Abstract
Abnormalities of mast cell structure or function may play prominent roles in irritable bowel syndrome (IBS) symptom genesis. Mast cells show close apposition to sensory nerves and release bioactive substances in response to varied stimuli including infection, stress, and other neuroendocrine factors. Most studies focus on patients who develop IBS after enteric infection or who report diarrhea‐predominant symptoms. Three topics underlying IBS pathogenesis have been emphasized in recent investigations. Visceral hypersensitivity to luminal stimulation is found in most IBS patients and may contribute to abdominal pain. Mast cell dysfunction also may disrupt epithelial barrier function which alters mucosal permeability potentially leading to altered bowel function and pain. Mast cell products including histamine, proteases, prostaglandins, and cytokines may participate in hypersensitivity and permeability defects, especially with diarrhea‐predominant IBS. Recent experimental evidence indicates that the pronociceptive effects of histamine and proteases are mediated by the generation of prostaglandins in the mast cell. Enteric microbiome interactions including increased mucosal bacterial translocation may activate mast cells to elicit inflammatory responses underlying some of these pathogenic effects. Therapies to alter mast cell activity (mast cell stabilizers) or function (histamine antagonists) have shown modest benefits in IBS. Future investigations will seek to define patient subsets with greater potential to respond to therapies that address visceral hypersensitivity, epithelial permeability defects, and microbiome alterations secondary to mast cell dysfunction in IBS.
Keywords: barrier function, IBS, mast cell, microbiome, visceral hypersensitivity
Abbreviations
- CCL
cytokine
- CGRP
calcitonin gene‐related peptide
- COX1/2
cyclooxygenase1/2
- CRH
corticotropin‐releasing hormone
- CXCL
cytokine of CXC chemokine family
- DRG
dorsal root ganglia
- FGF
fibroblast growth factor
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- IgE
immunoglobulin E
- IL‐1β
interleukin‐1β
- MAPK
extracellular signal‐regulated kinase
- NGF
nerve growth factor
- NK
neurokinin
- NO
nitric oxide
- PAR
protease‐activated receptor
- PGE2
prostaglandin E2
- PGH2
prostaglandin H2
- PLA2
phospholipase A2
- SCF
stem cell factor
- TGF‐β
transforming growth factor β
- TLR (1‐9)
tool‐like receptor
- TNFR
tumor necrosis factor receptor
- TNF‐α
tumor necrosis factor‐α
- TRPA1
transient receptor potential ankyrine 1
- TRPV1
transient receptor potential vanilloid 1
- VEGF
vascular endothelial growth factor
- VIP
vasoactive intestinal polypeptide
- VPF
vascular permeability factor
Content available: Author Interview
Key Points.
Abnormalities of epithelial barrier integrity resulting from activation by mast cell mediators may lead to increased mucosal permeability and development of visceral hypersensitivity.
Recent studies have focused on interaction between gastrointestinal mast cells and the enteric microbiome which can modulate gut inflammatory process underlying IBS symptom exacerbations.
Future studies addressing mast cell participation in IBS symptom genesis will define patient subsets who respond to treatments that reverse visceral hypersensitivity, permeability defects, and microbiome alterations in this condition.
1. INTRODUCTION
Irritable bowel syndrome (IBS) is the most prevalent gastrointestinal disorder and presents with abdominal pain and altered bowel habits. 1 IBS pathophysiology is heterogeneous and variable from patient to patient. Visceral hypersensitivity may underlie symptoms in large IBS subsets 2 , 3 Dysfunction of epithelial barrier function with increases in permeability may contribute to altered defecation and pain in IBS. 4 Alterations in gut bacterial populations are common and may participate in IBS pathophysiology. 5 Each of these factors interact with each other and other factors including bile acids, enteric and central nervous activity, and the immune system to produce IBS symptoms. 4 Better understanding of mechanisms underlying development of hypersensitivity, epithelial dysfunction, and gut dysbiosis in IBS will provide insight into symptom pathogenesis and facilitate drug discovery for improved treatment of this condition.
Mucosal mast cells are increased and show heightened activation in some IBS subsets. Mast cells can elicit visceral hypersensitivity, influence epithelial function, and interact with gut microbes providing a possible link between the neuroimmune system and other contributors to IBS pathogenesis. 6 The aims of this review are to describe gut mast cell biology, characterize mast cell abnormalities in IBS, detail roles of mast cell activity in visceral hypersensitivity, epithelial barrier function, and enteric microbial activity, and to speculate on the potential for future therapies targeting mast cell functions in IBS.
2. MAST CELLS IN THE GUT
2.1. Structural considerations
Mast cells represent up to 5% of gut mononuclear cells and are present in the mucosa, lamina propria, submucosa, smooth muscle, and serosa. On ultrastructural analyses, activated mast cells contain cytoplasmic granules with bioactive mediators (Figure 1). 7 Mast cells are derived from pluripotent bone marrow progenitors including CD34+/CD117+ cells. These differentiate into tissue mast cells after exposure to growth factors and other agents promoting maturation including interleukins (IL‐3, IL‐4, IL‐9, IL‐10, and IL‐33), transforming growth factor‐β (TGF‐β), nerve growth factor (NGF), stem cell factor (SCF), and the chemokine CXCL12. 8 Two subtypes of gut mast cells have been identified, mucosal mast cells (MCT) and connective tissue mast cells (MCTC). 7 , 9 , 10 Small intestinal mucosal mast cell density increases from the jejunum to the distal ileum; colon mast cells decrease from the cecum to the rectum. 11 Mast cell numbers increase from the mucosa villous tips to the bases of the crypts. Forty‐seven to 77% of mucosal mast cells are closely apposed to sensory nerve fibers in different gut regions. 12 Most nerve fibers adjacent to mast cells are unmyelinated and stain positive for neurotransmitters involved in gut sensation including calcitonin gene‐related peptide (CGRP) and substance P. 13
FIGURE 1.
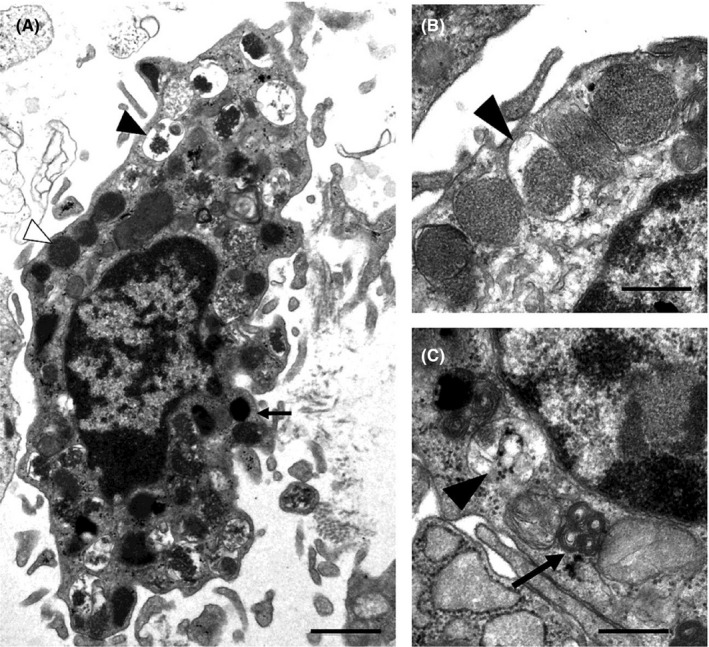
Ultrastructure of a mucosal mast cell is shown. Activated mast cells exhibit irregular plasma membranes and lipid bodies (arrow) and cytoplastic granules (A). Intact (white arrowhead) and degranulated (black arrowhead) granules are seen. On high magnification, mucosal mast cell cytoplasmic granules can show either crystalloid stricture (B) or scroll patterns (black arrow) (C). Enlarged empty and partly empty granules (black arrowhead) reflect piecemeal degranulation. Bars: 1 μm (A) and 0.5 μm (B, C). From Wouters et al. 7
2.2. Mast cell mediators
Gut mast cells release biologically active substances, which can be stratified into preformed, neo‐synthesized, and neo‐formed lipid mediators (Table 1). 9
TABLE 1.
Mast cell mediators
| Preformed mediators | Neo‐synthesized mediators | Neo‐formed lipid mediators |
|---|---|---|
|
Histamine Tryptases (α, β, γ) Chymase Carboxypeptidase‐A Heparin Chondroitin sulfates Cathepsin Major basic protein |
Cytokines (IL‐1, IL‐1R antagonist, IL‐3, IL‐4, IL‐5, IL‐6, IL‐8, IL‐9, IL‐10, IL‐11, IL‐12, IL‐13, IL‐14, IL‐15, IL‐16, IL‐18, TNF‐α, TNF‐β, INF‐γ) Growth factors (basic FGF, FGF2, TGF‐β1, SCF, GM‐CSF, M‐CSF, VGEF, VPF, NGF, NT‐3, LIF, LT‐β, MIF, EGF, PDGF‐AA, PDGF‐BB) Chemokines (CCL1, CCl2, CCL3, CCL3L1, CCL4, CCL5, CCL7, CCL8, CCL11, CCL13, CCL16, CCL17, CCL20, CCL22, CXCL1, CXCL2, CXCL3, CXCL8, CXCL10, XCL1) Other neo‐synthesized mediators (NO, superoxide, CRH, urocortin) |
PGD2 PGE2 PGF2 PGI2 TX LTB4 LTC4 LTD4 PAF |
Adapted from Buhner and Schemann. 30
2.2.1. Preformed mediators
Preformed mediators are stored in cytoplasmic granules and include histamine, proteases, and heparin which can be rapidly replenished after mast cell activation. 9 Contents of restored mast cell granules may be markedly different from the original mediator profile prior to degranulation. 14 Histamine is synthesized by histidine decarboxylase and influences gut motor function, fluid transport, and inflammation by action on submucosal and primary afferent neurons. 15 , 16 Activated mucosal MCT mast cells release relatively less histamine than cysteinyl leukotrienes, while connective tissue MCTC mast cells release higher levels of histamine and prostaglandin D2 (PGD2). 17 Proteases produced by MCTC cells include tryptase, chymase, and carboxypeptidase; the main protease produced by MCT cells is tryptase. 7 , 9 , 10 In addition to proteolytic activity, tryptase and other proteases cleave protease‐activated receptors (PARs), which regulate motility, pain perception, epithelial permeability and secretion, and inflammation. 18 , 19 , 20 PARs are expressed by neurons in dorsal root ganglia (DRG) and the myenteric plexus. Tryptase specifically activates PAR2. 21 Upon activation of PAR1 and PAR2 receptors, sensory neurons release CGRP and substance P which then elicit neurogenic pain.
2.2.2. Neo‐synthesized mediators
Neo‐synthesized mediators, including cytokines, chemokines, and growth factors, are produced by transcriptional activation after exposure to a mast cell stimulus. Cytokines synthesized by gut mast cells include those which are proinflammatory (IL‐1, IL‐3, IL‐4, IL‐5, IL‐6, IL‐12, IL‐13, IL‐16, IL‐18, tumor necrosis factor‐α [TNF‐α], interferon‐γ [IFN‐γ]), and anti‐inflammatory (IL‐10) and are produced within hours of activation. 22 Gut mast cell chemokines include CXCL8, MCP‐1 (CCL2), MIP‐1α (CCL3), MIP‐1β (CCL4), and CCL5. 23 In addition to participating in inflammation, cytokines (IL‐3, IL‐4, IL‐6, IL‐9, and IL‐10) and NGF participate in mast cell differentiation in rodents. 9 Growth factors secreted by gastrointestinal mast cells include fibroblast growth factor‐2 (FGF2), basic FGF, TGF‐β1, SCF, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), vascular endothelial growth factor (VEGF), vascular permeability factor (VPF), and NGF. 23 NGF regulates maturation, growth, and maintenance of central and peripheral neurons.
2.2.3. Neo‐formed lipid mediators
Neo‐formed lipid mediators synthesized after mast cell activation include eicosanoid compounds. Prostaglandin G2 is an arachidonic acid product converted by cyclooxygenases (COX) into an intermediary molecule, prostaglandin H2 (PGH2). There are three COX isoforms. COX1 is a constitutive form expressed in mast cells and is responsible for basal prostanoid synthesis. COX3 is a splice variant of COX1 mostly expressed in the brain and heart. COX2 is induced in several cell types by cytokines, hormones, and mitogens and elicits prostaglandin production in inflammation. Rapid increases in COX2 gene expression in inflamed tissues are followed by PGD2, PGE 2 , PGF 2 , PGI2, and thromboxane (TX) biosynthesis. In a rigorous study, immunoreactivities for mast cell COX2 and tryptase extensively overlapped in human and animal colonic tissues confirming a mast cell origin for mucosal prostaglandins. 24 Prostaglandin D synthase is responsible for PGD2 generation, which is abundantly released by mast cells and fibroblasts and regulates central and peripheral nerve function. 25 Many symptoms of IBS can be mimicked by exogenous prostaglandin administration, which are ameliorated by prostaglandin synthase inhibition. 26
Prostaglandin pathways overlap with nitric oxide (NO) processes. Activated mast cells express inducible nitric oxide synthase (iNOS); mast cell iNOS expression is increased after cytokine exposure. 27 In iNOS knockout mice, PGE2 formation after proinflammatory stimulation decreased by ~80% although COX2 protein expression was not impaired, confirming the importance of NO generation for prostaglandin synthesis. 28 NO increases COX2 activity by reacting with the heme‐component of the enzyme to increase prostaglandin synthesis and acts at transcriptional and translational levels to augment COX2 expression.
2.3. Mast cell activation
Stimuli including allergens, infections, stress, and neurotransmitters promote mast cell activation. For example, substance P increases mast cell histamine content and causes degranulation. 29 Alternatively, transmitters like somatostatin blunt mast cell function. 30 Mast cells are activated when antigens crosslink immunoglobulin E (IgE) to high‐affinity Fc epsilon receptors with subsequent degranulation and release of stored mediators (histamine, tryptase, proteoglycans) and subsequent leukotriene and PGD2 synthesis. Non‐IgE‐mediated mast cell activation occurs after exposure to neuropeptides, complement, physical stimuli, and infection.
2.3.1. Activated mast cell involvement in inflammation
Mast cells participate in inflammation by virtue of their proximity to nerve fibers, epithelial cells, and blood vessels. Sensorimotor dysfunction induced by inflammation may be mediated by proinflammatory cytokines and persists after resolution of the acute inflammatory response. 31 Mast cell mediators also contribute to recruiting neutrophils, macrophages, and T‐lymphocytes which then release additional pronociceptive mediators. In a study of pleurisy in rats, injection of isologous serum promoted neutrophil infiltration which peaked at 4 h and was followed by eosinophilic influx lasting 24–48 h. 32 Mast cell‐deficient (Ws/Ws) rats exhibited reduced neutrophil recruitment and myeloperoxidase activity in pleural lavage extracts which increased after local reconstitution with mast cells from wild‐type rat peritoneum. Mast cells support polarization of T‐lymphocyte responses through secretion of IL‐12, IFN‐γ, Th‐1, IL‐4, and IL‐6 and internalize and process antigens presented to T‐lymphocytes by MHC class II pathways. 33 Activated mast cells release TNF‐α, which binds T‐cell TNF receptor I (TNFRI) and TNFRII to regulate T‐lymphocyte activation. 34 Mast cells also contribute to B‐lymphocyte proliferation and differentiation into IgA‐secreting plasma cells by direct interaction with costimulatory proteins (CD40, CD40L) and secretion of IL‐5, IL‐6, and TGF‐β. 35
2.3.2. Activated mast cell involvement in gut neural function
Activated mast cells elicit nerve‐mediated sensorimotor responses that increase perception. Mast cell histamine and proteases extracted from supernatants of colon biopsy specimens from IBS patients activate enteric and primary afferent neurons in experimental models. 36 Using calcium imaging, mast cell degranulation activates DRG neurons in co‐culture. 37 Cell adhesion molecule 1 (CADM1) couples mast cells to sensory neurons. CADM1 blocking peptide or knockdown prevents mast cell degranulation and IL‐6 secretion. 38
2.3.3. Self‐amplification of mast cell activation and response
Mast cell activation can be stimulated by other mediators, reflecting self‐amplification of mast cell‐regulated processes that sustain inflammatory responses. 7 , 39 Chymase, tryptase, histamine, and IL‐29 promote inflammatory cell accumulation. 40 Chymase also is a potent chemoattractant for eosinophils, monocytes, and neutrophils by extracellular signal‐regulated kinase (ERK) and p38 mitogen‐activated protein kinase (MAPK) pathways. 41 These reciprocal interactions activate nerve‐mediated responses that modulate subsequent mast cell functions. 42
3. ROLE OF MAST CELLS IN IBS PATHOGENESIS
3.1. Mast cell abnormalities in IBS
Mast cell alterations are prominent in IBS, including changes in mast cell number, mediator release during stimulation, and proximity to nerve tissue. Studies show benefits of treating mast cell dysfunction in IBS subsets. Mutations in the tyrosine kinase Kit gene are described in IBS, suggesting a possible genetic basis for mast cell dysregulation. In one study, 13 of 19 IBS patients showed one or multiple Kit mutations including D 419 H and D 816V. 43
Some studies report mast cell increases in IBS, but cell counts overlap with healthy values. 44 Meta‐analyses report increased mast cell counts in the small intestine and colon of IBS patients with greater overall lamina propria area occupied by mast cells. 36 , 44 , 45 , 46 Mast cell numbers were similar in IBS and ulcerative colitis in remission in one study. 47 Regional colon mast cell differences are found, being higher in the cecum in one report. 48 Small intestinal mast cells were higher in 10 of 11 studies from one meta‐analysis and two systematic reviews. 44 , 45 There also are regional differences in small bowel distributions in IBS, being higher in the ileum than the duodenum and jejunum in one meta‐analysis. 45 Mast cell numbers correlated with bloating and dysmotility‐like dyspepsia in another study. 49
Mast cell increases have been related to specific IBS subsets. Female patients had higher mast cell numbers versus males in one report. 49 Increased lamina propria mast cells are described in those with chronic symptoms after Campylobacter‐induced gastroenteritis. 50 Some researchers propose that patients with postinfectious IBS selectively develop low‐grade mast cell responses, while others observe no mast cell elevations in IBS patients without prior infection. 51 In a recent review, no overall differences in mast cells were seen in postinfectious‐ versus non‐postinfectious‐IBS. 52 Some groups report higher cell counts in diarrhea‐predominant IBS (IBS‐D), while others also note prominent mast cells in constipation‐predominant IBS (IBS‐C). 52 , 53 , 54 , 55 In a meta‐analysis of 22 studies, mast cells were increased to similar degrees in both IBS‐C and IBS‐D patients in the descending (standardized mean difference 1.69, 95% CI 0.65–2.73, p = 0.001) and rectosigmoid (SMD 0.38, 95% CI 0.06–0.71, p = 0.02) colon. 44 A study of IBS‐D patients noted higher mast cell counts in those with lactose intolerance and symptoms versus asymptomatic patients who were lactose intolerant. 56
Mast cells show important morphologic differences in IBS. The proximity of mast cells to nerve endings is closer (within 5–10 microns) in IBS patients versus healthy controls, which correlates with abdominal pain severity. 36 , 51 , 55 , 57 , 58 Substance P containing nerve fibers and nerve endings that express TRPV1 show close proximity to ileal and colonic mast cells in postinfectious IBS. 6 , 51 , 59 These findings correlate with abdominal pain severity and frequency.
3.1.1. Alterations in mast cell mediator release and responses in IBS
Electron microscopic evidence of colonic mast cell activation is often observed in IBS, including increases in degranulation with labyrinthic arrays and clearing of individual granules. 36 , 55 In one study, 77% of IBS patients showed higher mast cell density including 150% increases in degranulating mast cells. 55
Mast cell mediators are increased in IBS. Mucosal biopsies in IBS exhibit higher stored mediators like histamine and tryptase and neo‐formed lipid mediators like PGE2 with associated increases in COX2 mRNA and protein expression (Figure 2). 24 , 36 Histamine, protease, and PGE2 release is increased in colon biopsy and fecal supernatants from IBS patients. 16 , 19 , 36 , 55 , 60 , 61 , 62 These findings have been related to increased tryptase and PAR2 mRNA and tryptase protein. 63 Tryptase expression is increased in IBS‐D versus IBS‐C and levels correlate with stool frequency and consistency in IBS‐D. 64 , 65 , 66 Serum cytokines including IL‐6, IL‐8, and TNF‐α are increased in some studies in IBS. 67 In one report, elevated mast cell NGF correlated with higher mast cell numbers. 68 In another IBS study, mucosal NGF, neurotrophic receptor tyrosine kinase 1 (NTRK1), and tropomyosin receptor kinase A (TrkA) expression were increased. 69 Mast cell NGF release can increase neuronal sprouting and neuroplastic changes in colon mucosa in IBS. 69 In colon mucosa from IBS‐D patients, mast cell numbers increase in association with upregulated tryptase, iNOS, and IL‐1β expression showing involvement of NO pathways in mast cell function. 27 Mast cell counts correlated with mucosal substance P and vasoactive intestinal polypeptide (VIP) content in female IBS patients in one report. 54 In another study, mucosal serotonin release and abdominal pain intensity correlated with higher mast cell numbers. 70
FIGURE 2.
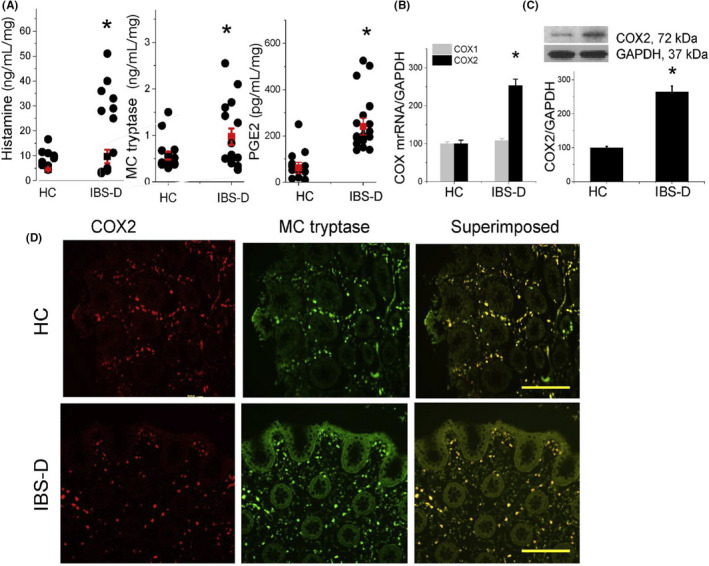
Proinflammatory mediators released by colonic mucosa of IBS‐D patients and healthy controls (HC) are shown. Some IBS‐D patients exhibit increased release of histamine, mast cell (MC) tryptase, and PGE2 (A). IBS‐D patients but not healthy controls (HCs) show increased COX2 mRNA (B) and COX2/GAPDH protein (C) expression. Immunofluorescence staining for COX2 (red) and MC tryptase (green) is shown for HCs and IBS‐D patients (D). Superimposed staining shows significant overlap of COX2 and MC tryptase immunoreactivity (yellow). Scale bar: 200 mm. From Grabauskas et al. 24
3.1.2. Activation of mast cells in IBS
Triggers for mast cell activation in animal models include gastrointestinal infection, food intolerance, and stress. 7 After remission of experimental colitis in C57BL/6 mice, increases in tryptase‐positive mast cells were associated with prolonged gastrointestinal transit. 71 In another report in guinea pigs, ileal and colon mast cells remained increased after Trichinella spiralis infection. 72 Supernatants from these regions increased mesenteric afferent nerve firing, an effect blunted by cromolyn disodium. Dietary fructooligosaccharide increases ileal mast cells and stimulates interleukin production in water avoidance‐stressed mice. 73 Modulators of stress effects on gut sensorimotor and immune function include glucocorticoids, VIP, substance P, corticotropin‐releasing hormone (CRH), neurotensin, adrenomedullin, and catecholamines. 74 Stress alters mast cell degranulation, increases PGE2 production, activates histamine and tryptase release, stimulates COX2 mRNA expressions, and impairs epithelial barrier function; together, these actions accelerate colon transit and increase fecal expulsion. 75
Stress pathways play prominent roles in mast cell activation in humans. Cold pain stress induces jejunal release of mast cell mediators in patients with food allergy. 76 CRH mediates stress‐induced disruption of human gut motor, epithelial barrier, and perceptual activity. 77 , 78 High numbers of CRH1 and CRH2 receptors are expressed by human colon mast cells. 77 , 79 CRH receptor stimulation elicits mast cell degranulation and releases cytokines and growth factors. 79 Stress‐ and CRH‐induced changes in intestinal motor and epithelial function are absent in mast cell‐deficient rodents and are abolished by mast cell stabilizers. 80 A recent review emphasized the ability of CRH1 and CRH1/CRH2 receptor antagonists to reduce stress‐induced mast cell activation in experimental models, but clinical studies of such therapies have been limited by unfavorable pharmacokinetics and formation of reactive metabolites. 75
3.1.3. Treatments that target mast cell pathways in IBS
Treatments to control mast cell activation or reduce actions of mast cell mediators have been studied in IBS. Disodium cromoglycate, a mast cell stabilizer that inhibits histamine and leukotriene release, decreased tryptase release and TLR2 and TLR4 expression in preliminary studies in IBS‐D. 81 In double‐blind trials, cromoglycate was superior to placebo in reducing symptoms in IBS patients with food intolerance. 82 , 83 , 84 A placebo‐controlled trial found that ketotifen, a mast cell stabilizer with histamine H1 antagonist properties, decreased IBS symptoms and improved quality of life but did not reduce mast cells. 85 , 86
Other studies suggest benefits of H1 antagonists in IBS. In a placebo‐controlled trial in 28 IBS patients, the H1 antagonist ebastine reduced abdominal pain and overall symptoms and improved quality of life. 87 A retrospective analysis from 307 children with functional gastrointestinal disorders reported symptom improvement with cyproheptadine—an antihistamine with anticholinergic and antiserotonergic properties. 88 Some propose that benefits of tricyclic antidepressants in IBS may result from histamine antagonism. 89
Other drugs which influence mast cell function have been proposed as IBS treatment. Mesalamine, an anti‐inflammatory agent which acts by COX and prostaglandin inhibition, reduced symptoms in some early studies in IBS‐D. 90 , 91 However, two more recent controlled trials in IBS failed to show benefit of mesalamine over placebo. 92 , 93 Corticosteroids were ineffective in one report, possibly due to an inability to affect mast cell appearance and degranulation. 94
4. PROPOSED MECHANISMS OF MAST CELL‐MEDIATED IBS SYMPTOM PATHOGENESIS
4.1. Mast cells and gut hypersensitivity
Depending on geography and symptom characteristics, heightened gut perception is reported by 20–94% of IBS patients in different investigations. 2 , 3 Hypersensitivity likely is influenced by mast cell activation and mediators as detailed in the following sections. Much of these data originate from animal and in vitro investigations which provide plausibility for mast cell‐induced visceral hypersensitivity in IBS.
4.1.1. Characterization of mast cell involvement in hypersensitivity development
Support for mast cell mediation of visceral hypersensitivity is offered by rodent models. Mast cell hyperplasia and increased granulation are found in hypersensitive rodents. 95 Mast cell‐deficient mice do not exhibit hypersensitivity to 2,4,6‐trinitrobenzene sulfonic acid (TNBS). Mast cell deficiency does not affect normal nociception to colon distention, but abolishes hypersensitivity evoked by IBS‐D colon biopsy supernatants. 24 , 96 Reconstitution of mast cell‐deficient mice with bone marrow‐derived mast cells from wild‐type mice restores the ability of IBS colon supernatants to elicit hypersensitivity, verifying mast cell participation for this potential mechanism for IBS symptoms. 24
Based on these animal studies, mast cell pathways have been proposed to modulate visceral hypersensitivity in IBS. 24 , 55 , 57 One study noted lower ileal and colonic mast cells in IBS patients with rectal hypersensitivity, while another noted no difference in cell counts in relation to sensation. 57 , 97
4.1.2. Mast cell mediators as potential triggers of hypersensitivity
Preformed mast cell mediators contribute to hypersensitivity in animal models. Histamine activation of afferent neurons adjacent to mast cells promotes sensitization to painful stimuli. Abdominal pain in IBS is proposed to result from TRPV1 sensitization after H1 receptor activation from findings of a study employing IBS rectal supernatants. 87 Intracolonic PAR2 agonist infusion promotes hypersensitivity to distention in rats and PAR2‐dependent mechanisms underlie hyperalgesia and increased sensory neuron calcium signaling in mice after exposure to IBS colon supernatants. 18 , 19 PAR2‐deficient mice do not develop hypersensitivity to supernatant exposure. Histamine, PAR2 agonists, and IBS colon supernatants fail to induce hypersensitivity in mast cell‐deficient mice (Figure 3). 24
FIGURE 3.
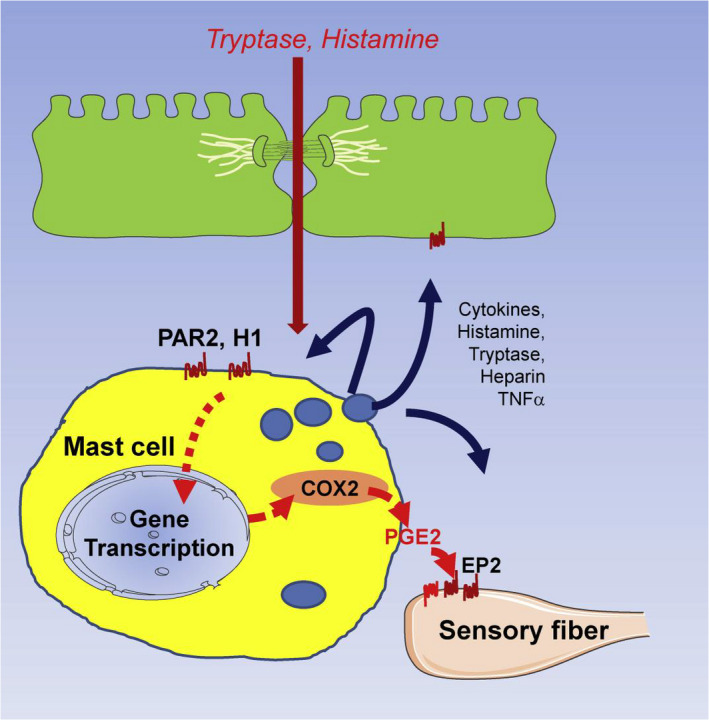
Role of PGE2 produced by mucosal mast cells in generating visceral hypersensitivity in IBS is shown. Proinflammatory mediators such as histamine, tryptase, and LPS are increased in IBS. These activate mast cell GPCRs (H1, PAR2, TLR4, etc.) which lead to degranulation of vesicular mediators (histamine, tryptas3, PGE2, etc.) and induce transcription activation of COX2 which increases synthesis of prostaglandins. Mast cells in close proximity to submucosal sensory nerve fibers release PGE2 which acts on sensory fiber EP2 receptors and potentiates action of pronociceptive mediators released by mechanical or chemical stimulation, leading to hypersensitivity. Histamine and tryptase are critical mediators released by mast cells to activate COX2 synthesis as blockade of either molecule prevents hypersensitivity development. However, histamine and tryptase are not the final mediators; rather their actions are dependent on PGE2 synthesized and released by mast cells. Histamine, tryptase, TNF‐α, and other mediators also may activate receptors on epithelial cells and enteric neurons causing dysmotility and epithelial barrier dysfunction via modulation of tight junction proteins. From Grabauskas et al. 24
Neo‐synthesized mediators and associated pathways also participate in hypersensitivity. IL‐1β and TNF‐α sensitize nociceptive neurons via p38 MAPK phosphorylation of Nav1.8, TRPV1, and transient receptor potential ankyrine‐1 (TRPA1) channels, which then induces hyperalgesia to mechanical and thermal stimuli. 98 , 99 Estrogen and an agonist of G‐protein coupled estrogen receptor (GPER) increase mast cell degranulation, tryptase and histamine release, and hypersensitivity in a rat stress model while ovariectomy decreases these activities. 100
Prostaglandin involvement in gut hypersensitivity is incompletely understood. PGE2 signaling directly sensitizes peripheral nociceptors in inflamed tissues by activating TRPV1, hyperpolarization‐activated cyclic nucleotide‐2 (HCN2), and tetrodotoxin‐resistant sodium channels on sensory neurons and induces hyperalgesia via protein kinase A‐ and C‐mediated activation of nuclear factor κB (NF‐κB) in DRG neurons. PGE2 production and COX2 expression are upregulated in IBS‐D colon supernatants. 24 , 55 , 61 This response is absent in mast cell‐deficient rats but is restored after reconstitution with bone marrow‐derived mast cells from wild‐type mice but not bone marrow mast cells from COX2‐(Ptgs2Y385F) mutant mice. 24 Mast cell PGE2 sensitizes gut afferent fibers to other mediators and participates in hypersensitivity in inflammation. PGE2 facilitates substance P and CGRP release, promotes IL‐6 and brain‐derived neurotrophic factor (BDNF) synthesis by DRG neurons, and enhances neuronal sensitivity to serotonin, bradykinin, and cytokines. 101 , 102 PGE2 alone enhances serotonin‐evoked currents in stomach‐ and ileum‐innervating afferent neurons. 103
Support for prostaglandins as final mediators of nociception come from studies showing that intracolonic histamine or tryptase causes delayed colon PGE2 increases which coincide with hypersensitivity development. Mast cell‐deficient and PtgS2Y38SF mutant mice develop hypersensitivity after PGE2 but not histamine or tryptase administration, confirming intermediary roles of histamine and proteases and final mediation by prostaglandins in rat models (Figure 3). 24 Similarly, prostaglandins may mediate hypersensitivity induced by cytokines as shown by the ability of COX2 inhibitors to block TNF‐α and IL‐1β‐induced nociceptor sensitization. 104
4.1.3. Reversal of mast cell‐mediated visceral hypersensitivity
Mast cell stabilizers and agents which target preformed mast cell mediators blunt hypersensitivity in animal studies. Stress‐induced hypersensitivity is prevented by the mast cell stabilizer doxantrazole in rats. 105 Mast cell stabilizer reductions in hypersensitivity are associated with lower mast cell degranulation and TLR4 mRNA and protein expression. 106 In rats, cromolyn sodium prevents hypersensitivity induced by supernatants from IBS‐D colon biopsies, histamine, and PAR2 agonism. 24 Hypersensitivity in stressed rats is prevented by the H1 antagonists, fexofenadine, and ebastine. 107 Likewise, the H1 antagonist olopatadine blunts hypersensitivity elicited by IBS‐D colon supernatants. 24
COX2 inhibition and other agents have impact on mast cell‐mediated hypersensitivity. In rats, the COX2 inhibitor celecoxib prevented hypersensitivity induced by IBS colon supernatants. 24 Bradykinin actions on serosal afferent nerves were blunted by the COX inhibitor naproxen but were restored by adding PGE2 in another report. 108 Hypersensitivity elicited by colonic PAR2 agonist infusion was prevented by a neurokinin‐1 antagonist in a different report. 18 Electroacupuncture reduced hypersensitivity in rats which was associated with decreased TLR4 mRNA and protein and mast cell degranulation. 106
These animal models offer plausible support for clinical observations in IBS. Ketotifen was shown in one investigation to reduce perception of distention in IBS patients with defined hypersensitivity (Figure 4). 85 In another controlled trial in IBS‐D, ketotifen reduced hypersensitivity to noxious distention versus placebo. 86
FIGURE 4.
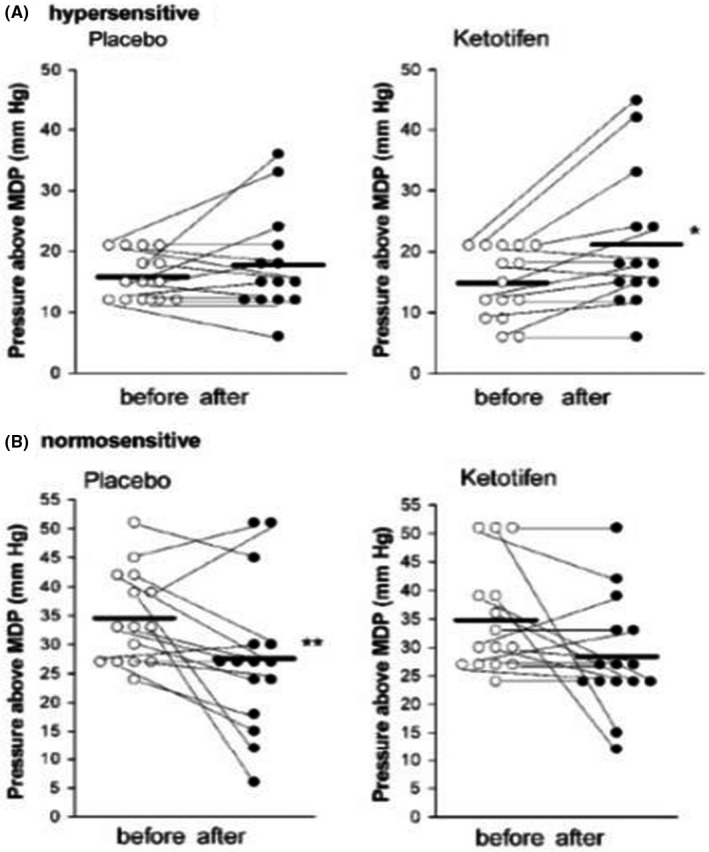
Thresholds for discomfort/pain during rectal distention before and after 8 weeks of treatment with placebo or ketotifen are shown for individual subjects with IBS with hypersensitivity (A) and without hypersensitivity (B). The horizontal lines represent mean thresholds for discomfort. *p = 0.015, **p = 0.024 versus before treatment. From Klooker et al. 85
4.2. Mast cells and gut epithelial function
Increased gut permeability is observed in some IBS subgroups (IBS‐D, postinfectious IBS) and is associated with altered bowel habits and increased abdominal pain. 4 Positive correlations of mast cell numbers with intestinal permeability defects have been reported mostly in animal studies as detailed in the following sections. 109 These findings support roles for mast cells in modulating epithelial dysfunction clinically observed in IBS.
4.2.1. Characterization of mast cell involvement in epithelial barrier dysfunction
Mast cell influences on the epithelial barrier have been demonstrated in rodent models. Increased mast cell mediators and gut permeability are noted after parasitic infection in rats. 110 Models of stress including water avoidance provide evidence for mast cell participation in epithelial barrier function. 80 , 111 , 112 , 113 Mast cell‐deficient mice models verify dependence of nerve‐mediated chloride secretion on mucosal mast cells. 114 Intestinal barrier alterations in mast cell‐deficient (Wsh) mice lead to reduced epithelial migration and permeability. 115 Claudin‐3 expression is linked to regulation of barrier function by mast cell protease‐4 (Mcpt‐4). Mcpt‐4‐deficient mice exhibit similar permeability alterations as Wsh mice, but reconstitution of Wsh mice with bone marrow mast cells from wild‐type mice but not Mcpt‐4‐deficient mice restores epithelial architecture and permeability. Water avoidance stress effects on epithelial function are seen in wild type but not mast cell‐deficient mice.
Epithelial barrier alterations with increased transcellular and paracellular mucosal permeability may underlie symptoms in some IBS subsets, especially relating to bowel habits. 60 , 111 , 116 , 117 Transcellular permeability across the rectal mucosa of IBS‐D patients measured with horseradish peroxidase correlates with mast cell numbers and increased tryptase activity, offering a clinical correlate to observations from animal studies. 111 , 116 , 117
4.2.2. Mast cell mediators as potential triggers of epithelial barrier dysfunction
Mast cell histamine, chymase, and PGD2 increase epithelial secretion and other mast cell products also impair epithelial function. 115 Proteases disrupt paracellular permeability by direct proteolysis and action on epithelial PAR receptors. Tryptase and chymase also cleave tight junction proteins including claudin‐1, claudin‐3, claudin‐5, and junctional adhesion molecule‐A (JAM‐A). 115 , 117 , 118 Elevated colon paracellular permeability in IBS‐D results from tryptase action on PAR2 receptors. PAR2 receptor‐mediated effects may involve calmodulin‐dependent activation of myosin light chain kinase (MLCK) or β‐arrestin‐dependent activation of cofilin, a regulatory protein that severs actin. 119 , 120 In knock out mice, microRNAs (MIR29) may regulate expression of tight junction proteins (cingulin, claudin‐1) and NFRF to increase intestinal permeability. 121 Mast cell‐dependent pathways involving substance P contribute to Clostridium difficile induced secretion in mice. 122
Human studies provide support for mast cell mediation of gut barrier function. In IBS‐D, tryptase levels correlate with epithelial tight junction ultrastructural changes including increases in dilated junctions and intercellular distance plus enhanced myosin phosphorylation, redistribution of tight junction zonula occludens‐1 (ZO‐1) and occludin from the membrane to the cytoplasmin, decreased ZO‐1 protein expression, and increased claudin‐2 expression. 66 , 123 Reductions in JAM‐A in IBS are associated with worse abdominal pain and longer symptom durations. 117 Tight junctions are disrupted by cytokines like TNF‐α that act by MLCK‐mediated myosin light chain phosphorylation and ZO‐1 and occludin reorganization. 124 A recent study demonstrated that intestinal tissues from patients with IBS‐D had increased levels of MIR29A. 121 Clinical studies in IBS indicate that the magnitude of barrier loss and mast cell activation correlate with pain severity. 116 , 125 Of 54 IBS‐D patients, 39% were found to have increased membrane permeability as measured by the lactulose/mannitol ratio. 126 Interestingly, the same group of patients also demonstrated increased visceral and thermal sensitivity. It is conceivable that increased permeability might allow access of luminal bacterial products into lamina proper which in turn stimulate sensory neurons to induce visceral hypersensitivity.
4.2.3. Reversal of mast cell‐mediated epithelial barrier dysfunction
Treatments targeting mast cell function can reverse epithelial abnormalities in animal and human models. The mast cell stabilizer doxantrazole reversed increased secretion and permeability in stressed rodents and reduced secretion elicited by substance P. 112 , 127 In a rat model of postinfectious IBS, Trichinella spiralis increased mast cells, altered cytokine production, enhanced permeability, and elicited hypersensitivity. 128 Barrier and perceptual effects of Trichinella spiralis were normalized by a PAR2 antagonist. In maternally separated rats, the sulfonylurea antidiabetic agent metformin inhibited loss of tight junction proteins and improved permeability and hypersensitivity. 129 Cromoglycate blocked increases in small intestinal permeability evoked by stress and CRH in healthy humans. 80
4.3. Mast cell interactions with microbiome
IBS patients exhibit gut microbiota alterations including increases in Firmicutes and reductions in Bacteroides species, but findings are inconsistent between studies and geographic regions. 5 Changes in bacterial populations may cause symptoms by altering cytokine levels. 130 Organisms like Enterococcus faecalis reduce in vitro mast cell degranulation. 131
4.3.1. Characterization of mast cell interactions with enteric flora
Interactions between mast cells and gut microbes may underlie some manifestations of IBS. Enteric bacteria promote mast cell histamine and protease release and activate inflammation through production of bile acids, organic acids, amino acids, phenols, polyunsaturated fatty acids, and short chain fatty acids. 132 Increased cellular translocation of bacteria promotes up‐regulation of mast cell signaling in IBS‐D. 60 Physical contact is not required for bacteria to activate mast cells; rather, bacterial toxins, metabolites such as histamine, and cell wall constituents accomplish this function after breaching the epithelial barrier. 133 Enteric flora modulate gut functions other than inflammation. Hypersensitivity to colonic distention of IBS patients can be transferred to rats through their fecal bacteria, demonstrating contributions of gut microbiota to sensorimotor dysfunction. 134
4.3.2. Mast cell mediators involved in interactions with enteric bacteria
Mast cell recognition of bacterial products involves activation of (i) TLR4 receptors by lipopolysaccharide (LPS), (ii) TLR5 receptors by flagellin, and (iii) TLR2 receptors by the gram‐positive bacterial component peptidoglycan. 60 Receptors for Clostridium difficile, Bordetella pertussis, and Vibrio cholerae toxins are expressed by mast cells. 135 Responses differ depending on the bacterial constituent. Microbial peptidoglycan triggers mast cell degranulation and cytokine release while LPS elicits cytokine release without degranulation. 136 Clostridium difficile toxin A binds to mast cell neurokinin‐1 receptors to cause gut secretion.
4.3.3. Reversal of mast cell‐associated gut microbiota interactions
Animal and in vitro studies of therapies with dual action on mast cells and enteric bacteria illustrate possible roles of mast cell‐microbiome interactions in gut illness. Ketotifen reduces enteritis in rodents exposed to Clostridium difficile toxin, blunts effects of Vibrio cholerae toxin in rat ileum, decreases epithelial passage of Escherichia coli and Salmonella typhimurium, and reverses effects of Salmonella to decrease occludin levels. 60 , 137 , 138 Miltefosine, a treatment of leishmaniasis, reverses hypersensitivity in maternally separated rats in association with microbiome alterations and reduced mast cell degranulation. 139 Also in this model, fungicides including fluconazole and nystatin reversed gut sensitivity. 140 The human mast cell line HMC‐1 released histamine in response to fungal antigens in this study.
Roles of mast cells in responding to therapies that modulate microbiome populations in IBS (probiotics, antibiotics, prebiotics, and fecal transplant) are poorly understood. However, foods which are high in fermentable oligosaccharides, disaccharides, monosachrides, and polyols (FODMAPs) increase visceral nociception by inducing gut dysbiosis and elevated fecal LPS level which mediate intestinal inflammation and barrier dysfunction, providing a potential mechanism for clinically observed IBS symptom exacerbation with FODMAP intake. 141 These abnormalities were reversed by low FODMAP diet. Subsequent studies in IBS‐D patients showed that low FODMAP diets normalize fecal LPS levels and improve IBS symptoms, accompanied by improved colon barrier functions and reduced mast cell activation. 142
5. CONCLUSIONS AND CLINICAL IMPLICATIONS
Prominent abnormalities in mast cell numbers, connectivity, and mediator release have been identified in IBS. Small intestine and colon mast cells show close apposition to sensory nerves, which modulate sensorimotor and secretory activities. Mast cells release preformed, neo‐synthesized, and neo‐formed lipid bioactive substances in response to stimuli including infection, stress, allergens, and neuroendocrine factors. Studies of mast cells in different IBS subsets have yielded inconsistent results, but research suggests that IBS that develops after an enteric infection or is diarrhea‐predominant most often has mast cell dysfunction.
Research has focused on three factors as contributors to IBS symptom development. Visceral hypersensitivity is detectable in many patients and may influence abdominal pain pathogenesis. Important recent investigations have defined prominent abnormalities of mast cell prostaglandin E2 synthesis which show interactions with histamine and tryptase release and which induce hypersensitivity in IBS‐D. Mast cell dysfunction with abnormal protease and cytokine release also produces epithelial barrier dysfunction in IBS, which alters mucosal permeability and may disrupt defecation patterns. Epithelial abnormalities frequently coexist with hypersensitivity in IBS, worsening abdominal pain in this disorder. Lastly, enteric microbe interactions with mast cells may affect symptom reports in some patients. This is evidenced by the observation that low FODMAP diet corrects gut dysbiosis and improves IBS symptoms. This is accompanied by reduction of mast cell activation and normalization of colonic barrier function. 142
Validating the importance of any purported pathogenic factor in IBS should include characterizing effective treatments which target underlying mechanistic defects. To date, treatments that alter mast cell activity (mast cell stabilizers) or function (histamine antagonists) have shown only modest benefits in IBS and are not widely adopted in clinical practice. Limitations of published studies include recruitment of small samples and poor experimental designs. Currently, no biomarkers are available to define mast cell causation of symptoms in specific IBS subsets. A blood panel that measures interleukins released by mast cells has shown 88% sensitivity and 86% specificity in distinguishing IBS patients from healthy controls, but these findings have not been specifically ascribed to mast cell abnormalities. 143 Studies in IBS and animal models suggest potential treatments to reverse visceral hypersensitivity, epithelial dysfunction, or microbial abnormalities. Novel pharmaceuticals have been proposed which reduce IBS symptoms by modifying mast cell activity include next‐generation histamine antagonists, anti‐Th2 cytokine antibodies, PAR antagonists, anti‐IgE antibodies, tyrosine kinase inhibitors, miRNA inhibitors or precursors, and dietary therapies. 126 , 144 , 145 Omalizumab, a medication that blocks IgE, elicited responses in a small study in IBS‐D. 146 A recent 12‐week controlled trial of palmitoylethanolamide and polydatin, two dietary compounds which synergistically reduce mast cell activation, reported reductions in abdominal pain in IBS patients without decreasing mast cell numbers. 147 Randomized trials of these and other therapies will define roles of mast cell dysfunction in well‐defined IBS subsets.
DISCLOSURE
No competing interests declared.
Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterology & Motility. 2022;34:e14339. doi: 10.1111/nmo.14339
Funding information
The studies were supported by the National institute if diabetes and digestive and kidney disease R01DK110436 and P30DK34933
Listen to the podcast here
REFERENCES
- 1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393‐1407.e5. doi: 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 2. Mertz H, Naliboff B, Munakara J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40‐52. [DOI] [PubMed] [Google Scholar]
- 3. Camilleri M, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camilleri M, Lasch K, Zhou K. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775‐G785. [DOI] [PubMed] [Google Scholar]
- 5. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157:97‐108. [DOI] [PubMed] [Google Scholar]
- 6. Hughes PA, Zola H, Penttila IA, et al. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066‐1074. [DOI] [PubMed] [Google Scholar]
- 7. Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155‐168. [DOI] [PubMed] [Google Scholar]
- 8. Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metcalfe DD, Baram D, Mekori MA. Mast cells. Physiol Rev. 1997;77:1033‐1079. [DOI] [PubMed] [Google Scholar]
- 10. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neural protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464‐4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol. 1999;120:129‐145. [DOI] [PubMed] [Google Scholar]
- 12. Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575‐585. [DOI] [PubMed] [Google Scholar]
- 13. Stead RH, Tomioka M, Quinonez G, et al. Intestinal mucosal mast cells in normal and nematode‐infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987;84:2975‐2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65‐78. [DOI] [PubMed] [Google Scholar]
- 15. Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut. 2006;55:445‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425‐1434. [DOI] [PubMed] [Google Scholar]
- 17. Heavey DJ, et al. Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat mucosa mast cells. J Immunol. 1988;140:1953‐1957. [PubMed] [Google Scholar]
- 18. Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase‐activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035‐1047. [DOI] [PubMed] [Google Scholar]
- 19. Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valdez‐Morales EE, Overington J, Guerrero‐Alba R, et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea‐predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol. 2013;108:1634‐1643. [DOI] [PubMed] [Google Scholar]
- 21. Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR‐2. J Biol Chem. 1997;272:4043‐4049. [DOI] [PubMed] [Google Scholar]
- 22. Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624‐631. [DOI] [PubMed] [Google Scholar]
- 23. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grabauskas G, Wu X, Gao J, et al. Prostaglandin E2, produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology. 2020;158:2195‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, et al. Role of prostaglandin D2 in mast cell activation‐induced sensitization of esophageal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G908‐G916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira SH. Prostaglandins, aspirin‐like drugs and analgesia. Nat New Biol. 1972;240:200‐203. [DOI] [PubMed] [Google Scholar]
- 27. An S, Zong G, Wang Z, et al. Expression of inducible nitric oxide synthase in mast cells contributes to the regulation of inflammatory cytokines in irritable bowel syndrome with diarrhea. Neurogastroenterol Motil. 2016;28:1083‐1093. [DOI] [PubMed] [Google Scholar]
- 28. Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric‐oxide synthase. J Biol Chem. 2000;275:13427‐13430. [DOI] [PubMed] [Google Scholar]
- 29. Shanahan F, Denburg JA, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985;135:1331‐1337. [PubMed] [Google Scholar]
- 30. Buhner S, Schemann M. Mast cell‐nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012;1822:85‐92. [DOI] [PubMed] [Google Scholar]
- 31. Collins SM, McHugh K, Jacobson K, et al. Previous inflammation alters the response of the rat colon to stress. Gastroenterology. 1996;111:1509‐1515. [DOI] [PubMed] [Google Scholar]
- 32. Nishida M, Uchikawa R, Tegoshi T, et al. Migration of neutrophils is dependent on mast cells in nonspecific pleurisy in rats. APMIS. 1999;107:929‐936. [DOI] [PubMed] [Google Scholar]
- 33. Theiner G, Gessner A, Lutz MB. The mast cell mediator PGD2 suppresses IL‐12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006;211:463‐472. [DOI] [PubMed] [Google Scholar]
- 34. Nakae S, Suto H, Kakurai M, et al. Mast cells enhance T cell activation: importance of mast cell‐derived TNF. Proc Natl Acad Sci U S A. 2005;1021:6467‐6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merluzzi S, Frossi B, Gri G, et al. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA‐secreting plasma cells. Blood. 2010;115:2810‐2817. [DOI] [PubMed] [Google Scholar]
- 36. Barbara G, Wang B, Stanghellini V, et al. Mast cell‐dependent excitation of visceral‐nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26‐37. [DOI] [PubMed] [Google Scholar]
- 37. De Jonge F, De Laet A, Van Nassauw L, et al. In vitro activation of murine DRG neurons by CGRP‐mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G178‐G191. [DOI] [PubMed] [Google Scholar]
- 38. Magadmi R, Meszaros J, Damanhouri ZA, Seward EP. Secretion of mast cell inflammatory mediators is enhanced by CADM1‐dependent adhesion to sensory neurons. Front Cell Neurosci. 2019;13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He S, Zhang H, Zeng X, Yang P. Self‐amplification mechansism of mast cell activation: a new look in allergy. Curr Mol Med. 2012;12:1329‐1339. [DOI] [PubMed] [Google Scholar]
- 40. He S, Zhang H, Chen H, et al. Expression and release of IL‐29 by mast cells and modulation of mast cell behavior by IL‐29. Allergy. 2010;65:1234‐1241. [DOI] [PubMed] [Google Scholar]
- 41. Terakawa M, Tomimori Y, Goto M, et al. Eosinophil migration induced by mast cell chymase is mediated by extracellular signal‐regulated kinase pathway. Biochim Biophys Res Commun. 2005;332:969‐975. [DOI] [PubMed] [Google Scholar]
- 42. Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635‐657. [DOI] [PubMed] [Google Scholar]
- 43. Frieling T, Meis K, Kolck U, et al. Evidence for mast cell activation in patients with therapy‐resistant irritable bowel syndrome. Z Gastroenterol. 2011;49:191‐194. [DOI] [PubMed] [Google Scholar]
- 44. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta‐analysis. Neurogastroenterol Motil. 2018;30(1):e13192. doi: 10.1111/nmo.13192 [DOI] [PubMed] [Google Scholar]
- 45. Robles A, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta‐analysis. Neurogastroenterol Motil. 2019;31:e13718. [DOI] [PubMed] [Google Scholar]
- 46. Burns G, et al. Evidence for local and systemic immune activation in functional dyspepsia and irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2019;114:429‐436. [DOI] [PubMed] [Google Scholar]
- 47. Ahn JY, et al. Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig Dis Sci. 2014;59:1001‐1011. [DOI] [PubMed] [Google Scholar]
- 48. O'Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449‐457. [DOI] [PubMed] [Google Scholar]
- 49. Cremon C, Gargano L, Morselli‐Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender‐dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392‐400. [DOI] [PubMed] [Google Scholar]
- 50. El‐Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large‐intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmulson M, et al. Microbiota, gastrointestinal infections, low‐grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence‐based review. Rev Gastroenterol Mex. 2014;79:96‐134. [DOI] [PubMed] [Google Scholar]
- 53. Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778‐1783. [DOI] [PubMed] [Google Scholar]
- 54. Sohn W, et al. Mast cell number, substance P and vasoactive intestinal polypeptide in irritable bowel syndrome with diarrhea. Scand J Gastroenterol. 2014;49:43‐51. [DOI] [PubMed] [Google Scholar]
- 55. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693‐702. [DOI] [PubMed] [Google Scholar]
- 56. Yang J, Fox M, Cong Y, et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39:302‐311. [DOI] [PubMed] [Google Scholar]
- 57. Park JH, Rhee P‐L, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71‐78. [DOI] [PubMed] [Google Scholar]
- 58. Di Nardo G, Barbara G, Cucchiara S, et al. Neuroimmune interactions at different intestinal sites are related to abdominal pain symptoms in children with IBS. Neurogastroenterol Motil. 2014;26:196‐204. [DOI] [PubMed] [Google Scholar]
- 59. Schemann M, Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013;144:698‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bednarska O, Walter SA, Casado‐Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153:948‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cenac N, Bautzova T, Le Faouder P, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology. 2015;149:433‐444. [DOI] [PubMed] [Google Scholar]
- 62. Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):41‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liang W‐J, Zhang G, Luo H‐S, et al. Tryptase and protease‐activated receptor 2 expression levels in irritable bowel syndrome. Gut Liv. 2016;10:382‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591‐599. [DOI] [PubMed] [Google Scholar]
- 65. Tooth D, Garsed K, Singh G, et al. Characterisation of faecal protease activity in irritable bowel syndrome with diarrhoea: origin and effect of gut transit. Gut. 2014;63:753‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martínez C, Lobo B, Pigrau M, et al. Diarrhoea‐predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160‐1168. [DOI] [PubMed] [Google Scholar]
- 67. Scully P, McKernan DP, Keohane J, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra‐intestinal co‐morbidity. Am J Gastroenterol. 2010;105:2235‐2243. [DOI] [PubMed] [Google Scholar]
- 68. Willot S, Gauthier C, Patey N, Faure C. Nerve growth factor content is increased in the rectal mucosa of children with diarrhea‐predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:734‐739. [DOI] [PubMed] [Google Scholar]
- 69. Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002‐1011. [DOI] [PubMed] [Google Scholar]
- 70. Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290‐1298. [DOI] [PubMed] [Google Scholar]
- 71. Kodani M, et al. Association between gastrointestinal motility and macrophage/mast cell distribution in mice during the healing phase after DSS‐induced colitis. Mol Med Rep. 2018;17:8167‐8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Song J, Zhang L, Bai T, et al. Mast cell‐dependent mesenteric afferent activation by mucosal supernatant from different bowel segments of guinea pigs with post‐infectious irritable bowel syndrome. J Neurogastroenterol Motil. 2015;21:236‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen B‐R, Du L‐J, He H‐Q, et al. Fructo‐oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress‐induced irritable bowel syndrome mouse model. World J Gastroenterol. 2017;23:8321‐8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ibeakanma C, Ochoa–Cortes F, Miranda–Morales M, et al. Brain‐gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098‐2108. [DOI] [PubMed] [Google Scholar]
- 75. Tache Y, Million M. Role of corticotropin‐releasing factor signaling in stress‐related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. 2015;21:8‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640‐648. [DOI] [PubMed] [Google Scholar]
- 77. Sagami Y. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin‐releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563‐568. [DOI] [PubMed] [Google Scholar]
- 79. Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin‐releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665‐7675. [DOI] [PubMed] [Google Scholar]
- 80. Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin‐releasing hormone increase intestinal permeability in humans by a mast cell‐dependent mechanism. Gut. 2014;63:1293‐1299. [DOI] [PubMed] [Google Scholar]
- 81. Lobo B, Vicario M, Martinez C, et al. Clinical improvement in IBS after disodium cromoglycate involves mast cell‐mediated toll‐like receptor signaling downregulation (abstract). Gastroenterology. 2011;140(Suppl 1):499‐500. [Google Scholar]
- 82. Lunardi C, et al. Double‐blind cross‐over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy. 1991;21:569‐572. [DOI] [PubMed] [Google Scholar]
- 83. Stefanini GF, Saggioro A, Alvisi V, et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995;30:535‐541. [DOI] [PubMed] [Google Scholar]
- 84. Leri O, Tubili S, De Rosa FG, et al. Management of diarrhoeic type of irritable bowel syndrome with exclusion diet and disodium cromoglycate. Inflammopharmacology. 1997;5:153‐158. [DOI] [PubMed] [Google Scholar]
- 85. Klooker TK, et al. The mast cell stabilizer ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213‐1221. [DOI] [PubMed] [Google Scholar]
- 86. Wang J, Wang Y, Zhou H, et al. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur J Gastroenterol Hepatol. 2020;32:706‐712. [DOI] [PubMed] [Google Scholar]
- 87. Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1‐mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150:875‐887. [DOI] [PubMed] [Google Scholar]
- 88. Modani S, Cortes O, Thomas R. Cyproheptadine use in children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2016;62:409‐413. [DOI] [PubMed] [Google Scholar]
- 89. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dorofeyev AE, Kiriyan EA, Vasilenko IV, Rassokhina OA, Elin AF. Clinical, endoscopical and morphological efficacy of mesalazine in patients with irritable bowel syndrome. Clin Exp Gastroenterol. 2011;4:141‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tuteja AK, Fang JC, Al‐Suqi M, Stoddard GJ, Hale DC. Double‐blind placebo‐controlled study of mesalamine in post‐infective irritable bowel syndrome—a pilot study. Scand J Gastroenterol. 2012;47:1159‐1164. [DOI] [PubMed] [Google Scholar]
- 92. Barbara G, Cremon C, Annese V, et al. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65:82‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lam C, et al. A mechanistic multicentre, parallel group, randomised placebo‐controlled trial of mesalazine for treatment of IBS with diarrhoea (IBS‐D). Gut. 2016;65:91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double‐blind, placebo‐controlled trial of prednisolone in post‐infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77‐84. [DOI] [PubMed] [Google Scholar]
- 95. La JH, Kim TW, Sung TS, et al. Role of mucosal mast cells in visceral hypersensitivity in a rat model of irritable bowel syndrome. J Vet Sci. 2004;5:319‐324. [PubMed] [Google Scholar]
- 96. Ohashi K, Sato Y, Kawai M, Kurebayashi Y. Abolishment of TNBS‐induced visceral hypersensitivity in mast cell deficient rats. Life Sci. 2008;82:419‐423. [DOI] [PubMed] [Google Scholar]
- 97. Braak B, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there a relationship? Am J Gastroenterol. 2012;107:715‐726. [DOI] [PubMed] [Google Scholar]
- 98. Jin X, Gereau RW. Acute p38‐mediated modulation of tetrodotoxin‐resistant sodium channels in mouse sensory neurons by tumor necrosis factor‐alpha. J Neurosci. 2006;26:246‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Binshtok AM, et al. Nociceptors are interleukin‐1 beta sensors. J Neurosci. 2008;28:14062‐14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu S, Wang X, Zhao J, et al. GPER‐mediated, oestrogen‐dependent visceral hypersensitivity in stressed rats is associated with mast cell tryptase and histamine expression. Fundam Clin Pharmacol. 2020;34:433‐443. [DOI] [PubMed] [Google Scholar]
- 101. St‐Jacques B, Ma W. Role of prostaglandin E2 in the synthesis of the pro‐inflammatory cytokine interleukin‐6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem. 2011;118:841‐854. [DOI] [PubMed] [Google Scholar]
- 102. Cruz Duarte P, St‐Jacques B, Ma W. Prostaglandin E2 contributes to the synthesis of brain‐derived neurotrophic factor in primary sensory neuron in ganglion explant cultures and in a neuropathic pain model. Exp Neurol. 2012;234:466‐481. [DOI] [PubMed] [Google Scholar]
- 103. Kim S, Jin Z, Lee G, et al. Prostaglandin potentiates 5‐HT responses in stomach and ileum innervating visceral afferent sensory neurons. Biochem Biophys Res Commun. 2015;456:167‐172. [DOI] [PubMed] [Google Scholar]
- 104. Cunha TM, Verri WA, Silva JS, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van den Wijngaard RM, et al. Susceptibility to stress induced visceral hypersensitivity in maternally separated rats in transferred across generations. Neurogastroenterol Motil. 2013;25:e780‐e790. [DOI] [PubMed] [Google Scholar]
- 106. Yang J, et al. The role of toll‐like receptor 4 and mast cell in the ameliorating effect of electroacupuncture on visceral hypersensitivity in rats. Neurogastroenterol Motil. 2019;31:e13583. [DOI] [PubMed] [Google Scholar]
- 107. Stanisor OI, van Diest SA, Yu Z, et al. Stress induced visceral hypersensitivity in maternally separated rats can be reversed by peripherally restricted histamine‐1‐receptor antagonists. PLoS One. 2013;8:e66884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Maubach KA, Grundy D. The role of prostaglandins in the bradykinin‐induced activation of serosal afferents of the rat jejunum in vitro. J Physiol. 1999;515:277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kamphuis JBJ, Guiard B, Leveque M, et al. Lactose and fructo‐oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology. 2020;158:652‐663. [DOI] [PubMed] [Google Scholar]
- 110. Ramage JK, Hunt RH, Perdue MH. Changes in intestinal permeability and epithelial differentiation during inflammation in the rat. Gut. 1988;29:57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lee H, Park JH, Park DI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19:244‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Demaude J, Salvador‐Cartier C, Fioramonti J, Ferrier L, Bueno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. . Am J Physiol Gastrointest Liver Physiol. 2000;278:G847‐G854. [DOI] [PubMed] [Google Scholar]
- 114. Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell‐deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Groschwitz KR, Ahrens R, Osterfeld H, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4‐dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381‐22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196‐201. [DOI] [PubMed] [Google Scholar]
- 117. Wilcz‐Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule‐A (JAM‐A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108:1140‐1151. [DOI] [PubMed] [Google Scholar]
- 118. Groschwitz KR, Wu D, Osterfeld H, Ahrens R, Hogan SP. Chymase‐mediated intestinal epithelial permeability is regulated by a protease‐activating receptor/matrix metalloproteinase‐2‐dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2013;304:G479‐G489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cenac N, et al. PAR2 activation alters colonic paracellular permeability in mice via IFN‐gamma‐dependent and ‐independent pathways. J Physiol. 2004;558:913‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jacob C, Yang P‐C, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease‐activated receptor 2 and beta‐arrestins. J Biol Chem. 2005;280:31936‐31948. [DOI] [PubMed] [Google Scholar]
- 121. Zhou QiQi, Costinean S, Croce CM, et al. MicroRNA 29 targets nuclear factor‐κB‐repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148:158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A‐induced enteritis in mice. Gastroenterology. 1998;114:956‐964. [DOI] [PubMed] [Google Scholar]
- 123. Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea‐predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736‐746. [DOI] [PubMed] [Google Scholar]
- 124. Shen Q, et al. Improving RhoA‐mediated intestinal epithelial permeability by continuous blood purification in patients with severe acute pancreatitis. Int J Artif Organs. 2013;36:812‐820. [DOI] [PubMed] [Google Scholar]
- 125. Bertiaux‐Vandaële N, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease bubtype. Am J Gastroenterol. 2011;106:2165‐2173. [DOI] [PubMed] [Google Scholar]
- 126. Zhou QiQi, Zhang B, Verne NG, et al. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang X, Sheng L, Guan Y, Qian W, Hou X. Synaptic plasticity: the new explanation of visceral hypersensitivity in rats with Trichinella spiralis infection. Dig Dis Sci. 2009;54:937‐946. [DOI] [PubMed] [Google Scholar]
- 128. Du L, Long Y, Kim JJ, et al. Protease activated receptor‐2 induces immune activation and visceral hypersensitivity in post‐infectious irritable bowel syndrome mice. Dig Dis Sci. 2019;64:729‐739. [DOI] [PubMed] [Google Scholar]
- 129. Li Y, et al. Metformin prevents colonic barrier dysfunction by inhibiting mast cell activation in maternal separation‐induced IBS‐like rats. Neurogastroenterol Motil. 2019;31:e13556. [DOI] [PubMed] [Google Scholar]
- 130. Camilleri M, Halawi H, Oduyebo I. Biomarkers as a diagnostic tool for irritable bowel syndrome: where are we? Expert Rev Gastroenterol Hepatol. 2017;11:303‐316. [DOI] [PubMed] [Google Scholar]
- 131. Kasakura K, et al. Commensal bacteria directly suppress in vitro degranulation of mast cells in a MyD88‐independent manner. Biosci Biotchnol Biochem. 2014;78:1669‐1676. [DOI] [PubMed] [Google Scholar]
- 132. Tana C, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512‐519. [DOI] [PubMed] [Google Scholar]
- 133. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135‐142. [DOI] [PubMed] [Google Scholar]
- 134. Crouzet L, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272‐e282. [DOI] [PubMed] [Google Scholar]
- 135. Moreno L, Gatheral T. Therapeutic targeting of NOD1 receptors. Br J Pharmacol. 2013;170:475‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll‐like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pothoulakis C, Karmeli F, Kelly CP, et al. Ketotifen inhibits Clostridium difficile toxin A‐induced enteritis in rat ileum. Gastroenterology. 1993;105:701‐707. [DOI] [PubMed] [Google Scholar]
- 138. Rocha MFG, Aguiar JEP, Sidrim JJC, et al. Role of mast cells and pro‐inflammatory mediators on the intestinal secretion induced by cholera toxin. Toxicon. 2003;42:183‐189. [DOI] [PubMed] [Google Scholar]
- 139. Botschuijver S, et al. Miltefosine treatment reduces visceral hypersensitivity in a rat model for irritable bowel syndrome via multiple mechanisms. Sci Rep. 2019;9:12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Botschuijver S, Roeselers G, Levin E, et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology. 2017;153:1026‐1039. [DOI] [PubMed] [Google Scholar]
- 141. Zhou S‐Y, Gillilland M, Wu X, et al. FODMAP diet modulates visceral nociception by lipopolysaccharide‐mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128:267‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Singh P, et al. High FODMAP diet causes barrier loss via liposaccharide mediated mast cell activation. JCI Insight. 2021;6:e146529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mujagic Z, et al. A novel biomarker panel for irritable bowel syndrome and the application in the general population. Sci Rep. 2016;6:26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Eswaran SL, Chey WD, Han‐Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS‐D. Am J Gastroenterol. 2016;111:1824‐1832. [DOI] [PubMed] [Google Scholar]
- 145. Dionne J, et al. A systematic review and meta‐analysis evaluating the efficacy of a gluten‐free diet and a low FODMAP diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol. 2018;113:1290‐1300. [DOI] [PubMed] [Google Scholar]
- 146. Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6‐17. [DOI] [PubMed] [Google Scholar]
- 147. Cremon C, Stanghellini V, Barbaro MR, et al. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther. 2017;45:909‐922. [DOI] [PubMed] [Google Scholar]


