Abstract
Brodalumab, an interleukin‐17 receptor A antagonist, is approved for treatment of moderate‐to‐severe plaque psoriasis in adults without response or with loss of response to other systemic therapies. In the United States, there is a boxed warning for brodalumab regarding suicidal ideation and behavior; however, no causal relationship between brodalumab and suicidality was established during pivotal trials. In the 2‐year pharmacovigilance data, no completed suicides or suicide attempts were reported. The most frequent adverse event (AE) was arthralgia. The safety profile of brodalumab is now being updated after 3 years of pharmacovigilance data. Here, we outline pharmacovigilance data reported to Ortho Dermatologics by patients and healthcare professionals in the United States from August 15, 2017, to August 14, 2020. Brodalumab exposure estimates were obtained by calculating the time between first and last prescription‐dispensing authorization dates. Data from 1854 patients were collected, and brodalumab exposure was estimated to be 2736 patient‐years. The most frequent AE was arthralgia (111 events; 0.04 events per patient‐year). One episode of suicide attempt was reported in a patient with a history of depression. No completed suicides were reported. There were 81 serious infections reported, none of which were fungal. Over the 3‐year period, 30 malignancies occurred in 25 patients, none of which were determined to be related to brodalumab. Three‐year pharmacovigilance data are consistent with the safety profile of brodalumab previously reported in long‐term analyses of clinical trials and the 2‐year pharmacovigilance data.
Keywords: drug reaction, pharmacology, psoriasis, quality of life, therapy—systemic
1. INTRODUCTION
Brodalumab, an interleukin‐17 receptor A antagonist, is indicated for treatment of moderate‐to‐severe plaque psoriasis in adults with inadequate response or loss of response to prior systemic therapy. 1 The safety profile of brodalumab was characterized in one phase 2 and three phase 3 trials (AMAGINE‐1/‐2/‐3) and has also been documented in 1‐ and 2‐year pharmacovigilance analyses. 1 , 2 , 3 , 4 The U.S. package insert for brodalumab includes a boxed warning regarding suicidal ideation and behavior, but a causal relationship between brodalumab and suicidal behavior (i.e., attempted or completed suicide) has not been identified. 1 Notably, these pivotal trials did not screen or exclude patients with a history of depression, suicidal behavior, or substance use disorders. 5
During clinical trials, the most commonly reported adverse events (AEs) with 120 weeks of treatment were arthralgia, headaches, diarrhea, oropharyngeal pain, and Candida infections. 6 In the 2‐year pharmacovigilance report, the most frequent AEs were arthralgia, headaches, diarrhea, depression, and injection‐site reactions. 7 No completed suicides, suicide attempts, or serious fungal infections were reported in that analysis. Herein, an update based on 3‐year pharmacovigilance data is provided, building directly on the data presented in the 2‐year pharmacovigilance report. 7
2. METHODS
This analysis includes data on AEs—reported by patients and healthcare professionals (HCPs) through pharmacovigilance channels—that were circulated to Ortho Dermatologics between August 15, 2017, and August 14, 2020. The most common AEs listed in the brodalumab package insert (incidence ≥1%), including arthralgia, headaches, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection‐site reactions, influenza, neutropenia, and Tinea infections, are assessed here. 1 In addition, clinical events of special interest are assessed with descriptive statistics and as exposure‐adjusted rates per patient‐year (PY). For the case of suicide attempt, we include details from the MedWatch pharmacovigilance report to contextualize the event.
Drug exposure was determined by calculating the time between the dates of the first prescription‐dispensing authorization and the last prescription authorization. Patients with the same prescription‐dispensing authorization dates for the initial and last prescription were excluded from the analysis. Notably, these pharmacovigilance reports typically lack important contextual information on the patients, such as prior psoriatic medications, time duration between prior therapy and initiation of brodalumab, and other relevant aspects of medical history. 7
Because the postmarketing data presented here were noninterventional and not collected as part of a clinical study, ethics approval and informed consent were not necessary.
3. RESULTS
3.1. AEs listed in brodalumab package insert: Real‐world experience
Data were collected from 1854 patients in the United States, and brodalumab exposure was estimated as 2736 PYs. During the 3‐year period, 19.6% of AEs were reported by HCPs and 80.4% by patients/non‐HCPs. There were 111 reports of arthralgia (0.04 events/PY; Table 1). Of these 111 patients, 53 continued and 25 discontinued brodalumab treatment; 33 actions were unknown. Similar to data from the 2‐year analysis, arthralgia was most frequently reported for patients aged 40–59 years (n = 51), followed by patients aged 60–90 years (n = 29). Of the 111 patients with arthralgia, 13 also reported myalgia, nine reported fatigue, and 28 had relevant comorbidities (e.g., psoriatic arthritis, possible arthritis, prior joint pain, or other autoimmune conditions).
TABLE 1.
U.S. pharmacovigilance monitoring of brodalumab through 3 years (August 15, 2017, to August 14, 2020)
| AE | Event, n (r) a | Event drug related, n b | Discontinued, n (%) c | Maintained, n (%) c | Action unknown/NA, n (%) c |
|---|---|---|---|---|---|
| Arthralgia | 111 (0.04) | 1 | 7 (48) | 20 (23) | 18 (30) |
| Fatigue | 44 (0.02) | 1 | 7 (16) | 20 (45) | 17 (39) |
| Diarrhea | 32 (0.01) | 0 | 7 (22) | 19 (59) | 6 (19) |
| Injection‐site reaction | 35 (0.01) | 2 | 1 (3) | 18 (51) | 16 (46) |
| Headache | 43 (0.02) | 0 | 6 (14) | 24 (56) d | 13 (30) |
| Myalgia | 28 (0.01) | 0 | 6 (21) | 16 (57) e | 6 (21) |
| Nausea | 28 (0.01) | 0 | 5 (18) | 17 (61) | 6 (21) |
| Oropharyngeal pain | 20 (0.01) | 0 | 2 (10) | 10 (50) f | 10 (50) |
| Influenza | 22 (0.01) | 1 | 8 (36) | 7 (32) | 7 (32) |
| Neutropenia | 1 | 0 | 0 | 1 | 0 |
| Tinea infection | 0 | 0 | — | — | — |
Abbreviations: AE, adverse event; NA, not applicable; r, exposure‐adjusted rate per patient‐year.
Number of patients experiencing AE, not total number of AEs.
Relatedness to brodalumab was based on company‐determined causality.
Treatment action taken upon AE occurrence. Percentage is event divided by total number of patients experiencing event.
One patient had the drug placed on hold.
One patient increased brodalumab dose.
One patient temporarily stopped taking the drug but planned to resume brodalumab treatment.
The brodalumab package insert also lists neutropenia, injection‐site reactions, headaches, fatigue, influenza, diarrhea, oropharyngeal pain, nausea, myalgia, and Tinea infections as common AEs. In this 3‐year analysis, mild neutropenia was reported in one patient with alcohol abuse and a history of doxycycline use, both of which are known contributors to neutropenia. Of 35 patients with injection‐site reactions, 18 continued and 1 discontinued brodalumab treatment; 16 actions were unknown. Most patients with AEs of headache (n/N = 27/43) and fatigue (n/N = 26/44) were female; in both groups, six patients discontinued brodalumab. Nine patients with headache also experienced fatigue. More than one‐third of patients experiencing fatigue also reported potentially contributory comorbidities (including anemia, arthritis, or hypothyroidism [n/N = 16/44]), and 11 were taking other medications associated with fatigue (e.g., central nervous system depressants or antihistamines). Among 22 influenza cases, 12 occurred between the months of October and May, which are associated with flu season. 8 Nearly all of the influenza cases (n/N = 20/22) were reported by patients/non‐HCPs. Few AE reports included details on patients' medical histories or medication lists, thereby limiting our understanding of potential contributory factors.
3.2. Clinical events of special interest
Clinical events of special interest as deemed by the reporter or company include serious infections, fungal infections, Crohn's disease, malignancies, depression, and suicide (Figure 1). 1 Although 81 serious infections were reported (0.03 events/PY), only three were considered related to brodalumab (57 were considered possibly related, seven unlikely related, and 10 unrelated to brodalumab treatment). The causes of four infections could not be assessed. Among the 81 patients developing serious infections, 31 continued brodalumab treatment and 26 discontinued treatment; 24 actions were unknown. No serious infections were reported as fungal in nature, although four nonserious fungal infections (0.0015 events/PY) led to brodalumab discontinuation (two oral fungal infections [one oral candidiasis reported as “yeast infection of the mouth”], one fungal infection of the fingernail, and one unclassified fungal infection). Among five reported cases of oral candidiasis, three patients continued treatment and two actions were unknown. No new cases of Crohn's disease were reported since the 2‐year pharmacovigilance data were published. One case of Crohn's disease was previously diagnosed in a patient who exhibited symptoms before initiating brodalumab. 7
FIGURE 1.
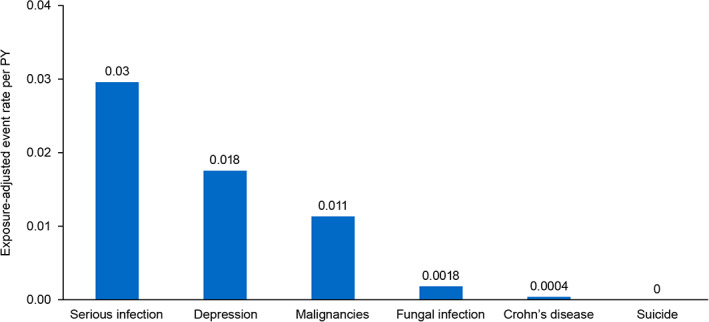
Exposure‐adjusted clinical events of special interest per PY. Exposure‐adjusted event rate per PY is the number of events per 2736 PYs of exposure. PY, patient‐year
Malignancy rates from a long‐term clinical trial study and 1‐ and 2‐year U.S. pharmacovigilance studies were 0.009, 0.01, and 0.008 events/PY, respectively. 7 During the 3‐year analysis, 30 cases of malignancy were reported and occurred in 25 patients (0.01 events/PY); these included 13 cases previously reported in the 2‐year analysis. Reported malignancies included ovarian, hepatic, lung, prostate, renal, and gallbladder cancers; one case of plasma cell myeloma; three other neoplasms (neck tumor, carcinoma removed from leg, and malignant tumor removed from arm); and other unspecified neoplasms. Dermatologic cancers included one keratoacanthoma‐type squamous cell carcinoma, five other squamous cell carcinomas, five basal cell carcinomas, and one malignant melanoma. None of the malignancies were determined to be related to brodalumab. Of the 25 patients with malignancies, nine continued brodalumab treatment, 12 discontinued treatment, and actions were unknown for four cases.
3.2.1. Depression and reported case of suicide attempt
There were 48 reports of patients experiencing depression, which, in four cases, was reported as related to brodalumab. No completed suicides were reported.
One patient case involving suicide attempt was reported by a physician (Figure 2). The patient received brodalumab 210 mg every 2 weeks for approximately 4 months; after a 2‐month interruption in therapy for unstated reasons, the patient resumed the previous treatment regimen. Approximately 3 months after restarting treatment (1 week after the most recent brodalumab dose), the patient reported a weeklong episode of depression and an attempt at self‐harm. The patient also mentioned previous depressive episodes in contexts of bereavement and substance use treatment. The encounter concluded with the physician recommending that the patient go to the emergency department for treatment. Later, the physician learned that the patient left the emergency department before being treated as the wait was too long and the patient felt better at that time. The physician then advised that the patient seek treatment with a psychiatrist or psychologist. Approximately 2 months later, the physician saw the patient again and learned that the patient had not yet seen a mental healthcare provider. During this encounter, the physician informed the patient that they would not resume brodalumab or any other treatment until the patient was seen by a psychiatrist or psychologist, and an initial report was received by Ortho Dermatologics, with a full follow‐up report received approximately 1 month later. The physician did not indicate any suspected causal relationship between brodalumab use and the patient's depressed mood or attempt at self‐harm.
FIGURE 2.
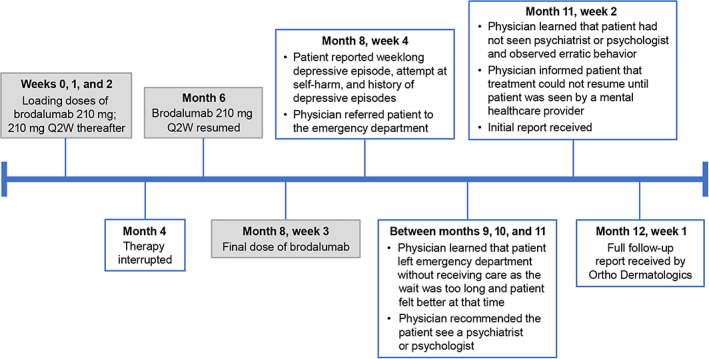
Timeline of events for patient with reported suicide attempt (2019–2020). Q2W, every 2 weeks
4. DISCUSSION
This analysis of 3‐year brodalumab pharmacovigilance data highlights the most frequent AEs listed in the package insert of brodalumab and clinical events of special interest reported from August 15, 2017, to August 14, 2020. The most frequently reported AE was arthralgia (111 reports), a finding consistent with data from clinical trials and 2‐year pharmacovigilance reports. Depression was documented in 48 patients; four of these cases were reported as related to brodalumab. One case of suicide attempt was reported in a patient with a history of depression, but no completed suicides were reported. Eighty‐one cases of serious infections were reported, none of which were fungal related. No trends could be concluded from the reported infections.
Previous analyses have documented the short‐term safety of brodalumab. In one study, brodalumab compared favorably to other biologics in terms of treatment discontinuation, although infection data were incomplete. 9 Another meta‐analysis reported that brodalumab carried a low risk of short‐term AEs versus other interleukin inhibitors. 10 The current report strengthens these analyses by documenting real‐world pharmacovigilance data through 3 years, including data related to serious infections and other clinical events of special interest. One case of Crohn's disease was documented during the 3‐year period, supporting the low risk found at 52 weeks in brodalumab clinical trials, during which one case was documented. 2 Although concern has arisen over case reports of inflammatory bowel disease following IL‐17‐inhibitor treatment, the data here are reassuring. 11 Overall, the 3‐year pharmacovigilance data support the long‐term safety of brodalumab in patients with psoriasis. Clinical events of special interest (such as malignancies) were uncommon and were largely determined to be unrelated to brodalumab.
Although a single episode involving suicide attempt occurred in a patient with a history of depression, evidence of a causal link between brodalumab and suicidality did not emerge. In the single case of suicide attempt, the physician did not report any suspected relationship between the patient's psychiatric symptoms and brodalumab treatment. The context of the patient's psychiatric symptoms, particularly the history of depressive episodes and the fact that symptoms occurred months after initiating brodalumab and continued months after stopping treatment, suggests that this event may not be primarily attributed to brodalumab.
There are limitations of this 3‐year report that should be considered. For instance, only AEs reported to Ortho Dermatologics were documented, and there are no comparison groups without brodalumab to aid interpretation of these findings. In addition, reported patient‐exposure estimates were based on prescription‐authorization dates because exact brodalumab administration dates were not available. Furthermore, data reported through pharmacovigilance channels often lack contextual information that would enhance interpretation of the relationship between the drug and AEs.
5. CONCLUSIONS
Selection of optimal therapy for patients with psoriasis is a complex decision, with the patient and HCP balancing patient‐specific factors with evidence of effectiveness and risk of AEs. 12 , 13 This 3‐year pharmacovigilance report supports the favorable safety profile of brodalumab established in previous analyses. The rate of serious infections was low, with no concerning trends. A suicide attempt was reported in a patient with a history of depression during the 3‐year period, but no completed suicides were reported. Overall, brodalumab was well tolerated, with no new safety concerns emerging after 3 years of pharmacovigilance monitoring.
CONFLICT OF INTEREST
ML is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB; and is a consultant for Aditum Bio, AnaptysBio, Almirall, Arcutis, Aristea, Arrive Technology, Avotres Therapeutics, BioMx, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Dr. Reddy, Evelo, Evommune, Facilitate International Dermatologic Education, Forte, Foundation for Research and Education in Dermatology, Helsinn, LEO Pharma, Meiji, Mindera, Pfizer, and Verrica. CL is or has been a consultant, investigator, or speaker for AbbVie, Actavis, Allergan, Amgen, Boehringer Ingelheim, Celgene, Cellceutix, Coherus, Corrona, Dermira, Eli Lilly, Galderma, Glenmark, Janssen, LEO Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Sienna, Stiefel, Sun Pharmaceutical, UCB, Vitae, and Wyeth. AA has served as a research investigator for and/or scientific advisor to AbbVie, BMS, Dermavant, Dermira, Incyte, Janssen, LEO Pharma, Lilly, Modmed, Novartis, Ortho Dermatologics (a division of Bausch Health US, LLC), Pfizer, Regeneron Pharmaceuticals, Sanofi, Sun Pharmaceutical, and UCB. NR and AJ are employees of Ortho Dermatologics (a division of Bausch Health US, LLC). FK is or has served as a research investigator, speaker, or member of an advisory board for AbbVie, Amgen, AstraZeneca, Brickell, Celgene, Dr. Reddy, Eli Lilly, Janssen, LEO Pharma, Menlo, Novartis, Ortho Dermatologics (a division of Bausch Health US, LLC), Pfizer, Regeneron, Sanofi, Sun Pharmaceutical, UCB, and XBiotech. BA and EG are employees of Bausch Health.
AUTHOR CONTRIBUTIONS
All authors meet International Committee of Medical Journal Editors criteria for authorship for this article. Mark Lebwohl has contributed to the conception of study. Mark Lebwohl has contributed to the design of study. Mark Lebwohl, Craig Leonardi, and Francisco Kerdel have contributed to the acquisition of data. Mark Lebwohl, Craig Leonardi, April Armstrong, Nicole Rawnsley, Binu Alexander, Earl Goehring, and Abby Jacobson have contributed to the analysis and interpretation of data. Nicole Rawnsley, Binu Alexander, and Abby Jacobson have contributed to drafting the manuscript. Mark Lebwohl, Craig Leonardi, April Armstrong, Nicole Rawnsley, Earl Goehring, Francisco Kerdel, and Abby Jacobson have contributed to critically revising the manuscript for important intellectual content. All authors have given final approval of the manuscript for publication and agree to be accountable for all aspects of the work.
ETHICS STATEMENT
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
ACKNOWLEDGMENTS
This study was sponsored by Ortho Dermatologics. Medical writing and editorial support were provided under the direction of the authors by Samantha Agron, MD, Rebecca Slager, PhD, Sherri Damlo, ELS, and Jenna Lewis, MA, ELS, of MedThink SciCom, and were funded by Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
Lebwohl M, Leonardi C, Armstrong A, et al. Three‐year U.S. pharmacovigilance report of brodalumab . Dermatologic Therapy. 2021;34(6):e15105. doi: 10.1111/dth.15105
Funding information Ortho Dermatologics
DATA AVAILABILITY STATEMENT
The data sets generated or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Siliq [package insert]. Bridgewater, NJ: Bausch Health US, LLC; 2017.
- 2. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318‐1328. [DOI] [PubMed] [Google Scholar]
- 3. Papp K, Menter A, Leonardi C, et al. Long‐term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE‐1). Br J Dermatol. 2020;183(6):1037‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2016;175(2):273‐286. [DOI] [PubMed] [Google Scholar]
- 5. Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81.e85‐89.e85. [DOI] [PubMed] [Google Scholar]
- 6. Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long‐term efficacy and safety of brodalumab in the treatment of psoriasis: 120‐week results from the randomized, double‐blind, placebo‐ and active comparator‐controlled phase 3 AMAGINE‐2 trial. J Am Acad Dermatol. 2020;82(2):352‐359. [DOI] [PubMed] [Google Scholar]
- 7. Lebwohl M, Leonardi C, Wu JJ, et al. Two‐year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11(1):173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The flu season. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/about/season/flu-season.htm. Updated May 6, 2021. Accessed June 25, 2021.
- 9. Xu G, Xia M, Jiang C, et al. Comparative efficacy and safety of thirteen biologic therapies for patients with moderate or severe psoriasis: a network meta‐analysis. J Pharmacol Sci. 2019;139(4):289‐303. [DOI] [PubMed] [Google Scholar]
- 10. Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short‐term efficacy and safety of IL‐17, IL‐12/23, and IL‐23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta‐analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fieldhouse KA, Ukaibe S, Crowley EL, Khanna R, O'Toole A, Gooderham MJ. Inflammatory bowel disease in patients with psoriasis treated with interleukin‐17 inhibitors. Drugs Context. 2020;9:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 13. Amin M, No DJ, Egeberg A, Wu JJ. Choosing first‐line biologic treatment for moderate‐to‐severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19(1):1‐13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated or analyzed during the current study are available from the corresponding author on reasonable request.


