Abstract
Different antioxidants including coenzyme Q10 (CoQ10) have been tried to treat idiopathic male infertility (IMI) with variable results. Therefore, this study aimed to determine the clinical and biochemical predictors of pregnancy outcome and time to pregnancy (TTP) in infertile men with idiopathic oligoasthenospermia (OA) pre‐ and post‐CoQ10 therapy. This prospective controlled clinical study included 178 male patients with idiopathic OA and 84 fertile men (controls). Patients received 200 mg of oral CoQ10 once daily for 6 months. Demographics, semen parameters, seminal CoQ10 levels, reactive oxygen species (ROS) levels, total antioxidant capacity (TAC), catalase (CAT), glutathione peroxidase (GPx), sperm DNA fragmentation (SDF) and body mass index were measured and compared at baseline and after 6 months. All participants were followed up for another 18 months for pregnancy outcome and TTP. CoQ10 therapy for 6 months significantly improved semen parameters, antioxidant measures and reduced SDF. The pregnancy rate was 24.2% and TTP was 20.52 ± 6.72 months in patients as compared to 95.2% and 5.73 ± 6.65 months in fertile controls. After CoQ10 therapy, CoQ10 level, sperm concentration, motility and ROS were independent predictors of pregnancy outcome and CoQ10 level, male age, sperm concentration, motility, ROS and GPx were independent predictors of TTP in patients. In conclusion, CoQ10 therapy of 6 months is a potential treatment for men with idiopathic OA. CoQ10 level, male age, semen parameters, ROS and GPx could potentially be used as diagnostic biomarkers for male fertility and predictors for pregnancy outcome and TTP in these patients.
Keywords: coenzyme Q10, idiopathic oligoasthenospermia, pregnancy, time to pregnancy
1. INTRODUCTION
Infertility is defined as the failure to achieve pregnancy after 12 months of regular unprotected sexual intercourse (Ko et al., 2014). It affects around 8%–15% of couples within the reproductive age globally, with half of these cases are associated with male factor. Male infertility could be attributed to varicocele, genital tract infections, congenital abnormalities, endocrine disorders and genetic, immunological and systemic diseases as well as environmental factors (Elsheikh et al., 2015). Oligoasthenospermia (OA) is defined as a reduction in sperm concentration below 15 million/ml and sperm progressive motility below 32% or total motility below 40% according to World Health Organization (WHO) 2010 5th criteria W. H. O, (2010).
Approximately 25% of infertility cases is of idiopathic origin (Punab et al., 2017). Potential mechanisms for idiopathic male infertility (IMI) and idiopathic OA include genetic, epigenetic, posttranslational modifications, sperm DNA fragmentation (SDF) and oxidative stress (OS)(Santi et al., 2018). A low level of reactive oxygen species (ROS) is necessary for several physiological processes, including sperm capacitation, hyperactivation, acrosomal reaction and fertilization (Gulcin, 2020; Gülçin et al., 2012). However, the overproduction of ROS causes an imbalance between oxidants and antioxidants leading to OS. Sperm cells are sensitive to OS due to the presence of unsaturated fatty acids which makes them prone to lipid peroxidation (Agarwal et al., 2006; Kose & Gulcin, 2021; Köse et al., 2015). Oxidative stress has been linked to reduced sperm membrane fluidity, motility, vitality, fertilization potential as well as high SDF (Kao et al., 2008; Nowicka‐Bauer & Nixon, 2020). Further, approximately 30%–80% of infertile men exhibits OS semen characteristics and, therefore, may serve as a potential biomarker of male fertility (Huang et al., 2018).
Another mechanism suggested for IMI is sperm DNA fragmentation (Selvam et al., 2020). Causes of SDF encompass extrinsic factors such as smoking, environmental toxins, radiation and chemotherapy as well as intrinsic factors such as defective germ cell maturation, leukocytes, abortive apoptosis and OS (Esteves et al., 2021). Elevated SDF has been associated with reduced sperm motility, recurrent abortions and reduced fertilization (Aktan et al., 2013; Alahmar et al., 2021). Additionally, SDF has been recently linked to increased incidence of genetic diseases, childhood malignancies and neurological disorders in offspring (Agarwal & Bui, 2017; Alahmar, 2019).
Seminal fluid is a major source of antioxidants that play key roles in protecting sperm from oxidative injury (Zini et al., 2009). The endogenous antioxidants include enzymatic antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S‐transferase (GST) and catalase (CAT), and non‐enzymatic antioxidants including urate, carnitine, glutathione, coenzyme Q10 (CoQ10) and vitamins C and E (Nakamura et al., 2010). Oral antioxidants have been tried to improve semen parameters, antioxidant capacity, SDF and fertility potential of men with IMI (Ahmadi et al., 2016). The treatment of men with unexplained idiopathic infertility, however, remains a challenge as different medications have been tried individually or in combination with inconsistent results (Alahmar, 2018; Majzoub & Agarwal, 2018). Some studies have reported that antioxidant therapy may be beneficial and improve several sperm parameters (Alahmar, Calogero, Singh, et al., 2021; Alahmar & Sengupta, 2021). Other studies, on the contrary, reported no improvements in semen parameters (Ahmadi et al., 2016; Alahmar, 2018). Further, there is a lack of consensus on the type, dosing, duration of treatment, target patient groups and the use of individual or combination antioxidants (Majzoub et al., 2017).
Coenzyme Q10 is a component of the mitochondrial respiratory chain with antioxidant properties that counteract lipid peroxidation and OS (Showell et al., 2014). In healthy males, seminal fluid CoQ10 concentrations positively correlate with sperm concentration and motility (Alahmar, Calogero, Singh, et al., 2021). We and others have reported improvement in sperm concentration and motility following CoQ10 therapy (Alahmar, 2019; Alahmar, Calogero, Sengupta, et al., 2021; Safarinejad, 2009). Further, our recent meta‐analysis (Vishvkarma et al., 2020) and another meta‐analysis (Lafuente et al., 2013) of three randomized controlled trials confirmed improvement of semen parameters but not improvement of pregnancy rates. Other studies, however, demonstrated no improvement in one or more of the seminal fluid parameters following CoQ10 therapy (Imamovic Kumalic & Pinter, 2014).
Many previous clinical studies on the effect of CoQ10 therapy in men with IMI had semen parameters improvement but not pregnancy as a primary endpoint. Further, the results of these studies were limited by a small number of participants, heterogeneity of the patients’ groups, a short period of follow‐up and the lack of exploration of the predictors of pregnancy outcomes (Lafuente et al., 2013; Safarinejad, 2009). Additionally, data on the impact of CoQ10 therapy on seminal antioxidant capacity, SDF and pregnancy outcomes are limited. Therefore, this study aimed to determine the clinical, antioxidant and other biochemical predictors of pregnancy outcome and time to pregnancy (TTP) in infertile men with idiopathic OA following 6 months of coenzyme Q10 therapy and another 18 months of follow‐up.
2. MATERIALS AND METHODS
2.1. Participants
In this prospective controlled clinical study, one hundred and seventy‐eight patients with idiopathic OA and 84 fertile men (controls) were recruited at the Fertility Clinic, Babyl, Iraq, from September 2018 to February 2019. Eight patients and five controls dropped out of the study and, therefore, were excluded. The participants underwent comprehensive fertility assessment by fertility specialists at the Fertility Clinic at baseline as well as during follow‐up visits. All patients received a daily dose of 200 mg of CoQ10 (as ubiquinol) (America Medic and Science AMS, WA, USA) as a single oral dose for 6 months (Balercia et al., 2009). The controls did not receive treatment and served as no treatment group. Clinical demographics, weight, height, body mass index (BMI), semen parameters, seminal CoQ10 level, ROS, TAC, GPx, CAT and SDF were measured compared at baseline and after 6 months. All participants were followed up for another 18 months for pregnancy outcome and TTP and follow‐up visits which were scheduled at 3‐month intervals. Sample size calculation was performed using 80% power and 5% level of significance and was 72 for each group. Study approval was obtained from the University of Sumer local research ethical committee (EC/2018/8866/8876/8878/8879).
2.2. Eligibility criteria
Patients had a history of infertility of at least one year in spite of regular unprotected intercourse and semen analysis shows OA. OA was defined according to the WHO 2010 (5th criteria) (W. H. O, 2010). Men with varicocele, genital infection, azoospermia, anatomical abnormalities, testicular injury or surgery, endocrine disease, renal, hepatic or other systemic illness, relevant medications, smoking, alcohol intake, recent antioxidant intake and the existence of female cause were excluded. Fertile controls enrolled in the study had a history of having had a child in the last 24 months, normal semen analysis, normal female fertility assessment and they were trying to get pregnant. All the participants provided informed consent before enrolment in the study.
2.3. Semen analysis
Semen samples were collected by masturbation following abstinence of 2–3 days. A special wide‐mouth container was used to collect semen, incubated at 37°C until semen was liquefied and then semen analysis was performed within an hour following the WHO manual criteria (5th edition, 2010) (W. H. O, 2010). Duplicate semen analyses were performed at baseline and after 6 months, and the average of the two values was used to analyse the results. The same investigator performed all semen analyses to optimize repeatability.
2.4. Measurement of seminal CoQ10 concentrations
Semin CoQ10 level was measured using high‐performance liquid chromatography (HPLC) using a UV detector at 275 nm and calculated using a published method (Li et al., 2006). Reversed‐phase HPLC with UV detection using coenzyme Q9 as the internal standard are utilized to obtain seminal CoQ10 level.
2.5. Seminal ROS measurement
Semen samples were centrifuged at 3000 rpm (1008 g) for 5 minutes to obtain seminal plasma and then were stored at −20°C. A manual method was used for ROS measurement as previously described by Venkatesh et.al. (Venkatesh et al., 2011). To 400 µl of liquefied neat semen, 10 µl of luminol (5‐amino‐2,3,‐dihydro‐1,4‐phthalazinedione; Sigma), prepared as 5 mM stock in dimethyl sulfoxide (DMSO), was added. Ten microlitres of 5 mM luminol in DMSO served as blank. Twenty‐five microlitres H2O2 with 10 µl luminol was used as a positive control. The luminol‐dependent chemiluminescence served as an indicator of ROS levels.
2.6. Measurement of seminal total antioxidant capacity (TAC), Glutathione peroxidase (GPx) and catalase (CAT) activity
TAC was estimated with a colorimetric method using the Total Antioxidant Capacity Assay Kit (#E‐BC‐K136, Elabscience, Texas, USA). Seminal plasma GPx activity was assessed using GPx Assay Kit (#E‐BC‐K096, Elabscience, Texas, USA), and seminal plasma CAT activity was assessed using CAT Assay Kit (#E‐BC‐K031, Elabscience, Texas, USA) using a colorimetric method and the protocol recommended by the manufacturer.
2.7. Sperm chromatin dispersion test
Sperm chromatin dispersion test was applied using the Halosperm kit (Halotech DNA, S.L. Madrid, Spain). The test principle is that sperm with SDF do not exhibit the halo of dispersed DNA loops that is observed in sperm without SDF, after denaturation of acid and removal of nuclear proteins. The nucleoids from spermatozoa with SDF show no or minimal dispersion halo. Bright‐field microscopy with Diff‐Quik staining was utilized to examine the halos. SDF, defined as the percentage ratio of sperm with SDF to total spermatozoa, was calculated using a previously published method (Alahmar et al., 2021; Zaazaa et al., 2018).
2.8. Statistical analysis
SPSS software (SPSS, v. 24, IBM, USA) was used for data analysis. Results were expressed as mean ± SD. Data normality was assessed using Shapiro–Wilk test and indicated a non‐normal distribution (p < 0.05). Wilcoxon signed‐rank test was used to compare pre‐ and post‐treatment values in patients and controls. Mann–Whitney U test was used to compare means for independent groups (patients and controls at baseline). Chi‐square test was used to compare proportions of family history, education and pregnancy outcome in patients and controls. Spearman's correlation coefficient was applied to find the relationships between seminal fluid parameters, antioxidant measures, CoQ10 level, SDF, age, BMI, pregnancy outcome and TTP in patients and controls. Univariate and multivariate logistic regressions were used to explore the predictors of pregnancy outcome in patients and controls (by estimating pre‐ and post‐values for each group). Univariate and multivariate Cox regression tests were used to perform survival analysis to estimate the predictors of TTP in patients and controls (by estimating pre‐ and post‐values for each group). Kaplan–Meier curve was used to examine the survival analysis between family history and education with TTP in patients and controls. P‐value lower than 0.05 was considered statistically significant.
3. RESULTS
3.1. CoQ10 therapy improved sperm parameters and antioxidant levels in infertile men as compared to baseline values with a pregnancy rate of 24.2%
Following 6 months of CoQ10 therapy, patients exhibited significant improvement in semen parameters and an increment in semen volume, concentration, total and progressive motility and normal morphology as compared to baseline (Table 1). The patients also demonstrated a significant increase in seminal antioxidant capacity and higher CoQ10 level, TAC, GPX, CAT, BMI and lower ROS and SDF after CoQ10 therapy. Family history was positive in 13.5%. The pregnancy rate was 24.2%, and TTP was 20.52 ± 6.72 months in the infertile patients’ group. The controls, on the contrary, demonstrated higher total motility, CoQ10 level, TAC, ROS, SDF and BMI and lower progressive motility, normal morphology, GPx and CAT after 6 months as compared to baseline. The improvement, however, was mild and levels remained within the normal range. Family history was positive in 4.8% in the control group. The pregnancy rate in controls was 95.2%, and TTP was 5.73 ± 6.65 months. As expected, infertile men had lower semen parameters, antioxidant capacity, pregnancy rate and higher SDF and TTP as compared to fertile controls.
TABLE 1.
Clinical, seminal and biochemical characteristics of patients and controls at baseline and after CoQ10 therapy
| Parameter |
Controls (No = 84) |
Patients (N = 178) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post |
Per cent change |
P value Pre vs post |
Pre | Post |
Per cent change |
P value Pre vs post |
P value patient vs controls baseline | |
|
Male age (year) |
31.69 ± 7.79 | 32.19 ± 7.7 |
1.58 |
0.001 | 29.46 ± 6.49 | 29.69 ± 6.4 | 0.78 | 0.06 | 0.06 |
|
Infertility duration (year) |
6.38 ± 3.55 | ||||||||
|
Volume (ml) |
3.00 ± .51 | 3.28 ± .060 |
9.33 |
0.058 | 2.88 ± 1.14 | 3.06 ± 0.28 | 6.25 | 0.001 | 0.37 |
|
Concentration (*106/ml) |
45.16 ± 21.13 | 47.61 ± 26.43 |
5.43 |
0.167 | 9.41 ± 3.53 | 9.70 ± 3.73 | 3.08 | 0.001 | 0.001 |
|
Progressive motility (%) |
46.88 ± 7.80 | 42.32 ± 7.07 |
−9.73 |
0.001 | 21.53 ± 6.89 | 28.84 ± 8.88 | 33.95 | 0.001 | 0.001 |
|
Total motility (%) |
65.66 ± 10.91 | 72.26 ± 12.01 |
10.05 |
0.001 | 29.03 ± 8.09 | 37.33 ± 11.41 | 28.59 | 0.001 | 0.001 |
|
Normal morphology (%) |
44.08 ± 7.47 | 39.64 ± 6.83 |
−10.07 |
0.001 | 36.98 ± 11.58 | 39.75 ± 6.72 | 7.49 | 0.047 | 0.001 |
|
CoQ10 level (ng/ml) |
60.62 ± 28.45 | 66.30 ± 31.48 |
9.37 |
0.000 | 44.28 ± 22.11 | 79.57 ± 17.46 | 79.70 | 0.001 | 0.001 |
|
ROS (×104 RLU/min/20million spermatozoa) |
0.10 ± 0.05 | 0.162 ± 0.018 |
62.00 |
0.001 | 4.62 ± 1.34 | 4.03 ± 1.16 | −12.77 | 0.001 | 0.001 |
|
TAC (mmol/L) |
1.80 ± 0.20 | 1.98 ± 0.22 |
10.00 |
0.001 | 0.89 ± 0.40 | 1.16 ± 0.45 | 30.34 | 0.001 | 0.001 |
|
GPx (U/ml) |
0.65 ± 0.07 | 0.58 ± 0.06 |
−10.77 |
0.001 | 0.23 ± 0.06 | 0.39 ± .044 | 69.57 | 0.001 | 0.001 |
|
CAT (U/ml) |
14.72 ± 2.40 | 13.22 ± 2.16 |
−10.19 |
0.001 | 10.25 ± 1.59 | 12.34 ± 1.69 | 20.39 | 0.001 | 0.001 |
|
SDF (%) |
15.38 ± 3.13 | 16.83 ± 3.63 |
9.43 |
0.001 | 34.78 ± 5.57 | 32.71 ± 7.19 | −5.95 | 0.001 | 0.001 |
|
Female age (year) |
25.54 ± 6.09 | 26.04 ± 6.09 |
1.96 |
0.001 | 23.36 ± 5.03 | 23.86 ± 5.03 | 2.14 |
0.01 |
0.002 |
|
BMI (Kg/m2) |
26.32 ± 5.43 | 27.63 ± 5.70 |
4.98 |
0.001 | 27.70 ± 4.82 | 29.92 ± 5.2 | 8.01 |
0.04 |
0.03 |
|
Family history Of male infertility |
Yes 4 (4.8%) No 80 (95.2%) |
24 (13.5%), 154 (86.5%) | 0.03 | 0.03 | |||||
| Education |
Primary 18 (21.4%) Secondary 45 (53.6%) Tertiary 21 (25.0%) |
Primary 127 (71.3%) Secondary 31 (17.4%) Tertiary 19 (10.7%) |
0.001 | 0.001 | |||||
|
Pregnancy (%) |
Yes 80 (95.2%) No 4 (4.8%) |
Yes 43 (24.2%) No 135 (75.8%) |
0.001 | 0.001 | |||||
|
TTP (month) |
5.73 ± 6.65 | 20.52 ± 6.72 | 0.001 | 0.001 | |||||
Abbreviations: Statistical tests: Wilcoxon signed‐rank test (for dependent samples), Mann–Whitney U (for independent samples), Chi‐square test to compare proportions.
ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; CoQ10, Coenzyme Q10; BMI, Body Mass Index; TTP: Time To Pregnancy.
3.2. Correlations between semen parameters, antioxidant measures, SDF and pregnancy outcome in patients and controls after 6 months
In patients, semen parameters (sperm concentration, progressive motility, total motility and normal morphology) correlated significantly with CoQ10 levels, antioxidant measures (ROS, TAC, GPx and CAT), SDF, BMI, female age, pregnancy rate and TTP after CoQ10 therapy (Table 2). Antioxidant measures correlated significantly with CoQ10 level, semen parameters, SDF, BMI and pregnancy measures. SDF correlated significantly with semen parameters, CoQ10 level, antioxidant measures, female age and pregnancy measures. Pregnancy rate and TTP correlated significantly with semen parameters, antioxidant measures, SDF and BMI. Controls, on the contrary, showed similar but weaker correlations between semen parameters and antioxidant measures, SDF, female age and BMI after 6 months of follow‐up. Many of the correlations between antioxidant measures and SDF, female age, BMI and pregnancy measures were not statistically significant (Table 3).
TABLE 2.
Correlations between semen parameters, antioxidants and time to pregnancy in patients post‐CoQ10 therapy
|
Male age |
Volume | Concentration |
Progressive motility |
Total motility |
Normal morphology |
CoQ10 level |
ROS | TAC | GPx | CAT | SDF |
Female age |
BMI | TTP | Pregnancy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
|
|
Male age |
NS |
−0.20 0.007 |
−0.42 0.000 |
−0.19 0.01 |
0.21 0.003 |
NS | NS | NS | NS |
−0.23 0.002 |
0.42 0.000 |
0.97 0.000 |
0.35 0.000 |
NS | NS | |
| Volume | NS | NS | NS | NS |
0.32 0.000 |
0.22 0.002 |
0.36 0.000 |
NS |
0.69 0.000 |
NS | NS | NS | NS | NS | NS | |
|
Sperm concentration |
−0.20 0.007 |
NS |
0.38 0.000 |
0.26 0.000 |
−0.41 0.000 |
0.20 0.006 |
0.16 0.02 |
0.35 0.000 |
NS |
0.60 0.000 |
−0.38 0.000 |
−0.20 0.007 |
−0.18 0.01 |
−0.65 0.000 |
−0.63 0.000 |
|
|
Progressive motility |
−0.42 0.000 |
NS |
0.38 0.000 |
0.44 0.000 |
NS |
0.44 0.000 |
0.42 0.000 |
0.41 0.000 |
NS |
0.61 0.000 |
−0.99 0.000 |
−0.39 0.000 |
−0.71 0.000 |
−0.35 0.000 |
−0.34 0.000 |
|
|
Total motility |
−0.19 0.01 |
NS |
0.26 0.000 |
0.44 0.000 |
NS |
0.46 0.000 |
0.28 0.000 |
0.47 0.000 |
NS |
0.46 0.000 |
−0.43 0.000 |
−0.16 0.03 |
−0.39 0.000 |
−0.42 0.000 |
−0.39 0.000 |
|
|
Normal morphology |
0.21 0.003 |
0.32 0.000 |
−0.41 0.000 |
NS | NS |
0.23 0.000 |
0.32 0.000 |
NS |
0.23 0.000 |
NS |
0.14 0.04 |
0.21 0.003 |
NS |
0.34 0.000 |
0.33 0.000 |
|
|
CoQ10 level |
NS |
0.22 0.002 |
0.20 0.006 |
0.44 0.000 |
0.46 0.000 |
0.23 0.000 |
0.73 0.000 |
0.61 0.000 |
0.38 0.000 |
0.52 0.000 |
−0.43 0.000 |
NS |
−0.32 0.000 |
NS | NS | |
| ROS | NS |
0.36 0.000 |
0.16 0.02 |
0.42 0.000 |
0.28 0.000 |
0.32 0.000 |
0.73 0.000 |
0.38 0.000 |
0.22 0.003 |
0.59 0.000 |
−0.41 0.000 |
NS |
−0.25 0.001 |
−0.17 0.02 |
0.17 0.02 |
|
| TAC | NS | NS |
0.35 0.000 |
0.41 0.000 |
0.47 0.000 |
NS |
0.61 0.000 |
0.38 0.000 |
0.17 0.01 |
0.66 0.000 |
−0.41 0.000 |
NS |
−0.46 0.000 |
−0.31 0.000 |
−0.28 0.000 |
|
| GPx | NS |
0.69 0.000 |
NS | NS | NS |
0.23 0.000 |
0.38 0.000 |
0.22 0.003 |
0.17 0.01 |
NS | NS | NS | NS | NS | NS | |
| CAT |
−0.23 0.002 |
NS |
0.60 0.000 |
0.61 0.000 |
0.46 0.000 |
NS |
0.52 0.000 |
0.59 0.000 |
0.66 0.000 |
NS |
−0.61 0.000 |
−0.21 0.004 |
−0.43 0.000 |
−0.49 0.000 |
−0.46 0.000 |
|
| SDF |
0.42 0.000 |
NS |
−0.38 0.000 |
−0.99 0.000 |
−0.43 0.000 |
0.14 0.04 |
−0.43 0.000 |
−0.41 0.000 |
−0.41 0.000 |
NS |
−0.61 0.000 |
0.39 0.000 |
0.72 0.000 |
0.35 0.000 |
0.35 0.000 |
|
|
Female age |
0.97 0.000 |
0.97 0.000 |
−0.20 0.007 |
−0.39 0.000 |
−0.16 0.03 |
0.21 0.003 |
NS | NS | NS | NS |
−0.21 0.004 |
0.39 0.000 |
0.32 0.000 |
NS | NS | |
| BMI |
0.35 0.000 |
NS |
−0.18 0.01 |
−0.71 0.000 |
−0.39 0.000 |
NS |
−0.32 0.000 |
−0.25 0.001 |
−0.46 0.000 |
NS |
−0.43 0.000 |
0.72 0.000 |
0.32 0.000 |
0.25 0.001 |
0.21 0.004 |
|
| TTP | NS | NS |
−0.65 0.000 |
−0.35 0.000 |
−0.42 0.000 |
0.34 0.000 |
NS |
−0.17 0.02 |
−0.31 0.000 |
−0.49 0.000 |
NS |
0.35 0.000 |
NS |
0.25 0.001 |
0.95 0.000 |
|
| Pregnancy | NS | NS |
−0.63 0.000 |
−0.34 0.000 |
−0.39 0.000 |
0.33 0.000 |
NS |
0.17 0.02 |
−0.28 0.000 |
NS |
−0.46 0.000 |
0.35 0.000 |
NS |
0.21 0.004 |
0.95 0.000 |
Abbreviations: r, Spearman correlation coefficient; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index; TTP, time to pregnancy; CoQ10, Coenzyme Q10; NS, non‐significant.
TABLE 3.
Correlations between semen parameters, antioxidants and time to pregnancy in controls post‐CoQ10 therapy
|
Male age |
Volume | Concentration |
Progressive motility |
Total motility |
Normal morphology |
CoQ10 level |
ROS | TAC | GPx | CAT | SDF |
Female age |
BMI | TTP | Pregnancy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
r P value |
|
|
Male age |
0.27 0.01 |
0.33 0.002 |
0.22 0.037 |
0.23 0.031 |
−0.38 0.000 |
0.25 0.01 |
NS | NS | NS |
0.24 0.02 |
NS |
0.99 0.000 |
−0.29 0.006 |
−.031 0.004 |
NS | |
| Volume |
0.27 0.01 |
NS | NS | NS | NS | NS |
0.25 0.02 |
0.25 0.01 |
0.23 0.03 |
NS | NS |
0.26 0.01 |
NS | NS | NS | |
|
Sperm concentration |
0.33 0.002 |
NS |
0.21 0.04 |
NS | NS | NS |
0.27 0.01 |
0.27 0.01 |
0.31 0.004 |
NS |
−0.25 0.02 |
0.32 0.003 |
−0.29 0.006 |
−.070 0.000 |
−0.34 0.001 |
|
|
Progressive motility |
0.22 0.03 |
NS |
0.21 0.04 |
0.98 0.000 |
NS |
0.90 0.000 |
0.79 0.000 |
0.79 0.000 |
0.72 0.000 |
0.88 0.000 |
−0.41 0.000 |
0.21 0.04 |
−0.86 0.000 |
NS | NS | |
|
Total motility |
0.23 0.031 |
NS | NS |
0.98 0.000 |
NS |
0.90 0.000 |
0.79 0.000 |
0.79 0.000 |
0.74 0.000 |
0.88 0.000 |
−0.31.004 |
0.22 0.04 |
−0.84 0.000 |
NS | NS | |
|
Normal morphology |
−0.38 0.000 |
NS | NS | NS | NS | NS |
0.21 0.04 |
0.22 0.04 |
NS | NS | NS |
−0.39 0.000 |
NS |
0.31 0.004 |
0.21 0.04 |
|
|
CoQ10 level |
0.25 0.019 |
NS | NS |
0.90 0.000 |
0.90 0.000 |
NS |
0.58 0.000 |
0.58 0.000 |
0.49 0.000 |
0.86 0.000 |
−0.59 0.000 |
0.25 | −0.85 | NS | NS | |
| ROS | NS |
0.25 0.02 |
0.27 0.01 |
0.79 0.000 |
0.79 0.000 |
0.21 0.04 |
0.58 0.000 |
0.99 0.000 |
0.98 0.000 |
0.74 0.000 |
NS | NS |
−0.62 0.000 |
NS | NS | |
| TAC | NS |
0.25 0.01 |
0.27 0.01 |
0.79 0.000 |
0.79 0.000 |
0.22 0.04 |
0.58 0.000 |
0.99 0.000 |
0.98 0.000 |
0.75 0.000 |
NS | NS |
−0.62 0.000 |
NS | NS | |
| GPx | NS |
0.23 0.03 |
0.31 0.004 |
0.72 0.000 |
0.74 0.000 |
NS |
0.49 0.000 |
0.98 0.000 |
0.98 0.000 |
0.67 0.000 |
NS | NS |
−0.54 0.000 |
NS | NS | |
| CAT |
0.24 0.02 |
NS | NS |
0.88 0.000 |
0.88 0.000 |
NS |
0.86 0.000 |
0.74 0.000 |
0.75 0.000 |
0.67 0.000 |
−.36 0.001 |
0.23 0.03 |
−0.78 0.000 |
NS | NS | |
| SDF | NS | NS |
−0.25 0.02 |
−0.41 0.000 |
−0.31 0.000 |
NS |
−0.59 0.000 |
NS | NS | NS |
−0.36 0.001 |
NS |
0.45 0.000 |
0.29 0.006 |
NS | |
|
Female age |
0.99 0.000 |
0.26 0.01 |
0.32 0.003 |
0.21 0.04 |
0.22 0.04 |
−0.39 0.000 |
0.25 0.02 |
NS | NS | NS |
0.23 0.03* |
NS |
−0.29 0.007 |
−0.29 0.006 |
NS | |
| BMI |
−0.29 0.006 |
NS |
−0.29 0.006 |
−0.86 0.000 |
−0.84 0.000 |
NS |
−0.85 0.000 |
−0.62 0.000 |
−0.62 0.000 |
−0.54 0.000 |
−0.78 0.000 |
0.45 0.000 |
−0.29 0.007 |
0.23 0.03 |
NS | |
| TTP |
−0.31 0.000 |
NS |
−0.70 0.000 |
NS | NS |
0.31 0.004 |
NS | NS | NS | NS | NS |
0.29 0.006 |
−0.29 0.006 |
0.23 0.03 |
0.37 0.000 |
|
| Pregnancy | NS | NS |
−0.34 0.001 |
NS | NS |
0.21 0.04 |
NS | NS | NS | NS | NS | NS | NS | NS |
0.37 0.000 |
Abbreviations: r, Spearman correlation coefficient; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index, TTP, time to pregnancy; CoQ10, Coenzyme Q10; NS, non‐significant.
3.3. Predictors of pregnancy outcome in patients and controls (pre and post)
Using univariate regression analysis, factors associated with pregnancy outcome in patients before CoQ10 therapy were sperm concentration, progressive and total motility, CoQ10 level, ROS, GPx, CAT, SDF, BMI and patient education (Table 4). After CoQ10 therapy, factors in patients were sperm concentration, progressive and total motility, normal morphology, TAC, CAT, SDF, BMI and patient education. In the multivariate logistic regression model, factors that independently predicted pregnancy outcome in patients before CoQ10 therapy were sperm progressive motility, CoQ10 level and patients’ education (Table 5). Post‐CoQ10 therapy, the independent factors in patients were CoQ10 level, sperm concentration, total motility and ROS. Univariate regression analysis for pregnancy outcome in controls at baseline and after 6 months showed that none of the variables of the study was associated with pregnancy outcome (Table 6). Using a multivariate regression model, factors that independently predicted pregnancy outcome in controls at baseline were male age and total motility. Following 6 months, none of the variables predicted pregnancy outcomes (Table 5).
TABLE 4.
Logistic regression analysis for predictors of pregnancy in patients
| Before CoQ10 therapy | After CoQ10 therapy | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male age | 0.95 | 0.9–1.01 | 0.1 | 0.95 | 0.9–1.01 | 0.1 |
| Infertility duration | 0.96 | 0.87–1.06 | 0.53 | 0.85 | 0.8–1.03 | 0.71 |
| Volume | 0.85 | 0.62–1.16 | 0.31 | 0.46 | 0.13–1.57 | 0.21 |
| Concentration | 1.15 | 1.04–1.27 | 0.006 | 1.75 | 1.47–2.08 | 0.001 |
| Progressive motility | 1.12 | 1.06–1.19 | 0.001 | 1.08 | 1.03–1.13 | 0.001 |
| Total motility | 1.09 | 1.05–1.15 | 0.001 | 1.1 | 1.05–1.14 | 0.001 |
| Normal morphology | 1.02 | 0.99–1.05 | 0.17 | 0.83 | 0.77–0.9 | 0.001 |
| CoQ10 level | 1.03 | 1.01–1.04 | 0.001 | 1.01 | 0.99–1.03 | 0.22 |
| ROS | 0.61 | 0.46–0.81 | 0.001 | 1.32 | 0.97–1.79 | 0.07 |
| TAC | 1.64 | 0.72–3.7 | 0.23 | 3.6 | 1.6–8.1 | 0.001 |
| GPx | 0.001 | 0.001–0.44 | 0.03 | 0.33 | 0.01–7.32 | 0.77 |
| CAT | 1.25 | 1.01–1.56 | 0.04 | 1.97 | 1.51–2.5 | 0.001 |
| SDF | 0.93 | 0.87–0.99 | 0.02 | 0.9 | 0.86–0.95 | 0.001 |
| Female age | 0.93 | 0.86–1.01 | 0.06 | 0.93 | 0.87–1.01 | 0.06 |
| BMI | 0.92 | 0.86–0.99 | 0.03 | 0.9 | 0.87–0.99 | 0.03 |
| Family history | 1.24 | 0.43–3.5 | 0.68 | 1.24 | 0.4–3.5 | 0.68 |
| Education | 3.3 | 2.01–5.5 | 0.001 | 3.3 | 2.01–5.5 | 0.001 |
Abbreviations: OR, odds ratio; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index; CoQ10, Coenzyme Q10.
TABLE 5.
Multivariate Logistic regression analysis for predictors of pregnancy in patients and controls
| Controls | Patients | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | OR | After 6 months | OR | Baseline | OR | After CoQ10 therapy | OR |
| Male age | 6.9* | Progressive motility | 1.77** | Sperm Concentration*** | 1.55 | ||
| Total motility | 0.88* | CoQ10 | 0.87** | Total motility** | 1.09 | ||
| Education | 3.9*** | CoQ10* | 0.93 | ||||
| ROS** | 2.7 | ||||||
Abbreviations: OR, odds ratio; ROS, reactive oxygen species; CoQ10, Coenzyme Q10.
p < 0.05
p < 0.01
p < 0.001.
TABLE 6.
Logistic regression analysis for predictors of pregnancy in controls
| Baseline | After 6 months | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male age | 1.02 | 0.89–1.17 | 0.7 | 1.02 | 0.89–1.17 | 0.7 |
| Volume | 0.49 | 0.06–3.6 | 0.49 | 2.1 | 0.37–12.1 | 0.38 |
| Concentration | 1.02 | 0.96–1.08 | 0.42 | 1.45 | 0.45–4.65 | 0.53 |
| Progressive motility | 0.92 | 0.8–1.05 | 0.23 | 0.91 | 0.78–1.05 | 0.21 |
| Total motility | 0.94 | 0.85–1.03 | 0.19 | 0.94 | 0.87–1.03 | 0.2 |
| Normal morphology | 1.02 | 0.87–1.14 | 0.98 | 0.85 | 0.72–1.02 | 0.08 |
| CoQ10 level | 0.97 | 0.93–1.01 | 0.16 | 0.97 | 0.94–1.01 | 0.15 |
| ROS | 1882.4 | 0.001–13700 | 0.17 | 0.68 | 0.01–1.15 | 0.69 |
| TAC | 2.6 | 0.01–397.9 | 0.7 | 2.4 | 0.02–2.8 | 0.6 |
| GPx | 82.5 | 0.001–26882 | 0.56 | 0.05 | 0.01–1.3 | 0.47 |
| CAT | 0.74 | 0.48–1.14 | 0.18 | 0.72 | 0.44–1.16 | 0.17 |
| SDF | 1.01 | 0.85–1.2 | 0.85 | 0.87 | 0.65–1.16 | 0.35 |
| Female Age | 1.01 | 0.85–1.2 | 0.85 | 1.01 | 0.85–1.2 | 0.85 |
| BMI | 1.1 | 0.91–1.34 | 0.29 | 1.1 | 0.9–1.3 | 0.29 |
| Family history | 0.001 | 0.001–1.1 | 0.99 | 0.1 | 0.05–1.1 | 0.9 |
| Education | 3.7 | 0.68–20.9 | 0.12 | 3.7 | 0.68–20.9 | 0.12 |
Abbreviations: OR, odds ratio; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index; CoQ10, Coenzyme Q10.
3.4. Predictors of time to pregnancy in patients and controls (pre and post)
Univariate Cox regression for TTP in patients before CoQ10 therapy demonstrated that factors associated with TTP were sperm concentration, progressive motility, total motility, CoQ10 level, ROS, GPx, CAT, SDF, BMI and patients’ education all in the condition before CoQ10 treatment (Table 7). Following CoQ10 therapy, factors that predicted TTP in patients were sperm concentration, progressive and total motility, CAT, SDF, BMI and patient education. Additionally, normal morphology and TAC were also associated with TTP. Using multivariate Cox regression, factors that independently predicted TTP in patients were age, sperm concentration, progressive motility, CoQ10 level and patient education (Table 8). After CoQ10 therapy, independent predictors of TTP were male age, sperm concentration, total motility, CoQ10 level, ROS and GPx. Kaplan–Meier curve for patients showed that patient education was associated with TTP (p < 0.001). Kaplan–Meier curve for family history of male infertility versus TTP in patients demonstrated no association between family history and TTP (P value = 0.67) (Figure 1‐A and 1‐C).
TABLE 7.
Univariate Cox regression analysis for predictors of time to pregnancy in patients
| Before CoQ10 therapy | After CoQ10 therapy | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Male age | 0.96 | 0.91–1.01 | 0.12 | 0.96 | 0.91–1.01 | 0.12 |
| Infertility duration | 0.97 | 0.89–1.06 | 0.59 | 0.88 | 0.81–1.01 | 0.64 |
| Volume | 0.9 | 0.68–1.18 | 0.45 | 0.47 | 0.16–1.38 | 0.17 |
| Concentration | 1.13 | 1.04–1.24 | 0.003 | 1.5 | 1.4–1.7 | 0.001 |
| Progressive motility | 1.11 | 1.06–1.17 | 0.001 | 1.07 | 1.03–1.12 | 0.001 |
| Total motility | 1.09 | 1.04–1.13 | 0.001 | 1.09 | 1.05–1.13 | 0.001 |
| Normal morphology | 1.02 | 0.99–1.04 | 0.16 | 0.84 | 0.79–0.9 | 0.001 |
| CoQ10 level | 1.03 | 1.01–1.04 | 0.001 | 1.01 | 0.99–1.02 | 0.15 |
| ROS | 0.63 | 0.49–0.8 | 0.001 | 1.3 | 0.99–1.7 | 0.05 |
| TAC | 1.69 | 0.83–3.41 | 0.14 | 3.4 | 1.7–6.8 | 0.001 |
| GPx | 0.0005 | 0.001–0.005 | 0.03 | 0.3 | 0.01–266.6 | 0.75 |
| CAT | 1.25 | 1.02–1.51 | 0.02 | 1.83 | 1.5–2.23 | 0.001 |
| SDF | 0.93 | 0.88–0.98 | 0.01 | 0.91 | 0.87–0.95 | 0.001 |
| Female age | 0.94 | 0.88–1.01 | 0.08 | 0.94 | 0.88–1.00 | 0.08 |
| BMI | 0.92 | 0.87–0.98 | 0.01 | 0.93 | 0.99–0.98 | 0.01 |
| Family history | 1.2 | 0.47–3.08 | 0.68 | 1.2 | 0.47–3.08 | 0.68 |
| Education | 2.5 | 1.7–3.5 | 0.001 | 2.51 | 1.7–3.5 | 0.001 |
Abbreviations: OR, odds ratio; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index; CoQ10, Coenzyme Q10.
TABLE 8.
Multivariate Cox regression analysis for predictors of time to pregnancy in patients and controls
| Controls | Patients | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | HR | After 6 months | HR | Baseline | HR | After CoQ10 therapy | HR |
| Normal morphology | 0.96* | Male age | 1.04* | Male age | 1.006* | Male age | 1.05* |
| ROS | 0.001* | Sperm Concentration | 0.66*** | Sperm Concentration | 1.13* | Sperm Concentration | 1.46*** |
| GPx | 44392** | Progressive motility | 1.03* | Progressive motility | 1.62*** |
Total motility |
1.06* |
| CAT | 0.59** | ROS | 1.833** | CoQ10 | 0.9** | CoQ10 | 0.94** |
| Education | 1.54* | GPx | 0.001* | Education | 3.14*** | ROS | 1.96** |
| GPx | 761837* | ||||||
Abbreviations: HR, hazard ratio; ROS, reactive oxygen species; GPx, glutathione peroxidase; CAT, catalase; CoQ10, Coenzyme Q10.
p < 0.05,
p < 0.01,
p < 0.001.
FIGURE 1.
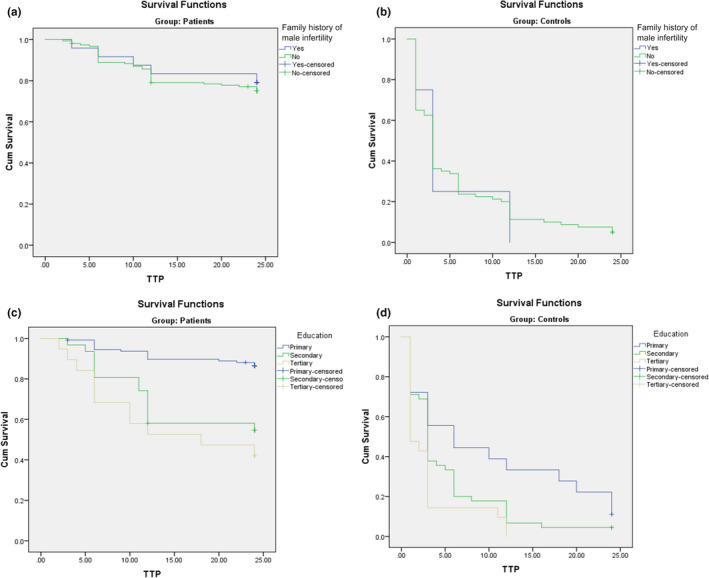
A. Kaplan–Meier curve for family history of male infertility versus time to pregnancy (TTP) in patients; B. Kaplan–Meier curve for family history of male infertility versus time to pregnancy (TTP) in controls; C. Kaplan–Meier curve for education versus time to pregnancy (TTP) in patients; D. Kaplan–Meier curve for education versus time to pregnancy (TTP) in controls
Univariate Cox regression in controls at baseline showed that male age and education were the only predictors of TTP in this group (Table 9). After 6 months, the predictors of TTP were male age, sperm concentration, normal morphology, SDF and education. Multivariate Cox regression in controls at baseline demonstrated that the independent predictors of TTP were sperm normal morphology, ROS, GPx, CAT and education (Table 8). After 6 months, the independent predictors in controls were male age, sperm concentration, progressive motility, ROS and GPx. Kaplan–Meier curve for controls demonstrated that education was associated with TTP (p < 0.01). Kaplan–Meier curve for family history of male infertility versus TTP in controls demonstrated no association between family history and TTP (P value = 0.88) (Figure 1‐B and 1‐D).
TABLE 9.
Univariate Cox regression analysis for predictors of time to pregnancy in controls
| Baseline | After 6 months | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Male age | 1.03 | 1.01–1.06 | 0.03 | 1.03 | 1.00–1.06 | 0.03 |
| Volume | 0.65 | 0.40–1.05 | 0.07 | 1.12 | 0.8–1.7 | 0.29 |
| Concentration | 1.01 | 0.99–1.01 | 0.12 | 1.03 | 1.02–1.04 | 0.001 |
| Progressive motility | 1.01 | 0.97–1.03 | 0.92 | 1.00 | 0.97–1.03 | 0.96 |
| Total motility | 1.002 | 0.98–1.02 | 0.97 | 1.00 | 0.98 | 0.9 |
| Normal morphology | 0.99 | 0.95–1.02 | 0.54 | 0.96 | 0.93–0.99 | 0.01 |
| CoQ10 level | 0.99 | 0.99–1.01 | 0.73 | 1.01 | 0.99–1.01 | 0.96 |
| ROS | 3.1 | 0.05–187.7 | 0.58 | 552.2 | 0.003–11319 | 0.31 |
| TAC | 1.96 | 0.66–5.7 | 0.22 | 1.7 | 0.62–4.6 | 0.29 |
| GPx | 10.9 | 0.42–282.7 | 0.15 | 10.4 | 0.25–425.9 | 0.21 |
| CAT | 0.98 | 0.89–1.07 | 0.71 | 0.99 | 0.89–1.09 | 0.85 |
| SDF | 0.92 | 0.85–1.01 | 0.06 | 0.93 | 0.87–0.99 | 0.04 |
| Female age | 1.04 | 1.01–1.08 | 0.053 | 1.04 | 1.00–1.08 | 0.05 |
| BMI | 0.98 | 0.94–1.02 | 0.47 | 0.98 | 0.95–1.02 | 0.47 |
| Family history | 0.94 | 0.34–2.5 | 0.9 | 0.94 | 0.34–2.5 | 0.9 |
| Education | 1.5 | 1.1–2.1 | 0.01 | 1.5 | 1.1–2.1 | 0.01 |
Abbreviations: HR, hazard ratio; ROS, reactive oxygen species; TAC, total antioxidant capacity; GPx, glutathione peroxidase; CAT, catalase; SDF, sperm DNA fragmentation; BMI, body mass index; CoQ10, Coenzyme Q10.
4. DISCUSSION
Men with IMI represent a real challenge in medical practice as the exact mechanisms underlying semen abnormalities are unknown. Further, several therapies have been tried to improve semen measures and men's fertility potential with variable results (Imamovic Kumalic & Pinter, 2014). The rationale for some of these therapeutics such as oral antioxidants including CoQ10 is based on the proposed association between IMI and OS and SDF and lower seminal antioxidant capacity in infertile men (Agarwal et al., 2019). Data on the predictors of pregnancy and time to pregnancy in men with idiopathic OA before and after receiving oral antioxidants are limited. To our knowledge, this study is the first study to explore these predictors in men with idiopathic OA before and after CoQ10 therapy.
Our study demonstrated a beneficial effect for CoQ10 therapy of 6 months on improving semen parameters and antioxidant capacity in men with idiopathic OA as compared to fertile controls.
The main improvement was in sperm concentration, motility, normal morphology, markers of antioxidant capacity and reduction in SDF following CoQ10 treatment. Our findings are consistent with previous studies which have reported similar improvement in men with IMI (Balercia et al., 2009; Safarinejad, 2009). In a randomized clinical trial on 228 men with idiopathic OA, treatment with CoQ10 (200 mg/day) was associated with improvement in semen parameters, and these parameters also correlated with antioxidant capacity (Safarinejad et al., 2012). Another clinical trial that involved treatment with CoQ10 (200 mg/day) for 3 months in men with idiopathic oligoasthenoteratospermia (OAT) reported an increment in sperm motility, CoQ10, CAT and SOD (Nadjarzadeh et al., 2014). Further, a randomized double‐blind placebo‐controlled study observed an increase in forward and total motility after 6 months of CoQ10 treatment (Balercia, 2004), which may suggest that a longer treatment regimen may be more effective in improving sperm parameters. Our previous studies have also demonstrated a beneficial effect for CoQ10 on sperm concentration, motility as well as antioxidant capacity(Alahmar, 2019; Alahmar, Calogero, et al., ; Alahmar & Sengupta, ). However, in one RCT in men with idiopathic OAT who received CoQ10 for 3 months, there was no improvement in semen parameters following CoQ10 therapy (Nadjarzadeh et al., 2011). The control group also showed mild improvement in semen parameters and antioxidant capacity and correlations between semen parameters and other study variables, but these correlations were weak correlations. The enhancement of semen parameters and antioxidant capacity observed in our study could be attributed to higher CoQ10 level, the antioxidant properties of CoQ10 and its role in mitochondrial chain reaction kinetic, higher levels of seminal antioxidant which counteract OS as well as the longer duration of treatment as compared to shorter periods in other studies.
We have also identified higher SDF in infertile patients and significant correlations between semen parameters and antioxidant measures, SDF, BMI and pregnancy outcomes. These correlations were stronger in patients with IMI as compared to fertile controls.
Our findings are consistent with previous studies which reported correlations between semen parameters and antioxidant measures such as CAT, TAC, GPx, ROS and seminal CoQ10 level (Alahmar & Sengupta, ; Nadjarzadeh et al., 2014). High SDF level has been observed in infertile men and correlated with CoQ10 level and semen parameters (Alahmar, Calogero, Sengupta, et al., 2021). These findings are supported by the observations of reduced antioxidant capacity and higher SDF levels among infertile men (Huang et al., 2018; Safarinejad, 2012). Further, CoQ10 therapy resulted in improvement in antioxidant capacity and reduced SDF levels (Alahmar, Calogero, Sengupta, et al., 2021; Kumar & Sharma, 2010). Obesity and high BMI also correlate with semen parameters, OS and high SDF levels among infertile men (Dubeux et al., 2016). Correlation of pregnancy rate with semen parameters and antioxidant capacity have been reported previously as well as an increase in pregnancy rate following antioxidant treatment in infertile men (Huang et al., 2018). A study reported that men with elevated seminal ROS levels have a sevenfold decrease in conception rates when compared to men having low ROS (Aitken et al., 1991). Male and female age, as well as BMI, may reduce semen parameters and clinical pregnancy rate (Dubeux et al., 2016). The correlations between semen parameters and antioxidant capacity and SDF may establish the foundation for the use of oral antioxidants including CoQ10 in the treatment of infertile men with IMI and idiopathic OA to enhance their pregnancy outcomes. Further, these measures could be also used as diagnostic biomarkers for male fertility and pregnancy outcome.
The pregnancy rate in patients in the current study was 24.2%, and TTP was 20.52 ± 6.72 months following 6 months of CoQ10 therapy and another 18 months of follow‐up. We have also identified many independent predictors for pregnancy and TTP.
Our results are consistent with the results of an uncontrolled study in men with idiopathic OAT treated with CoQ10 300 mg twice daily for 12 months that reported a pregnancy rate of 34.1% and time to pregnancy of 8.4 ± 4.7 months (Safarinejad, 2012). Another RCT in men with IMI reported a pregnancy rate of 10% in patients following CoQ10 therapy (200 mg/day) for 6 months and a period of follow‐up of 9 months (Balercia et al., 2009). In contrast, a systematic review and meta‐analysis that looked at several studies that supplemented infertile men with CoQ10 did not observe an increase in pregnancy rates (Lafuente et al., 2013). Although the findings of this meta‐analysis are in contrast to our study and others, the number of events included in the meta‐analysis is relatively small, and both live births and pregnancy rates were not the primary outcomes of the included trials. The high pregnancy rate in men with idiopathic OA after CoQ10 therapy could be attributed to improvement in semen parameters, antioxidant capacity and reduction in OS and SDF and, therefore, enhanced fertility potential in these patients.
In multivariate logistic regression, factors that independently predicted pregnancy in patients before and after CoQ10 therapy in our study were CoQ10 level and sperm motility. Additional factors that independently predicted pregnancy post‐CoQ10 therapy were sperm concentration and ROS. Our results are in agreement with previous studies which showed an association between sperm concentration, motility, normal morphology and pregnancy outcome (Aboutorabi et al., 2018; Jedrzejczak et al., 2008). Semen analysis and semen parameters, however, have limitations as WHO reference values of semen analysis were obtained from fertile couples, unequal distribution of population and inability to assess sperm function and fertilization (Agarwal et al., 2018). Therefore, additional biomarkers of sperm function and male fertility are essential. In our study, antioxidant measures also correlated and predicted pregnancy. Studies have reported lower levels of antioxidants in infertile men (Huang et al., 2018) as well as higher pregnancy rates following oral antioxidant therapy including CoQ10 (Ahmadi et al., 2016; Majzoub & Agarwal, 2018). Our previous studies have also demonstrated lower antioxidant measures and higher SDF in infertile men with idiopathic OA or OAT, and these abnormalities were ameliorated with CoQ10 therapy (Alahmar, Calogero, Sengupta, et al., 2021; Alahmar, Calogero, Singh, et al., 2021). High levels of SDF have been linked to IMI, abnormal semen parameters, pregnancy loss and poor fertilization (Arafa et al., 2020). Further, different cut‐off values from 4% to 56% have been proposed for SDF prediction of pregnancy in infertile men (Agarwal et al., 2020). Obesity and high BMI have been associated with IMI, poor semen parameters, OS and reduced fertilization and pregnancy rates (Palmer et al., 2012). Our findings suggest that CoQ10 level, sperm motility and ROS could be diagnostic biomarkers for male fertility as well as predictors of pregnancy outcome in men with idiopathic OA with CoQ10 therapy.
In multivariate Cox regression, factors that independently predicted TTP in patients before and after CoQ10 treatment were male age, sperm concentration, sperm motility and CoQ10 level. Additional factors that predicted TTP post‐therapy were sperm concentration, ROS and GPx. Our results are consistent with a follow‐up study on 501 couples that showed longer TTP and lower fecundability odds ratios (FORs) were associated with normal sperm morphology, male age and female BMI (Buck Louis et al., 2014). Further, a multicentre study demonstrated that sperm concentration, normal morphology and multiple anomalies index (MAI) can predict pregnancy and TTP among infertile couples (Slama et al., 2002). Elevated ROS can be associated with a sevenfold decrease in pregnancy rate (Aitken et al., 1991). High SDF levels among infertile men were associated with idiopathic infertility, recurrent IUI failure, recurrent pregnancy loss and IVF/ICSI outcomes (Cho & Agarwal, 2018). The association between obesity and high BMI with longer TTP could be attributed to abnormal semen parameters, OS, low testosterone/estradiol ratio and increased SDF among infertile men with obesity (Le et al., 2020). A study has also reported a link between CoQ10 intake and altered serum testosterone level which was attributed to the antioxidant properties of CoQ10 that protect against gonadal toxicity (Banihani, 2018). Our previous study, however, reported the lack of altered hormonal profile post‐CoQ10 therapy in men with IMI (Alahmar, Calogero, et al., ). A lower level of education was a significant factor in the occurrence of infertility in our patient group, which also correlated with pregnancy outcomes. A lower level of education has previously been linked to infertility in males (Moridi et al., 2019). The link between a low level of education and infertility could be attributed to lack of awareness about reproductive organs and fertilization physiology, factors that may cause infertility, early diagnosis and treatment and the available treatment options and health care facilities (Mahanta, 2016). Our results point out that male age, sperm concentration, motility, ROS and GPx could be used as diagnostic biomarkers as well as independent predictors of TTP in men with idiopathic OA with CoQ10 therapy. Therefore, our study has highlighted the possible role of CoQ10 in improving semen parameters, seminal antioxidant status and SDF in men with idiopathic OA. Potential predictors of pregnancy and time to pregnancy have also been suggested. Our observations are consistent with previous studies, which have reported similar results of antioxidants in men with IMI (Arafa et al., 2020; Balercia et al., 2009; Imamovic Kumalic & Pinter, 2014). Previous studies have also explored the link between dietary intake of CoQ10 and semen parameters and male fertility (Torres‐Arce et al., 2021; Vishvkarma et al., 2020). Another study, however, reported the lack of association between dietary sources of CoQ10 and semen measures among infertile men (Tiseo et al., 2017). Although dietary assessment of CoQ10 could be useful, we and others have not assessed it due to the subjective nature of dietary questionnaires with a potential recall bias and also it can be limited by the complex nature of multiple dietary micronutrients (Mirmiran et al., 2021).
Limitations of our study include a smaller number of controls in comparison with patients and the lack of placebo arm due to ethical considerations although we have used fertile controls as no treatment group. Another limitation is that the participants were recruited from one location, so our findings may not be generalized as there are genetic, racial and geographical variations in semen parameters; so further multicentre studies are warranted to consolidate the evidence provided in this study.
5. CONCLUSIONS
Our findings demonstrate that 6 months of CoQ10 therapy significantly increase CoQ10 levels in seminal plasma and improve semen parameters, antioxidant capacity and SDF with a pregnancy rate of 24.2% in men with idiopathic OA. CoQ10 level, male age, semen parameters, ROS and GPx could be used as diagnostic biomarkers for male fertility and predictors for pregnancy outcome and time to pregnancy in these men. Further, CoQ10 therapy for 6 months could be a potential therapy for men with idiopathic OA and may enhance their fertility and pregnancy outcomes.
6. Funding statement
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest associated with this study.
ETHICAL APPROVAL
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the local ethical committee at the University of Sumer, Iraq (EC/2018/8866/8876/8878/8879), and all participants consented for participation in the study.
Alahmar, A. T. , & Naemi, R. (2022). Predictors of pregnancy and time to pregnancy in infertile men with idiopathic oligoasthenospermia pre‐ and post‐coenzyme Q10 therapy. Andrologia, 54, e14385. 10.1111/and.14385
Correction added on 10 February 2022 after first online publication: Corrections have been made in this article.
DATA AVAILABILITY STATEMENT
Data will be available on request from the authors.
REFERENCES
- Aboutorabi, R. , Zamani, S. , Zarrin, Y. , & Mostafavi, F. S. (2018). A Survey on Main Semen Parameters in Natural Pregnancy and Intrauterine Insemination: Are There Any Significant Differences? American Journal of Men’s Health, 12(3), 617. 10.1177/1557988316647966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, A. , & Bui, A. D. (2017). Oxidation‐reduction potential as a new marker for oxidative stress: Correlation to male infertility. Investigative and Clinical Urology, 58(6), 385–399. 10.4111/icu.2017.58.6.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, A. , Majzoub, A. , Baskaran, S. , Panner Selvam, M. K. , Cho, C. L. , Henkel, R. , Finelli, R. , Leisegang, K. , Sengupta, P. , Barbarosie, C. , Parekh, N. , Alves, M. G. , Ko, E. , Arafa, M. , Tadros, N. , Ramasamy, R. , Kavoussi, P. , Ambar, R. , Kuchakulla, M. , … & Shah, R. (2020). Sperm DNA Fragmentation: A New Guideline for Clinicians. The World Journal of Men's Health, 38(4), 412. 10.5534/wjmh.200128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, A. , Parekh, N. , Selvam, M. K. P. , Henkel, R. , Shah, R. , Homa, S. T. , Ramasamy, R. , Ko, E. , Tremellen, K. , Esteves, S. , Majzoub, A. , Alvarez, J. G. , Gardner, D. K. , Jayasena, C. N. , Ramsay, J. W. , Cho, C. L. , Saleh, R. , Sakkas, D. , Hotaling, J. M. , … Harlev, A. (2019). Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. The World Journal of Men's Health, 37(3), 296–312. 10.5534/wjmh.190055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, A. , Prabakaran, S. , & Allamaneni, S. S. S. R. (2006). Relationship between oxidative stress, varicocele and infertility: a meta‐analysis. Reprod Biomed Online, 12(5), 630–633. 10.1016/S1472-6483(10)61190-X [DOI] [PubMed] [Google Scholar]
- Agarwal, A. , Qiu, E. , & Sharma, R. , (2018). Laboratory assessment of oxidative stress in semen. Arab Journal of Urology, 16(1), 77–86. 10.1016/J.AJU.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi, S. , Bashiri, R. , Ghadiri‐Anari, A. , & Nadjarzadeh, A. (2016). Antioxidant supplements and semen parameters: An evidence based review. International Journal of Reproductive BioMedicine, 14(12), 729–736. 10.29252/ijrm.14.12.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken, R. , Irvine, D. , & Wu, F. (1991). Prospective analysis of sperm‐oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. American Journal of Obstetrics and Gynecology, 164(2), 542–551. 10.1016/S0002-9378(11)80017-7 [DOI] [PubMed] [Google Scholar]
- Aktan, G. , Doğru‐Abbasoğlu, S. , Küçükgergin, C. , Kadıoğlu, A. , Özdemirler‐Erata, G. , & Koçak‐Toker, N. (2013). Mystery of idiopathic male infertility: is oxidative stress an actual risk? Fertility and Sterility, 99(5), 1211–1215. 10.1016/j.fertnstert.2012.11.045 [DOI] [PubMed] [Google Scholar]
- Alahmar, A. T. (2018). The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clinical and Experimental Reproductive Medicine, 45(2), 57–66. 10.5653/cerm.2018.45.2.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar, A. (2019). Role of oxidative stress in male infertility: An updated review. Journal of Human Reproductive Sciences, 12(1), 4–18. 10.4103/jhrs.JHRS_150_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar, A. T. (2019). The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clinical and Experimental Reproductive Medicine, 46(3), 112–118. 10.5653/cerm.2019.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar, A. , Calogero, A. E. , Sengupta, P. , & Dutta, S. (2021). Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. The World Journal of Men’s Health, 39(2), 346. 10.5534/wjmh.190145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar, A. , Calogero, A. E. , Singh, R. , Cannarella, R. , Sengupta, P. , & Dutta, S. (2021). Coenzyme Q10, oxidative stress, and male infertility: A review. Clinical and Experimental Reproductive Medicine, 48(2), 97–104. 10.5653/cerm.2020.04175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar, A. T. , & Sengupta, P. (2021). Impact of coenzyme Q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic infertility. Biological Trace Element Research, 199(4), 1246–1252. 10.1007/s12011-020-02251-3 [DOI] [PubMed] [Google Scholar]
- Alahmar, A. T. , Sengupta, P. , Dutta, S. , & Calogero, A. E. (2021). Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clinical and Experimental Reproductive Medicine, 48(2), 150–155. 10.5653/CERM.2020.04084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa, M. , Agarwal, A. , Majzoub, A. , Panner Selvam, M. K. , Baskaran, S. , Henkel, R. , & Elbardisi, H. (2020). Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants, 9(3), 219– 10.3390/antiox9030219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balercia, G. (2004). Coenzyme q10 supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertility and Sterility, 81(1), 93–98. 10.1016/j.fertnstert.2003.05.009 [DOI] [PubMed] [Google Scholar]
- Balercia, G. , Buldreghini, E. , Vignini, A. , Tiano, L. , Paggi, F. , Amoroso, S. , Ricciardo‐Lamonica, G. , Boscaro, M. , Lenzi, A. , & Littarru, G. (2009). Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo‐controlled, double‐blind randomized trial. Fertility and Sterility, 91(5), 1785–1792. 10.1016/j.fertnstert.2008.02.119 [DOI] [PubMed] [Google Scholar]
- Banihani, S. A. (2018). Effect of Coenzyme Q 10 Supplementation on Testosterone. Biomolecules, 8(4), 10.3390/BIOM8040172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis, G. M. , Sundaram, R. , Schisterman, E. F. , Sweeney, A. , Lynch, C. D. , Kim, S. , Maisog, J. M. , Gore‐Langton, R. , Eisenberg, M. L. , & Chen, Z. (2014). Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertility and Sterility, 101(2), 453–462. 10.1016/j.fertnstert.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, C.‐L. , & Agarwal, A. (2018). Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab Journal of Urology, 16(1), 21–34. 10.1016/j.aju.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubeux, V. T. , Renovato, T. , Esteves, A. C. , André, L. , de Oliveira, A. , & Penna, I. A. (2016). The impact of obesity on male fecundity: a Brazilian study. JBRA Assisted Reproduction, 20(3), 137–141. 10.5935/1518-0557.20160031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSheikh, M. G. , Hosny, M. B. , Elshenoufy, A. , Elghamrawi, H. , Fayad, A. , & Abdelrahman, S. (2015). Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: A prospective, randomized trial. Andrology, 3(5), 864–867. 10.1111/andr.12086 [DOI] [PubMed] [Google Scholar]
- Esteves, S. C. , Zini, A. , Coward, R. M. , Evenson, D. P. , Gosálvez, J. , Lewis, S. E. M. , Sharma, R. , & Humaidan, P. (2021). Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia, 53(2), e13874. 10.1111/and.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcin, İ. (2020). Antioxidants and antioxidant methods: an updated overview. Archives of Toxicology, 94(3), 651–715. 10.1007/S00204-020-02689-3 [DOI] [PubMed] [Google Scholar]
- Gülçin, I. , Elmastaş, M. , & Aboul‐Enein, H. Y. (2012). Antioxidant activity of clove oil – A powerful antioxidant source. Arabian Journal of Chemistry, 5(4), 489–499. 10.1016/J.ARABJC.2010.09.016 [DOI] [Google Scholar]
- Huang, C. , Cao, X. , Pang, D. , Li, C. , Luo, Q. , Zou, Y. , Feng, B. , Li, L. , Cheng, A. , & Chen, Z. (2018). Is male infertility associated with increased oxidative stress in seminal plasma? A‐meta analysis. Oncotarget, 9(36), 24494–24513. 10.18632/oncotarget.25075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamovic Kumalic, S. , & Pinter, B. (2014). Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. BioMed Research International, 2014, 10.1155/2014/426951. 426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejczak, P. , Taszarek‐Hauke, G. , Hauke, J. , Pawelczyk, L. , & Duleba, A. J. (2008). Prediction of spontaneous conception based on semen parameters. International Journal of Andrology, 31(5), 499–507. 10.1111/J.1365-2605.2007.00799.X [DOI] [PubMed] [Google Scholar]
- Kao, S.‐H. , Chao, H.‐T. , Chen, H.‐W. , Hwang, T. I. S. , Liao, T.‐L. , & Wei, Y.‐H. (2008). Increase of oxidative stress in human sperm with lower motility. Fertility and Sterility, 89(5), 1183–1190. 10.1016/j.fertnstert.2007.05.029 [DOI] [PubMed] [Google Scholar]
- Ko, E. Y. , Sabanegh, E. S. , & Agarwal, A. (2014). Male infertility testing: reactive oxygen species and antioxidant capacity. Fertility and Sterility, 102(6), 1518–1527. 10.1016/j.fertnstert.2014.10.020 [DOI] [PubMed] [Google Scholar]
- Kose, L. P. , & Gulcin, İ. (2021). Evaluation of the antioxidant and antiradical properties of some phyto and mammalian lignans. Molecules, 26(23), 7099. 10.3390/molecules26237099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köse, L. P. , Gülçin, I. , Gören, A. C. , Namiesnik, J. , Martinez‐Ayala, A. L. , & Gorinstein, S. (2015). LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Industrial Crops and Products, 74, 712–721. 10.1016/J.INDCROP.2015.05.034 [DOI] [Google Scholar]
- Kumar, A. , & Sharma, S. S. (2010). NF‐kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochemical and Biophysical Research Communications, 394(2), 360–365. 10.1016/j.bbrc.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Lafuente, R. , González‐Comadrán, M. , Solà, I. , López, G. , Brassesco, M. , Carreras, R. , & Checa, M. A. (2013). Coenzyme Q10 and male infertility: a meta‐analysis. Journal of Assisted Reproduction and Genetics, 30(9), 1147–1156. 10.1007/s10815-013-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, M. , Nguyen, D. , Le, D. , & Tran, N. (2020). Impact of body mass index and metabolic syndrome on sperm DNA fragmentation in males from infertile couples: A cross‐sectional study from Vietnam. Metabolism Open, 7, 10.1016/J.METOP.2020.100054. 100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Shi, Y. , Chen, S. , Li, W. , Shang, X. , & Huang, Y. (2006). Determination of coenzyme Q10 in human seminal plasma by high‐performance liquid chromatography and its clinical application. Biomedical Chromatography, 20(10), 1082–1086. 10.1002/bmc.645 [DOI] [PubMed] [Google Scholar]
- Mahanta, A. (2016). Impact of Education on Fertility: Evidence from a Tribal Society in Assam, India. International Journal of Population Research, 2016, 1–7. 10.1155/2016/3153685 [DOI] [Google Scholar]
- Majzoub, A. , & Agarwal, A. (2018). Systematic review of antioxidant types and doses in male infertility : Benefits on semen parameters, advanced sperm function, assisted reproduction and live‐birth rate. Arab Journal of Urology, 16(1), 113–124. 10.1016/j.aju.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub, A. , Agarwal, A. , & Esteves, S. C. (2017). Antioxidants for elevated sperm DNA fragmentation: a mini review. Translational Andrology and Urology, 6(S4), S649–S653. 10.21037/tau.2017.07.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran, P. , Bahadoran, Z. , & Gaeini, Z. (2021). Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. International Journal of Endocrinology and Metabolism, 19(3), 10.5812/IJEM.108170. 108170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moridi, A. , Roozbeh, N. , Yaghoobi, H. , Soltani, S. , Dashti, S. , Shahrahmani, N. , & Banaei, M. (2019). Etiology and risk factors associated with infertility. Int J Women’s Health Reprod Sci, 7(3), 346–353. 10.15296/ijwhr.2019.57 [DOI] [Google Scholar]
- Nadjarzadeh, A. , Sadeghi, M. R. , Amirjannati, N. , Vafa, M. R. , Motevalian, S. A. , Gohari, M. R. , Akhondi, M. A. , Yavari, P. , & Shidfar, F. (2011). Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: A randomized double‐blind, placebo controlled trial. Doi. Org, 34(8), e224–e228. 10.3275/7572 [DOI] [PubMed] [Google Scholar]
- Nadjarzadeh, A. , Shidfar, F. , Amirjannati, N. , Vafa, M. R. , Motevalian, S. A. , Gohari, M. R. , Nazeri Kakhki, S. A. , Akhondi, M. M. , & Sadeghi, M. R. (2014). Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double‐blind randomised clinical trial. Andrologia, 46(2), 177–183. 10.1111/and.12062 [DOI] [PubMed] [Google Scholar]
- Nakamura, B. N. , Lawson, G. , Chan, J. Y. , Banuelos, J. , Cortés, M. M. , Hoang, Y. D. , Ortiz, L. , Rau, B. A. , & Luderer, U. (2010). Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age‐dependent manner. Free Radical Biology and Medicine, 49(9), 1368–1379. 10.1016/j.freeradbiomed.2010.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka‐Bauer, K. , & Nixon, B. (2020). Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants, 9(2), 134. 10.3390/antiox9020134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, N. O. , Bakos, H. W. , Fullston, T. , & Lane, M. (2012). Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis, 2(4), 253. 10.4161/SPMG.21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punab, M. , Poolamets, O. , Paju, P. , Vihljajev, V. , Pomm, K. , Ladva, R. , Korrovits, P. , & Laan, M. (2017). Causes of male infertility: A 9‐year prospective monocentre study on 1737 patients with reduced total sperm counts. Human Reproduction, 32(1), 18–31. 10.1093/humrep/dew284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarinejad, M. R. (2009). Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. The Journal of Urology, 182(1), 237–248. 10.1016/j.juro.2009.02.121 [DOI] [PubMed] [Google Scholar]
- Safarinejad, M. R. (2012). The effect of coenzyme Q 10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: An open‐label prospective study. International Urology and Nephrology, 44(3), 689–700. 10.1007/s11255-011-0081-0 [DOI] [PubMed] [Google Scholar]
- Safarinejad, M.R. , Safarinejad, S. , & Shafiei, N. S. S. (2012). Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: A double‑blind, placebo‐controlled, randomized study. Journal of Urology, 188, 526‑531. [DOI] [PubMed] [Google Scholar]
- Santi, D. , Spaggiari, G. , & Simoni, M. (2018). Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management – meta‐analyses. Reproductive BioMedicine Online, 37(3), 315–326. 10.1016/j.rbmo.2018.06.023 [DOI] [PubMed] [Google Scholar]
- Selvam, M. K. P. , Sengupta, P. , & Agarwal, A. (2020). Sperm DNA fragmentation and male infertility. In Genetics of male infertility (pp. 155–172). Springer. [Google Scholar]
- Showell, M. G. , Mackenzie‐Proctor, R. , Brown, J. , Yazdani, A. , Stankiewicz, M. T. , & Hart, R. J. (2014). Antioxidants for male subfertility. Cochrane Database of Systematic Reviews, 10.1002/14651858.CD007411.pub3 [DOI] [PubMed] [Google Scholar]
- Slama, R. , Eustache, F. , Ducot, B. , Jensen, T. , Jørgensen, N. , Horte, A. , Irvine, S. , Suominen, J. , & Andersen, A. (2002). Time to pregnancy and semen parameters: a cross‐sectional study among fertile couples from four European cities. Human Reproduction, 17(2), 503–515. 10.1093/HUMREP/17.2.503 [DOI] [PubMed] [Google Scholar]
- Tiseo, B. C. , Gaskins, A. J. , Hauser, R. , Chavarro, J. E. , & Tanrikut, C. (2017). Coenzyme Q10 Intake From Food and Semen Parameters in a Subfertile Population. Urology, 102, 100–105. 10.1016/j.urology.2016.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Arce, E. , Vizmanos, B. , & Babio, N. (2021). Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology, 10(3),241. 10.3390/biology10030241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh, S. , Shams, M. , Dudeja, S. , Kumar, R. , & Dada, R. (2011). Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Archives of Gynecology and Obstetrics, 283, 121–126. 10.1007/s00404-010-1645-4 [DOI] [PubMed] [Google Scholar]
- Vishvkarma, R. , Alahmar, A. , Gupta, G. , & Rajender, S. (2020). Coenzyme Q10 effect on semen parameters: Profound or meagre? Andrologia, 52(6), e13570. 10.1111/and.13570 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2010). WHO laboratory manual for the examination and processing of human semen. 5th ed.
- Zaazaa, A. , Adel, A. , Fahmy, Y. , Elkhiat, Y. , Awaad, A. , & Mostafa, T. (2018). Effect of varicocelectomy and/or mast cells stabilizer on sperm DNA fragmentation in infertile patients with varicocele. Andrology, 6(1), 146–150. 10.1111/andr.12445 [DOI] [PubMed] [Google Scholar]
- Zini, A. , San Gabriel, M. , & Baazeem, A. (2009). Antioxidants and sperm DNA damage: a clinical perspective. Journal of Assisted Reproduction and Genetics, 26(8), 427–432. 10.1007/s10815-009-9343-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request from the authors.


