Abstract
Childhood trauma (CT) is frequent in patients with alcohol use disorder (AUD) and may impact on adult drinking behaviour and treatment outcome. This study aimed to investigate the structural correlates of CT in AUD, focusing on the amygdala, which plays a crucial role in the neurobiology of trauma. We hypothesized reduced amygdala volume and reduced structural connectivity as quantified by fractional anisotropy (FA) and by number of streamlines in those AUD patients with a history of moderate to severe CT (AUD‐CT). T1‐weighted MP2RAGE and diffusion‐weighted imaging (DWI) 3‐Tesla MRI‐scans were acquired in 41 recently abstinent patients with AUD. We compared bilateral amygdala volume and structural connectivity (FA and number of streamlines) of pathways emanating from the amygdala between AUD‐CT (n = 20) and AUD without CT (AUD‐NT, n = 21) using a mixed model multivariate analysis of variance (MANCOVA) controlling for age and gender. AUD‐CT displayed reduced FA and reduced number of streamlines of amygdalar tracts. There were no differences regarding amygdala volume. The severity of physical abuse, a subscale of the childhood trauma questionnaire, was negatively correlated with FA and with number of streamlines. AUD‐CT and AUD‐NT differ regarding structural connectivity of pathways projecting to and from the amygdala, but not regarding amygdala volume. Those alterations of structural connectivity in AUD‐CT may represent a distinguishable neurobiological subtype of AUD, which might be associated with the complex clinical picture and poorer outcome that patients with CT and AUD often present.
Keywords: alcohol use disorder, amygdala, childhood trauma, DTI, emotion
Patients with alcohol use disorder (AUD) and a history of childhood trauma (CT) differ regarding structural connectivity of pathways projecting to and from the amygdala but not regarding amygdala volume compared with patients with AUD without CT. Thus, patients with AUD and CT may represent a distinguishable neurobiological subtype of AUD, which might be associated with the complex clinical picture and poorer outcome that patients with CT and AUD often present.
1. INTRODUCTION
Child maltreatment such as physical, emotional, or sexual abuse, is the most prevalent cause of childhood trauma (CT) 1 and has repeatedly been shown to have a negative impact on mental health outcomes in adulthood. 2 , 3 The majority of maltreated children experience polyvictimization and report multiple types of CT experiences. 4 Child maltreatment has a detrimental and enduring effect on affective states in later life, in the way that traumatic events in early life increases the risk of experiencing increased negative affect and decreased positive affect in adulthood. 5 Furthermore, there is considerable evidence to suggest that adverse early‐life experiences have a profound effect on the developing brain. 6 The exact pathophysiological mechanism underlying these neuronal changes remains unclear, although alterations of stress systems, particularly of the hypothalamo–pituitary–adrenal (HPA) axis, seems to plays a crucial role. 7 Repeated early‐life stress leads to enhanced levels of glucocorticoids, the HPA axis' end product, which can cause both structural and functional abnormalities in the developing brain and also influences emotion regulation and learning processes. 8 Prolonged exposure to any type of stressor during childhood may thus result in brain injury, particularly in those regions that are sensitive to stress during brain development such as the amygdala, hippocampus, and prefrontal cortex. The amygdala–hippocampus–medial prefrontal circuit is a well‐understood circuits in processing of fear and stress 9 and there is evidence that connectivity of those system is impaired in patients with history of childhood trauma. 10 These long‐lasting effects of CT on the developing brain and affective states may be contributing factors to increasing the risk for developing various psychiatric disorders and being associated with poorer treatment outcome of those disorders. 11 While the prevalence rates of CT is about 10% among the general population, 12 overall prevalence of traumatic experiences have been found to be up to 55% in patients with alcohol use disorder (AUD). 4 CT is associated with early initiation of alcohol use, earlier age at AUD onset, 4 longer duration, more severe AUD symptoms, and poorer treatment outcomes including higher risk of relapse. 11 , 13
CT is a risk factor for the development of AUD. Therefore, AUD and a history of exposure to CT frequently co‐occur. Furthermore, AUD and CT share many neurobiological harmful consequences. 14 For instance, both exposures have deleterious effects on the brain especially during the sensitive developmental period, and particularly on the amygdala and the hippocampus, which has been shown in several cross‐sectional and longitudinal studies. 14 , 15 , 16 , 17 CT and AUD have both been associated with grey matter loss of the hippocampus and especially the amygdala. 18 , 19 One previous study investigated the impact of alcohol and CT on the development of substructures of the amygdala and the hippocampus in an adolescent sample pointing to both volumetric increases and decreases of substructures. Those alterations were amongst other influenced by age and severity of traumatic experiences. 14 However, grey matter alterations in adults with CT and in adults with AUD extend to further limbic and prefrontal brain regions such as the orbitofrontal cortex, the hippocampus, the nucleus accumbens, and the insula. 18 , 20 , 21 This suggests a pathology on a network level rather than an isolated pathology of the amygdala. On the behavioural level, adults with CT and AUD both show dysregulation in affect processing and affect regulation, which might be associated with alterations of the amygdala.
MRI‐diffusion weighted imaging‐based tractography is a method that reconstructs neuronal pathways, hereby enabling an assessment of white matter microstructure (mostly quantified by means of fractional anisotropy, FA). Multiple studies demonstrated widespread reductions of FA in adults with AUD. 19 , 21 , 22 Similarly, in adults with a history of CT recent studies started to provide accumulating evidence for FA reductions in various pathways of the brain. 23 , 24 , 25 Those pathways partially overlap with pathways that have been shown to be impaired in AUD. 21 , 23
Besides consistent findings of reduced amygdala volume in both patients with AUD and in adults with CT, structural connectivity of the amygdala has not been investigated specifically in AUD patients with CT. Even though AUD and CT often co‐occur and represent a subtype that differs regarding onset, progression and treatment outcome of AUD, 4 , 11 , 13 to date no study has investigated whether AUD patients with CT differ from AUD patients without CT regarding structural connectivity, nor on the behavioural level regarding affect processing. Differentiating these effects would contribute to the better understanding of the neuronal and behavioural correlates of this psychopathology, which, in turn, may help develop better treatment strategies for this high‐risk population.
The aim of this study was to compare the neurobiology of recently abstinent AUD patients with and without a history of moderate to severe CT. Using MRI‐based imaging, we assessed amygdala volume and structural connectivity of pathways emanating from the amygdala. Structural connectivity was assessed using fractional anisotropy (FA), a measure of white matter microstructure, and number of streamlines, a macroscopic and geometric tract measure. 21 We hypothesized reduced bilateral amygdala volume, reduced FA, and a reduced number of streamlines in AUD patients with a history of moderate to severe CT compared with AUD patients without CT. As alterations of the amygdala may be directly associated with affect processing, we further assume that AUD patients with moderate to severe CT show increased negative affect and less positive affect.
2. MATERIALS AND METHODS
2.1. Participants
All patients were participating in a randomized‐controlled, double‐blind, clinical trial investigating the effects of an alcohol‐specific inhibition training on relapse. 26 An additional subsample of 49 right‐handed patients was recruited to participate in a longitudinal multimodal MRI‐study investigating the neuronal correlates of this inhibition training. Only baseline and preintervention data were included in the present analyses and are described in detail. All patients had been abstinent for at least 4 weeks prior to MRI measurement and were attending a 12‐week abstinence‐oriented residential treatment program for AUD in a specialized treatment centre in Switzerland (Clinic Suedhang). Included patients fulfilled the main diagnosis of AUD according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5 27 ) and were 18 to 61 years of age. Other severe substance use disorders (except nicotine; Drug Use Identification Test DUDIT ≥25 per substance 28 ), diagnosed neurocognitive disorders (e.g., Korsakoff syndrome), current medical conditions excluding participation (e.g. acute infectious disease), current treatment with benzodiazepines or methylphenidate, contraindications to perform an MRI or inability to read and understand the participant's information led to exclusion. Three subjects had to be excluded because of technical problems during MR scan, which resulted in incomplete data and five patients because of missing DTI measurement. The final sample consisted of 41 patients (14 females, 27 males) with an average age of 43 years (SD 9.50) and an average duration of problematic alcohol consumption of 12 years (SD 9.73). Of the 41 patients included, two patients did not receive any medication. Medication ranged from nutritional supplements, vitamins, and antidepressant medication to medication for somatic problems. A detailed sample description is shown in Table 1.
TABLE 1.
Demographic, baseline, and clinical variables of interest
| AUD‐NT (N = 21) | AUD‐CT (N = 20) | p values | |
|---|---|---|---|
| Females/males | 5/16 | 11/9 | 0.153 |
| Age | 43.33 (8.60) | 43.05 (10.32) | 0.925 |
| Relationship (yes/no) | 10/11 | 11/9 | 0.636 |
| Years of education | 14.31 (3.73) | 14.25 (3.82) | 0.960 |
| Employment (yes/no) | 11/10 | 12/8 | 0.623 |
| CTQ | 35.23 (9.86) | 59.00 (16.23) | <0.001 |
| Nr. of detox | 2.50 (2.79) | 3.90 (3.25) | 0.315 |
| Days of abstinence | 33.67 (14.94) | 27.61 (13.45) | 0.195 |
| Years of probl. drinking | 10.10 (7.45) | 13.45 (11.15) | 0.162 |
| Age of first illness | 33.24 (11.30) | 29.60 (11.44) | 0.312 |
| AUDIT | 24.81 (6.85) | 25.10 (7.96) | 0.901 |
| BSCL GSI | 0.95 (0.57) | 1.63 (0.75) | 0.002 |
| PSS (yes/no) | 4/17 | 8/12 | 0.141 |
| BDI II | 11.85 (7.94) | 19.30 (10.46) | 0.015 |
| BAI | 5.05 (5. 350) | 12.91 (12.71) | 0.016 |
Note: AUD‐NT: patients with alcohol use disorder and no experience of childhood trauma; AUD‐CT: patients with alcohol use disorder and childhood trauma; Nr. detox: Number of previous detoxifications; Years of probl. drinking: years of problematic drinking; AUDIT: Alcohol Use Disorders Identification Test; BAI: Beck Anxiety Inventory; CTQ: Childhood Trauma Questionnaire: BSCL GSI: Global severity index of the Brief Symptom Check List; BDI II: Beck Depression Inventory; PSS: PTSD Symptom Scale (total score higher than 13, suspected posttraumatic stress disorder).
All participants provided written informed consent and were reimbursed for participation with 50 Swiss Francs. The study was approved by the local ethics committee (KEK‐number: 2016‐00988) and registered at Clinicaltrials.gov (NCT02968537) and the Swiss National Clinical Trials Portal (SNCTP000002043). For more details on procedure, tasks, materials, and questionnaires used in the main study, see Tschuemperlin et al. 26
2.2. Procedure and measurements
At treatment admission, eligible patients were informed about the study and asked to participate. After assessing inclusion/exclusion criteria, written informed consent was obtained and a baseline measurement during the second treatment week followed including the assessment of all descriptive data, the clinical interview, and questionnaires. AUD diagnosis was verified with the Diagnostic Expert System for Psychiatric Disorders (DIA‐X 29 ), the AUD part adapted to DSM‐5 and self‐rating Alcohol Use Disorders Identification Test (AUDIT) to assess the severity of drinking problems. 30 Alongside with alcohol‐specific variables, depressive and anxiety symptoms were assessed using the Beck Depression Inventory (BDI‐II 31 ) and the Beck Anxiety Inventory (BAI 32 ), and general clinically significant symptoms were assessed by the global severity index (GSI) of the Brief Symptom Check List (BSCL 33 ). Post‐traumatic stress symptoms were assessed with the PTSD Screening Scale (PSS 34 ). The PSS is a self‐reported questionnaire to screen for symptoms of posttraumatic stress disorder. A total score higher than 13 indicates on likelihood of PTSD.
2.3. Assessment of childhood trauma
History of CT was assessed with the Childhood Trauma Questionnaire, Short‐Form (CTQ‐SF 35 ). The CQ is a 28‐item screening tool to assess the history of CT with five subscales. Five different dimension of childhood mistreatment are measured: physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect. Each dimension of maltreatment is represented by five items rating the frequency of maltreatment experiences on a 5‐point Likert‐type scale ranging from 1 (never true) to 5 (very often true). The total score reflects the severity of overall trauma exposure. The subsequent classification of the severity of experienced maltreatment in each scale ranged from none to low, low to moderate, moderate to severe, and severe to extreme exposure. 36 According to the severity of CT experience, participants were divided into a nontrauma group (AUD‐NT, n = 21) and a trauma group (AUD‐CT, n = 20). Patients who experienced moderate to extreme emotional, physical, or sexual abuse (CTQ subscales) during childhood were allocated to the AUD‐CT group, whereas patients who reported none to low or moderate maltreatment in these dimensions were allocated to AUD‐NT group (see Table 1; Table S1).
2.4. Assessment of positive and negative affect
The Positive and Negative Affect Schedule (PANAS) is a widely used self‐report measure developed by Watson et al. 37 to assess two broad domains of affect, termed Positive Affect (PA) and Negative Affect (NA). Both PA and NA represent largely independent constructs ranging from low to high levels of emotional experience. Negative affect is related to self‐reported stress, poor coping, health complaints, and frequency of unpleasant events. Positive affect is related to social activity and satisfaction and the frequency of pleasant events.
2.5. MRI‐data acquisition
MRI data were acquired at the Department of Diagnostic and Interventional Neuroradiology, University Hospital of Bern, with a 3 Tesla Siemens MAGNETOM Prisma scanner and with a 64‐channel head/neck coil. For high‐resolution T1‐weighted structural images, we used a bias‐field corrected MP2RAGE sequence with the following parameters: 256 Slices, field of view (FoV) = 256 × 256, matrix = 256 × 256 matrix, voxel size = 1 mm3, repetition time (TR) = 5000 ms, echo time (TE) = 2.98 ms, inversion time T1 = 700 ms, and T2 = 2500 ms. The MP2RAGE sequence generated two gradient echo images (INV1 and INV2) and a T1‐weighted image (UNI). Diffusion weighted images (DWI) were acquired using a spin‐echo echo‐planar sequence with one nongradient weighted b0 volume and 64 noncollinear directions with b value = 1000 s/mm2. Parameters were 60 Slices, FoV = 269 × 269, matrix = 122 × 122, voxel size = 2.2 mm3, TR = 6200 ms, TE = 69 ms.
2.6. Computation of amygdala volumes
We used FSL‐FIRST implemented in FSL6.0 for model‐based segmentation and registration of the bilateral amygdala using a Bayesian appearance model by measuring the probabilistic relationships between shape and GM intensity. 38 In advance, we used the INV2 volumes for robust brain extraction with FSL‐BET with R‐option and applied a binary brain mask to the T1‐weighted UNI volumes with FSLMATHS. Then we run FSL‐FIRST for segmentation of the bilateral amygdala using the brain extracted UNI volumes as input. Volumes were finally determined in cubic millimetre using FSL‐STATS with the all_fast_firstseg files as input and a threshold of 17.5–18.5 for the left amygdala, 44.5–45.5 for the right amygdala.
2.7. Computation of tracts emanating from or passing through the amygdala
We preprocessed DWI‐data using ExploreDTI 4.8.6 39 as described in previous publications. 21 , 40 In short, a subjection motion and distortion correction and an echo planar imaging (EPI) correction to the brain extracted MP2RAGE UNI image was performed resulting in the DWI‐images being in the same undistorted space as the MP2RAGE images. 41 Whole‐brain tractography was executed fitting a single diffusion tensor model to DWI data. Termination criteria were angle threshold of >45° and FA < 0.2.
A whole‐brain structural connectivity matrix was computed using network analysis tool implemented in ExploreDTI. We used the Automated Anatomical Labeling (AAL) atlas as template with 90 distinct region of interests (ROI) in MNI space. 42 First, EPI corrected DWI files were normalized to MNI space, second the AAL atlas was transformed to native space using the inverse of the acquired normalization matrix, and third deterministic tractography performed in native space using the EPI corrected DWI files. Tractography was performed between every inversely transformed AAL‐ROI pair using the PASS criteria. The two resulting 90 × 90 connectivity matrices contained information about (1) the number of streamlines, (2) FA, (3) mean diffusion (MD), (4) radial diffusion (RD), and (5) axial diffusion (AD). We used MATLAB R2020a for further extraction of the tracts passing through either the left or the right amygdala (see Figure 1).
FIGURE 1.

Computation of bilateral amygdala volumes and whole brain connectivity matrix with 90 regions of interest for computation of tracts passing through or emanating from the amygdala
2.8. Statistical analyses
Statistical analyses were performed using Statistical Package for Social Sciences SPSS 26.0 (SPSS, Inc., Chicago, Illinois). Demographics between AUD‐NT and AUD‐CT were compared using t‐tests for continuous variables or tests for dichotomous variables. Mixed‐model MANCOVA controlling for age and gender with the between‐factor group (AUD‐CT vs. AUD‐NT), the within‐factor hemisphere (left, right) and the dependent variables comprising the different brain variables (amygdala volume, amygdala tract (FA), amygdala tract (number of streamlines) were used. Significant main effects were followed up with post‐hoc unimodal analyses of variance (ANOVAs). In case of significant group differences of FA, we additionally explored group differences of the following complementary diffusion properties: mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). Three separate mixed‐modal analyses of covariance (ANCOVAs) controlling for age and gender with the between‐factor group (AUD‐CT vs. AUD‐NT) and the within‐factor hemisphere (left, right) were calculated for MD, RD, and AD. Results are displayed in Table S2 and Figure S1.
To assess the association of amygdala volumes, structural amygdala connectivity (FA, number of streamlines), negative and positive affect with CTQ subscales emotional, sexual and physical abuse, we performed exploratory correlation coefficients. Exploratory correlational analysis were not corrected for multiple comparisons and included all patients of the two groups. Group differences (AUD‐CT vs. AUD‐NT) in negative and positive affect were further analysed with unpaired t‐tests.
All tests were two tailed, and a probability of <0.05 was considered statistically significant. Effect sizes were reported as η 2.
3. RESULTS
3.1. Study population
The two patient groups (AUD‐CT and AUD‐NT) did not differ regarding sociodemographic, alcohol specific variables or suspected posttraumatic stress disorder except for higher depression and anxiety scores, as well as higher scores in the global severity index (GSI) of the BSCL and CTQ subscales in the AUD‐CT group (see Table 1; for CTQ subscales, see Table S1). Analysis regarding affective experience revealed that patients of the AUD‐CT group showed significant higher scores of the negative affect (PANAS negative affect: t = −3.272; df = 32.51; p = 0.003) and trend towards lower scores of positive affect (PANAS positive affect: t = 1.769; df = 39; p = 0.085) compared with the AUD‐NT group.
3.2. Group comparisons
3.2.1. Amygdala
The mixed model MANCOVA revealed a significant overall main effect of group showing a large effect size (F(3, 35) = 3.877, p = 0.017, η 2 = 0.249). Post‐hoc unimodal ANOVAs demonstrated that AUD‐CT patients showed reduced FA of amygdala tracts (AUD‐NT > AUD‐CT; F(1, 37) = 8.962, p = 0.005, η 2 = 0.194), as well as reduced number of streamlines of amygdala tracts (AUD‐NT > AUD‐CT; F(1, 37) = 8.002, p = 0.008, η 2 = 0.178) compared with the AUD‐NT (Figure 2). Results remained significant when controlling for depression (BDI‐total) and anxiety (BAI‐total) (main effect of group: p = 0.041; FA of amygdala tracts: p = 0.015; number of streamlines of amygdala tracts: p = 0.013).
FIGURE 2.
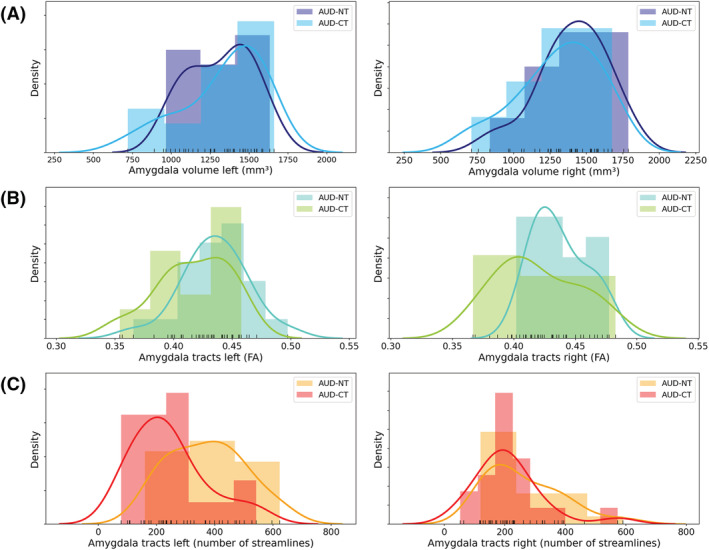
Group differences in amygdala volume and structural connectivity of the amygdala between AUD patients with childhood trauma (AUD‐CT) and without childhood trauma (AUD‐NT). (A) No group differences regarding amygdala volume, (B) AUD‐CT showed reduced FA of amygdala tracts, (C) AUD‐CT showed reduced number of streamlines of amygdala tracts
Additional exploratory group comparisons of MD, RD, and AD between AUD‐CT and AUD‐NT showed reduced AD (F(1, 37) = 5.4, p = 0.03) in AUD‐CT compared with AUD‐NT but no effects in MD and RD (for complete analysis, see Table S2 and Figure S2).
3.3. Exploratory analyses
Additional exploratory correlation analysis including all patients of the two groups showed a negative association between the bilateral amygdala tracts (FA and number of streamlines) and the severity of physical abuse (CTQ's physical abuse subscale) but not with sexual or emotional abuse, nor with physical or emotional neglect (Table 2; Figure S2). Amygdala volume was not significantly correlated with any of the CTQ subscales. Furthermore, there was a significant correlation between negative affect and the severity of all the CTQ subscales (abuse and neglect), whereas there was only a negative association between positive affect and subscale of sexual abuse of the CTQ. However, neither positive and negative affect nor any other alcohol specific variables were associated with the neuronal correlates (all p > 0.05) except of age of first illness (FA amygdala left: r = 0.315, p = 0.045) and years of problematic alcohol consumption (no of streamlines amygdala left: r = −0.351, p = 0.024).
TABLE 2.
Correlation analyses of CTQ subscores and neuronal variables and positive and negative affect
| CTQ | CTQ | CTQ | |
|---|---|---|---|
| Physical abuse | Sexual abuse | Emotional abuse | |
| Amygdala volume left | r = 0.248, p = 0.117 | r = 0.276, p = 0.080 | r = −0.035, p = 0.828 |
| Amygdala volume right | r = −0.021, p = 0.895 | r = 0.026, p = 0.873 | r = 0.059, p = 0.716 |
| Amygdala tracts left (no. streamlines) | r = −0.477, p = 0.002 | r = −0.187, p = 0.241 | r = −0.281, p = 0.076 |
| Amygdala tracts right (no. streamlines) | r = −0.311, p = 0.048 | r = 0.131, p = 0.415 | r = 0.172, p = 0.282 |
| Amygdala tracts left (FA) | r = −0.389, p = 0.012 | r = −0.267, p = 0.092 | r = −0.199, p = 0.212 |
| Amygdala tracts right (FA) | r = −0.409, p = 0.008 | r = −0.087, p = 0.587 | r = −0.059, p = 0.716 |
| PANAS: positive affect | r = 0.051, p = 0.753 | r = −0.265, p = 0.095 | r = −0.078, p = 0.628 |
| PANAS: negative affect | r = 0.325, p = 0.038 | r = 0.316, p = 0.044 | r = 0.372, p = 0.020 |
Note: CTQ: Childhood Trauma Questionnaire; No.: number of; PANAS: The Positive and Negative Affect Schedule; The two CTQ neglect subscales did not significantly correlate with any of the neuronal variables (all p > 0.146) except of the negative affect measured with the PANAS (CTQ emotional neglect: r = 0.424, p = 0.006; CTQ physical neglect: r = 0.350, p = 0.025).
4. DISCUSSION
We found reduced FA and a reduced number of streamlines emanating from the amygdala in AUD‐CT as compared with AUD‐NT. This suggests that CT is related to a reduction of connection pathways of the amygdala in this subgroup. Amygdala volumes did not differ between AUD‐CT and AUD‐NT suggesting that our findings of decreased structural connectivity are independent from grey matter morphology of the amygdala.
Bearing in mind that AUD (in comparison with healthy controls) as such is associated with alterations of FA, 19 , 21 , 22 it is relevant that we found pronounced FA reductions (large effect size of η 2 = 0.194) of structural connectivity of amygdalar pathways in AUD‐CT as compared with AUD‐NT. Thus, there seems to be an independent or even an additive effect of CT on white matter microstructural alterations in AUD‐CT. We complemented FA, a measure of white matter microstructure with number of streamlines, a volumetric tract measure 21 which was reduced in AUD‐CT as well (effect size of η 2 = 0.178). Our finding of reduced streamlines fits results of exploratory analyses of further diffusion properties showing reductions of AD in AUD‐CT pointing to an axonal pathology. 43 Thus, the observed reduction of number of streamlines in AUD‐CT, which was further associated with the severity of experienced abuse, seems to be associated with axonal properties that are independent from grey matter volumes of bilateral amygdalae.
The amygdala is essential for emotion regulation and memory; thus, alterations in this region might affect emotion processing, which appears to be a predictor for poorer treatment outcome in AUD. 44 We found increased negative affect and lower positive affect measured with the PANAS in patients of the AUD‐CT group, which was associated with the severity of CT but not with the neuronal correlates. Negative affect is related to self‐reported stress, poor coping, health complaints, and frequency of unpleasant events while positive affect is related to social activity and satisfaction and the frequency of pleasant events. This is in line with findings showing that higher prevalence and maintenance and early onset of general psychopathology following child maltreatment is often linked to disruption in emotion regulation. 45 Brain regions that are essential for emotion regulation can be roughly divided in two functional categories: emotion‐generating or emotion‐processing regions (i.e., amygdala and insula) and emotion‐regulatory regions (i.e. dorsomedial prefrontal cortex and dorsal anterior cingulate cortex). Neuroimaging studies investigating emotion regulation show that CT is associated with elevated response in the amygdala and other areas of the salience network in response to negative affect. 45 However, subjects with CT seem to be able to down‐regulate the amygdala after negative affect to the same extent as subjects without CT, but they use regions involved in effortful control to a greater degree to do so. McLaughlin et al. suggest that greater effort might be required to modulate heightened amygdala responses. 45 Interestingly, our neuroanatomical findings of reduced amygdala connectivity are complemented by the self‐rating reports, which indicate that patients with CT experience more negative emotions and less positive affect than patients without CT. Such an enhanced experience of negative emotions might be expected as the psychological counterpart of an elevated amygdala response. 45 The connectivity deficits observed in the AUD‐CT subgroup might then indicate that emotion‐regulatory regions in this subgroup have to exert their regulatory power within a reduced or poorly developed network. Additionally, it has been reported that especially the experience of positive affect is associated with positive treatment outcome in patients with AUD, 46 while difficulties regulating both positive and negative emotions carry a heightened risk of engaging in coping‐oriented alcohol use during drinking episodes. 47
In AUD, impaired emotion regulation contributes to the development and severity of the disorder and predicts poor treatment outcome. 48 Even though impaired emotion regulation is associated with increased amygdala activation, in substance use disorders, such findings are not consistently observed. According to a review on the neural circuitry, the emotion regulation disturbance in substance use disorder might rather be related to impairments of prefrontal functioning to down‐regulate amygdala activity, rather than from increased amygdala reactivity to emotional stimuli. 48 Furthermore, in AUD, impairments in functional resting state connectivity and white matter tract integrity between emotion‐generating and emotion‐processing regions (e.g., amygdala) and regulatory regions (e.g., regions of the PFC and ACC) might contribute to the described impaired down‐regulation of emotion‐generating regions. As AUD‐CT is a subgroup of our AUD patient sample, the typical AUD findings and the typical CT findings might be cumulated.
Subjects with CT and AUD show functional and structural impairments resulting in increased amygdala reactivity and enhanced negative affect on the one hand, as well as impairments of prefrontal regions failing to down‐regulate heightened amygdala response on the other hand. Therefore, this subgroup might profit from combined treatment approaches such as (i) interventions for emotion regulation that normalize prefrontal functioning and strengthen functional connectivity or the integrity of white matter tracts between emotion regulatory and emotion‐generating regions and (ii) treatments that dampen amygdala reactivity, reduce the experience of negative affect and enhance structural connectivity of emotion‐generating and emotion‐processing region.
Our samples of AUD‐CT and AUD‐NT did not differ regarding the volume of the amygdala. Reduced amygdala volume in patients with AUD as well as in adults with CT have been reported consistently. 18 , 19 , 49 However, the inexistent difference between the two groups suggests that the effect of severe AUD on amygdala volume may be more pronounced than any effect caused by CT. Pronounced neurotoxic effects of alcohol on grey matter including the amygdala are well described. 19 On the other hand, there is increasing evidence for white matter microstructural alterations in adults with traumatic childhood experiences. 23 , 24 , 25 Thus, it is possible that diffusion weighted MRI‐derived measures of fibre tracts contribute to specifically distinguish AUD‐CT from AUD‐NT whilst this is not the case for amygdala volume.
The overall prevalence of almost 49% of AUD patients experiencing moderate to extreme trauma exposure during childhood in our inpatient sample with severe AUD is in line with a study by Huang and colleagues reporting a prevalence of up to 55%. 4 Sexual, physical, and emotional abuse and physical and emotional neglect often co‐occur within individuals and show a dose–response relationship of the number and severity of childhood trauma types and the risk of psychiatric comorbidities. According to the high prevalence of CT in this patient population and the evidence of its association with poor treatment outcome, the history of CT should be systematically assessed in patients with AUD to tailor evidence‐based interventions for this high‐risk population as proposed above.
This study has some limitations that need to be addressed. First, the research design and the results do not allow to establish a causal role of CT in the development of AUD. Second, CT was only assessed with one questionnaire; thus, recall bias may affect the reporting of abuse incidents. We further did not assess the age when trauma occurred, which has an influence on the subsequent developmental processes. Third, although not statistically significant, there are three times more male patients in the AUD‐NT group than female patients, whereas in the AUD‐T group, the gender ratio is equally distributed. Fourth, amygdala volumes are not unitary structures. Each structure is composed of several subregions representing diverse functions, which may be different between the investigated groups. 14 Our ROI approach did not take subcompartments into account. Fifth, regarding tractography, the general limitations apply: directionality, monosynaptic and polysynaptic connections, the type of neurotransmitters, and neurobiological specificity of findings cannot be assessed. Sixth, both grey matter volume and white matter microstructure are influenced by severity of AUD and by comorbidities such as depression severity and anxiety. Our samples did differ regarding these variables. However, controlling for BDI and BAI scores, results remained significant. Additionally, the lack of a control group prevents the investigation of reduced amygdala volume in patients, which has been shown in numerous studies in patients with AUD and CT. Thus, future studies should include a control group with and without CT to investigate distinct effects of CT and AUD on amygdala volume.
In conclusion, our findings demonstrate relatively high rates of CT in a sample of patients with severe AUD, highlighting the need for greater care in assessing and classifying these subjects, which may contribute to a better understanding of the complex clinical picture that maltreated subjects often present. The subgroup of patients with AUD and CT did not only display a heightened negative emotionality but also neuronal alterations: The neuronal findings indicate that traumatization in AUD may induce structural alterations of amygdala connectivity. This suggests that AUD patients with CT represent a distinguishable neurobiological subtype, which might need distinct treatment approaches.
AUTHOR CONTRIBUTION
LS, MS, and FM were responsible for the study concept and design. LS, MS, FM, MG, RW, AF, TB, ND, HB, and RT contributed to the acquisition of data. ND, TB, and LS performed the analysis. LS, TB, MS, MG, ND, SV, and FM assisted with data analysis and interpretation of findings. LS and TB drafted the manuscript. LS, MS, FM, SV, TB, MG, and ND provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Supporting information
Table S1: Group comparisons of CTQ subscales between the AUD patients with and without experience of childhood trauma
Table S2: Group differences between AUD‐NT and AUD‐CT regarding DTI measures computed such as MD, RD, and AD were explored using ANCOVAs controlling for age and gender
Figure S1: Group differences in additional diffusion characteristics of amygdala tracts between AUD patients with childhood trauma (AUD‐CT) and without childhood trauma (AUD‐NT). A) No group differences regarding MD. B) No group differences regarding RD. C) AUD‐CT showed reduced FA of amygdala tracts C) AUD‐CT showed significantly reduced AD of amygdala tracts.
Figure S2: Scatter plots of significant negative correlations of amygdala tracts data (FA and number of streamlines) with CTQ physical abuse subscale (non‐normal distribution).
ACKNOWLEDGEMENTS
We thank Sara Lustenberger, Miranda Germann, Elena Leumann, Kirstin Schürch, Sophia Vögtli, Manuela Wüthrich, and Benjamin Erb for excellent research assistance. The study was funded by a grant from the Swiss Foundation for Alcohol Research (SSA‐Nr.: 283), by the Swiss National Science Foundation (SNF; No: 105319_159286), and by the Novartis‐Foundation for Medical‐Biological Research (No: 19A063). Furthermore, this work was funded in part by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation—GRK2350/1, Project‐ID 324164820). Tobias Bracht received a scholarship from the Adrian et Simone Frutiger Foundation. Open Access Funding provided by Universitat Bern.
Soravia LM, Denier N, Moggi F, et al. Reduced structural connectivity of the amygdala is associated with childhood trauma in adult patients with alcohol use disorder. Addiction Biology. 2022;27 (3): e13164. doi: 10.1111/adb.13164
Leila M. Soravia and Niklaus Denier shared first authorship.
Maria Stein and Tobias Bracht shared last authorship.
[Correction added on 8 April 2022, after first online publication: CSAL funding statement has been added.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Hodgdon HB, Suvak M, Zinoviev DY, Liebman RE, Briggs EC, Spinazzola J. Network analysis of exposure to trauma and childhood adversities in a clinical sample of youth. Psychol Assess 2019;31(11):1294–1306. doi: 10.1037/pas0000748 [DOI] [PubMed] [Google Scholar]
- 2. Zaorska J, Kopera M, Trucco EM, Suszek H, Kobyliński P, Jakubczyk A. Childhood trauma, emotion regulation, and pain in individuals with alcohol use disorder. Front Psych 2020;11:1132. doi: 10.3389/fpsyt.2020.554150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schückher F, Sellin T, Fahlke C, Engström I. The impact of childhood maltreatment on age of onset of alcohol use disorder in women. Eur Addict Res 2018;24(6):278–285. doi: 10.1159/000494766 [DOI] [PubMed] [Google Scholar]
- 4. Huang MC, Schwandt ML, Ramchandani VA, George DT, Heilig M. Impact of multiple types of childhood trauma exposure on risk of psychiatric comorbidity among alcoholic inpatients. Alcohol Clin Exp Res 2012;36(6):1099–1107. doi: 10.1111/j.1530-0277.2011.01695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turiano NA, Silva NM, McDonald C, Hill PL. Retrospective reports of childhood misfortune are associated with positive and negative affect in adulthood: exploring the moderating role of control beliefs. Int J Aging Hum Dev 2017;84(3):276–293. doi: 10.1177/0091415016688480 [DOI] [PubMed] [Google Scholar]
- 6. Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65:18‐28. [PubMed] [Google Scholar]
- 7. Cassiers LL, Sabbe BG, Schmaal L, Veltman DJ, Penninx BW, Van Den Eede F. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: a systematic review of neuroimaging findings. Front Psych 2018;9:329. doi: 10.3389/fpsyt.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear‐related disorders. Nat Rev Neurosci 2017;18(1):7–19. doi: 10.1038/nrn.2016.155 [DOI] [PubMed] [Google Scholar]
- 9. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010;35(1):169–191. doi: 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cisler JM. Childhood trauma and functional connectivity between amygdala and medial prefrontal cortex: a dynamic functional connectivity and large‐scale network perspective. Front Syst Neurosci 2017;11:29. doi: 10.3389/fnsys.2017.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenfield SF, Kolodziej ME, Sugarman DE, Muenz LR, Vagge LM, He DY, Weiss RD History of abuse and drinking outcomes following inpatient alcohol treatment: a prospective study. Drug Alcohol Depend 2002;67(3):227–234. doi: 10.1016/S0376-8716(02)00072-8 [DOI] [PubMed] [Google Scholar]
- 12. Stoltenborgh M, van IJzendoorn MH, Euser EM, Bakermans‐Kranenburg MJ. A global perspective on child sexual abuse: meta‐analysis of prevalence around the world. Child Maltreat 2011;16(2):79–101. doi: 10.1177/1077559511403920 [DOI] [PubMed] [Google Scholar]
- 13. Schäfer I, Langeland W, Hissbach J, Luedecke C, Ohlmeier MD, Chodzinski C, Kemper U, Keiper P, Wedekind D, Havemann‐Reinecke U Childhood trauma and dissociation in patients with alcohol dependence, drug dependence, or both—a multi‐center study. Drug Alcohol Depend 2010;109(1–3):84–89. doi: 10.1016/j.drugalcdep.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 14. Phillips RD, De Bellis MD, Brumback T, de Bellis MD, Clausen AN, Clarke‐Rubright EK, Haswell CC, Morey RA Volumetric trajectories of hippocampal subfields and amygdala nuclei influenced by adolescent alcohol use and lifetime trauma. Transl Psychiatry 2021;11(1):1–13. doi: 10.1038/s41398-021-01275-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silveri MM, Dager AD, Cohen‐Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev 2016;70:244–259. doi: 10.1016/j.neubiorev.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson S, Bair J, Thomas KM, Iacono WG. Problematic alcohol use and reduced hippocampal volume: a meta‐analytic review. Psychol Med 2017;47(13):2288–2301. doi: 10.1017/S0033291717000721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kribakaran S, Danese A, Bromis K, Kempton MJ, Gee DG. Meta‐analysis of structural MRI studies in pediatric PTSD and comparison with related conditions. Biol Psychiatry: Cogn Neurosci Neuroimagin 2020;5(1):23–34. doi: 10.1016/j.bpsc.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel‐wise meta‐analysis. Am J Psychiatry 2014;171(8):854–863. doi: 10.1176/appi.ajp.2014.13101427 [DOI] [PubMed] [Google Scholar]
- 19. Fritz M, Klawonn AM, Zahr NM. Neuroimaging in alcohol use disorder: From mouse to man. J Neurosci Res 2019. Advance online publication. doi: 10.1002/jnr.24423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Begemann MJH, Schutte MJL, van Dellen E, van Dellen E, Abramovic L, Boks MP, van Haren NEM, Mandl RCW, Vinkers CH, Bohlken MM, Sommer IEC Childhood trauma is associated with reduced frontal gray matter volume: a large transdiagnostic structural MRI study. Psychol Med 2021:1–9. doi: 10.1017/S0033291721002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bracht T, Soravia L, Moggi F, Stein M, Grieder M, Federspiel A, Tschümperlin R, Batschelet HM, Wiest R, Denier N The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl Psychiatry 2021;11(1):267. doi: 10.1038/s41398-021-01384-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Santis S, Bach P, Perez‐Cervera L, de Santis S, Pérez‐Cervera L, Cosa‐Linan A, Weil G, Vollstädt‐Klein S, Hermann D, Kiefer F, Kirsch P, Ciccocioppo R, Sommer WH, Canals S Microstructural white matter alterations in men with alcohol use disorder and rats with excessive alcohol consumption during early abstinence. JAMA Psychiat 2019;76(7):749–758. doi: 10.1001/jamapsychiatry.2019.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeRosse P, Ikuta T, Karlsgodt KH, Szeszko PR, Malhotra AK. History of childhood maltreatment is associated with reduced fractional anisotropy of the accumbofrontal ‘reward’ tract in healthy adults. Brain Imaging Behav 2020;14(2):353–361. doi: 10.1007/s11682-020-00265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tendolkar I, Martensson J, Kuhn S, Klumpers F, Fernandez G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum Brain Mapp 2018;39(3):1283–1290. doi: 10.1002/hbm.23916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olson EA, Overbey TA, Ostrand CG, Pizzagalli DA, Rauch SL, Rosso IM. Childhood maltreatment experiences are associated with altered diffusion in occipito‐temporal white matter pathways. Brain Behav 2020;10(1):e01485. doi: 10.1002/brb3.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tschuemperlin RM, Stein M, Batschelet HM, Moggi F, Soravia LM. Learning to resist the urge: a double‐blind, randomized controlled trial investigating alcohol‐specific inhibition training in abstinent patients with alcohol use disorder. Trials 2019;20(1):402. doi: 10.1186/s13063-019-3505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Association AP . Diagnostic and Statistical Manual of Mental Disorders, (DSM‐5®). Vol 5. American Psychiatric Pub; 2013. [Google Scholar]
- 28. Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 2005;11(1):22–31. doi: 10.1159/000081413 [DOI] [PubMed] [Google Scholar]
- 29. Wittchen HU, Pfister H. Instruktionsmanual zur Durchführung von DIA‐X‐Interviews. Swets Test Services; 1997. [Google Scholar]
- 30. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 31. Beck AT, Steer RA, Brown GK. BDI‐II Beck Depressions‐Inventar 2. Auflage. Harcourt; 2006. [Google Scholar]
- 32. Beck AT, Steer R. Beck anxiety inventory (BAI). Überblick über Reliabilitäts‐und Validitätsbefunde von klinischen und außerklinischen Selbst‐und Fremdbeurteilungsverfahren. 1988.
- 33. Franke G. Brief Symptom Checklist (BSCL). Manual Göttingen. Hogrefe‐Verlag; 2017. [Google Scholar]
- 34. Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self‐report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assess 1997;9(4):445–451. doi: 10.1037/1040-3590.9.4.445 [DOI] [Google Scholar]
- 35. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- 36. Häuser W, Schmutzer G, Brähler E, Glaesmer H. Misshandlungen in Kindheit und Jugend. Dtsch Arztebl. 2011;108(17):287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54(6):1063. 1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 38. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leemans A, Jeurissen B, Sijbers J, Jones DK. ExporeDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. In: Proceedings of the International Society for Magnetic Resonance in Medicine 17th Annual Meeting. Honolulu Hawaii; 2009:3536. [Google Scholar]
- 40. Denier N, Walther S, Schneider C, Federspiel A, Wiest R, Bracht T. Reduced tract length of the medial forebrain bundle and the anterior thalamic radiation in bipolar disorder with melancholic depression. J Affect Disord 2020;274:8–14. doi: 10.1016/j.jad.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 41. Leemans A, Jones DK. The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61(6):1336–1349. doi: 10.1002/mrm.21890 [DOI] [PubMed] [Google Scholar]
- 42. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 43. Budde MD, Xie M, Cross AH, Song S‐K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion‐regulation skills predict alcohol use during and after cognitive–behavioral therapy for alcohol dependence. J Consult Clin Psychol 2011;79(3):307. 318. doi: 10.1037/a0023421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child maltreatment and neural systems underlying emotion regulation. J am Acad Child Adolesc Psychiatry 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serafini K, Malin‐Mayor B, Nich C, Hunkele K, Carroll KM. Psychometric properties of the Positive and Negative Affect Schedule (PANAS) in a heterogeneous sample of substance users. Am J Drug Alcohol Abuse 2016;42(2):203–212. doi: 10.3109/00952990.2015.1133632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paulus DJ, Heggeness LF, Raines AM, Zvolensky MJ. Difficulties regulating positive and negative emotions in relation to coping motives for alcohol use and alcohol problems among hazardous drinkers. Addict Behav 2021;115:106781. doi: 10.1016/j.addbeh.2020.106781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilcox CE, Pommy JM, Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. Am J Psychiatry 2016;173(4):344–361. doi: 10.1176/appi.ajp.2015.15060710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paquola C, Bennett MR, Lagopoulos J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment—a meta‐analysis and review. Neurosci Biobehav Rev 2016;69:299–312. doi: 10.1016/j.neubiorev.2016.08.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Group comparisons of CTQ subscales between the AUD patients with and without experience of childhood trauma
Table S2: Group differences between AUD‐NT and AUD‐CT regarding DTI measures computed such as MD, RD, and AD were explored using ANCOVAs controlling for age and gender
Figure S1: Group differences in additional diffusion characteristics of amygdala tracts between AUD patients with childhood trauma (AUD‐CT) and without childhood trauma (AUD‐NT). A) No group differences regarding MD. B) No group differences regarding RD. C) AUD‐CT showed reduced FA of amygdala tracts C) AUD‐CT showed significantly reduced AD of amygdala tracts.
Figure S2: Scatter plots of significant negative correlations of amygdala tracts data (FA and number of streamlines) with CTQ physical abuse subscale (non‐normal distribution).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


