Abstract
Objectives
Recent evidence suggests that Sort1 promotes carcinogenesis and tumor progression in multiple types of cancers. This study investigates the role of Sort1 in hepatocellular carcinoma (HCC).
Methods
The differentially expressed gene was screened through GEO and TCGA databases. The Sort1 gene was identified and its expression was then verified by TCGA and HCCDB (a database of hepatocellular carcinoma expression atlas) databases. The Human Protein Atlas database was used to assess the gene expression in tissues. The TCGA and KM-plotter databases were used to study the relationship between Sort1 and HCC. The correlation between Sort1 and immune cells was evaluated through the TIMER database. GO and KEGG enrichment analysis was used to investigate the possible mechanism. The role of Sort1 in cell proliferation and invasion of HCC was further explored through in vitro experiments.
Result
The differentially expressed molecule obtained from database screening was Sort1. Its expression was higher in cancer tissues than in paracancerous ones, and it was mainly located in the cytoplasm. The TCGA, KM-plotter databases, and our study data showed that low expression of Sort1 in HCC patients had better overall survival (OS), progression-free survival (PFI), and disease-specific survival (DSS). Further analysis indicated a significant correlation between Sort1 expression and immune cell infiltration. The gene set enrichment analysis (GSEA) analysis showed that Sort1 affected the biological events of HCC by participating in the WNT, TGF-BETA, JAK, STAT, and CALCIUM signaling pathways. In vitro, cytological experiments demonstrated reduced expression of PCNA, Ki-67, Vimentin, N-cadherin, and MMP-9 mRNA after knocking down Sort1, although E-cadherin expression was promoted. Overall, these processes reduced the ability of proliferation and invasion of HCC cells.
Conclusion
Downregulation of Sort1 can prolong the OS, PFI, and DSS of HCC patients. Furthermore, due to its link with immune cell infiltration, the Sort1 gene represents a potentially novel predictive biomarker of HCC. The growth of HCC can be significantly inhibited by interfering with Sort1; therefore, these results provide a potential target for developing anticancer strategies for HCC.
1. Introduction
Hepatocellular carcinoma (HCC) is a type of primary liver cancer that ranks sixth in incidence among all malignant tumors and third in mortality worldwide [1, 2]. The occurrence of hepatocellular carcinoma is a complex process involving multiple genes and steps that are linked to risk factors such as alcohol consumption, aflatoxin, nonalcoholic fatty liver disease, and hepatitis B and C viruses [3, 4]. Diagnosis for HCC usually occurs during the late stages of the condition, resulting in a poor prognosis. At present, HCC is mostly treated by surgery, intrahepatic intervention, targeted therapy, and others. Although these commonly used clinical treatments can prolong the survival time of patients, they have limitations and could not significantly reduce the recurrence and mortality of liver cancer [1, 5]. The occurrence and development of HCC have been found to be linked with a variety of oncogenes, tumor suppressor genes, and signaling pathways, with some examples being the RAS mitogen-activated protein kinase (RAS/RAF/MAPK) and the receptor tyrosine kinase signaling pathways [6]. However, ideal tumor markers that would enable HCC to be diagnosed at an early stage or even to predict prognosis are yet to be available, and the molecular pathogenesis is also being poorly understood. Hence, exploring tumor markers that could assist the early diagnosis and prognosis of HCC would be of great clinical significance.
Sortilin 1 (Sort1) is an important lipid metabolism regulatory gene. In 2010, through a genome-wide association study (GWAS), the Sort1 gene was first proved to be related to the metabolism of low-density lipoprotein, and the gene exists in chromosome lp13.3 in [7]. Located in the trans-Golgi network (TGN), the Sort1 gene is largely involved in the directional transport of various proteins in lysosomes, although part of Sort1 can also occur on the plasma membrane where it is involved in receptor-mediated endocytosis [8]. Transformed cells display rewired metabolism, with an increased rate of lipid synthesis being a key feature of this altered metabolism. In this context, aberrant lipid biosynthesis is involved in cancer migration, invasion, and the induction of tumor angiogenesis [9]. Previous studies have demonstrated the link of abnormal lipid metabolism with tumor occurrence and development. For example, Broadfield et al. [10] showed that fat induces glucose metabolism in nontransformed hepatocytes and promotes liver tumorigenesis; Yang et al. [11] showed that miR-760 negatively drives fat metabolism by targeting c-Myc and exerts an anticancer effect in esophageal squamous cell carcinoma. As the liver is an important organ for lipid metabolism in the human body, it is meaningful to study hepatocellular carcinoma from the perspective of lipid metabolism-related genes.
We screened the TCGA database and found that the Sort1 gene is differentially expressed, which is linked to HCC prognosis. We evaluated the expression of the gene and its role in predicting the survival rate of HCC patients. In addition, the effects of Sort1 on tumor cell behavior and the underlying mechanisms were uncovered through bioinformatics analyses and in vitro experiments.
2. Materials and Methods
2.1. Data Analysis
Using the GEOquery package from the GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi), two RNA expression datasets, GSE84402 and GSE89377 (containing normal and tumor tissues), were downloaded. The probes corresponding to multiple molecules for one probe were removed. When encountering probes corresponding to the same molecule, only the probe with the largest signal value is retained. The differentially expressed genes dataset of hepatocellular carcinoma was then obtained from the TCGA database (https://tcga-data.nci.nih.gov/) before making a Venn diagram based on the intersection of the three datasets. Eventually, differentially expressed genes related to HCC prognosis were identified after applying a ∣log2FC∣ > 1 and a p value < 0.05 as parameters. The result is Sort1.
To investigate how Sort1 and other clinical characteristics, such as age, gender, and disease stage, influenced HCC prognosis, a forest map and both univariate and multivariate Cox regression analyses were applied to display the 95% confidence interval (CI) of each variable, the Hazard Ratio (HR), and the p values. The “Survminer” and “Survival” packages in R (Version 4.0.3) were implemented for analyzing Kaplan–Meier (KM) survival curves, with the latter subsequently generated with the Kaplan–Meier Plotter (https://kmplot.com). Basically, this involved applying the log-rank test for gene expression in liver cancer to produce c curves. The risk score and predictive accuracy of Sort1 were eventually compared by TimeROC analysis [12–14].
The Tumor Immunity Estimation Resource (TIMER) (https://cistrome.shinyapps.io/Timer) was used to determine how immune cell infiltration in HCC patients was related to the transcription level of Sort1. In addition, differentially expressed genes related to Sort1 gene transcription were analyzed using LinkedOmics (https://www.linkedomics.org/login.php) functional module.
2.2. Cell Lines
This study used five hepatocellular carcinoma cell lines (Huh7, HepG2, Hep3B, LO2, and MHCC97H) obtained from the Shanghai Chinese Academy of Sciences Cell Bank.
2.3. Reverse Transcription Quantitative PCR Method to Detect Sort1-Encoding mRNA
After grinding 100 mg of tumor and paracancerous tissues, obtained from the patients pathologically diagnosed with primary liver cancer included in the study, total RNA was extracted with 1 ml of Trizol lysis solution. From the resulting RNA, a corresponding cDNA was synthesized with the reverse transcription kit before performing real-time fluorescence quantitative PCR. For this purpose, the following primers were used for Sort1: forward: 5′-CAGTCCAAGCTATATCGAAG TGAGG-3′; reverse: 5′-AAGATGGTGTTGTCTG ATCCCCATTT-3′; β-actin, 5′-AGCCTCGCCT TTGCCGA-3′ and 5′-CTGGTGCCTGGGG CG-3′ were selected as forward and reverse primers, respectively. Finally, the relative expression of the targeted genes was determined based on the 2−△△Ct method to obtain the transcription level of Sort1 in both sets of tissues.
2.4. In Vitro Cytological Experiments
2.4.1. CCK Assay for Cell Viability
The Cell Counting Kit-8 Assay (CCK8) was used as specified by the manufacturer to quantify cell proliferation. After seeding 1500 cells into a 96-well plate, the CCK-8 solution was added to each well on the following day. This was followed by a 4 h incubation under 5% CO2 at 37°C before recording light absorbance values at 450 nm with a microplate reader (BioRad). The experiment was repeated three times to obtain the mean values of the three experiments.
2.4.2. Colony Formation Assay
For this assay, after seeding the cells (500 cells/well) into 6-well culture plates, the cells were gently shaken prior to incubation for 10 days at 37°C and 5% CO2. After incubation, removal of the medium was followed by cell staining using 0.1% crystal violet (Sigma, St. Louis, MO). Cells were observed under a microscope and the number of positive colonies (>40 μm in diameter) was counted. The experiment was repeated three times, with the colony-forming ability of each cell type recorded each time.
2.4.3. Transwell Migration Assay
Cell migration was analyzed using Transwell chambers (Bd Biosciences, San Jose, CA, USA). To the upper chamber, 200 ul of serum-free DMEM containing 5 × 104 cells was added, while to the lower one, DMEM containing 10% FBS was added. This was followed by a 24 h incubation, after which invading cells on the underside of the membrane were fixed with methanol before staining using crystal violet (Beyotime). An inverted microscope was used to capture Images, with invading cells counted at three different positions. The experiment was performed in triplicate, and the average value of the three experiments was taken.
2.4.4. Transwell Invasion Assay
In this assay, serum-free DMEM at 4°C was used to dilute Matrigel (BD Biosciences, San Jose, USA) 1 : 8 before using the mixture with 50 μl coated polycarbonate filters (8 μm; Corning, NY, USA). After overnight incubation at 37°C, 5 × 105cells, in 200 μl of serum-free DMEM, were seeded into the upper chamber, while to the lower one, 500 μl of DMEM, supplemented with 10% FBS, was added. The cells were allowed to grow under 5% CO2 at 37°C, and after 24 h, paraformaldehyde was used to fix the upper chamber prior to staining using 0.5% crystal violet. Eventually, noninvading cells were removed and surface cells were counted under a microscope.
2.5. Western Blot Detection
PBS at 4°C was used to wash the cells twice before performing cell lysis in cold RIPA buffer to which protease inhibitors had been added. The extracted proteins had their concentrations determined using the BCA protein assay kit (Pierce, Rockford, IL, USA) before denaturing. Proteins were separated in 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membrane blocking was first carried out at room temperature for 1 h using 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST). After overnight incubation at 4°C with the primary antibody, the membranes were washed three times with TBST before a second 1 h incubation at room temperature with the secondary antibody (anti-rabbit IgG). TBST was finally used to wash the membrane three times before visualizing the target protein using an ECL reagent (EMD Millipore, MA, USA).
2.6. Statistical Analysis
For statistical analyses, SPSS (version 24.0; SPSS, Inc., Chicago, IL, USA) and R (version 4.0.1; https://www.r-project.org/) were used. Results for continuous data were first expressed as the mean ± standard deviation, with the significance of differences in means assessed by Student's t-tests. Differences in Sort1 expression between normal and tumor tissues were determined by Wilcoxon's tests, with the Kruskal–Wallis test also used to assess the association of clinical stage and Sort1 expression. Kaplan–Meier curves were used to determine survival outcomes, and correlations were evaluated based on Spearman's correlation coefficient. For all tests, comparisons were considered to be statistically significant at p < 0.05.
3. Results
3.1. Sort1 Expression Was Elevated in Hepatocellular Carcinoma
We first obtained the differentially expressed gene Sort1 through Venn diagram analysis between the two datasets, GSE84402 and GSE89377, and the TCGA database (Figure 1(a)). At the same time, it was found by pan-cancer analysis that the Sort1 gene has high expression and low expression in all tumors (Figure 1(b)). Searches made on the TCGA database indicated that Sort1 was upregulated in HCC tumor tissues compared with paracancerous ones (Figures 1(c) and 1(d)), with similar differences in mRNA levels found after 12 HCC research cohorts from the HCCDB database were analyzed (Figures 1(e) and 1(f)).
Figure 1.
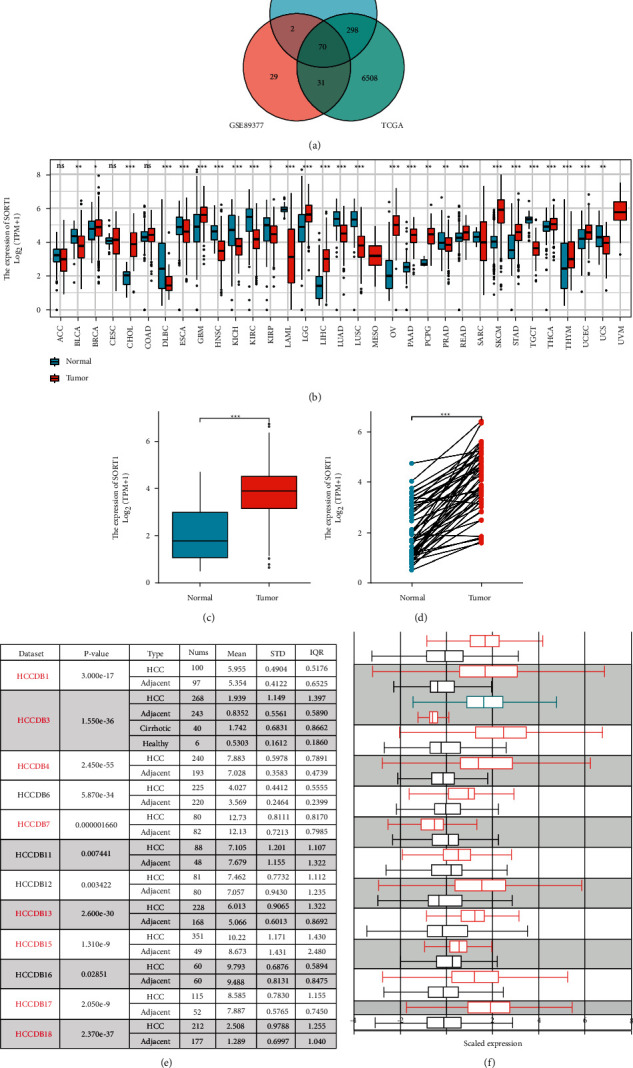
Expression of Sort1 was elevated in hepatocellular carcinoma. (a) Venn diagrams of genes from the three datasets. (b) Sort1 expression in pan-cancer analysis. (c) Sort1 expression in tumor tissues was high for unpaired HCC (n = 398) and paracancerous tissues (n = 50) in the TCGA database. (d) Sort1 expression in tumor tissues was high for paired hepatocellular carcinoma (n = 50) and paracancerous tissues (n = 50) in the TCGA database. (e, f) For HCCDB, significantly higher Sort1 transcription was observed in HCC tissues as opposed to adjacent normal ones. Differences were statistically significant at ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.2. Expression of Sort1 at Tissue Level in the Human Protein Atlas Database
After analyzing the Human Protein Atlas database to determine Sort1 expression, it was observed that the gene was mostly located in the cell cytoplasm (Figures 2(a) and 2(c)). The results of immunohistochemistry further indicated that Sort1 was more expressed in the cancer tissues, especially in poorly differentiated tumors, compared with the paracancerous ones.
Figure 2.
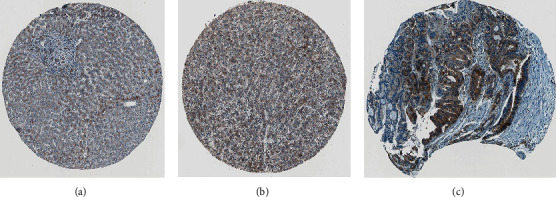
Expression of Sort1 at the tissue level in the Human Protein Atlas database. Immunohistochemical staining of (a) Sort1 in normal tissues; (b) low Sort1 expression in HCC tissues; and (c) Sort1 expression in HCC tissues.
3.3. Assessing the Prognostic Value of Sort1 in HCC
To investigate the association of Sort1 expression and clinical data (age, pathology classification (pTNM, including pT, pN, and pM stages), tumor grades, AFP, albumin level, and presence or absence of vascular invasion) and OS in HCC patients, univariate and multivariate Cox regression analyses were used. A significant association between pM stage (p value = 0.017), pT stage (p.value < 0.001), Sort1 expression (p value = 0.048), and OS was found based on univariate Cox analysis. Multivariate analysis also highlighted the significance of Sort1 expression (p value = 0.008), indicating that Sort1 could be a prognostic factor for HCC (Figure 3(a)). On stratifying clinical factors using Kaplan–Meier (KM) plots, it was observed that low Sort1 expression was a better prognostic factor. These results were supported by previous reports that Sort1 is an “oncogene” in HCC (Figure 3(b)). Sort1 expression was also related to survival outcomes of the HCC cohort based on the Kaplan–Meier plotter's liver cancer RNA-seq database and plotted Kaplan–Meier survival curves as in this case. High Sort1 expression had poorer OS, PFI, and DSS than those with low expression. In particular, OS and PFI had statistical significance (p < 0.05) (Figure 3(c)). In addition, as AFP, pT, pN, and pM stages of HCC increased, the Sort1 expression decreased (Figure 3(d)), suggesting that Sort1 could potentially act as a biomarker for HCC disease progression. Furthermore, risk score and the predictive accuracy of ASF1B were compared by ROC analysis. The results showed that Sort1 expression can predict the 1-year, 3-year, and 5-year survival. The AUC under the ROC curve was 0.679, 0.563, and 0.558, respectively (Figure 3(e)). These findings indicate that Sort1 has a predictive role for the prognosis of HCC.
Figure 3.
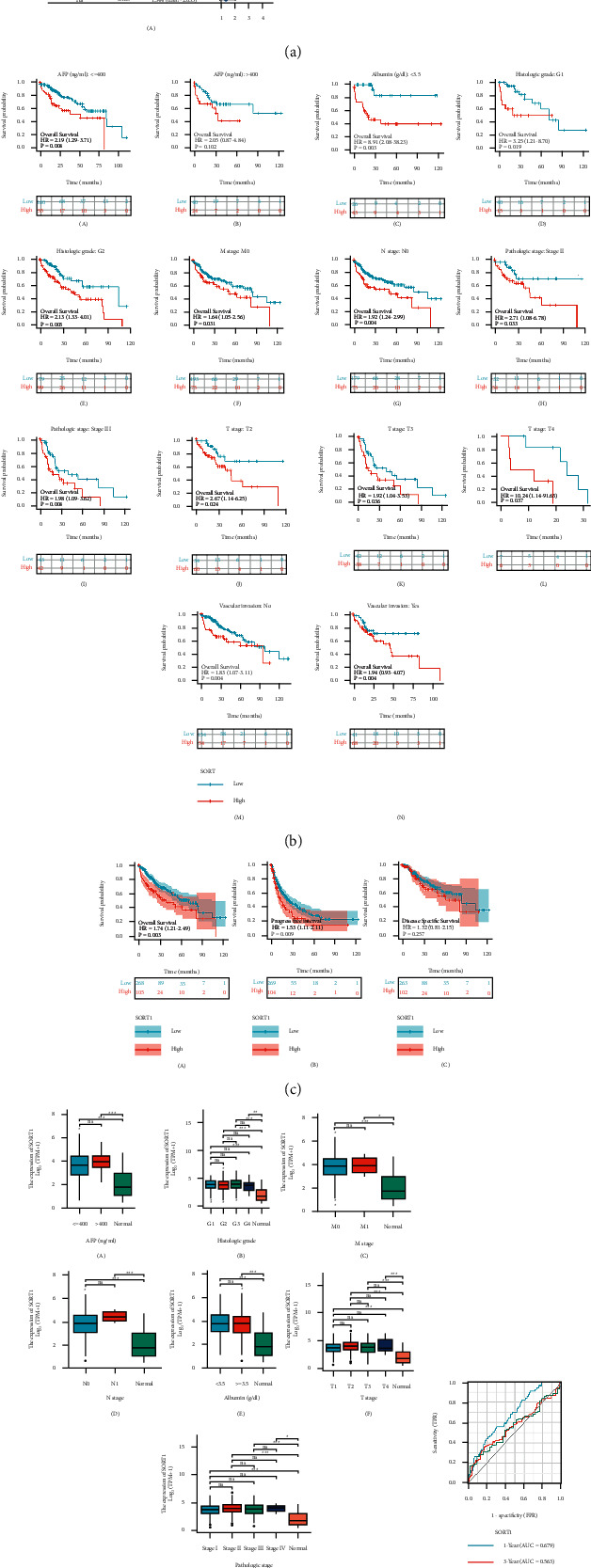
Poor survival was predicted for hepatocellular carcinoma patients with high Sort1 expression. (a) Forest plot analysis of risk factors for HCC overall survival. (b) The relationship between OS and Sort1 expression for different groups of HCC patients was stratified using the Kaplan–Meier curve. (c) Overall survival (OS), progression-free survival (PFI), and disease-specific survival (DSS) in relation to Sort1 expression in HCC patients. (d) Sort1 expression was correlated with AFP level, tumor stage, and albumin level in hepatocellular carcinoma. (e) Transient ROC analysis of Sort1 expression in HCC. “ns” means p < 0.05; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
3.4. Sort1 Expression and Immune Cells Infiltration Based on the TIMER Database
In order to assess the association of Sort1 expression and different types of immune cell infiltration in HCC, bar graphs from the TIMER database were constructed. Overall, the gene's expression was found to be positively correlated with some of the most infiltrating immune cells, such as mast cells, Th1 cells, Th2 cells, NK CD56 bright cells, macrophages, and T helper cells (Figure 4(a)). The influence of Sort1 on the tumor microenvironment (TME) was further assessed by determining the relationship between specific immune cells and Sort1. The results showed a positive correlation between the gene and the infiltration levels of T helper cells, NK CD56 bright cells, and macrophages. However, a negative correlation was observed with the infiltration levels of DCs, cytotoxic T cells, NK CD56dim cells, Tgd, and pDCs (Figure 4(b)). Furthermore, the results also indicated good correlations between Sort1 expression and molecules such as PDCD1, CD274, and CTLA-4 that are involved in immune checkpoints (Figure 4(c)). Altogether, these results suggest a certain correlation between Sort1 expression and immune cell infiltration, with the tumor microenvironment of HCC likely to be involved in allowing cancer cells to evade the immune system. These findings can form the basis of future research.
Figure 4.
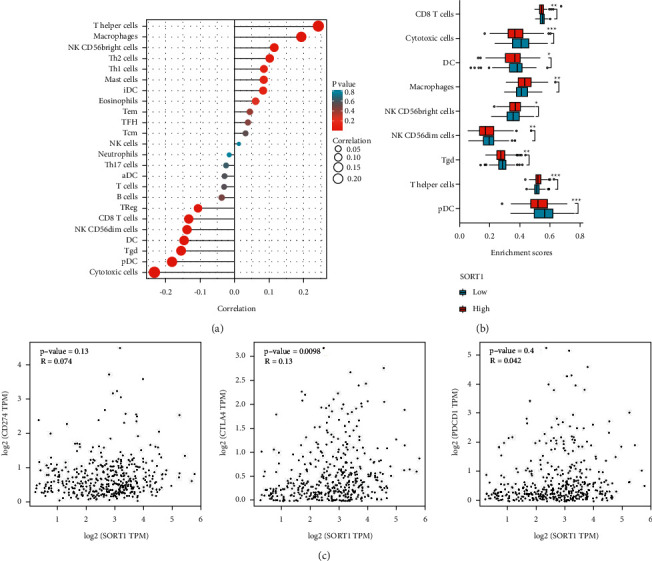
Sort1 expression and immune cell infiltration based on the TIMER database. (a) Positive correlation between immune cells infiltration and Sort1 in the TIMER database. (b) Significant correlation between infiltration of immune cells in HCC and Sort1 expression. (c) Scatter plot showing Sort1 correlation with CTLA-4, CD274, and PDCD1.
3.5. The Coexpression Network of Sort1 Suggests a Potential Function of Sort1 in HCC
The coexpression network of Sort1 in the hepatocellular carcinoma (HCC) group was analyzed using LinkedOmics to determine the biological significance of the gene. Genes that were positively or negatively correlated with Sort1 expression were shown in the heatmap (Figure 5(a)). By analyzing these genes, it was found that Sort1 is associated with the upregulation of HCC risk factors while downregulating those that protect against HCC. In addition, Sort1 is involved in HCC occurrence and development. Gene set enrichment analysis (GSEA) further showed that Sort1 could affect HCC prognosis by influencing the WNT, TGF-BETA, JAK, STAT, and CALCIUM signaling pathways (Figure 5(b)).
Figure 5.
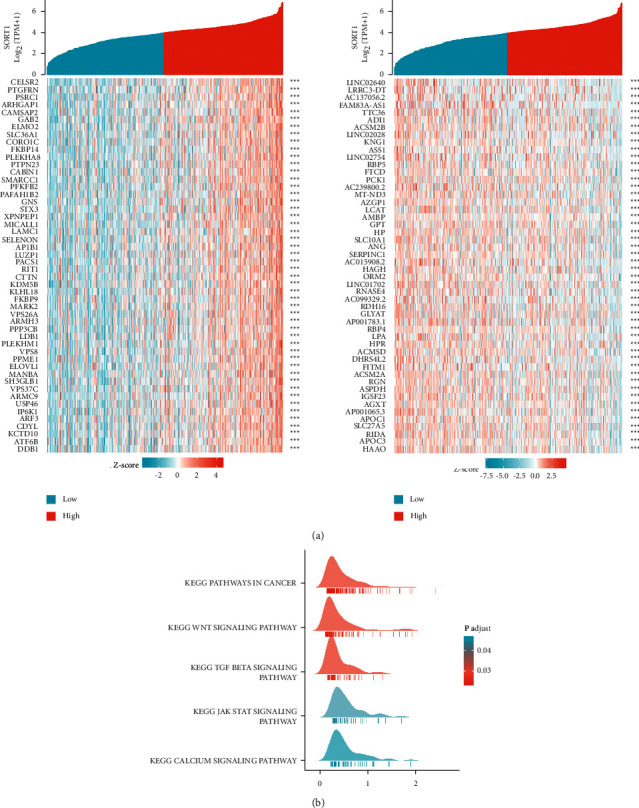
Analysis of genes coexpressed with Sort1 (LinkedOmics). (a) Cotranscribed genes that were upregulated or downregulated by Sort1. (b) Ridge plot of GSEA analysis.
3.6. Effects of Sort1 Knockdown on Proliferation and Clonogenic Ability of HCC Cells
Fluorescence quantitative PCR was used to assess Sort1 expression levels in HCC cell lines and human normal hepatocytes (THLE-2) cells. The expression levels were significantly increased in HCC cell lines (MHCC97H, LO2, Hep3B, HepG2, and Huh7), with the highest expression level being in HepG2 cells (Figure 6(a)). Therefore, in the subsequent knockdown experiments, HepG2 cells were selected. HepG2 was transfected with a lentiviral interference vector (shRNA-Sort1) targeting Sort1. The results of quantitative fluorescence PCR showed that the transfection of shRNA-Sort1 could significantly reduce Sort1 expression compared with the control group (Control-shRNA) (Figure 6(b)). Next, the effects of Sort1 knockdown on the proliferation of hepatoma cells HepG2 were investigated using the CCK-8 assay. In this case, the results indicated the inhibition of HepG2 proliferation after transfection of shRNA-Sort1 (3 days) compared with the control (Control-shRNA). After 4 days of culture, knockdown of Sort1 inhibited the proliferation ability of HepG2 cells more significantly (Figure 6(c)). Finally, based on the colony formation experiment, it was observed that the transfection of shRNA-Sort1 resulted in a significant inhibition in the ability of HepG2 cells to form colonies, especially in comparison to the control (Control-shRNA) (Figure 6(d)).
Figure 6.
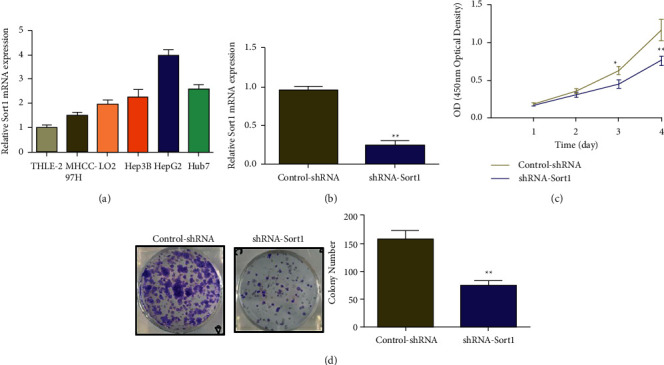
Knocking down Sort1 significantly inhibited proliferation and colony formation in HCC cells. (a) Sort1 expression levels in HCC cell lines and human normal hepatocytes (THLE-2) determined by real-time PCR. (b) Effects of shRNA-Sort1 transfection on Sort1 expression in HepG2 cells determined by fluorescent quantitative PCR. (c) Effect of Sort1 knockdown on HepG2 proliferation determined by CCK-8 assay. (d) Effects of Sort1 knockdown on the clonogenic ability of HepG2 cells based on the colony formation assay. ∗p < 0.05 and ∗∗p < 0.01.
3.7. Effects of Sort1 Knockdown on the Invasion and Migration of HCC Cells
The high mortality of liver cancer can be attributed to metastasis, especially in the advanced stage of liver cancer. Therefore, Transwell was used to determine how Sort1 influenced the ability of cell invasion and migration. It was found that compared with the control (Control-shRNA), a significant reduction in the migration and invasion abilities of HepG2 cells occurred after transfection with shRNA-Sort1 (Figure 7).
Figure 7.
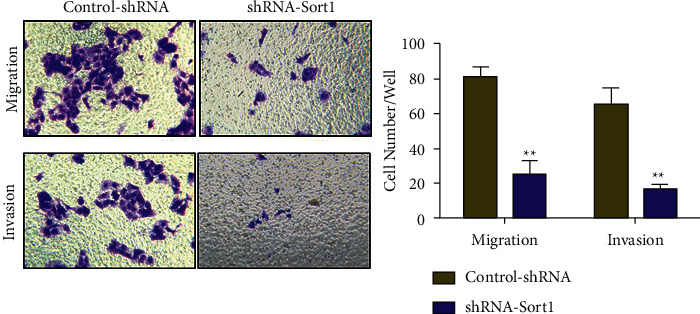
HepG2's invasion and migration abilities were significantly inhibited after Sort1 knockdown. ∗∗p < 0.01.
3.8. Effects of Sort1 Knockdown on the Expression of Molecules Related to Cell Proliferation and Invasion
Following the above results, real-time quantitative PCR was applied to examine further the underlying mechanism through which Sort1 knockdown inhibited the cell proliferation and invasion. The results indicated that, in comparison with the control (Control-shRNA), knocking down Sort1 inhibited the proliferation and invasion of HCC cells and the resulting low Sort1 also significantly reduced the mRNA levels of intracellular Ki-67 and PCNA (Proliferating Cell Nuclear Antigen) (Figure 8(a)). In addition, as opposed to the control (Control-shRNA), Sort1 knockdown significantly promoted E-cadherin expression while inhibiting those of MMP-9 mRNA, Vimentin, and N-cadherin. However, the gene knockdown did not affect MMP-2 expression (Figure 8(b)). Thus, the results suggested that the expression of molecules related to invasion and proliferation were significantly inhibited by Sort1 knockdown.
Figure 8.
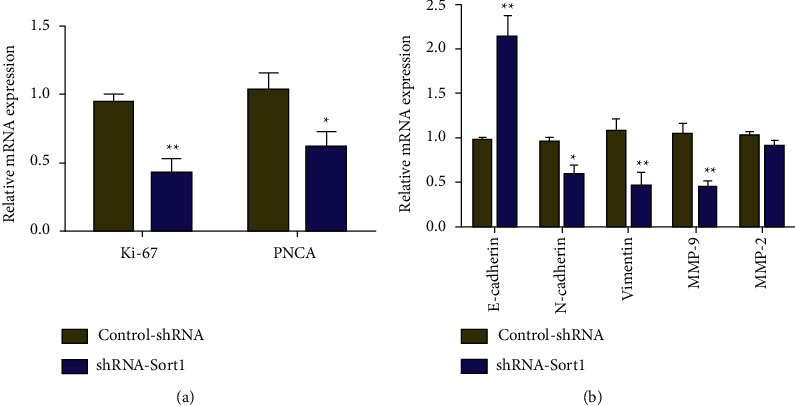
The expression of genes related to proliferation and invasion of HepG2 cells was significantly inhibited by Sort1 knockdown. ∗p < 0.05 and ∗∗p < 0.01.
4. Discussion
HCC, as a common primary liver cancer, has increased in prevalence in recent years [15]. Currently, hepatectomy, liver transplantation, and local ablation remain the most effective curative methods, but HCC patients still have a low 5-year survival rate [16, 17]. In fact, by the time they are diagnosed, many HCC patients already reach the middle and advanced stages and often have severe liver cirrhosis, thus making them unsuitable for surgical resection or liver transplantation [18]. In contrast, the more popular immunotherapy can reverse the immune escape of tumors by inhibiting or activating certain immune checkpoints [19, 20]. Relevant clinical studies have confirmed that immune checkpoint therapy is an effective means of treating tumors [21, 22]. Therefore, finding new sensitive molecular markers and therapeutic targets is crucial for improving the prognosis of HCC patients.
As sequencing and omics technologies developed, it became possible to better understand the mechanism of HCC and identify target genes that are of potential diagnostic and therapeutic value [23]. In our study, we screened HCC genes from GEO and TCGA data and identified the differentially expressed molecule Sort1 from the differential genes. Previous studies have shown that Sort1 acts as an oncogene that is linked with poor prognosis in gastric [24], prostate [25], and colorectal cancers [26], but there is no relevant study on a similar mechanism in HCC. We verified the ability of reduced Sort1 expression in inhibiting HCC proliferation and invasion, with the findings expected to reflect the potential importance of using Sort1 to assess the prognosis of HCC.
We obtained the common upregulated differentially expressed gene Sort1 by taking the intersection of the three datasets obtained from the GEO and TCGA data and presented them in Venn diagrams. By analyzing Sort1 expression based on multiple databases and bioinformatics analyses, it was observed that most cancers, including liver cancer, abnormally expressed this gene. This result was supported by existing literature [27–29]. RNA-seq data in TCGA and corresponding clinical data were analyzed to further determine how Sort1 and HCC were related. In this case, Cox regression analysis demonstrated that Sort1 could represent a risk factor for HCC prognosis, with high expression of the gene being linked to poor prognosis. The Sort1 expression was associated with the progression of tumor T stage and overall disease progression.
Currently, there is an increasing interest in immunotherapy in the treatment of HCC. Previous studies have shown that tumor-infiltrating lymphocytes independently predict the status of sentinel lymph nodes and the survival of cancer patients [30, 31]. By mining public data, it was found that Sort1 expression was correlated with immune cell infiltration, with a positive correlation with most infiltrating immune cells such as mast cells, Th1 cells, Th2 cells, NK CD56 bright cells, macrophages, and T helper cells among others. In contrast, a negative correlation with the infiltration levels of DC, cytotoxic T cells, NK CD56dim cells, Tgd, and pDC were observed. Furthermore, molecules such as CTLA-4, CD274, and PDCD1 that are involved in immune checkpoints were also correlated with Sort1 expression. Thus, by highlighting the significant relationship between immune cell infiltration and Sort1 expression, the results suggest not only that Sort1 is involved in the HCC tumor microenvironment but also that this process could be important for allowing tumor cells to evade the immune system.
When analyzing genes that were significantly associated with Sort1 expression in HCC, it was observed that these genes were also abnormally expressed, with most of them being linked with the overall survival of HCC cells. It is quite likely that Sort1 interacts with these genes to establish a regulatory network that eventually promotes HCC occurrence and development. Gene set enrichment analysis (GSEA) further revealed that Sort1 could be involved in the WNT, TGF-BETA, JAK, STAT, and CALCIUM signaling pathways, resulting in the different prognosis of HCC, which are also associated with the high proliferation of HCC. The pathological features are consistent with other hyperproliferative cancers [32].
Predicting outcomes and discovering key factors in the biological mechanisms leading to adverse outcomes are two important parts of cancer research [33]. Based on the above research results, we found that Sort1 may be involved in the poor prognosis of HCC through a certain pathway mechanism. Therefore, we further verified the expression of Sort1 in HCC through cytological experiments. It was found that Sort1 expression was higher in liver cancer cell lines (Huh7, HepG2, Hep3B, LO2, and MHCC97H) than that on normal human hepatocytes (THLE-2), with the highest expression level occurring in HepG2 cells. Hence, Sort1 was knocked down in HepG2 cells, which reduced the proliferation and invasion of the cells, suggesting that this gene is important to maintain tumorigenic activity in vitro. The expression of Ki-67, PCNA, N-cadherin, E-cadherin, Vimentin, and MMP-9 mRNA was assessed by real-time PCR to investigate the underlying mechanism behind HCC suppression after Sort1 knockdown. Ki-67 is a proliferating cell-associated nuclear antigen. Previous studies have shown that Ki-67 expression and tumor lymph node metastasis are two independent prognostic factors for disease-free survival and overall survival in HCC patients, which may help decision-making of adjuvant therapy [34]. PCNA is an important factor representing DNA replication [35]. Gramantieri et al. [36] suggested that in human hepatocellular carcinoma with cirrhosis, cell proliferation involving P21 during DNA repair depends on PCNA. Gan et al. [37] found that RARγ-induced downregulation of E-cadherin induced HCC cells to invade and metastasize, and tumor metastasis and poor surgical outcome were linked with reduced expression of N-cadherin in cancer cells [38]. Similarly, in HCC, Huang et al. [39] found that CMTM6 interacted with and stabilized vimentin to promote migration, invasion, and Epithelial-Mesenchymal Transition (EMT). Finally, as complex matrix metalloproteinases, MMP-9 is involved in tumor cell invasion and metastasis by degrading extracellular matrix (ECM) components [40]. In this study, knocking down of Sort1 significantly promoted E-cadherin expression and suppressed the mRNA levels of Ki-67, PCNA, N-cadherin, Vimentin, and MMP-9 mRNA. Based on the results, it is likely that Sort1 is involved in various pathological events of HCC through its ability to regulate cell proliferation and invasion.
5. Conclusion
In conclusion, this study provides various types of evidence to support the significance of Sort1 in HCC development, especially in its value as a potential biomarker of HCC progression. Interfering with Sort1 significantly inhibited HCC growth by influencing the ability of cells to proliferate and invade. This study provides a potential target for developing anticancer strategies against HCC.
Acknowledgments
This study was supported by the Lianyungang Haiyan Project (No. 2021-ZD-001) and Doctor of Innovation and Entrepreneurship in Jiangsu Province (JSSCBS20211608).
Data Availability
The data are available from the corresponding author upon request via e-mail (yuancla@163.com).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Anwanwan D., Singh S. K., Singh S., Saikam V., Singh R. Challenges in liver cancer and possible treatment approaches. Biochimica et Biophysica Acta (BBA)—Reviews on Cancer . 2020;1873(1) doi: 10.1016/j.bbcan.2019.188314.188314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Rowe J., Ghouri Y., Mian I. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. Journal of Carcinogenesis . 2017;16 doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alqahtani A., Khan Z., Alloghbi A., Said Ahmed T., Ashraf M., Hammouda D. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina . 2019;55(9) doi: 10.3390/medicina55090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labgaa I., Taffé P., Martin D., et al. Comparison of partial hepatectomy and transarterial chemoembolization in intermediate-stage hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer . 2020;9(2):138–147. doi: 10.1159/000505093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimri M., Satyanarayana A. Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers . 2020;12(2) doi: 10.3390/cancers12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musunuru K., Strong A., Frank-Kamenetsky M., et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature . 2010;466 doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafsen C., Kjolby M., Nyegaard M., et al. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metabolism . 2014;19(2):310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Baenke F., Peck B., Miess H., Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Disease Models and Mechanisms . 2013;6 doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broadfield L. A., Duarte J. A. G., Schmieder R., et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis. Cancer Research . 2021;81(8) doi: 10.1158/0008-5472.CAN-20-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Zhang C., Tie H., Luo J., Wang Y., Wu Q. miR‐760 exerts an antioncogenic effect in esophageal squamous cell carcinoma by negatively driving fat metabolism via targeting c‐Myc. Journal of Cellular Biochemistry . 2020;121(4):2950–2961. doi: 10.1002/jcb.29540. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z., Zhang Y., Dang Q., et al. Genomic alteration characterization in colorectal cancer identifies a prognostic and metastasis biomarker: fam83A|Ido1. Frontiers in Oncology . 2021;11 doi: 10.3389/fonc.2021.632430.632430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Liu L., Guo C., et al. Tumor suppressor gene mutations correlate with prognosis and immunotherapy benefit in hepatocellular carcinoma. International Immunopharmacology . 2021;101 doi: 10.1016/j.intimp.2021.108340.108340 [DOI] [PubMed] [Google Scholar]
- 14.Neumann J., Heinemann V., Engel J., Kirchner T., Stintzing S. The prognostic impact of CDX2 correlates with the underlying mismatch repair status and BRAF mutational status but not with distant metastasis in colorectal cancer. Virchows Archiv . 2018;473(2):199–207. doi: 10.1007/s00428-018-2360-y. [DOI] [PubMed] [Google Scholar]
- 15.Maluccio M., Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA: A Cancer Journal for Clinicians . 2012;62(6):394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y., Han Q., Zhao H., Zhang J. The mechanisms of HBV-induced hepatocellular carcinoma. Journal of Hepatocellular Carcinoma . 2021;8:435–450. doi: 10.2147/JHC.S307962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Wu Y.-S., Chen D., Lin H. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Management and Research . 2019;11:5711–5724. doi: 10.2147/CMAR.S189777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woeste M. R., Geller A. E., Martin R. C. G., Polk H. C. Optimizing the combination of immunotherapy and trans-arterial locoregional therapy for stages B and C hepatocellular cancer. Annals of Surgical Oncology . 2021;28(3):1499–1510. doi: 10.1245/s10434-020-09414-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Kim B.-H., Lee D., Shin E. Genomic alterations in signet ring and mucinous patterned colorectal carcinoma. Pathology, Research and Practice . 2019;215(10) doi: 10.1016/j.prp.2019.152566.152566 [DOI] [PubMed] [Google Scholar]
- 20.Inthagard J., Edwards J., Roseweir A. K. Immunotherapy: enhancing the efficacy of this promising therapeutic in multiple cancers. Clinical Science . 2019;133(2):181–193. doi: 10.1042/CS20181003. [DOI] [PubMed] [Google Scholar]
- 21.Reyes S. J., González K. B., Rodríguez C., et al. Actualización general de inmunoterapia en cáncer. Revista Medica de Chile . 2020;148(7):970–982. doi: 10.4067/S0034-98872020000700970. [DOI] [PubMed] [Google Scholar]
- 22.Abu Eid R. Editorial: advances in head and neck cancer immunology and immunotherapy. Frontiers in Oncology . 2019;9 doi: 10.3389/fonc.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhanasekaran R., Nault J.-C., Roberts L. R., Zucman-Rossi J. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology . 2019;156(2):492–509. doi: 10.1053/j.gastro.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang M., Yao W., Shi B., et al. Circular RNA hsa_circ_0110389 promotes gastric cancer progression through upregulating SORT1 via sponging miR-127-5p and miR-136-5p. Cell Death and Disease . 2021;12(7):p. 639. doi: 10.1038/s41419-021-03903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson I. R. D., Parkinson-Lawrence E. J., Keegan H., et al. Endosomal gene expression: a new indicator for prostate cancer patient prognosis? Oncotarget . 2015;6(35):37919–37929. doi: 10.18632/oncotarget.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blondy S., Talbot H., Saada S., et al. Overexpression of sortilin is associated with 5‐FU resistance and poor prognosis in colorectal cancer. Journal of Cellular and Molecular Medicine . 2021;25(1):47–60. doi: 10.1111/jcmm.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smirnov D. A., Foulk B. W., Doyle G. V., Connelly M. C., Terstappen L. W. M. M., O’Hara S. M. Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Research . 2006;66(6):2918–2922. doi: 10.1158/0008-5472.CAN-05-4003. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Huang J., Jiang M., Chen Q., Jiang Z., Feng H. CAMK1 phosphoinositide signal-mediated protein sorting and transport network in human hepatocellular carcinoma (HCC) by biocomputation. Cell Biochemistry and Biophysics . 2014;70(2):1011–1016. doi: 10.1007/s12013-014-0011-8. [DOI] [PubMed] [Google Scholar]
- 29.Xiao D., Wu J., Zhao H., Jiang X., Nie C. RPP25 as a prognostic-related biomarker that correlates with tumor metabolism in glioblastoma. Frontiers in Oncology . 2022;11 doi: 10.3389/fonc.2021.714904.714904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azimi F., Scolyer R. A., Rumcheva P., et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. Journal of Clinical Oncology . 2012;30(21):2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 31.Leone V., Ali A., Weber A., Tschaharganeh D. F., Heikenwalder M. Liver inflammation and hepatobiliary cancers. Trends in Cancer . 2021;7(7):606–623. doi: 10.1016/j.trecan.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Couri T., Pillai A. Goals and targets for personalized therapy for HCC. Hepatology International . 2019;13(2):125–137. doi: 10.1007/s12072-018-9919-1. [DOI] [PubMed] [Google Scholar]
- 33.Quan Y., Xu M., Cui P., Ye M., Zhuang B., Min Z. Grainyhead-like 2 promotes tumor growth and is associated with poor prognosis in colorectal cancer. Journal of Cancer . 2015;6(4):342–350. doi: 10.7150/jca.10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King K. L., Hwang J. J., Chau G. Y., et al. Ki‐67 expression as a prognostic marker in patients with hepatocellular carcinoma. Journal of Gastroenterology and Hepatology . 1998;13(3):273–279. doi: 10.1111/j.1440-1746.1998.01555.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang S.-C. PCNA: a silent housekeeper or a potential therapeutic target? Trends in Pharmacological Sciences . 2014;35(4):178–186. doi: 10.1016/j.tips.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Gramantieri L., Trerè D., Chieco P., et al. In human hepatocellular carcinoma in cirrhosis proliferating cell nuclear antigen (PCNA) is involved in cell proliferation and cooperates with P21 in DNA repair. Journal of Hepatology . 2003;39(6):997–1003. doi: 10.1016/s0168-8278(03)00458-6. [DOI] [PubMed] [Google Scholar]
- 37.Gan W.-J., Wang J.-R., Zhu X.-L., et al. RARγ-induced E-cadherin downregulation promotes hepatocellular carcinoma invasion and metastasis. Journal of Experimental and Clinical Cancer Research . 2016;35(1):p. 164. doi: 10.1186/s13046-016-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan D.-Q., Wei S., Liu C., et al. Reduced N-cadherin expression is associated with metastatic potential and poor surgical outcomes of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology . 2012;27(1):173–180. doi: 10.1111/j.1440-1746.2011.06847.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang X., Xiang L., Wang B., et al. CMTM6 promotes migration, invasion, and EMT by interacting with and stabilizing vimentin in hepatocellular carcinoma cells. Journal of Translational Medicine . 2021;19(1):p. 120. doi: 10.1186/s12967-021-02787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakamoto Y., Mafune K., Mori M., et al. Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. International Journal of Oncology . 2000;17:237–243. doi: 10.3892/ijo.17.2.237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon request via e-mail (yuancla@163.com).


