Abstract
There is growing evidence that immune signalling may be involved in both the causes and consequences of alcohol abuse. Toll‐like receptor (TLR) expression is increased by alcohol consumption and is implicated in AUD, and specifically TLR7 may play an important role in ethanol consumption. We administered the TLR7‐specific agonist imiquimod in male and female Long–Evans rats to determine (1) gene expression changes in brain regions involved in alcohol reinforcement, the nucleus accumbens core and anterior insular cortex, in rats with and without an alcohol history, and (2) whether TLR7 activation could modulate operant alcohol self‐administration. Interferon regulatory factor 7 (IRF7) was dramatically increased in both sexes at both 2‐ and 24‐h post‐injection regardless of alcohol history and TLR3 and 7 gene expression was increased as well. The proinflammatory cytokine TNFα was increased 24‐h post‐injection in rats with an alcohol self‐administration history, but this effect did not persist after four injections, suggesting molecular tolerance. Ethanol consumption was increased 24 h after imiquimod injections but did not occur until the third injection, suggesting adaptation to repeated TLR7 activation is necessary for increased drinking to occur. Notably, imiquimod reliably induced weight loss, indicating that sickness behaviour persisted across repeated injections. These findings show that TLR7 activation can modulate alcohol drinking in an operant self‐administration paradigm and suggest that TLR7 and IRF7 signalling pathways may be a viable druggable target for treatment of AUD.
Keywords: alcohol, AUD, imiquimod, IRF7, self‐administration, TLR7
There is growing evidence that Toll‐like receptor 7 (TLR7) may be involved in the cause and consequence of alcohol use disorder. In these experiments, we found that repeated TLR7 activation using the drug imiquimod increased alcohol consumption the day following exposure and greatly increased gene expression of interferon regulatory factor 7 in both males and females. Repeated TLR7 activation was required to induce changes in drinking in both sexes, suggesting that adaptations within the TLR7 and IRF7 signaling pathways are able to modulate consumption of alcohol in a self‐administration paradigm.
1. INTRODUCTION
There is growing evidence that immune signalling is involved in both the development and persistence of alcohol use disorder (AUD1 1 ). Increased expression of proinflammatory genes such as Toll‐like receptors (TLRs) and cytokines have been found in the brains of postmortem alcoholics 2 , 3 , 4 and chronic alcohol consumption is associated with increased levels of cytokines in circulation. 5 In animal models, alcohol has been shown to increase expression of neuroimmune signalling molecules in the brain, 6 , 7 , 8 , 9 which have in turn been shown to increase voluntary alcohol consumption. 10 , 11 , 12 , 13 Thus, neuroimmune signalling may serve as a positive feedback loop in AUD, being both a cause and consequence of excessive alcohol intake. 1
TLRs are a major component of immune signalling that were discovered to mediate responses to pathogens and necrotic cell damage. For example, TLR4 was first found to respond to bacterial endotoxin, and endosomal TLR3 and 7 respond to double and single‐stranded RNA, respectively, primarily produced by viruses. The brain is generally sterile, but recent studies have examined endogenous TLR agonist signalling in brain contributing to induction of neuronal TLR in a baseline state. 9 Activation of TLRs induces signalling through adapters, MyD88 (all TLR except TLR3) and/or TRIF (TLR3 and 4), ultimately resulting in nuclear translocation and induction of proinflammatory genes including cytokines and interferons. 4 TLRs also have important roles in normal brain functioning. For example, TLR3, 7, and 8 regulate axonal growth, dendritic pruning, and neuronal morphology. 14 , 15 Thus, TLR signalling contributes to neurobiology as well as their known roles in the immune system.
While studies have found increased expression of multiple TLRs in post‐mortem human brain of AUD patients, 16 preclinical studies indicate a complex relationship with alcohol drinking. An initial experiment linking TLRs to alcohol consumption found that the TLR4 agonist and bacterial endotoxin LPS increased voluntary intake of alcohol in a two‐bottle choice model in multiple mouse strains. 17 Interestingly, other studies found that TLR4 knockout and antagonism had little effect on ethanol consumption across both rats and mice, 18 , 19 suggesting that other immune factors contribute to the increase in drinking. TLR3 knockout male mice consumed less alcohol in a two‐bottle choice paradigm, 20 and recent studies have shown that TLR3 activation is capable of driving alcohol consumption in male rats 13 and mice. 10 , 11 TLR7 was recently shown to drive voluntary ethanol consumption as mice given 10 injections of the TLR7/8 small molecule agonist R848, which only activates TLR7 (i.e. does not activate TLR8) in rodents, 21 subsequently drank more alcohol in a two‐bottle choice procedure. 12 Voluntary alcohol drinking may assess different circuitry as compared with operant self‐administration, with the latter better assessing the rewarding properties of alcohol. 22 Nonetheless, work from our lab showed that TLR3 activation produced a similar increase in operant alcohol self‐administration and rapid increases in TLR3 gene expression in the insular cortex and nucleus accumbens, 13 a brain circuit known to be involved in the regulation of alcohol drinking and interoceptive sensitivity to alcohol. 23 , 24 Further, these increases in TLR3 gene expression were positively correlated with TLR7 gene expression in the insular cortex but not the nucleus accumbens. Thus, TLR agonists induce increases in expression of multiple TLR receptors adding to the complexity of interpreting responses to TLR specific agonists.
While initial studies examining TLR signalling and alcohol tended to focus solely on males, when female subjects have been included, sex differences have been found. TLR3 activation via poly(I:C) in female mice did not increase alcohol consumption as has been seen in males, and the time course of the subsequent immune response differs with females showing a delayed response. 10 , 11 Interestingly, both sexes displayed decreased drinking in TLR2 knockout mice while only MyD88 knockout males increased voluntary alcohol consumption. 19 At present, the effects of TLR7 modulation in females has yet to be examined. TLR7 makes for a particularly interesting target in females as it is located on the X chromosome and escapes X‐inactivation in immune cells and thus may be more highly expressed in females at baseline. 25 , 26 As such, the goals of the present study, were to examine (1) gene expression changes in the nucleus accumbens core (AcbC) and anterior insular cortex (AI) in alcohol naïve male and female Long–Evans rats following administration of the TLR7 agonist imiquimod and (2) whether imiquimod modulates alcohol self‐administration in male and female rats and the consequences of multiple imiquimod injections on self‐administration and gene expression. Gene expression analyses focused on TLR signalling pathways in the AcbC and AI to highlight downstream signalling and immune molecules that may play an important role in the TLR7‐mediated increase in alcohol consumption.
2. MATERIALS AND METHODS
2.1. Animals
Adult male and female Long–Evans rats (Envigo‐Harlan, Indianapolis, IN) were delivered at 7 weeks old and were handled daily for 1 week prior to the start of the experiment. All rats were doubled housed in ventilated cages in same‐sex pairs. Rats had ad libitum access to food and water in the home cage unless noted. The rats were kept in a temperature‐ and humidity‐controlled colony room that ran on a 12‐h light/dark cycle (lights on at 07:00). All experiments were conducted during the light cycle. Animals were under the care of the veterinary staff from the Division of Comparative Medicine at UNC‐Chapel Hill. All of the procedures followed the guidelines established by the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
2.2. Drugs
Ethanol (95% v/v; Pharmco‐AAPER, Shelbyville, KY) was diluted in distilled water. Imiquimod (Sigma‐Aldrich CO. LLC, Saint Louis, MO, USA; Lot# 25236) was dissolved in 45% hydroxypropyl‐beta‐cyclodextrin (Acros Organics, Geel, Belgium), which was also used for control injections. Imiquimod was injected intraperitoneal (IP) at a volume of 1 ml/kg.
2.3. Apparatus
Self‐administration chambers (Med Associates Inc., St. Albans, VT) were individually located within sound attenuating chambers with an exhaust fan to circulate air and mask outside sounds. Chambers were fitted with a retractable lever on the opposite walls (left and right) of the chamber. There was a cue light above each lever and liquid receptacles in the centre panels adjacent to both levers. Responses on the left (i.e., active) lever resulted in cue light illumination, stimulus tone, and delivery of 0.1 ml of solution across 1.66 s via a syringe pump into the left receptacle once the response requirement was met. Responses on the right (inactive) lever had no programmed consequence. The chambers also had infrared photobeams which divided the floor into four zones to record general locomotor activity throughout each session.
2.4. EtOH self‐administration training
Self‐administration sessions (30 min) took place 5 days per week (M‐F) with the active lever on a fixed ratio 2 schedule of reinforcement such that every second response resulted in delivery of EtOH. 27 A sucrose‐fading procedure was used in which EtOH was gradually added to the 10% (w/v) sucrose solution. The exact order of exposure was as follows: 2% (v/v) EtOH/10% (w/v) sucrose, 2E/10S, 5E/10S, 10E/10S, 10E/5S, 15E/5S, 15E/2S, 20E/2S, 20E, 15E. Following sucrose fading, unsweetened EtOH/15% (w/v) was the reinforcer for the remainder of the study. At the end of each session, wells were inspected to ensure that rats had consumed all fluid.
2.5. Brain tissue collection and sectioning
Brains were rapidly extracted and flash frozen with isopentane (Sigma‐Aldrich, MI). Brains were stored at −80°C until brain region sectioning. Brains were sectioned on a cryostat (−20°C) up to a predetermined bregma coordinate for each region of interest (ROI). Then, a micropunch tool was used to collect tissue specific to each brain region. ROIs were separated by left and right hemispheres, and all real‐time reverse transcription polymerase chain reaction (RTPCR) experiments used the right hemisphere when separated. Brain tissue was stored at −80°C until real‐time RTPCR analysis. For all experiments where tissue was collected 24‐h post‐imiquimod injection (experiments 2–4), spleens were weighed as an index of the inflammatory response.
2.6. Tissue processing and real‐time RTPCR
RNA was extracted from brain tissue using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer's instructions. RLT lysis buffer containing β‐mercaptoethanol (Sigma Aldrich) was used for tissue homogenization. RNA concentration and purity for each sample were determined using a spectrophotometer (Nanodrop 2000, ThermoScientific). RNA was reverse transcribed into cDNA using the either the QuantiNova Reverse Transcription Kit (Qiagen) or Superscript III First‐Strand Synthesis System (Invitrogen, Waltham, MA, USA) according to the manufacturer's instructions. Following reverse transcription, all samples were diluted 1:10 with nanopure water (200 μl total) and stored at −20°C before RT‐PCR experiments. Real‐time RTPCR was conducted using a QuantStudio3 (ThermoFisher) for all experiments. Using a 96‐well plate, each sample was run in triplicate using 10‐μl total volume per well with the following components: PowerUp Sybr green dye (ThermoFisher, containing ROX dye for passive reference), forward and reverse primers (Eton Biosciences Inc., NC, USA), and cDNA template. The PCR was run with an initial activation for 10 min at 95°C, followed by 40 cycles of the following: denaturation (95°C for 15 s), annealing (60°C for 30 s), and extension (72°C for 45 s). Melt curves were obtained for all experiments to verify synthesis of a single amplicon. All primer sequences are displayed in Table 1.
TABLE 1.
Primers used for PCR analysis
| Target | Forward | Reverse |
|---|---|---|
| Β‐actin | CTACAATGAGCTGCGTGTGGC | CAGGTCCAGACGCAGGATGGC |
| TLR3 | AAGACGCTACAGCTTTCCTG | TGTGTGTCAGCTTCAAATGGC |
| TLR7 | GCCTTCAAGAAAGATGCCATTG | ACCATCGAAACCCAAGGACTC |
| IRF3 | GCTGCGAGTCTCAACTACTG | TCCTCAGCTAATCGCAACAC |
| IRF7 | TAACTTACCACCCCCAGAGG | CCTAGGGACATACCCTGTGT |
| MyD88 | CGACGCCTTCATCTGCTACTGC | CCACCACCATGCGACGACAC |
| TRIF | CCCTGTCATTTCTTGAGCGT | GGGAGATTTAGCAACGCACT |
| IL‐1β | AGGACCCAAGCACCTTCTTT | AGACAGCACGAGGCATTTTT |
| TNFα | GTCCCAACAAGGAGGAGAAGTT | CTCCGCTTGGTGGTTTGCTA |
Note: Forward and reverse primers used for real‐time RTPCR analyses.
2.7. Experiments
2.7.1. Experiment 1: Central gene expression 2‐h post‐imiquimod injection
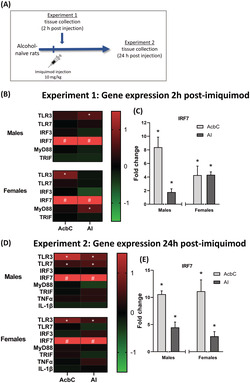
Male and female naive rats were injected with imiquimod or vehicle (0 or 10 mg/kg, IP; n = 8/dose/sex). To examine the short‐term consequences of imiquimod, rats were sacrificed 2‐h post‐injection (Figure 1A). Brain tissue was collected for RT‐PCR analysis. Spleen weights were not collected for this experiment as they were not expected to differ 2‐h post‐imiquimod.
FIGURE 1.
Experiment 1 and 2 gene expression. (A) Rats were given an injection of either imiquimod (10 mg/kg, IP) or vehicle and tissue was collected either 2 h (Experiment 1) or 24 h (Experiment 2) post‐injection. (B) Experiment 1 gene expression changes in the nucleus accumbens core (AcbC) and anterior insula (AI) induced by imiquimod relative to controls visualized as a heatmap. (C) As IRF7 changes were much greater than seen in other genes these data are shown separately as fold change from vehicle controls (where vehicle group = 0 fold change). (D) Experiment 2 gene expression changes visualized as a heatmap. At 24‐h post‐injection, TLR3 was reliably increased across both brain regions and sexes. (E) IRF7 gene expression remained high 24‐h post‐injection. *p < 0.05 compared with vehicle control, #p < 0.05 compared with control and value is greater than the heatmap scale
2.7.2. Experiment 2: Central gene expression 24‐h post‐imiquimod injection
The design of this experiment was identical to that of Experiment 1 except that rats (n = 8/dose/sex) were sacrificed 24‐h post‐injection and spleen weights were collected as an index of the inflammatory response (Figure 1A).
2.7.3. Experiment 3: Effect of EtOH self‐administration history on gene expression 24 h after a single imiquimod injection
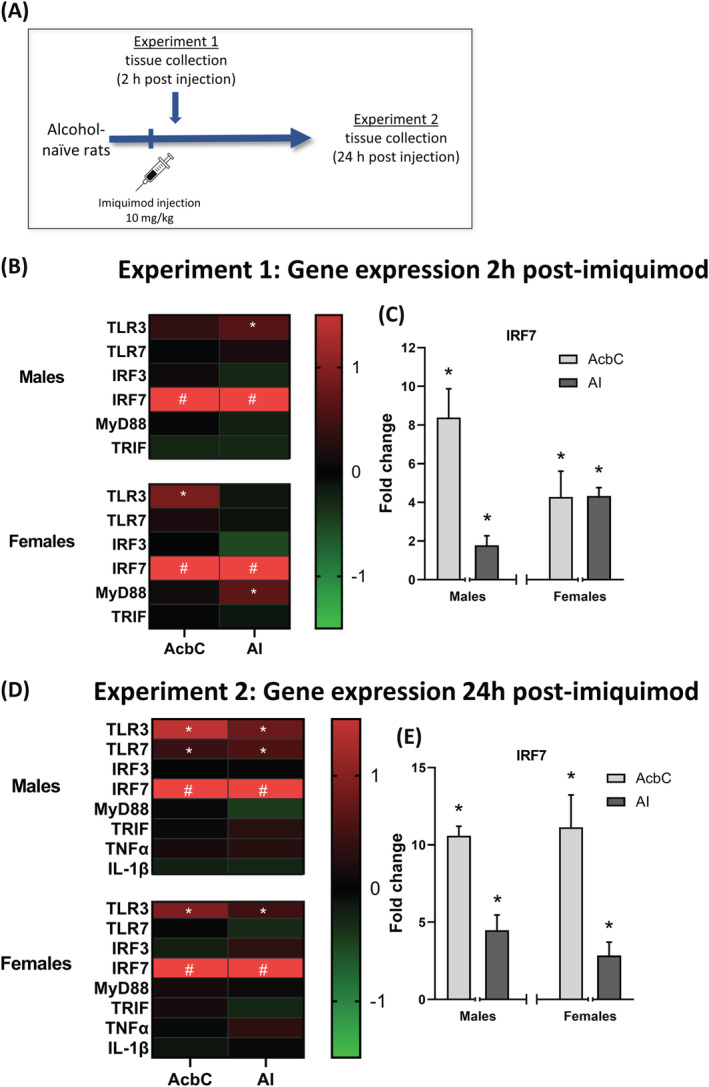
After daily self‐administration of 15% ethanol for 24 days, male and female Long–Evans rats were injected with imiquimod (0 or 10 mg/kg, IP; n = 10/dose/sex) and sacrificed 24‐h post‐injection (Figure 2A). Brains and tissue were collected for RT‐PCR analysis and spleen weights were recorded.
FIGURE 2.
Experiment 3 behaviour and gene expression. (A) For Experiment 3, rats trained on ethanol self‐administration were given an injection of imiquimod (10 mg/kg, IP) or vehicle and tissue was collected 24‐h post‐injection. (B,C) Alcohol intake and locomotor rate on the day of injection. (D) weight change between the day of injection and the following day. (E) Experiment 3 gene expression changes in the (AcbC) and AI induced by imiquimod relative to controls visualized as a heatmap. (F) IRF7 gene expression was greatly increased in both sexes and targets after a single imiquimod injection in rats with a self‐administration history. *p < 0.05 compared with vehicle control, #p < 0.05 compared with control and value is greater than the heatmap scale. & indicates a main effect of sex
2.7.4. Experiment 4: Effects of repeated imiquimod injections on EtOH self‐administration and gene expression 24‐h post‐imiquimod injection
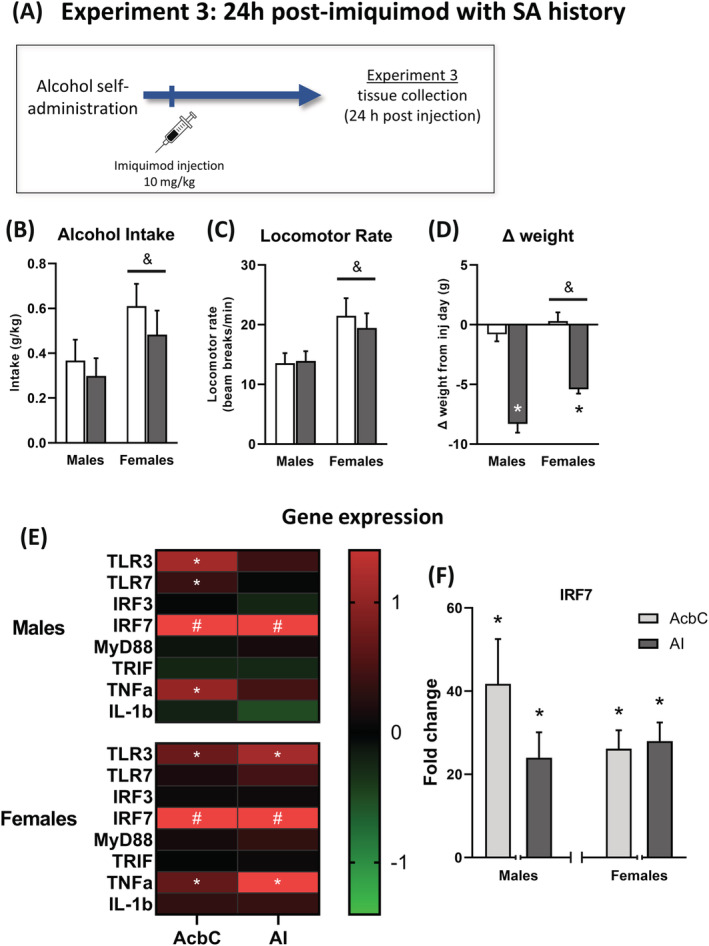
Male and female rats expressing stable self‐administration of 15% ethanol after daily self‐administration for 24 days were injected with imiquimod (0 or 10 mg/kg, IP; n = 12/dose/sex) once every 15 days for a total of 4 injections with 14 self‐administration days between each injection (Figure 3A). On injection days, imiquimod was administered 2 h prior to a standard 30‐min self‐administration session. The 15 days in‐between injections were standard self‐administration sessions, as described above. For the figures and analyses, the first 3 days post‐injection are used for analysis as self‐administration returned to control levels. The 15 days between injections was used to ensure that there was no residual drug effect. Alcohol intake during the operant session is shown as the primary outcome measurement, and number of active lever presses are shared in Figure S1. Rats were sacrificed 24 h after the fourth imiquimod injection, spleens were weighed, and brain was collected for RT‐PCR analysis.
FIGURE 3.
Experiment 4 self‐administration behaviour. (A) In Experiment 4, rats were given imiquimod injections (10 mg/kg, IP) before daily self‐administration sessions then tissue was collected 24 h after the last injection. Baseline data are the average of the three training days prior to the first imiquimod injection, and each injection day is represented as a dotted line with subsequent days designated (e.g., post 1 is the following day). (B) Alcohol intake was unaffected by the first injection but subsequent injections reduced alcohol intake on the day of injection and increased intake the day after the third injection. (C) Like the reductions in drinking, locomotor reductions were present on injection day starting with the second injection. Finally, imiquimod resulted in weight loss after every injection in both sexes (D). ^ indicates main effect of day, & indicates main effect of sex, and * indicates a significant difference between imiquimod and control on this day when data are collapsed across sex
2.8. Statistical analyses
2.8.1. Gene expression
We used the 2^ΔΔCt method to determine fold change relative to controls. 28 β‐actin was used as the housekeeping gene for all targets and β‐actin Ct value did not significantly differ between groups in any case. Fold changes were normalized so that average control fold change equaled 0. For all gene expression analyses, the tissue from males and females and from each brain region was processed separately; thus, sex and region were not included as a factor in analyses. T‐tests were used to determine the effect of imiquimod relative to controls within each sex. Graphs are displayed as expression relative to control subjects with 1 representing the average fold change of controls. Samples were removed from analysis in case of experimenter error or if determined to be a statistical outlier (greater than 2 std. dev. from the mean). All gene expression data including control values are reported as % change from control in Tables S1–S4.
2.8.2. Spleen weights
Spleen weights were analysed via two‐way analysis of variance (ANOVA) comparing effects of imiquimod and sex.
2.8.3. Behavioural analyses
In order to take into account IMI‐induced weight changes, our primary measure in the self‐administration studies was alcohol intake, calculated from rat body weight and the number of reinforcers delivered. Alcohol lever responses are presented in Figure S1. For the test days and subsequent three self‐administration sessions, all behaviours of interest (alcohol intake, locomotor activity, change in weight, and alcohol lever responses) were analysed by three‐way ANOVAs for each injection day and the proceeding 3 days (repeated measure) in order to determine the immediate and short‐term effects of imiquimod and sex on behaviour. Behaviour on the fourth and final injection day was analysed via two‐way ANOVA. Post‐hoc analysis (Tukey) was used to determine differences between specific days of training.
3. RESULTS
3.1. Experiment 1: Central gene expression 2‐h post‐imiquimod injection
This study investigated the response to the small molecule selective TLR7 agonist imiquimod, in both males and females 2 h after injection. The gene expression (fold change) analysis is illustrated by the heatmap in Figure 1B (see Table S1 for data values). AcbC. At 2‐h post‐IMI, IRF7 was greatly increased in both males (9.4‐fold; t(11) = 7.38, p < 0.01) and females (5.3‐fold; t(14) = 3.13, p < 0.01; Figure 1C). TLR3 gene expression was elevated 1.7‐fold following IMI only in females (t(11) = 4.04, p < 0.01). AI. In contrast, in the AI TLR3 expression was increased 1.7‐fold only in males (t(12) = 2.77, p < 0.05). IRF7 was again greatly increased in both males (2.8‐fold; t(12) = 2.99, p < 0.05) and females (5.3‐fold; t(11) = 6.88, p < 0.0001; Figure 1C). MyD88 was increased in females only in the AI (1.5‐fold; t(13) = 2.77, p < 0.05).
3.2. Experiment 2: Central gene expression 24‐h post‐imiquimod injection
The gene expression (fold change) analysis is illustrated by the heatmap in Figure 1D (see Table S2 for data values). AcbC. 24‐h post‐imiquimod, TLR3 gene expression was increased the AcbC in both males (2.2‐fold: t(14) = 3.03, p < 0.01) and females (1.9‐fold: t(12) = 4.83, p < 0.001), while TLR7 expression was increased specifically in males (1.4‐fold: t(12) = 2.41, p < 0.05; see Figure 1E). Similar to Experiment 1, IRF7 expression was greatly increased in both brain regions in males (11.6‐fold: t(10) = 13.69, p < 0.0001) and females (12.2‐fold: t(12) = 4.58, p < 0.01; Figure 1E). AI. In the AI both males and females had increased expression of TLR3 (male 1.8‐fold: t(14) = 2.70 p < 0.05; female 1.6‐fold: t(13) = 2.32, p < 0.05), while like in the AcbC TLR7 expression was increased only in males (1.8‐fold: t(14) = 2.27, p < 0.05; Figure 1E). IRF7 gene expression was once again greatly increased in the AI in both males and females (males 5.5‐fold: t(10) = 3.13, p < 0.05; females 3.8‐fold: t(10) = 2.26, p < 0.05; Figure 1E). These findings suggest that a single dose of IMI induces IRF7 mRNA, a direct downstream transcription factor target of TLR7, in both AcbC and AI at 2 h. that persists for at least 24 h, whereas IRF3, TNFα or IL‐1β are not altered. These findings are consistent with the small molecule IMI activating brain TLR7 receptors.
Increased spleen weights are commonly used to assess systemic immune responses. At 24‐h post‐injection spleen weights were increased relative to body weight as compared with controls (Table 2; F(1,28) = 5.31, p < 0.05), indicative of induction of an inflammatory response, while there was no effect of sex.
TABLE 2.
Spleen weights
| Study | Sex | Vehicle | Imiquimod |
|---|---|---|---|
| Experiment 2 | Males | 2.17 ± 0.06 | 2.32 ± 0.04 a |
| Females | 2.23 ± 0.08 | 2.44 ± 0.11 a | |
| Experiment 3 | Males | 2.01 ± 0.05 | 2.36 ± 0.06 a |
| Females | 2.25 ± 0.06 | 2.73 ± 0.16 a | |
| Experiment 4 | Males | 1.54 ± 0.04 | 2.12 ± 0.07 a |
| Females | 1.77 ± 0.04 | 2.35 ± 0.09 a |
Note: Spleen weights adjusted to body weight for Experiments 2–4 (calculated as weight of spleen (g) − body weight (g) * 1000).
Main effect of imiquimod, bold indicates a main effect of sex. p < 0.05.
3.2.1. Experiment 3: Behaviour and weights
During the self‐administration session 2‐h post‐injection on the imiquimod injection day, the two‐way ANOVAs showed a main effect of sex as females showed greater alcohol intake (F(1,36) = 5.02, p < 0.05) and locomotor rate (F(1,36) = 8.94, p < 0.01) than males, but there was no main effect of drug, indicating that imiquimod did not reduce drinking or were locomotor rates (Figure 2B,C). However, the following day rats lost weight (F(1,36) = 110.3, p < 0.0001) as indicated by a main effect of drug, indicating that imiquimod induced sickness (Figure 2D). Males lost more weight than females (F(1,36) = 10.13, p < 0.01), likely due to their larger initial size.
Effect of EtOH self‐administration history on gene expression 24 h after a single imiquimod injection
AcbC: In rats with a history of alcohol self‐administration, TLR3 gene expression was increased in the AcbC in both males (2.4‐fold; t(18) = 3.49, p < 0.01) and females (1.7‐fold; t(17) = 3.42, p < 0.01). A small but significant 1.4‐fold increase in TLR7 was found only in males (t(17) = 2.23, p < 0.05). Imiquimod induced expression of the proinflammatory cytokine TNFα in males (1.9‐fold; t(16) = 2.84, p < 0.05) and females (1.6‐fold; t(17) = 2.88, p < 0.01). IRF7 gene expression was greatly increased in both males (42.7‐fold; t(18) = 3.88, p < 0.01; Figure 2D) and females (27.2‐fold; t(17) = 5.57, p < 0.0001). AI: In the AI, TLR3 gene expression was increased 2‐fold specifically in females (t(18) = 3.30, p < 0.01). IRF7 expression was again greatly increased in both sexes (males 25‐fold; t(18) = 4.19, p < 0.001 females 29‐fold; t(19) = 5.92, p < 0.0001). Again, expression of TNFα was increased specifically in females (2.3‐fold; t(16) = 3.61, p < 0.01). See Table S3 for data values.
Spleen weights
Imiquimod again resulted in increased spleen sizes 24‐h post‐injection (F(1,35) = 19.22, p < 0.0001; Table 2). Further, after a history of alcohol self‐administration females had larger higher weight relative to body weight as compared with males (F(1,35) = 9.56, p < 0.01; Table 2).
3.2.2. Experiment 4: Effects of repeated imiquimod injections on EtOH self‐administration 24‐h post‐imiquimod
Self‐administration: Alcohol intake
During the first injection round a three‐way ANOVA found a main effect of test day (F(3,132) = 8.13, p < 0.0001). There were significant two‐way interactions between day and sex (F(3,132) = 5.52, p < 0.01) and imiquimod and day (F(3,132) = 2.91, p < 0.05), as well as a three‐way interaction (F(3,132) = 2.91, p < 0.05). However, post‐hoc analyses did not find differences induced by imiquimod on any given day. On the second injection round there was a main effect of test day (F(3,132) = 20.4, p < 0.0001) and an interaction between imiquimod and day (F(1,132) = 7.41, p < 0.0001). Post‐hoc analyses found a reduction in alcohol intake on the injection day (p < 0.001). On the third injection round there was an effect of day (F(3,132) = 30.24, p < 0.0001) and females generally showed higher intake than males (F(3,132) = 14.21, p < 0.001). There was also an imiquimod by day interaction (F(3,132) = 26.83, p < 0.0001) and post‐hoc tests indicated that imiquimod reduced intake on the injection day while also increasing intake the next day (ps < 0.01). Finally, on the final injection day (injection #4) a two‐way ANOVA found a significant main effect of sex (F(1,44) = 5.47, p < 0.05) with lower alcohol intake in males than females. Overall, Figure 3B shows that on the day of injection, imiquimod reduced alcohol intake on the second and third injection rounds while increasing intake 24 h after the third injection. Additionally, these effects were observed in males and females, suggesting a lack of sex specificity.
Locomotor activity
During the first injection round, there were main effects of day (F(3,132) = 5.88, p < 0.001), sex (F(3,132) = 16.89, p < 0.001), and imiquimod (F(3,132) = 6.23, p < 0.05), with females showing greater activity and imiquimod reducing activity in general. There was also a day × sex interaction (F(3,132) = 5.21, p < 0.01). For the second round, there were main effects of day (F(3,132) = 9.12, p < 0.001) and sex (F(3,132) = 5.38, p < 0.05) with females again showing a generally higher level of activity than males. There were also two‐way interactions between day and sex (F(3,132) = 2.95, p < 0.05) and day and imiquimod (F(3,132) = 5.80, p < 0.0001), with post‐hoc analyses finding that imiquimod reduced locomotor activity on the second injection day (p < 0.0001). During the third injection round, there were again main effects of day (F(3,132) = 11.96, p < 0.0001) and sex (F(3,132) = 13.15, p < 0.001) as well as a main effect of imiquimod (F(3,132) = 5.88, p < 0.05) where the drug generally reduced locomotor activity. There was also a day by imiquimod interaction (F(3,132) = 5.67, p < 0.01) and post‐hoc analysis again found reduced activity on the injection day (p < 0.001). On the fourth and final injection day, imiquimod again reduced activity (F(1,44) = 15.28, p < 0.001) and females had higher locomotor activity than males (F(1,44) = 19.87, p < 0.0001). Overall imiquimod reduced locomotor activity in both sexes, and this effect became more pronounced starting with the second injection.
Change in weight
In the days following the first injection, there was an initial loss of weight in the rats that received imiquimod followed by weight gain resulting in a significant main effect of day (F(2,88) = 30.79, p < 0.0001). There was also an effect of sex (F(1,44) = 9.85, p < 0.05) likely due to the size difference between males and females. This same outcome occurred again with the second round (day: F(2,88) = 43.76, p < 0.0001; sex: F(1,44) = 47.22, p < 0.0001) and there was a trend for an effect of imiquimod (p = 0.061). There were also significant day by imiquimod (F(2,88) = 6.62, p < 0.01) and imiquimod by sex (F(1,4) = 9.89, p < 0.01) interactions. Post‐hoc analyses found that imiquimod resulted in lower weight than controls across all three post‐injection days (ps < 0.0001). The days following the third injection showed the same pattern of results (day: F(2,88) = 54.05, p < 0.0001; sex: F(1,44) = 70.94, p < 0.0001; trend for effect of imiquimod [p = 0.051]; day by sex interaction: F(2,88) = 5.49, p < 0.01; imiquimod by sex interaction: F(1,44) = 9.89, p < 0.01). Overall imiquimod resulted in weight loss in both sexes that was of greater magnitude in males.
Effects of repeated imiquimod injections on gene expression 24‐h post‐imiquimod
AcbC.In rats trained to self‐administer alcohol, 24 h after four repeated imiquimod injections, TLR3 was increased in both males (2.6‐fold; t(19) = 3.15, p < 0.01) and females (2.1‐fold; t(21) = 2.89, p < 0.05). TLR7 gene expression was increased 1.9‐fold specifically in females (t(22) = 4.73, p < 0.001), but the increase did not reach the level of significance in males (p = 0.057). As has been seen in all of our experiments, IRF7 gene expression was greatly increased in both males (23.4‐fold; t(17) = 6.56, p < 0.0001) and females (16.5‐fold; t(19) = 4.73, p < 0.0001; Figure 4D). MyD88 was also increased specifically in males in the AcbC (t(19) = 2.32, p < 0.05). AI. TLR3 gene expression was increased in the AI in both males (1.6‐fold; t(19) = 3.02, p < 0.01) and females (2.1‐fold; t(19) = 2.77, p < 0.05). As was seen in the AcbC, TLR7 gene expression was increased specifically in females (1.5‐fold; t(20) = 2.39, p < 0.05) but not in males. Lastly, IRF7 was greatly increased in both sexes (males 15.5‐fold; t(19) = 6.31, p < 0.0001: females 18.7‐fold; t(21) = 6.20, p < 0.0001; Figure 4C). See Table S4 for data values.
FIGURE 4.
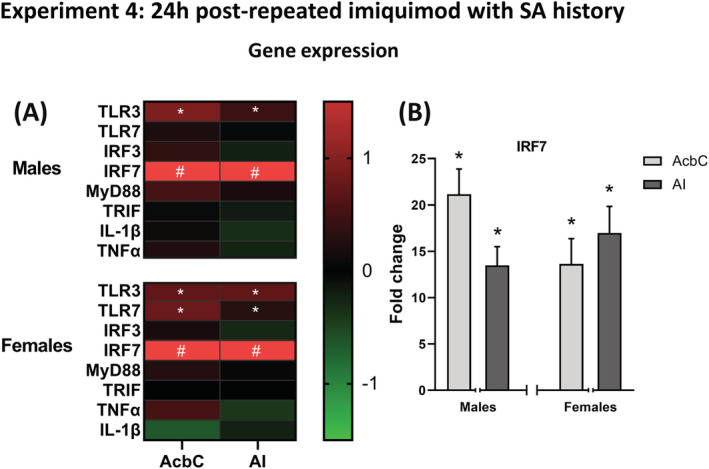
Experiment 4 gene expression. (A) Experiment 4 gene expression changes in the (AcbC) and AI induced by imiquimod relative to controls visualized as a heatmap, showing consistent increases in TLR3 and IRF7 across brain regions in both sexes. (B) Multiple injections of imiquimod resulted in greatly increased expression of IRF7 in both brain regions and sexes. *p < 0.05 compared with vehicle control, #p < 0.05 compared with control and value is greater than the heatmap scale
3.2.3. Spleen weights
Imiquimod again resulted in increased spleen weights (F(1,44) = 83.77, p < 0.0001), indicating an inflammatory response. Also, as was seen in Experiment 3, a history of alcohol self‐administration resulted in larger spleens in females relative to males (F(1,44) = 13.74, p < 0.001; Table 2).
4. DISCUSSION
The role of TLR7 signalling in operant alcohol self‐administration behaviour has not been well established. Thus, we examined gene expression changes induced by administration of the TLR7 receptor agonist imiquimod and its effects on operant self‐administration in male and female rats. In naïve rats, we first determined what genes are modulated by TLR7 activation in the AcbC and AI, two brain regions previously implicated in alcohol seeking and self‐administration. 13 , 29 , 30 , 31 , 32 In both sexes, we observed a profound induction of IRF7 in both brain regions at 2 and 24 h as well as increases in expression of TLR3 and 7, particularly at 24 h. In male and female rats with a self‐administration history, a single injection of imiquimod did not significantly reduce alcohol drinking 2 h after injection (Figure 2); 24 h later, this group showed an increase in IRF7 that was higher than in the alcohol naïve rats and again increases in TLR3 and 7 genes were observed, as well as an increase in the proinflammatory cytokine TNFα. Finally, in the last experiment, we examined the consequences of multiple imiquimod injections on alcohol self‐administration. Rats were insensitive to the first imiquimod injection, but imiquimod reduced drinking on injection day starting with the second injection, and after three injections drank more alcohol the following day. In general there were no sex differences in the effects of imiquimod on alcohol self‐administration (Figure 3). Interestingly, after four imiquimod injections IRF7 and TLR3 expression were increased in males and females in both the AcbC and AI with TLR7 also being increased in both regions in females, but TNFα was not changed from baseline, suggesting the possibility of a molecular adaptation.
After acute administration of imiquimod in naïve rats, at 2‐h post‐injection, we found that TLR3 gene expression was increased in the AI in males, but in females, the increase was in the AcbC (Figure 1B). By 24 h, TLR3 was increased in both brain regions in both sexes, and TLR7 expression was significantly elevated in the males as well (Figure 1C). Thus, we found a similar pattern in the AI in females and in the AcbC in males. Further studies will be needed to determine how the neuroimmune response differs across the factors of immune‐inducing agent, brain region, and sex. TLR signalling, and specifically TLR3 and TLR7 activation, has been shown to induce microglial activation and chemotaxis 4 , 33 and can modulate neuronal morphology, activity and synaptic plasticity. 14 , 34 , 35 TLR7 is primarily expressed in microglia whereas TLR3 is expressed in neurons, astrocytes, and microglia 36 , 37 , 38 ; thus, the induction of TLR7 only in males at 24 h may be indicative of microglial activation or priming specifically in the males that is not present in females. Alternatively, the time course of TLR7 activation may not be adequately captured by the study's timepoints, either increasing and resolving between 2 and 24 h or delayed past 24 h. IRF7 expression was increased by imiquimod across all of the 2 and 24 h timepoints and in both brain regions, in agreement with findings after administration of the TLR7 agonist R848 in male mice. 12 IRF7 signalling is thought to be downstream of MyD88, 14 thus it is surprising that MyD88 was increased only in the AI in females at 2 h post‐injection. However, in an experiment where TLR7 agonist R848 was administered, MyD88 was increased in male mice at 8‐h post‐injection but was resolved to baseline at 24 h (in agreement with our findings), and female mice have yet to be examined. In fact, in that work, nearly all TLR‐related targets were elevated at 8 h (TLR7, TLR4, MyD88, IRF7, TRIF, IRF3) and resolved by 24 h save for IRF7, suggesting that the 2 h time point used in the present work may be too early for detection of peak immune induction of many of our targets which have previously been shown to be important in affecting alcohol intake in mice. 10 , 11 This time point was chosen as imiquimod has a half‐life of 2 h 39 and has been shown to induce fever by 2 h at a lower dose of 5 mg/kg, 40 but it may be that some aspects of inflammatory gene expression peak significantly later. Indeed, with said lower dose of imiquimod, TNFα and IL‐6 expression were increased at 6 h but not 2 h in the rat hypothalamus, 40 so the peak immune response may occur later than the peak blood concentration of imiquimod. Additionally, it is possible that rats and mice differ in the time course of the response to TLR7 activation, and the TLR7 agonists used did differ between our study and the Warden et al. studies 10 , 11 ; however, the finding that IRF7 remained elevated 24‐h post‐injection in both species is an interesting consistent finding. Lastly, in the 24‐h group, we did not find changes in proinflammatory cytokines TNFα or IL‐1β in either brain region. We did not examine proinflammatory cytokines at the 2 h timepoint in Experiment 1 as that timepoint was chosen to target TLR signalling molecules rather than cytokines.
In rats that had an alcohol self‐administration history, we found that acute imiquimod 2 h before the session did not significantly alter alcohol intake nor locomotor rate (Experiment 3, Figure 2B,C). However, imiquimod did likely induce sickness as rats lost weight overnight and had enlarged spleens at time of sacrifice 24 h later (Figure 2D; Table 2), both of which indicate induction of an immune response. The timepoint post‐injection is the same between Experiments 2 and 3, and though we do not directly compare the two studies, it appears that the alcohol history resulted in a greater IRF7 induction (Figure 1E vs. Figure 2F; 20‐ to 40‐fold vs. 13‐ to 20‐fold, respectively). As has been well established in the literature, microglia can be primed by an initial stress or pharmacological challenge, including alcohol, resulting in a stronger response to a subsequent challenge. 35 , 41 , 42 , 43 Thus, it may be that the alcohol self‐administration history primed microglia resulting in enhanced neuroimmune response to imiquimod. Additionally, IRF7 itself is thought to have a role in priming the immune response, so it may be that the history of alcohol self‐administration induced changes in microglia that led to a sensitized IRF7 response after imiquimod challenge. 44 There is some precedent for alcohol history to result in increased expression of IRF7 after chronic alcohol administration in adolescent rats 9 ; further study will need to be done to determine whether a similar response occurs in adults and the alcohol dose range and administration schedules that would promote such priming effects. Curiously, while the IRF7 response appeared to be heightened in both brain regions in both sexes, consistent with what was found in naïve rats, the TLR expression was not as consistently elevated 24 h after imiquimod in these rats with a self‐administration history. In males, TLRs 3 and 7 were elevated in the AcbC but were not increased in the AI, while in females TLR3, but not TLR7, was increased in both brain regions (Figure 2E). These adaptions may be due to induction of molecular tolerance due to the history of alcohol self‐administration. Previous studies have found reduced expression of these targets with repeated administration of immune challenge as compared with a single dose, 12 and alcohol may be functioning similarly in a sex‐ and region‐specific manner. As has been seen previously, imiquimod and R848 TLR7 activation in vitro, central gene expression of proinflammatory cytokine TNFα was increased in both brain regions in females and in the AcbC in males. 36 , 45 Notably, we did not see increased TNFα in alcohol naïve rats in Experiment 2, suggesting that the alcohol history primed the neuroimmune response resulting in greater proinflammatory cytokine expression.
As an effect on drinking was not observed 2 h following imiquimod injection in Experiment 3, Experiment 4 was designed to more thoroughly characterize imiquimod effects on self‐administration behaviour (Figure 3A). For simplicity, first we will discuss self‐administration on the day of injection (depicted by dotted lines). Consistent with Experiment 3, there were no changes in drinking 2 h following a single imiquimod injection (Figure 3B). However, following the second, third, and fourth injection, we found reduced drinking 2‐h post‐imiquimod suggesting adaptation to repeated imiquimod exposure. Reductions in locomotor activity 2 h following the imiquimod injection mirrored the reductions in drinking suggesting that both effects may be due to the induction of sickness behaviour (Figure 3C). Notably, the first injection did not induce weight loss that reached the level of statistical significance (Figure 3D), which combined with the above suggests that initial tolerance in respect to behaviour, molecular expression, and sickness is overcome with repeated exposure.
On the days following the first two imiquimod injections rats did not increase their alcohol intake relative to controls, while drinking increased after the third injection (Figure 3B). These changes were independent of sex. This increase in alcohol intake after repeated exposure to a TLR7 agonist is somewhat consistent with the other existing experiment which found increased home cage alcohol intake after administering a TLR7 agonist (R848) for 10 days every other day. After a two‐week incubation period mice were tested using an every other day drinking in the dark (EODID) model and exhibited a sustained increase in alcohol intake. 12 Though we did not observe sustained increases in drinking, our design differed in that it utilized operant self‐administration and drinking sessions occurred 5 days/week. It is important to note differences between the two studies in both the pattern and amount of drinking, as the EODID procedure used by Grantham et al. resulted in consumption of 5–10 g/kg in control rats across 24 h every other day, while in the present study, control rats consumed approximately 0.7–1.0 g/kg five consecutive days a week. Our lab previously found increased intake after an 18‐day incubation period in operant self‐administration when male rats were given 3 mg/kg of the TLR3 agonist poly(I:C), 13 but similarly to the findings in the present experiment the increased intake was not sustained. Additionally, a limitation of the present work is that a single dose of imiquimod was assessed; therefore, it is possible that prolonged increase in drinking may be observed using a higher dose. It is noteworthy that few studies examining TLR activation have explicitly examined females, though interestingly peak cytokine levels induced by TLR3 activation by poly(I:C) increased drinking in male mice while reducing intake in females in an EODOD model. 10 , 11
In Experiment 4, tissue was collected from subjects after the fourth imiquimod injection. Spleens in animals that received imiquimod were much larger than vehicle controls likely due to the cumulative amount of imiquimod administered alongside possible potentiation resulting from a history of drinking. As was seen in all other experiments, IRF7 expression levels were greatly elevated in both brain regions in males and females (Figure 4B). However, IRF7 levels were not as highly expressed as observed in Experiment 3 where rats had a self‐administration history after a single imiquimod injection (Figure 3I, 12‐ to 22‐fold vs. 25‐ to 40‐fold). Molecular tolerance to repeated TLR7 agonism has been reported 12 , 36 and may be responsible for this difference, though a direct comparison within a single study is needed for confirmation. In support of this idea, TNFα was not elevated after 4 imiquimod injections (Figure 4A) as was observed in Experiment 3 (Figure 2H). The pattern of TLR gene expression was different within each sex as well, with males exhibiting increased TLR3 expression in both the AcbC and AI as opposed to just the AcbC, and no changes to TLR7. Females, instead of showing increased TLR3 in both brain regions after a single imiquimod injection (Figure 2E), had increased expression of both TLR3 and TLR7. It should be noted that the TLR7 gene is encoded on the X chromosome and is known to escape X inactivation, 25 thus it is possible that it is induced at higher levels in females. Brain region‐specific actions of TLRs may be playing an important role in modulating behaviour as TLRs are known to have roles outside of antigen recognition. 14 , 34 , 35 , 46 The specifics of these changes will have to be teased apart with studies that manipulate TLRs and their downstream signalling molecules across brain regions and sexes to determine whether they may play a causal role in regulating drinking behaviour.
5. CONCLUSION
In summary, we have shown that TLR7 activation reliably induces IRF7 gene expression in both males and females. Further, we demonstrated a role for TLR7 activation in modulation of alcohol self‐administration with increased consumption after repeated injections. It is noteworthy that some dependent measures, such as proinflammatory cytokines and TLR gene expression, drinking, and locomotor activity, changed with repeated injections while increased IRF7 gene expression was always found. We found few sex differences induced by imiquimod, though the lack of direct sex comparisons in the gene expression data limits interpretation. A focused experimental approach explicitly designed to assess sex differences in gene and protein expression will be important and may find TLR‐related differences between males and females. Interestingly, in a prior study with resiquimod a general trend of reduced inflammatory response with repeated injections was found, which fits well with our TNFα results. 36 The cell‐type specificity of the adaptions we observed are not yet known, and it is likely that interactions between multiple cell types are responsible for modulation of behavioural output. As TLR7 is primarily expressed in microglia and is known to modulate neuronal development, function, and morphology, 14 , 34 it will be important to consider changes that occur within certain cell types rather than simply as a whole in a given brain region. Further, this study examined two brain regions whose interactions are known to modulate alcohol seeking 32 , 47 ; thus, an important future direction will be understanding how changes in one region affect the other as well as other relevant brain regions. Imiquimod itself is used as an immune inducer, sold as a topical cream for skin conditions 48 and used as adjunctive treatment with vaccines 49 and with cancer treatments. 50 , 51 , 52 These actions are thought to occur through induction of interferons, particularly interferon‐alpha. Thus, it is possible that the molecular cascade involved in interferon induction may be more important in regulation of alcohol‐related behaviours than that of classic proinflammatory cytokines such as IL‐1β and TNFα as were examined here. A greater understanding of the molecular underpinnings of how TLR activation can modulate alcohol drinking will be critical in working towards development of treatment strategies for AUD.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
DFL contributed to design of experiments, collected and analysed tissue, analysed and interpreted all results, and was a major contributor in writing the manuscript. WL collected tissue, conducted RTPCR analyses, and interpreted results. SEL collected and analysed behavioural data, collected tissue, analysed RTPCR results, interpreted results, and contributed to writing the manuscript. JL conducted RTPCR analyses and contributed to interpretation of results. KV collected behavioural data, contributed to interpretation of results, and contributed to writing the manuscript. KG contributed to interpretation of results and contributed to writing the manuscript. RPV was a contributor in the design of the experiments and contributed to interpretation of results. FTC was a major contributor in the design of the experiments and contributed to interpretation of results. JB was a major contributor in the design of the experiments, contributed to interpretation of results, and was a major contributor in writing the manuscript.
ETHICS STATEMENT
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill and conducted in accordance with National Institutes of Health regulations for the care and use of animals.
Supporting information
Figure S1: Experiment 3 lever responses. Active lever responses on injection days and three days after the first three injections. After the first injection a three way ANOVA found main effects of day [F(3,132) = 8.37, p < 0.0001] and imiquimod [F(1,44) = 28.75, p < 0.0001] and an interaction between day and imiquimod [F(3,132) = 14.33, p < 0.0001]. On the second injection round there were again main effects of day [F(3,132) = 18.13, p < 0.0001] and imiquimod [F(1,44) = 28.75, p < 0.0001] and an interaction between day and imiquimod [F(3,132) = 6.07, p < 0.001] with a significant reduction in lever presses on the injection day (p < 0.0001). For the third injection round there were again main effects of day [F(3,132) = 25.57, p < 0.0001] and imiquimod [F(1,44) = 6.59, p < 0.05] and an interaction between day and imiquimod [F(3,132) = 25.75, p < 0.0001] and a reduction in lever presses on the injection day (p < 0.0001). Finally, on the fourth injection day a two‐way ANOVA found a main effect of imiquimod [F(1,44) = 6.64, p < 0.05] and a significant interaction [F(1,44) = 8.34, p < 0.01] with post‐hoc testing showing males that received imiquimod had reduced lever presses relative to male controls. ^ indicates main effect of day, & indicates main effect of sex, and * indicates a significant difference between imiquimod and control on this day when data are collapsed across sex.
Data S1.Supporting information
ACKNOWLEDGEMENTS
This work was supported in part by the National Institute on Alcohol Abuse and Alcoholism (AA025713, AA020024, AA020023, AA011605, AA019767), the National Institute on Aging (AG072894) of the National Institutes of Health, and the Bowles Center for Alcohol Studies. DFL was supported by F32AA029289 and AA007573. KAG was supported by R25GM08956.
Lovelock DF, Liu W, Langston SE, et al. The Toll‐like receptor 7 agonist imiquimod increases ethanol self‐administration and induces expression of Toll‐like receptor related genes. Addiction Biology. 2022;27 (3): e13176. doi: 10.1111/adb.13176
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on request.
REFERENCES
- 1. Coleman LG, Crews FT. Innate immune signaling and alcohol use disorders. In: The Neuropharmacology of Alcohol. Springer; 2018:369‐396. doi: 10.1007/164_2018_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He J, Crews FT. Increased MCP‐1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349‐358. doi: 10.1016/j.expneurol.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll‐like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73(7):602‐612. doi: 10.1016/j.biopsych.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman LG, Zou J, Crews FT. Microglial‐derived miRNA let‐7 and HMGB1 contribute to ethanol‐induced neurotoxicity via TLR7. J Neuroinflammation. 2017;14(1):1‐15. doi: 10.1186/s12974-017-0799-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5(1):83‐91. doi: 10.1007/s11481-009-9185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emanuele NV, LaPaglia N, Kovacs EJ, Emanuele MA. The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: reproductive implications. Cytokine. 2005;30(3):109‐115. doi: 10.1016/j.cyto.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 7. Qin L, He J, Hanes RN, Pluzarev O, Hong J‐S, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflamm. 2008;5(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gano A, Doremus‐Fitzwater TL, Deak T. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res. 2016;1646:62‐72. doi: 10.1016/j.brainres.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crews FT, Walter TJ, Coleman LG, Vetreno RP. Toll‐like receptor signaling and stages of addiction. Psychopharmacology. 2017;234(9‐10):1483‐1498. doi: 10.1007/s00213-017-4560-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warden AS, Azzam M, DaCosta A, et al. Toll‐like receptor 3 dynamics in female C57BL/6J mice: Regulation of alcohol intake. Brain, Behav, Immun. 2019;77:66‐76. doi: 10.1016/j.bbi.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warden AS, Azzam M, DaCosta A, et al. Toll‐like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain, Behav, Immun. 2019;77:55‐65. doi: 10.1016/j.bbi.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grantham E, Warden A, McCarthy G, et al. Role of Toll‐like receptor 7 (TLR7) in voluntary alcohol consumption. Brain, Behav, Immun. 2020;89:423‐432. doi: 10.1016/j.bbi.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Randall PA, Vetreno RP, Makhijani VH, Crews FT, Besheer J. The Toll‐like receptor 3 agonist poly (I: C) induces rapid and lasting changes in gene expression related to glutamatergic function and increases ethanol self‐administration in rats. Alcohol Clin Exp Res. 2019;43(1):48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hung Y‐F, Chen C‐Y, Shih Y‐C, Liu H‐Y, Huang C‐M, Hsueh Y‐P. Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. J Cell Biol. 2018;217(8):2727‐2742. doi: 10.1083/jcb.201712113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cameron JS, Alexopoulou L, Sloane JA, et al. Toll‐like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27(47):13033‐13041. doi: 10.1523/JNEUROSCI.4290-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vetreno RP, Qin L, Coleman LG Jr, Crews FT. Increased Toll‐like receptor‐MyD88‐NFκB‐proinflammatory neuroimmune signaling in the orbitofrontal cortex of human alcohol use disorder. Alcohol Clin Exp Res. 2021;45(9):1747‐1761. doi: 10.1111/acer.14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blednov Y, Benavidez JM, Geil C, Perra S, Morikawa H, Harris R. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25:S92‐S105. doi: 10.1016/j.bbi.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris RA, Bajo M, Bell RL, et al. Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J Neurosci. 2017;37(5):1139‐1155. doi: 10.1523/JNEUROSCI.2002-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blednov YA, Black M, Chernis J, Da Costa A, Mayfield J, Harris RA. Ethanol consumption in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res. 2017;41(3):516‐530. doi: 10.1111/acer.13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blednov YA, Da Costa A, Mayfield J, Harris RA, Messing RO. Deletion of Tlr3 reduces acute tolerance to alcohol and alcohol consumption in the intermittent access procedure in male mice. Addict Biol. 2021;26(2):e12932. doi: 10.1111/adb.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Govindaraj RG, Manavalan B, Basith S, Choi S. Comparative analysis of species‐specific ligand recognition in Toll‐like receptor 8 signaling: a hypothesis. PLoS ONE. 2011;6(9):e25118. doi: 10.1371/journal.pone.0025118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson A, Neill J, Costall B. Strain differences in ethanol preference and reinforced behaviour: a comparison of two‐bottle choice and operant self‐administration paradigms. Behav Pharmacol. 1997;8(1):37‐46. doi: 10.1097/00008877-199702000-00004 [DOI] [PubMed] [Google Scholar]
- 23. Jaramillo AA, Van Voorhies K, Randall PA, Besheer J. Silencing the insular‐striatal circuit decreases alcohol self‐administration and increases sensitivity to alcohol. Behav Brain Res. 2018;348:74‐81. doi: 10.1016/j.bbr.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J. Functional role for cortical‐striatal circuitry in modulating alcohol self‐administration. Neuropharmacol. 2018;130:42‐53. doi: 10.1016/j.neuropharm.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Souyris M, Cenac C, Azar P, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855. doi: 10.1126/sciimmunol.aap8855 [DOI] [PubMed] [Google Scholar]
- 26. Souyris M, Mejía JE, Chaumeil J, Guéry J‐C. Female predisposition to TLR7‐driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin Immunopathol. 2019;41(2):153‐164. doi: 10.1007/s00281-018-0712-y [DOI] [PubMed] [Google Scholar]
- 27. Randall PA, Stewart RT, Besheer J. Sex differences in alcohol self‐administration and relapse‐like behavior in Long–Evans rats. Pharmacol Biochem Behav. 2017;156:1‐9. doi: 10.1016/j.pbb.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25(4):402‐408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29. Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self‐administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250‐258. [PubMed] [Google Scholar]
- 30. Hodge CW, Samson HH, Chappelle AM. Alcohol self‐administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21(6):1083‐1091. doi: 10.1097/00000374-199709000-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grodin EN, Sussman L, Sundby K, et al. Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(12):1022‐1031. doi: 10.1016/j.bpsc.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 32. Haaranen M, Schäfer A, Järvi V, Hyytiä P. Chemogenetic stimulation and silencing of the insula, amygdala, nucleus accumbens, and their connections differentially modulate alcohol drinking in rats. Front Behav Neurosci. 2020;14:189. doi: 10.3389/fnbeh.2020.580849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ifuku M, Buonfiglioli A, Jordan P, Lehnardt S, Kettenmann H. TLR2 controls random motility, while TLR7 regulates chemotaxis of microglial cells via distinct pathways. Brain Behav Immun. 2016;58:338‐347. doi: 10.1016/j.bbi.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 34. Hung Y‐F, Chen C‐Y, Li W‐C, Wang T‐F, Hsueh Y‐P. Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav Immun. 2018;72:101‐113. doi: 10.1016/j.bbi.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 35. Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017;122:56‐73. doi: 10.1016/j.neuropharm.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michaelis KA, Norgard MA, Levasseur PR, et al. Persistent Toll‐like receptor 7 stimulation induces behavioral and molecular innate immune tolerance. Brain Behav Immun. 2019;82:338‐353. doi: 10.1016/j.bbi.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist‐induced neuroinflammation and neurodegeneration. J Neuroinflamm. 2012;9(1):1‐18. doi: 10.1186/1742-2094-9-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Town T , Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double‐stranded RNA via TLR3. J Immunol. 2006;176(6):3804‐3812. doi: 10.4049/jimmunol.176.6.3804 [DOI] [PubMed] [Google Scholar]
- 39. ASHSP . Drug Information 2012. 2012.
- 40. Damm J, Wiegand F, Harden L, Gerstberger R, Rummel C, Roth J. Fever, sickness behavior, and expression of inflammatory genes in the hypothalamus after systemic and localized subcutaneous stimulation of rats with the Toll‐like receptor 7 agonist imiquimod. Neuroscience. 2012;201:166‐183. doi: 10.1016/j.neuroscience.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 41. Walter TJ, Vetreno RP, Crews FT. Alcohol and stress activation of microglia and neurons: brain regional effects. Alcohol Clin Exp Res. 2017;41(12):2066‐2081. doi: 10.1111/acer.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marshall SA, Geil CR, Nixon K. Prior binge ethanol exposure potentiates the microglial response in a model of alcohol‐induced neurodegeneration. Brain Sciences. 2016;6(2):16. doi: 10.3390/brainsci6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crews FT, Vetreno RP. Stress and alcohol priming of brain Toll‐like receptor signaling in alcohol use disorder. Alcohol Alcohol. 2018;53(6):639‐641. doi: 10.1093/alcalc/agy061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ning S, Pagano J, Barber G. IRF7: activation, regulation, modification and function. Genes Immun. 2011;12(6):399‐414. doi: 10.1038/gene.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hemmi H, Kaisho T, Takeuchi O, et al. Small anti‐viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway. Nat Immunol. 2002;3(2):196‐200. doi: 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 46. Kubo Y, Yanagawa Y, Matsumoto M, Hiraide S, Togashi H. Enhanced depressive‐like behaviors after Toll‐like receptor 7 stimulation in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 2013;33(2):41‐47. [PubMed] [Google Scholar]
- 47. Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J. Functional role for suppression of the insular‐striatal circuit in modulating interoceptive effects of alcohol. Addict Biol. 2018;23(5):1020‐1031. doi: 10.1111/adb.12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bubna AK. Imiquimod‐Its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47(4):354. doi: 10.4103/0253-7613.161249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang AJ, Li C, To KK, et al. Toll‐like receptor 7 agonist imiquimod in combination with influenza vaccine expedites and augments humoral immune responses against influenza A (H1N1) pdm09 virus infection in BALB/c mice. Clin Vaccine Immunol. 2014;21(4):570‐579. doi: 10.1128/CVI.00816-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune‐mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18(24):6748‐6757. doi: 10.1158/1078-0432.CCR-12-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dewan MZ, Vanpouille‐Box C, Kawashima N, et al. Synergy of topical Toll‐like receptor 7 agonist with radiation and low‐dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18(24):6668‐6678. doi: 10.1158/1078-0432.CCR-12-0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222(1):357‐368. doi: 10.1111/j.1600-065X.2008.00604.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Experiment 3 lever responses. Active lever responses on injection days and three days after the first three injections. After the first injection a three way ANOVA found main effects of day [F(3,132) = 8.37, p < 0.0001] and imiquimod [F(1,44) = 28.75, p < 0.0001] and an interaction between day and imiquimod [F(3,132) = 14.33, p < 0.0001]. On the second injection round there were again main effects of day [F(3,132) = 18.13, p < 0.0001] and imiquimod [F(1,44) = 28.75, p < 0.0001] and an interaction between day and imiquimod [F(3,132) = 6.07, p < 0.001] with a significant reduction in lever presses on the injection day (p < 0.0001). For the third injection round there were again main effects of day [F(3,132) = 25.57, p < 0.0001] and imiquimod [F(1,44) = 6.59, p < 0.05] and an interaction between day and imiquimod [F(3,132) = 25.75, p < 0.0001] and a reduction in lever presses on the injection day (p < 0.0001). Finally, on the fourth injection day a two‐way ANOVA found a main effect of imiquimod [F(1,44) = 6.64, p < 0.05] and a significant interaction [F(1,44) = 8.34, p < 0.01] with post‐hoc testing showing males that received imiquimod had reduced lever presses relative to male controls. ^ indicates main effect of day, & indicates main effect of sex, and * indicates a significant difference between imiquimod and control on this day when data are collapsed across sex.
Data S1.Supporting information
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on request.


