Abstract
Some evidence suggests that males and females may differ in their responses to acute cannabis effects, including subjective drug effects and behavioural effects, and cannabinoid pharmacokinetics. This is significant given current changes to cannabis‐related policies and, in consequence, increased cannabis accessibility. The present study combines data from two randomized controlled trials to investigate possible differences among males (n = 21) and females (n = 19) in the acute effects of vaporized cannabis containing 13.75 mg Δ9‐tetrahydrocannabinol (THC), with and without cannabidiol (CBD; 13.75 mg). To control for differences in the timing of assessments, peak (or peak change from baseline) scores were calculated for a range of measures including subjective drug effects, cognitive performance, cardiovascular effects, and plasma concentrations of THC, CBD, and their respective primary metabolites. While THC elicited robust and significant changes in all but one outcome measure relative to placebo, relatively few sex differences were observed after controlling for BMI and plasma THC concentrations. Relative to females, males performed better overall on a divided attention task (DAT) and had higher peak plasma concentrations of 11‐nor‐9‐carboxy‐THC (11‐COOH‐THC). Males and females did not differ with respect to plasma concentrations of any other analyte, subjective drug effects, or cardiovascular measures. These data indicate an absence of systematic sex differences in acute cannabis effects given a moderate dose of vaporized cannabis. They do not preclude the possibility that sex differences may emerge with higher THC doses or with other commonly used routes of administration (e.g., orally administered oils or edibles).
Keywords: cannabis, CBD, sex differences, THC
The present study combines data from two randomized controlled trials to investigate possible differences among males (n = 21) and females (n = 19) in the acute effects of vaporized cannabis containing 13.75 mg Δ9‐tetrahydrocannabinol (THC), with and without cannabidiol (CBD; 13.75 mg). While THC elicited robust and significant changes in all but one outcome measure relative to placebo, relatively few sex differences were observed after controlling for BMI and plasma THC concentrations.
1. INTRODUCTION
Recent changes to cannabis‐related policy and law in many countries have increased community access to a diverse range of cannabinoid products and reduced perceptions of harm associated with cannabis use. 1 , 2 Adult, non‐medical (‘recreational’) cannabis use is now legal in Canada and Uruguay as well as 18 U.S. states, the District of Columbia, and 2 territories 3 while medical cannabis is legal in a growing number of jurisdictions worldwide. There were an estimated 192 million past‐year cannabis users in 2018, with an almost two‐fold increase in the prevalence of daily or near‐daily cannabis use in the United States over the period 2009–2018. 4 With these changes there are concerns around possible public health ramifications such as increased prevalence of driving under the influence of cannabis (DUIC) and cannabis use disorder (CUD). 5 , 6
While men continue to use cannabis at higher rates than women, 4 , 7 there is evidence that this gender gap is narrowing. 7 , 8 Some authors have described a ‘telescoping’ effect whereby women progress more rapidly than men from initiation of cannabis use to onset of CUD. 9 , 10 Notably, women are increasingly using cannabis for medical purposes 11 and report different effects and patterns of use when compared with men. 12 In a recent survey of medical cannabis users in Illinois, women were more likely than men to increase their cannabis use after obtaining a medical cannabis certification and to discontinue use of prescription medications while using medical cannabis. 13 One study observed that men used cannabis more frequently and in higher quantities and tended to report increased appetite while women tended to report decreased appetite and were more likely to report nausea and anxiety as withdrawal symptoms. 12 Only minor sex differences, however, were observed in the therapeutic effects of medical cannabis. 12
Studies in laboratory animals consistently demonstrate that females are more sensitive to cannabinoid effects than males. 11 However, laboratory studies in humans have obtained less consistent results. No sex differences were observed in simulator driving performance after male and female participants smoked a joint containing approximately 22.9 mg THC, even after excluding data for the 44.4% of women who did not smoke all of the joint. 14 Cooper and Haney 15 observed greater abuse‐related effects (‘take again’ and ‘good’) in female participants following smoked cannabis but no differences in magnitude of intoxication. Matheson 16 also failed to observe sex differences in subjective drug effects following smoked cannabis. While female participants exhibited lower concentrations of delta‐9‐tetrahydrocannabinol (THC) and its primary metabolite 11‐nor‐9‐carboxy‐THC (11‐COOH‐THC) than male participants, females also tended to smoke less of the joint than males which suggests that this difference was simply due to reduced THC consumption. 16 In another recent study, 17 female participants smoked less of a joint than male participants while experiencing similar subjective effects, although this was only apparent in a combined alcohol‐cannabis condition. When cannabis was administered alone, no sex differences were observed either in amount of cannabis smoked or in pharmacological or subjective effects. By contrast, a recent study by Scholler et al 18 reported subtle sex differences in the acute effects of oral and vaporized cannabis, with female participants exhibiting greater sensitivity to several subjective drug effects (e.g. ‘drug effect’, ‘anxious/nervous’) and higher peak blood 11‐OH‐THC concentrations when compared with male participants.
The present study further investigated sex differences in the acute effects of vaporized cannabis in a sample of healthy, occasional cannabis users. Data from two within‐subjects, randomized and placebo‐controlled trials utilizing comparable experimental designs were combined and subsequently analysed to test for sex differences in measures of driving and cognitive performance, subjective drug effects, physiological measures and pharmacokinetic parameters following controlled cannabis vaporization. Understanding sex differences in acute cannabis effects may assist with public health messaging and the management of potential risks associated with increased cannabis accessibility.
2. METHODS
Data from two randomized, placebo‐controlled trials involving healthy, occasional cannabis users (cannabis use <2 times/week and >10 lifetime exposures) were pooled and harmonized. Both studies assessed cognitive performance, subjective drug effects, physiological measures, and cannabinoid pharmacokinetics (secondary outcome measures) for up to 6 h post drug administration. Study 1 was completed at the Royal Prince Alfred Hospital in Sydney, Australia, 19 while study 2 was completed at the Faculty of Psychology and Neuroscience at Maastricht University, the Netherlands. 20 In study 1, participants received THC‐dominant, THC/CBD‐equivalent or placebo cannabis via vaporization over three separate sessions. In study 2, participants received THC‐dominant, THC/CBD‐equivalent, CBD‐dominant or placebo cannabis via vaporization over four separate sessions. In both studies, THC and CBD doses were 13.75 mg, experimental sessions were separated by a washout period of at least 1 week, and the order in which study drugs were administered was counterbalanced. A computer‐generated, block randomization schedule was used to assign participants to a treatment order in both studies.
2.1. Participants
Participants were recruited via advertisements, Facebook and word of mouth and underwent an initial eligibility screen following by a comprehensive medical and psychiatric evaluation. All participants provided written informed consent and received compensation for participants. Inclusion criteria were similar across both studies: (1) aged 18–65 (study 1); aged 20–50 (study 2); (2) cannabis use <2 times/week in the last 12 months and >10 lifetime exposures; (3) body mass index (BMI) between 20 and 28 kg/m2 (study 2 only), and; (4) in possession of a valid driver license with at least 4 years driving experience (study 1) or with at least 2 years driving experience and driving >2000 km/year (study 2). Exclusion criteria across both studies included: (1) current or history of psychiatric disorder; (2) any prior significant adverse response to cannabis; (3) cannabis dependence (study 1) or history of drug abuse or addiction (study 2); (4) pregnant/nursing; (5) current use of medications known to affect driving, and; (6) hypertension or cardiac dysfunction. Both studies received ethical approval and were conducted in accordance with the Helsinki Declaration.
2.2. Study drug
In study 1, THC‐dominant (11% THC, <1% CBD), THC/CBD‐equivalent (11% THC, 11% CBD) and placebo (<1% THC & CBD) cannabis types were obtained from Tilray (BC, Canada). In study 2, THC‐dominant (22% THC, <1% CBD), CBD‐dominant (9% CBD, <1% THC) and placebo (<0.2% total cannabinoid content) cannabis types were obtained from Bedrocan (Netherlands). Study drugs in study 2 were weighed and combined to deliver target doses of 13.75‐mg THC and CBD, equivalent to the target doses administered in study 1. Thus, across both studies, participants received maximum doses of 13.75‐mg THC (THC condition), 13.75‐mg THC and 13.75‐mg CBD (THC/CBD condition), and 13.75‐mg CBD (CBD condition; study 2 only). Study drugs were vaporized at 200°C (Mighty Medic, Storz & Bickel) according to a controlled inhalation procedure as previously described. 19 , 20 In brief, participants inhaled cannabis vapour at fixed intervals (inhale for 3–5 s, hold for 3 s, exhale and rest for 30 s) for a minimum of 5 min (study 1) or 10 inhalations (study 2), or, if vapour was still visible in exhaled breath at this point, until vapour was no longer visible. Research staff and participants were blind to the randomization schedule and the study drug which was prepared in advance by unblinded personnel and provided to research staff on the morning of the experimental session in an opaque contained labelled with the participant code and test day number.
2.3. Experimental sessions
At the start of each test day, a zero‐blood alcohol concentration was confirmed via breathalyser (Alcotest 5510, Dräger, Germany) and oral fluid was screened (DrugWipe 5 s, Securetec, Germany; DrugTest 5000, Dräger) for the presence of cannabis, cocaine, opiates, amphetamine, methamphetamine, or MDMA. After baseline physiological and subjective drug effect measurements, a catheter was inserted into the participant's non‐dominant arm and a baseline blood sample was collected. Participants then inhaled the study drug via vaporization. In study 1, cognitive tests were conducted at 20‐ and 200‐min post‐vaporization while blood samples and physiological measurements were collected at 10‐, 60‐, 120‐ and 180‐min post‐vaporization. Subjective drug effects were assessed at 15‐, 60‐, 120‐, 180‐ and 240‐min post‐vaporization. In study 2, cognitive tests were conducted at 5‐, 135‐ and 205‐min post‐vaporization, while blood samples and physiological measurements were collected at 0 min (i.e., immediately post vaporization) and at 25, 130, 200 and 320‐min post‐vaporization. Subjective drug effects were assessed at 0‐, 25‐, 130‐, 200‐ and 240‐min post‐vaporization.
2.4. Outcome measures
Outcome measures were consistent across both studies and have been described in detail previously. 19 , 20 To account for differences across studies in the timing of certain measurements, peak change from baseline or peak score (where no baseline measurement was collected) was quantified for each outcome measure. In addition to the outcome measures reported here, both studies also examined driving performance at multiple timepoints. These data are excluded from the present analyses as data collection methods varied considerably, with study 1 using a driving simulator and study 2 assessing on‐road driving under real‐world conditions.
2.5. Data analysis
Demographics were obtained for male (n = 21) and female (n = 19) participants and compared using independent sample t tests or Mann Whitney U tests depending on whether data were normally distributed. Linear mixed‐effect models were used to test for an effect of condition (THC, THC/CBD, CBD or placebo) and sex (male, female) on each outcome measure. The restricted maximum likelihood method was used, and a first order autoregressive covariance structure was selected based on Schwarz Bayesian Information Criterion (BIC) model fit values. Two models were tested; model 1 included condition, sex and condition*sex as fixed factors, a random intercept, and BMI as a covariate to control for differences in body weight. Model 2 included change from baseline to peak THC concentration as an additional covariate. Model 2 was not applied to cannabinoid concentrations given expected collinearity between THC and other analyte concentrations. Bonferroni‐corrected pairwise comparisons were used to quantify sex differences if and when a significant main effect of sex or a significant sex*condition interaction was observed. All statistical analyses were conducted using SPSS v24. Pairwise comparisons were two‐tailed tests, and statistical significance was set as p < 0.05. Analyses excluding data from the CBD condition in study 2 did not differ meaningfully from the results presented here.
3. RESULTS
3.1. Participant characteristics
Participant characteristics are described in Table 1. Males (n = 21) and females (n = 19) did not differ significantly in BMI or frequency of cannabis use in the 3 months prior to study admission. Males did, however, weigh more than females (74.4 vs. 62.5 kg, p < 0.01) and had significantly more years of driving history (8.8 vs. 5.5, p < 0.01). Males were also slightly older than females (25.8 vs. 23.5 years, p = 0.02) All participants had at a minimum completed high school education. Males were more likely than females to consume alcohol on a weekly basis (25 vs. 17.5%) and were more likely to have ever driven under the influence of cannabis (25 vs. 10%).
TABLE 1.
Participant characteristics
| Males (n = 21) | Females (n = 19) | P value | |
|---|---|---|---|
| Age | 25.8 (3.6) | 23.5 (4.0) | 0.02 |
| BMI | 23.6 (4.5) | 22.0 (3.2) | 0.16 |
| Weight | 74.35 (14.7) | 62.48 (9.7) | 0.004 |
| Cannabis use in previous 3 months (no. occasions) | 12.7 (13.6) | 8.6 (10.0) | 0.28 |
| Years of driving | 8.8 (4.4) | 5.5 (2.6) | 0.023 |
| % High school or Higher Education | 100 | 100 | ‐ |
| Lifetime incidence of DUIC (%) | 25 | 10 | ‐ |
| Weekly alcohol use (%) | 25 | 17.5 | ‐ |
Note: DUIC = driving under the influence of cannabis.
3.2. Cognitive performance (Figure 1)
FIGURE 1.
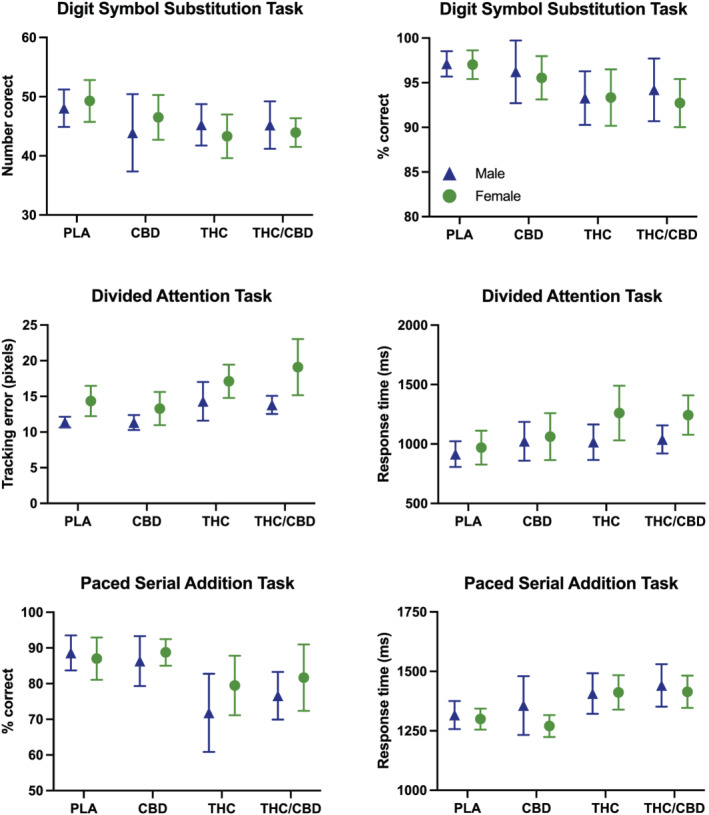
Cognitive performance data by condition in male and female participants. Data points represent mean peak scores, and error bars show 95% confidence intervals. PLA = placebo; CBD = CBD‐dominant (study 2 only); THC = THC‐dominant; THC/CBD = THC/CBD‐equivalent
As Table 2 shows, there was a significant effect of treatment on all outcome measures except for no. attempted trials on the DSST. A significant effect of sex was observed only for tracking error on the DAT in both models 1 (p < 0.01) and 2 (p < 0.05), with females exhibiting increased tracking error (i.e., worse performance) relative to males in the THC (both models: p = 0.04) and THC/CBD (both models: p = 0.01) conditions.
TABLE 2.
Results from mixed‐effects models
| Outcome measure | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Condition | Sex | Condition * sex | Condition | Sex | Condition * sex | |
| Cognitive DSST | ||||||
| DSST | ||||||
| No. attempted | 0.105 | 0.914 | 0.236 | 0.073 | 0.763 | 0.156 |
| % Correct | 0.016 | 0.663 | 0.913 | 0.022 | 0.686 | 0.918 |
| DAT | ||||||
| Tracking error | <0.001 | 0.009 | 0.897 | <0.001 | 0.018 | 0.659 |
| Response time | 0.011 | 0.056 | 0.307 | 0.016 | 0.074 | 0.234 |
| PSAT | ||||||
| Response time | <0.001 | 0.526 | 0.860 | <0.001 | 0.297 | 0.635 |
| Number correct | <0.001 | 0.230 | 0.957 | <0.001 | 0.230 | 0.957 |
| Subjective | ||||||
| Strength | <0.001 | 0.551 | 0.761 | <0.001 | 0.356 | 0.792 |
| Liking | <0.001 | 0.428 | 0.486 | <0.001 | 0.181 | 0.390 |
| Stoned | <0.001 | 0.970 | 0.739 | <0.001 | 0.755 | 0.458 |
| Sedated | <0.001 | 0.488 | 0.588 | <0.001 | 0.781 | 0.345 |
| Anxious | <0.001 | 0.384 | 0.744 | <0.001 | 0.733 | 0.479 |
| Confident to Drive | <0.001 | 0.188 | 0.824 | <0.001 | 0.298 | 0.784 |
| Physiological | ||||||
| Systolic BP | <.001 | 0.614 | 0.696 | <0.001 | 0.679 | 0.780 |
| Diastolic BP | .016 | 0.803 | 0.106 | 0.040 | 0.736 | 0.134 |
| HR | <0.001 | 0.427 | 0.254 | <0.001 | 0.422 | 0.229 |
| Cannabinoid concentrations | ||||||
| THC | <0.001 | 0.681 | 0.843 | — | — | — |
| 11‐OH‐THC | <0.001 | 0.906 | 0.814 | — | — | — |
| 11‐COOH‐THC | <0.001 | 0.007 | 0.226 | — | — | — |
| CBD | <0.001 | 0.283 | 0.889 | — | — | — |
| 7‐OH‐CBD a | <0.001 | 0.789 | 0.861 | — | — | — |
| 7‐COOH‐CBD a | <0.001 | 0.660 | 0.041 | — | — | — |
Data available from study 2 only, BP blood pressure, HR heart rate. Model 1 includes BMI as a covariate; model 2 includes BMI and peak change from baseline THC concentration as covariates.
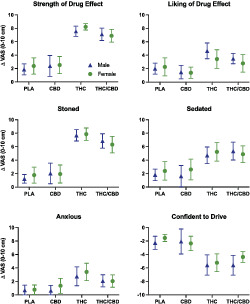
3.3. Subjective drug effects (Figure 2)
FIGURE 2.
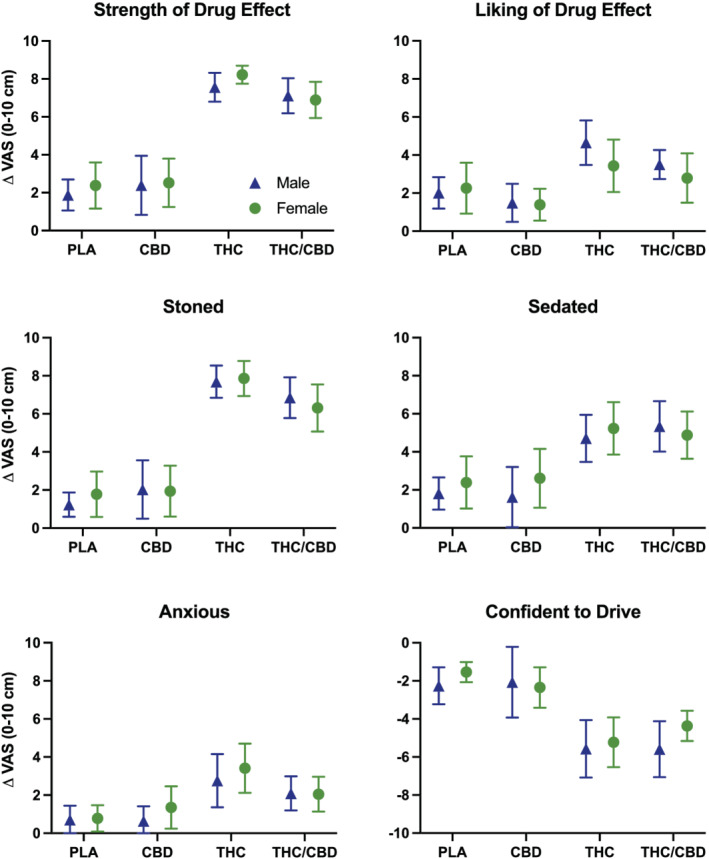
Subjective drug effects by condition in male and female participants. Data points represent mean peak change from baseline (visual analog scales; 0–10 cm), and error bars show 95% confidence intervals. Ratings of ‘confident to drive’ have been inverted so that negative scores indicate reduced confidence in driving ability relative to baseline. PLA = placebo; CBD = CBD‐dominant (study 2 only); THC = THC‐dominant; THC/CBD = THC/CBD‐equivalent
The main effect of treatment was significant for all subjective drug effect measures. There was no effect of sex or a condition*sex interaction for any outcome measure.
3.4. Physiological effects (Figure 3)
FIGURE 3.
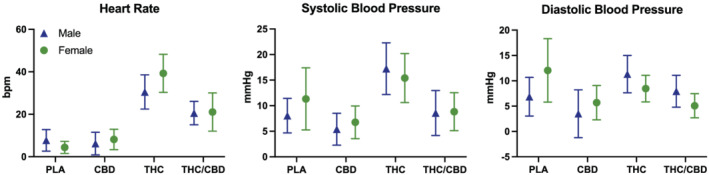
Cardiovascular effects by condition in male and female participants. Data points represent mean peak change from baseline, and error bars show 95% confidence intervals. PLA = placebo; CBD = CBD‐dominant (study 2 only); THC = THC‐dominant; THC/CBD = THC/CBD‐equivalent
There was a significant effect of treatment on both blood pressure and heart rate. There was no effect of sex or a condition*sex interaction.
3.5. Cannabinoid pharmacokinetics (Figure 4)
FIGURE 4.
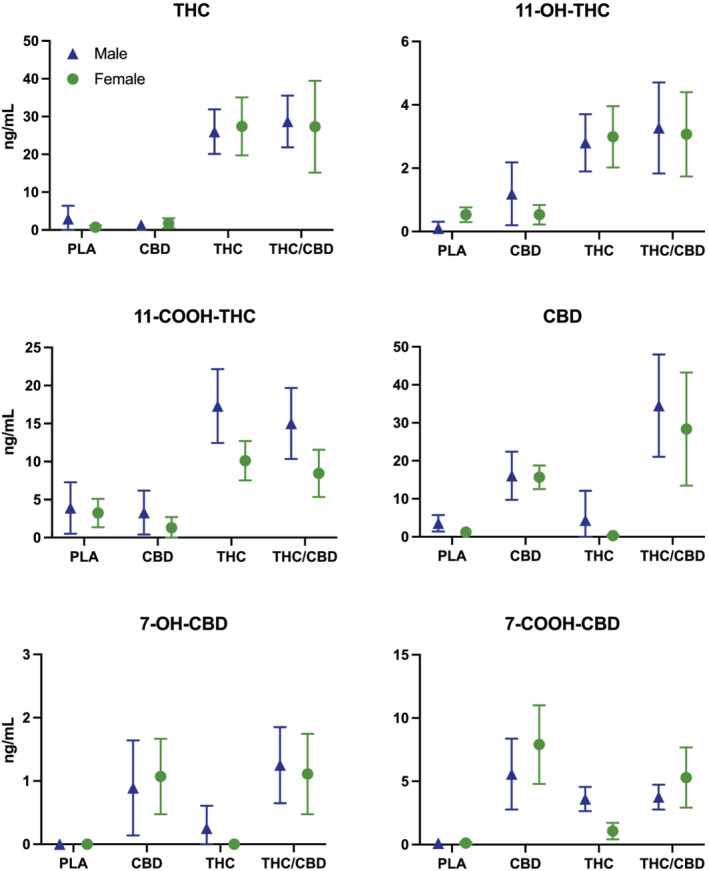
Plasma cannabinoid concentrations by condition in male and female participants. Data points represent mean peak change from baseline, and error bars show 95% confidence intervals. PLA = placebo; CBD = CBD‐dominant (study 2 only); THC = THC‐dominant; THC/CBD = THC/CBD‐equivalent
The main effect of treatment was highly significant for all plasma cannabinoid concentrations. There was a significant effect of sex on 11‐COOH‐THC (p < 0.01), with males showing higher concentrations than females in the THC (p < 0.01) and THC/CBD conditions (p = 0.01). There was also a significant condition*sex interaction for 7‐COOH‐CBD (p = 0.04), although none of the pairwise comparisons reached statistical significance.
4. DISCUSSION
The present analysis was designed to investigate possible sex differences in acute cannabis effects on a range of behavioural and pharmacological outcome measures across two placebo‐controlled and randomized human laboratory studies. Overall, and as expected, we found evidence for robust acute cannabis effects on the majority of outcome measures examined. We did not, however, find evidence for systematic sex differences in acute cannabis effects even after controlling for BMI and plasma THC concentrations. Overall, females performed worse on the divided attention task (DAT) when compared with males, and this did not vary as a function of treatment. Relative to females, male participants had higher peak plasma concentrations of 11‐COOH‐THC but not THC nor 11‐0H‐THC following THC administration.
In a recent study which also pooled data from multiple controlled, human laboratory studies, Sholler, et al 18 reported increased peak subjective ratings of ‘drug effect’, ‘anxious/nervous’, ‘heart racing’, and ‘restless’ among female participants relative to males. Sex differences in anxiety‐related effects remained significant after controlling for body weight and blood THC, 11‐OH‐THC and 11‐COOH‐THC, suggesting that females may be more susceptible than males to the anxiogenic effects of cannabis, and specifically, THC. In the present analysis, sex did not appear to influence ratings of ‘anxious’ or any other subjective drug effect measure. One important point to note is that participants in the studies analysed by Sholler et al. 18 had not used cannabis for ≥30 days prior to randomization. On average, female participants had not used cannabis for 210 days, and males had not used cannabis for 180 days; the range in responses for both males (30–7655 days) and females (30–1825) indicates that it had in fact been years since some participants had last used cannabis. It is therefore likely that these participants would have been significantly more sensitive to acute cannabis effects than the participants analysed in the present study. Moreover, significant sex differences only emerged in the high dose conditions (20–25 mg THC), whereas participants in the present study received a maximum dose of 13.75 mg THC. This would suggest that sex differences in acute cannabis effects may be subtle in nature and less prominent at lower doses.
Despite strong preclinical evidence for sex differences in cannabinoid pharmacokinetics and pharmacodynamics, 11 findings from other human laboratory studies have not always been consistent. In one study which looked at sex differences in the acute effects of cannabis concentrates following ad libitum administration, 21 male participants had significantly higher mean plasma THC concentrations than females immediately after inhalation (489.88 vs. 135.08 ng/ml), ostensibly indicating greater cannabis intake. However, plasma THC concentrations did not differ significantly at 1‐h post‐inhalation, and males and females exhibited similar plasma 11‐OH‐THC concentrations at both the immediate and 1‐h assessment timepoints. Males and females also exhibited similar levels of intoxication and impairment at both timepoints. As plasma cannabinoid concentrations peak and begin to decline rapidly within this time window (<20 min post‐inhalation), 22 it is possible that the difference in plasma THC concentrations seen immediately post‐inhalation was driven at least in part by variability in the time of blood sampling. The time at which the first assessment occurred did vary substantially (M = 13.49 min, SD = 7.44 min), and males returned to the mobile testing lab 4.54 min earlier than females on average, although the authors note that this difference was driven largely by a single female outlier who returned at 47 min.
In agreement with the findings reported here, both Cooper et al. 15 and Matheson et al. 16 failed to observe sex differences in subjective drug effects following smoked cannabis administration. However, female participants did exhibit stronger abuse‐related effects (‘take again’ and ‘good’) when compared with males, 15 and females smoked less of the joint and had lower peak THC and 11‐COOH‐THC concentrations relative to males. 16 Here, males also had higher peak plasma 11‐COOH‐THC concentrations than females; however, neither THC nor 11‐OH‐THC concentrations differed by sex. Interpretation of sex differences in cannabinoid concentrations is complicated with inhaled routes of administration due to dose titration effects which commonly occur when individuals alter their inhalation topography (e.g., intake volume, depth of inhalation) in order to control their drug intake. 22 , 23 The use of a strict vapour inhalation protocol (whereby participants were told exactly when to inhale, hold long to hold their breath for, and how long to pause in between inhalations) in the present studies aimed to minimize variability in cannabis intake, as did the use of vaporization over smoking which is a less efficient delivery method due to loss of drug to sidestream smoke. 24 , 25 , 26 Nonetheless, even with these controls in place, it is impossible to eliminate dose titration effects completely when cannabis is administered via inhalation.
Compared with inhaled routes, oral administration may provide a better window through which to observe potential sex differences due to elimination of the variance in dosing introduced by dose titration effects. Yet, relatively few studies have investigated sex differences following oral cannabis administration, and extant findings are mixed. A retrospective analysis 27 revealed dose‐dependent sex differences in subjective drug effects following acute administration of oral THC, although there was little clarity around the direction of these effects. For instance, while a 5‐mg dose elicited more pronounced effects in female participants, a 15‐mg dose elicited stronger effects in males. No significant differences were observed with the highest dose (25–30 mg), and females exhibited greater subjective responses relative to males on several measures (e.g., ‘good effect’, ‘high’) under placebo conditions, making it somewhat difficult to draw any meaningful conclusions. Contrary to prior studies involving smoked cannabis, non‐cannabis using men in another study were found to be more sensitive than women to the intoxicating effects of cannabis (2.5–10 mg oral THC) than women, as evidenced by stronger ratings of ‘high’. 28
An investigation into potential CBD modulation of THC pharmacokinetics in which oral THC (10 mg) was administered either as a pure isolate or as a cannabis extract in combination with CBD (5.4 mg) revealed a significantly higher area under the curve (AUC) for THC, 11‐OH‐THC and CBD (but not 11‐COOH‐THC) in female participants when compared with males. Higher maximum THC, 11‐OH‐THC, 11‐COOH‐THC and CBD concentrations (Cmax) as well as shorter times to maximum THC, 11‐OH‐THC and CBD (but not 11‐COOH‐THC) concentrations (Tmax) were also observed for females relative to males. 29 These findings appear to offer strong evidence for sex differences in cannabinoid pharmacokinetics with orally administered THC, yet a more recent study was unable to replicate them. 30 While females exhibited a significantly higher Cmax relative to males (2.36 vs. 1.39 ng/ml) after a 5 mg dose of oral THC in a fasted state (treatment A), Cmax did not differ between sexes in any of the other three treatments examined (B: 5 mg THC + high‐fat meal; C: 10 mg THC + fasted; D: 10 mg THC + high‐fat meal). No sex differences were observed for any other pharmacokinetic parameters, including AUC and Tmax, in any of the four treatments. 30 Neither study corrected pharmacokinetic values for body weight, leaving few hints as to why such disparate results were obtained.
Overall, the present analyses suggest that sex differences in acute cannabis effects, given a modest THC dose and administration via vaporization to individuals with a history of prior but occasional cannabis use, are trivial, if indeed they are present at all. This does not preclude the possibility that sex differences might emerge with higher THC doses, particularly when given to cannabis‐naïve individuals, as some evidence suggests. 18 There are several key strengths to this study, including the use of a comprehensive dataset involving an equal number of males and females from a diverse range of backgrounds, with one study being conducted in Australia and the other in the Netherlands. The use of a tightly controlled dosing protocol and equivalent THC and CBD concentrations in the cannabis types used in the two studies are also major strengths. Notably, this is the only study to date to have examined sex differences in the acute effects of cannabis containing THC with and without CBD in equal proportions. In addition, this analysis benefits from having a wide range of outcome measures, including measures of cognitive performance, subjective drug effects and plasma cannabinoid concentrations.
There are also several limitations that warrant mention. First and foremost, as this analysis was exploratory in nature, neither of the studies analysed here were specifically powered to detect sex differences, which as other studies suggest may be subtle in magnitude. The exploratory nature of these analyses is the reason why we elected to use a Bonferroni adjustment for pairwise comparisons, although we recognize this may be overly conservative. Second, our use of peak change scores rather than complete data sets may have reduced our statistical power and therefore our ability to detect sex differences. We chose to use peak change scores so as to be able to harmonize datasets from separate studies across which the timing of assessments varied. That all main effects of treatment remained highly significant where they were so in the original studies suggests that any loss of statistical power was minimal and potentially negated by the use of a larger dataset with very similar group sizes. Third, although the two studies analysed here involved equivalent doses of THC and CBD, a range of different cannabis strains (chemovars) from two different producers were used to deliver these active doses. Despite attempts to minimize systematic variance by using a placebo‐controlled and within‐subjects design, this may have introduced an additional source of variance into these data. Fourth, due to differences in the timing of blood sampling across the two studies, we were unable to examine pharmacokinetic parameters beyond peak concentration (Cmax) for each analyte. Given mixed findings in previous studies, 29 , 30 this is something that clearly warrants investigation in future research. Finally, we note that participants in study 2 were on average younger and had a lower BMI than participants in study 1. Although frequency of cannabis use did not differ in these two cohorts, such differences in age and BMI should nonetheless be taken into account.
5. CONCLUSION
In conclusion, the findings reported here suggest that men and women who use cannabis occasionally exhibit relatively few differences in their responses to acute cannabis effects after controlling for differences in BMI and plasma THC concentrations. These findings do not preclude the possibility that men and women may experience acute cannabis effects differently with higher THC doses and with other product formulations (e.g., oils and edibles).
CONFLICT OF INTEREST
TA has received speaker's honoraria from Legalwise Seminars and the International College of Cannabinoid Medicine. ISM has received research funding from the National Health and Medical Research Council of Australia and salary and research funding from the Lambert Initiative during the conduct of the study. He currently acts as a consultant to Kinoxis Therapeutics, Psylo and Emiria and has received a speaker's honorarium from Janssen. Professor McGregor holds various patents relating to cannabinoid and non‐cannabinoid therapeutics and has acted as an expert witness in legal cases involving the use of medical and non‐medical use of cannabis. NL has received funding for unrelated research from Camurus. The other authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
TA and IM were responsible for the study concept and design. TA, FV and RK contributed to the acquisition of data. TA conducted the data analysis and interpretation of findings. TA drafted the manuscript. IM, RK and JR provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
ACKNOWLEDGEMENTS
This research was supported by The Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded independent research centre at the University of Sydney.
Arkell TR, Kevin RC, Vinckenbosch F, et al. Sex differences in acute cannabis effects revisited: Results from two randomized, controlled trials. Addiction Biology. 2022;27 (2): e13125. doi: 10.1111/adb.13125
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rudy AK, Barnes AJ, Cobb CO, Nicksic NE. Attitudes about and correlates of cannabis legalization policy among U.S. young adults. J Am Coll Health. 2020;69(8):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mark Anderson D, Hansen B, Rees DI. Medical Marijuana Laws and Teen Marijuana Use. Am Law Econ Rev. 2015;17(2):495‐528. [Google Scholar]
- 3. National Conference on State Legislatures . State Medical Marijuana Laws. Accessed July 26, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Published 2021. Updated 07/14/2021.
- 4. United Nations . World Drug Report 2020 (Sales No. R.20.XI.6). 2020.
- 5. Cerda M, Mauro C, Hamilton A, et al. Association Between Recreational Marijuana Legalization in the United States and Changes in Marijuana Use and Cannabis Use Disorder From 2008 to 2016. JAMA Psychiat. 2020;77(2):165‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu V, Leung J, Hall W, Stjepanovic D, Degenhardt L. Public health impacts to date of the legalisation of medical and recreational cannabis use in the USA. Neuropharmacology. 2021;193:108610. [DOI] [PubMed] [Google Scholar]
- 7. Hemsing N, Greaves L. Gender norms, roles and relations and cannabis‐use patterns: a scoping review. Int J Environ Res Public Health. 2020;17:(3):947. 10.3390/ijerph17030947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapman C, Slade T, Swift W, Keyes K, Tonks Z, Teesson M. Evidence for Sex Convergence in Prevalence of Cannabis Use: A Systematic Review and Meta‐Regression. J Stud Alcohol Drugs. 2017;78(3):344‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM‐IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan SS, Secades‐Villa R, Okuda M, et al. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130(1–3):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper ZD, Craft RM. Sex‐Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology. 2018;43(1):34‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuttler C, Mischley LK, Sexton M. Sex Differences in Cannabis Use and Effects: A Cross‐Sectional Survey of Cannabis Users. Cannabis Cannabinoid Res. 2016;1(1):166‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruce D, Grove TJ, Foster E, Shattell M. Gender Differences in Medical Cannabis Use: Symptoms Treated, Physician Support for Use, and Prescription Medication Discontinuation. J Womens Health (Larchmt). 2021;30(6):857‐863. [DOI] [PubMed] [Google Scholar]
- 14. Anderson BM, Rizzo M, Block RI, Pearlson GD, O'Leary DS. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42(1):19‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper ZD, Haney M. Investigation of sex‐dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matheson J, Sproule B, Di Ciano P, et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl). 2020;237(2):305‐316. [DOI] [PubMed] [Google Scholar]
- 17. Wright M, Wickens CM, Di Ciano P, et al. Sex differences in the acute pharmacological and subjective effects of smoked cannabis combined with alcohol in young adults. Psychol Addict Behav. 2021;35(5):536‐552. [DOI] [PubMed] [Google Scholar]
- 18. Sholler DJ, Strickland JC, Spindle TR, Weerts EM, Vandrey R. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict Biol. 2021;26(4):e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arkell TR, Lintzeris N, Kevin RC, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)‐induced impairment of driving and cognition. Psychopharmacology (Berl). 2019;263(9):2713‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG. Effect of Cannabidiol and Delta9‐Tetrahydrocannabinol on Driving Performance: A Randomized Clinical Trial. Jama. 2020;324(21):2177‐2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson LP, Gust CJ, Ellingson JM, et al. Investigating sex differences in acute intoxication and verbal memory errors after ad libitum cannabis concentrate use. Drug Alcohol Depend. 2021;223:108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huestis MA, Smith ML. Cannabinoid Markers in Biological Fluids and Tissues: Revealing Intake. Trends Mol Med. 2018;24(2):156‐172. [DOI] [PubMed] [Google Scholar]
- 23. Leung J, Stjepanovic D, Dawson D, Hall WD. Do Cannabis Users Reduce Their THC Dosages When Using More Potent Cannabis Products? A Review. Front Psych. 2021;12(163):630602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spindle TR, Cone EJ, Schlienz NJ, et al. Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open. 2018;1(7):e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gieringer D, St. Laurent J, Goodrich S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J Cannabis Therapeut. 2004;4(1):7‐27. [Google Scholar]
- 26. Lanz C, Mattsson J, Soydaner U, Brenneisen R. Medicinal Cannabis: In Vitro Validation of Vaporizers for the Smoke‐Free Inhalation of Cannabis. PLoS ONE. 2016;11(1):e0147286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fogel JS, Kelly TH, Westgate PM, Lile JA. Sex differences in the subjective effects of oral Delta(9)‐THC in cannabis users. Pharmacol Biochem Behav. 2017;152:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32(6):1391‐1403. [DOI] [PubMed] [Google Scholar]
- 29. Nadulski T, Pragst F, Weinberg G, et al. Randomized, double‐blind, placebo‐controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9‐tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27(6):799‐810. [DOI] [PubMed] [Google Scholar]
- 30. Lunn S, Diaz P, O'Hearn S, et al. Human Pharmacokinetic Parameters of Orally Administered Delta(9)‐Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res. 2019;4(4):255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


