Abstract
Undernutrition limits reproduction through inhibition of gonadotropin‐releasing hormone (GnRH)/luteinizing hormone (LH) secretion. Because KNDy neurons coexpress neuropeptides that play stimulatory (kisspeptin and neurokinin B [NKB]) and inhibitory (dynorphin) roles in pulsatile GnRH/LH release, we hypothesized that undernutrition would inhibit kisspeptin and NKB expression at the same time as increasing dynorphin expression. Fifteen ovariectomized lambs were either fed to maintain pre‐study body weight (controls) or feed‐restricted to lose 20% of pre‐study body weight (FR) over 13 weeks. Blood samples were collected and plasma from weeks 0 and 13 were assessed for LH by radioimmunoassay. At week 13, animals were killed, and brain tissue was processed for assessment of KNDy peptide mRNA or protein expression. Mean LH and LH pulse amplitude were lower in FR lambs compared to controls. We observed lower mRNA abundance for kisspeptin within KNDy neurons of FR lambs compared to controls with no significant change in mRNA for NKB or dynorphin. We also observed that FR lambs had fewer numbers of arcuate nucleus kisspeptin and NKB perikarya compared to controls. These findings support the idea that KNDy neurons are important for regulating reproduction during undernutrition in female sheep.
Keywords: dynorphin, kisspeptin, LH, neurokinin B, undernutrition, xxxx, fx1
1. INTRODUCTION
Reproduction is vital for the preservation of mammalian species and is influenced by numerous environmental cues through the central regulation of gonadotropin‐releasing hormone (GnRH) secretion. Sufficient energy intake is one such external factor that can determine reproductive capacity, as illustrated by undernutrition delaying puberty onset, 1 , 2 , 3 prolonging postpartum anestrus, 4 , 5 , 6 and inhibiting ovulatory cycles. 7 Although caloric restriction has been shown to inhibit GnRH release, and in turn reduce luteinizing hormone (LH) secretion, 8 GnRH neurons are devoid of receptors for metabolic signals such as leptin and insulin. 9 , 10 , 11 , 12 , 13 Thus, reduced energy availability during undernutrition is likely relayed to GnRH neurons through as of yet to be fully elucidated afferent neuronal inputs consisting of reduced excitatory drive and/or an elevated inhibitory drive.
It is well‐established that kisspeptin signaling is essential for reproductive success. Initial evidence of this importance came from genetic mutations in humans and mice that resulted in impaired fertility. 14 , 15 Given that kisspeptin has elicited either GnRH or LH secretion in all species studied to date 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and that the vast majority of GnRH neurons coexpress the receptor for kisspeptin, Kiss1R, 16 , 24 , 25 , 26 kisspeptin is considered to be a key stimulatory element of GnRH/LH secretion. Kisspeptin perikarya primarily reside in the arcuate nucleus (ARC) 27 and the anteroventral periventricular nucleus/rostral periventricular area of the third ventricle of rodent species, 28 , 29 , 30 or in the preoptic area of non‐rodent species. 31 The ARC kisspeptin population highly coexpresses two other neuropeptides relevant to reproduction, neurokinin B (NKB) and dynorphin, 31 and were thus termed KNDy neurons. 32 Similar to kisspeptin, NKB is considered to play a stimulatory role in GnRH/LH secretion because deletion of NKB in humans leads to infertility, 33 and multiple groups have shown that activation of the NKB receptor, neurokinin 3 receptor (NK3R), increases LH secretion. 34 , 35 , 36 , 37 However, unlike kisspeptin, NKB appears to stimulate GnRH neurons indirectly via local activation of KNDy neurons, which highly coexpress NK3R. 38 Dynorphin, on the other hand, is known for mediating the inhibitory influence of progesterone on GnRH/LH secretion, 39 which could be directly exerted on GnRH neurons and/or on KNDy neurons given that both cell types express receptors for dynorphin, kappa‐opioid receptors. 40 , 41 Together with evidence indicating that at least 45%–60% of GnRH neurons receive input from KNDy neurons, 42 this population of cells has been suggested to regulate the pulsatile nature of GnRH/LH secretion. 43
There is growing evidence indicating that KNDy neurons may also play a key role in mediating the effects of energy insufficiency on GnRH neurons during chronic undernutrition. Given that ARC kisspeptin neurons, which are presumptively KNDy neurons, express receptors for both leptin and insulin, 13 , 44 , 45 , 46 they possess the potential to directly detect changes in energy balance and influence GnRH/LH secretion. Work conducted in ovariectomized (OVX) rodents has shown that extended periods of nutritional restriction (2–6 weeks) reduces ARC mRNA abundance of kisspeptin. 47 , 48 In addition, studies using chronic feed restriction for 6–10 months in OVX adult sheep have also demonstrated that undernutrition inhibits ARC kisspeptin mRNA abundance. 45 In support of this, our recent work in young (less than 1 year of age), castrated male sheep demonstrates that a shorter duration of feed restriction (approximately 13 weeks) is sufficient to reduce mRNA and protein expression of ARC kisspeptin. 49 Similar to kisspeptin, ARC NKB mRNA abundance in rodents 47 , 48 and protein expression in young male sheep 49 are reduced with chronic undernutrition. However, unlike kisspeptin and NKB, there has been no report of the impact of undernutrition on dynorphin expression in sheep, and the current rodent data for dynorphin are inconsistent because chronic undernutrition reduces ARC mRNA for dynorphin in mice 48 but has no effect on mRNA for dynorphin in rats. 47
With such a high degree of coexpression, it is likely that the impact of undernutrition on ARC kisspeptin, NKB, and dynorphin resides largely within KNDy neurons. However, there has yet to be a report detailing the impact of chronic undernutrition on these reproductively relevant neuropeptides using methods to detect both mRNA and protein within the same animal. Given their stimulatory (kisspeptin and NKB) and inhibitory (dynorphin) roles in GnRH/LH secretion, we hypothesized that chronic undernutrition, which we have shown suppresses LH secretion in young, castrated male sheep, 49 would inhibit expression of kisspeptin and NKB at the same time as stimulating dynorphin expression in the ARC and within KNDy neurons of OVX sheep. In the present study, we used OVX sheep, a longstanding animal model in reproduction, 50 to directly examine the nutritional effects on LH secretion apart from changes in sensitivity to gonadal steroids. In addition, in the present study, we used maintenance fed females as controls. Because this is a period when lambs are typically growing, maintaining body weights during this period represents a mild form of growth restriction, but provides a relatively constant metabolic background devoid of additional growth‐related cues with which to compare our feed restricted animals. Furthermore, because we previously reported that ARC dynorphin is undetectable when using immunohistochemistry in young male and female sheep, 40 , 49 in the present study we use a relatively new fluorescent in situ hybridization technique, RNAscope, to simultaneously assess mRNA for kisspeptin, NKB, and dynorphin together with classic immunohistochemistry for protein detection of kisspeptin and NKB in the ARC of young, OVX sheep during chronic feed restriction.
2. MATERIALS AND METHODS
2.1. Institutional Review Board Statement
All procedures were approved by the North Carolina State University Animal Care and Use Committee (#17‐020‐B) and followed the National Institutes of Health guidelines for use of animals in research.
2.2. Animals
Fifteen Suffolk ewe lambs from single, twin, or triplet pregnancies were approximately 4–5 months of age at the start of this study, which was conducted from July through October 2019. Prior to the study, ewes were group‐housed in an open barn for a minimum of 14 days, received ad libitum access to water, and were fed hay supplemented with the same diet used during the experiment (Mule City Specialty Feeds; crude protein, 12%; crude fat, 2.5%; crude fiber, 5.0%; 3.28 Mcal kg−1). Once moved indoors for the study, ewe lambs were housed individually (2.32 m2 per pen) except for when pair housed (1.39 m2 per pen) 7 days prior to and 14 days after OVX, performed as described previously 37 at the North Carolina State University School of Veterinary Medicine. No corpora lutea were present on any ovaries at the time of OVX. Animals were housed in raised‐floor penning made of polyvinyl chloride or stainless steel with a clear view of other sheep, were fed once daily with the experiment diet, were allowed ad libitum access to water, and provided with indoor lighting automated to mimic natural photoperiod.
2.3. Experimental design
Thirty days after OVX, animals were placed into one of two groups, fed to maintain (FM) pre‐study body weight (n = 7) or feed‐restricted (FR) to lose 20% of pre‐study body weight by week 13 (n = 8), with animals from single, twin, and triplet pregnancies present in each group. All animals were weighed weekly, and the amount of feed was adjusted on an individual animal basis to achieve the desired body weight. Biweekly, blood samples (3–4 mL per sample) were taken via jugular venipuncture every 12 min for 4.5 h (276 min), and plasma samples were stored at −20°C until assayed for LH by a previously established radioimmunoassay. 49 At the end of the experiment (week 13), ewes were given heparin (20,000 U, i.v.) and killed with an i.v. overdose of sodium pentobarbital (Euthasol; Patterson Veterinary). Heads were removed and perfused via the carotid arteries with four liters of 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (pH 7.4) containing 0.1% sodium nitrite. Blocks of hypothalamic tissue were removed and stored in 4% PFA for 24 h at 4°C and transferred to a 20% sucrose solution until sectioning. Frozen coronal sections were cut at 45–50 μm with a freezing microtome into a series of five vials and stored in cryopreservative solution until RNAscope and immunohistochemistry.
2.4. RNAscope in situ hybridization
For detection of mRNA for kisspeptin, NKB, and dynorphin, RNAscope was conducted similarly to that reported previously. 49 Briefly, RNAscope was performed on four medial ARC hemisections (at least 250 μm apart) per animal using the RNAscope Multiplex Fluorescent Reagent Kit v2 (Advanced Cell Diagnostics; catalog. no. 323100), and all incubations at 40°C were completed using an ACD HybEZ II Hybridization System with EZ‐Batch Slide System (Advanced Cell Diagnostics; catalog. no. 321710). On day 1, hemisections were incubated in 0.1 m phosphate buffered saline (PBS) (pH 7.4) on a rocking shaker at 4°C overnight. On day 2, hemisections were mounted onto Superfrost/Plus microscope slides (Fisher Scientific), allowed to air dry for 2 h, and then the slides were heated on a slide warmer to 60°C for 90 min. Next, slides were incubated in 4% PFA at 4°C for 1 h, rinsed four times in 0.1 m PBS (5 min per rinse), and then incubated in increasing concentrations of ethanol (50%, 70%, 100%, and 100%) for 5 min at each concentration. Then slides were air‐dried at room temperature (RT) for 5 min followed by incubation in hydrogen peroxide solution (Advanced Cell Diagnostics; catalog. no. 322335) for 10 min at RT. Next, slides were briefly rinsed three times with deionized water, incubated with target retrieval solution (Advanced Cell Diagnostics; catalog. no. 322001) for 10 min at 94°C, and rinsed three times in deionized water followed by submersion in 100% ethanol three times and then allowed to air dry. A hydrophobic barrier was created around each hemisection using an ImmEdge Pen (Advanced Cell Diagnostics; catalog. no. 310018), and slides were stored overnight at RT. On day 3, sections were treated with RNAscope Protease III (Advanced Cell Diagnostics; catalog. no. 322337) for 30 min at 40°C. Probes for target genes and positive controls were mixed at a concentration of 50:1:1 for the channel 1 probe, channel 2 probe, and channel 3 probe, respectively, and all probe solutions, including the negative control solution, were heated to 40°C for 10 min in a water bath and cooled to RT before application. Following Protease III, tissue was incubated with RNAscope target probes from Advanced Cell Diagnostics (NKB, Oa‐TAC3‐O1, catalog. no. 481411; dynorphin, Oa‐PDYN‐O1‐C2, catalog. no. 481421‐C2; kisspeptin, Oa‐KISS1‐C3, catalog. no. 497471‐C3) or control probes from Advanced Cell Diagnostics (positive controls: Oa‐POLR2A, catalog. no. 516171; Oa‐PPIB, catalog. no. 457031‐C2; Oa‐UBC‐C3, catalog. no.516181‐C3; negative control: 3‐plex Negative Control Probe, catalog. no. 320871) for 2 h at 40°C. Next, slides were washed twice (2 min each) at RT with 1 × Wash Buffer (Advanced Cell Diagnostics; catalog. no. 310091) followed by sequential tissue application and incubation of the following at 40°C with 2 min washes using 1 × Wash Buffer between applications: RNAscope Multiplex FL v2 AMP 1 (Advanced Cell Diagnostics; catalog. no. 323101) for 30 min, RNAscope Multiplex FL v2 AMP 2 (Advanced Cell Diagnostics; catalog. no. 323102) for 30 min, and RNAscope Multiplex FL v2 AMP 3 (Advanced Cell Diagnostics; catalog. no. 323103) for 15 min. Following the final incubation with AMP 3, slides were rinsed twice (2 min each) with 1 × Wash Buffer at RT followed by tissue application of RNAscope Multiplex FL v2 HRP C1 (Advanced Cell Diagnostics; catalog. no. 323104) for 15 min at 40°C. Next, sections were incubated with Opal 690 (Fisher Scientific; catalog. no. NC1605064) in RNAscope TSA buffer (Advanced Cell Diagnostics; catalog. no. 322809) at a final concentration of 1:1500 for 30 min at 40°C. Following two rinses (2 min each) with 1 × Wash Buffer at RT, RNAscope Multiplex FL v2 HRP Blocker (Advanced Cell Diagnostics; catalog. no. 323107) was applied to tissue for 15 min at 40°C. Slides were then rinsed twice (2 min each) with 1 × Wash Buffer at RT followed by tissue application of RNAscope Multiplex FL v2 HRP‐C2 (Advanced Cell Diagnostics; catalog. no. 323106) for 15 min at 40°C. Sections were next incubated with Opal 570 (Fisher Scientific; catalog. no. NC1601878) in RNAscope TSA buffer at a final concentration of 1:1500 for 30 min at 40°C, and followed by two rinses (2 min each) in 1 × Wash Buffer at RT. Then, RNAscope Multiplex FL v2 HRP Blocker was applied to tissue for 15 min at 40°C. Finally, slides were then rinsed twice (2 min each) with 1 × Wash Buffer at RT followed by tissue application of RNAscope Multiplex FL v2 HRP‐C3 (Advanced Cell Diagnostics; catalog. no. 323106) for 15 min at 40°C. Sections were next incubated with Opal 520 (Fisher Scientific; catalog. no. NC1601877) in RNAscope TSA buffer at a final concentration of 1:1500 for 30 min at 40°C, and followed by two rinses (2 min each) in 1 × Wash Buffer at RT. Then, RNAscope Multiplex FL v2 HRP Blocker was applied to tissue for 15 min at 40°C. Finally, slides were coverslipped with Invitrogen ProLong Gold Antifade Mountant (Fisher Scientific; catalog. no. P36930) and stored at 4°C until image acquisition.
2.5. Immunohistochemistry
Dual‐label immunohistochemistry was completed for free‐floating hypothalamic hemisections for the detection of kisspeptin and NKB using primary antisera validated for use in sheep. 49 , 51 Three medial‐caudal ARC sections (at least 250 μm apart) were selected per animal from a series of every fifth hypothalamic section. On day 1, sections were washed 12 times (5 min each) in 0.1 m PBS at RT. Subsequent steps were also performed at RT. Sections were incubated in 0.1 m PBS containing 1% hydrogen peroxide (H2O2) for 10 min followed by three washes in 0.1 m PBS (5 min each). This was followed by incubation in 0.1 m PBS solution with 0.4% Triton X‐100 (Sigma‐Aldrich) and 20% normal goat serum (NGS) (Jackson ImmunoResearch Laboratories, Inc.) for 1 h. Then, sections were incubated with primary antibody for detection of kisspeptin peptide (1:50,000; rabbit anti‐kisspeptin; Gift from I. Franceschini, #566) diluted in 0.1 m PBS containing 0.4% Triton X‐100 and 20% NGS overnight for 16 h. On day 2, sections were washed in 0.1 m PBS three times (5 min each) and subsequently incubated in biotinylated goat anti‐rabbit IgG (1:400; Vector Laboratories; catalog. no. BA‐1000) in 0.1 m PBS with 0.4% Triton X‐100 and 20% NGS for 1 h. After washing three times in 0.1 m PBS (5 min each), tissue was sequentially incubated in Vectastain ABC (1:600 diluted in 0.1 m PBS; Vector Laboratories; catalog. no. PK‐6100), Biotinyl‐Tyramide (1:250 diluted in 0.1 m PBS to 1 μL mL−1 of 3% H2O2; Perkin Elmer; catalog. no. NEL700A), and DyLight 488 green conjugated to streptavidin (1:200 diluted in 0.1 m PBS; Thermo Fisher Scientific; catalog. no. 21832), for 1 h, 10 min, and 1 h, respectively, with three rinses in 0.1 m PBS (5 min each) between each step. Tissue was covered to prevent light exposure following the application of fluorescent dyes. After DyLight application, tissue was rinsed four times in PBS (5 min each) and incubated in 0.1 m PBS with 0.4% Triton X‐100 and 20% NGS for 1 h followed by incubation in rabbit anti‐NKB (1:250; Phoenix Pharmaceuticals, Inc.; catalog. no. H‐046‐26) diluted in 0.1 m PBS with 0.4% Triton X‐100 and 4% NGS overnight for 16 h. On day 3, tissue was washed in 0.1 m PBS three times (5 min each) and incubated in goat anti‐rabbit Alexa‐555 (1:200; Thermo Fisher Scientific; catalog. no. A‐21428) diluted in 0.1 m PBS with 0.4% Triton X‐100 and 20% NGS for 1 h. After finally washing four times in 0.1 m PBS (5 min each), tissue sections were mounted on slides, air dried, coverslipped using gelvatol, and stored in the dark at 4°C.
2.6. Data analysis
2.6.1. LH assay
Luteinizing hormone concentrations were measured in duplicate for each sample with a radioimmunoassay using 50–200 μL of plasma and reagents purchased from the National Hormone and Peptide Program as described previously. 52 Assay sensitivity was 0.20 ng per tube with the intra‐ and inter‐assay coefficients of variation being 8.26% and 12.60%, respectively. Pulses were identified using three previously described criteria 53 : (1) a peak must exceed assay sensitivity; (2) a peak must occur within two data points of the previous nadir; and (3) a peak must exceed a 95% confidence interval (CI) of the previous and following nadirs.
2.6.2. RNAscope in situ hybridization
Imaging of mRNA for kisspeptin, NKB, and dynorphin was used to identify ARC cell numbers of kisspeptin neurons, NKB neurons, dynorphin neurons, and KNDy neurons. Images from two non‐overlapping confocal z‐stack images, one dorsal‐medial and one ventral‐medial, were captured at 1‐μm optical sections from ARC sections (four hemisections per animal) using a Zeiss 880 confocal laser scanning microscope equipped with a Plan Apochromat 20×/0.8 dry objective with consistent acquisition settings for all images and images were acquired by an observer blinded to treatment within 3 weeks following RNAscope in situ hybridization. Following acquisition, each image was opened using Zen 2.3 SP1 Black (Zeiss), where individual cells were marked using ScreenMarker MFC Application 1.0.0.1 (Uptodown), ensuring that each cell was only counted once. Then, the blinded observer used Fiji/ImageJ 54 to quantify the average number of cells expressing each transcript, as well as the percentage of cells that coexpressed multiple transcripts. In addition, images of individual KNDy neurons (39–40 cells per animal), which expressed mRNA for kisspeptin, NKB, and dynorphin, were used to determine the integrated density of each transcript within KNDy neurons. Confocal z‐stack images of randomly selected KNDy cells that encompassed each cell were captured at 1 μm optical sections through the cell with a Zeiss 880 confocal laser scanning microscope equipped with a Plan Apochromat 63×/1.4 oil objective with acquisition settings held constant for all images and images were acquired by an observer blinded to treatment within 3 weeks following RNAscope in situ hybridization. Following image acquisition, an observer blinded to treatment using Fiji/ImageJ converted images to 8‐bit and applied a region of interest (312 × 312 pixels) directly over each cell to determine integrated density. An automatic minimum threshold was recorded for each channel corresponding to its specific label in all optical slices to calculate an average threshold for each channel. These respective averages were used as the fixed threshold intensity for integrated density analysis to normalize results across treatment. Three optical slices from the center of each cell, as determined by the extent of detectable signal throughout the cell, were used for analysis with the sum of the integrated density values calculated per cell and then averaged per animal for statistical comparison.
2.6.3. Immunohistochemistry
To assess the number of kisspeptin‐positive, NKB‐positive, and neurons coexpressing both peptides following immunohistochemistry for protein detection, ARC tissue sections were imaged using a MIF Olympus VS120 Slide Scanner. Images were taken by an experimenter blinded to treatment using a U PlanS Apochromat 20×/0.75 dry objective with consistent acquisition settings for all images and neurons from each hemisection were counted by an experimenter blinded to treatment using OlyVIA, version 2.9.1 (Olympus), and the number of cells per hemisection was averaged per animal for statistical comparison.
2.7. Statistical analysis
Outlier analysis was conducted on mean LH from week 0 using the Igewicz and Hoalglin's robust test for multiple outliers (two sided test) with an outlier criterion modified z score (≥ 3.5) (online outlier test; Contchart Software). Average body weights were analyzed using repeated‐measures ANOVA allowing for effects of time and treatment and with a first‐order autoregressive covariance structure. The covariance structure was selected using Akaike information criterion. Between group comparisons were run within each week using an F test. The percent change in bodyweight is also reported. Mean LH concentrations, LH pulse amplitude, and the log‐transformed average LH interpulse interval were each analyzed using repeated measures two‐way ANOVA allowing for effects of time and treatment and a variance components structure on the covariance matrix. Analyses of cell numbers (RNAscope and immunohistochemistry), percent coexpression (RNAscope and immunohistochemistry), and integrated density (RNAscope) were conducted using a one‐tailed, unpaired Student's t test with Satterthwaite's approximation to adjust for unequal variances. All values are reported as means and 95% CIs. Model fits were assessed via visual inspection of residual diagnostics. p < 0.05 was considered statistically significant. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Body weights
Average weekly body weights from FM OVX lambs and FR OVX lambs throughout the study are illustrated in Figure 1. The repeated‐measures model found a strong interaction between week and treatment (F 13,169 = 9.54, p < 0.001). This interaction term can be explained by the increasing difference in body weights between treatment groups through the duration of the study. At the beginning of the experiment (week 0), there was no evidence to suggest that total body weights were different (F 1,169 =0.15, p = 0.703) between FM OVX lambs (30.99; 95% CI = 28.52–33.47 kg) and FR OVX lambs (31.65; 95% CI = 29.33–33.97 kg). Beginning at week 6 through to the end of the study, total body weights were lower (F 1,169 = 6.94; p < 0.05) in FR OVX lambs compared to FM OVX lambs. At week 13 (day of tissue collection), the average total body weight was higher (F 1,169 = 22.82, p < 0.001) in FM OVX lambs (33.07; 95% CI = 30.59–35.55 kg) compared to FR OVX lambs (24.86; 95% CI = 22.545–27.180 kg), and, as designed, the average percent change in pre‐study body weight for the FM and FR OVX lambs at week 13 was 7.35% (95% CI = 2.35%–12.36%) and −21.36% (95% CI = −23.43% to 19.28%), respectively.
FIGURE 1.
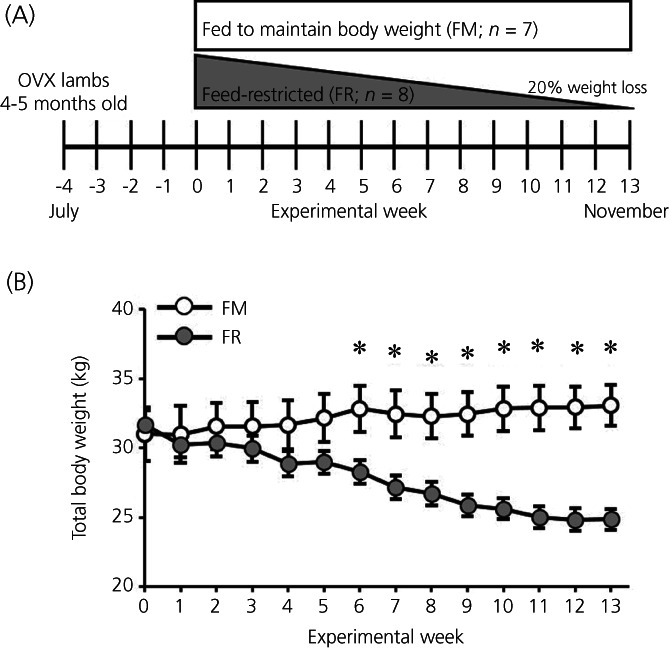
(A) Schematic illustration of the experimental design where young (4–5 month old) female sheep were ovariectomized (OVX) in the month of July, which was 30 days prior to week 0, when they were placed into either a fed to maintain pre‐study body weight group (FM; n = 7) or a feed‐restricted to lose 20% of their pre‐study body weight (FR; n = 8) by week 13 group (November). (B) Mean ± SEM weekly total body weights were recorded throughout the study and feed intake was adjusted weekly to achieve the desired weight change in each group. A repeated‐measures two‐way ANOVA found a significant interaction between the effects of treatment and time, and fed and feed‐restricted groups were compared weekly via F tests for simple effects. From week 6 to week 13, average body weights were lower (*p < 0.05) in FR animals compared to FM animals
3.2. LH data
Representative LH pulse profiles from a FM and a FR OVX lamb taken at the beginning (week 0) and end (week 13) of the study are shown in Figure 2. At week 0, a time when all animals exhibited equivalent body weights, one animal (FM #19008) was identified as an outlier based on mean LH concentrations (#19008, 4.28 ng mL−1; all other animals, 17.61 ± 1.2 ng mL−1). Data from this ewe were excluded from all further analysis. Quantification of mean LH concentration, LH interpulse interval, and LH pulse amplitude for FM and FR OVX lambs at weeks 0 and 13 are illustrated in Figure 3. There was an interaction of time × treatment for mean LH concentration (F 1,12 = 5.31, p = 0.040). The interaction term for the LH pulse amplitude was not quite sufficiently large to meet p < .05 for statistical significance (F 1,12 = 3.78, p = 0.076). The effect is significant at the p < 0.1, and post‐hoc tests were still run. Although not different at week 0, at week 13 the mean LH concentration (Figure 3A) and LH pulse amplitude (Figure 3B) were lower (F 1,12 = 9.79, p = .009 and F 1,12 = 7.94, p = 0.016, respectively) in FR OVX lambs compared to FM OVX lambs. No significant differences between groups were detected for LH interpulse interval (Figure 3C) (F1,12 = 0.27, p = 0.6144).
FIGURE 2.
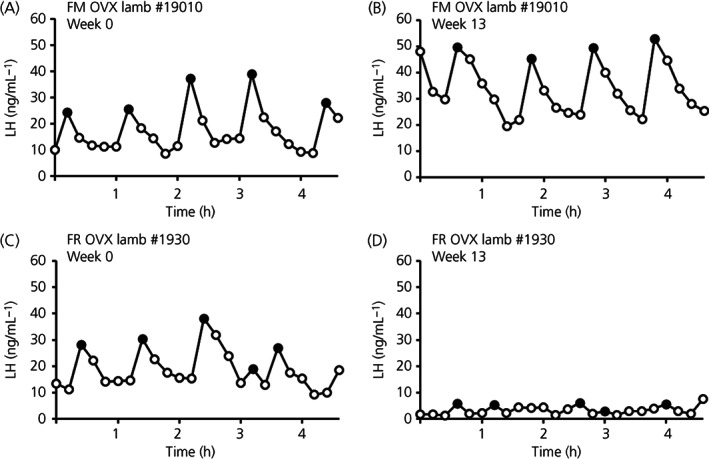
Representative luteinizing hormone (LH) pulse profiles at week 0 (A,C) and week 13 (B,D) for a fed to maintain (FM) (A,B) ovariectomized (OVX) lamb and a feed‐restricted (FR) (C,D) OVX lamb. LH pulses are denoted by closed circles
FIGURE 3.
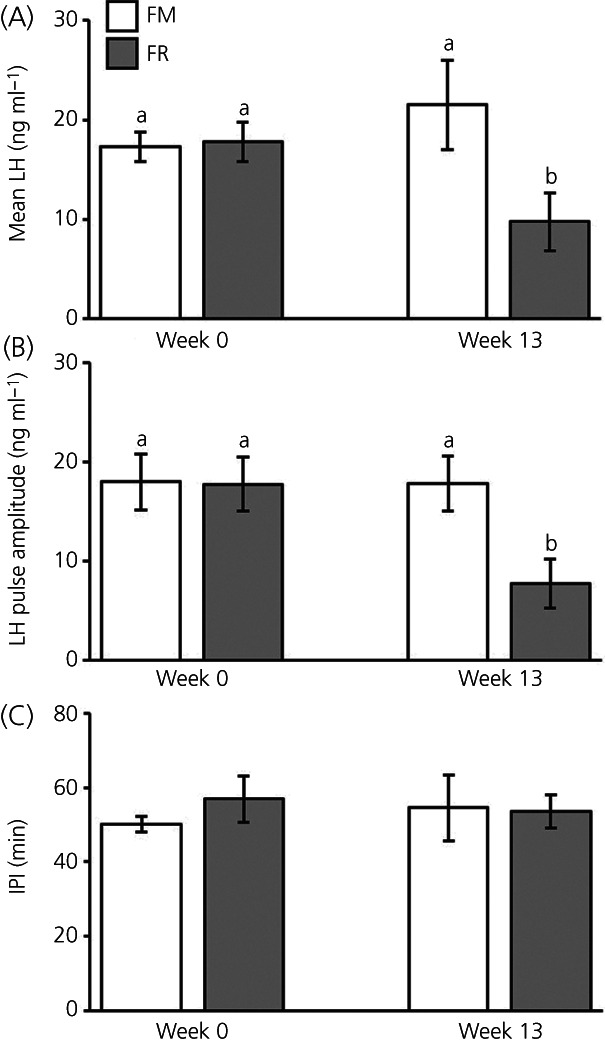
Mean ± SEM concentrations of luteinizing hormone (LH) (A), LH pulse amplitude (B), and LH interpulse interval (IPI) (C) from fed to maintain (FM; n = 6) and feed‐restricted (FR; n = 8) ovariectomized (OVX) lambs. Mean LH was significantly lower in FR OVX lambs compared to FM OVX lambs at week 13 (F 1,12 = 9.79, p = 0.009), but not at week 0. LH pulse amplitude was significantly lower in FR OVX lambs compared to FM OVX lambs at week 13 (F 1,12 = 7.94, p = 0.016, respectively), but not at week 0. IPI was not significantly different between groups at either time point (F 1,12 = 0.27, p = 0.6144). Statistical significance (p < 0.05) is indicated by differing superscripts and was determined using a repeated measure two‐way ANOVA
3.3. Kisspeptin data
We assessed mRNA for kisspeptin in the ARC between FM and FR OVX lambs using RNAscope. Cells expressing mRNA for kisspeptin were readily visible in the ARC of both FM OVX lambs (Figure 4A) and FR OVX lambs (Figure 4B). The average number of kisspeptin cells (Figure 4C) was lower in FR OVX lambs compared to FM OVX lambs (difference of means = 55.42, SE = 28.2, t 9.5 = 1.96, p = 0.040), but the percentage of kisspeptin neurons that coexpressed NKB and dynorphin in the FM and FR animals did not differ (difference of means = 0.44, SE = 9.87, t 10.4 = .05, p = 0.483) and was 76.0% (SE = 6.45) and 76.4% (SE = 7.72), respectively. The expression of mRNA for kisspeptin within KNDy cells (Figure 4D) was lower (difference of means = 2,807,598, SE = 915,588, t 10.7 = 3.07, p = 0.006) in FR OVX lambs compared to FM OVX lambs.
FIGURE 4.
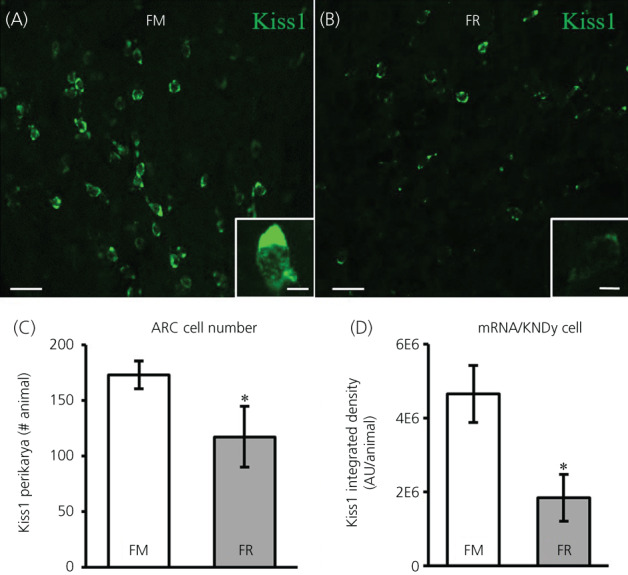
Kisspeptin mRNA (Kiss1) in the arcuate nucleus (ARC) of fed to maintain (FM) and feed‐restricted (FR) ovariectomized (OVX) lambs. Confocal images (20×) of Kiss1 cells (green) from a FM (A) and a FR (B) OVX lamb. Insets: 1 μm optical sections; 63× objective. (C) Mean ± SEM number of Kiss1 perikarya in the ARC was significantly different (difference of means = 55.42, SE = 28.2, t 9.5 = 1.96, p = 0.040) between FM (n = 6) and FR (n = 8) OVX lambs. (D) Mean ± SEM integrated density of mRNA for Kiss1 within KNDy neurons was significantly lower (difference of means = 2,807,598, SE = 915,588, t 10.7 = 3.07, p = 0.006) in FR OVX lambs compared to FM OVX lambs. Scale bars = 50 μm. Inset scale bars = 10 μm. Statistical significance (*p < 0.05) was determined using a one‐tailed, unpaired Students t test
In addition, we assessed kisspeptin peptide expression in the ARC between FM and FR OVX lambs using immunohistochemistry. Perikarya and neuronal fibers expressing peptide for kisspeptin were readily visible in the ARC of both FM OVX lambs (Figure 5A) and FR OVX lambs (Figure 5B). Analysis of kisspeptin‐immunopositive perikarya revealed that FR OVX lambs had fewer (difference of means = 34.54, SE = 14.66, t 12 = 2.36, p = 0.018) numbers of kisspeptin cell bodies compared to FM OVX lambs (Figure 5C). The percentage of kisspeptin neurons that coexpressed NKB in the FM and FR animals did not differ (difference of means = 0.48, SE = 5.23, t 10.4 = .09, p = 0.465) and was 73.9 (SE = 4%) and 73.4 (SE = 4%), respectively.
FIGURE 5.
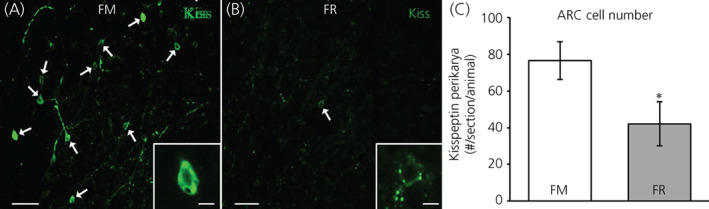
Kisspeptin peptide in the arcuate nucleus (ARC) of fed to maintain (FM) and feed‐restricted (FR) ovariectomized lambs. (A,B) Representative confocal images (1 μm optical section; 20× objective) from a FM (A) and a FR (B) OVX lamb. Individual kisspeptin perikarya (green) are indicated with white arrows. Insets: 1 μm optical sections; 40× objective. (C) Mean ± SEM number of kisspeptin perikarya in the ARC was significantly lower (difference of means = 34.54, SE = 14.66, t 12 = 2.36, p = 0.018) in FR OVX lambs (n = 8) compared to FM OVX lambs (n = 6). Scale bars = 50 μm. Inset scale bars = 10 μm. Statistical significance (*p < 0.05) was determined using a one‐tailed, unpaired Students t test
3.4. NKB data
We assessed mRNA for NKB in the ARC between FM and FR OVX lambs using RNAscope. Cells expressing mRNA for NKB were readily visible in the ARC of both FM OVX lambs (Figure 6A) and FR OVX lambs (Figure 6B). Although NKB cell numbers were reduced by 23% (Figure 6C), this difference was not statistically significant (difference of means = 38.28, SE = 24.96, t 9.7 = 1.53, p = 0.079). The percentage of NKB neurons that coexpressed kisspeptin and dynorphin in the FM and FR animals was not different (difference of means = 13.81, SE = 10.88, t 11.8 = 1.27, p = 0.114) and was 81.4 (SE = 8%) and 67.6 (SE = 8%), respectively. Likewise, the expression of mRNA for NKB within KNDy cells (Figure 6D) was not different (difference of means = −1,112,851, SE = 1,008,531, t 11.7 = −1.12, p = 0.8581) between groups.
FIGURE 6.
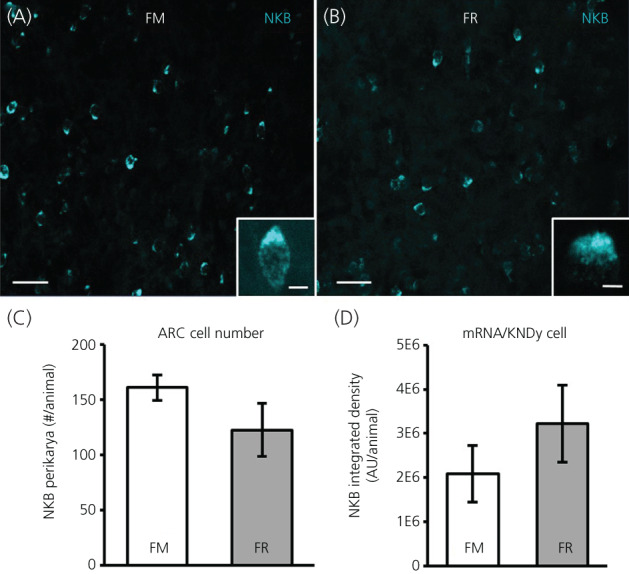
NKB mRNA in the arcuate nucleus (ARC) of fed to maintain (FM) and feed‐restricted (FR) ovariectomized (OVX) lambs. Confocal images (20×) of NKB cells (blue) from a FM (A) and a FR (B) OVX lamb. Insets: 1 μm optical sections; 63× objective. (C) Mean ± SEM number of NKB perikarya in the ARC was not significantly different (difference of means = 38.28, SE = 24.96, t 9.7 = 1.53, p = 0.079) between FM (n = 6) and FR (n = 8) OVX lambs. (D) Mean ± SEM integrated density of mRNA for NKB within KNDy neurons was not significantly different (difference of means = −1,112,851, SE = 1,008,531, t 11.7 = −1.12, p = 0.8581) between FM and FR OVX lambs. Scale bars = 50 μm. Inset scale bars = 10 μm. Statistical significance was determined using a one‐tailed, unpaired Students t test
In addition, we assessed protein for NKB in the ARC between FM and FR OVX lambs using immunohistochemistry. Perikarya and neuronal fibers expressing protein for NKB were readily visible in the ARC of both FM OVX lambs (Figure 7A) and FR OVX lambs (Figure 7B). Analysis of NKB‐immunopositive perikarya revealed that there was a statistically significant difference (difference of means = 29.42, SE = 15.77, t 11.9 = 1.87, p = 0.043) with FR OVX lambs having fewer numbers of NKB cell bodies compared to FM OVX lambs (Figure 7C). The percentage of NKB neurons that coexpressed kisspeptin in the FM and FR animals was different (difference of means = 12.34, SE = 4.49, t 10.4 = 2.75, p = 0.010) and was 72.5 (SE = 4%) and 60.1 (SE = 3%), respectively.
FIGURE 7.
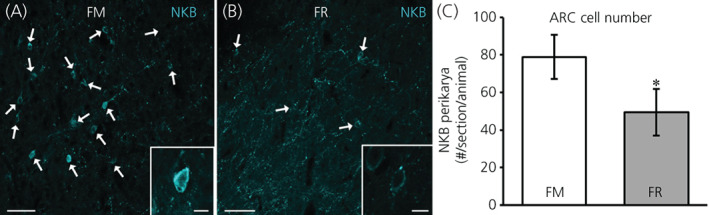
NKB protein in the arcuate nucleus (ARC) of fed to maintain (FM) and feed‐restricted (FR) ovariectomized lambs. (A,B) Representative confocal images (1 μm optical section; 20× objective) from a FM (A) and a FR (B) OVX lamb. Individual NKB perikarya (blue) are indicated with white arrows. Insets: 1 μm optical sections; 40× objective. (C) Mean ± SEM number of NKB perikarya in the ARC was significantly lower (difference of means = 29.42, SE = 15.77, t 11.9 = 1.87, p = 0.043) in FR OVX lambs (n = 8) compared to FM OVX lambs (n = 6). Scale bars = 50 μm. Inset scale bars = 10 μm. Statistical significance (*p < 0.05) was determined using a one‐tailed, unpaired Students t test
3.5. Dynorphin data
We assessed mRNA for dynorphin in the ARC between FM and FR OVX lambs using RNAscope. Unlike that previously reported for dynorphin protein, 40 , 49 cells expressing mRNA for dynorphin were readily visible in the ARC of both FM OVX lambs (Figure 8A) and FR OVX lambs (Figure 8B). The average number of dynorphin cells (Figure 8C) were not different (difference of means = 13.35, SE = 34.35, t 11.5 = 0.39, p = 0.352) between groups, but the percentage of dynorphin neurons that coexpressed kisspeptin and NKB in the FM and FR animals was different (difference of means = 20.91, SE = 7.61, t 8.67 = 2.75, p = 0.012) and was 67.0 ± 3% and 46.1% ± 8%, respectively. The expression of mRNA for dynorphin in KNDy cells (Figure 8D) was not different (difference of means = −85,149, SE = 172,570, t 12 = −0.49, p = 0.685) between groups.
FIGURE 8.
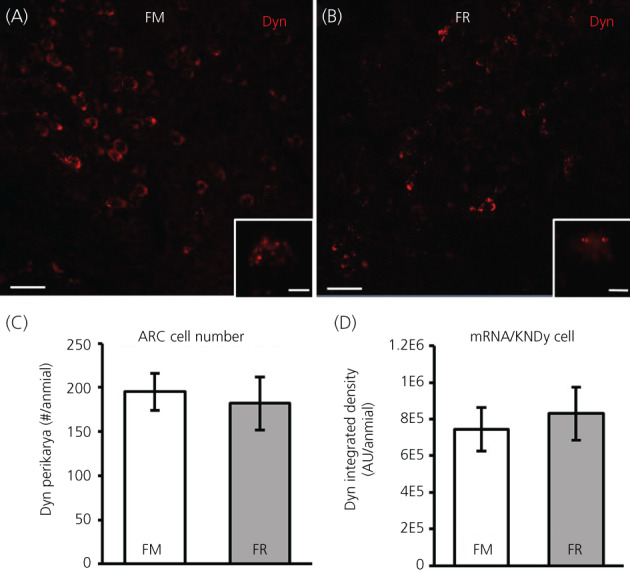
Dynorphin (Dyn) mRNA in the arcuate nucleus (ARC) of fed to maintain (FM) and feed‐restricted (FR) ovariectomized (OVX) lambs. Confocal images (20×) of Dyn cells (red) from a FM (A) and a FR (B) OVX lamb. Insets: 1 μm optical sections; 63× objective. (C) Mean ± SEM number of Dyn perikarya in the ARC was not significantly different (difference of means = 13.35, SE = 34.35, t 11.5 = 0.39, p = 0.352) between FM (n = 6) and FR (n = 8) OVX lambs. (D) Mean ± SEM integrated density of mRNA for Dyn within KNDy neurons was not significantly different (difference of means = −85,149, SE = 172,570, t 12 = −0.49, p = 0.685) between FM and FR OVX lambs. Scale bars = 50 μm. Inset scale bars = 10 μm. Statistical significance was determined using a one‐tailed, unpaired Students t test
4. DISCUSSION
The data obtained in the present study provide evidence indicating that chronic undernutrition differentially regulates kisspeptin, NKB, and dynorphin within KNDy neurons of female sheep. Chronic feed‐restriction in OVX lambs, which produced a significant reduction in LH secretion, resulted in the reduction in the total number of ARC cells expressing mRNA for kisspeptin, less mRNA for kisspeptin within KNDy neurons, and fewer ARC neurons expressing peptide for kisspeptin. Furthermore, although this model of undernutrition in ewe lambs did not alter the number of ARC neurons expressing mRNA for NKB nor the abundance of mRNA for NKB within KNDy neurons, there were fewer ARC neurons expressing protein for NKB with undernutrition. By contrast, we did not observe an impact of feed restriction on either total number of ARC cells expressing mRNA for dynorphin or on the abundance of mRNA for dynorphin within KNDy neurons.
Chronic undernutrition has been shown to impair reproductive success through the central inhibition of GnRH release from the hypothalamus which, in turn, reduces LH secretion. 8 , 55 Following previous models as an approach to feed‐restriction in young, gonadectomized sheep, 2 , 56 , 57 we first established a model of chronic undernutrition in wethers that reduced mean LH concentrations and LH interpulse interval but did not inhibit LH pulse amplitude. 49 In the present study, we observed that feed‐restriction in OVX lambs inhibits mean LH concentrations and LH pulse amplitude but does not inhibit LH interpulse interval. Given that the present study used the same model of undernutrition and pulse identification criteria as our previous study, 49 we consider that this may constitute a sex difference in the central mechanism governing the suppression of LH secretion during undernutrition. Furthermore, although the central mechanism by which undernutrition impairs reproduction is shared between young and adult sheep, a much longer duration of feed restriction is required to suppress LH secretion in adult ewes, 58 presumably because of a larger metabolic reserve (i.e., adipose tissue) in adult animals. Moreover, although we have used LH concentrations as an index for GnRH secretion, there is the possibility that undernutrition could act at the level of the pituitary to reduce gonadotrope responsiveness to GnRH. However, studies in rats 59 and sheep 60 have demonstrated normal LH secretion following administration of exogenous GnRH during undernutrition. Taken together with evidence that GnRH neurons are devoid of receptors for leptin and insulin, 9 , 10 , 11 , 12 , 13 the reduction in LH secretion with feed restriction very likely reflects a central suppression of GnRH secretion mediated through other afferent neurons.
Although it is generally accepted that KNDy neurons are important for GnRH/LH pulse generation, the role that these neurons play in mediating the negative impact of undernutrition on the reproductive axis is less well developed. Given that kisspeptin has been shown to stimulate GnRH and/or LH secretion in all mammalian species to date 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and that the vast majority of GnRH neurons express Kiss1R, 16 , 24 , 25 , 26 kisspeptin is considered to provide direct stimulatory drive to GnRH neurons. With up to 60% of GnRH neurons receiving input from KNDy neurons 42 and the majority of ARC kisspeptin neurons (presumptive KNDy neurons) expressing receptors for peripheral metabolic hormones such as leptin and insulin, 13 , 44 , 45 , 46 kisspeptin within KNDy neurons may serve as a key component of the nutritional regulation of GnRH release. Previous reports in rodents 47 , 48 and in sheep 45 using a single probe for kisspeptin demonstrated that chronic undernutrition reduces ARC mRNA abundance for kisspeptin. More recently, through the use of RNAscope, we have shown that chronic undernutrition in castrated male sheep reduces mRNA for kisspeptin within kisspeptin/NKB expressing cells. 49 However, although only 52%–65% of kisspeptin cells expressed NKB in castrated males, 49 possibly reflecting the lower degree to which males coexpress kisspeptin and NKB in the ARC, 35 , 61 we were unable to draw a definitive conclusion about the impact of chronic undernutrition on KNDy neurons because we did not assess dynorphin expression. In the present study using OVX lambs, we observed that undernutrition reduces the number of ARC cells that express mRNA and peptide for kisspeptin, which aligns with our previous report in castrated male sheep. 49 Furthermore, we report novel evidence that feed restriction inhibits mRNA abundance of kisspeptin within KNDy neurons and propose that this reduction led to fewer detectable ARC kisspeptin cells using RNAscope and immunohistochemistry. Although it is assumed that these changes in mRNA and peptide occur within the same cell, further work examining mRNA and peptide within the same sample (i.e., RNAscope combined with immunohistochemistry) is needed to determine whether this change occurs specifically within KNDy neurons and/or within non‐KNDy neurons.
Similar to kisspeptin, NKB also plays a stimulatory role in GnRH/LH secretion. 34 , 35 , 36 , 37 However, unlike kisspeptin, the actions of NKB are assumed to be mediated, at least in part, via ARC kisspeptin neurons given that they express NK3R, whereas GnRH neurons do not. 38 With the vast majority of NKB perikarya residing in the ARC, 38 , 62 , 63 there is growing evidence to suggest that NKB neurons in the ARC play an important role in regulating GnRH/LH secretion during undernutrition. Work in female rodents has shown that a 2–4‐week period of chronic undernutrition reduces mRNA for NKB in the ARC. 47 , 48 Our recent report in male sheep also showed that feed restriction for 13 weeks inhibits the number of ARC cells that express mRNA and protein for NKB. 49 In the present study, using the same undernutrition paradigm that we first established in males, 49 we did not detect a significant reduction in ARC cells that express mRNA for NKB, but did observe a significant reduction in ARC cells expressing NKB protein, albeit not to the degree that we reported in the males. In addition, in the present study, we demonstrate that the mRNA abundance for NKB within KNDy neurons is unaltered with undernutrition. Because the impact of undernutrition on LH secretion appears to differ between male and female sheep, in that feed restriction reduces LH pulse frequency in males 49 but reduces LH pulse amplitude in females (present study), we postulate that the preserved LH pulsatility in feed restricted female sheep is a result of conserved mRNA for NKB within the ARC, as well as a sufficient number of cells expressing protein for NKB, which highlights the possibility for sex differences in NKB signaling and regulation of NKB cells during undernutrition. Furthermore, work in young female pigs using short‐term (10 days) feed restriction designed to hinder weight gain (but not induce weight loss) has shown an increase in mean LH, LH pulse amplitude, and ARC mRNA abundance for NKB. 64 Although the data may appear paradoxical (i.e., feed restriction increases LH secretion), taken together with data obtained in the present study indicating that feed restriction reduces mean LH, LH pulse amplitude, and ARC NKB protein, they support the existing idea that NKB signaling may be the driving stimulus for GnRH pulse initiation 65 , 66 and leave open the possibility that NKB may also play a role in GnRH pulse amplitude in females. Further work is needed to determine central mechanisms governing the difference in NKB signaling that may exist between sexes, species, and models of undernutrition.
Dynorphin is considered to play a dominant role in mediating progesterone negative feedback 39 and may act directly on GnRH neurons and/or ARC kisspeptin neurons given both express kappa‐opioid receptors. 40 , 41 Although substantial data exist to support the idea that KNDy‐derived dynorphin may be part of the “pulse generator” and act to terminate GnRH pulses, 67 much less is known about the role dynorphin may play to regulate GnRH/LH secretion during undernutrition. In mice, chronic undernutrition has been shown to reduce and increase ARC mRNA abundance for dynorphin in OVX and OVX+ oestradiol (E2) mice, respectively. 48 However, work in OVX and OVX + E2 rats failed to find a change in ARC mRNA for dynorphin with undernutrition. 47 In the present study, we observed that feed restriction in OVX lambs did not alter the total number of ARC cells expressing mRNA for dynorphin or the abundance of mRNA for dynorphin in KNDy neurons. Because we previously were unable to detect dynorphin protein by immunohistochemistry in young male or female sheep, 40 , 49 we must rely on mRNA assessment alone to suggest that dynorphin may not play a role in the nutritional regulation of GnRH/LH secretion independent of sex steroids in female sheep. However, given that blockade of dynorphin signaling elicits an increase in LH secretion in prepubertal OVX + E2 lambs even in the absence of detectable ARC dynorphin immunostaining, 40 it is tempting to speculate that a similar mechanism may exist for dynorphin signaling by which undernutrition may impair GnRH/LH secretion in the presence of E2, aligning with ovarian hormones playing both activational 68 and organizational 69 roles in the hypothalamus. Moreover, given that dynorphin cells also reside in hypothalamic areas outside of the ARC, 70 further examination of dynorphin signaling during undernutrition in non‐ARC regions in both the presence and absence of E2 is warranted.
Although the data obtained in the present study provide clear evidence that undernutrition reduces kisspeptin within KNDy neurons, it is still unknown whether the effect of chronic undernutrition is exerted directly on KNDy neurons or whether afferent neuronal networks are responsible for relaying energy status to these key reproductive neurons. There is evidence that ARC kisspeptin neurons (presumptive KNDy neurons) express receptors for leptin, 44 , 45 , 46 although leptin administration in 48 h fasted adult sheep 12 and normally fed rats 47 does not induce expression of p‐STAT3, a cellular marker of functional leptin receptors, in ARC kisspeptin neurons. Thus, for longer periods of feed restriction in young animals, it is still unknown whether leptin is capable of directly activating ARC kisspeptin neurons. In addition, more recent work in sheep has revealed that ARC kisspeptin neurons also express insulin receptors, 13 although it has yet to be shown that insulin administration during undernutrition can activate kisspeptin neurons. Although direct metabolic regulation should not be ruled out, recent findings in sheep 71 and mice 72 showing that the majority of ARC kisspeptin neurons express melanocortin 3 receptors support the idea that KNDy neurons may be governed by neurons within the ARC that have classically been associated with regulating energy homeostasis (i.e., pro‐opiomelanocortin and agouti‐related peptide neurons). Furthermore, although larger litter sizes during pregnancy result in lower birth weights in sheep, 73 the data obtained in the present study are not sufficient to determine whether litter size has a long‐term impact on hypothalamic neurons during undernutrition and this warrants further investigation.
In conclusion, the data obtained in the present study support the hypothesis that undernutrition acts through KNDy neurons to reduce GnRH/LH secretion in young, OVX lambs. Furthermore, the data raise the possibility that differences exist in the central mechanism by which undernutrition and metabolic status regulates pulsatile LH secretion in male and female sheep. Although we did not find evidence to support a role for dynorphin during chronic feed restriction, we cannot rule out the possibility of a role for dynorphin in the presence of E2. Additionally, the role of circulating concentrations of markers for metabolic status such as leptin and insulin and the expression of their respective receptors in interneurons that are important for controlling GnRH/LH secretion may be of importance in the future investigation of chronic undernutrition. Thus, future studies will be needed to fully elucidate the central mechanisms underlying the nutrition‐induced suppression of GnRH release.
AUTHOR CONTRIBUTIONS
KaLynn Harlow: Data curation; formal analysis; investigation; methodology; project administration; writing – original draft; writing – review and editing. Max Griesgraber: Data curation; investigation; methodology; project administration. Andrew Seman: Investigation; methodology. Sydney Shuping: Investigation; methodology; project administration. Jeffrey Sommer: Methodology; project administration; supervision. Emily H. Griffith: Formal analysis. Stanley M. Hileman: Conceptualization; data curation; formal analysis; funding acquisition; project administration; resources; software; supervision; writing – review and editing. Casey Nestor: Conceptualization; data curation; formal analysis; funding acquisition; project administration; resources; software; supervision; writing – original draft; writing – review and editing.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by USDA National Institute of Food and Agriculture Hatch Project 1012905, by the North Carolina Agricultural Foundation, Inc., and by National Institute of Food and Agriculture Grant no. 2019–67016‐29408 (to SMH and CCN). We thank Tabatha Wilson (North Carolina State University Metabolic Education Unit) for care of animals, Dr Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH, and Dr Isabelle Franceschini for the kind gift of kisspeptin antisera. In addition, we thank Maggie Cummings, Haley Johnson, and Alyssa Valentine for their assistance with conducting this research (animal care, blood collection/processing, tissue collection, and/or preparing laboratory solutions). We also acknowledge the use of the Cellular and Molecular Imaging Facility (CMIF) at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation.
Harlow K, Griesgraber MJ, Seman AD, et al. The impact of undernutrition on KNDy (kisspeptin/neurokinin B/dynorphin) neurons in female lambs. Journal of Neuroendocrinology. 2022;34(6):e13135. doi: 10.1111/jne.13135
Funding information National Institute of Food and Agriculture, Grant/Award Numbers: 2019‐67016‐29408, Hatch 1012905
DATA AVAILABILITY STATEMENT
All data are available from corresponding author upon reasonable request.
REFERENCES
- 1. Glass AR, Swerdloff RS. Nutritional influences on sexual maturation in the rat. Fed Proc. 1980;39(7):2360‐2364. [PubMed] [Google Scholar]
- 2. Foster DL, Olster DH. Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology. 1985;116(1):375‐381. doi: 10.1210/endo-116-1-375 [DOI] [PubMed] [Google Scholar]
- 3. Day M, Imakawa K, Zalesky D, Kittok R, Kinder JE. Effects of restriction of dietary energy intake during the prepubertal period on secretion of luteinizing hormone and responsiveness of the pituitary to luteinizing hormone‐releasing hormone in heifers. J Anim Sci. 1986;62(6):1641‐1648. [DOI] [PubMed] [Google Scholar]
- 4. Wiltbank J, Rowden W, Ingalls J, Geegoey K, Koch RM. Effect of energy level on reproductive phenomena of mature Hereford cows. J Anim Sci. 1962;21(2):219‐225. [Google Scholar]
- 5. Wiltbank J, Rowden W, Ingalls J, Zimmerman D. Influence of post‐partum energy level on reproductive performance of Hereford cows restricted in energy intake prior to calving. J Anim Sci. 1964;23(4):1049‐1053. [Google Scholar]
- 6. Lents C, White F, Ciccioli N, Wettemann R, Spicer L, Lalman D. Effects of body condition score at parturition and postpartum protein supplementation on estrous behavior and size of the dominant follicle in beef cows. J Anim Sci. 2008;86(10):2549‐2556. [DOI] [PubMed] [Google Scholar]
- 7. Richards MW, Wettemann RP, Schoenemann HM. Nutritional anestrus in beef cows: body weight change, body condition, luteinizing hormone in serum and ovarian activity. J Anim Sci. 1989;67(6):1520‐1526. doi: 10.2527/jas1989.6761520x [DOI] [PubMed] [Google Scholar]
- 8. I'Anson H, Manning JM, Herbosa CG, et al. Central inhibition of gonadotropin‐releasing hormone secretion in the growth‐restricted hypogonadotropic female sheep. Endocrinology. 2000;141(2):520‐527. [DOI] [PubMed] [Google Scholar]
- 9. Finn PD, Cunningham MJ, Pau K‐YF, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139(11):4652‐4662. [DOI] [PubMed] [Google Scholar]
- 10. Hâkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18(1):559‐572. doi: 10.1523/JNEUROSCI.18-01-00559.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin‐releasing hormone neuronal function. Endocrinology. 2009;150(6):2805‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louis GW, Greenwald‐Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG Jr. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152(6):2302‐2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cernea M, Phillips R, Padmanabhan V, Coolen LM, Lehman MN. Prenatal testosterone exposure decreases colocalization of insulin receptors in kisspeptin/neurokinin B/dynorphin and agouti‐related peptide neurons of the adult ewe. Eur J Neurosci. 2016;44(8):2557‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Roux N, Genin E, Carel J‐C, Matsuda F, Chaussain J‐L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1‐derived peptide receptor GPR54. Proc Natl Acad Sci. 2003;100(19):10972‐10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 16. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS‐1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264‐272. [DOI] [PubMed] [Google Scholar]
- 17. Navarro VM, Castellano JM, Fernández‐Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS‐1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone‐releasing activity of KiSS‐1 peptide. Endocrinology. 2004;145(10):4565‐4574. doi: 10.1210/en.2004-0413 [DOI] [PubMed] [Google Scholar]
- 18. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci. 2005;102(6):2129‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lents CA, Heidorn NL, Barb CR, Ford JJ. Central and peripheral administration of kisspeptin activates gonadotropin but not somatotropin secretion in prepubertal gilts. Reproduction. 2008;135(6):879‐887. [DOI] [PubMed] [Google Scholar]
- 20. Mason AO, Greives TJ, Scotti M‐AL, et al. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52(4):492‐498. doi: 10.1016/j.yhbeh.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayasena CN, Nijher GMK, Chaudhri OB, et al. Subcutaneous injection of Kisspeptin‐54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes Tachyphylaxis. J Clin Endocrinol Metab. 2009;94(11):4315‐4323. doi: 10.1210/jc.2009-0406 [DOI] [PubMed] [Google Scholar]
- 22. Magee C, Foradori CD, Bruemmer JE, et al. Biological and anatomical evidence for Kisspeptin regulation of the hypothalamic‐pituitary‐gonadal Axis of estrous horse mares. Endocrinology. 2009;150(6):2813‐2821. doi: 10.1210/en.2008-1698 [DOI] [PubMed] [Google Scholar]
- 23. Ohkura S, Takase K, Matsuyama S, et al. Gonadotrophin‐releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813‐821. doi: 10.1111/j.1365-2826.2009.01909.x [DOI] [PubMed] [Google Scholar]
- 24. Herbison AE, d'Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin‐releasing hormone neurons. Endocrinology. 2010;151(1):312‐321. doi: 10.1210/en.2009-0552 [DOI] [PubMed] [Google Scholar]
- 25. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001‐1012. [DOI] [PubMed] [Google Scholar]
- 26. Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype‐specific regulation by 17β‐estradiol. Mol Cell Endocrinol. 2013;367(1):85‐97. doi: 10.1016/j.mce.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073‐4077. [DOI] [PubMed] [Google Scholar]
- 29. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686‐3692. [DOI] [PubMed] [Google Scholar]
- 30. Herbison AE. Estrogen positive feedback to gonadotropin‐releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin‐3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836‐3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494‐4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metabol. 2011;300(1):E202‐E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nestor CC, Briscoe AMS, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin‐releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1‐12. doi: 10.1111/j.1365-2826.2009.01930.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin‐releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959‐2967. [DOI] [PubMed] [Google Scholar]
- 40. Lopez JA, Bedenbaugh MN, McCosh RB, et al. Does dynorphin play a role in the onset of puberty in female sheep? J Neuroendocrinol. 2016;28(12). 10.1111/jne.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ‐Opioid receptor is Colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367‐2379. doi: 10.1210/en.2015-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nestor CC, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL. Regulation of GnRH pulsatility in ewes. Reproduction. 2018;156(3):R83‐R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS‐1 neurones are direct targets for leptin in the Ob/Ob mouse. J Neuroendocrinol. 2006;18(4):298‐303. [DOI] [PubMed] [Google Scholar]
- 45. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151(5):2233‐2243. doi: 10.1210/en.2009-1190 [DOI] [PubMed] [Google Scholar]
- 46. Cravo RM, Margatho LO, Osborne‐Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37‐56. doi: 10.1016/j.neuroscience.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. True C, Kirigiti M, Kievit P, Grove K, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23(11):1099‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang JA, Yasrebi A, Snyder M, Roepke TA. The interaction of fasting, caloric restriction, and diet‐induced obesity with 17β‐estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol Cell Endocrinol. 2016;437:35‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merkley CM, Renwick AN, Shuping SL, Harlow K, Sommer JR, Nestor CC. Undernutrition reduces kisspeptin and neurokinin B expression in castrated male sheep. Reprod Fertil. 2020;1(1):21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore N. The ovariectomized ewe: its contribution to controlled breeding. Aust J Biol Sci. 1988;41(1):15‐22. [PubMed] [Google Scholar]
- 51. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co‐express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225‐230. [DOI] [PubMed] [Google Scholar]
- 52. Whisnant C, Goodman R. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39(5):1032‐1038. [DOI] [PubMed] [Google Scholar]
- 53. Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286‐1290. [DOI] [PubMed] [Google Scholar]
- 54. Schindelin J, Arganda‐Carreras I, Frise E, et al. Fiji: an open‐source platform for biological‐image analysis. Nat Methods. 2012;9(7):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prasad BM, Conover CD, Sarkar DK, Rabii J, Advis J‐P. Feed restriction in prepubertal lambs: effect on puberty onset and on in vivo release of luteinizing‐hormone‐releasing hormone, neuropeptide Y and beta‐endorphin from the posterior‐lateral median eminence. Neuroendocrinology. 1993;57(6):1171‐1181. [DOI] [PubMed] [Google Scholar]
- 56. Hileman SM, Lubbers LS, Jansen HT, Lehman MN. Changes in hypothalamic estrogen receptor‐containing cell numbers in response to feed restriction in the female lamb. Neuroendocrinology. 1999;69(6):430‐437. [DOI] [PubMed] [Google Scholar]
- 57. Adam CL, Findlay P, Kyle C, Young P, Mercer J. Effect of chronic food restriction on pulsatile luteinizing hormone secretion and hypothalamic neuropeptide Y gene expression in castrate male sheep. J Endocrinol. 1997;152(2):329‐337. [DOI] [PubMed] [Google Scholar]
- 58. Backholer K, Bowden M, Gamber K, Bjørbæk C, Iqbal J, Clarke IJ. Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology. 2010;91(1):27‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bronson F. Effect of food manipulation on the GnRH‐LH‐estradiol axis of young female rats. Am J Physiol Regul Integr Comp Physiol. 1988;254(4):R616‐R621. [DOI] [PubMed] [Google Scholar]
- 60. Ebling FJP, Kushler RH, Foster DL. Pulsatile LH secretion during sexual maturation in the female sheep: photoperiodic regulation in the presence and absence of ovarian steroid feedback as determined in the same individual. Neuroendocrinology. 1990;52(3):229‐237. [DOI] [PubMed] [Google Scholar]
- 61. Hrabovszky E, Sipos MT, Molnár CS, et al. Low degree of overlap between Kisspeptin, neurokinin B, and Dynorphin Immunoreactivities in the infundibular nucleus of Young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978‐4989. doi: 10.1210/en.2012-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goubillon M‐L, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B‐expressing neurons as an highly estrogen‐receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218‐4225. [DOI] [PubMed] [Google Scholar]
- 63. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534‐541. [DOI] [PubMed] [Google Scholar]
- 64. Thorson JF, Prezotto LD, Adams H, et al. Energy balance affects pulsatile secretion of luteinizing hormone from the adenohypophesis and expression of neurokinin B in the hypothalamus of ovariectomized gilts. Biol Reprod. 2018;99(2):433‐445. doi: 10.1093/biolre/ioy069 [DOI] [PubMed] [Google Scholar]
- 65. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin‐releasing hormone secretion. Endocrinology. 2010;151(8):3479‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin‐releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859‐11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259‐4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406‐5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pereira LS, Gobbo DR, Ferreira JGP, JD H‐J, Sa SI, Bittencourt JC. Effects of ovariectomy on inputs from the medial preoptic area to the ventromedial nucleus of the hypothalamus of young adult rats. J Anat. 2021;238(2):467‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835‐1842. [DOI] [PubMed] [Google Scholar]
- 71. Merkley CM, Shuping SL, Sommer JR, Nestor CC. Evidence that agouti‐related peptide may directly regulate Kisspeptin neurons in male sheep. Metabolites. 2021;11(3):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sweeney P, Bedenbaugh MN, Maldonado J, et al. The melanocortin‐3 receptor is a pharmacological target for the regulation of anorexia. Sci Transl Med. 2021;13(590):eabd6434. doi: 10.1126/scitranslmed.abd6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gootwine E, Spencer T, Bazer F. Litter‐size‐dependent intrauterine growth restriction in sheep. Animal. 2007;1(4):547‐564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from corresponding author upon reasonable request.


