Dear Editor,
We read with great interest the recent article by Scangos and colleagues on their closed-loop deep brain stimulation (DBS) study that targeted ventral capsule/ventral striatum (VC/VS) for the treatment of major depressive disorder (MDD) in a single participant [1]. The study consisted of an open-loop stage (Stage I) and a closed-loop stage (Stage II). In the open-loop stage, gamma power in bilateral amygdala was identified as a biomarker for the high symptom state using cross-validated logistic regression models. VC/VS was identified as an upstream stimulation target based on sophisticated structural (diffusion-based tractography) and functional mapping (stimulation-evoked potentials). In the closed-loop stage, a DBS system was implanted to detect gamma activity in the right amygdala and to stimulate the right VC/VS in a closed loop to reduce amygdala gamma activity and alleviate symptoms of depression. The participant experienced a precipitous drop in symptom severity in the first week of closed-loop stimulation compared to the week prior and remained in a low symptom state for the majority of the closed-loop stimulation period. The authors suggested that while immediate benefits of DBS to VC/VS have been repeatedly demonstrated, these effects are difficult to sustain. With a closed-loop system, the acute benefit of stimulation can be maximized, and attenuation of its efficacy can be avoided with infrequent stimulation. Overall, the work effectively integrates multiple experimental and engineering techniques, which exemplifies the future of personalized psychiatric treatment using closed-loop DBS. We are impressed with the sustained clinical outcome of closed-loop DBS in this n-of-1 study, and we suggest that the precise mechanism underlying the successful intervention could be further elucidated with a dynamical systems approach and a closer examination of the nonlinear relation between gamma activity and symptom severity.
First, analyzing the impact of closed-loop stimulation on depressive symptoms as a bistable dynamical system may provide novel insight into the therapeutic mechanism. In Scangos et al., 2021 [1], Fig. 2i demonstrates the key clinical outcome, i.e., precipitous symptom reduction in the first week of closed-loop stimulation compared to the week before the onset of closed-loop stimulation. Such precipitous change is often a sign of phase transition in bistable or multistable nonlinear dynamical systems [2]. This observation is particularly interesting in the context of the Stage I results of the study, where Scangos et al. demonstrated that the symptomology of the patient fluctuated between two distinct states (Fig. 1b and Fig. 2c of the original paper [1]), i.e., a high symptom state and a low symptom state (Fig. 1a, reproduced using data from the GitHub repository of the paper). Thus, it is clear that the participant was in the high symptom state during the week before the onset of closed-loop stimulation: an average of 77.33 for the visual analog scale for depression (VAS-D) and an average of 16 for the 6-item Hamilton Depression Rating Scale, (HAMD-6) [1]. The participant transitioned to the low symptom state during the week after the onset of closed-loop stimulation (average VAS-D of 25.5, average HAMD-6 of 3.4). From a dynamical system perspective, we propose that closed-loop stimulation triggered an immediate transition from the high symptom state to the low symptom state at its onset, leading to a precipitous symptom drop, and continued to destabilize the high symptom state (Fig. 1b, red ball) and/or stabilize the low symptom state (blue ball) in the following months. Whether there exists long-term destabilization of the high symptom state needs to be examined in the context of the intrinsic (baseline) symptom dynamics of the participant. For this purpose, we modeled the mood of the patient as a Markov chain with two mood states and quantified the probability of staying within each state and transitioning between states (Fig.1c and d for intrinsic versus closed loop-controlled dynamics respectively). At baseline, the low symptom state was more common (denoted by node size) and persistent (self-loop of the blue node) than the high symptom state, and with a lower probability of transitioning to the high symptom state (edge from blue to red node) than the reverse (Fig. 1c). By comparison, closed-loop stimulation increased the relative stability of the low symptom state to the high symptom state, shown as an overall increase of transition probability towards the low symptom state and an overall decrease of transition probability towards the high symptom state (Fig. 1d). From the dynamical system perspective, the increased probability of transitioning to the low symptom state may better quantify the therapeutic impact of closed-loop stimulation than the magnitude of immediate symptom change as reported by Scangos et al., 2021 (the magnitude of immediate change corresponds to the horizontal distance between the blue and the red ball in Fig. 1b, whereas the stability of the states corresponds to the size of the basins around each ball). Our analyses suggest that the closed-loop stimulation served to reduce the stability of the high symptom state and facilitated the transition to the low symptom state by reshaping the landscape of symptom dynamics (Fig. 1b). Designing stimulation to facilitate state transition may require very low control energy by tapping into the intrinsic symptom dynamics, analogous to the control of chaotic dynamical systems with small perturbations [3]. Future research could distinguish between neural activity for maintaining the present state versus activity that corresponds to a state transition, and closed-loop stimulation could be used to prevent state transition out of the low symptom state.
Fig. 1. Closed-loop stimulation destabilizes the high symptom state.
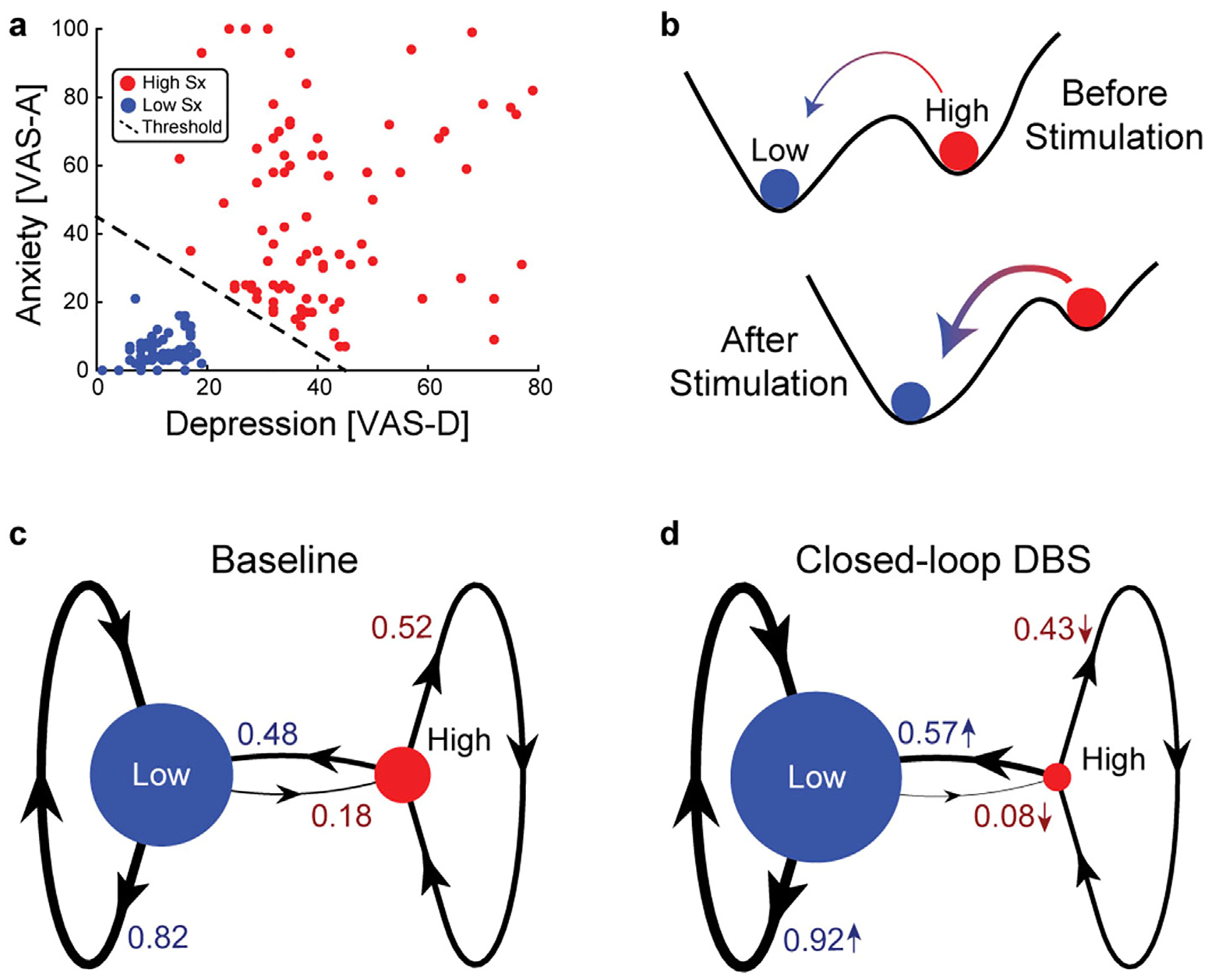
(a) High and low symptom (Sx) states were classified during the baseline using symptom data and state labels provided in the GitHub repository for Scangos et al., 2021. The visual analog scale for anxiety (VAS–A) and depression (VAS-D) were used for classifying a low symptom (blue) and high symptom (red) state. Classification boundary (dashed line) was (VAS-A + VAS-D) > 45. (b) Dynamic bistability of the symptom states can be understood as an energy landscape where the size of the valleys (i.e., basins of attraction) reflect state stability and the peak between the valleys (i.e., separatrix) influences transition probability. The symptom dynamics during the Stage I 10-day baseline period (c) and the closed-loop deep brain stimulation (DBS) period (d) were modeled as Markov chains with two symptom states. With stimulation, the low symptom state became more likely (greater node size) and more stable (thicker self-loop) with decreased transition probability to the high symptom state. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Second, it is unclear how gamma activity in the amygdala mediates the effect of closed-loop stimulation on symptom improvement. Our analysis of the source data [1] suggests that symptom severity may depend nonlinearly on amygdala gamma power. In Stage I (10-d baseline) of the study, Scangos and colleagues identified amygdala gamma power as the most predictive feature of symptom states using logistic regressions and demonstrated that stimulation trials that improved symptoms were associated with decreased gamma activity (n = 2), whereas stimulation trials that did not improve symptoms were associated with increased gamma activity (n = 3) (see Fig. 1h of Scangos et al., 2021). In Fig. 2, we plotted the relation between one of the six best features (right amygdala contact 1 high gamma) reported in Fig. 1c of Scangos et al., 2021 [1] and the corresponding symptom scores. We found, to our surprise, that gamma power was negatively correlated with symptom scores for all six neural features (Spearman correlation, all p < 0.05 after FDR correction). A closer look revealed that symptoms peaked when gamma power was around 10 (rank), which separates the gamma-symptom relation into two regimes (delineated by the dashed line in Fig. 2a and b): when gamma power was low (left of the dashed line), symptoms increased with gamma power, but when gamma power was higher (right of the dashed line), symptoms decreased, then plateaued with gamma power. From a dynamical systems perspective, these are clear signs of nonlinearity and state-dependency in the gamma-symptom relation. In the closed-loop stimulation stage of Scangos et al., 2021 [1], the gamma detection threshold was set to be 0.8% of the full amplitude, i.e., triggering (presumed) gamma-suppressing stimulation when the gamma amplitude was already low. Thus, it is likely that the closed-loop stimulation had controlled the gamma oscillation within the low amplitude regime where suppressing gamma would indeed lead to symptom improvement. We suggest that examination of amygdala gamma activity prior to and following each closed-loop stimulation event will help verify target engagement [4], and in turn, improve the design of closed-loop DBS. Further elucidating the neural mechanisms with which gamma oscillations influence symptoms in conjunction with other cognitive functions may help us better understand the long-term consequences of gamma suppression in normal emotion processing.
Fig. 2. The nonlinear relation between gamma power and symptom severity in Stage I baseline.
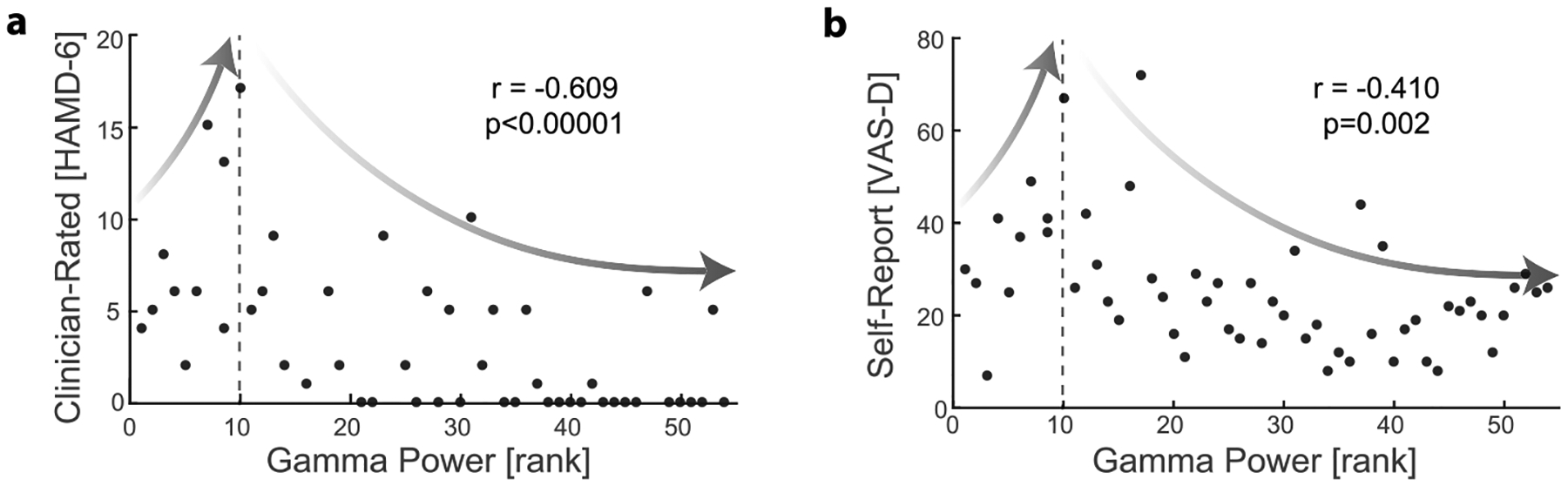
This figure is created using the source data of Fig. 1 of Scangos et al., 2021 [1]. The relation between high gamma power (rank) in the right amygdala (lead 1) to (a) clinician-rated depression severity (HAMD-6) and (b) self-reported depression severity (VAS-D). While six features for gamma power were correlated to symptom severity (Fig. 1c of Scangos et al., 2021 [1]), the relationship was quantitatively the same for each feature. The right amygdala was selected as this site was used for closed-loop DBS. Spearman correlation coefficients (r) and the corresponding p-values are displayed. When gamma power was low (left to the dashed line), both VAS-D and HAMD increased with gamma power (r = 0.710, p = 0.021 for VAS-D, and r = 0.505, p = 0.137 for HAMD-6). However, as gamma increased past a critical value (dashed line), the symptom scores began to decrease with gamma power and eventually plateaued.
In summary, we provided a dynamical systems interpretation of the primary findings regarding the role of closed-loop DBS in modulating symptom dynamics and pointed to the potentially complex role of amygdala gamma activity in mediating that effect. The existence of bistable symptom states is a hallmark of nonlinear dynamics since linear systems do not exhibit more than one stable state. Bistability is ubiquitous in human behavior, perception, and brain dynamics [2] and operates across spatiotemporal scales [5]. The control of bistable systems requires new concepts and tools [6], and we hope that our novel interpretation of Scangos et al., 2021 offers a glimpse into how this paradigm can be translated into clinical application. In addition, we found the relation between gamma power and symptom severity to be nonlinear such that gamma suppression may be clinically beneficial only when the overall gamma activity is very low. Therefore, the role of gamma activity in amygdala in this closed-loop system should be examined before and after each stimulation event to help address these open questions. Finally, the long-term consequences on emotional processing are unknown. This closed-loop DBS paradigm may lead to suppression of gamma power in the amygdala that could negatively impact emotional cognition [7]; alternatively, the closed-loop DBS paradigm in the long term may inadvertently incentivize the presumed to be pathological gamma power in the amygdala in order to trigger the therapeutic effect of VC/VS stimulation. We hope this letter brings new perspectives for the continued improvement of stimulation design for the treatment of psychiatric disorders.
Acknowledgments
This work was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH101547, and R01MH111889 awarded to FF and K99MH126161 awarded to JR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: FF is the lead inventor of IP filed by UNC. FF is the founder, shareholder, and chief science officer of Pulvinar Neuro, which did not play any role in the writing of this article. FF has received honoraria from the following entities in the last twelve months: Sage Therapeutics, Academic Press, Insel Spital, and Strategic Innovation. MZ and JR have nothing to declare.
Contributor Information
Mengsen Zhang, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Carolina Center for Neurostimulation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Justin Riddle, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Carolina Center for Neurostimulation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Flavio Frohlich, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Carolina Center for Neurostimulation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Cell Biology and Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Neuroscience Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
References
- [1].Scangos KW, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med 2021;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kelso JAS. Multistability and metastability: understanding dynamic coordination in the brain. Philosophical Transactions Royal Soc B Biological Sci 2012;367:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boccaletti S, Grebogi C, Lai YC, Mancini H, Maza D. The control of chaos: theory and applications. Phys Rep 2000;329:103–97. [Google Scholar]
- [4].Kurmann R, Gast H, Schindler K, Fröhlich F. Rational design of transcranial alternating current stimulation: Identification, engagement, and validation of network oscillations as treatment targets. Clin Transl Neurosci July 2018. 10.1177/2514183X18793515. [DOI] [Google Scholar]
- [5].Zhang M, Beetle C, Kelso JAS, Tognoli E. Connecting empirical phenomena and theoretical models of biological coordination across scales. J Roy Soc Interface 2019;16:20190360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McKinley J, et al. Third party stabilization of unstable coordination in systems of coupled oscillators. J Phys Conf Ser 2021;2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci 2002;22:9502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


