Abstract
Rationale
Dental calculus (mineralised dental plaque) is composed primarily of hydroxyapatite. We hypothesise that the carbonate component of dental calculus will reflect the isotopic composition of ingested simple carbohydrates. Therefore, dental calculus carbonates may be an indicator for sugar consumption, and an alternative to bone carbonate in isotopic palaeodiet studies.
Methods
We utilised Fourier transform infrared attenuated total reflectance analysis to characterise the composition and crystallisation of bone and dental calculus before isotope analysis of carbonate. Using a Sercon 20‐22 mass spectrometer coupled with a Sercon GSL sample preparation system and an IsoPrime 100 dual inlet mass spectrometer plus Multiprep device to measure carbon, we tested the potential of dental calculus carbonate to identify C4 resources in diet through analysis of δ13C values in paired bone, calculus and teeth mineral samples.
Results
The modern population shows higher δ13C values in all three tissue carbonates compared to both archaeological populations. Clear differences in dental calculus δ13C values are observed between the modern and archaeological individuals suggesting potential for utilising dental calculus in isotope palaeodiet studies. The offset between dental calculus and either bone or enamel carbonate δ13C values is large and consistent in direction, with no consistent offset between the δ13C values for the three tissues per individual.
Conclusions
Our results support dental calculus carbonate as a new biomaterial to identify C4 sugar through isotope analysis. Greater carbon fractionation in the mouth is likely due to the complex formation of dental calculus as a mineralized biofilm, which results in consistently high δ13C values compared to bone and enamel.
1. INTRODUCTION
Investigations into past diets frequently draw upon direct isotopic measurements of the surviving body tissues of consumers. Bone and teeth most commonly survive in archaeological contexts but soft tissues (e.g. skin), hair and fingernails can also provide dietary information if they are preserved. Dental calculus (mineralised dental plaque) has recently received attention for its potential to reveal aspects of past diets, primarily through the analysis of biomolecules – proteins, DNA 1 , 2 – and micro‐debris trapped within the mineral matrix. 3 Only a few studies have explored the potential for isotopic analysis of dental calculus but they have mainly analysed bulk carbon (δ13C) and nitrogen (δ15N) isotope compositions of the organic matrix with mixed results. 4 , 5 , 6 , 7 , 8 One recent study specifically targeted the inorganic fraction of calculus, suggesting that this component may have more validity as a palaeodiet proxy; however, the study was limited in terms of the methodology as well as the small number of samples analysed. 8 The present study goes further to explicitly explore the potential utility of δ13C in dental calculus mineral as a dietary proxy for identifying C4 sugar (maize/cane). Building on previous studies applying Fourier transform infrared (FTIR) spectroscopy to characterise dental calculus composition, 9 , 10 we apply this analysis to systematically characterise the composition and crystallisation of dental calculus. This study presents the first analysis and interpretation of dental calculus FTIR results as an assessment of diagenesis in line with what has been previously undertaken for bone and enamel carbonate. It goes on to present the first comparison of dental calculus carbonate with both bone and enamel carbonate to determine whether dental calculus carbonate is a suitable substrate for identifying C4 resource consumption. In addition, the results obtained here are used to determine if there is any intra‐individual variation among tissues. This analysis is notable for analysing material from archaeological populations as well as modern individuals, with the latter rarely incorporated into archaeological studies.
The current understanding of diet in palaeodietary studies utilising stable isotopes (δ13C, δ15N) is mainly based on the analysis of bone and dentine collagen (δ13C, δ15N) as well as enamel and bone carbonate (δ13C). 11 , 12 Bone and dentine collagen δ13C values represent the protein sources in the diet, but a proportion of collagen can be synthesised from lipids or carbohydrates. Bone and enamel carbonate δ13C values, however, reflect whole diets including carbohydrates, lipids and proteins. 13 Palaeodietary reconstruction using carbon isotope values is useful in distinguishing between the consumption of C3 (low δ13C value) and C4 (high δ13C value) terrestrial resources as well as between marine (high δ13C value) and terrestrial (low δ13C value) foods. Nitrogen isotope values provide information on the main protein sources of diet, for example, differentiating between plant‐rich protein and animal‐rich protein diets. 14
Previously, C4 cane sugar consumption has only been detected under specific circumstances, such as when sugarcane is an indigenous crop 15 or inferred where sugarcane may have been used as animal fodder, in which case the C4 signal is acquired by herbivores. 16 Neither maize nor cane sugar would have been available to the medieval populations considered here. 17 The post‐medieval period between the 17th and 19th centuries, however, saw an increase in the consumption of cane sugar in England due to Britain's colonisation of the West Indies in the 17th century. 18 , 19 Although maize was available in post‐medieval England, historical sources indicate that it was considered to be suitable for animal fodder or famine relief food, particularly for the Irish poor. 20 , 21 However, a recent analysis of dental calculus micro‐debris of 36 individuals from the middle‐class Cross Street population revealed that two individuals had the presence of maize (not included in this study). 3 Maize is therefore another possible source of C4 carbohydrate for the post‐medieval population; however, sugar will have been more ubiquitous. Although hypothesised in some bone collagen isotope studies of post‐medieval populations in England, 22 , 23 , 24 , 25 the consumption of cane sugar has been hard to detect despite it being a key element of the dietary staple. In this study, it is hypothesised that dental calculus carbonate could offer a novel and more sensitive way of identifying cane sugar/maize than the traditional isotopic methods focusing on bone.
1.1. Dental calculus and potential for isotopic analysis
Calculus is frequently preserved in archaeological contexts and has been found to survive on the teeth of late Pliocene hominins 26 and Miocene apes dating up to 8 to 12 million years ago. 27 Dental calculus results from the calcification of plaque biofilms that accumulate and mineralise during life; however, the mechanism and rate by which dental calculus forms are still not completely understood. 28 Of the two types of dental calculus, supragingival and subgingival, the present study attempted to target supragingival dental calculus for all analyses.
Plaque formation on the supragingival surface of teeth begins when microorganisms, overwhelmingly bacteria, colonise the pellicle on the tooth surface. These bacteria obtain their nutrients primarily from the amino acids, proteins, glycoproteins and peptides from saliva to grow, confluence and produce a biofilm. 29 During the production of the biofilm, extracellular polymer synthesis occurs resulting in glucans and fructans from sucrose (refined carbohydrate) metabolism becoming part of the plaque matrix. 29 , 30 Plaque may begin to harden after about ten days to form dental calculus which then builds up over time. Depending on an individual's hygiene, diet and lifestyle, the deposition of plaque begins soon after tooth eruption, and the quantity increases over time, ceasing at death when the production of saliva stops. Mineralisation of dental plaque only occurs in the presence of saliva when an individual is alive. 31 , 32 Calculus formation is facilitated by alkaline conditions in the mouth which in turn increases the precipitation of minerals from the saliva. 33 Mineralisation also depends on the food being consumed, salivary flow, oral hygiene and the genetics of the individual. 34 The mineral composition of calculus varies according to the concentration of calcium and phosphorus, the presence of calcification promoters such as urea, fluoride and silicon 35 and presumably also by the bicarbonate composition in the saliva – itself a function of the rate of carbohydrate metabolism. 30
Dental calculus is composed of about 20% organic and 80% inorganic constituents. 29 Calculus deposits generally contain the inorganic mineral calcium phosphate: crystalline forms of hydroxyapatite, octacalcium phosphate and whitlockite in varying quantities, with hydroxyapatite usually being the most abundant (ca 58%), which has high levels of carbonate. 36 The organic matrix contains trapped proteins, glycoproteins, plant fibres, lipids and carbohydrates. 29 Unlike the carbonate component of bone and enamel, which derives from blood bicarbonate, 37 supragingival calculus derives its carbonate from the precipitated bicarbonate of salivary fluids. 38 Experiments have indicated that salivary fluids derive bicarbonate from two sources in the body: (i) transfer from the blood and (ii) bicarbonate that is produced in the cells of the salivary glands. 39 The salivary gland bicarbonate is from carbon dioxide (CO2) resulting from the secretory activity of the salivary gland cell (based on the equilibrium CO2 + H2O ⇌ HCO3 − + H+) during the action of microbes when metabolising carbohydrates in the mouth. The concentration of bicarbonate is greatly increased during food intake and mastication. 30 , 40
As previously mentioned, dental calculus formation is, in part, linked to the consumption of high levels of carbohydrates due to sugars that are eventually converted to CO2. 29 , 32 Similarly, consumption of carbohydrate‐containing foods has been associated with the formation of dental caries. It has been determined that dental caries develops due to a dissolution of the enamel by the action of acids that are produced in the mouth by the fermentation of dietary carbohydrates by oral bacteria, particularly Streptococcus mutans. 41 As is evident by the link between sugar consumption and dental caries, oral microbial communities preferentially metabolise the most bioavailable components of dietary carbohydrate. 42 , 43 Therefore, it is likely that 13C‐enriched CO2 generated from microbial carbohydrate metabolism in the mouth and from the blood via the salivary glands is incorporated into calculus carbonate. The hypothesis, therefore, is that calculus carbonate will be more responsive than bone apatite to the consumption of C4 cane sugar/maize in post‐medieval England, which has been difficult to identify using other isotope methods.
To fully assess the potential for dental calculus in palaeodietary studies, and particularly for the possibility of determining C4 cane sugar/maize consumption for post‐medieval England, isotope analysis on dental calculus, bone and enamel obtained from the same individuals was conducted in a broad range of populations.
2. MATERIALS AND METHODS
2.1. Materials
Bone, enamel and dental calculus samples were collected from 57 individuals for bone collagen, bone carbonate, enamel carbonate and calculus carbonate analysis. The three different populations sampled for this study were as follows: (i) 22 individuals from medieval burial populations from England, including Southwell Cemetery, Nottinghamshire (SCN), St Peter's Cemetery, Leicester (SPL) and Nun's Field, Chester (NFC) dating between the 7th and 15th centuries CE, (ii) 15 post‐medieval individuals buried in the Cross Street Chapel cemetery, Manchester (CSM), England, dating to the 18th–19th centuries CE and (iii) 20 modern individuals (FAC), who died between 1996 and 2016, from the William M. Bass Donated Skeletal Collection housed in the Department of Anthropology, University of Tennessee, USA. Additional information on the ethical considerations and treatment conditions for the modern materials can be found in the supporting information.
For medieval and post‐medieval individuals, the first priority was to sample individuals with dental calculus. Ribs were preferentially sampled; however, where ribs were not available, long bones were sampled. In the case of Southwell and St Peter's Cemetery, only mandibles were available to sample. For the modern individuals, the majority of samples were pedal phalanges, two were 12th ribs and one was a hand phalanx. These samples were selected for two reasons. First, these particular elements are numerous and relatively non‐diagnostic compared to other elements, and therefore preferable for destructive analysis in order to preserve the majority of the skeleton for future research. Second, in a large study testing all the skeletal elements from numerous individuals specifically from the Bass Collection, 44 these elements consistently yielded full STR profiles, indicating good biomolecular preservation.
Previous isotope analysis has revealed that medieval populations consumed C3 terrestrial resources. 45 Similarly, diet for post‐medieval individuals has been found to have been dominated by C3 terrestrial resources, although C4 cane sugar/maize is known to have been part of their diet or available. 25 , 46 Finally, the modern population represents North American individuals, who almost certainly consumed an abundance of C4 sugar and maize. Currently, the intake of sugar refined from C4 plants such as corn and cane syrup makes up to 78% of the sugar consumed in the United States and forms around 16% of the total calories consumed; however, in some cases it exceeds 35%. 47 The modern group, therefore, provides a control for diets rich in C4 sources.
2.2. Methods
2.2.1. FTIR with attenuated total reflectance (ATR)
Sample preparation and analysis of 57 bone and 52 dental calculus samples were executed according to the Kontopoulos et al 48 method. We carried out FTIR‐ATR analysis before acetic acid treatment. The use of ATR during FTIR analysis has the advantage of being generally insensitive to sample thickness. 49 Bones were cleaned prior to analysis using a sterile scalpel blade and dental calculus samples were rinsed with deionised water to remove dirt and contaminating material. Once cleaned, the samples were ground using an agate mortar and pestle. The powdered samples were then sieved through Endecotts woven stainless steel mesh sieves with an aperture size of 20 and 50 μm so that only grains between 20 and 50 μm particle size would be collected. Spectral analyses were performed using OPUS software (Bruker). Spectra were collected in 144 scans, in the 400–4000 cm−1 wavenumber range, with a spectral resolution of 4 cm−1 and zero‐filling factor of 4. Each sample was measured in triplicate and ca 2–3 mg of powder was pressed onto the diamond crystal and measured. We cleaned the instrument's crystal and arm's tip with tissue paper soaked in propanol before each measurement. Baseline correction and spectra normalisation were carried out using OPUS software as reported in Kontopoulos et al. 48 We followed the established bone FTIR analysis method for dental calculus samples as there is no agreed standard for the latter yet. We used two quality parameters, the infrared splitting factor (IRSF) and the carbonate‐to‐phosphate ratios (C/P), to assess our samples. IRSF is used to evaluate the crystallinity (structural order) within the mineral component of the bone while the C/P ratio is a measure of diagenesis that reflects the changes to the carbonate in bioapatite crystals relative to the phosphate content ratio in a bone sample. We calculated IRSF indices following the method of Weiner and Bar‐Yosef 50 that measures the heights of the double peaks of the phosphate antisymmetric bending frequency between 550 and 650 cm−1, 51 divided by the trough between them:
The C/P ratio was estimated using the method of Wright and Schwarcz 52 by dividing the main v 3 carbonate peak height with the main v 3 phosphate vibrational band:
For bone, the peaks were at 565 cm−1 (v 4PO4 3−), 605 cm−1 (v 4PO4 3−), 1035 cm−1 (v 3PO4 3−) and 1035 cm−1 (v 3CO3 2−). Therefore, IRSF and C/P ratio were calculated as follows:
For dental calculus, the absorption peaks used followed the measurements of Hayashizaki et al, 9 i.e. 580 cm−1 (v 4PO4 3−), 600 cm−1 (v 4PO4 3−), 595 cm−1 (v 3PO4 3−) and 870 cm−1 (v 3CO3 2−), resulting in IRSF and C/P ratios being calculated as follows:
We also utilised the mean values of a modern bovine bone as a reference throughout.
2.2.2. Bone collagen extraction
Lipids were removed from all modern material prior to collagen and carbonate analysis following Colonese et al. 53 The samples were rinsed six times in a 2:1 dichloromethane–methanol solvent solution (3 × 2 mL), ultrasonicated for 15 min and centrifuged (850g) for 10 min. They were then rinsed with deionised water and dried at room temperature. Collagen extraction from the 57 bone samples followed the Longin 54 method modified by Brown et al. 55 Each bone sample was cleaned using a scalpel to remove contaminants from the outer layer of bone. Following this, bone chunks of ca 300–500 mg were demineralised in 8 mL of 0.6 M hydrochloric acid (HCl), agitated twice daily. The acid was changed every two days until demineralisation was complete. Next, the supernatant was removed, and the samples were rinsed thrice using deionised water and then gelatinised using HCl (pH 3) at 80°C for 48 h. Next, the supernatant liquid containing the collagen was filtered using Ezee™ filters to remove unwanted particulate matter from the collagen solution and was then frozen for a minimum of 12 h at −20°C before being freeze‐dried for 48 h. Collagen yields were estimated by dividing the collagen mass after filtration by the original bone mass after cleaning.
2.2.3. Bone preparation for carbonate analysis
Bone carbonate analysis followed a procedure adapted from Snoeck and Pellegrini 56 and Pellegrini and Snoeck. 57 The bones were cleaned using a sterile scalpel blade to remove dirt and contaminating material. The cleaned bones were crushed using an agate mortar and pestle. Approximately 7.5 mg of bone powder was required for each sample. To remove secondary minerals from the samples, the weighed samples were dissolved in 15 mL of calcium acetate ((CH3COO)2Ca) buffered 1 M solution (ratio 1:2) and placed on a roller rocker for about 30 min. After treatment, the samples were rinsed six times with deionised water, placed in a freezer for 24 h and freeze‐dried for 24 h to remove all the water and isolate the apatite. Centrifuge tubes (15 mL) containing the treated samples were reweighed and the mass loss generated by the treatment was measured by subtracting the original weight of the tube.
2.2.4. Enamel preparation for carbonate analysis
Enamel carbonate preparation followed the method described in Miller et al. 58 We separated a section of enamel, approximately 3 mm wide that spanned from the cervical margin to the cusp, from the tooth crown. In order to minimise contamination, we cleaned all tools prior to use and between samples. All surfaces of the enamel samples were lightly drilled with an abrasive drill bit, set at the lowest speed to avoid heating. Any evident cracks, as well as cut surfaces, were lightly drilled. Each enamel chip was placed in a 2 mL Eppendorf vial with deionised water and sonicated for 3 min to remove any fine powder. In cases where water remained cloudy after sonication, repeat washes were performed. Enamel was then finely ground using an agate mortar and pestle to a particle size of less than 50 μm. Possible diagenetic contaminants were removed by soaking the enamel powder in 0.1 mL of 0.1 M acetic acid per 1 mg of enamel and agitated for 10 min. The acid was removed by repeated rinsing in distilled water five times and centrifuging for 2 min at 13 700g between rinses. We froze the samples for 24 h and then freeze‐dried them for 24 h to remove all the water and isolate the apatite. The tubes containing the treated samples were reweighed and the mass loss generated by the treatment was measured by subtracting the original weight of the tube.
2.2.5. Dental calculus preparation for carbonate analysis
Methods of separating carbonate from calculus prior to isotope analysis have not been as extensively explored as they have been for bone and enamel. Therefore, our methods followed the only existing published protocols in order to facilitate comparisons and we used the methods outlined in Price et al 8 with modifications from Crisp et al 59 to enhance the removal of the organic component of dental calculus. Before carbonate analysis, the optimal time for the calculus to be submerged in NaClO (bleach) was checked using the protocol of Crisp et al. 59 The bleach was expected to oxidise amino acids rendering them unavailable for analysis. The study of Crisp et al 59 noted that ostrich eggshells coarse grain (500–1000 μm) free amino acid samples took a long time to demineralise, probably due to the reduced surface area to volume ratio; therefore they decided not to use powdered particles >500 μm in size in further experiments.
In light of the preceding recommendations by Crisp et al, 59 for this study, the dental calculus samples were first washed in deionised water four times in order to remove any soil loosely adhering to them. The clean calculus was left to dry at room temperature and then powdered with an agate pestle and mortar to <500 μm. Since dental calculus is much richer in organics than shells, 60 , 61 considering that for powdered shells, after 72 h, all amino acids accessible by 50 mL of 12% (w/v) NaClO had been removed, the powdered samples in this study were submerged in 50 mL of 12% (w/v) NaClO per milligram of sample for 96 h. The samples were agitated every 24 h to ensure that the whole calculus matrix was exposed to the bleach. The bleach was removed by pipette and spotted onto coloured tissue paper to test if it was still active. The dental calculus was then washed five times in distilled water, with a sixth wash with HPLC‐grade methanol to reduce any leftover bleach and then air‐dried overnight. To extract the mineral in dental calculus, ca 1.5 mg of each bleached sample was reacted with 0.2 mL per 5 mg of (CH3COO)2Ca buffered 1 M solution (ratio 1:2; pH ~ 4.5) and placed on a roller rocker for about 40 min to remove exogenous carbonates. The samples were cleaned with distilled water four times and dried at 40°C.
2.3. Analytical measurements
All δ13C and δ15N ratios are expressed using the delta notation (δ) in parts per thousand (‰) relative to international standards, VPDB for δ13C and atmospheric N2 (AIR) for δ15N, using the following equation:
where i E and j E denote the heavier and lighter isotopes, respectively. 62
2.3.1. Bone collagen
Approximately 0.5 mg of freeze‐dried retentate was weighed out into 4 mm × 3.2 mm tin capsules and combusted alongside international standards in an elemental analyser/isotope ratio mass spectrometer: a Sercon 20‐22 mass spectrometer coupled with a Sercon GSL sample preparation system module at BioArch, University of York. All samples were analysed in duplicate. The duplicate sample 1σ reproducibility was <0.2‰ for both δ15N and δ13C values. Accuracy for bone collagen was determined at the University of York by measurements of international standard reference materials within each analytical run. These were: IAEA 600, δ13Craw = −27.65 ± 0.09‰, δ13Ctrue = −27.77 ± 0.04‰, δ15Nraw = 0.92 ± 0.21‰, δ15Ntrue = 1 ± 0.2‰; IAEA N2, δ15Nraw = 20.35 ± 0.13‰, δ15Ntrue = 20.3 ± 0.2‰; IA Cane, δ13Craw = −11.77 ± 0.09‰, δ13Ctrue = −11.64 ± 0.03‰. The overall uncertainties on the measurements of each sample were calculated based on the method of Kragten 63 by combining uncertainties in the values of the international reference materials and those determined from repeated measurements of samples and reference materials. These are expressed as one standard deviation. The maximum uncertainty for all samples across all runs was <0.2‰ for both δ13C and δ15N values. In addition, a homogenised bovine bone extracted and analysed within the same batch as the samples produced the following average values: δ13C = −23.01 ± 0.13‰; δ15N = 6.21 ± 0.44‰. This was comparable to the overall mean value from 50 separate extracts of this bone sample, which produced values of δ13C = −22.97 ± 0.19‰ and δ15N = 6.19 ± 0.30‰.
2.3.2. Bone, enamel and dental calculus carbonates
All carbonate analysis was carried out using an IsoPrime 100 dual inlet mass spectrometer plus Multiprep device at the Natural Environment Research Council's National Environmental Isotope Facility (Keyworth, Nottingham, UK). Approximately 50–100 μg of each sample was loaded into glass vials and sealed with septa. The vials were evacuated, and anhydrous phosphoric acid was delivered to the carbonate at 90°C. The evolved CO2 was collected for 15 min (from the point of acid delivery), cryogenically cleaned and passed to the mass spectrometer. Accuracy for bone, enamel and dental calculus carbonates was determined by measurements of the Keyworth Carrara Marble (KCM), the laboratory's carbonate reference material (average KCM δ13C = 2.00 ± 0.02‰), calibrated to NBS19 and NBS18 certified reference material. Accuracy or systematic error (u [bias]) was determined to be ± 0.01 for δ13C values on the basis of the difference between the observed and known δ13C values of the check standard KCM and the long‐term standard deviation of this check standard. The total analytical uncertainty was estimated to be ± 0.11‰ for the carbonate analysis calculated using the method recommended in Szpak et al 64 by combining uncertainties in the values of the laboratory's carbonate reference material and those determined from repeated measurements of unknown samples. In addition, three homogenised bovine bones extracted and analysed within the same batch as the samples produced the δ13C average values of −16.01 ± 0.08‰.
2.4. Suess effect correction
All the modern tissue δ13C values were corrected for the Suess effect (Table S4) which is defined as the global decrease of 14C and 13C relative to 12C in atmospheric CO2 which occurred primarily due to fossil fuel burning since the Industrial Revolution. 65 A time‐dependent correction per year to each sample was applied according to Graven et al, 65 normalised up to 2015. For all individuals who died after 2015, the 2015 normalisation was utilised.
2.5. Statistical analysis
Statistical analysis was carried out using R statistics, 66 PAST statistics 67 and IBM SPSS statistics version 26. 68 Non‐parametric statistics were used to compare isotope values among the population groups because of the non‐normal distribution of some data as indicated by Kolmogorov–Smirnov and Shapiro–Wilk tests. The tests were executed using the non‐parametric equivalent of a one‐way analysis of variance and the non‐parametric equivalent of the independent T‐test, the Kruskal–Wallis test and the Mann–Whitney test, respectively.
3. RESULTS AND DISCUSSION
In total, 57 samples, namely modern (n = 20), medieval (n = 22) and post‐medieval (n = 15), were subjected to bone collagen, bone carbonate, enamel carbonate and calculus carbonate isotope analysis. FTIR‐ATR analysis was performed on 57 bones and 52 dental calculus samples. Tooth enamel is generally assumed to be resistant to diagenetic alteration because of its structure, 69 , 70 and since there was not enough material for FTIR analysis as well as isotope analysis, FTIR‐ATR analysis on enamel samples was not performed. Raw isotope data and information for all remains are presented in Table S1.
3.1. FTIR‐ATR analysis of bone and dental calculus
Diagenesis in bones and dental calculus was assessed using FTIR‐ATR, but dental calculus was insufficient to use for both FTIR and isotope analysis in five of the individuals studied. FTIR data from all sites in this study are provided in Table S3 and plotted in Figures 1 and 2. Analysis and interpretation of FTIR results for dental calculus for assessment of diagenesis before isotope analysis are still in the exploratory stage. Only Price et al 8 have previously measured IRSF values in archaeological dental calculus before isotope analysis. The values for C/P ratio and IRSF that indicate that a bone is unaltered are not expected to be relevant to understanding dental calculus preservation quality. Further work is still required to study the FTIR‐ATR spectra in dental calculus and to identify the exact phosphates and carbonate spectra that can be used specifically for dental calculus diagenesis analysis. Therefore, dental calculus parameters are provided only for building a database for dental calculus data that can be used to further analyse calculus structure and its diagenetic alteration (Table S3). The parameters found in this study, however, cannot be compared to those of the study of Price et al 8 because that study did not use the same FTIR‐ATR methodology.
FIGURE 1.
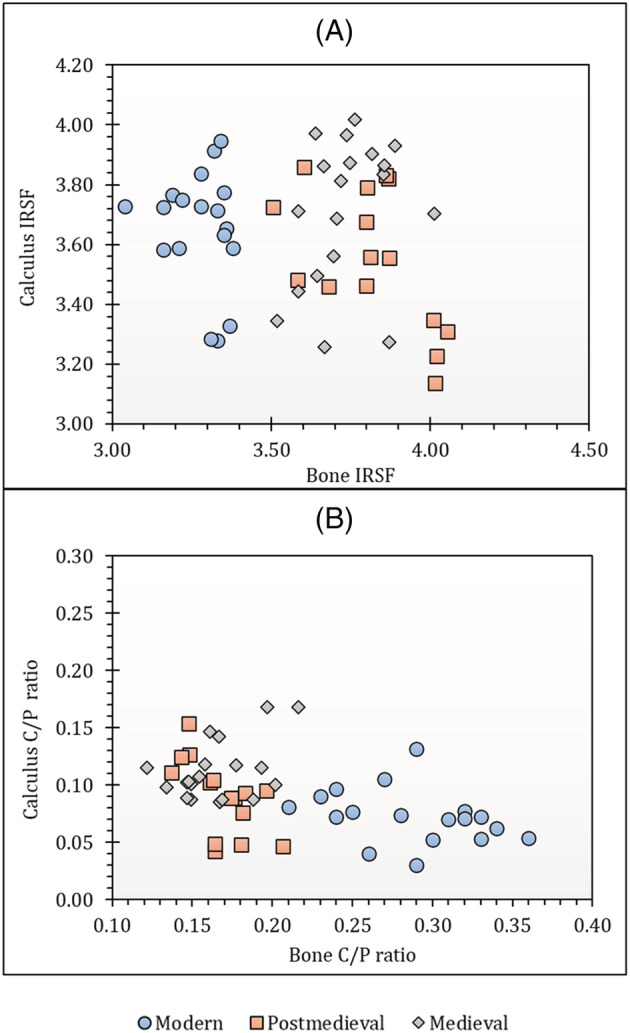
Comparisons between (A) bone IRSF and dental calculus IRSF and (B) bone C/P ratio and calculus C/P ratio [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
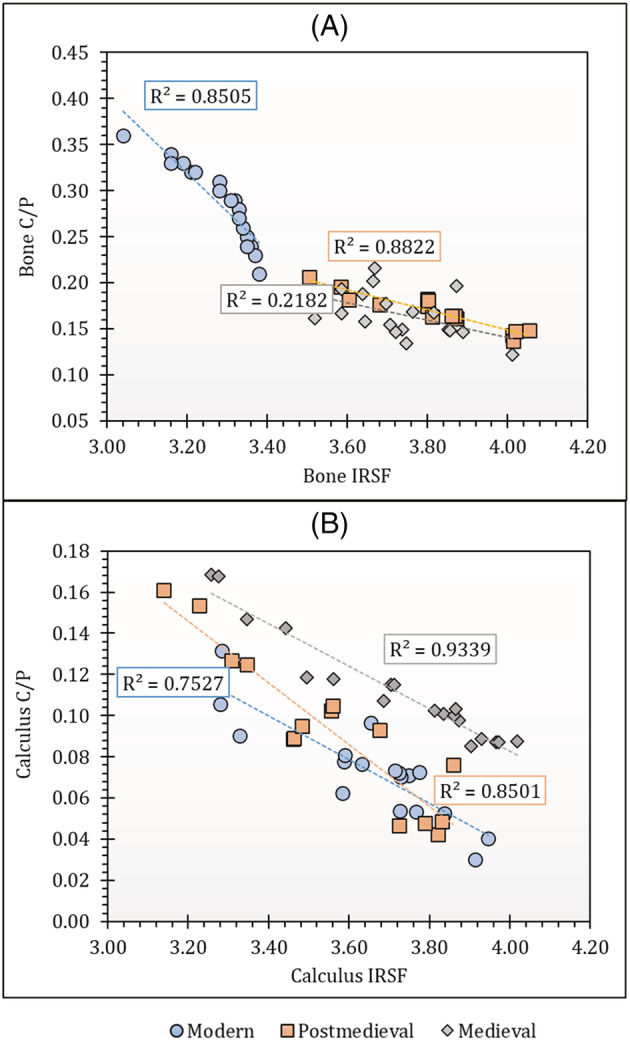
Plots of (A) bone IRSF versus bone C/P ratio and (B) calculus IRSF versus calculus C/P ratio [Color figure can be viewed at wileyonlinelibrary.com]
3.1.1. Infrared splitting factor
The crystallinity (IRSF) values in modern bones ranged from 3.04 to 3.38 (mean = 3.28 ± 0.09; see Table S3; Figure 1A), slightly lower than the value (IRSF = 3.357 ± 0.007) that was obtained for the modern bones in Kontopoulos et al. 71 The crystallinity in all the archaeological samples in this project range from 3.50 to 4.05 (medieval mean = 3.74 ± 0.12; post‐medieval mean = 3.82 ± 0.17), higher than both the samples in Kontopoulos et al 71 and the modern bones from this study. Alteration induced by age‐related diagenesis is demonstrated for any bone if its crystallinity is higher than that of the modern samples. The study by Kontopoulos et al 72 revealed that archaeological samples with IRSF values greater than 4.2 did not preserve DNA; therefore, all archaeological bone samples with values higher than 4.2 were excluded from this study on the assumption that they may have poor bioapatite preservation as well. The crystallinity values for modern dental calculus samples ranged from 3.28 to 3.95 (mean = 3.66 ± 0.19) and those for archaeological samples ranged from 3.14 to 4.02 (medieval mean = 3.71 ± 0.24; post‐medieval mean = 3.55 ± 0.23). The mean IRSF values for all groups in this study are higher than the value (mean = 3.2 ± 0.2) observed by Price et al, 8 but this could be due to the small sample size (n = 7 of 28) they analysed in their study. For the present study, only grains between 20 and 50 μm particle size were used and were sampled using the ATR technique with FTIR. Price et al 8 did not use ATR nor did they consider the effect of sample particle size on FTIR measurements as observed previously in bones. 48 There seems to be no clear relationship or a constant offset between each individual's bone and calculus IRSF values (Figure 1A).
3.1.2. Carbonate‐to‐phosphate ratio (C/P)
The modern bone CO3/PO4 absorbance ratios range from 0.21 to 0.36 (mean = 0.28 ± 0.04) and those for the archaeological samples range from 0.12 to 0.22 (medieval mean = 0.17 ± 0.03; post‐medieval mean = 0.17 ± 0.02) indicating remarkable similarity between the two archaeological sets of samples (Table S3; Figure 1B). The C/P values for all of the archaeological bones fell below the mean C/P values that were obtained from modern bones analysed in this study as well as those that have been previously obtained in modern unaltered bone (mean C/P = 0.24 ± 0.003) 71 indicating a loss of the carbonate fraction from the bone apatite.
The C/P ratios for modern dental calculus samples ranged from 0.03 to 0.13 (mean = 0.07 ± 0.02) and those for archaeological samples ranged from 0.04 to 0.17 (medieval mean = 0.11 ± 0.03; post‐medieval mean = 0.09 ± 0.03) (Table S3). Similarly, there seems to be no relationship between the individual bone and calculus C/P values (Figure 1B). Hayashizaki et al 9 revealed that the carbonate content in dental calculus was higher when compared to other biological apatites such as bone, enamel and dentine; therefore, the C/P ratios in dental calculus were expected to be higher in this study. However, the C/P ratios of dental calculus in this study are lower than that of bone, suggesting that there could be other contributing factors. Dental calculus contains non‐apatitic calcium phosphates, 9 which are not present in other normal mineralised tissues. 73 Dental calculus may therefore have an inherent higher phosphate content since, in addition to non‐apatitic calcium phosphates, it also has hydroxyapatite leading to a potentially lower C/P ratio relative to bone. The mean value of specific phosphorus concentration in human rib bone weights has been found to be 8.42 ± 2.14% of dry bone weight 74 whereas that of dental calculus has been found to be 19%. 75 This strongly suggests that phosphates may be the cause of the lower C/P values in dental calculus when compared to bone.
Moreover, it was also observed that unlike in bone, the most recent calculus samples have the lowest C/P ratio followed by the post‐medieval samples and then finally the medieval samples. This pattern seems to indicate that the C/P ratio of dental calculus increases with the age of the sample. On the other hand, Hayashizaki et al 9 revealed that carbonate content in dental calculus depended on its location in the mouth such that the lower anterior teeth have higher carbonate content compared to the upper posterior teeth. All the calculus in this study was collected from posterior teeth, but randomly from either the mandible or maxilla. Therefore, if differences in carbonate content can occur due to the location of where calculus was formed, 9 it is possible that the apparent trend in C/P ratios observed in this study could simply be a function of where each sample was formed (Figure 1B).
3.1.3. IRSF and C/P relationship
Overall, the bone IRSF and C/P ratios display a very strong inverse correlation for modern and post‐medieval bone samples (Figure 2A). The weaker correlation in medieval samples may relate to the varied ages of the burials (7th to 16th century) as well as the different environments from which they were recovered. Environmental factors that degrade samples differ from site to site and, even in the same setting, the type of burial can influence how environmental factors interact with archaeological remains. Moreover, the longer the remains are buried, the greater the diagenetic alteration. 76 , 77 Alteration of the carbonate content in skeletal material has also been shown to be site‐specific. 78 , 79 Both the modern and post‐medieval populations were each obtained from a single site while the medieval samples were collected from three sites. There is, however, a strong inverse correlation for dental calculus samples in all periods (Figure 2B). Since the relationship is negative for both bones and dental calculus (Figure 2), there is a general trend of increasing IRSF values with a reduction in C/P values reflecting the loss of carbonate with increasing crystallinity. This is in keeping with the previously reported work on bones. 80
3.2. Isotopes
Collagen quality was assessed using the established collagen quality criteria. 81 , 82 All samples in this study produced sufficient collagen for mass spectrometry (Table S1), with collagen yields ranging between 2.7% and 24.0% (mean = 14.5%). The atomic C:N values ranged from 3.1 to 3.4 and are within the range of C:N of 2.9 to 3.6 that DeNiro 83 specified as acceptable. Furthermore, the percentage of carbon and nitrogen in all the collagen samples from bone fell within the elemental percentages reported for modern mammalian bone collagen by Ambrose 81 that ranged between 15.3% and 47% and between 5.5% and 17.3% by weight for carbon and nitrogen, respectively.
The mean bone collagen δ13C values for medieval, post‐medieval and modern populations are −19.7 ± 0.4‰, −19.8 ± 0.5‰ and −14.0 ± 0.6‰ while their mean δ15N values are 11.7 ± 0.9‰, 11.8 ± 1.1‰ and 10.8 ± 0.6‰ respectively. In addition, the mean bone carbonate δ13C values for the same populations are −13.4 ± 0.8‰, −14.3 ± 1.0‰ and −9.3 ± 1.1‰ while their mean enamel carbonate δ13C values are −13.6 ± 1.0‰, −13.3 ± 1.0‰ and −6.8 ± 1.1‰ respectively (Figure S1; Table 1). There are statistically significant differences in both δ13C and δ15N values between populations as determined by the Kruskal–Wallis H test (bone collagen δ13C: X 2(2) = 38.295, p < 0.001; δ15N: X 2(2) = 14.730, p = 0.001; bone carbonate δ13C: X 2(2) = 41.149, p < 0.001; calculus carbonate δ13C: X 2(2) = 37.615, p < 0.001; enamel carbonate δ13C: X 2(2) = 38.489, p < 0.001). The post hoc comparisons from the Kruskal–Wallis test revealed that for all tissue δ13C values and bone collagen δ15N values there were significant differences between the modern population and both the medieval and post‐medieval populations. All modern tissues had higher mean δ13C values and lower mean δ15N values than both the medieval and post‐medieval populations (Table 1). There was, however, no statistically significant difference between the medieval and the post‐medieval populations (Table S5).
TABLE 1.
Descriptive statistics for all δ13C (‰) and δ15N (‰) isotope values being investigated in this study. Contains isotope data, produced by the British Geological Survey, UKRI
| Site | N | Min. | Max. | Mean ± 1σ | Range | |
|---|---|---|---|---|---|---|
|
Bone δ13Ccoll (‰) |
Medieval | 22 | −20.9 | −18.9 | −19.7 ± 0.4 | 1.9 |
| Post‐medieval | 15 | −20.7 | −19.1 | −19.8 ± 0.5 | 1.6 | |
| Modern | 20 | −15.3 | −12.4 | −14.0 ± 0.6 | 2.9 | |
|
Bone δ15Ncoll (‰) |
Medieval | 22 | 10.1 | 13.7 | 11.7 ± 0.9 | 3.6 |
| Post‐medieval | 15 | 9.5 | 13.0 | 11.8 ± 1.1 | 3.5 | |
| Modern | 20 | 9.6 | 12.0 | 10.8 ± 0.6 | 2.4 | |
|
Bone δ13Ccarb (‰) |
Medieval | 22 | −14.8 | −12.1 | −13.4 ± 0.8 | 2.7 |
| Post‐medieval | 15 | −15.7 | −12.6 | −14.3 ± 1.0 | 3.1 | |
| Modern | 20 | −11.0 | −7.1 | −9.3 ± 1.1 | 3.9 | |
| Enamel δ13Ccarb (‰) | Medieval | 22 | −15.2 | −12.1 | −13.6 ± 1.0 | 3.1 |
| Post‐medieval | 15 | −14.8 | −11.9 | −13.3 ± 1.0 | 2.8 | |
| Modern | 20 | −9.2 | −4.6 | −6.8 ± 1.1 | 4.6 | |
| Calculus δ13Ccarb (‰) | Medieval | 22 | −12.7 | −8.0 | −9.7 ± 1.2 | 4.7 |
| Post‐medieval | 15 | −11.4 | −6.1 | −9.2 ± 1.4 | 5.3 | |
| Modern | 20 | −7.0 | −1.2 | −4.1 ± 1.8 | 5.8 |
3.2.1. Correlations between tissues
When considered as a collective dataset (the medieval, post‐medieval and modern populations together), there is a strong correlation between bone carbonate δ13C values and calculus carbonate δ13C values and between enamel δ13C values and calculus δ13C values (enamel δ13C and calculus δ13C: R 2 = 0.7206; bone δ13C and calculus δ13C: R 2 = 0.7159; see Figures 3A and 3B). When analysed as discrete populations, however, there is no correlation between either calculus carbonate δ13C values and bone carbonate δ13C values or calculus carbonate δ13C values and enamel carbonate δ13C values (Figures 3A and 3B). A similar phenomenon was observed by Eerkens et al 7 in their analysis of paired calculus and bone carbonates from North American hunter‐gatherers and northeastern African agriculturalists. Eerkens et al 7 noted that strong correlations are observed when analysing the collective data of individuals consuming distinct diets; however, correlations between bones and calculus within sites were weaker because of a limited range of isotopic values that were found when individuals were consuming similar diets, and this study agrees with that conclusion.
FIGURE 3.
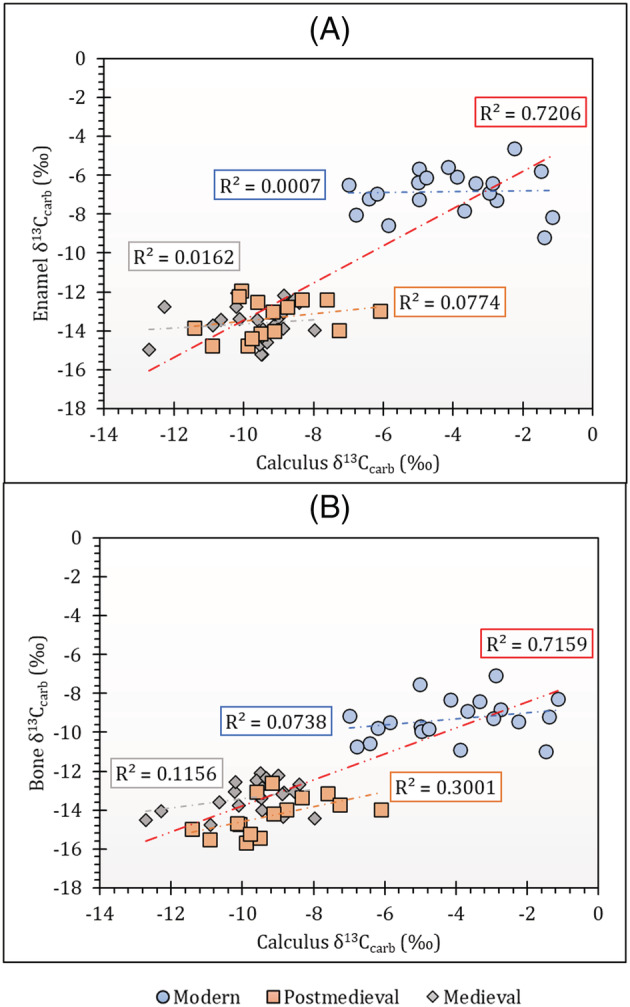
Graphs showing δ13C correlations between (A) calculus carbonate and enamel carbonate and (B) calculus carbonate and bone carbonate for separate modern, medieval and post‐medieval population groups. Each trendline is coded according to the symbol colour for each population and the red trendline represents the collective dataset in each graph. Contains isotope data, produced by the British Geological Survey, UKRI [Color figure can be viewed at wileyonlinelibrary.com]
3.2.2. Carbonate δ13C tissue offsets
As expected, the modern population shows higher δ13C values in all three tissue carbonates compared to both the medieval and post‐medieval populations. Except for four individuals (FAC 10, FAC 13, FAC 20 and NFC 80), the offset in dental calculus and either bone or enamel carbonate δ13C values is usually large and consistent in direction, i.e. except FAC 20, in all populations, dental calculus δ13C values are always significantly higher (Figure 4). The modern, medieval and post‐medieval carbonate △13Ccalculus‐enamel and △13Ccalculus‐bone spacing are listed in Table S2. However, there is no consistent offset between the δ13C values for the three tissues per individual (Table S2; Figure 4). No constant offsets were observed in Price et al, 8 the one other study that has also examined calculus mineral and bone carbonate. Despite this, the authors suggested that their average calculus–bone offset of 3.1‰ was probably due to the isotopic exchange between saliva and atmospheric CO2.
FIGURE 4.
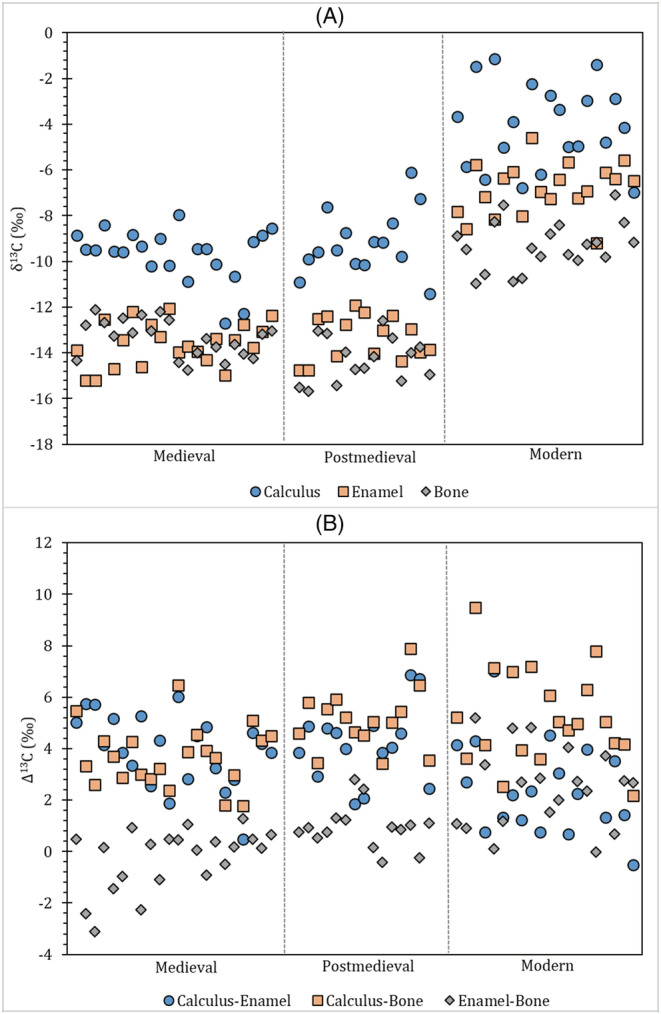
(A) Carbon isotopes by individual data trends and (B) carbon isotope offsets (difference, Δ) by individual data trends. Contains isotope data, produced by the British Geological Survey, UKRI [Color figure can be viewed at wileyonlinelibrary.com]
In contrast to Price et al, 8 we suggest that a direct comparison between different tissues' isotopic composition is not strictly possible as there may be a difference between the isotopic composition of carbon preserved in bone, teeth and dental calculus. For example, the isotopic composition of carbon in bone carbonate and enamel carbonate has been investigated by Warinner and Tuross, 84 who compared bone and enamel carbonate of pigs raised on controlled diets containing either raw maize or nixtamalized maize for 13 weeks. The results revealed a significant difference between bone and enamel carbonate in the animals with the δ13C values in enamel being higher by 2.2‰ and 2.3‰ in the nixtamalized and raw diets, respectively. Since simultaneously forming bone and enamel carbonate were used, the offset could not be attributed to ‘preferential or differential digestion since the carbonate in both the enamel and bone apatite was deposited from dissolved blood bicarbonate during the same experiment’. 84 Considering that enamel, once formed, cannot be remodelled, whereas bone undergoes constant remodelling, the authors suggested that the enamel carbonate and bone carbonate of adult animals, therefore, represented ‘temporally segregated isotopic deposition events’. Therefore, since enamel reflects diet at the time of formation (childhood diet) and bone represents an average from the last few years of life, 85 , 86 we propose that the inconsistent offsets between enamel and bone are most likely to be due to each individual's different childhood and adult diets.
Additionally, we propose that, unlike bone and enamel, the isotopic fractionation in dental calculus may not be associated simply with metabolism and food sources. In the case of dental calculus, which is formed from a living biofilm, the isotopic fractionation would likely increase with calculus biofilm thickness, similar to what has been observed in other biofilms. 87 However, it is also notable that in modern individuals the extent of fractionation is lower than in medieval and post‐medieval populations. Although this study does not have the details on the thickness of the dental calculus biofilm, it is proposed here that greater oral hygiene may lead to less well‐established calcified biofilms. Therefore, it is suggested that since the mechanisms involved in the formation of dental calculus, fractionation effects and turnover time in this tissue are different from that of enamel and bone carbonate, a constant offset is not expected between dental calculus carbonate and either bone carbonate or enamel carbonate.
Further, we disagree with the suggestion by Price et al 8 that the calculus carbonate δ13C values were consistently higher than that of bone carbonate due to the exchange with inhaled atmospheric CO2 (which has a δ13C value of −7‰) and saliva. Although we agree that it is impossible to exclude atmospheric CO2 from the sample being analysed, other studies have established that δ13Cbreath values are reflective of ingested food. 88 , 89 Therefore, we argue that since the δ13Cbreath values reflect the isotopic signature of the food ingested and if the calculus δ13C values are affected by breath CO2 then they will reflect the isotope composition of the food consumed. This is, of course, in addition to the bicarbonate derived from saliva that derives carbonate from the blood and the action of microbes when metabolising carbohydrates in the mouth. 30 , 40
A final consideration is the potential impact of chemical pre‐treatment steps prior to calculus carbonate isotope analysis. Calculus carbonate is often observed to be enriched in 13C compared to bone and enamel carbonate; however, NaClO treatment to remove organics can impact carbon isotope composition as the process induces the adsorption of atmospheric CO2 which results in secondary carbonate incorporation. 57 The subsequent acetic acid treatment carried out here mitigates this by removing such secondary carbonates, but see Pellegrini and Snoeck. 57 Further work is required to fully understand pre‐treatment effects on dental calculus. However, what is clear is that calculus δ13C values track those of bone and enamel in populations analysed here, i.e. calculus δ13C values rise in tandem with bone and enamel δ13C values in modern versus archaeological populations (Figure 4), suggesting that any impact of pre‐treatment is likely to be minimal.
3.2.3. Dietary interpretation from the three types of tissues
Generally, the carbon isotope values for the modern North American population are extremely high for all tissue types and consistently higher than those of the medieval and post‐medieval populations. Unlike the archaeological populations from England, the modern individuals are influenced by the dominant consumption of C4 foods like fructose, maize and its by‐products (e.g. corn syrup). 47 The medieval and post‐medieval diets, on the other hand, were dominated by C3 foods. 17 , 18
Overall, the mean collagen results for medieval and post‐medieval individuals suggest a diet dominated by C3 terrestrial resources while that of the modern individuals is dominated by C4 resources as expected. The relatively low δ15N values in all populations including the modern individuals suggest that the diets were most likely to have been based on terrestrial rather than marine foods. To further investigate the consumption of C4 resources, all individuals were examined using the Froehle et al 90 multivariate isotope model which incorporates δ13Cbone‐collagen, δ15Nbone‐collagen and δ13Cbone‐carbonate data to reconstruct diet. Using published archaeological data, Froehle et al 90 generated two functions that describe how the test samples varied in terms of isotopic data with the function scores produced from these 2 functions enabling the plotting of data into five discrete clusters of dietary types seen in Figure 5. Seven individuals (SPL 226; SPL 552; SPL 1384; NFC 91; CSM 2.34; CSM 5.09; and CSM 41) in the archaeological population plot within Cluster 4, which corresponds to a total diet of about 30% C4 foods and about 35% C4 protein in addition to C3 foods (Figure 5). An additional 8 individuals (SCN 33; SPL 1063; SPL 1248; NFC 13; NFC 60; CSM 2.31; CSM 5.16; and CSM 37) plot within the area where Clusters 1 and 4 overlap, suggesting that these individuals most likely consumed some C4 plants, although it is not clear how much (Figure 5). In contrast, based on the carbon isotope values of enamel, a distinctive C4 signature is observed in the modern enamel as expected; however, the C4 signature is not so clear with both the medieval and post‐medieval populations. The average δ13C values of −13.6 ± 1.0‰ and −13.3 ± 1.0‰ for medieval and post‐medieval samples, respectively, are consistent with the uptake of C3 resources. Tooth δ13Cenamel values are expected to fall between −17‰ and −13‰ for a pure C3‐based diet. 91 The average δ13Cenamel values for both the medieval and post‐medieval populations are therefore consistent with the typical spectrum of a terrestrial diet in England characterised by high consumption of C3 resources as suggested in historical sources. 17 , 18
FIGURE 5.
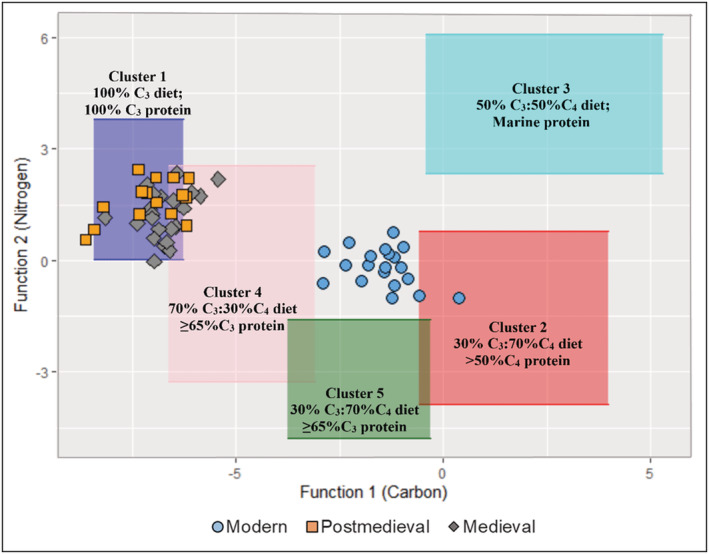
F1 and F2 discriminant function values for all individuals plotted against previously generated dietary clusters (see Froehle et al 90 ). Contains isotope data, produced by the British Geological Survey, UKRI [Color figure can be viewed at wileyonlinelibrary.com]
Although there are some suggestions of C4 food consumption using the Froehle et al 90 multivariate isotope model, the use of carbon isotope ratios on dental calculus carbonate should be able to determine if the post‐medieval populations were consuming C4 resources (cane sugar/maize). The mean calculus carbonate δ13C values for medieval, post‐medieval and modern populations are −9.7 ± 1.2‰, −9.2 ± 1.4‰ and −4.1 ± 1.8‰, respectively. If these δ13C values are interpreted as akin to bone carbonate and enamel carbonate values for individuals who consumed an abundance of C4 resources (e.g. bone carbonate modern samples for this study = −9.3 ± 1.1‰; enamel carbonate modern samples for this study = −6.8 ± 1.1‰; Tykot et al 11 study C4 bone apatite = −9.8 ± 1.0‰; C4 tooth enamel = −8.7 ± 2.3‰), the archaeological populations' calculus δ13C values would suggest high inclusion of C4 terrestrial resources in their diet. However, the calculus δ13C values for archaeological populations are less than those found in modern individuals who in this case are known to have consumed C4 foods in large quantities. It should be noted, however, there are slightly higher dental calculus δ13C values in some post‐medieval individuals that suggest consumption of a more 13C‐enriched carbohydrate such as cane sugar/maize.
There are statistically significant differences in δ13Ccalculus‐enamel, δ13Ccalculus‐bone and δ13Cenamel‐bone values between populations as determined by the Kruskal–Wallis H test (δ13Ccalculus‐enamel: X 2(2) = 8.199, p = 0.017; δ13Ccalculus‐bone: X 2(2) = 12.43, p = 0.001; δ13Cenamel‐bone: X 2(2) = 27.24, p < 0.001). The post hoc comparisons from the Kruskal–Wallis test (Table 2) revealed no significant differences between the modern and post‐medieval populations but significant differences between the medieval and post‐medieval populations in the calculus–bone offsets. The similarity between the post‐medieval data and the modern data supports that the former was consuming some C4 foods; however, this similarity is less clear cut as the post‐medieval population was consuming a C3 diet with mixed C3/C4 simple carbohydrates, whereas the modern diet is mixed C3/C4 with predominantly C4 simple carbohydrates. On the other hand, the differences observed between the medieval and post‐medieval populations suggest that there was no input of C4 carbohydrate consumption in the medieval population.
TABLE 2.
P‐values for the post hoc δ13C differences for all populations. The significance level is 0.05 and the significance values have been adjusted by the Bonferroni correction for multiple tests. Contains isotope data, produced by the British Geological Survey, UKRI
| Sample 1–sample 2 | Calculus–enamel δ13C | Calculus–bone δ13C | Enamel–bone δ13C |
|---|---|---|---|
| Modern–post‐medieval | 0.015 | 0.796 | 0.030 |
| Modern–medieval | 0.014 | 0.000 | 0.000 |
| Post‐medieval–medieval | 0.847 | 0.002 | 0.010 |
The causes for the differences between bone and enamel are explained in greater detail below.
3.2.4. Causes of diet differences between bone and enamel
The modern individuals' enamel carbonate δ13C values for this study are consistently enriched in 13C relative to bone carbonate, whereas enamel and bone carbonate for medieval and post‐medieval individuals are broadly similar. Throughout an individual's life bone is constantly remodelled and reflects diet ingested over a number of years prior to death, depending on the skeletal element. 85 In contrast, enamel in teeth forms over a relatively short time with the materials used in the present study (M1, M2 and M3) completing formation at age 2.5–3, 7–8 and 12–16 years, respectively. 86 Consequently, the differences observed between these two tissues in modern individuals would be heavily influenced by changes in diet due to age. The enamel carbonate δ13C values for the rest of the population are consistent with the well‐documented consumption of sugary foods among children and adolescents. 92 The total daily energy from added sugars for the American adolescent and teenage population group has been found to be higher than that of adults – approximately 13.1% to 17.5% among children compared to 11.2% to 14.5% among adults. 92 , 93 Therefore, Americans consuming more sugar during childhood could create significant offsets between bone and tooth δ13C values.
In the case of the medieval and the post‐medieval individuals, the enamel and the bone δ13C values are the same because not only are the bones and enamel tapping from the same pool of bicarbonates in the blood during formation, but also the children were not exposed to sugary foods, unlike their modern counterparts. Unlike modern individuals, the archaeological children's diet did not differ substantially from their adult diet, at least in terms of consumption of C4 resources.
4. CONCLUSIONS
In this study, stable isotope analysis of dental calculus was performed alongside bone and enamel from archaeological samples from England and modern samples from the William M. Bass Donated Skeletal Collection at the University of Tennessee, USA. Diagenesis of dental calculus samples was also assessed, and the calculus diagenetic parameters were compared with those of bone. In general, the availability of modern unaltered calculus enabled this study to introduce FTIR parameters that can be used to investigate diagenesis in dental calculus. It is accepted that the diagenetic process of dental calculus is not fully understood. Although we cannot yet demonstrate whether the archaeological dental samples used here were diagenetically altered or not, this study has provided a large body of data to the dental calculus FTIR dataset. Nevertheless, it is believed that the pre‐treatments that were performed before analysis removed the majority of any diagenetically altered carbonate material. In addition, the unaltered dental calculus revealed a similar pattern of higher calculus carbonate δ13C values compared to their bone and enamel values.
Previous studies applying isotope analysis to dental calculus to investigate diet have faced several challenges. This study argues that with additional studies, isotopic analysis of dental calculus carbonates could prove to be a potential method to use in the identification of C4 resources. This study effectively produced results that show differences in diet between different populations, whereby higher δ13C values found in modern calculus samples confirmed that those people were consuming more C4 foods than the English archaeological populations. Furthermore, the data are showing that among the English archaeological populations, there is clearly some C4 carbohydrates integrated into the post‐medieval diet. Additionally, it has been established that, within individuals, dental calculus δ13C values were (mostly) consistently higher than those of enamel or bone; however, the offset (difference, Δ) in δ13C values between the three tissues was not consistent. This could be due to differences in diet at the time of tissue formation or carbon fractionation in the mouth versus the body. The calculus δ13C values will most certainly be high due to the sugars that are being metabolised in the mouth as well as the effect of more fractionation in this much more poorly biologically controlled environment compared to that of bone and enamel. Using information from previous breath studies, this study has also demonstrated that it is highly unlikely that atmospheric CO2 is causing higher δ13C values as suggested by a previous study 8 as δ13Cbreath values are reflective of the whole diet. We instead argue that calculus carbonate δ13C values are highly likely to represent the carbohydrates consumed. In spite of these conclusions, there is an awareness that the formation process and composition of dental calculus are highly variable between individuals, and therefore there are still a number of gaps in knowledge that need to be addressed before its full potential as a viable tool for palaeodietary studies can be realised. For instance, the effect of pre‐treatment methods and the potential issue of the presence of particles (inorganic debris) in dental calculus are still outstanding, and more information is required to understand how the micro‐debris affects the overall isotope values found in dental calculus. We recommend controlled feeding experiments to clearly observe the effect of different carbohydrates on dental calculus carbon isotope ratios.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/rcm.9286.
Supporting information
Figure S1: Human δ13C and δ15N values from modern, post‐medieval and medieval individuals
Table S1: Enamel carbonate, calculus carbonate, bone collagen, bone carbonate isotope data and collagen quality indicators for all samples analysed. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S2: Individual δ13C isotope offsets in this study. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S3: Summary of bone and dental calculus FTIR‐ATR data displaying the average of data measured in triplicate ‐ IRSF: Infrared splitting factor; C/P: carbonate‐to‐phosphate ratio.
Table S4: Modern carbon isotope data with required adjustments/corrections. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S5: Post hoc results for all populations. The significance level is 0.05 and the significance values have been adjusted by the Bonferroni correction for multiple tests. Contains Isotope Data, produced by the British Geological Survey, UKRI
ACKNOWLEDGMENTS
This work was supported by the Arts & Humanities Research Council (grant number AH/L503848/1) through the White Rose College of the Arts & Humanities with analysis subsidised by the Department of Archaeology, University of York. All carbonate analysis undertaken at the NERC National Environmental Isotope Facility was funded by a NERC Isotope Geoscience Facilities Steering Committee grant (IP‐1801‐0618). Many thanks go to the William M. Bass Donated Skeletal Collection housed in the Department of Anthropology, University of Tennessee, USA, for allowing us to sample the modern individuals. Thanks are also due to CFA Archaeology and Earthworks Archaeology for giving permission to analyse the medieval and post‐medieval populations studied here. The authors are also grateful to Pre‐Construct Archaeological Services Ltd and the University of Leicester Archaeological Services (ULAS) who allowed A.R. to sample the medieval populations as part of this and other research. Special mention goes to Matthew von Tersch for his assistance with FTIR‐ATR and mass spectrometry at the University of York.
Chidimuro B, Mundorff A, Speller C, et al. Isotope analysis of human dental calculus δ13CO3 2−: Investigating a potential new proxy for sugar consumption. Rapid Commun Mass Spectrom. 2022;36(11):e9286. doi: 10.1002/rcm.9286
Contributor Information
Blessing Chidimuro, Email: b.chidimuro@reading.ac.uk.
Amy Mundorff, Email: amundorff@utk.edu.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Warinner C, Hendy J, Speller C, et al. Direct evidence of milk consumption from ancient human dental calculus. Sci Rep. 2014;4(1):7104. doi: 10.1038/srep07104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendy J, Warinner C, Bouwman A, et al. Proteomic evidence of dietary sources in ancient dental calculus. Proc Biol Sci. 2018;285(1883):20190977. doi: 10.1098/rspb.2018.0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacKenzie L, Speller CF, Holst M, Keefe K, Radini A. Dental calculus in the industrial age: Human dental calculus in the post‐medieval period, a case study from industrial Manchester. Quat Int. 2021. doi: 10.1016/j.quaint.2021.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott GR, Poulson SR. Stable carbon and nitrogen isotopes of human dental calculus: A potentially new non‐destructive proxy for paleodietary analysis. J Archaeol Sci. 2012;39(5):1388‐1393. doi: 10.1016/j.jas.2011.09.029 [DOI] [Google Scholar]
- 5. Poulson SR, Kuzminsky SC, Scott GR, Standen VG, Arriaza B, Muñoz I, Dorio L Paleodiet in northern Chile through the Holocene: Extremely heavy δ15N values in dental calculus suggest a guano‐derived signature? J Archaeol Sci. 2013;40(12):4576‐4585. doi: 10.1016/j.jas.2013.07.009 [DOI] [Google Scholar]
- 6. Salazar‐Garcia DC, Richards MP, Nehlich O, Henry AG. Dental calculus is not equivalent to bone collagen for isotope analysis: A comparison between carbon and nitrogen stable isotope analysis of bulk dental calculus, bone and dentine collagen from the same individuals from the medieval site of El Raval (Alicante, Spain). J Archaeol Sci. 2014;47:70‐77. doi: 10.1016/j.jas.2014.03.026 [DOI] [Google Scholar]
- 7. Eerkens JW, de Voogt A, Dupras TL, Rose SC, Bartelink EJ, Francigny V. Intra‐ and inter‐individual variation in δ13C and δ15N in human dental calculus and comparison to bone collagen and apatite isotopes. J Archaeol Sci. 2014;52:64‐71, doi: 10.1016/j.jas.2014.08.020 [DOI] [Google Scholar]
- 8. Price SDR, Keenleyside A, Schwarcz HP. Testing the validity of stable isotope analyses of dental calculus as a proxy in paleodietary studies. J Archaeol Sci. 2018;91:92‐103. doi: 10.1016/j.jas.2018.01.008 [DOI] [Google Scholar]
- 9. Hayashizaki J, Ban S, Nakagaki H, Okumura A, Yoshii S, Robinson C. Site specific mineral composition and microstructure of human supra‐gingival dental calculus. Arch Oral Biol. 2008;53(2):168‐174. doi: 10.1016/j.archoralbio.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 10. Satpathy A, Mohanty G, Bose A, Mohanty R, Niyogi P, Rautray TR. Fourier transform infra‐red spectroscopic analysis of supragingival and subgingival human dental calculus. Indian J Forensic Med Toxicol. 2019;13(4):1870‐1875. doi: 10.5958/0973-9130.2019.00589.9 [DOI] [Google Scholar]
- 11. Tykot RH, van der Merwe NJ, Hammond N. Stable isotope analysis of bone collagen, bone apatite, and tooth enamel in the reconstruction of human diet. Archaeol Chem. 1996;355‐365. doi: 10.1021/bk-1996-0625.ch025 [DOI] [Google Scholar]
- 12. Beaumont J, Atkins E‐C, Buckberry J, et al. Comparing apples and oranges: Why infant bone collagen may not reflect dietary intake in the same way as dentine collagen. Am J Phys Anthropol. 2018;167(3):524‐540. doi: 10.1002/ajpa.23682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambrose SH, Norr L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In: Lambert JB, Grupe G, ed. Prehistoric Human Bone: Archaeology at the Molecular Level. Berlin: Springer; 1993:1‐37. doi: 10.1007/978-3-662-02894-0_1 [DOI] [Google Scholar]
- 14. Schwarcz HP, Schoeninger MJ. Stable isotopes of carbon and nitrogen as tracers for paleo‐diet reconstruction. In: Baskaran M, ed. Handbook of Environmental Isotope Geochemistry. : Vol I. Berlin: Springer; 2012:725‐742, doi: 10.1007/978-3-642-10637-8_34 [DOI] [Google Scholar]
- 15. Ambrose SH, Butler BM, Hanson DB, Hunter‐Anderson RL, Krueger HW. Stable isotopic analysis of human diet in the Marianas archipelago, western Pacific. Am J Phys Anthropol. 1997;104(3):343–361. doi: [DOI] [PubMed] [Google Scholar]
- 16. Alexander MM, Gerrard CM, Gutiérrez A, Millard AR. Diet, society, and economy in late medieval Spain: Stable isotope evidence from Muslims and Christians from Gandía, Valencia. Am J Phys Anthropol 2015;156(2):263‐273. doi:10.1002/ajpa.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woolgar CM. Food in Medieval England: Diet and Nutrition. Oxford: Oxford University Press; 2009. [Google Scholar]
- 18. Oddy DJ. The paradox of diet and health: England and Scotland in the nineteenth and twentieth centuries. In: Fenton A, ed. Order and Disorder: The Health Implications of Eating and Drinking in the Nineteenth and Twentieth Centuries. Edinburgh: Tuckwell Press; 2000:45‐63. [Google Scholar]
- 19. Sugar WJ. The World Corrupted: From Slavery to Obesity. London: Hachette; 2017. [Google Scholar]
- 20. Holland JH. Food and fodder plants. Bull Misc Inform Kew; 1919;1919(1/2):1‐84. doi: 10.2307/4107656 [DOI] [Google Scholar]
- 21. Ó'Gráda C. The Great Irish Famine. Cambridge: Cambridge University Press; 1989. doi: 10.1007/978-1-349-08269-8 [DOI] [Google Scholar]
- 22. Trickett MA. A tale of two cities: Diet, health and migration in post‐medieval Coventry and Chelsea through biographical reconstruction, osteoarchaeology and isotope biogeochemistry. Doctoral thesis: Durham University; 2006. http://etheses.dur.ac.uk/1330/ [Google Scholar]
- 23. Nitsch EK, Humphrey LT, Hedges REM. The effect of parity status on δ15N: Looking for the ‘pregnancy effect’ in 18thh and 19th century London. J Archaeol Sci. 2010;37(12):3191‐3199. doi: 10.1016/j.jas.2010.07.019 [DOI] [Google Scholar]
- 24. Nitsch EK, Humphrey LT, Hedges REM. Using stable isotope analysis to examine the effect of economic change on breastfeeding practices in Spitalfields, London, UK. Am J Phys Anthropol. 2011;146(4):619–628. doi: 10.1002/ajpa.21623 [DOI] [PubMed] [Google Scholar]
- 25. Bleasdale M, Ponce P, Radini A, et al. Multidisciplinary investigations of the diets of two post‐medieval populations from London using stable isotopes and microdebris analysis. Archaeol Anthropol Sci. 2019;11(11):6161‐6181. doi:10.1007/s12520‐019‐00910‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blumenschine RJ, Peters CR, Masao FT, et al. Late Pliocene homo and hominid land use from Western Olduvai Gorge, Tanzania. Science. 2003;299(5610):1217‐1221. doi: 10.1126/science.1075374 [DOI] [PubMed] [Google Scholar]
- 27. Hershkovitz I, Kelly J, Latimer B, Rothschild BM, Simpson S, Polak J, Rosenberg M Oral bacteria in miocene Sivapithecus. J Hum Evol. 1997;33(4):507‐512. doi: 10.1006/jhev.1997.0149 [DOI] [PubMed] [Google Scholar]
- 28. Wilkins EM. Clinical practice of the dental hygienist. 10thed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 29. Lieverse AR. Diet and the aetiology of dental calculus. Int J Osteoarchaeol. 1999;9(4):219‐232. doi: [DOI] [Google Scholar]
- 30. Hicks J, Garcia‐Godoy F, Flaitz C. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent. 2003;28(1):47‐52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20 [DOI] [PubMed] [Google Scholar]
- 31. MacPhee T, Cowley G. Essentials of Periodontology and Periodontics. Oxford: Blackwell Scientific Publications; 1975. [Google Scholar]
- 32. Buckley S, Usai D, Jakob T, Radini A, Hardy K. Dental calculus reveals unique insights into food items, cooking and plant processing in prehistoric Central Sudan. PLoS One. 2014;9(7):e100808. doi: 10.1371/journal.pone.0100808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hillson SW. Diet and dental disease. World Archaeol. 1979;11(2):147‐162. doi: 10.1080/00438243.1979.9979758 [DOI] [PubMed] [Google Scholar]
- 34. Hardy K, Blakeney T, Copeland L, Kirkham J, Wrangham R, Collins M. Starch granules, dental calculus and new perspectives on ancient diet. J Archaeol Sci. 2009;36(2):248‐255. doi: 10.1016/j.jas.2008.09.015 [DOI] [Google Scholar]
- 35. Jin Y, Yip H‐K. Supragingival calculus: Formation and control. Crit Rev Oral Biol Med. 2002;13(5):426‐441. doi: 10.1177/154411130201300506 [DOI] [PubMed] [Google Scholar]
- 36. Goldman HM. Periodontal disease, part IV: Calculus and other etiologic factors. Compend Contin Educ Dent. 1986;7(270–1):274‐278. [PubMed] [Google Scholar]
- 37. Krueger HW, Sullivan CH. Models for carbon isotope fractionation between diet and bone. Stable Isotopes Nutr. 1984:205‐220. doi: 10.1021/bk-1984-0258.ch014 [DOI] [Google Scholar]
- 38. Jepsen S, Deschner J, Braun A, Schwarz F, Eberhard J. Calculus removal and the prevention of its formation. Periodontol 2000. 2011;55:167‐188. doi: 10.1111/j.1600-0757.2010.00382.x [DOI] [PubMed] [Google Scholar]
- 39. Wechsler A. The secretion of bicarbonate in saliva. MSc thesis, McGill University Montreal. 1959. Accessed May 4, 2020. https://central.bac-lac.gc.ca/.item?id=TC-QMM-112109&op=pdf&app=Library&oclc_number=892518560 [Google Scholar]
- 40. Sand HF. Source of the bicarbonate of saliva. J Appl Physiol. 1951;4(2):66‐76. doi: 10.1152/jappl.1951.4.2.66 [DOI] [PubMed] [Google Scholar]
- 41. Touger‐Decker R, van Loveren C. Sugars and dental caries. Am J Clin Nutr. 2003;78(4):881S‐892S. doi: 10.1093/ajcn/78.4.881S [DOI] [PubMed] [Google Scholar]
- 42. Sheiham A, James WPT. A reappraisal of the quantitative relationship between sugar intake and dental caries: The need for new criteria for developing goals for sugar intake. BMC Public Health. 2014;14(1):863. doi: 10.1186/1471-2458-14-863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mant M, Roberts C. Diet and dental caries in post‐medieval London. Int J Hist Archaeol. 2015;19(1):188‐207. doi: 10.1007/s10761-014-0286-x [DOI] [Google Scholar]
- 44. Mundorff A, Davoren JM. Examination of DNA yield rates for different skeletal elements at increasing post mortem intervals. Forensic Sci Int Genet. 2014;8(1):55‐63. doi: 10.1016/j.fsigen.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 45. Müldner G, Richards MP. Fast or feast: Reconstructing diet in later medieval England by stable isotope analysis. J Archaeol Sci. 2005;32(1):39‐48. doi: 10.1016/j.jas.2004.05.007 [DOI] [Google Scholar]
- 46. Beaumont J, Geber J, Powers N, Wilson A, Lee‐Thorp J, Montgomery J. Victims and survivors: Stable isotopes used to identify migrants from the great Irish famine to 19th century London. Am J Phys Anthropol. 2013;150(1):87‐98. doi: 10.1002/ajpa.22179 [DOI] [PubMed] [Google Scholar]
- 47. Jahren AH, Bostic JN, Davy BM. The potential for a carbon stable isotope biomarker of dietary sugar intake. J Anal At Spectrom. 2014;29(5):795‐816. doi: 10.1039/C3JA50339A [DOI] [Google Scholar]
- 48. Kontopoulos I, Presslee S, Penkman K, Collins MJ. Preparation of bone powder for FTIR‐ATR analysis: The particle size effect. Vib Spectrosc. 2018;99:167‐177. doi: 10.1016/j.vibspec.2018.09.004 [DOI] [Google Scholar]
- 49. Kazarian SG, Chan KLA. Applications of ATR‐FTIR spectroscopic imaging to biomedical samples. Biochim Biophys Acta 2006;1758(7):858‐867. doi: 10.1016/j.bbamem.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 50. Weiner S, Bar‐Yosef O. States of preservation of bones from prehistoric sites in the Near East: A survey. J Archaeol Sci. 1990;17(2):187‐196. doi: 10.1016/0305-4403(90)90058-D [DOI] [Google Scholar]
- 51. Li C, Ge X, Li G, Gao Q, Ding R. A facile hydrothermal method for synthesis of submillimeter‐long octacalcium phosphate and hydroxyapatite as drug carriers with sustained release behaviors. Adv Powder Technol. 2014;25(6):1661‐1666. doi: 10.1016/j.apt.2014.06.001 [DOI] [Google Scholar]
- 52. Wright LE, Schwarcz HP. Infrared and isotopic evidence for diagenesis of bone apatite at dos Pilas, Guatemala: Palaeodietary implications. J Archaeol Sci. 1996;23(6):933‐944. doi: 10.1006/jasc.1996.0087 [DOI] [Google Scholar]
- 53. Colonese AC, Lucquin A, Guedes EP, Thomas R, Best J, Fothergill BT, Sykes N, Foster A, Miller H, Poole K, Maltby M, von Tersch M, Craig OE The identification of poultry processing in archaeological ceramic vessels using in‐situ isotope references for organic residue analysis. J Archaeol Sci. 2017;78:179‐192. doi: 10.1016/j.jas.2016.12.006 [DOI] [Google Scholar]
- 54. Longin R. New method of collagen extraction for radiocarbon dating. Nature. 1971;230(5291):241‐242. doi: 10.1038/230241a0 [DOI] [PubMed] [Google Scholar]
- 55. Brown TA, Nelson DE, Vogel JS, Southon JR. Improved collagen extraction by modified Longin method. Radiocarbon. 1988;30(2):171‐177. doi: 10.1017/S0033822200044118 [DOI] [Google Scholar]
- 56. Snoeck C, Pellegrini M. Comparing bioapatite carbonate pre‐treatments for isotopic measurements: Part 1—Impact on structure and chemical composition. Chem Geol. 2015;417:394‐403. doi: 10.1016/j.chemgeo.2015.10.004 [DOI] [Google Scholar]
- 57. Pellegrini M, Snoeck C. Comparing bioapatite carbonate pre‐treatments for isotopic measurements: Part 2—Impact on carbon and oxygen isotope compositions. Chem Geol. 2016;420:88‐96. doi: 10.1016/j.chemgeo.2015.10.038 [DOI] [Google Scholar]
- 58. Miller AV, Fernandes R, Janzen A, et al. Sampling and pre‐treatment of tooth enamel carbonate for stable carbon and oxygen isotope analysis. J Vis Exp. 2018;138(138):58002. doi:10.3791/58002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crisp M, Demarchi B, Collins M, Morgan‐Williams M, Pilgrim E, Penkman K. Isolation of the intra‐crystalline proteins and kinetic studies in Struthio camelus (ostrich) eggshell for amino acid geochronology. Quat Geochronol. 2013;16:110‐128. doi: 10.1016/j.quageo.2012.09.002 [DOI] [Google Scholar]
- 60. Brooks AS, Hare PE, Kokis JE, Miller GH, Ernst RD, Wendorf F. Dating pleistocene archaeological sites by protein diagenesis in ostrich eggshell. Science. 1990;248(4951):60‐64. doi: 10.1126/science.248.4951.60 [DOI] [PubMed] [Google Scholar]
- 61. Mackie M, Radini A, Speller CF. The sustainability of dental calculus for archaeological research. In: Favreau J, Patalano R, ed. Shallow Pasts, Endless Horizons: Sustainability & Archaeology: Proceedings of the 48th Annual Chacmool Archaeology Conference. 2017:74‐81. http://eprints.whiterose.ac.uk [Google Scholar]
- 62. Roberts P, Fernandes R, Craig OE, et al. Calling all archaeologists: Guidelines for terminology, methodology, data handling, and reporting when undertaking and reviewing stable isotope applications in archaeology. Rapid Commun Mass Spectrom. 2018;32(5):361‐372. doi: 10.1002/rcm.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kragten J. Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst. 1994;119(10):2161‐2165. doi: 10.1039/an9941902161 [DOI] [Google Scholar]
- 64. Szpak P, Metcalfe JZ, Macdonald RA. Best practices for calibrating and reporting stable isotope measurements in archaeology. J Archaeol Sci Rep. 2017;13:609‐616. doi: 10.1016/j.jasrep.2017.05.007 [DOI] [Google Scholar]
- 65. Graven H, Allison CE, Etheridge DM, et al. Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci Model Dev. 2017;10(12):4405‐4417. doi:10.5194/gmd‐10‐4405‐2017 [Google Scholar]
- 66. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Accessed April 4, 2021. https://www.r-project.org/
- 67. Hammer Ø, Harper DAT, Ryan PD, Others. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- 68. IBM . IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corporation; 2019. [Google Scholar]
- 69. Koch PL, Tuross N, Fogel ML. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J Archaeol Sci. 1997;24(5):417‐429. doi: 10.1006/jasc.1996.0126 [DOI] [Google Scholar]
- 70. Lee‐Thorp JA. Preservation of biogenic carbon isotopic signals in Plio‐Pleistocene bone and tooth mineral. In: Ambrose SH, Katzenberg MA, ed. Biogeochemical Approaches to Paleodietary Analysis. Boston, MA: Springer US; 2002:89‐115. doi: 10.1007/0-306-47194-9_5 [DOI] [Google Scholar]
- 71. Kontopoulos I, Penkman K, McAllister GD, et al. Petrous bone diagenesis: A multi‐analytical approach. Palaeogeogr Palaeoclimatol Palaeoecol. 2019;518:143‐154. doi: 10.1016/j.palaeo.2019.01.005 [DOI] [Google Scholar]
- 72. Kontopoulos I, Penkman K, Mullin VE, et al. Screening archaeological bone for palaeogenetic and palaeoproteomic studies. PLoS One. 2020:15(6):e0235146. doi: 10.1371/journal.pone.0235146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laskus A, Kolmas J. Ionic substitutions in non‐apatitic calcium phosphates. Int J Mol Sci. 2017;18(12). 10.3390/ijms18122542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tzaphlidou M, Zaichick V. Calcium, phosphorus, calcium‐phosphorus ratio in rib bone of healthy humans. Biol Trace Elem Res. 2003;93(1‐3):63‐74. doi: 10.1385/BTER:93:1-3:63 [DOI] [PubMed] [Google Scholar]
- 75. Nasution AH, Amatanesia DD. Correlation of salivary phosphorous level to dental calculus accumulation on patients of the periodontology installation in dental hospital of USU. J Phys Conf Ser. 2018;1116:052044. doi: 10.1088/1742-6596/1116/5/052044 [DOI] [Google Scholar]
- 76. Nielsen‐Marsh CM, Hedges REM. Patterns of diagenesis in bone I: The effects of site environments. J Archaeol Sci. 2000:27(12):1139‐1150. doi: 10.1006/jasc.1999.0537 [DOI] [Google Scholar]
- 77. Nielsen‐Marsh C, Gernaey A, Turner‐Walker G, Hedges R, Pike AWG, Collins M. The chemical degradation of bone. In: Cox M, Mays S, eds. Human Osteology: Archaeology and Forensic Science. Cambridge: Cambridge University Press; 2000:548. [Google Scholar]
- 78. Kohn MJ, Schoeninger MJ, Barker WW. Altered states: Effects of diagenesis on fossil tooth chemistry. Geochim Cosmochim Acta. 1999;63(18):2737‐2747. doi: 10.1016/S0016-7037(99)00208-2 [DOI] [Google Scholar]
- 79. Trueman CN, Privat K, Field J. Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr Palaeoclimatol Palaeoecol. 2008;266(3‐4):160‐167. doi: 10.1016/j.palaeo.2008.03.038 [DOI] [Google Scholar]
- 80. Dal Sasso G, Asscher Y, Angelini I, Nodari L, Artioli G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci Rep. 2018;8(1):12025. doi: 10.1038/s41598-018-30642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci. 1990;17(4):431‐451. doi: 10.1016/0305-4403(90)90007-R [DOI] [Google Scholar]
- 82. van Klinken GJ. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J Archaeol Sci. 1999;26(6):687‐695. doi: 10.1006/jasc.1998.0385 [DOI] [Google Scholar]
- 83. DeNiro MJ. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature. 1985;317(6040):806‐809. doi: 10.1038/317806a0 [DOI] [Google Scholar]
- 84. Warinner C, Tuross N. Alkaline cooking and stable isotope tissue‐diet spacing in swine: Archaeological implications. J Archaeol Sci. 2009;36(8):1690‐1697. doi: 10.1016/j.jas.2009.03.034 [DOI] [Google Scholar]
- 85. Hedges REM, Clement JG, Thomas CDL, O'connell TC. Collagen turnover in the adult femoral mid‐shaft: Modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol. 2007;133(2):808‐816. doi: 10.1002/ajpa.20598 [DOI] [PubMed] [Google Scholar]
- 86. Schuurs A. Pathology of the Hard Dental Tissues. Hoboken, NJ: Blackwell Publishing; 2013, doi: 10.1002/9781118702659 [DOI] [Google Scholar]
- 87. Staal M, Thar R, Kühl M, et al. Different carbon isotope fractionation patterns during the development of phototrophic freshwater and marine biofilms. Biogeosciences. 2007:4;613‐626. doi:10.5194/bg‐4‐613‐2007 [Google Scholar]
- 88. Ayliffe LK, Cerling TE, Robinson T, et al. Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia. 2004;139(1):11‐22. doi: 10.1007/s00442-003-1479-x [DOI] [PubMed] [Google Scholar]
- 89. Passey BH, Robinson TF, Ayliffe LK, et al. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. J Archaeol Sci. 2005;32(10):1459‐1470. doi: 10.1016/j.jas.2005.03.015 [DOI] [Google Scholar]
- 90. Froehle AW, Kellner CM, Schoeninger MJ. Multivariate carbon and nitrogen stable isotope model for the reconstruction of prehistoric human diet. Am J Phys Anthropol. 2012;147(3):352‐369. doi: 10.1002/ajpa.21651 [DOI] [PubMed] [Google Scholar]
- 91. Krigbaum J. Neolithic subsistence patterns in northern Borneo reconstructed with stable carbon isotopes of enamel. J Anthropol Archaeol. 2003;22(3):292‐304. doi: 10.1016/S0278-4165(03)00041-2 [DOI] [Google Scholar]
- 92. Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS Data Brief. 2013;1–8. https://www.cdc.gov/nchs/data/databriefs/db122.pdf. Accessed 2 October 2021 [PubMed]
- 93. Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005. –2008. NCHS Data Brief 2012;1–8. https://www.cdc.gov/nchs/data/databriefs/db87.pdf. Accessed 2 October 2021 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Human δ13C and δ15N values from modern, post‐medieval and medieval individuals
Table S1: Enamel carbonate, calculus carbonate, bone collagen, bone carbonate isotope data and collagen quality indicators for all samples analysed. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S2: Individual δ13C isotope offsets in this study. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S3: Summary of bone and dental calculus FTIR‐ATR data displaying the average of data measured in triplicate ‐ IRSF: Infrared splitting factor; C/P: carbonate‐to‐phosphate ratio.
Table S4: Modern carbon isotope data with required adjustments/corrections. Contains Isotope Data, produced by the British Geological Survey, UKRI.
Table S5: Post hoc results for all populations. The significance level is 0.05 and the significance values have been adjusted by the Bonferroni correction for multiple tests. Contains Isotope Data, produced by the British Geological Survey, UKRI
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


