Abstract
Objective
Variation in bronchiolitis management by race and ethnicity within emergency departments (EDs) has been described in single-center and prospective studies, but large-scale assessments across EDs and inpatient settings are lacking. Our objective is to describe the association between race and ethnicity and bronchiolitis management across 37 US free-standing children’s hospitals from 2015 to 2018.
Methods
Using the Pediatric Health Information System, we analyzed ED and inpatient visits from November 2015 to November 2018 of children with bronchiolitis 3 to 24 months old. Rates of use for specific diagnostic tests and therapeutic measures were compared across the following race/ethnicity categories: 1) non-Hispanic white (NHW); 2) non-Hispanic black (NHB); 3) Hispanic; and 4) other. Sub-analyses of ED patients only and children <1 year old were performed. Mixed-effect logistic regression was performed to compare the adjusted odds of receiving specific test/treatment using NHW children as the reference group.
Results
A total of 134,487 patients met inclusion criteria (59% male, 28% NHB, 26% Hispanic). Adjusted analysis showed that NHB children had higher odds to receive medication associated with asthma (OR=1.27, 95% CI=1.22–1.32) and lower odds to receive diagnostic tests (blood cultures, complete blood counts, viral testing, chest x-rays, OR=0.78, 95% CI=0.75–0.81) and antibiotics (OR=0.58, 95% CI=0.52–0.64) than NHW children. Hispanic children had lower odds to receive diagnostic testing (OR=0.94, 95% CI=0.90–0.98), asthma-associated medication (OR=0.92, 95% CI=0.88–0.96), and antibiotics (OR=0.74, 95% CI=0.66–0.82) compared to NHW children.
Conclusion
NHB children more often receive corticosteroid and bronchodilator therapies; NHW children more often receive antibiotics and chest radiography. Given that current guidelines generally recommend supportive care with limited diagnostic testing and medical intervention, these findings among NHB and NHW children represent differing patterns of overtreatment. The underlying causes of these patterns require further investigation.
Keywords: Bronchiolitis, Health Disparities, Pediatrics
Introduction:
Bronchiolitis is one of the most common reasons for hospitalization in children under 12 months.1 Current American Academy of Pediatrics (AAP) guidelines recommend supportive oxygen and hydration as necessary with limited diagnostic testing; antibiotics are given if a secondary bacterial infection is suspected.2 Studies show that patients often receive corticosteroids, albuterol, and antibiotics - treatments that are not recommended per national guidelines nor found effective in bronchiolitis - and that use of these interventions varies widely across hospitals. These studies also show variable use of diagnostic testing, including viral testing, chest x-rays (CXR), and complete blood counts (CBCs). 3–6 This high variability in bronchiolitis management results in potentially avoidable exposure to painful tests and medication side effects as well as likely overutilization of resources, whose cost may be passed on to patient families. Consequently, children with bronchiolitis may experience an increased burden of disease by being subjected to unnecessary healthcare interventions.7,8
Part of the variability in bronchiolitis management may be explained by differences in healthcare provided to individuals from different racial or ethnic groups, possibly representing a disparity in care. Health disparities encompass preventable differences in the burden of disease experienced by individuals from socially disadvantaged populations.9 Previous studies conducted primarily in emergency departments (EDs), single-sites, or in prospective cohorts have identified significant differences in diagnostic test use and medication use among children with bronchiolitis from different racial or ethnic backgrounds.4,5,10,11 Studies of hospitalized children with bronchiolitis focus primarily on race and ethnic differences in admission and transfer rates and generally do not analyze management patterns.12–14 Analyzing resource utilization data from a large, multi-center patient cohort is necessary to understand better the potential association of patient race and ethnicity with care patterns in bronchiolitis.
Given that hundreds of thousands of children across the US receive medical care for bronchiolitis annually, identifying possible disparities in bronchiolitis management associated with a patient’s race and/or ethnicity may highlight an opportunity for reducing unnecessary variations in care.15 Our objective is to describe the association between race and ethnicity and bronchiolitis diagnostic and treatment modalities across US free-standing children’s hospitals.
Patients and Methods
Data Source
This study is a retrospective, cross-sectional cohort study using the Pediatric Health Information System (PHIS) database (Children’s Hospital Association, Overland Park, KS). The PHIS database contains deidentified administrative data, detailing demographics, diagnostics, procedures, and pharmacy billing from 49 free-standing children’s hospitals across the United States. A joint effort between the Children’s Hospital Association and participating hospitals ensures data quality16. The study was determined to be non-human subjects by the University of Nebraska Institutional Review Board.
Patient Population
Eligible patients included children 3 to 24 months old who were discharged between November 1, 2015, and November 1, 2018, with a bronchiolitis diagnosis. All initial ED and hospital admissions of patients were included if they met one of the following criteria:
All Patient Refined Diagnosis-Related Groups (APR-DRG) for bronchiolitis (code 138);
a diagnosis of acute bronchiolitis (International Classification of Diseases, Tenth Revision code J21.0, J21.1, J21.8, or J21.9). 11
Exclusion criteria included the presence of ICD-10 codes associated with RSV pneumonia (J12.1), a chronic complex condition, a length of stay over ten days, any readmission during the study period, bacterial co-infection, or any of the PHIS variables for transfer into the Intensive Care Unit or any dependance on mechanical ventilation. Chronic complex conditions, according to Feudtner et al.17, include respiratory diagnoses such as congenital malformations, cystic fibrosis, bronchopulmonary dysplasia, or chronic lung disease, but not asthma or reactive airway disease. Secondary ED visits and hospital readmissions were excluded as they were unlikely to represent clinical practices associated with initial presentation for bronchiolitis and thus be beyond the focus of the present study. Bacterial co-infection was determined by an independent analysis of secondary diagnoses listed for bronchiolitis encounters. The independent analysis involved two independent clinicians deciding what secondary diagnoses were bacterial in origin, with 99% agreement. A third coder adjudicated the 1% of diagnosis codes remaining.18 The full list of identified bacterial ICD-10 codes for exclusion is listed in Table S1.
Study Variables
The measured exposure was patient race and/or ethnicity. Race and ethnicity in PHIS are categorized separately as race (White, Black, Asian, Pacific Islander, American Indian, Other) and ethnicity (Hispanic or Latino, Not Hispanic or Latino). The unadjusted analysis represented calculations using four race/ethnicity groups based on the combination of these two variables: Non-Hispanic White (NHW), Non-Hispanic Black (NHB), Hispanic, and other. Other covariates used in the adjusted analysis were as follows: age at admission, payor type (public, private, or other), APR-DRG severity level, median income quartile (for zip code of residence), site or hospital visited, year of presentation, transfer status (source of admission), and rural-urban commuting area (RUCA).19,20
Outcome Variables
The primary outcomes were the use of specific diagnostic tests and medications (Table S2). Selected diagnostic tests were identified using clinical transaction classification (CTC) codes and included CXR, CBCs, blood culture, and viral testing. Medications examined included systemic corticosteroids, albuterol, and antibiotics.
Statistical Analysis
The number of bronchiolitis cases at each site was broken down by race and ethnicity to ensure all sites had more than ten total cases in the four race/ethnicity categories; those with less than ten cases were removed from the analysis. Rates of use for diagnostic tests and medications were individually compared by patient race and ethnicity using mixed-effect logistic regression models controlling for all covariates and clustering by hospital using a random intercept for site. Two sub-analyses were performed: 1) the model was restricted to encounters where the patient presented to the ED and was released from the ED to test the association among ED-only patients21,22, and 2) the model was restricted to just those encounters among children under one year of age to evaluate whether the suspicion of asthma drove treatment differences. The demographics by race/ethnicity were compared using a chi-square statistical analysis. Statistical analysis was performed using R version 3.5.1 (R Core Team, Vienna, Austria).
Results
A total of 134,487 bronchiolitis cases were included in the final cohort (Figure 1). Twelve children’s hospital sites of the original 49 hospitals in the PHIS database, representing 3,775 cases, were not included due to insufficient populations of NHW, NHB, or Hispanic cases. Demographic characteristics of the final cohort by race and ethnicity are summarized in Table 1. A majority of the cohort patients were male, between 3–12 months old, and were from an urban zip code. NHW children were nearly evenly split on type of insurance, but the majority of all other groups were on public health insurance. NHW children also transferred into the center more often than NHB or Hispanic children, but most cases presented to the center without being transferred. Two sites were removed from the crude and adjusted viral testing analysis and one site removed from the crude and adjusted blood culture analysis due to no reported tests in the PHIS database. Overall, 12.4% of patients received viral testing, and 21.4% received a CXR. In total, 28.3% of patients were treated with albuterol, 8.6% received systemic corticosteroids, and 2.6% received antibiotics.
Figure 1.
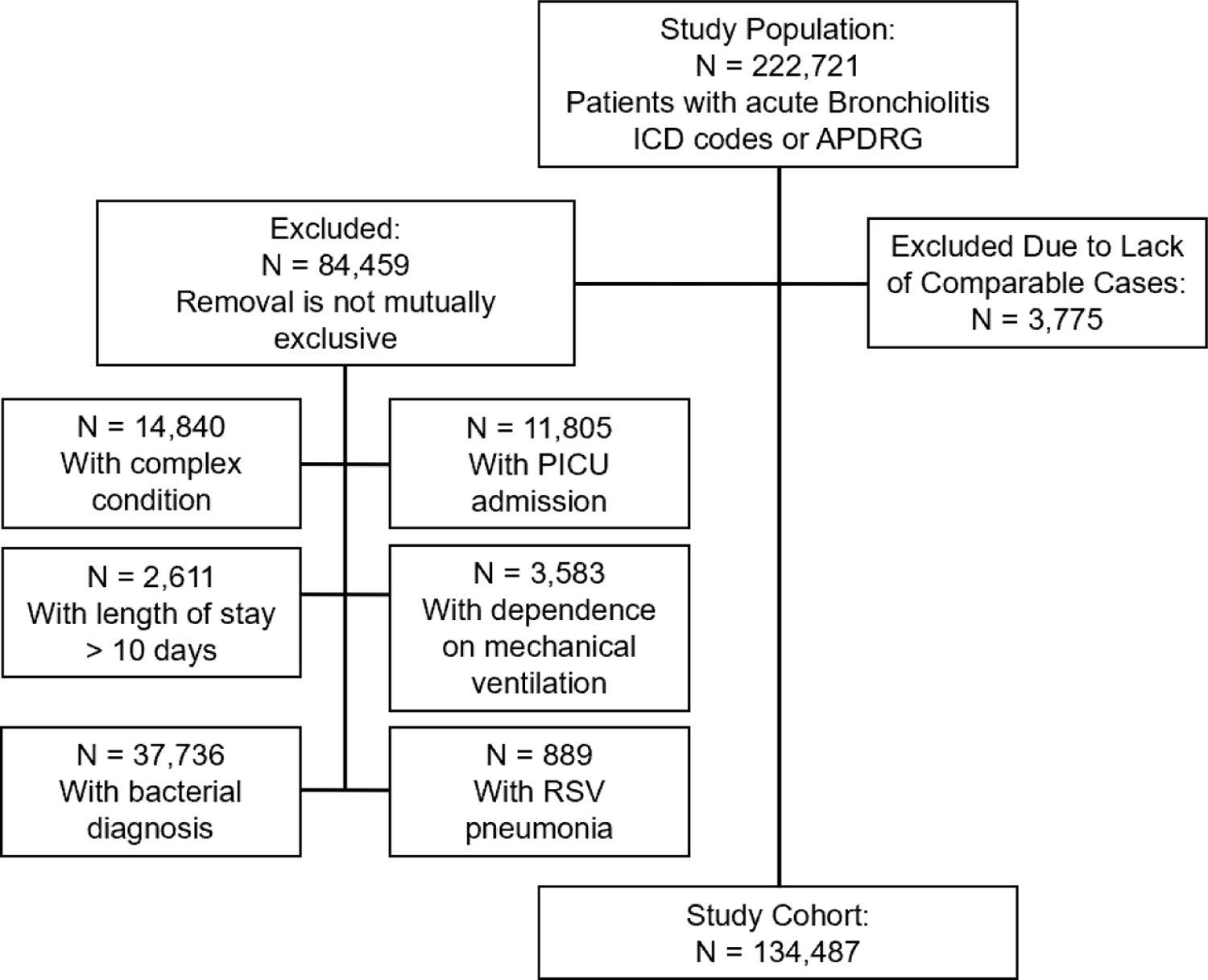
Cohort flow diagram.
Table 1.
Demographic Information of Study Population by Race and Ethnicity
| NHW | NHB | Hispanic | Other | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | ||
| n | 46918 | 34.9 | 38731 | 28.8 | 35643 | 26.5 | 13195 | 9.81 | |
| Year | <0.001 | ||||||||
| 2015 | 3558 | 7.6 | 3879 | 10.0 | 3026 | 8.5 | 1105 | 8.4 | |
| 2016 | 15926 | 33.9 | 13168 | 34.0 | 12054 | 33.8 | 4334 | 32.8 | |
| 2017 | 16429 | 35.0 | 13009 | 33.6 | 12200 | 34.2 | 4629 | 35.1 | |
| 2018 | 11005 | 23.5 | 8675 | 22.4 | 8363 | 23.5 | 3127 | 23.7 | |
| Admit Age (months) | <0.001 | ||||||||
| 3–12 | 33991 | 72.4 | 28490 | 73.6 | 25695 | 72.1 | 9029 | 68.4 | |
| Sex | <0.001 | ||||||||
| Male | 27805 | 59.3 | 22635 | 58.4 | 21291 | 59.7 | 7985 | 60.5 | |
| Female | 19094 | 40.7 | 16093 | 41.6 | 14351 | 40.3 | 5176 | 39.2 | |
| Payor | <0.001 | ||||||||
| Private | 22622 | 48.2 | 4212 | 10.9 | 3925 | 11.0 | 3491 | 26.5 | |
| Public | 22275 | 47.5 | 32618 | 84.2 | 29141 | 81.8 | 8993 | 68.2 | |
| Other | 2021 | 4.3 | 1901 | 4.9 | 2577 | 7.2 | 711 | 5.4 | |
| Median Income Quartile (MIQ) | <0.001 | ||||||||
| Q4 | 4635 | 9.9 | 15082 | 38.9 | 8827 | 24.8 | 2890 | 21.9 | |
| Q3 | 10200 | 21.7 | 10347 | 26.7 | 10220 | 28.7 | 2924 | 22.2 | |
| Q2 | 13018 | 27.7 | 7615 | 19.7 | 9456 | 26.5 | 3462 | 26.2 | |
| Q1 | 17987 | 38.3 | 5317 | 13.7 | 6532 | 18.3 | 3605 | 27.3 | |
| Unknown | 1078 | 2.3 | 370 | 1.0 | 608 | 1.7 | 314 | 2.4 | |
| RUCA | <0.001 | ||||||||
| Isolated | 802 | 1.7 | 128 | 0.3 | 91 | 0.3 | 68 | 0.5 | |
| Small Rural | 1511 | 3.2 | 272 | 0.7 | 381 | 1.1 | 152 | 1.2 | |
| Large Rural | 2829 | 6.0 | 397 | 1.0 | 838 | 2.4 | 205 | 1.6 | |
| Urban | 40698 | 86.7 | 37564 | 97.0 | 33725 | 94.6 | 12456 | 94.4 | |
| Unknown | 1078 | 2.3 | 370 | 1.0 | 608 | 1.7 | 314 | 2.4 | |
| AP-DRG Severity | <0.001 | ||||||||
| Extreme | 30 | 0.1 | 8 | 0.0 | 17 | 0.0 | 9 | 0.1 | |
| Major | 823 | 1.8 | 462 | 1.2 | 639 | 1.8 | 265 | 2.0 | |
| Moderate | 4254 | 9.1 | 2778 | 7.2 | 2921 | 8.2 | 1175 | 8.9 | |
| Minor | 41788 | 89.1 | 35465 | 91.6 | 32052 | 89.9 | 11737 | 89.0 | |
| Transfer Status | <0.001 | ||||||||
| Yes | 1436 | 3.1 | 493 | 1.3 | 550 | 1.5 | 557 | 4.2 | |
| No | 45482 | 96.9 | 38238 | 98.7 | 35093 | 98.5 | 12638 | 95.8 | |
| Diagnostics | |||||||||
| CBC | 2562 | 5.5 | 1403 | 3.6 | 2040 | 5.7 | 776 | 5.9 | |
| Blood Culture | 1550 | 3.3 | 798 | 2.1 | 1211 | 3.4 | 422 | 3.2 | |
| Viral Testing | 6170 | 13.2 | 4129 | 10.7 | 4510 | 12.7 | 1695 | 12.8 | |
| CXR | 10831 | 23.1 | 7064 | 18.2 | 8246 | 23.1 | 2654 | 20.1 | |
| Therapies | |||||||||
| Albuterol | 11723 | 25.0 | 12386 | 32.0 | 9617 | 27.0 | 4281 | 32.4 | |
| Systemic Steroids | 3750 | 8.0 | 3469 | 9.0 | 3094 | 8.7 | 1216 | 9.2 | |
| Antibiotics | 1536 | 3.3 | 701 | 1.8 | 789 | 2.2 | 407 | 3.1 | |
Overall adjusted outcomes by race/ethnicity are summarized in Table 2. In adjusted analyses, in comparison to NHW, NHB had lower odds of receiving chest radiography, undergoing a CBC, viral testing, or having a blood culture obtained. Hispanic children, when compared to NHW children, also showed a small decrease in the odds of receiving a CBC, CXR, and viral testing. Compared to NHW children, the adjusted odds of receiving albuterol were greater in NHB children and slightly lower in Hispanic children. NHW children had greater odds of receiving antibiotics than both NHB and Hispanic children.
Table 2.
Overall Adjusted Outcomes by Race and Ethnicity
| aOR (95% CI) | ||||
|---|---|---|---|---|
| NHW | NHB | Hispanic | Other | |
| CBC | 1 (ref.) |
0.72 (0.66–0.78) | 0.90 (0.82–1.00) | 1.00 (0.91–1.10) |
| Blood Culture | 0.68 (0.61–0.75) | 0.90 (0.83–0.98) | 0.93 (0.82–1.05) | |
| Viral Testing | 0.78 (0.74–0.82) | 0.89 (0.83–0.95) | 0.99 (0.92–1.03) | |
| CXR | 0.78 (0.75–0.82) | 0.94 (0.89–0.98) | 0.97 (0.92–1.03) | |
| Albuterol | 1.30 (1.26–1.36) | 0.94 (0.90–0.99) | 1.02 (0.97–1.07) | |
| Systemic Steroids | 1.17 (1.10–1.24) | 0.86 (0.80–0.92) | 0.89 (0.82–0.96) | |
| Antibiotics | 0.58 (0.52–0.64) | 0.74 (0.66–0.82) | 0.84 (0.74–0.95) | |
When analyzing the subset of children presenting and discharged from the ED for bronchiolitis (n= 102,592), similar associations between race/ethnicity and diagnostic test use and medical treatments persisted with only a few exceptions (Table 3). Specifically, one difference by race/ethnicity among ED children was in the adjusted odds of receiving albuterol, with Hispanic children now having similar odds as NHW children. A subset of children under the age of 1 (n = 97,205) showed trends similar to the overall odds of receiving any of the tests or medications by race/ethnicity (Table 4).
Table 3.
ED Only Adjusted Outcomes by Race and Ethnicity
| aOR (95% CI) | |||||
|---|---|---|---|---|---|
| NHW | NHB | Hispanic | Other | ||
| CBC | 1 (ref.) | 0.71 (0.66–0.77) | 0.90 (0.83–0.97) | 1.00 (0.90–1.10) | |
| Blood Culture | 0.71 (0.60–0.84) | 0.97 (0.83–1.18) | 1.08 (0.88–1.34) | ||
| Viral Testing | 0.79 (0.74–0.84) | 0.93 (0.86–1.00) | 1.08 (0.98–1.18) | ||
| CXR | 0.79 (0.76–0.84) | 0.94 (0.90–0.98) | 0.98 (0.93–1.04) | ||
| Albuterol | 1.32 (1.26–1.39) | 0.99 (0.94–1.05) | 1.05 (0.98–1.12) | ||
| Systemic Steroids | 1.15 (1.06–1.24) | 0.90 (0.82–0.98) | 0.92 (0.82–1.02) | ||
| Antibiotics | 0.76 (0.61–0.95) | 0.75 (0.58–0.96) | 0.89 (0.67–1.17) | ||
Table 4.
Under Age One Adjusted Outcomes by Race and Ethnicity
| aOR (95% CI) | |||||
|---|---|---|---|---|---|
| NHW | NHB | Hispanic | Other | ||
| CBC | 1 (ref.) | 0.74 (0.68–0.82) | 0.85 (0.78–0.94) | 0.96 (0.85–1.08) | |
| Blood Culture | 0.74 (0.63–0.80) | 0.92 (0.82–1.03) | 0.93 (0.80–1.09) | ||
| Viral Testing | 0.80 (0.76–0.85) | 0.93 (0.87–1.00) | 0.98 (0.90–1.07) | ||
| CXR | 0.80 (0.76–0.84) | 0.89 (0.84–0.94) | 0.94 (0.87–1.00) | ||
| Albuterol | 1.31 (1.24–1.37) | 0.89 (0.84–0.94) | 1.00 (0.94–1.07) | ||
| Systemic Steroids | 1.16 (1.06–1.27) | 0.76 (0.68–0.83) | 0.83 (0.73–0.94) | ||
| Antibiotics | 0.59 (0.52–0.68) | 0.72 (0.63–0.83) | 0.78 (0.67–0.92) | ||
Discussion:
Our analysis of more than 130,000 bronchiolitis ED and hospital visits across free-standing children’s hospitals found that NHB and Hispanic children with bronchiolitis were less likely to receive viral testing, CBC, CXR, and antibiotics than NHW children and that NHB children were more likely to receive albuterol and systemic steroids than NHW children. While diagnostically NHB children received care more in alignment with the AAP bronchiolitis guidelines (i.e., fewer CXRs and CBCs), treatments given were more consistent with a diagnosis of asthma than of bronchiolitis. This trend persists after excluding encounters over the age of 1, which minimizes the likelihood of diagnostic confusion with asthma. These findings are particularly relevant as treatment guidelines for bronchiolitis are well established and clearly indicate the lack of clinical evidence to support any of these interventions.2 It is important to note, however, that our investigation aimed not to determine the efficacy of these treatments but to describe patterns in the diagnostic and treatment methods for bronchiolitis received by children by race/ethnicity. Additionally, the data presented here reflects overall lower utilization of these management methods since previously described work.23
One possible explanation for the finding that NHB children received more albuterol and corticosteroids could be the heterogeneity in bronchiolitis presentation producing difficulty in distinguishing it from asthma in young children.24,25 However, our study looks specifically at patients below the age of 24 months with a sub-analysis of children less than one year, which are age groups where we anticipate less diagnostic confusion. Our findings, particularly among children <1 year old, demonstrate that, even when diagnosed with bronchiolitis, children more often received management in line with asthma if they were NHB or Hispanic.
A greater understanding of the outcomes of asthma could explain the asthma therapy in bronchiolitis as well. Literature supports the association of bronchiolitis as a child and a future diagnosis of asthma,26,27 as well as NHB children experiencing more asthma cases,28,29 and worse outcomes associated with asthma exacerbations.30,31 The similar clinical presentation could be associated with the finding that healthcare providers more often diagnose asthma in young NHB and Hispanic children with acute wheezing in ambulatory settings, therefore possibly causing more aggressive treatment of the wheezing in bronchiolitis with asthma therapy.32,33 Akinbami, et al. examined a group of 946 children aged 3–17 who reported a wheezing episode in the last 12 months and found, regardless of severity level, minority children were more likely to have been diagnosed with asthma by a health care professional.32 The study authors suggest this discrepancy may be due to greater awareness of asthma symptoms by NHB children and their families or a greater prevalence of family history of asthma in NHB families. Both of these interpretations could result in more aggressive treatment of bronchiolitis with asthma therapies. Roberts, et al. found that NHB children aged 1–5 years who reported wheeze in the previous 12 months were more than twice as likely than their NHW peers to be diagnosed with asthma.33 In both these studies, authors theorized that practitioners’ perception of patient illness, rather than objective data, influenced diagnosis disparities. Zook, et al. revealed that in patients diagnosed with asthma presenting to a pediatric emergency department, NHB and Hispanic patients were more likely to receive corticosteroids than their NHW counterparts.34 This finding may be in part due to poorer outcomes for NHB and Hispanic patients who are diagnosed with asthma impacting a healthcare provider’s prescription decisions.
However, while an aggressive approach to bronchiolitis therapy could be rooted in a desire to ensure all possible root causes of the wheezing have been addressed, a diagnosis of asthma in children below 24 months of age is not common. Asthma prevalence in the US among patients aged 0–4 years is 3.8% compared to 9.6% in children aged 5–11 years and 12.5% in children 12 years old and older.
The variations in care experienced by NHW children with bronchiolitis are consistent with previous pediatric physician behavior reports. Prior studies have demonstrated that primary care pediatricians were more likely to diagnose acute respiratory tract infections (ARTI) in NHW children when compared to NHB children and that they were also more likely to prescribe antibiotics for ARTI’s in NHW children.35 Previous studies have also revealed that NHB children in pediatric emergency departments are less likely to receive antibiotics than their NHW counterparts for ARTI’s.6 In these studies, the authors conclude that disparate diagnostic and treatment behaviors based on race more commonly represented overtreatment of NHW patients rather than undertreatment of NHB patients. This phenomenon of overtreatment for NHW patients has also been described in the use of head CT for patients with minor head trauma, CXR in the evaluation of asthmatics, and ultrasound and CT use in evaluating abdominal pain associated with constipation in the ED. 34,36,37 In these studies, authors suggest that overtreatment of NHW children may be associated with parental anxiety and perceived parental preference. Our study findings are consistent with a provider practice that favors the overtreatment of NHW patients. However, we cannot determine whether viral testing, CXR and antibiotic administration were influenced by parental anxiety or parental preference.
The establishment and distribution of guidelines have been shown to decrease overall utilization of incorrect therapies and diagnostics.11 Understanding why physicians break from well-established protocol is key to addressing over-utilization of resources. Clinical decision-making in pediatric medicine is complex and impacted by several factors, including clinical assessment, implicit bias, perceived parental pressure, explicit parental request, and self-perception of accuracy.38 Furthermore, the care provided for pediatric patients in the ED can be affected by primary care provided before ED presentation.3,39 Prior studies have found that parents of NHW children with bronchiolitis more often seek medical care prior to their emergency department visit, which matches the predominance of transfer patients in our study being NHW.4 Since not all previous medical encounters translate directly to an ED or hospital admission and data from our cohort does not include specific information about the outpatient healthcare encounter preceding the initial ED visit or hospitalization, we cannot determine if differences in preadmission practice patterns may be influencing subsequent care.
Practitioners’ implicit bias may play a significant role in the disparities in bronchiolitis management identified in this study. Implicit bias, defined as attitudes or stereotypes that can affect behavior in an unconscious manner, is one form of racism highlighted in the AAP’s statement on the impact of racism on child and adolescent health.40 In this policy statement, the AAP highlights the negative impact that racism as a social determinant of health has on children, adolescents, and their families.41 In particular, the provision of high-quality healthcare can be impacted by racism and can result in health disparities. Clinicians in the United States demonstrate pro-white implicit bias at the same rate as individuals in the general population. 42 These racial and ethnic implicit biases have been shown to impact patient health outcomes.42–47 Although race is a social construct with no biological significance, 48 as a medical community, healthcare providers continue to ascribe clinical health outcomes to specific racial groups.
While other causes of management variation are challenging to address, acknowledging a possible bias can allow for the construction of system and policy support to limit its effect proactively and is explicitly highlighted as an important action item in the AAP’s racism in healthcare policy statement.41 The AAP goes on to recommend research that focuses on determining the consequences of discrimination on healthcare provision and health outcomes and on reducing implicit bias as a means of improving the quality and safety of healthcare delivery. Ku, et al in 2018 identify that since mortality associated with variations in bronchiolitis care is rare, future quality improvement studies within the ED should focus on making patient-oriented care equitable.15 While further work needs to be done to confirm that the variations along racial and ethnic lines seen in our cohort are due to implicit provider bias, this difference in care still represents an opportunity for reducing unnecessary variation in patient care. Future quality improvement projects may do well to consider incorporating metrics and interventions targeted at limiting health disparities in addition to standardizing practice in general.
Limitations
This study has limitations. Data used was obtained from an administrative and billing dataset that does not contain detailed clinical information about the patient encounter. The visits were identified using diagnosis and procedure codes, which entail the risk of misclassification of patients. The observed differences in odds of resource utilization between NHB and NHW children in our cohort could be due to systematic differences in initial healthcare utilization that may influence subsequent clinical management. The reported APR-DRG within the database may not be representative of actual disease severity. It is unknown how race/ethnicity information is collected within the included Children’s Hospitals. Local variations in how race and ethnicity are elicited and reported within institutions could result in data inconsistencies within PHIS records. Reported race and ethnicity are also not inclusive and represent gross generalizations whereby diverse communities may be lumped together, ignoring subsets of populations. Finally, this study was limited to free-standing Children’s hospitals, which may not represent other ED and inpatient settings.
Conclusion
Among children seen in ED and inpatient settings for bronchiolitis, NHB children more often received corticosteroid and bronchodilator therapies, and NHW children more often received antibiotics. The underlying causes for these disparities in bronchiolitis management remain uncertain. Future prospective studies and quality improvement initiatives should focus on identifying and addressing underlying factors contributing to these health disparities.
Supplementary Material
Funding Sources/Disclosures:
RM, EK, and AH receive support from the Office of the Director of the National Institutes of Health under award UG1OD024953. The remaining authors did not receive funding. Funded by the National Institutes of Health (NIH). There are no financial relationships relevant to this article to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Prior Presentations: None
Conflict of Interest: RM from time to time provides expert review for legal matters. All other authors report no conflicts of interest.
Supplemental Information Linked to the online version of the paper at Wiley-Blackwell:
• Table S1, Table S2
References
- 1.Cangiano G, Nenna R, Frassanito A, et al. Bronchiolitis: Analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol 2016;51(12):1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014;134(5):e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 3.Valet RS, Gebretsadik T, Carroll KN, et al. Increased healthcare resource utilization for acute respiratory illness among Latino infants. J Pediatr 2013;163(4):1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago J, Mansbach JM, Chou SC, et al. Racial/ethnic differences in the presentation and management of severe bronchiolitis. J Hosp Med 2014;9(9):565–572. [DOI] [PubMed] [Google Scholar]
- 5.Bjur KA, Wi CI, Ryu E, et al. Socioeconomic Status, Race/Ethnicity, and Health Disparities in Children and Adolescents in a Mixed Rural-Urban Community-Olmsted County, Minnesota. Mayo Clin Proc 2019;94(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal MK, Johnson TJ, Chamberlain JM, et al. Racial and Ethnic Differences in Antibiotic Use for Viral Illness in Emergency Departments. Pediatrics 2017;140(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009;123(3):1003–1010. [DOI] [PubMed] [Google Scholar]
- 8.Wernroth ML, Fall K, Svennblad B, et al. Early Childhood Antibiotic Treatment for Otitis Media and Other Respiratory Tract Infections Is Associated With Risk of Type 1 Diabetes: A Nationwide Register-Based Study With Sibling Analysis. Diabetes Care 2020;43(5):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disparities | Healthy People 2020 https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities. Published 2021. Accessed.
- 10.Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics 2014;133(3):e730–737. [DOI] [PubMed] [Google Scholar]
- 11.Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics 2014;133(1):e1–7. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki K, Blackshear C, Burns PA, Hobbs CV. Racial/Ethnic Disparity in the Incidence of Bronchiolitis Requiring Hospitalization. Clin Infect Dis 2020. [DOI] [PubMed]
- 13.Bourgeois FT, Monuteaux MC, Stack AM, Neuman MI. Variation in emergency department admission rates in US children’s hospitals. Pediatrics 2014;134(3):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle TP, Macias CG, Wu S, et al. Characterizing Avoidable Transfer Admissions in Infants Hospitalized for Bronchiolitis. Hosp Pediatr 2020. [DOI] [PMC free article] [PubMed]
- 15.Ku BC, Chamberlain JM, Shaw KN. Quality Improvement and Safety in Pediatric Emergency Medicine. Pediatr Clin North Am 2018;65(6):1269–1281. [DOI] [PubMed] [Google Scholar]
- 16.Callahan TJ, Bauck AE, Bertoch D, et al. A Comparison of Data Quality Assessment Checks in Six Data Sharing Networks. eGEMs (Generating Evidence & Methods to improve patient outcomes) 2017;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews C, S LM, Kerns E, McCulloh R, Alverson B. The Association of Seasonality With Resource Use in a Large National Cohort of Infants With Bronchiolitis. Hosp Pediatr 2021;11(2):126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health 2005;95(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hailu A, VanEenwyk J. Guidelines for using rural-urban classification systems for public health assessment. Olympia, WA: Department of Health 2009.
- 21.Howard LM, Thurm C, Dantuluri K, et al. Parenteral Antibiotic Use Among Ambulatory Children in United States Children’s Hospital Emergency Departments. Open Forum Infectious Diseases 2020;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akenroye AT, Thurm CW, Neuman MI, et al. Prevalence and predictors of return visits to pediatric emergency departments. Journal of Hospital Medicine 2014;9(12):779–787. [DOI] [PubMed] [Google Scholar]
- 23.Ralston S, Parikh K, Goodman D. Benchmarking Overuse of Medical Interventions for Bronchiolitis. JAMA Pediatr 2015;169(9):805–806. [DOI] [PubMed] [Google Scholar]
- 24.Hancock DG, Charles-Britton B, Dixon DL, Forsyth KD. The heterogeneity of viral bronchiolitis: A lack of universal consensus definitions. Pediatr Pulmonol 2017;52(9):1234–1240. [DOI] [PubMed] [Google Scholar]
- 25.Dumas O, Hasegawa K, Mansbach JM, Sullivan AF, Piedra PA, Camargo CA. Severe bronchiolitis profiles and risk of recurrent wheeze by age 3 years. Journal of Allergy and Clinical Immunology 2019;143(4):1371–1379.e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jartti T, Mäkelä MJ, Vanto T, Ruuskanen O. The Link Between Bronchiolitis and Asthma. Infectious Disease Clinics of North America 2005;19(3):667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Current Opinion in Allergy & Clinical Immunology 2013;13(2):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asthma prevalence and control characteristics by race/ethnicity--United States, 2002. MMWR Morb Mortal Wkly Rep 2004;53(7):145–148. [PubMed] [Google Scholar]
- 29.Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatric pulmonology 1999;28(6):394–401. [DOI] [PubMed] [Google Scholar]
- 30.Guilbert T, Zeiger RS, Haselkorn T, et al. Racial Disparities in Asthma-Related Health Outcomes in Children with Severe/Difficult-to-Treat Asthma. The Journal of Allergy and Clinical Immunology: In Practice 2019;7(2):568–577. [DOI] [PubMed] [Google Scholar]
- 31.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. Journal of Allergy and Clinical Immunology 2014;134(3):547–553.e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics 2005;115(5):1254–1260. [DOI] [PubMed] [Google Scholar]
- 33.Roberts EM. Racial and ethnic disparities in childhood asthma diagnosis: the role of clinical findings. J Natl Med Assoc 2002;94(4):215–223. [PMC free article] [PubMed] [Google Scholar]
- 34.Zook HG, Payne NR, Puumala SE, Ziegler KM, Kharbanda AB. Racial/Ethnic Variation in Emergency Department Care for Children With Asthma. Pediatr Emerg Care 2019;35(3):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics 2013;131(4):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natale JE, Joseph JG, Rogers AJ, et al. Cranial computed tomography use among children with minor blunt head trauma: association with race/ethnicity. Arch Pediatr Adolesc Med 2012;166(8):732–737. [DOI] [PubMed] [Google Scholar]
- 37.Caperell K, Pitetti R, Cross KP. Race and acute abdominal pain in a pediatric emergency department. Pediatrics 2013;131(6):1098–1106. [DOI] [PubMed] [Google Scholar]
- 38.Conway PH, Edwards S, Stucky ER, Chiang VW, Ottolini MC, Landrigan CP. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics 2006;118(2):441–447. [DOI] [PubMed] [Google Scholar]
- 39.Grech CK, Laux MA, Burrows HL, Macy ML, Pomeranz ES. Pediatric Emergency Department Resource Utilization among Children with Primary Care Clinic Contact in the Preceding 2 Days: A Cross-Sectional Study. J Pediatr 2017;188:245–251.e242. [DOI] [PubMed] [Google Scholar]
- 40.Staats C, Capatosto K, Tenney L, Mamo S. Implicit Bias Review The Ohio State University Kirwan Institute;2017. [Google Scholar]
- 41.Trent M, Dooley DG, Dougé J, HEALTH SOA, PEDIATRICS COC, ADOLESCENCE CO. The Impact of Racism on Child and Adolescent Health. Pediatrics 2019;144(2). [DOI] [PubMed] [Google Scholar]
- 42.Blair IV, Steiner JF, Fairclough DL, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. Ann Fam Med 2013;11(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Ryn M, Burgess DJ, Dovidio JF, et al. THE IMPACT OF RACISM ON CLINICIAN COGNITION, BEHAVIOR, AND CLINICAL DECISION MAKING. Du Bois Rev 2011;8(1):199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013;28(11):1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall WJ, Chapman MV, Lee KM, et al. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am J Public Health 2015;105(12):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health 2012;102(5):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabin JA, Greenwald AG. The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am J Public Health 2012;102(5):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nature Genetics 2004;36(11):S13–S15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


