Abstract
Background
Hong Kong maintained low circulation of SARS-CoV-2 until a major community epidemic of the omicron (B.1.1.529) sublineage BA.2 began in January, 2022. Both mRNA (BNT162b2 [Fosun Pharma-BioNTech]) and inactivated CoronaVac (Sinovac, Beijing, China) vaccines are widely available; however, vaccination coverage has been low, particularly in older adults aged 70 years or older. We aimed to assess vaccine effectiveness in this predominantly infection-naive population.
Methods
In this observational study, we used individual-level case data on mild or moderate, severe or fatal, and fatal disease in patients hospitalised with COVID-19 along with census information and coverage data of BNT162b2 and CoronaVac. We used a negative binomial model, adjusting for age, sex, and calendar day to estimate vaccine effectiveness of one, two, and three doses of both BNT162b2 and CoronaVac vaccines, and relative effectiveness by number of doses and vaccine type.
Findings
Between Dec 31, 2020, and March 16, 2022, 13·2 million vaccine doses were administered in Hong Kong's 7·4-million population. We analysed data from confirmed cases with mild or moderate (n=5566), severe or fatal (n=8875), and fatal (n=6866) COVID-19. Two doses of either vaccine protected against severe disease and death within 28 days of a positive test, with higher effectiveness among adults aged 60 years or older with BNT162b2 (vaccine effectiveness 89·3% [95% CI 86·6–91·6]) compared with CoronaVac (69·9% [64·4–74·6]). Three doses of either vaccine offered very high levels of protection against severe or fatal outcomes (97·9% [97·3–98·4]).
Interpretation
Third doses of either BNT162b2 or CoronaVac provide substantial additional protection against severe COVID-19 and should be prioritised, particularly in older adults older than 60 years and others in high-risk populations who received CoronaVac primary schedules. Longer follow-up is needed to assess duration of protection across different vaccine platforms and schedules.
Funding
COVID-19 Vaccines Evaluation Program, Chinese Center for Disease Control and Prevention.
Introduction
Hong Kong (population 7·4 million) has pursued a COVID-19 elimination strategy since January, 2020, involving stringent physical distancing measures, border entry restrictions, isolation of cases, quarantine of close contacts, and the use of personal protective measures.1 Consequently, the disease had been largely controlled until December, 2021, with four previous epidemic waves resulting in 12 631 cases (<2 per 1000) and 213 deaths (<3 per 100 000). Since February, 2021, both inactivated (CoronaVac [Sinovac, Beijing, China]) and mRNA (BNT162b2 [Fosun Pharma-BioNTech]) vaccines have been widely available with residents older than 5 years offered the choice of either. However, by January, 2022, two-dose vaccine coverage had only reached 46% in adults aged 70–79 years of age and 18% in those aged 80 years and older.
A major community epidemic of the SARS-CoV-2 omicron (B.1.1.529) variant sublineage BA.2 began in early January, 2022, resulting in 741 708 laboratory confirmed cases, 441 945 cases positive by rapid antigen tests, and 8856 deaths until April 15, 2022.2 Vaccination coverage has since increased but remains low in older people, with two-dose coverage at 62% in those aged 80 years and older by June 27, 2022. Third vaccine doses were recommended first for priority groups and then for members of the general public older than 18 years on Jan 1, 2022, to be given 6 months after the second dose.3 As of April 18, 2022, third dose uptake has been highest in those aged 40–59 years (61%) and lower in older adults (39% in those aged 70–79 years and 15% in those aged ≥80 years). Efforts to increase uptake are underway, including reducing the duration between first and second doses for care-home residents; extending vaccination clinic operating hours; and deploying vaccine outreach teams to care homes, housing estates, and residents with reduced mobility.4
International data have shown that vaccination with BNT162b2 reduces the frequency of severe outcomes and, to a lesser extent, infection for variants circulating before omicron.5, 6, 7, 8 Waning protection has been observed in multiple contexts, in particular against infection,9, 10, 11 and studies have provided early indications of reduced effectiveness of BNT162b2 against omicron.12, 13 Evidence on vaccine performance against the more transmissible omicron sublineage BA.2 remains scarce, as are data on the performance of the inactivated CoronaVac vaccine against previously circulating variants. Some observational evidence suggests strong and durable protection against severe disease and death from both vaccines, with transient protection against milder symptomatic disease.14, 15, 16, 17 With a largely infection-naive population and two COVID-19 vaccines in widespread use, Hong Kong represents a unique environment for monitoring vaccine effectiveness against omicron lineage BA.2. We aimed to estimate vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac, their relative effectiveness, and the additional protection offered by third doses against mild and moderate infections, severe disease, and death.
Research in context.
Evidence before this study
A systematic review by Higdon and colleagues identified 22 efficacy studies for 15 COVID-19 vaccine candidates and 107 observational studies describing performance of eight COVID-19 vaccines. Their review included 86 studies of the vaccine effectiveness of BNT162b2 (Fosun Pharma-BioNTech) and six studies of CoronaVac (Sinovac, Beijing, China) effectiveness. Four BNT162b2 studies and none of the CoronaVac studies were done in areas with circulation of the omicron (B.1.1.529) variant. We searched medRxiv, PubMed, and SSRN using the following search terms: “((vaccine effectiveness) AND (omicron) AND (BA.2)) AND ((BNT162b2) OR (Comirnaty) OR (Coronavac))”, restricting the search from Nov 24, 2021, to March 16, 2022, to coincide with when the omicron variant was reported to WHO and the cutoff for inclusion in our study. We found no published articles and 32 preprints, five of which estimated vaccine effectiveness using clinical outcome data. Of these, only two studies estimated mRNA vaccine effectiveness against the BA.2 sublineage (both in Qatar). The authors reported vaccine effectiveness estimates of BNT162b2 against COVID-19 hospitalisation and death in the range of 70–80% any time after the second dose, and greater than 90% after the third dose. No estimates of the CoronaVac vaccine against BA.2 have been reported to date. Because of previously low SARS-CoV-2 circulation, no previous estimates of COVID-19 vaccine effectiveness in Hong Kong have been published. Given that both CoronaVac and BNT162b2 are widely in use, the BA.2 sublineage is in circulation, and population immunity is almost entirely vaccine-derived, Hong Kong represents a unique environment for monitoring vaccine effectiveness, and vaccine performance might be expected to vary from that of other settings.
Added value of this study
To our knowledge, we present the first assessment of the vaccine effectiveness of mRNA and inactivated vaccines, and relative effectiveness of three versus two doses, against the omicron BA.2 sublineage, in an immunologically-naive population. Recipients of at least two doses of BNT162b2 vaccine had at least 85% vaccine effectiveness and three doses of either BNT162b2 or CoronaVac had greater than 95% vaccine effectiveness against severe or fatal outcomes, irrespective of age. Greater protection was observed among those who received two doses of BNT162b2 compared with two doses of CoronaVac across all age groups. Third vaccine doses were associated with a relative effectiveness versus two doses of 68–97% against severe and fatal outcomes, with the caveat that third doses were recently administered (within a median of 44–61 days), and the vaccine effectiveness might wane. These findings are the first estimates of vaccine effectiveness from Hong Kong and will therefore provide important contributions to vaccination policy in areas where two-dose and three-dose vaccine coverage in older adults remains low.
Implications of all the available evidence
Our results show the importance and urgency of achieving high vaccination coverage in a population that has acquired minimal protection from natural infection, particularly in those most at risk, with a preference for BNT162b2 in a two-dose schedule. Older adults (>60 years) and those in high-risk groups who have received two doses of an inactivated vaccine are strongly recommended to receive a third dose to obtain high levels of protection. A third dose of either an inactivated or mRNA vaccine provides high protection from severe and fatal COVID-19, and innovative public health policies to improve coverage in older adults should be urgently followed to minimise avoidable COVID-19 morbidity and mortality. Additional, longer-term research is needed to understand the duration of protection associated with different vaccines, including heterologous schedules.
Methods
Study design and population
In this observational study, we assessed vaccine effectiveness of the BNT162b2 and CoronaVac vaccines using an ecological study design, previously used in Israel.18 The study population was Hong Kong residents aged 20 years and older. The population vaccinated with zero, one, two or three doses of either vaccine at risk at a given time was derived using vaccination programme and census data. Information on all laboratory-confirmed SARS-CoV-2 infections was obtained from individual-level surveillance data provided by the Hong Kong Centre for Health Protection and linked to clinical outcome data provided by the Hospital Authority of Hong Kong.
This project received approval from the Institutional Review Board of the University of Hong Kong (UW 20-341). Informed consent was not required.
Procedures
Extensive PCR testing for SARS-CoV-2 is done in public hospitals, community test centres, and private laboratories in Hong Kong. Testing is free-of-charge or low cost and required for those with COVID-19-like symptoms or following contact tracing based on exposure history or residential location. Regular screening is also required for those in certain professions, in particular those working with older adults or vulnerable people. Positive rapid test results have been recognised as confirmed infections since Feb 25, 2022. Data on all confirmed cases between Dec 31, 2021, and March 16, 2022, were extracted and cases that were classified as imported—ie, detected in on-arrival quarantine—were excluded because of their non-representative histories of SARS-CoV-2 exposure and vaccination. Individuals with missing age or sex information were excluded, as well as vaccinated individuals with missing information on vaccine type or date of any vaccine dose. Sequencing of a subset of cases each day indicated that less than 1% of cases and deaths during the fifth wave occurred with the variant B.1.617.2 (delta), with the remaining infections attributed to omicron sublineage BA.2 (Poon L, University of Hong Kong, personal communication).19
Until mid-February, 2022, all patients with SARS-CoV-2 infections were admitted to hospitals regardless of symptoms. After this point hospitalisation was reserved for patients with more severe disease, and patients with milder disease were required to isolate at Government quarantine facilities or at home. Electronic medical records from patients attending hospitals managed by the Hospital Authority of Hong Kong are stored in the centralised clinical data analysis and reporting system, including information on demographics, laboratory results, and clinical data.20 We extracted records of all hospitalisations with confirmed COVID-19 between Dec 31, 2021, and March 16, 2022, from data provided on April 14, 2022, to capture all deaths within 28 days of laboratory confirmation, including those with mild or moderate disease before Feb 16, 2022, and severe disease or death at any time. Records were regularly updated and the worst condition during hospitalisation was documented as either mild (non-fatal, non-serious, and non-critical), serious (oxygen supplement of 33 litres per min), or critical (admitted to an intensive care unit [ICU], intubated, requiring extracorporeal membrane oxygenation [ECMO], or in shock). Deaths within 28 days of a positive SARS-CoV-2 test were considered COVID-19 fatalities. We defined severe disease as any serious or critical condition and combined this definition with COVID-19 fatality to form the severe or fatal outcome (appendix p 1). This categorisation aimed to minimise misclassification bias arising from coding anomalies whereby oxygen supplementation or other clinical information requiring manual data entry might have been omitted from patient records, and to include individuals who died from COVID-19-related causes before meeting the criteria for serious or critical episodes due to health-care capacity becoming overwhelmed.
Data on the estimated population size at the end of 2021 by age (years) were obtained from the Census and Statistics Department of the Hong Kong Government. Data on the number of people vaccinated with BNT162b2 or CoronaVac vaccines each day since Feb 22, 2021, are available in a vaccination database provided by the Department for Health. We extracted data on all vaccinations that had occurred up to March 16, 2022, by age, sex, and the type and date of receipt of each vaccine dose on April 12, 2022 (appendix pp 2–3). Individuals with laboratory-confirmed SARS-CoV-2 infection who received vaccines other than BNT162b2 or CoronaVac, a mixed primary series (one dose of BNT162b2 and one dose of CoronaVac), or a third dose that was different from the primary series were excluded from the analysis. Individuals with known previous SARS-CoV-2 infection were also excluded.
Statistical analysis
We estimated incidence rate ratios (IRRs) according to the number of vaccine doses received (none, one, two, or three) for each of the mild or moderate, severe or fatal, and fatal COVID-19 outcomes. Data were stratified by age group (20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, ≥80 years), sex, vaccine type, and calendar day throughout the study period. Vaccination status was categorised according to the date of vaccination plus a 14-day lag for all doses, to allow for the delay in immune response to vaccination. Daily numbers of people in each vaccination category were inferred from the uptake data assuming that individuals received the same vaccine for first and second dose (aligned with Hong Kong guidelines), and using aggregate data by age on vaccine switching for the third dose. The population at risk in each stratum was matched to the report date of cases, and individuals with previous SARS-CoV-2 infection within each group were removed from the population at risk at each timepoint. This process was repeated for each outcome of interest. IRRs were estimated in adults younger than 60 years and in those aged 60–69 years, 70–79 years, and 80 years or older for all outcomes, using negative binomial regression models for the daily counts of cases, adjusting for age group, sex, and calendar day and including the logarithm of person-time as an offset term in the model to account for differing numbers at risk within each strata. Each stratified daily case count was considered as a single observation, resulting in a total of 7448 observations across all age groups. Vaccine effectiveness was defined as (1–IRR) × 100%. We performed sensitivity analyses calculating incidence per calendar week and assuming a 7-day lag instead of 14 days for immune response to vaccination. All analyses were done with R (version 4.1.1).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Dec 31, 2021, and March 16, 2022, 962 557 people had confirmed SARS-CoV-2 infection. Of these, 5566 (0·6%) people were recorded as having mild or moderate disease between Dec 31, 2021, and Feb 15, 2022, and were included in the analysis, after excluding an additional 37 790 (3·9%) mild cases occurring between Feb 16 and March 16, 2022, due to changes in admission criteria. 40 (<0·1%) cases were listed as mild but with fatal outcomes; these individuals were included in the severe or fatal outcomes group. During the entire study period severe or fatal disease occurred in 8875 (0·9%) people and 6866 (0·7%) deaths occurred in 462 638 762 person-days (figure 1 ; appendix p 4). 30 reinfected cases were excluded, along with two individuals with unknown age and nine individuals with differing numbers of doses administered according to different datasets and who we therefore considered of unknown vaccination status.
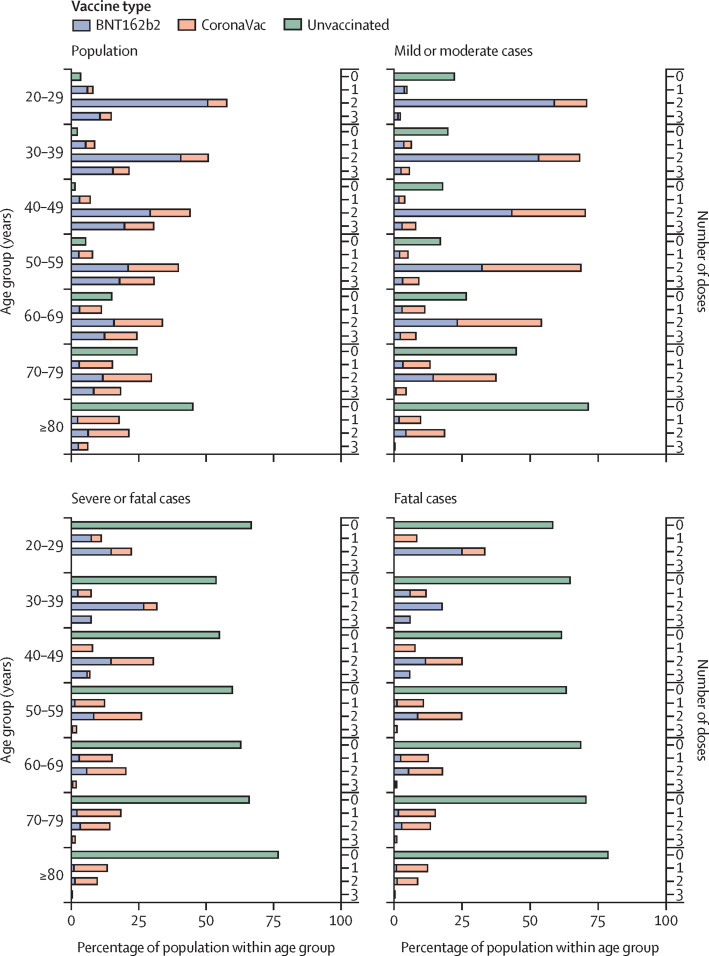
Figure 1.
Daily incidence of cases and deaths by vaccination status
(A) All confirmed COVID-19 cases. (B) Mild or moderate cases in the early part of the fifth wave before Feb 15, 2022. (C) Severe or fatal cases. (D) Deaths throughout the fifth wave in Hong Kong. Severe disease was defined as having ever been listed as serious or critical during hospitalisation for COVID-19 or having a fatal outcome within 28 days of positive test. Vaccination status was categorised according to the number of doses received plus a 14-day lag for all doses, to allow for the immune response to vaccination. Mild cases were only included up until Feb 15, 2022, to account for change in admission criteria.
Up to March 16, 2022, 13·2 million vaccine doses had been administered. Severe disease or death occurred a median of 167 days (IQR 76–209) days after the second vaccination in those vaccinated with two doses of BNT162b2, and 125 days (47–166) among those who received two doses of CoronaVac (table 1 ). Those with severe and fatal outcomes after a third dose tested positive a median of 44 days (28–56) after vaccination with BNT162b2 and 61 days (33–101) after vaccination with CoronaVac (table 1). Severe disease and death occurred predominantly in the unvaccinated population (figure 2 ).
Table 1.
Participant characteristics
| Mild or moderate disease (n=5566)* | Severe or fatal disease (n=8875) | Fatal disease (n=6866) | ||
|---|---|---|---|---|
| Age, years | ||||
| 20–49 | 3198 (57·5%) | 170 (1·9%) | 81 (1·2%) | |
| 50–69 | 1620 (29·1%) | 1214 (13·7%) | 764 (11·1%) | |
| ≥70 | 748 (13·4%) | 7491 (84·4%) | 6021 (87·7%) | |
| Sex | ||||
| Male | 2383 (42·8%) | 5322 (60·0%) | 4152 (60·5%) | |
| Female | 3183 (57·2%) | 3553 (40·0%) | 2714 (39·5%) | |
| Vaccination status† | ||||
| No doses | 1402 (25·2%) | 6413 (72·3%) | 5204 (75·8%) | |
| One dose | ||||
| BNT162b2 | 157 (2·8%) | 126 (1·4%) | 81 (1·2%) | |
| CoronaVac | 227 (4·1%) | 1143 (12·9%) | 794 (11·6%) | |
| Two doses | ||||
| BNT162b2 | 2169 (39·0%) | 242 (2·7%) | 149 (2·2%) | |
| CoronaVac | 1274 (22·9%) | 870 (9·8%) | 596 (8·7%) | |
| Three doses | ||||
| BNT162b2 | 125 (2·2%) | 28 (0·3%) | 16 (0·2%) | |
| CoronaVac | 212 (3·8%) | 53 (0·6%) | 26 (0·4%) | |
| Median number of days between last vaccine dose and positive SARS-CoV-2 test result‡ | ||||
| One dose | ||||
| BNT162b2 | 27 (21–35) | 21 (18–31) | 21 (17–29) | |
| CoronaVac | 29 (21–35) | 22 (17–31) | 22 (17–32) | |
| Two doses | ||||
| BNT162b2 | 181 (150–216) | 167 (76–209) | 172 (92–217) | |
| CoronaVac | 179 (146–209) | 125 (47–166) | 122 (47–164) | |
| Three doses | ||||
| BNT162b2 | 31 (20–48) | 44 (28–56) | 50 (43–70) | |
| CoronaVac | 39 (25–66) | 61 (33–101) | 65 (32–106) | |
Data are n (%) or median (IQR). Includes confirmed COVID-19 cases in Hong Kong classified as having mild or moderate disease between Dec 31, 2021, and Feb 15, 2022; and severe or fatal disease or fatal disease between Dec 31, 2021 and 16 March 2022.
Number of mild or moderate cases occurring before Feb 16, 2022, due to changes in admission criteria.
Number of doses plus 14-day lag.
Median time since vaccination among those for whom 14 days had passed since latest dose.
Figure 2.
Vaccine status, age group, and vaccine type
We found some protection against mild or moderate disease from two doses of either CoronaVac or BNT162b2 in adults aged 20–59 years (table 2 ). Both vaccines were estimated to have high effectiveness against severe disease in adults aged 20–59 years, in whom vaccine effectiveness was estimated to be 96·3% (95% CI 94·9–97·3) for two doses of BNT162b2 and 91·7% (88·7–94·0) for two doses of CoronaVac (table 2). The difference in vaccine effectiveness was greater for older adults, with higher effectiveness among adults aged 60 years or older who received two doses of BNT162b2 (89·3% [86·6–91·6]) compared with those who received two doses of CoronaVac (69·9% [64·4–74·6]). When disaggregated further by age, we estimated that vaccine effectiveness was 91·1% (86·9–94·0) for two doses of BNT162b2 and 79·3% (71·8–85·0) for two doses of CoronaVac in those aged 60–69 years, reducing to 86·9% (80·5–91·3%) for two doses of BNT162b2 and 58·2% (45·1–68·2) for two doses of CoronaVac among those aged 80 years or older (table 2). Findings were similar for death; in adults aged 80 years or older two doses of BNT162b2 offered a higher level of protection against fatal disease (90·3% [84·9–93·9%]) compared with two doses of CoronaVac (63·0% [50·3–72·5]).
Table 2.
Vaccine effectiveness by dose and vaccine type in all ages and within age categories against COVID-19
|
One dose |
Two doses |
Three doses |
||||
|---|---|---|---|---|---|---|
| BNT162b2 | CoronaVac | BNT162b2 | CoronaVac | BNT162b2 | CoronaVac | |
| Mild or moderate disease | ||||||
| 20–59 years | 39·9% (24·8–52·3) | 32·7% (14·4–47·6) | 35·1% (26·6–42·5) | 25·1% (14·7–34·3) | 73·5% (66·6–79·2) | 51·0% (39·6–60·4) |
| ≥60 years | None* | None* | None* | None* | 70·2% (53·3–82·0) | 32·4% (8·3–51·0) |
| Severe or fatal disease | ||||||
| 20–59 years | 95·4% (90·7–98·1) | 74·8% (63·7–82·8) | 96·3% (94·9–97·3) | 91·7% (88·7–94·0) | 98·6% (97·5–99·3) | 98·8% (97·5–99·5) |
| 60–69 years | 70·0% (51·8–82·0) | 54·2% (36·4–67·3) | 91·1% (86·9–94·0) | 79·3% (71·8–85·0) | 98·9% (97·3–99·6) | 97·4% (95·2–98·7) |
| 70–79 years | 72·2% (56·7–82·6) | 29·2% (7·4–46·1) | 89·8% (85·1–93·1) | 74·3% (66·5–80·3) | 99·0% (97·4–99·7) | 95·4% (92·2–97·4) |
| ≥80 years | 75·0% (61·1–84·2) | 39·0% (20·9–53·0) | 86·9% (80·5–91·3) | 58·2% (45·1–68·2) | 97·1% (93·8–98·7) | 97·3% (94·9–98·7) |
| Death | ||||||
| 20–59 years | 96·7% (90·9–99·2) | 78·2% (64·9–86·9) | 96·8% (95·1–98·0) | 93·3% (89·9–95·6) | 99·2% (97·9–99·7) | 99·4% (98·1–99·9) |
| 60–69 years | 77·6% (59·9–88·4) | 65·6% (49·8–76·8) | 92·7% (88·6–95·4) | 84·3% (77·8–89·0) | 99·0% (97·2–99·8) | 99·0% (97·3–99·8) |
| 70–79 years | 80·5% (66·3–89·2) | 45·3% (25·1–60·3) | 92·3% (88·0–95·2) | 76·7% (68·5–82·8) | 99·4% (97·9–99·9) | 97·0% (94·2–98·6) |
| ≥80 years | 78·7% (65·5–87·0) | 44·8% (26·9–58·4) | 90·3% (84·9–93·9) | 63·0% (50·3–72·5) | 97·5% (94·2–99·0) | 97·9% (95·7–99·1) |
Data are effectiveness (95% CI).
No evidence of protection based on a negative or very small positive point estimate and wide CIs.
We compared the two-dose schedules of both vaccines and found differences between BNT162b2 and CoronaVac for mild disease in younger adults (relative vaccine effectiveness of BNT162b2 vs CoronaVac 11·5% [95% CI 0·4–21·3]), but we could not generate robust relative vaccine effectiveness estimates for mild disease in older age groups. Compared with CoronaVac, two doses of BNT162b2 offered better protection against severe or fatal disease in adults younger than 60 years (relative vaccine effectiveness 52·3% [95% CI 29·8–67·8%]) and in those aged 60 years or older (59·8% [51·1–67·1]). Findings were similar for death in those aged 20–59 years (relative vaccine effectiveness 49·8% [15·5–70·5]) and in those aged 60 years or older (62·5% [52·9–70·3]).
We estimated that three recent doses of any vaccine (median time between third dose and onset 44 days for BNT162b2 and 61 days for CoronaVac; table 1) offered very high protection against severe disease (97·9% [95% CI 97·3–98·4]) and death (98·6% [98·0–99·0]), which was sustained within all age groups (appendix p 5). Vaccine effectiveness estimates were similar for both vaccines against severe and fatal outcomes (table 2). We estimated three doses of BNT162b2 to have a vaccine effectiveness of 73·5% (66·6–79·2) against mild or moderate disease in adults aged 20–59 years, whereas for three doses of CoronaVac we estimated the vaccine effectiveness to be 51·0% (39·6–60·4) against the same outcome (table 2). Vaccine effectiveness estimates that were calculated and adjusted for each week of the study period, rather than calendar day; or which considered a 7 day rather than 14 day duration between vaccination and immune response, yielded qualitatively similar vaccine effectiveness results but often with less precision, particularly for one-dose schedules (appendix pp 6–7).
We estimated the relative effect of three doses versus two doses of each vaccine type (table 3 ). For mild or moderate disease we found an additional benefit of a third dose of BNT162b2 in adults aged 20–59 years (relative vaccine effectiveness 59·8% [95% CI 49·7–68·1]) and in adults aged 60 years or older (71·6% [55·6–82·8%]) who had previously received two doses of BNT162b2 (table 3). A third dose of CoronaVac also increased protection in adults aged 20–59 years (35·7% [22·1–47·3]) and in adults aged 60 years or older (46·9% [29·6–60·6]) who had received two doses of CoronaVac (table 3). For severe or fatal disease we found an additional benefit of a third dose in adults of all ages for both vaccine types, with a relative vaccine effectiveness of 64·9% (29·3–84·4) for three versus two doses of BNT162b2, and 87·9% (79·5–93·3%) for three versus two doses of CoronaVac among those aged 80 years or older (table 3). Additional protection against death was offered by a third dose in all ages for both vaccines (table 3).
Table 3.
Relative vaccine effectiveness of three doses versus two doses of BNT162b2 and CoronaVac against COVID-19
| BNT162b2 | CoronaVac | |
|---|---|---|
| Mild or moderate disease | ||
| 20–59 years | 59·8% (49·7–68·1) | 35·7% (22·1–47·3) |
| ≥60 years | 71·6% (55·6–82·8) | 46·9% (29·6–60·6) |
| Severe or fatal disease | ||
| 20–59 years | 60·1% (24·2–81·0) | 85·2% (67·2–94·4) |
| 60–69 years | 84·5% (62·8–94·8) | 85·6% (72·7–93·1) |
| 70–79 years | 88·3% (69·5–96·6) | 76·9% (63·9–86·0) |
| ≥80 years | 64·9% (29·3–84·4) | 87·9% (79·5–93·3) |
| Mortality | ||
| 20–59 years | 71·2% (25·5–91·6) | 91·0% (61·0–97·9) |
| 60–69 years | 84·2% (54·1–96·3) | 92·5% (79·3–98·2) |
| 70–79 years | 90·0% (66·5–98·4) | 82·6% (68·6–91·5) |
| ≥80 years | 61·8% (16·4–84·9) | 88·6% (79·1–94·4) |
Data are effectiveness (95% CI).
Discussion
We used detailed population-level data on the vaccination programme in Hong Kong and individual-level COVID-19 case data to estimate vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac vaccines in a largely infection-naive population during the fifth wave of COVID-19 in Hong Kong. Two or three doses of BNT162b2 or three doses of CoronaVac provided a very high level of protection against severe disease and death in all ages. We found a reduction in vaccine effectiveness among two-dose CoronaVac recipients, in particular for those aged 80 years or older. Some protection against mild or moderate disease was restored with third doses for both vaccines, but we were only able to estimate vaccine effectiveness for a shorter period since administration of third vaccine doses, and it is unclear how long this protection will last.
A case fatality rate of over 9% was observed in the older than 75 years throughout the study period. Although the precise relationship between immune response and clinical outcome is uncertain, the Hong Kong population had little pre-existing naturally or vaccine-derived humoral immunity to the omicron sublineage BA.2 before the beginning of the fifth wave.21 Previous SARS-COV-2 infection has been shown to reduce fatality due to delta or omicron by approximately half (hazard ratio 0·47 [95% CI 0·32–0·68]) in vaccinated individuals and approximately five times (0·18 [0·06–0·57]) in unvaccinated individuals.22 Therefore, the high death rates observed in Hong Kong might be at least partly attributed to the older population remaining largely unvaccinated and infection-naive, combined with health-system congestion. Furthermore, because available data only identified those who died within 28 days of testing positive, deaths from other causes in which COVID-19 disease was incidental or contributory could also have been included within these estimates. In the hospitalisation data used in our study, we found few serious or critical but non-fatal cases. We expect that this finding was a consequence of hospital overload and triage, whereby perhaps only the most serious cases were admitted to ICU or EMCO facilities, but considering the magnitude of health-system disruption we cannot exclude information bias. We therefore applied a broad definition of severe case to account for these variations.
Almost all sequenced SARS-CoV-2 isolates during Hong Kong's fifth wave were of the omicron sublineage BA.2.19, 23, 24 Our overall findings are largely consistent with existing vaccine effectiveness evidence against this sublineage. A study25 in Qatar estimated that third-dose vaccine effectiveness for BNT162b2 against BA.2 was 43·7% (95% CI 36·5–50·0) in the first month and begins to decline again in the following weeks, with substantially improved protection against severe outcomes (6-week vaccine effectiveness 90·9% [78·6–96·1]). Similarly, a study of vaccine effectiveness in the USA26 estimated vaccine effectiveness of two doses of any mRNA vaccine against severe omicron disease, defined as COVID-19 requiring invasive mechanical ventilation or in-hospital death, to be 79% (66–87), a median of 256 days after the second dose, and three-dose vaccine effectiveness to be 94% (88–97), a median of 60 days after the third dose.
Despite the overall consistency between our results and those presented in other studies, vaccine effectiveness could have been overestimated in our study. Reasons for vaccine hesitancy in Hong Kong have varied throughout the pandemic; however, hesitancy has typically been most prominent among adults older than 60 years, and associated with underlying health conditions.27 Healthy vaccinee bias, by which vaccine recipients are healthier than their unvaccinated peers, might inflate the estimates in this setting. We could not formally assess this hypothesis with available data but our estimates are similar to those of other studies using alternative designs and we anticipate the magnitude of overestimation is unlikely to be substantial.12, 25 To address potential differences between vaccinated and unvaccinated cohorts, we also estimated a relative vaccine effectiveness of three versus two doses of each vaccine type; because individuals within these cohorts all chose to be vaccinated, they are more likely similar to each other in terms of baseline characteristics than their unvaccinated peers.28 We found that a third dose of either vaccine provided additional protection, reiterating the public health value of a third dose for minimising the risk of severe disease and death but also for reducing health-system congestion and public concern.
Our finding that three doses of CoronaVac are needed for older adults to achieve high levels of protection is consistent with WHO recommendations for this group.29 However, the estimates presented are likely to be affected by time since vaccination, in that typically more time has passed since administration of second than third doses, which have only been widely available in Hong Kong since the beginning of Jan, 2022. Data from Malaysia15 comparing the duration of protection of the BNT162b2 and CoronaVac vaccines show more rapid waning of protection following CoronaVac after two doses, in particular for mild and moderate outcomes. Furthermore, two-dose immunogenicity data from Hong Kong indicate lower humoral and cellular responses following CoronaVac than with BNT162b2 vaccination but whether inactivated vaccines given in three-dose schedules will provide similar protection to the mRNA vaccines in the long term is unclear. However, evidence from our analyses that three doses of inactivated vaccine provide a high level of protection against severe COVID-19 disease, at least in the short term, is reassuring.30
Our study has several limitations. First, we used census data to construct the source population, but any differential population movement by vaccine status could affect the validity of our estimates. Furthermore, we estimated vaccine effectiveness in real-time and there might have been some delay in recording events, or missed unreported infections, which could underestimate case numbers and overestimate the denominator population-at-risk. Second, there are some differences in testing requirements by vaccine status, particularly for those required to regularly test because of occupation. However, we expect that estimates of vaccine effectiveness against severe outcomes will be only marginally susceptible to biases related to testing requirements. Third, we assumed that the second vaccine type matched the first, as per local guidelines, however a small number of people may have received mixed schedules. Fourth, our severe COVID-19 outcome included oxygen supplementation or therapy, which are coded using the 9th edition of the International Classification of Diseases, requiring medical staff to manually enter these procedures into electronic medical records with the potential for imperfect data entry and capture and under-ascertainment of these procedures. Finally, in Hong Kong there was a clear preference for the BNT162b2 vaccine in younger age groups and for CoronaVac in older adults. We have addressed this confounding in estimates presented by stratifying by age and adjusting estimates by 10-year age categories, sex, and calendar day. However, some residual confounding by age is possible in the vaccine platform-specific estimates and other factors might confound the relationship between vaccine status, type, and risk of infection that cannot be accounted for in this design.
Our findings indicate that two-dose schedules of both BNT162b2 and CoronaVac vaccines offer strong protection against severe disease and death; however, we found higher levels of protection among those who received two doses of BNT162b2 compared with those who received two doses of CoronaVac, particularly in older age groups. Three doses of either vaccine offered very high levels of protection for older adults against severe outcomes, with no differences observed across vaccine types. Our results show the importance of vaccination in an adult population that has acquired minimal protection from natural infection. Increasing uptake of third vaccine doses will be important, particularly in older adults who have received two doses of CoronaVac. Further investigation of the durability of protection provided by both vaccines is warranted and planned.
Data sharing
Data on all vaccinations in Hong Kong by day and age are publicly available online (https://www.covidvaccine.gov.hk/en/dashboard). The clinical outcome data were extracted from the Hospital Authority database in Hong Kong and vaccine dose sequence for vaccinated cases were extracted from the eSARS COVID-19 surveillance database provided by the Centre for Health Protection. Restrictions apply to the availability of these data, used under license for this study. The hospitalisation and surveillance data were derived from records in the e-record system managed by the Hospital Authority and other databases by the Centre for Health Protection in Hong Kong and are restricted for reasons of patient consent. Data access can be discussed with the corresponding author or by approaching the Hospital Authority or Centre for Health Protection directly.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on July 28, 2022
Declaration of interests
BJC reports honoraria from AstraZeneca, Fosun Pharma, GlaxoSmithKline, Moderna, Pfizer, Roche, and Sanofi Pasteur. JN was previously employed by and owns shares in Sanofi. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This project was supported by the Chinese Center for Disease Control and Prevention-sponsored COVID-19 Vaccines Evaluation Program (COVEP). BJC is supported by the Theme-based Research Scheme (T11-712/19-N) from the Research Grants Council from the University Grants Committee of Hong Kong, and an Research Grants Council Senior Research Fellowship (HKU SRFS2021-7S03). The authors thank the Hong Kong Government and Hospital Authority for the timely sharing of COVID-19 vaccination and case data. The authors thank Julie Au for administrative support.
Contributors
The study was designed by MEMM, JN, GML. and BJC. The underlying data were verified by YL, EHYL and MEMM, and data analyses were done by MEMM and YL. MEMM wrote the first draft of the manuscript, which was revised by JN and BJC. All authors interpreted data, provided critical review and revision of the text, and approved the final version of the manuscript.
Supplementary Material
References
- 1.Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong Kong Government Latest situation of COVID-19 (as of April 15, 2022) 2022. https://www.chp.gov.hk/files/pdf/local_situation_covid19_en.pdf
- 3.Hong Kong Government Third dose COVID-19 vaccination arrangements for persons under certain groups. 2021. Nov 3, 2021. https://www.info.gov.hk/gia/general/202111/03/P2021110300536.htm
- 4.Tsang J. Coronavirus: vaccine outreach teams to visit ‘all Hong Kong care facilities by Friday’, at home jabs to be offered to residents with mobility issues. South China Morning Post. March 13, 2022 https://www.scmp.com/news/hong-kong/health-environment/article/3170304/coronavirus-vaccine-outreach-teams-visit-all-hong [Google Scholar]
- 5.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higdon MM, Wahl B, Jones CB, et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. medRxiv. 2022 doi: 10.1101/2021.09.17.21263549. published online March 6. (preprint). [DOI] [Google Scholar]
- 9.Goldberg Y, Mandel M, Bar-On YM, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;375 doi: 10.1136/bmj-2021-067873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119451. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022 doi: 10.1038/s41591-022-01701-w. published online Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suah JL, Husin M, Keng Tok PS, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. medRxiv. 2022 doi: 10.1101/2022.01.15.22269326. published online Jan 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suryatma A, Anasi R, Hananto M, et al. Effectiveness of the inactivated COVID-19 vaccine (CoronaVac) in adult population in Bali, Indonesia. medRxiv. 2022 doi: 10.1101/2022.02.02.22270351. published online Feb 4. (preprint). [DOI] [Google Scholar]
- 17.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesfin Y, Chen D, Bond H, et al. Epidemiology of infections with SARS-CoV-2 omicron BA.2 variant in Hong Kong, January–March 2022. medRxiv. 2022 doi: 10.1101/2022.04.07.22273595. published online April 14. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sing C-W, Woo Y-C, Lee ACH, et al. Validity of major osteoporotic fracture diagnosis codes in the Clinical Data Analysis and Reporting System in Hong Kong. Pharmacoepidemiol Drug Saf. 2017;26:973–976. doi: 10.1002/pds.4208. [DOI] [PubMed] [Google Scholar]
- 21.Chen L-L, Syed MUA, Chan W-M, et al. Contribution of low population immunity to the severe omicron BA.2 outbreak in Hong Kong. Nat Portf. 2022 doi: 10.21203/rs.3.rs-1512533/v1. published online April 14. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok K-H, Wong S-C, Chan W-M, et al. Co-circulation of two SARS-CoV-2 variant strains within imported pet hamsters in Hong Kong. Emerg Microbes Infect. 2022;11:689–698. doi: 10.1080/22221751.2022.2040922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng VC-C, Ip JD, Chu AW-H, et al. Rapid spread of SARS-CoV-2 omicron subvariant BA.2 in a single-source community outbreak. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. medRxiv. 2022 doi: 10.1101/2022.03.13.22272308. published online March 13. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenforde M, Self W, Gaglani M, et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021—January 2022. MMWR Morb Mortal Wkly Rep. 2022 doi: 10.15585/mmwr.mm7112e1. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao J, Cheung JK, Wu P, Ni MY, Cowling BJ, Liao Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: serial cross-sectional surveys. Lancet Reg Health West Pac. 2022;23 doi: 10.1016/j.lanwpc.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMenamin ME, Bond HS, Sullivan SG, Cowling BJ. Estimation of relative vaccine effectiveness in influenza: a systematic review of methodology. Epidemiology. 2022;33:334–345. doi: 10.1097/EDE.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . World Health Organization; Sept 2, 2021. The Sinovac-CoronaVac COVID-19 vaccine: what you need to know.https://www.who.int/news-room/feature-stories/detail/the-sinovac-covid-19-vaccine-what-you-need-to-know [Google Scholar]
- 30.Peng Q, Zhou R, Wang Y, et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on all vaccinations in Hong Kong by day and age are publicly available online (https://www.covidvaccine.gov.hk/en/dashboard). The clinical outcome data were extracted from the Hospital Authority database in Hong Kong and vaccine dose sequence for vaccinated cases were extracted from the eSARS COVID-19 surveillance database provided by the Centre for Health Protection. Restrictions apply to the availability of these data, used under license for this study. The hospitalisation and surveillance data were derived from records in the e-record system managed by the Hospital Authority and other databases by the Centre for Health Protection in Hong Kong and are restricted for reasons of patient consent. Data access can be discussed with the corresponding author or by approaching the Hospital Authority or Centre for Health Protection directly.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on July 28, 2022