Abstract
Climate change will lead to more frequent and more severe fires in some areas of boreal forests, affecting the distribution and availability of late‐successional forest communities. These forest communities help to protect globally significant carbon reserves beneath permafrost layers and provide habitat for many animal species, including forest‐dwelling caribou. Many caribou populations are declining, yet the mechanisms by which changing fire regimes could affect caribou declines are poorly understood. We analyzed resource selection of 686 GPS‐collared female caribou from three ecotypes and 15 populations in a ~600,000 km2 region of northwest Canada and eastern Alaska. These populations span a wide gradient of fire frequency but experience low levels of human‐caused habitat disturbance. We used a mixed‐effects modeling framework to characterize caribou resource selection in response to burns at different seasons and spatiotemporal scales, and to test for functional responses in resource selection to burn availability. We also tested mechanisms driving observed selection patterns using burn severity and lichen cover data. Caribou avoided burns more strongly during winter relative to summer and at larger spatiotemporal scales relative to smaller scales. During the winter, caribou consistently avoided burns at both spatiotemporal scales as burn availability increased, indicating little evidence of a functional response. However, they decreased their avoidance of burns during summer as burn availability increased. Burn availability explained more variation in caribou selection for burns than ecotype. Within burns, caribou strongly avoided severely burned areas in winter, and this avoidance lasted nearly 30 years after a fire. Caribou within burns also selected higher cover of terrestrial lichen (an important caribou food source). We found a negative relationship between burn severity and lichen cover, confirming that caribou avoidance of burns was consistent with lower lichen abundance. Consistent winter avoidance of burns and severely burned areas suggests that caribou will experience increasing winter habitat loss as fire frequency and severity increase. Our results highlight the potential for climate‐induced alteration of natural disturbance regimes to affect boreal biodiversity through habitat loss. We suggest that management strategies prioritizing protection of core winter range habitat with lower burn probabilities would provide important climate‐change refugia for caribou.
Keywords: boreal forest, burn severity, caribou, climate change, fire, functional response, habitat, late‐successional forest, resource selection
INTRODUCTION
The frequency, duration, timing, and magnitude of ecological disturbances, collectively known as a disturbance regime, are changing rapidly in response to human‐induced climate change across the globe (Sergio et al., 2018; Turner, 2010). Changes to natural disturbance regimes vary widely across space and time, are difficult to predict, and potentially lead to novel environmental conditions (Flannigan et al., 2009). Rapidly shifting disturbance regimes can alter ecosystem states in unpredictable and non‐linear ways (Seidl et al., 2017). How species respond to future changes in environmental conditions is a central question for ecologists, managers, and conservationists (Sutherland et al., 2013).
There are few places experiencing changes to disturbance regimes more rapidly than in North America's boreal forests, where temperatures are rising at a rate twice the global average (Callaghan et al., 2004). Wildfire has shaped boreal ecosystems for millennia and remains their dominant source of disturbance (Flannigan et al., 2009; Stocks et al., 2001). Boreal forest fires create a diversity of tree stand ages, physical structure, successional trajectories and species compositions (Burton et al., 2008; Dale et al., 2001). Climate warming is expected to increase the frequency, severity, duration, and spatial extent of fires in some areas of boreal forests, especially western North America, yet models predict spatial variability in these changes due to variation in precipitation, vegetation, soil composition, and fuel load (de Groot et al., 2013; Kasischke et al., 2010; Weber & Flannigan, 1997). Larger, more frequent, and more severe fires in boreal forests will affect the distribution and availability of late‐successional communities and alter habitat for boreal biodiversity that rely on these areas (Joly et al., 2012).
Characterizing habitat selection patterns helps ecologists to understand how animals respond to changing disturbance regimes, and their habitat needs. Resource selection analysis (RSA) clarifies how animals respond to a variety of disturbances, including human development (e.g., Hebblewhite & Merrill, 2008; Martin et al., 2010), fires (DeMars et al., 2019), and insect outbreaks (Rota et al., 2014). RSAs estimate the relative strength of animal selection for (or avoidance of) environmental resources and the relative probability (or intensity) of animal occurrence in a given spatiotemporal extent by comparing resources at locations used by animals to resources at “available” locations that could have been used (Johnson et al., 2006; Manly et al., 2002). Therefore, RSAs estimate the multivariate Hutchinsonian niche (Hutchinson, 1957), defined as habitat, for a given species (Hirzel & Le Lay, 2008; Holt, 2009), and behavioral avoidance of any resources (e.g., fire disturbance) leads to an indirect loss of habitat (e.g., Hirzel & Le Lay, 2008). For wide‐ranging species, defining available habitat using a movement‐based approach, such as a step selection function (SSF) accounts for the changing availability of resources in space and time (Thurfjell et al., 2014), exemplified by dynamic fire disturbance in the boreal forest (Avgar et al., 2016).
To predict animal habitat selection in response to future changes in disturbance regimes, we must first understand how selection varies across the full range of conditions that animals encounter. Variation in behavior across such a gradient of resource availability is known as a functional response in resource selection (Aarts et al., 2013; Matthiopoulos et al., 2011; Mysterud & Ims, 1998). Increased fire frequency in parts of western North America's boreal forests would decrease the availability of spruce‐dominated (Picea spp.) late‐successional habitats, which may transition to deciduous forests, shrubs, or even to a grassland state in some portions of the region (Barber et al., 2018; Rupp et al., 2000). Functional responses improve predictions of resource selection under these novel conditions (Matthiopoulos et al., 2011) and can help to identify thresholds in behavioral responses to disturbances that serve as targets for management and recovery (Beyer et al., 2013).
As a long‐lived and wide‐ranging species whose ecology is inextricably linked to fire, the forest‐dwelling caribou (Rangifer tarandus) is an iconic indicator of changing disturbance regimes and their effects on boreal biodiversity (Bichet et al., 2016; Festa‐Bianchet et al., 2011). Most populations of forest‐dwelling caribou in western North America are declining and listed as threatened under Canada's federal Species at Risk Act (SARA), while others are classified under SARA as species of special concern (Ray et al., 2015). The primary hypothesis for explaining caribou population declines in Canada's southern boreal forest is that habitat loss and fragmentation from human disturbance have facilitated increased predation on caribou (Festa‐Bianchet et al., 2011; Sorensen et al., 2008). For most populations inhabiting northern boreal forests, such as those in Alaska (AK), Yukon (YT), and Northwest Territories (NT), human disturbance is considerably lower and fire remains the major source of habitat alteration (Neufeld et al., 2020). In these areas, the degree to which changing fire regimes will affect caribou resource selection and drive population dynamics is unclear, and is a pressing challenge for conserving caribou and the boreal biodiversity they represent (Bichet et al., 2016).
Forest‐dwelling caribou have coexisted with fire for thousands of years. Fire heavily influences the abundance and distribution of boreal forest lichen (Payette et al., 2000), potentially resulting in direct bottom‐up effects on caribou through food limitation in winter in areas with very large burn footprints. Terrestrial lichens provide the bulk (usually >50%) of the diet for many northern caribou in winter, when the availability of high‐protein forage is limited (Joly & Cameron, 2018; Person et al., 1980; Thomas et al., 1996). Lichen is easily destroyed by fire due to its low moisture content, and takes multiple decades to recover to sufficient biomass for caribou foraging (Coxson & Marsh, 2001; Joly et al., 2003; Morneau & Payette, 1989). Therefore, caribou generally avoid burns in winter (e.g., Joly et al., 2003; Rettie & Messier, 2000; Schaefer & Pruitt, 1991) due to the negative effects of fire on lichen. However, caribou may benefit from some post‐fire habitat conditions, especially during the summer. Early seral vegetation in burns may provide crucial protein for caribou during summer, the period of peak nutritional demand for adult females (Brown & Mallory, 2007), and caribou resource selection studies during summer have shown more variable responses to burns (DeMars et al., 2020). The relationship between lichen cover and burn severity is less clear, but increasing severity could exacerbate the negative effects of fire on caribou resource selection in winter if it has strong effects on lichen abundance and regeneration (Russell & Johnson, 2019).
Here, we used hierarchical mixed‐effects RSAs to test for mechanisms by which changing fire disturbance regimes could affect resource selection, and potentially exacerbate existing population declines of forest‐dwelling caribou. We tested the overall hypothesis that caribou avoid burned areas, but predicted that factors such as season, spatiotemporal scale, burn severity, and availability of burns influenced the strength of avoidance. Within this working hypothesis, we addressed two main questions: (1) How do caribou alter their resource selection of burns across seasons, spatiotemporal scales, and the wide range of spatiotemporal fire frequency in western North America's boreal forests? (2) How does burn severity across and within burns drive caribou resource selection?
For Question 1, our analyses included GPS location data from 15 caribou populations and ~600,000 km2 of western Canada and eastern AK (Figure 1). We predicted that caribou would avoid burns more strongly in winter in part due to the negative effects of fire on lichen. We also tested for a functional response to burns, where caribou alter their relative selection for burns across the range of burn availability in our study area. Based on past studies showing caribou avoidance of human disturbance declines as human disturbance density increased (Holbrook et al., 2019; Mumma et al., 2019), we predicted that caribou avoidance of burns would decline in areas with more burns. In addition, we tested whether caribou ecotype or burn availability explained more variation in relative selection for burns. We refer to Question 1 analyses as the burn perimeter RSA in corresponding subsections.
FIGURE 1.
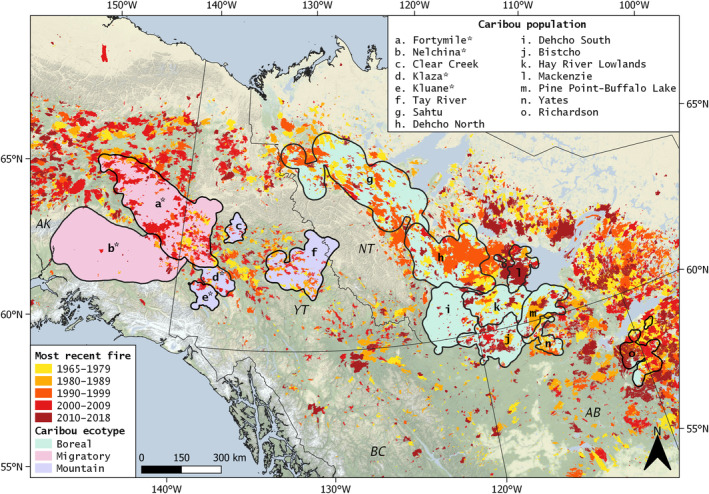
Map of fire history and location of 15 population ranges from three ecotypes of forest‐dwelling caribou (Rangifer tarandus) that were included in analyses. Asterisks denote four populations included in the within‐burn Resource selection analysis (RSA)
To answer Question 2, we conducted two separate analyses. The first analysis focused on how different levels of burn severity influenced caribou resource selection relative to unburned areas across the same 15 populations as Question 1. We refer to this analysis as the burn severity RSA. Caribou management decisions that consider the influence of disturbances on caribou populations primarily rely on polygonal fire perimeter data, overlooking variation in burn severity within burn perimeters, including the presence of completely unburned forest patches that may act as refuges and important food sources (Johnstone & Chapin, 2006b; Skatter et al., 2017). We tested the hypothesis that the caribou avoidance of burned areas was influenced by burn severity due to its possible negative effects on lichen cover and regeneration. Alternatively, fire may destroy lichen regardless of its severity, in which case we would predict that severity would be less important for caribou resource selection. We predicted the stronger effects of burn severity on caribou during winter because winter caribou diets include more lichens and fewer forbs and graminoids (Brown & Mallory, 2007) that flourish in recently burned areas. Conversely, because caribou select protein‐rich forbs and deciduous shrubs in the summer, we predicted weaker avoidance of burns in the summer (Denryter et al., 2017).
The second analysis under Question 2 aimed to test how burn severity and other conditions within burns influence caribou resource selection at a finer spatiotemporal scale through their effects on lichen abundance and distribution. This fine‐scale analysis included a subset of four populations in AK and YT for which we had previously developed, satellite‐derived data on percentage cover of terrestrial lichens and burn severity (Macander et al., 2020; Figure 1). We predicted that avoidance of severely burned areas during the winter would continue longer after a fire than in the summer due to the long post‐fire recovery time of lichens (Jandt et al., 2008). We also predicted that caribou would avoid areas deeper within burn perimeters (Joly et al., 2003). Finally, we predicted that the strength of avoidance of severely burned areas and areas deeper within burns would decrease as lichen and vegetation recovered over time. We refer to this analysis as the within‐burn RSA.
METHODS
We conducted three separate sets of RSAs for forest‐dwelling caribou responses to fire disturbance in northwestern North America (Figure 2). Below, we first describe our burn perimeter RSA focused on caribou responses to burns, along with functional responses to burns. We then provide details on our burn severity and within‐burn RSAs.
FIGURE 2.
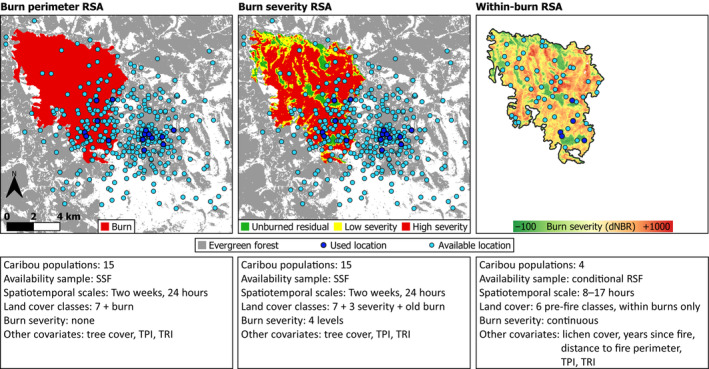
Example of spatial distribution of used and available caribou locations, and different burn characteristics for three separate resource selection analyses of female caribou (Rangifer tarandus) in eastern Alaska and western Canada. Panels show a 1998 fire in the Kluane caribou range, Yukon (YT), and GPS locations from one caribou during February 2014. Evergreen forest is the reference land cover category for the left and centre panels, and pre‐fire evergreen forest is the reference land cover category for the right panel. All other land cover categories are not shown. For simplicity, the fourth burn severity category (“regrowth”) is not shown in the middle panel
Study area
We analyzed resource selection in caribou from 15 populations across eastern AK, YT, NT and northern Alberta (AB). Each population was exposed to relatively low human disturbance (~2%–20% of range disturbed by humans, including 500‐m buffer, Johnson et al., 2020). Our populations included migratory (Rangifer tarandus granti, n = 2), mountain woodland (Rangifer tarandus caribou, n = 4), and boreal woodland (R. t. caribou, n = 9) caribou ecotypes (Ray et al., 2015). Estimated mean fire return intervals varied widely by dominant tree species, but were <100 years for northern AB boreal ranges (Johnstone & Chapin, 2006a; Larsen, 1997), ~40–200 years in southern NT (Bothwell et al., 2004; Larsen, 1997), and 100–200 years in YT and eastern AK (Kasischke et al., 2010). Interior sections of eastern AK and YT consisted of rolling hills, rugged peaks, subalpine and alpine areas, and large forested river valleys. In the boreal ranges of northern AB and NT, topography is gently rolling, except in localized upland areas and a few deeply incised river valleys. Common tree species throughout the study area include spruces (Picea mariana, P. glauca), poplars (Populus tremuloides, P. balsamifera), jack pine (Pinus banksiana), tamarack (Larix laricina), and birch (Betula papyrifera).
Capture and data summary
Caribou were generally captured from a helicopter by net gun and were subsequently fitted with GPS collars following approved federal, provincial, state, and territorial animal care protocols and permits (Appendix S1: Table S1). Prior to filtering and analyses, our data set included 1,804,829 GPS locations from 721 GPS‐collared female caribou from 15 populations whose collars collected data from between 2006 and 2019 (Appendix S1: Table S2).
Burn perimeter RSA
We filtered GPS location data to create separate data sets for relocation intervals of 2 weeks and 24 h. From this point forwards, we refer to these time periods as spatiotemporal scales because the relocation interval determined both the spatial and temporal extent of the domain available to an animal (e.g., Mahoney et al., 2018). These spatiotemporal scales roughly represent opposite ends of Johnson's (1980) third‐order selection (within an individual's seasonal range). We further divided these two data sets into two seasons, defining summer as 25 May to 5 October and winter as 6 October to 5 May based on general patterns in movement rates across populations (Appendix S1: Figure S1; please refer to Appendix S1: Table S2 for details on analysis subsets). The spatial extent of available habitat varied widely across ecotypes and populations, reflecting different movement behaviors. For example, the mean distances between consecutive locations (step lengths) at the 2‐week and 24‐h spatiotemporal scales during the summer were 71.6 and 7.6 km, respectively, for the migratory Fortymile population, versus 5.4 and 1.9 km for the relatively sedentary boreal Dehcho South population (Appendix S1: Figure S2). We explicitly accounted for variation in movement behavior across individuals and populations by sampling availability from step length and turning angle distributions fit for each individual at these two spatiotemporal scales.
We used point‐based SSFs in a generalized linear mixed modeling (GLMM) framework (Muff et al., 2019) to analyze caribou resource selection across the 15 caribou populations. This approach divides an animal's movement path into discrete steps based on a user‐defined time interval, restricting resource availability in the model by the animal's current location in space and time. Using the R package amt, version 0.1.2 (Signer et al., 2019), we generated 10 available locations per used location by making random draws from gamma distributions fitted to used step lengths and von Mises distributions fitted to turning angles between consecutive used locations (Signer et al., 2019). Each set of one used location and 10 available locations represented a stratum.
Our GLMMs accounted for correlated observations within individual caribou and within populations and for differences in sample sizes across individuals and populations (Gillies et al., 2006). Random coefficients allowed the effect of a covariate on resource selection to vary by individual caribou, population, or both (Muff et al., 2019). We estimated selection coefficients for each covariate using a Poisson regression with stratum‐specific intercepts, which is a likelihood equivalent of a conditional logistic regression often used in SSFs (McCullagh & Nelder, 1989; Muff et al., 2019). Within conditional Poisson GLMMs, we treated stratum‐specific intercepts as random effects with a fixed large variance using the R package glmmTMB, version 1.0.2.1 (Brooks et al., 2017), following Muff et al. (2019).
Because we were interested in estimating resource selection responses across and within populations while accounting for varying responses and sample sizes across individuals, our models included random coefficients at the population and individual level for every covariate. Each candidate model included all possible covariates (described below) that we hypothesized would affect caribou resource selection. We used Akaike's Information Criteria (AIC) to assess support for including linear versus non‐linear (i.e., second‐order) covariate terms in the models. Given caribou populations, animals, and a matched set of used and available locations, we used the following Poisson function (Muff et al., 2019) to estimate the relative intensity of use at each time point :
| (1) |
where is a stratum‐specific random intercept (with variance fixed at 106) for individual animal within population at time , is the transpose of the covariate vector selection coefficients estimated for a vector of covariates , is a vector of population‐ and individual‐level random coefficients, and is a subvector of covariates from . All used available RSAs estimate relative probabilities (or relative intensities in a Poisson regression) of selection that are proportional, but not equivalent, to true probabilities of selection (Manly et al., 2002).
We tested for functional responses in selection for burns by including an interaction between the burn landcover category and the average seasonal burn availability for each animal (Matthiopoulos et al., 2011). We estimated average seasonal burn availability by calculating the proportion of available locations at each movement step (i.e., stratum) that fell within a burn, and averaging over all steps along an animal's seasonal movement path. To test the effect of ecotype on relative selection for burns, we i'cluded a model with an interaction between ecotype and the burn landcover category. We used AIC to select the top model for each combination of season and scale from a candidate set that included models with linear or second‐order polynomial functional responses, those with the burn: ecotype interaction, and those without interaction terms.
Environmental covariates
We used burn perimeter polygons from the Alaska Large Fire Database (Kasischke et al., 2002) from 1965–2018 and from the Canada National Fire Database (Stocks et al., 2003) from 1965–2018. We excluded burn perimeters from fires that occurred prior to 1965 because not all regions reported burn perimeter data from this period. State, provincial and federal agencies typically rely on simple burn perimeters in caribou management plans (e.g., Environment Canada, 2012). Our models included land cover, tree cover, and indices of terrain ruggedness and terrain position to account for these additional habitat attributes. We used percentage tree cover data estimated for year 2000 (Hansen et al., 2013). We derived terrain indices from ~30‐m resolution elevation data from NASA's Shuttle Radar Topography Mission (≤60° N; Farr et al., 2007), the National Elevation Dataset (>60° N, >120° W; Gesch et al., 2002), and the Canadian Digital Elevation Model (>60° N, ≤120° W; Natural Resources Canada, 2015).
We used land cover data from a 30‐m resolution, Landsat‐based product with separate land cover classes estimated for each year from 1984–2014 (Wang et al., 2019). The 10 land cover classes were: evergreen forest, deciduous forest, shrubs, grass, sparse vegetation, barren, fen, bog, shallows/littoral, water. We collapsed barren, bog, and shallows/littoral into an “other” category, added in a “burn” category for all locations within burns (regardless of time since fire), and assigned evergreen forest as the reference land cover category. For caribou locations in unburned areas, we annotated land cover values from the year the animal was present unless it was after 2014, in which case we used the 2014 land cover value.
Burn severity RSA
We tested the degree to which caribou responded to different levels of burn severity relative to unburned areas outside burn perimeters by replacing the burn land cover category in our functional response models with five categories of burn severity. The levels for burn severity were regrowth areas within burns, residual unburned areas within burns, burns from <1985 (with no available burn severity data), low‐severity burns, and high‐severity burns. We defined cutoffs for burn severity categories below in burn severity RSA covariates following categories in Key and Benson (2006). Model coefficients for all five burn severity categories represented selection relative to unburned evergreen forest. After splitting burns into these five categories, we only had sufficient sample sizes for model convergence at the 24‐h spatiotemporal scale.
Burn severity RSA covariates
Aside from the addition of burn severity categories, models with categorical burn severity retained the same suite of covariates as the burn perimeter RSA above. For fires that occurred between 1985 and 2015, we used differenced normalized burn ratio (dNBR) burn severity data that were derived from Landsat image pairs collected the year preceding and the year following the fire year (Loboda et al., 2018). We classified burn severity into four severity categories by collapsing Key and Benson's (2006) seven categories. We defined dNBR values within burn perimeters between −500 and −101 as “regrowth” (1.8% of available locations within burn perimeters from 1985–2015 across both seasons at the 24‐h scale). These areas were likely to be dominated by herbaceous and deciduous shrub vegetation that was exposed to low‐severity burn and recovered quickly to exceed pre‐fire productivity (Key & Benson, 2006). We defined dNBR values between −100 and +99 as “residual unburned patches” (12.5%), which represented areas within burn perimeters with little to no change in productivity between pre‐ and post‐fire productivity. “Low severity” (39.4%) encompassed dNBR values between +100 and +439, while “high severity” (46.2%) included dNBR between +440 and +1300. We classified locations within burns from 1965–1984, for which we had no burn severity data, as “old burns.” We excluded locations within burns that occurred after 2015 because we lacked burn severity data for these burns.
Within‐burn RSA
We analyzed fine‐scale resource selection within burned areas for four populations in eastern AK and western YT (Appendix S1: Table S2, Figure 1) within the spatial domain of a previously developed model of terrestrial lichen cover (Macander et al., 2020). Prior to analysis, we filtered GPS locations to an interval of one location every 5–8 h. This relocation interval maximized sample size of locations within burns while avoiding dropping populations (e.g., Clear Creek, Tay River) with longer intervals between locations from the analysis. We used burn perimeters to constrain availability in a static (not movement‐based) RSA, randomly sampling 10 available locations within the same burn perimeter containing the corresponding used location. We defined a stratum as all used and available locations within a single burn for an individual‐year‐season. We modeled resource selection within burns using conditional Poisson GLMMs, which allowed for multinomial strata with a varying number of used points per stratum ( from Equation 1). Because >90% of locations in this analysis were from the Fortymile population, we estimated random coefficients at the individual level but not at the population level. We used interaction terms to account for our hypotheses that time since the most recent fire would affect caribou responses to burn severity (severity:time_since_fire) and distance within burn perimeter (dist_within_perimeter:time_since_fire).
Within‐burn covariates
We restricted this analysis to locations that occurred within burns from 1985–2015 for which we had burn severity data. We used percentage cover of terrestrial lichens estimated for year 2015 (Macander et al., 2020), which fell within the temporal range of most of our caribou location data. We estimated distance within burn perimeter by calculating the distance from each location within a burn to the burn perimeter, so larger distances indicated locations that were deeper within a burn. Time since fire represented the amount of time (in years) elapsed between the fire and the caribou GPS location time stamp. We used Wang et al.’s (2019) land cover layer to estimate pre‐burn land cover (for the year preceding the fire) within burn perimeters. We lumped “water” into the “other” land cover category because it was extremely rare in the spatial domain of this analysis.
Model validation
For the burn perimeter and burn severity RSAs, we evaluated all models using a cross‐validation procedure where we iteratively withheld one population as a test data set (Roberts et al., 2017) and fitted models to the remaining 14 populations. We estimated predicted values for the test data sets using fixed‐effects terms (omitting random coefficients) from fitted models. Within each stratum, we ranked predictions from used locations against those from available locations (from 1 to 11, i.e., 1 used and 10 available locations). We tallied used locations across all strata and calculated the Spearman rank correlation (rs) for each withheld population to test whether higher ranking bins included more used locations (Fortin et al., 2009). We used a similar cross‐validation procedure for within‐burn RSA models, but divided the data set into 10 random folds (instead of withholding by population), each with an equal number of individuals.
RESULTS
After thinning and filtering our data, models in the burn perimeter and burn severity RSAs included between 9551 and 266,768 GPS locations from between 539 and 685 caribou, depending on season and spatiotemporal scale, from 15 populations (Appendix S1: Table S3A). Our within‐burn RSA models included 13,295 GPS locations from 148 caribou in winter and 7918 GPS locations from 107 caribou in summer from four populations (Appendix S1: Table S3B). The median time between successive locations across all individuals included in the within‐burn RSA after excluding locations with burn severity dNBR values below −500 and above +1100 (Key & Benson, 2006) was 10.4 h in summer and 12.5 h in winter. Across all three analyses, we excluded random coefficients at the individual level for all land cover categories except burn because they often prevented model convergence. All final models within an analysis included the same set of random coefficients.
General patterns in caribou use of burns across populations and ecotypes
Caribou use of burns throughout the year varied widely across caribou ecotypes and populations. Boreal caribou populations generally spent a higher proportion of time in burned areas during the summer and early fall than during the rest of the year (Appendix S1: Figure S3). For example, 75% of caribou GPS locations from the Yates population in AB/NT between mid‐April and mid‐November were in burns versus 26%–62% between December and March. However, the Mackenzie population in NT almost exclusively used burns all year, as very little (~15%) of their annual range remained unburned. Peak caribou use of burns in mountain populations typically occurred in April and May (7%–34% of annual burn use), with low use of burns during September and October (2%–7% of annual burn use). There were dissimilar temporal patterns of burn use between the two migratory populations (Fortymile and Nelchina) in AK/YT. Nelchina caribou only used burns during the winter (fire was virtually absent from its summer range), while Fortymile used burns throughout the year except during the weeks prior to and immediately following calving (Appendix S1: Figure S3).
Burn perimeter RSA
Summary of non‐burn‐related covariates
In all four combinations of seasons (summer, winter) and spatiotemporal scales (2 weeks, 24 h), caribou avoided areas with higher tree cover (β summer,2weeks = −0.41 ± 0.11; β summer,24h = −0.27 ± 0.10 [SE]; β winter,2weeks = −0.60 ± 0.05; β winter,24h = −0.36 ± 0.05). Negative quadratic terms for tree cover during winter indicated that the strength of avoidance increased as tree cover increased (please refer to Appendix S1: Table S4A for remaining coefficient estimates). Relative to unburned evergreen forest, caribou avoided “other” land cover (category including barren, bog, and shallows/littoral land cover types) across all seasons and scales, and avoided shrubs and grass land cover types except during summer at the 2‐week scale (Appendix S1: Table S4A). Significant positive coefficients for terrain position index in all four models ( range: 0.05 to 0.09, Appendix S1: Table S4A) indicated that caribou selected ridgetops and avoided incised valleys. Caribou avoided more rugged terrain at all seasons and spatiotemporal scales (β range: −0.82 to −0.34, Appendix S1: Table S4A).
Summary of burn‐related coefficients
Fixed‐effects coefficients for burns indicated that caribou generally avoided burns ( range: −0.99 to −0.31; Appendix S1: Table S4A) relative to the reference category of evergreen forest. Caribou consistently avoided burns during winter at both spatiotemporal scales across nearly all populations, but avoidance was generally stronger at the larger 2‐week spatiotemporal scale (Figure 3, Appendix S1: Table S5). During summer, caribou avoidance of burns at both scales in most populations was weaker than during winter.
FIGURE 3.
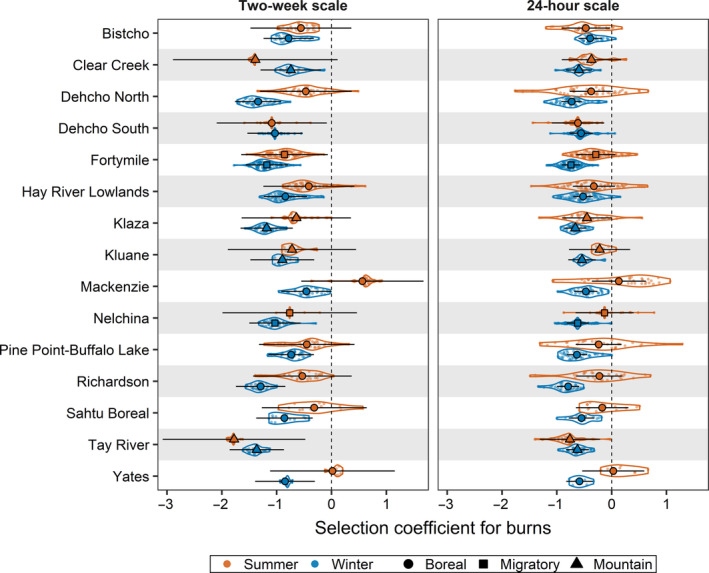
Violin plot of individual‐ and population‐level selection coefficients (conditional modes) for burns, shown by season and spatiotemporal scale, for female caribou (Rangifer tarandus) across 15 populations in western Canada and eastern Alaska. All coefficient values are relative to unburned evergreen forest, the reference land cover category. Violins with small points show the distribution of individual‐level coefficients, while bold circles and lines indicate population‐level coefficients for burns with their 95% confidence intervals (calculated using the sum of conditional and fixed‐effects variances)
Functional response in burn perimeter RSA
Burn availability explained more variation in relative selection for burns than ecotype (Appendix S1: Table S6). The top models during winter at both spatiotemporal scales included a second‐order polynomial functional response to burns (Figure 4; Appendix S1: Table S6), in which selection for burns initially increased as burn footprint increased but leveled off at higher levels of burn availability (i.e., 60%–70% of seasonal range burned; winter 2 weeks: burn:burnavailability = 0.53 ± 0.08, burn:burnavailability 2 = −0.15 ± 0.04; winter 24 h: burn:burnavailability = 0.21 ± 0.03, burn:burnavailability 2 = −0.07 ± 0.02. During summer, the top model at both scales included a linear functional response to burn availability, indicating that caribou decreased their avoidance of burns as burn availability increased (summer 2 weeks: burn:burnavailability = 0.76 ± 0.09, summer 24 h: burn:burnavailability = 0.46 ± 0.05).
FIGURE 4.
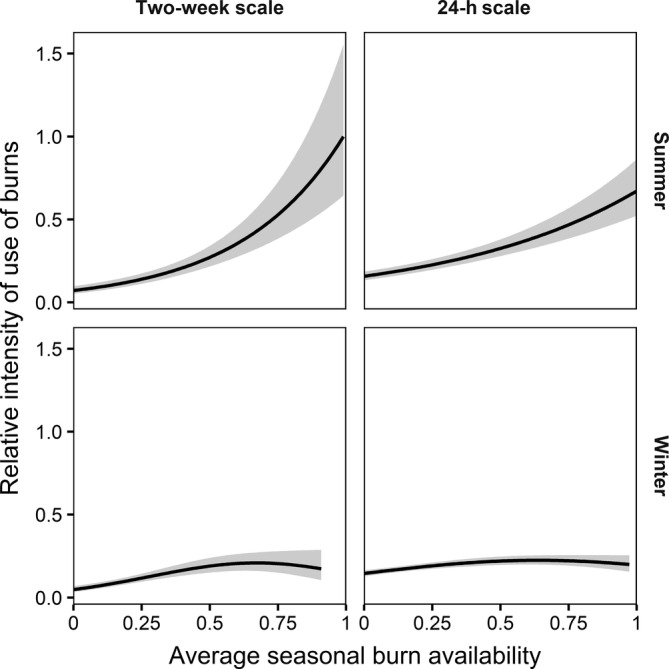
Functional responses to burns for female caribou (Rangifer tarandus) across 15 populations in western Canada and eastern Alaska shown by season and spatiotemporal scale. Gray‐shaded region indicates 95% confidence interval. Predictions and 95% confidence intervals (shaded regions) are based on fixed effects only. All other model covariates were assumed to be equally available. Continuous covariates were held at their mean values (pooled across all observations) and all non‐burn land cover covariates were set to 0 (reference category of evergreen forest)
Burn severity RSA
Our second analysis modeled caribou resource selection in response to different levels of burn severity relative to unburned evergreen forest across the same 15 populations as above. These models replaced simple burn perimeters from the first analysis with five levels of burn severity but retained the same suite of non‐burn‐related covariates (Appendix S1: Table S4). Coefficients for non‐burn‐related covariates only changed slightly from the those (average change of <3.0%) in the burn perimeter RSA (Appendix S1: Table S4A), confirming that there was no evidence of confounding with burn severity.
We found stronger avoidance of low‐ and high‐severity burns during winter than during summer ( summer,lowseverity = −0.26 ± 0.06, winter,lowseverity = −0.46 ± 0.07; summer,highseverity = −0.50 ± 0.06, winter,highseverity = −1.07 ± 0.08; Figure 5; Appendix S1: Table S4B). Relative to the reference category of unburned evergreen forest, fixed‐effects coefficients showed avoidance of all levels of burn severity during winter except unburned residuals. During winter, caribou avoided high‐severity burned areas more than low‐severity burned areas, old burns and regrowth areas, and avoided low‐severity burned areas more than unburned residuals. During summer, fixed‐effects coefficients indicated avoidance of all burn severity levels relative to the unburned evergreen forest, but the strength of avoidance was weaker than during the winter.
FIGURE 5.
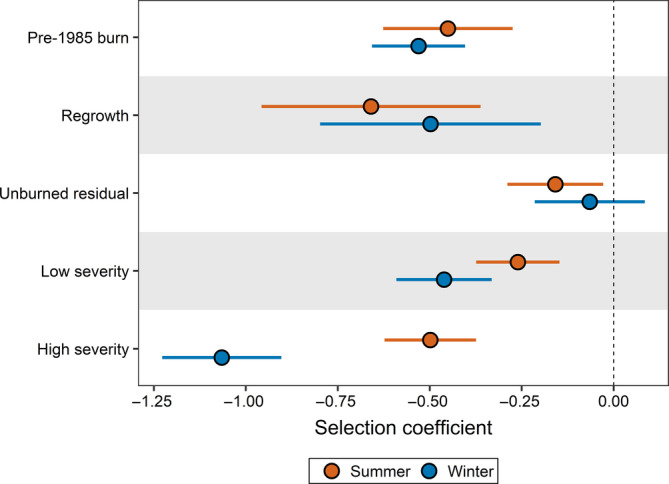
Fixed‐effects selection coefficients and their 95% confidence intervals for different levels of burn severity at the 24‐h scale, shown by season, for female caribou (Rangifer tarandus) across 15 populations in western Canada and eastern Alaska. All coefficient values are relative to unburned evergreen forest, the reference land cover category
Population‐level coefficients showed that all caribou populations avoided high‐severity burned areas and most populations avoided low‐severity burned areas relative to unburned evergreen forests during winter (Appendix S1: Table S7). During summer, caribou showed weak avoidance of high‐ and low‐severity areas relative to unburned evergreen forest, and there were fewer differences between burn severity levels.
Within‐burn RSA
Our third analysis modeled fine‐scale caribou resource selection within burns in response to a suite of burn characteristics across four populations (two migratory and two mountain ecotypes) in AK and YT. Within burns, caribou consistently selected areas with higher percent cover of terrestrial lichen (β summer,lichen = 0.35 ± 0.04, β summer,lichen 2 = −0.03 ± 0.01; β winter,lichen = 0.40 ± 0.03, β winter,lichen 2 = −0.04 ± 0.01) and areas closer to perimeters (β summer,d_perimeter = −0.58 ± 0.06, β summer,d_perimeter 2 = 0.06 ± 0.01; β winter,d_perimeter = −0.40 ± 0.04, β winter,d_perimeter 2 = 0.03 ± 0.01) during both summer and winter (Figure 6; Appendix S1: Table S4C). During winter, caribou avoided areas within burns that were more severely burned (Figure 6), but avoidance attenuated with increasing time since fire (Figure 6). Burn severity was a weak driver of resource selection within burns during the summer (Figure 6). During winter, our model predicted that relative intensity of use for high‐severity areas did not reach the relative intensity of use for unburned residuals until nearly 30 years after a fire (Figure 6). Finally, caribou selected areas closer to burn perimeters, avoiding areas deeper within burns during both seasons (Figure 6). We did not find evidence that this pattern weakened with increasing time since fire. We found a negative relationship between 2015 lichen cover and burn severity regardless of time since fire, except for dNBR values classified as post‐fire regrowth, i.e., below −100 (Appendix S1: Figure S4).
FIGURE 6.
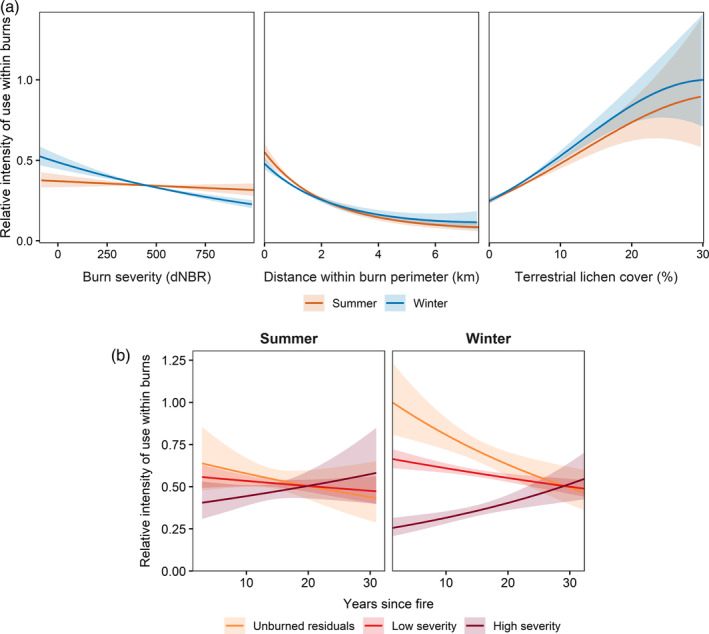
Predicted effects of burn severity, distance within burn perimeter, terrestrial lichen cover (a), and time since fire (b) on the relative intensity of use within burn perimeters for female caribou (Rangifer tarandus) across four populations in eastern Alaska and western Yukon. In panel (b), differenced normalized burn ratio (dNBR) values for unburned residuals, low‐severity, and high‐severity categories were held constant at 0, 270, and 900, respectively. Predictions and 95% confidence intervals (shaded regions) are based on fixed effects only. All other model covariates were assumed to be equally available. Continuous covariates other than severity were held at their mean values (pooled across all observations) and pre‐fire land cover were set to 0 (reference category of evergreen forest)
Model validation
Models from our burn perimeter and burn severity RSAs showed better predictive performance for winter than summer, while the within‐burn RSA models showed similar performance across seasons (Appendix S1: Table S8, Figure S5). The mean (±SD) r s across each of the 15 withheld populations was 0.77 ± 0.28 in summer and 0.96 ± 0.06 in winter at the 2‐week scale and 0.79 ± 0.28 in summer and 0.94 ± 0.17 in winter at the 24‐h scale in the burn perimeter RSA. The mean r s in the burn severity RSA was 0.82 ± 0.31 in summer and 0.98 ± 0.05 in winter. In the within‐burn RSA, the mean r s across the 10 withheld folds was 0.80 ± 0.13 in summer and 0.82 ± 0.11 in winter.
DISCUSSION
Our results provide strong support for the prevailing paradigm that caribou avoid burned areas very consistently across spatiotemporal scales during winter. Caribou generally avoided burns during the summer, but their responses were much more variable. Our analyses of burn severity and fine‐scale burn characteristics help to clarify the mechanisms driving these seasonal patterns in resource selection and confirmed that increasing fire severity will decrease lichen cover. Consistently strong avoidance of burns during winter at both spatiotemporal scales across a wide gradient of burn availability suggests that increasing fire frequency will accelerate habitat loss for caribou across huge swaths of North America's boreal forests.
The large spatial extent of our analysis and wide variability across our 15 caribou populations in their exposure to burns greatly increased our scope of inference for resource selection behavior compared with existing RSA studies focused on one or few populations inhabiting a limited geographic area. Caribou more strongly avoided burns at larger spatiotemporal scales in many populations, supporting the idea that a species’ primary limiting factors (e.g., predation risk) drive selection at coarser scales, while selection at finer scales may be influenced by multiple factors such as local food availability (Rettie & Messier, 2000; Spitz et al., 2019). Strong avoidance of burns during winter, but weaker avoidance during summer, corroborates previous studies on migratory caribou in AK (Joly et al., 2003, 2007, 2010) and boreal woodland caribou in Quebec (Courtois et al., 2007) that attributed burn avoidance to decreases in lichen cover, their main winter forage. Stronger caribou avoidance of burns during winter compared with other seasons is also consistent with studies of mountain woodland caribou in AB (Robinson et al., 2010) and boreal woodland caribou in NT (DeMars et al., 2020). Consistent avoidance of burns across a gradient of burn availability and across spatiotemporal scales implies that caribou will continue to avoid burns and experience habitat loss as fire frequency increases.
Our results cast a more complex picture of the relationship between caribou resource selection and fire in the summer. During summer, caribou avoided burns at the larger (2‐week) scale but showed weaker to no avoidance of burns at the smaller (24‐h) scale and exhibited positive functional responses to burns at both scales (detailed in the following section). Our results suggest that weaker avoidance of burns in the summer by adult female caribou may reflect a shift from a lichen‐dominated winter diet to a more diverse, protein‐rich diet to help meet increased nutritional demands after calving (Parker et al., 2009). Deciduous shrubs such as willow (Salix spp.) and birch (Betula spp.) are among the most important forage species for forest‐dwelling caribou in summer (Boertje, 1984; Denryter et al., 2017), and are particularly abundant early in post‐fire successional forests (Schaefer & Pruitt, 1991). Furthermore, variation in burn severity within burn perimeters may provide a diverse suite of forbs, deciduous shrubs, and fungi that are important in summer caribou diet (Thompson et al., 2015) yet are unavailable in winter. We speculate that the need for protein‐rich forage during summer (White et al., 2014) may override any potential increase in predation risk associated with burns (Robinson et al., 2010).
Our analyses of caribou resource selection responses to burn severity suggest that the effects of fire on species reliant on late‐successional boreal forest communities are more nuanced than that revealed by quantifying responses merely to burns (presence only) or to time since fire. Our burn perimeter RSA relied on fire databases that included unburned residuals within burn perimeters (Skatter et al., 2017). Our finding that caribou selection for these unburned residuals was relatively high compared with other burn severity categories in the burn severity RSA (Figure 5) indicated that our estimates of burn avoidance in the burn perimeter RSA may be somewhat conservative. Burn severity is the proportion of organic matter consumed by a fire (Keeley, 2009), which can drive biodiversity across species and scales and affect a diverse array of ecological processes governing post‐fire vegetation recovery in forest ecosystems (Romme et al., 2011). For example, burn severity levels have been shown to affect seed germination and net seedling establishment of dominant boreal tree species (Johnstone & Chapin, 2006a), relative abundance of birds species in western Montana (Smucker et al., 2005), species richness and abundance of ground beetles in northeast Alberta's boreal forest (Koivula & Spence, 2006). Here, we identified a clear negative relationship between burn severity and lichen cover. This result corroborates previous work in Alberta, in which lichen cover was negatively correlated with burn severity in jack pine forests (Pinno & Errington, 2016). Caribou avoidance of areas with high burn severity and low lichen cover during winter, coupled with the observed negative relationship between burn severity and lichen cover in AK and YT (Appendix S1: Figure S4), supports the supposition that lichen destruction by severe fires contributes to the lack of functional response to burns during that season.
Legacy effects of pre‐burn forest characteristics can affect post‐fire vegetation trajectories, future fire conditions, and biodiversity (Johnstone et al., 2010; Romme et al., 2011). We found that pre‐burn land cover may be an important predictor of fine‐scale caribou resource selection within burns in summer, presumably through its effects on post‐burn successional trajectory. Strong selection of pre‐burn grasslands and shrubs relative to pre‐burn evergreen forests within burns during summer might reflect more abundant graminoids (e.g., Eriophorum spp.), forbs, and deciduous shrubs in these areas after a fire (Jandt et al., 2008; Schaefer & Pruitt, 1991). More detailed data on pre‐ and post‐fire land cover could provide additional information on caribou selection responses to successional trajectory. In addition, increased deadfall in burned evergreen forests might impede caribou movement and contribute to stronger avoidance of those areas relative to pre‐burn grasslands.
Predicted increases in fire frequency in the central and western portions of the boreal forest will lead to younger forest stands, reducing the average time that forest tracts exist in a mature state and potentially decreasing caribou food availability, especially in winter (Rupp et al., 2006). Depending on factors such as soil type, soil moisture, and fire timing, more frequent and/or more severe fires may result in post‐fire successional trajectories dominated by deciduous species or even graminoids (Roland et al., 2019; Stralberg et al., 2018). Several studies based on projections from climate models have predicted broad‐scale shifts in successional trajectories that will produce novel conditions in North America's boreal forests (e.g., Rupp et al., 2000; Stralberg et al., 2018). Although there is considerable uncertainty around rates of predicted changes to forest composition and structure resulting from climate warming and changing fire regimes (Roland et al., 2019), more frequent fires in parts of boreal forest would result in younger, deciduous‐dominated vegetation communities favored by other ungulates such as moose (Alces alces) and deer (Odocoileus spp.). Our results show that caribou tend to avoid these land cover types relative to evergreen forests, especially in winter (Appendix S1: Table S4). Collectively, this suggests that important consequences of increasing fire frequency and severity and its effects on boreal biodiversity will be through the direct loss of late‐successional vegetation communities, and their resources (e.g., lichen in winter for caribou), and through land cover change and compounding effects on future fire.
It is important to understand how animals that are reliant on late‐successional forests might alter their selection of burned areas as fire frequency increases in the future, and how these changes may affect habitat use. For example, California spotted owls (Strix occidentalis occidentalis), a subspecies of spotted owl adapted to relatively small patches of severe fires, more strongly avoided severely burned areas when a higher proportion of their home ranges were severely burned (Jones et al., 2020). If caribou maintain strong avoidance of burns as the footprint of burns within their ranges increases, they will experience increasing habitat loss. Alternatively, caribou might relax their avoidance, indicating less habitat loss as fire availability increases. However, if burned habitat is of lower quality, increasing use of burns could ultimately have negative demographic impacts for caribou.
Many studies have used climate projections to predict future declines in caribou habitat quality and distribution based on present avoidance of burns by caribou (e.g., Barber et al., 2018; Gustine et al., 2014; Rupp et al., 2006). However, our functional response results in summer showed decreasing avoidance of burns as burn availability increased, to the point where relative selection of burns was equal to or greater than the selection of evergreen forests. The difference in functional response to burns between seasons may stem from seasonal differences in diet composition and nutritional demands. Caribou may also be constrained in their ability to avoid burns at extremely high levels of burn availability (Beyer et al., 2010). During winter, caribou rely on old growth habitats with sufficient lichen abundance and may be unable to shift to burned areas where lichen has been destroyed. As burn frequency and overall burn footprint increases, some burns may be adequate substitutes for unburned areas during summer because they can provide a diverse suite of protein‐rich forage. Our functional response models suggest that, at least in winter, future fires are likely to continue to result in increasing indirect habitat loss.
Several additional factors may also contribute to variation in relative selection for burns beyond burn availability and seasonal diet differences. During the winter, increased sunlight and wind exposure within burns may impede caribou movement and foraging due to snow density and surface crust thickness (Schaefer & Pruitt, 1991). In addition, historical exposure to burns in Quebec helped to predict caribou responses to forest harvest in Quebec, and may influence relative selection for burns in our study area (Lafontaine et al., 2019). We found that any potential effects of ecotype on caribou responses to burns were outweighed by seasonal burn availability.
Caribou are threatened or endangered across a large portion of northern North America. Winter range habitat loss, fragmentation, and degradation, due to both wildfire and human development, is increasing and is projected to continue to increase under climate‐change scenarios. Given that caribou have low winter range fidelity, (Faille et al., 2010; Joly et al., 2021), our results suggest that large areas should be considered for the conservation of caribou winter ranges. Managers can combine our resource selection models with wildfire risk assessments to identify core caribou wintering areas that also have low burn probabilities (Stockdale et al., 2019) These climate‐change refugia (sensu Stralberg et al., 2020) could then receive enhanced protection from the potential impacts of industrial development and be strategically targeted for fire suppression efforts when possible. This approach would also benefit other boreal biodiversity (Bichet et al., 2016) that depends on the climate‐change “slow lane” (Morelli et al., 2020).
Given their large ranges and reliance on late‐successional vegetation, forest‐dwelling caribou are important umbrellas of broad‐scale biodiversity (Bichet et al., 2016) and indicators of boreal carbon stocks, which account for roughly one‐third of the world's terrestrial carbon (Pan et al., 2011). Although most carbon beneath older, wetter forests is typically protected from combustion, shallower organic matter layers in warmer, drier, and younger forests allow fires to release more carbon, which could shift North American boreal region from a net sink to a net source of carbon (Walker et al., 2019). The area affected by greater fire frequency in boreal forests (de Groot et al., 2013) will probably dwarf the area harvested by the forestry industry, even though continued forestry and energy development throughout the region are main causes of population declines for many boreal species (Venier et al., 2014). As fire frequency increases, species that require late‐successional communities may retreat to climate refugia such as mountains and peatlands (Stralberg et al., 2020). Protecting late‐successional habitats that experience fires of increasing frequency and considerable spatiotemporal unpredictability is a major conservation challenge and underscores the need to minimize negative effects of new human disturbance in remaining mature forests. In addition, our study also has implications for other types of boreal forest disturbances, such as insect outbreaks, that may interact with fire to affect late‐successional communities (Bradshaw et al., 2009; Labadie et al., 2021). Future work directly linking animal demography to habitat selection in response to both fire and human disturbance would provide a clearer picture of the degree to which fire may affect boreal biodiversity.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We acknowledge support from the University of Montana, Alaska Department of Fish and Game, Alberta Environment and Parks, US Bureau of Land Management, Government of the Northwest Territories Department of Environment and Natural Resources, US National Park Service, and Yukon Government's Department of Environment. First Nations partners Sambaa K'e Dene Band, Fort Simpson Métis Local, The Denendeh Harvesters Committee of Líídlíí Kue First Nation (Fort Simpson), Jean Marie River First Nation, Pehdzeh Ki First Nation (Wrigley), Nahanni Butte Dene Band, Acho Dene Koe Band (Fort Liard) and Ka'a’gee Tu First Nation (Kakisa) supported boreal caribou collaring studies in the Northwest Territories. ECP received support from National Aeronautics and Space Agency's (NASA) Earth and Space Science Fellowship, Wildlife Conservation Society Canada's W. Garfield Weston Graduate Fellowship. MH and ECP acknowledge funding from NASA's Arctic Boreal Vulnerability Experiment grant no. NNX15AW71A. MH acknowledges funding from the National Science Foundation's Navigating the New Arctic grant #2127272.
Palm, Eric C. , Suitor Michael J., Joly Kyle, Herriges Jim D., Kelly Allicia P., Hervieux Dave, Russell Kelsey L. M., Bentzen Torsten W., Larter Nicholas C., and Hebblewhite Mark. 2022. “Increasing Fire Frequency and Severity Will Increase Habitat Loss for a Boreal Forest Indicator Species.” Ecological Applications 32(3): e2549. 10.1002/eap.2549
Handling Editor: Jacob R. Goheen
Funding information National Aeronautics and Space Administration, Grant/Award Number: NNX15AW71A
DATA AVAILABILITY STATEMENT
Data and code (ecpalm, 2021) are available in Zenodo at https://doi.org/10.5281/zenodo.5601986.
REFERENCES
- Aarts, G. , Fieberg J., Brasseur S., and Matthiopoulos J.. 2013. “Quantifying the Effect of Habitat Availability on Species Distributions.” Journal of Animal Ecology 82: 1135–45. [DOI] [PubMed] [Google Scholar]
- Avgar, T. , Potts J. R., Lewis M. A., and Boyce M. S.. 2016. “Integrated Step Selection Analysis: Bridging the Gap between Resource Selection and Animal Movement.” Methods in Ecology and Evolution 7: 619–30. [Google Scholar]
- Barber, Q. E. , Parisien M.‐A., Whitman E., Stralberg D., Johnson C. J., St‐Laurent M.‐H., DeLancey E. R., et al. 2018. “Potential Impacts of Climate Change on the Habitat of Boreal Woodland Caribou.” Ecosphere 9: e02472. [Google Scholar]
- Beyer, H. L. , Haydon D. T., Morales J. M., Frair J. L., Hebblewhite M., Mitchell M., and Matthiopoulos J.. 2010. “The Interpretation of Habitat Preference Metrics under Use‐Availability Designs.” Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, H. L. , Ung R., Murray D. L., and Fortin M. J.. 2013. “Functional Responses, Seasonal Variation and Thresholds in Behavioural Responses of Moose to Road Density.” Journal of Applied Ecology 50: 286–94. [Google Scholar]
- Bichet, O. , Dupuch A., Hébert C., Le Borgne H., and Fortin D.. 2016. “Maintaining Animal Assemblages through Single‐Species Management: The Case of Threatened Caribou in Boreal Forest.” Ecological Applications 26: 612–23. [DOI] [PubMed] [Google Scholar]
- Boertje, R. D. 1984. “Seasonal Diets of the Denali Caribou Herd, Alaska.” Arctic 37: 161–5. [Google Scholar]
- Bothwell, P. M. , de Groot W. J., Dubé D. E., Chowns T., Carlsson D. H., and Stefner C. N.. 2004. “Fire Regimes in Nahanni National Park and the Mackenzie Bison Sanctuary, Northwest Territories, Canada.” In Proceedings of the 22nd Tall Timbers Fire Ecology Conference: Fire in Temperate, Boreal, and Montane Ecosystems 43–54. Tallahassee, FL: Tall Timbers Research Station. [Google Scholar]
- Bradshaw, C. J. A. , Warkentin I. G., and Sodhi N. S.. 2009. “Urgent Preservation of Boreal Carbon Stocks and Biodiversity.” Trends in Ecology and Evolution 24: 541–8. [DOI] [PubMed] [Google Scholar]
- Brooks, M. E. , Kristensen K., Koen A. M., van Benthem J., Berg C. W., Nielsen A., Skaug H. J., Maechler M., and Bolker B. M.. 2017. “glmmTMB Balances Speed and Flexibility among Packages for Zero‐Inflated Generalized Linear Mixed Modeling.” The R Journal 9: 378–400. [Google Scholar]
- Brown, G. , and Mallory F.. 2007. A Review of Ungulate Nutrition and the Role of Top‐Down and Bottom‐up Forces in Woodland Caribou Population Dynamics. Technical Bulletin 934. Research Triangle Park, NC: National Council for Air and Stream Improvement, Inc. [Google Scholar]
- Burton, P. J. , Parisien M., Hicke J. A., Hall R. J., and Freeburn J. T.. 2008. “Large Fires as Agents of Ecological Diversity in the North American Boreal Forest.” International Journal of Wildland Fire 17: 754–67. [Google Scholar]
- Callaghan, T. V. , Björn L. O., Chernov Y., Chapin T., Christensen T. R., Huntley B., Ims R. A., et al. 2004. “Effects of Changes in Climate on Landscape and Regional Processes, and Feedbacks to the Climate System.” Ambio 33: 459–68. [DOI] [PubMed] [Google Scholar]
- Courtois, R. , Ouellet J.‐P., Breton L., Gingras A., and Dussault C.. 2007. “Effects of Forest Disturbance on Density, Space Use, and Mortality of Woodland Caribou.” Ecoscience 14: 491–8. [Google Scholar]
- Coxson, D. S. , and Marsh J.. 2001. “Lichen Chronosequences (Postfire and Postharvest) in Lodgepole Pine (Pinus contorta) Forests of Northern Interior British Columbia.” Canadian Journal of Botany 1464: 1449–64. [Google Scholar]
- Dale, V. H. , Joyce L. A., McNulty S., Neilson R. P., Ayres M. P., Flannigan M. D., Hanson P. J., et al. 2001. “Climate Change and Forest Disturbances: Climate Change Can Affect Forests by Altering the Frequency, Intensity, Duration, and Timing of Fire, Drought, Introduced Species, Insect and Pathogen Outbreaks, Hurricanes, Windstorms, Ice Storms, or Landslides.” Bioscience 51: 723–34. [Google Scholar]
- de Groot, W. J. , Flannigan M. D., and Cantin A. S.. 2013. “Climate Change Impacts on Future Boreal Fire Regimes.” Forest Ecology and Management 294: 35–44. [Google Scholar]
- DeMars, C. , Hodson J., Kelly A., Lamontagne E., Smith L., Groenewegen K., Davidson T., Behrens S., Cluff D., and Gurarie E.. 2020. Influence of Land Cover, Fire and Human Disturbance on Habitat Selection by Boreal Caribou in the NWT.
- DeMars, C. A. , Serrouya R., Mumma M. A., Gillingham M. P., McNay R. S., and Boutin S.. 2019. “Moose, Caribou, and Fire: Have we Got it Right Yet?” Canadian Journal of Zoology 879: 866–79. [Google Scholar]
- Denryter, K. A. , Cook R. C., Cook J. G., and Parker K. L.. 2017. “Straight from the caribou's (Rangifer tarandus) Mouth: Detailed Observations of Tame Caribou Reveal New Insights into Summer‐Autumn Diets.” Canadian Journal of Zoology 95: 81–94. [Google Scholar]
- ecpalm . 2021. ecpalm/caribou_ssf_fire: v1.0.0 (v1.0.0) [Data Set]. Zenodo. 10.5281/zenodo.5601986 [DOI]
- Environment Canada . 2012. Recovery Strategy for the Woodland Caribou (Rangifer tarandus Caribou), Boreal Population, in Canada. Species at Risk Act Recovery Strategy Series.
- Faille, G. , Dussault C., Ouellet J. P., Fortin D., Courtois R., St‐Laurent M. H., and Dussault C.. 2010. “Range Fidelity: The Missing Link between Caribou Decline and Habitat Alteration?” Biological Conservation 143: 2840–50. [Google Scholar]
- Farr, T. G. , Rosen P. A., Caro E., Crippen R., Duren R., Hensley S., Kobrick M., et al. 2007. “The Shuttle Radar Topography Mission.” Reviews of Geophysics 45: RG2004. [Google Scholar]
- Festa‐Bianchet, M. , Ray J. C., Boutin S., Côté S. D., and Gunn A.. 2011. “Conservation of Caribou (Rangifer tarandus) in Canada: An Uncertain Future.” Canadian Journal of Zoology 89: 419–34. [Google Scholar]
- Flannigan, M. D. , Krawchuk M. A., de Groot W. J., Wotton M. B., and Gowman L. M.. 2009. “Implications of Changing Climate for Global Wildland Fire.” International Journal of Wildland Fire 18: 483–507. [Google Scholar]
- Fortin, D. , Fortin M.‐E., Beyer H. L., Duchesne T., Courant S., and Dancose K.. 2009. “Group‐Size‐Mediated Habitat Selection and Group Fusion–Fission Dynamics of Bison under Predation Risk.” Ecology 90: 2480–90. [DOI] [PubMed] [Google Scholar]
- Gesch, D. , Oimoen M., Greenlee S., Nelson C., Steuck M., and Tyler D.. 2002. “The National Elevation Dataset.” Photogrammetric Engineering and Remote Sensing 68: 5–32. [Google Scholar]
- Gillies, C. S. , Hebblewhite M., Nielsen S. E., Krawchuk M. A., Aldridge C. L., Frair J. L., Saher D. J., Stevens C. E., and Jerde C. L.. 2006. “Application of Random Effects to the Study of Resource Selection by Animals.” Journal of Animal Ecology 75: 887–98. [DOI] [PubMed] [Google Scholar]
- Gustine, D. D. , Brinkman T. J., Lindgren M. A., Schmidt J. I., Rupp T. S., and Adams L. G.. 2014. “Climate‐Driven Effects of Fire on Winter Habitat for Caribou in the Alaskan‐Yukon Arctic.” PLoS One 9: e100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, M. C., P. V. Potapov, R. Moore, M. Hancher, S. A. A. Turubanova, A. Tyukavina, D. Thau, et al. 2013. “High‐Resolution Global Maps of 21st‐Century Forest Cover Change.” Science 342: 850–3. [DOI] [PubMed] [Google Scholar]
- Hebblewhite, M. , and Merrill E.. 2008. “Modelling Wildlife‐Human Relationships for Social Species with Mixed‐Effects Resource Selection Models.” Journal of Applied Ecology 45: 834–44. [Google Scholar]
- Hirzel, A. H. , and Le Lay G.. 2008. “Habitat Suitability Modelling and Niche Theory.” Journal of Applied Ecology 45: 1372–81. [Google Scholar]
- Holbrook, J. D. , Olson L. E., Decesare N. J., Hebblewhite M., and Squires J. R.. 2019. “Functional Responses in Habitat Selection: Clarifying Hypotheses and Interpretations.” Ecological Applications 29: e01852. [DOI] [PubMed] [Google Scholar]
- Holt, R. D. 2009. “Bringing the Hutchinsonian Niche into the 21st Century: Ecological and Evolutionary Perspectives.” Proceedings of the National Academy of Sciences of the United States of America 106: 19659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, G. E. 1957. “Concluding Remarks.” Cold Spring Harbor Symposia on Quantitative Biology 22: 415–27. [Google Scholar]
- Jandt, R. , Joly K., Meyers R. C., and Racine C.. 2008. “Slow Recovery of Lichen on Burned Caribou Winter Range in Alaska Tundra: Potential Influences of Climate Warming and Other Disturbance Factors.” Arctic, Antarctic, and Alpine Research 40: 89–95. [Google Scholar]
- Johnson, C. A. , Sutherland G. D., Neave E., Leblond M., Kirby P., Superbie C., and McLoughlin P. D.. 2020. “Science to Inform Policy: Linking Population Dynamics to Habitat for a Threatened Species in Canada.” Journal of Applied Ecology 57: 1314–27. [Google Scholar]
- Johnson, C. J. , Nielson S. E., Merrill E. H., McDonald T. L., and Boyce M. S.. 2006. “Resource Selection Functions Based on Use‐Availability Data: Theoretical Motivation and Evaluation Methods.” Journal of Wildlife Management 70: 347–57. [Google Scholar]
- Johnson, D. H. 1980. “The Comparison of Usage and Availability Measurements for Evaluating Resource Preference.” Ecology 61: 65–71. [Google Scholar]
- Johnstone, J. F. , and Chapin F. S.. 2006a. “Fire Interval Effects on Successional Trajectory in Boreal Forests of Northwest Canada.” Ecosystems 9: 268–77. [Google Scholar]
- Johnstone, J. F. , and Chapin F. S.. 2006b. “Effects of Soil Burn Severity on Post‐Fire Tree Recruitment in Boreal Forest.” Ecosystems 9: 14–31. [Google Scholar]
- Johnstone, J. F. , Hollingsworth T. N., Chapin F. S., and Mack M. C.. 2010. “Changes in Fire Regime Break the Legacy Lock on Successional Trajectories in Alaskan Boreal Forest.” Global Change Biology 16: 1281–95. [Google Scholar]
- Joly, K. , Bente P., and Dau J.. 2007. “Response of Overwintering Caribou to Burned Habitat in Northwest Alaska.” Arctic 60: 401–10. [Google Scholar]
- Joly, K. , and Cameron M. D.. 2018. “Early Fall and Late Winter Diets of Migratory Caribou in Northwest Alaska.” Rangifer 38: 27–38. [Google Scholar]
- Joly, K. , Chapin F. S., and Klein D. R.. 2010. “Winter Habitat Selection by Caribou in Relation to Lichen Abundance, Wildfires, Grazing, and Landscape Characteristics in Northwest Alaska.” Ecoscience 17: 321–33. [Google Scholar]
- Joly, K. , Dale B. W., Collins W. B., and Adams L. G.. 2003. “Winter Habitat Use by Female Caribou in Relation to Wildland Fires in Interior Alaska.” Canadian Journal of Zoology 81: 1192–201. [Google Scholar]
- Joly, K. , Duffy P. A., and Rupp T. S.. 2012. “Simulating the Effects of Climate Change on Fire Regimes in Arctic Biomes: Implications for Caribou and Moose Habitat.” Ecosphere 3: 36. [Google Scholar]
- Joly, K. , Gurarie E., Hansen D. A., and Cameron M. D.. 2021. “Seasonal Patterns of Spatial Fidelity and Temporal Consistency in the Distribution and Movements of a Migratory Ungulate.” Ecology and Evolution 11: 8183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G. M. , Kramer H. A., Whitmore S. A., Berigan W. J., Tempel D. J., Wood C. M., Hobart B. K., et al. 2020. “Habitat Selection by Spotted Owls after a Megafire Reflects their Adaptation to Historical Frequent‐Fire Regimes.” Landscape Ecology 35: 1199–213. [Google Scholar]
- Kasischke, E. S. , Verbyla D. L., Rupp T. S., McGuire A. D., Murphy K. A., Jandt R., Barnes J. L., et al. 2010. “Alaska's Changing Fire Regime—Implications for the Vulnerability of its Boreal Forests.” Canadian Journal of Forest Research 40: 1313–24. [Google Scholar]
- Kasischke, E. S. , Williams D., and Barry D.. 2002. “Analysis of the Patterns of Large Fires in the Boreal Forest Region of Alaska.” International Journal of Wildland Fire 11: 131–44. [Google Scholar]
- Keeley, J. E. 2009. “Fire Intensity, Fire Severity and Burn Severity: A Brief Review and Suggested Usage.” International Journal of Wildland Fire 18: 116–26. [Google Scholar]
- Key, C. H. , and Benson N. C.. 2006. “Landscape Assessment: Sampling and Analysis Methods.” In FIREMON: Fire Effects Monitoring and Inventory System. USDA Forest Service General Technical Report RMRS‐GTR‐164‐CD. edited by Lutes D. C., Keane R. E., Caratti J. F., Key C. H., Benson N. C., Sutherland S., and Gangi L. J., 1–55. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. [Google Scholar]
- Koivula, M. , and Spence J. R.. 2006. “Effects of Post‐Fire Salvage Logging on Boreal Mixed‐Wood Ground Beetle Assemblages (Coleoptera, Carabidae).” Forest Ecology and Management 236: 102–12. [Google Scholar]
- Labadie, G. , McLoughlin P. D., Hebblewhite M., and Fortin D.. 2021. “Insect‐Mediated Apparent Competition between Mammals in a Boreal Food Web.” Proceedings of the National Academy of Sciences of the United States of America 118: e2022892118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine, A. , Drapeau P., Fortin D., Gauthier S., Boulanger Y., and St‐Laurent M.. 2019. “Exposure to Historical Burn Rates Shapes the Response of Boreal Caribou to Timber Harvesting.” Ecosphere 10: e02739. [Google Scholar]
- Larsen, C. P. S. 1997. “Spatial and Temporal Variations in Boreal Forest Fire Frequency in Northern Alberta.” Journal of Biogeography 24: 663–73. [Google Scholar]
- Loboda, T. V. , Chen D., Hall J. V., and He J.. 2018. ABoVE: Landsat‐derived Burn Scar dNBR across Alaska and Canada, 1985‐2015. Oak Ridge, TN: ORNL DAAC. 10.3334/ORNLDAAC/1564 [DOI] [Google Scholar]
- Macander, M. , Palm E. C., Frost G. V., Herriges J. D., Nelson P. R., Roland C., Russell K. L. M., et al. 2020. “Lichen Cover Mapping for Caribou Ranges in Interior Alaska and Yukon.” Environment Research Letters 15: 055001. [Google Scholar]
- Mahoney, P. J. , Liston G. E., LaPoint S., Gurarie E., Mangipane B., Wells A. G., Brinkman T. J., et al. 2018. “Navigating Snowscapes: Scale‐Dependent Responses of Mountain Sheep to Snowpack Properties.” Ecological Applications 28: 1715–1729. [DOI] [PubMed] [Google Scholar]
- Manly, B. F. J. , McDonald L. L., Thomas D. L., McDonald T. L., and Erickson W. P.. 2002. Resource Selection by Animals: Statistical Design and Analysis for Field Studies. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Martin, J. , Basille M., van Moorter B., Kindberg J., Allainé D., and Swenson J. E.. 2010. “Coping with Human Disturbance: Spatial and Temporal Tactics of the Brown Bear (Ursus arctos).” Canadian Journal of Zoology 88: 875–83. [Google Scholar]
- Matthiopoulos, J. , Hebblewhite M., Aarts G., and Fieberg J.. 2011. “Generalized Functional Responses for Species Distributions.” Ecology 92: 583–9. [DOI] [PubMed] [Google Scholar]
- McCullagh, P. , and Nelder J.. 1989. Generalized Linear Models, 2nd ed. London: Chapman and Hall. [Google Scholar]
- Morelli, T. L. , Barrows C. W., Ramirez A. R., Cartwright J. M., Ackerly D. D., Eaves T. D., Ebersole J. L., et al. 2020. “Climate‐Change Refugia: Biodiversity in the Slow Lane.” Frontiers in Ecology and the Environment 18: 228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morneau, C. , and Payette S.. 1989. “Postfire Lichen‐Spruce Woodland Recovery at the Lmit of the Boreal Forest in Northern Quebec.” Canadian Journal of Botany 67: 2770–82. [Google Scholar]
- Muff, S. , Signer J., and Fieberg J.. 2019. “Accounting for Individual‐Specific Variation in Habitat‐Selection Studies: Efficient Estimation of Mixed‐Effects Models Using Bayesian or Frequentist Computation.” Journal of Animal Ecology 89: 80–92. [DOI] [PubMed] [Google Scholar]
- Mumma, M. A. , Parker K. L., Gillingham M. P., and Johnson C. J.. 2019. “Functional Responses to Anthropogenic Linear Features in a Complex Predator‐Multi‐Prey System.” Landscape Ecology 9: 2575–97. [Google Scholar]
- Mysterud, A. , and Ims R. A.. 1998. “Functional Responses in Habitat Use: Availability Influences Relative Use in Trade‐Off Situations.” Ecology 79: 1435–41. [Google Scholar]
- Natural Resources Canada . 2015. Canadian Digital Elevation Model. https://open.canada.ca/data/en/dataset/7f245e4d-76c2-4caa-951a-45d1d2051333.
- Neufeld, B. T. , Superbie C., Greuel R. J., Perry T., Tomchuk P. A., Fortin D., and McLoughlin P. D.. 2020. “Disturbance‐Mediated Apparent Competition Decouples in a Northern Boreal Caribou Range.” Journal of Wildlife Management 85: 254–70. [Google Scholar]
- Pan, Y. , Birdsey R. A., Fang J., Houghton R., Kauppi P. E., Kurz W. A., Phillips O. L., et al. 2011. “A Large and Persistent Carbon Sink in the World's Forests.” Science 333: 988–93. [DOI] [PubMed] [Google Scholar]
- Parker, K. L. , Barboza P. S., and Gillingham M. P.. 2009. “Nutrition Integrates Environmental Responses of Ungulates.” Functional Ecology 23: 57–69. [Google Scholar]
- Payette, S. , Bhiry N., Delwaide A., and Simard M.. 2000. “Origin of the Lichen Woodland at its Southern Range Limit in Eastern Canada: The Catastrophic Impact of Insect Defoliators and Fire on the Spruce‐Moss Forest.” Canadian Journal of Forest Research 30: 288–305. [Google Scholar]
- Person, S. J. , Pegau R. E., White R. G., and Luick J. R.. 1980. “In Vitro and Nylon‐Bag Digestibilities of Reindeer and Caribou Forages.” The Journal of Wildlife Management 44: 613–22. [Google Scholar]
- Pinno, B. D. , and Errington R. C.. 2016. “Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests.” Forests 7: 83. [Google Scholar]
- Ray, J. C. , Cichowski D. B., St‐Laurent M.‐H., Johnson C. J., Petersen S. D., and Thompson I. D.. 2015. “Conservation Status of Caribou in the Western Mountains of Canada: Protections under the Species at Risk Act, 2002‐2014.” Rangifer 35: 49–80. [Google Scholar]
- Rettie, W. J. , and Messier F.. 2000. “Hierarchical Habitat Selection by Woodland Caribou: Its Relationship to Limiting Factors.” Ecography 23: 466–78. [Google Scholar]
- Roberts, D. R. , Bahn V., Ciuti S., Boyce M. S., Elith J., Guillera‐Arroita G., Hauenstein S., et al. 2017. “Cross‐Validation Strategies for Data with Temporal, Spatial, Hierarchical, or Phylogenetic Structure.” Ecography 40: 913–29. [Google Scholar]
- Robinson, H. S. , Hebblewhite M., Decesare N. J., Whittington J., Bradley M., and Musiani M.. 2010. “The Effect of Fire on Spatial Separation between Wolves and Caribou.” Rangifer 20: 277–94. [Google Scholar]
- Roland, C. A. , Schmidt J. H., Winder S. G., Stehn S. E., and Nicklen E. F.. 2019. “Regional Variation in Interior Alaskan Boreal Forests Is Driven by Fire Disturbance, Topography, and Climate.” Ecological Monographs 89: e01369. [Google Scholar]
- Romme, W. H. , Boyce M. S., Gresswell R., Merrill E. H., Minshall G. W., Whitlock C., and Turner M. G.. 2011. “Twenty Years after the 1988 Yellowstone Fires: Lessons about Disturbance and Ecosystems.” Ecosystems 14: 1196–215. [Google Scholar]
- Rota, C. T. , Rumble M. A., Millspaugh J. J., Lehman C. P., and Kesler D. C.. 2014. “Space‐Use and Habitat Associations of Black‐Backed Woodpeckers (Picoides arcticus) Occupying Recently Disturbed Forests in the Black Hills, South Dakota.” Forest Ecology and Management 313: 161–8. [Google Scholar]
- Rupp, S. T. , Starfield A. M., and Stuart Chapin F.. 2000. “A Frame‐Based Spatially Explicit Model of Subarctic Vegetation Response to Climatic Change: Comparison with a Point Model.” Landscape Ecology 15: 383–400. [Google Scholar]
- Rupp, T. S. , Olson M., Adams L. G., Dale B. W., Joly K., Henkelman J., Collins W. B., and Starfield A. M.. 2006. “Simulating the Influence of Various Fire Regimes on Caribou Winter Habitat.” Ecological Applications 16: 1730–43. [DOI] [PubMed] [Google Scholar]
- Russell, K. L. M. , and Johnson C. J.. 2019. “Post‐Fire Dynamics of Terrestrial Lichens: Implications for the Recovery of Woodland Caribou Winter Range.” Forest Ecology and Management 434: 1–17. [Google Scholar]
- Schaefer, J. A. , and Pruitt W. O.. 1991. “Fire and Woodland Caribou in Southeastern Manitoba.” Wildlife Monographs 116: 3–39. [Google Scholar]
- Seidl, R. , Thom D., Kautz M., Martin‐Benito D., Peltoniemi M., Vacchiano G., Wild J., et al. 2017. “Forest Disturbances under Climate Change.” Nature Climate Change 7: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergio, F. , Blas J., and Hiraldo F.. 2018. “Animal Responses to Natural Disturbance and Climate Extremes: A Review.” Global and Planetary Change 161: 28–40. [Google Scholar]
- Signer, J. , Fieberg J., and Avgar T.. 2019. “Animal Movement Tools (Amt): R Package for Managing Tracking Data and Conducting Habitat Selection Analyses.” Ecology and Evolution 9: 880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatter, H. G. , Charlebois M. L., Eftestøl S., Tsegaye D., Colman J. E., Kansas J. L., Flydal K., and Balicki B.. 2017. “Living in a Burned Landscape: Woodland Caribou (Rangifer tarandus caribou) Use of Postfire Residual Patches for Calving in a High Fire – Low Anthropogenic Boreal Shield Ecozone.” Canadian Journal of Zoology 95: 975–84. [Google Scholar]
- Smucker, K. M. , Hutto R. L., and Steele B. M.. 2005. “Changes in Bird Abundance after Wildfire: Importance of Fire Severity and Time since Fire.” Ecological Applications 15: 1535–49. [Google Scholar]
- Sorensen, T. , McLoughlin P. D., Hervieux D., Dzus E., Nolan J., Wynes B., and Boutin S.. 2008. “Determining Sustainable Levels of Cumulative Effects for Boreal Caribou.” The Journal of Wildlife Management 72: 900–5. [Google Scholar]
- Spitz, D. B. , Hebblewhite M., and Stephenson T. R.. 2019. “Habitat Predicts Local Prevalence of Migratory Behaviour in an Alpine Ungulate.” Journal of Animal Ecology 89: 1032–44. [DOI] [PubMed] [Google Scholar]
- Stockdale, C. , Barber Q., Saxena A., and Parisien M.. 2019. “Examining Management Scenarios to Mitigate Wildfire Hazard to Caribou Conservation Projects Using Burn Probability Modeling.” Journal of Environmental Management 233: 238–48. [DOI] [PubMed] [Google Scholar]
- Stocks, B. J. , Mason J. A., Todd J. B., Bosch E. M., Wotton B. M., Amiro B. D., Flannigan M. D., et al. 2003. “Large Forest Fires in Canada, 1959–1997.” Journal of Geophysical Research 108: 8149. [Google Scholar]
- Stocks, B. J. , Wotton B. M., Flannigan M. D., Fosberg M. A., Cahoon D. R., and Goldammer J. G.. 2001. “Boreal Forest Fire Regimes and Climate Change.” In Remote Sensing and Climate Modelling: Synergies and Limitations, Vol 7, 233–46. Dordrecht, The Netherlands: Kluwer Academic. [Google Scholar]
- Stralberg, D. , Arseneault D., Baltzer J. L., Barber Q. E., Bayne E. M., Boulanger Y., Brown C. D., et al. 2020. “Climate‐Change Refugia in Boreal North America: What, where, and for how Long?” Frontiers in Ecology and the Environment 18: 261–70. [Google Scholar]
- Stralberg, D. , Wang X., Parisien M.‐A., Robinne F.‐N., Sólymos P., Mahon C. L., Nielsen S. E., and Bayne E. M.. 2018. “Wildfire‐Mediated Vegetation Change in Boreal Forests of Alberta, Canada.” Ecosphere 9: e02156. [Google Scholar]
- Sutherland, W. J. , Freckleton R. P., Godfray H. C. J., Beissinger S. R., Benton T., Cameron D. D., Carmel Y., et al. 2013. “Identification of 100 fundamental ecological questions.” Journal of Ecology 101: 58–67. [Google Scholar]
- Thomas, D. C. , Edmonds E. J., and Brown W. K.. 1996. “The Diet of Woodland Caribou Populations in West‐Central Alberta the.” Methods 9: 1–4.9245336 [Google Scholar]
- Thompson, I. D. , Wiebe P. A., Mallon E., Rodger A. R., Fryxell J. M., Baker J. A., and Reid D.. 2015. “Factors Influencing the Seasonal Diet Selection by Woodland Caribou (Rangifer tarandus Tarandus) in Boreal Forests in Ontario.” Canadian Journal of Zoology 93: 87–98. [Google Scholar]
- Thurfjell, H. , Ciuti S., and Boyce M. S.. 2014. “Applications of Step‐Selection Functions in Ecology and Conservation.” Movement Ecology 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, M. G. 2010. “Disturbance and Landscape Dynamics in a Changing World.” Ecology 91: 2833–49. [DOI] [PubMed] [Google Scholar]
- Venier, L. A. , Thompson I. D., Fleming R., Malcolm J., Aubin I., Trofymow J. A., Langor D., et al. 2014. “Effects of Natural Resource Development on the Terrestrial Biodiversity of Canadian Boreal Forests.” Environmental Review 490: 457–90. [Google Scholar]
- Walker, X. J. , Baltzer J. L., Cumming S. G., Day N. J., Ebert C., Goetz S., Johnstone J. F., et al. 2019. “Increasing Wildfires Threaten Historic Carbon Sink of Boreal Forest Soils.” Nature 572: 520–3. [DOI] [PubMed] [Google Scholar]
- Wang, J. A. , Sulla‐Menashe D., Woodcock C. E., Sonnentag O., Keeling R. F., and Friedl M. A.. 2019. “Extensive Land Cover Change across Arctic‐Boreal Northwestern North America from Disturbance and Climate Forcing.” Global Change Biology 26: 807–22. [DOI] [PubMed] [Google Scholar]
- Weber, M. G. , and Flannigan M. D.. 1997. “Canadian Boreal Forest Ecosystem Structure and Function in a Changing Climate: Impact on Fire Regimes.” Environmental Reviews 5: 145–66. [Google Scholar]
- White, R. G. , Russell D. E., and Daniel C. J.. 2014. “Simulation of Maintenance, Growth and Reproduction of Caribou and Reindeer as Influenced by Ecological Aspects of Nutrition, Climate Change and Industrial Development Using an Energy‐Protein Model.” Rangifer 34: 1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data and code (ecpalm, 2021) are available in Zenodo at https://doi.org/10.5281/zenodo.5601986.


