriasis as from 2008. Recently, clinical trials have demonstrated that even a single DMF therapy has a satisfactory effectiveness thus the new formulation, containing only this molecule (marketed with the name Skilarence), has been approved. The therapeutic dose ranges between 30 and 720 mg daily. 2 According to the local guidelines, in moderate to severe forms of psoriasis a conventional systemic therapy is indicated before starting a biological treatment. 3
We performed a retrospective data review based on our clinical experience, including all the patients affected by psoriasis treated with DMF in five dermatologic clinics in Emilia‐Romagna, a Northern Italian region, with the aim of assessing the effectiveness and safety of DMF in a real‐life setting. PASI50 and PASI75 achievement, as well as the occurrence of side effects, were recorded and their association with demographic and clinical data, comorbidities, and concomitant therapies was assessed. We also evaluated the retention rate of patients on treatment, corresponding to the interruption of the therapy due to side effects or loss of efficacy. To the best of our knowledge, this is the first real‐life study describing the use of DMF in a heterogeneous cohort of psoriatic patients, giving the opportunity to address its management in daily practice.
2. MATERIAL AND METHODS
2.1. Study design and setting
We performed a retrospective, multi‐center study enrolling successively all patients who underwent treatment with DMF and performed at least two visits comprehensive of clinical and lab assessments in the same center. Topical therapy (emollients, topical steroids, and vitamin D derivates) was permitted, as per normal clinical practice. The retrospective observation included a 30‐month period (from May 2018 to October 2020). The assessments were performed as in clinical practice every 4 weeks until week 16, then every 8 weeks (range 6–12 weeks depending on the clinical need, every visit was identified in the database by “T” followed by the number of weeks).
Each of the five centers involved (Bologna, Ferrara, Modena, Parma, and Reggio Emilia) has a dedicated outpatient psoriasis service. The current study was approved by the local Ethics Committee (protocol 649/2020/Oss/AOUFe) and informed consent was obtained by the subjects participating in this research.
2.2. Data collection and analysis
The following data were extracted from anonymized electronical medical records: demographics (gender, age), body mass index (BMI), presence of comorbidities (defined as any disease other than psoriasis reported by the patient), concomitant therapies, psoriasis duration, previous psoriasis therapies, presence of psoriatic arthritis (PsA), duration and daily dosage of DMF treatment, clinical and/or laboratory side effects, disease severity measured with Psoriatic Area Severity Index (PASI) before starting therapy at T0 and PASI achieved at every control visit or discontinuation of the treatment due to side effects or loss of efficacy.
We assessed which variables (between patient demographics, BMI, psoriasis history and severity, median DMF daily dose) might influence treatment response in terms of PASI50 and PASI75 at T12 and the occurrence of side effects. Moreover, we analyzed the retention rate, as the probability of maintaining the treatment over time, in order to provide an index of overall drug effectiveness, safety, patient satisfaction, and treatment compliance.
Data collection did not affect the treatment administered to patients since it is a retrospective study.
2.3. Statistical analysis
The Shapiro–Wilk test was used to test for normality of distribution of the continuous variables. In cases of symmetry distributions, the variables were represented with the mean and SD and comparisons were assessed using Student t‐test. In case of non‐normal distribution, the variables were represented with the median value and interquartile range [IQR] and comparisons were assessed using Wilcoxon Mann Whitney test. Categorical data were expressed as total numbers and percentages. Statistical comparisons of categorical variables were assessed using either Pearson's χ2 test or Fisher's exact test. Kaplan Meier curve was used to evaluate the patients' adherence to therapy. Analyses were performed using Stata 15.1 SE (Stata Corporation, College Station, TX). A p‐value < 0.05 was defined as statistically significant.
3. RESULTS
3.1. Study population
Our study included 103 patients, 72 (70.1%) men and 31 women (29.9%), with a mean age ± DS of 57.29 ± 15.36 years, a BMI of 26.38 ± 4.51, and a mean psoriasis history of 20.01 ± 14.69 years. Ten patients (9.7%) had been previously treated with a biological drug. Sixteen patients (15.53%) had PsA as well and 78 (75.72%) had other comorbidities. Most of the patients had more than one comorbidity (Table 1). Sixty patients (58.25%) were taking concomitant therapies due to comorbidities.
TABLE 1.
Patients' characteristics
| Patients features | |
|---|---|
| Demographics | |
| Gender (103 patients), n. (%) |
72 Male (70.1%) 31 Female (29.9%) |
| Mean BMI ± SD | 26.4 ± 4.5 |
| Age (years), ± SD | 57.3 ± 15.4 |
| Pso duration (years) ± SD | 20.0 ± 14.7 |
| Patients with comorbidities, n. (%) | 78 (75.72) |
| Patients taking concomitant therapies, n. (%) |
0 Drug: 43 1 Drug: 24 >2 Drugs: 36 |
| Patients treated with previous biological therapy, n. (%) | 10 Patients (9.7) |
| Arthritis n. (%) |
Yes 16 (15.5) No 87 (84.5) |
| Mean PASI at T0 ± SD | 11.2 ± 7.5 |
| Specific comorbidities a | |
| Cardiovascular, n. patients | 42 Hypertension |
| Metabolic, n. patients | 16 Dyslipidemia, 13 diabetes |
| Gastrointestinal, n. patients | 6 Gastritis/esophagitis/previous ulcer |
| Renal, n. patients | 6 Chronic renal failure |
| Respiratory, n. patients |
1 Chronic obstructive pulmonary disease 1 asthma |
| Psychiatric, n. patients |
1 Depression 1 Schizophrenic 2 Psychosis |
| Neurologic, n. patients |
1 Axonal polineruopathy 1 Retrobulbar optic neuritis 1 Parkinson |
| Dermatologic, n. patients |
1 Atopic dermatitis 1 Pemphigoid |
| Cancer, n. patients | 19 Malignant neoplasms (including 2 non melanoma skin cancer) |
| Infectious, n. patients |
2 Chronic HCV infection 2 Chronic HBV infection 2 Latent Tuberculosis Infection (LTBI) |
Abbreviation: BMI, body mass index.
Patients may suffer from more than one comorbidity.
3.2. Effectiveness
The median PASI at T0 was 10 (6.2 14), the median PASI reached at T12 was 4 (2 7) (T12 was reached by 69 patients) and at T26 it was 2 (1 4) (T26 was reached by 53 patients).
At T12, 54 patients (53.39% of the included patients, 79.71% of the 69 patients who were still in treatment at T12) reached PASI50 while 24 (23.30 or 34.78%, considering the whole population or the patients being treated at T12, respectively) PASI75. At T26 a further 18 patients (17.47% out of all included patients or 33.96% out of 53 patients still in treatment) reached PASI75. Thus, 42 patients (40.77 or 79.24%, based on the population considered) reached overall PASI75.
3.3. Treatment duration and daily dose
The median duration of treatment was 24 (8 36) weeks (range 4–130 weeks), 53 patients reached T26 and the mean dose taken per day was 262.13 ± 190.94 mg (Table 2).
TABLE 2.
Effectiveness, daily dose, and side effects of DMF therapy
| DMF therapy | |
|---|---|
| Efficacy | |
| Mean PASI at T12 ± SD | 5.2 ± 4.9 |
| Mean PASI at T 26 ± SD | 2.5 ± 2.7 |
| PASI 50 reached at T12, n. (%) | 55 (53.4%) |
| PASI 75 reached at T12, n. (%) | 24 (23.3%) |
| PASI 75 reached at T26, n. (%) | 18 (17.5%) |
| Mean duration (weeks) ± SD | 23.6 ± 18.0 |
| Mean dosage (mg) ± SD | 262.1 ± 190.9 |
| Side effects | |
| Patients with clinical side effects, n. (%) | 63 (61.2%) |
| Patients with laboratory side effects, n. (%) | 16 (15.5%) |
| Clinical side effects (total/ n. patients) | 117/63 |
| Laboratory side effects (total/ n. patients) | 17/16 |
| Therapy interrupted, n. (%) | 53 (51.4%) |
| Mean time interruption (weeks) | 14.9 ± 10.6 |
| Side effects in detail a | |
| Gastrointestinal, n. patients |
33 Diarrhea 28 Epigastralgia 25 Nausea 4 Stipsi 1 Vomit |
| Cardiovascular, n. patients | 17 Flushing |
| Neurological, n. patients |
5 Headache 1 Nightmares 1 Oral paresthesia 1 Panic attacks |
| Renal, n. patients | 1 Acute renal impairment |
| Laboratory side effects, n. patients |
9 Lymphopenia 5 Hypereosinophilia 1 Neutropenia 1 Increased of transaminases 1 Increase of creatinine |
Patients may experience more than one side effect.
Only one patient was concomitantly treated with another systemic drug (imbrication of DMF and cyclosporine in the first 4 weeks due to a previous long therapy with this drug), while no patients received phototherapy.
For all patients included, the starting dose of DMF was in accordance with the data sheet, namely 30 mg/day in the first week, then increasing to 60 at week 2 and up to 90 mg at week 3. Subsequently a dose of 120 mg was recommended; afterwards the dose had theoretically to increase by 120 mg every week. No patients needed a different dose prescription due to chronic renal failure or for other reasons. The patients were advised to take the tablets with food. The first follow‐up was usually scheduled at T4. Since most of the side effects (listed below) were reported between T4 and T12, when the patients were taking a dose ranging from 120 to 360 mg, variations in dosing regimen occurred more often in this timeframe. In order to either prevent the onset of side effects or to induce their resolution, the following strategies were usually adopted: (i) daily dose reduction, especially when side effects occurred, (ii) maintenance of the same dose for more than 1 week, (iii) slower increase of the dosage prescription, for example combining doses of 30 and 120 mg (i.e., 1 × 120 mg tablet +1 or 2 30 mg tablet in the same administration from week 5) (Figure 1). Sometimes the patients performed self‐adjustments of the dose with respect to their subjective tolerance.
FIGURE 1.
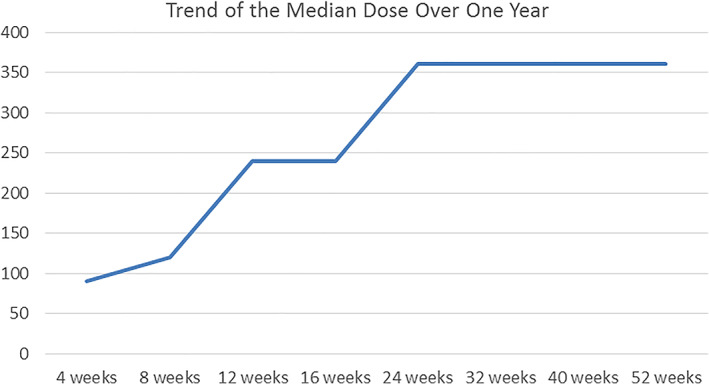
Median dose of DMF over 1 year of therapy. DMF, dimethyl fumarate
The dose was not further increased if control of the disease had been reached but, during the period of observation, this occurred only in five patients.
3.4. Safety and retention rate
Side effects occurred in 63 patients (61.16%), whereas 35 patients (33.98%) had more than one. Diarrhea was the most frequently observed and occurred in 33 patients (32.03%), followed by epigastralgia in 28 patients (27.18%), nausea in 25 (24.27%), flushing in 17 (16.50%), headache in 5 (4.8%) or other symptoms in five patients (4.85%). Seventeen patients showed an alteration of laboratory tests, all of them were transient: 9 lymphopenia (8.73%), (7 mild, 1 moderate and 1 severe), 5 hyper‐eosinophilia (4.85%), 1 neutropenia, 1 increase of transaminases and 1 increase of creatinine (0.97%) (Table 2). Since in some cases side effects were transitory, in 50 of the 103 (48.54%) patients the treatment is ongoing at the time of writing.
Analyzing the retention rate, assuming that all dropouts were due to side effects, we can observe that 51.45% of patients were still treated at T26, then this number decreased as described in the curve, but the follow‐up was not homogeneous for all the patients observed, due to the different times in which they started the therapy (Figure 2).
FIGURE 2.
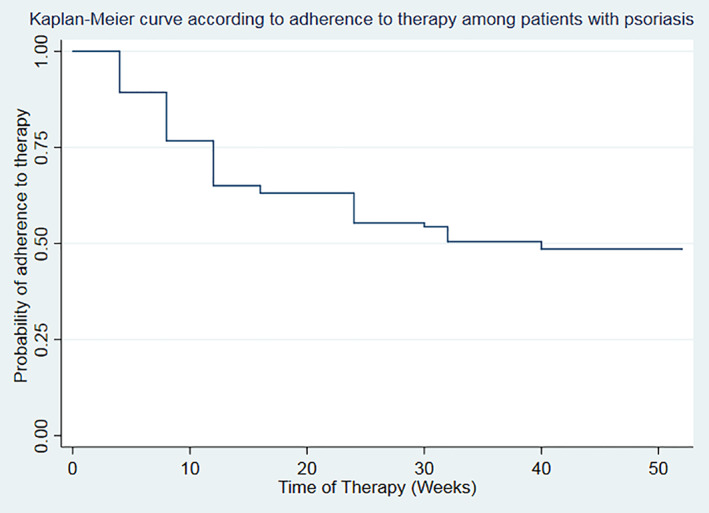
Retention rate represented as adherence to the therapy by a Kaplan–Meier curve
Safety assessment did not reveal reactivation of concomitant infection (HBV, HCV, or LTBI) or recurrence of oncological disease, although the follow‐up was limited.
Considering the particular period in which we performed this retrospective data collection, we also reported that only one patient, a 63‐year old female, was hospitalized for SARS‐CoV‐2 related interstitial pneumonia and discharged after 25 days (the Emilia Romagna region had a high‐Rt index during most phases of the pandemic). 4
3.5. Associations with effectiveness, safety, and retention rate
With reference to variables associated with DMF effectiveness, patients who achieved PASI50 at T12 had a lower BMI (p = 0.011) and were taking a higher daily dose (p < 0.001) than those who did not. The treatment discontinuation had a significant association with a higher BMI and a lower median daily dose, suggesting that DMF withdrawal more often occurred in the initial phases of treatment. Statistically, the occurrence of side effects was associated with the absence of concomitant therapies (p = 0.019) while, on the other hand, it was also associated with two or more concomitant therapies in a manner close to statistical significance (p = 0.051) (supplementary Table 1).
No other variables, including patient demographics and comorbidities, PASI at T0 and psoriasis duration, were found to be significantly associated either with effectiveness or with safety of the treatment (supplementary Table 1).
4. DISCUSSION
Fumarates are a group of small molecules often used as first‐line systemic psoriasis treatment, showing an acceptable safety profile for long‐term therapy. 5 The mechanism of action of fumarates is related to the inhibition of NF‐kB translocation, which leads to reduced inflammatory cytokine production and induction of pro‐apoptotic events, inhibition of keratinocyte proliferation, reduced expression of adhesion molecules, and diminished inflammatory response. Recently, some authors found that DMF enhanced the ratio of Treg cells to Th17 cells. 6 , 7 , 8 Such events are not completely understood and more research is needed to better profile fumaric esters' mechanism of action. Compared with other available therapies, fumarates are characterized by a slow onset of efficacy. 9
Our experience was consistent with this. In fact, at T12 about 23% of patients, which corresponds to about 35% of those still in treatment, reached PASI75. More than 40%, or almost 80% considering the patients who were continuing the treatment, achieved this endpoint considering a longer time frame, namely T26. This suggests that DMF, although less rapid and effective than biological drugs, guarantees an appreciable therapeutic response over longer times. DMF effectiveness, assessed by PASI50, appeared significantly related to lower BMI and to higher daily dose. The daily dosage should be gradually increased, compatibly with tolerability, in order to determine the best therapeutic response. On the other hand, when a satisfactory therapeutic effect is achieved, further dose increase does not seem necessary 10 and, doses much lower than the maximum indicated, such as 360 mg per day, seem to be effective for long‐term treatments. 11 Moreover, when a clinically relevant improvement has been reached, it is possible to gradually reduce the daily dose. 10 Our real life experience confirms these issues. In our population, the mean daily dose was 262.13 mg, albeit with a large standard deviation, which was much lower than the maximum allowable dose (720 mg). Because of the relatively short duration of follow‐up, only five of the patients included had a reduction of the dosage after the remission of psoriasis was achieved.
Since the median dose taken is linked to the duration of the treatment, we assume that long‐term therapy is more likely to lead to psoriasis improvement.
During the period analyzed, adverse events occurred in 79 patients (76.69%), considering both clinical and laboratory adverse events. The most common were diarrhea, epigastralgia, nausea, and flushing. Acute renal impairment was observed in one patient suffering from chronic renal failure, who underwent dehydration because of diarrhea and did not resume the therapy.
The events observed are consistent with those reported by other authors. DMF is the active ingredient of both Skilarence and Tecfidera. The latter is indicated for the treatment of multiple sclerosis (MS) so we discuss our results with respect to both neurological and dermatological fields. 12 , 13 , 14 In a head‐to‐head randomized and placebo‐controlled trial the side effects of treatment were observed in 84% of patients treated with Fumaderm or DMF, compared with 59.9% receiving placebo; the most common were gastro‐intestinal events and flushing. 2
The most common laboratory side effects that occurred in our patients were lymphopenia and hyper‐eosinophilia. It is noteworthy that one patient had neutropenia after 56 weeks of treatment, which resolved at week 78 after a reduction of the dose; neutropenia recurred again at week 96, but the patient continues the therapy. Lymphopenia has also been observed among the main laboratory side effects in other studies. 2 Goldman reported that neutropenia occurred in 7.8% of patients affected by MS, while the occurrence of lymphopenia was related to age. According to this study, neutropenia was more common in males and appeared to be independent of lymphopenia. 15
In our experience, the occurrence of side effects was statistically related with both the absence of concomitant treatment and two or more treatments. This finding suggests that further studies are needed to understand the tolerance of DMF in relation to the interactions with concomitant treatments. To date it is known that DMF does not interact with cytocrome P450, but it is metabolized by unspecific esterases and then excreted mainly by breath. It also has a short half‐life (the apparent terminal elimination half‐life of monomethyl fumarate is about 2 h), so DMF is not considered to have direct drug–drug interactions. 16
Based on our data, subjective tolerance is due to unknown variables. When side effects appear the dose may be temporarily reduced to the last tolerated dose. 17
In our experience we observed that, in some cases, after a dose reduction, a subsequent further increase was better tolerated. With the aim of optimizing the benefits and risks of the treatment, all physicians often scheduled a slow increase of dosage from T4. Another strategy adopted was to combine 30 and 120 mg tablets, with the aim to prevent or decrease the occurrence of side effects.
As previously reported, the maintenance dose was empirically found case by case by the physician, following the subjective tolerance referred by the patient. 18
In a survey performed in patients affected by MS, the duration of dose reduction ranges from 1 week to 3 months for upper and lower gastrointestinal side effects. 19 Side effects have shown to be the main reason for DMF discontinuation. In our experience, 53 patients (51.45%) discontinued the therapy because of them. In connection with this, therapy discontinuation was inversely related to the median daily dose, suggesting that if the patients tolerate the side effects and continue the therapy, it is less probable it will be interrupted over time. Furthermore, the side effects, as well as the subsequent treatment interruption, mostly occurred during the first weeks of treatment.
In a previous prospective study, it was observed that 43% of patients interrupted the therapy with Fumaderm due to an adverse event, in most cases gastrointestinal disorders, rarely because of alterations in blood count or hematology. 2 , 20 , 21 , 22
Although the follow‐up is limited, we noted that the dropouts occurred mostly in the first 5 months of treatment. Thus, this frame time could be considered as crucial to predict long‐term adherence to treatment. The high occurrence of interruption of the treatment could be related to the fact that DMF was proposed as first therapy to most of patients.
A particular aspect of our study is that a consistent number of patients treated with DMF have an oncological history and, in accordance with the absence of contra‐indication, none of them had a recurrence of malignancy during the follow‐up. Long‐term safety data about the use of fumarates in such patients are not available to date in the literature.
The study findings should be interpreted in the light of some limitations. This was an observational study without a control group and with a relatively limited observation time. This latter aspect is determined by the recent introduction of the drug in Italy. However, since according to our experience the first weeks are the most critical both for the occurrence of side effects and for the definition of the most appropriate daily dosage, the study duration appears adequate to focus on these key issues. The multicentric nature of the study cannot ensure homogeneity of treatment schemes and modalities of patients' assessment. Due to its retrospective design, the periods of observation were heterogeneous and not simultaneous for all patients included. We considered the dose taken and the occurrence of side effects at specific times, which coincided with the planned visits, without assessing them on a daily basis. Due to the differences in the duration of observation, not all patients reached the maintenance dose period, but some are still in the increasing phase: this significantly influences the results (in particular in terms of median dose, side effects, and treatment discontinuation). Moreover, most of the side effects were symptoms, and an established assessment of their severity is missing. Concomitant topical treatments, in terms of actives, treatment regimens and duration, have not been considered.
In conclusion, the prescription of a systemic drug for plaque psoriasis has to take into account some factors related to the patient such as demographics, the supposed compliance to the treatment, the co‐morbidity and co‐medication, but also the negative impact that psoriasis has on quality of life. The DMF guarantees easy handling and a large field of use, especially in patients affected by multiple comorbidities. On the basis of our experience, it is conceivable that customizing the dose to the specific habits of every patient, for example dietary habits, may allow a better adherence to the treatment and further optimize its benefit/risk ratio.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING INFORMATION
Open Access Funding provided by Universita degli Studi di Ferrara within the CRUI‐CARE Agreement. WOA Institution: Universita degli Studi di Ferrara Blended DEAL: CARE.
Supporting information
Supplementary table 1 Statistics analysis of variables observed in the study, significant values are reported in bold.
ACKNOWLEDGMENTS
Honorarium, grant, or other form of payment was not given to anyone of the authors to produce the manuscript. All authors made substantive intellectual contributions to the published study and each author listed on the manuscript has seen and approved the submission of the manuscript. Open Access Funding provided by Universita degli Studi di Ferrara within the CRUI‐CARE Agreement. [Correction added on May 24, 2022, after first online publication: CRUI funding statement has been added.]
Corazza M, Odorici G, Conti A, et al. Dimethyl fumarate treatment for psoriasis in a real‐life setting: A multicentric retrospective study. Dermatologic Therapy. 2021;34(5):e15066. 10.1111/dth.15066
Funding information WOA Institution: Universita degli Studi di Ferrara Blended DEAL: CARE; Universita degli Studi di Ferrara
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Brück J, Dringen R, Amasuno A, Pau‐Charles I, Ghoreschi K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018;27:611‐624. [DOI] [PubMed] [Google Scholar]
- 2. Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate‐to‐severe chronic plaque psoriasis: a randomized, double‐blind, Fumaderm® ‐ and placebo‐controlled trial (BRIDGE). Br J Dermatol. 2017;176:615‐623. [DOI] [PubMed] [Google Scholar]
- 3. Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31:774‐790. [DOI] [PubMed] [Google Scholar]
- 4. Ministero della Salute, https://www.salute.gov.it/portale/nuovocoronavirus
- 5. Regione Emilia‐Romagna, https://salute.regione.emilia-romagna.it/ssr/strumenti-e-informazioni/ptr/elaborati/94-linee-guida-psoriasi-2018luglio/20finale.pdf.
- 6. Reszke R, Szepietowski JC. A safety evaluation of dimethyl fumarate in moderate‐to‐severe psoriasis. Expert Opin Drug Saf. 2020;19:373‐380. [DOI] [PubMed] [Google Scholar]
- 7. Sulaimani J, Cluxton D, Clowry J, et al. Dimethyl fumarate modulates the Treg‐Th17 cell axis in patients with psoriasis. Br J Dermatol. 2021;184:495‐503. [DOI] [PubMed] [Google Scholar]
- 8. Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, Clement B. The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci. 2018;39:1‐12. [DOI] [PubMed] [Google Scholar]
- 9. de Jong R, Bezemer AC, Zomerdijk TP, van de Pouw‐Kraan T, Ottenhoff TH, Nibbering PH. Selective stimulation of T helper 2 cytokine responses by the anti‐psoriasis agent monomethylfumarate. Eur J Immunol. 1996;26:2067‐2074. [DOI] [PubMed] [Google Scholar]
- 10. Mrowietz U, Barker J, Boehncke W‐H, et al. Clinical use of dimethyl fumarate in moderate‐to‐severe plaque‐type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3‐14. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency. Available at: https://www.ema.europa.eu/en/documents/product‐information/skilarence‐epar‐product‐information_en.pdf
- 12. Sator P, Loewe R, Zamani O, et al. Dimethyl fumarate is efficacious in severe plaque psoriasis: post hoc analysis from the BRIDGE trial in Austria. Wien Klin Wochenschr. 2019;131:485‐492. [DOI] [PubMed] [Google Scholar]
- 13. Devonshire V, Lapierre Y, Macdonell R, et al. The global adherence project (GAP): a multicenter observational study on adherence to disease‐modifying therapies in patients with relapsing‐remitting multiple sclerosis. Eur J Neurol. 2011;18:69‐77. [DOI] [PubMed] [Google Scholar]
- 14. Giovannoni G, Southam E, Waubant E. Systematic review of disease‐modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18:932‐946. [DOI] [PubMed] [Google Scholar]
- 15. Lucchetta RC, Tonin FS, Borba HHL, et al. Disease‐modifying therapies for relapsing‐remitting multiple sclerosis: a network meta‐analysis. CNS Drugs. 2018;32:813‐826. [DOI] [PubMed] [Google Scholar]
- 16. Goldman MD, Dwyer L, Coleman R, Sohn M‐W, Stuve O. Patient‐specific factors modulate leukocyte response in dimethyl Fumarate treated MS patients. PLoS One. 2020;15:e0228617. 10.1371/journal.pone.0228617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aubets J, Jansat JM, Salva M, et al. No evidence for interactions of dimethylfumarate (DMF) and its main metabolite monomethylfumarate (MMF) with human cytochrome P450 (CYP) enzymes and the P‐glycoprotein (P‐gp) drug transporter. Pharmacol Res Perspect. 2019;7:e00540. 10.1002/prp2.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Theodore Phillips J, Erwin AA, Agrella S, et al. Consensus management of gastrointestinal events associated with delayed‐ release dimethyl fumarate: a Delphi study. Neurol Ther. 2015;4:137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoefnagel JJ, Thio HB, Willemze R, Bavinck JNB. Long‐term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149:363‐369. [DOI] [PubMed] [Google Scholar]
- 20. Campbell TL, Lefaux BJ, Mayer LL, et al. Nursing management of gastrointestinal adverse events associated with delayed‐release dimethyl fumarate: a global Delphi approach. J Neurosci Nurs. 2020;52:72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker A, Adamczyk C, Kellerer K, et al. Fumaderm® in daily practice for psoriasis: dosing, efficacy and quality of life F. Br J Dermatol. 2014;171:1197‐1205. [DOI] [PubMed] [Google Scholar]
- 22. O'Gorman J, Russell HK, Li J, et al. Effect of aspirin pretreatment or slow dose titration on Flushing and gastrointestinal events in healthy volunteers receiving delayed‐release dimethyl Fumarate. Clin Ther. 2015;37:1402‐1419.e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1 Statistics analysis of variables observed in the study, significant values are reported in bold.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


