Abstract
The ketogenic diet (KD) is a low-carbohydrate, high-fat and adequate-protein diet. As a diet mimicking fasting, it triggers the production of ketone bodies (KBs) and brings the body into a state of ketosis. Recent and accumulating studies on humans and animal models have shown that KD is beneficial to neurodegenerative diseases through modulating central and peripheral metabolism, mitochondrial function, inflammation, oxidative stress, autophagy, and the gut microbiome. Complicated interplay of metabolism, gut microbiome, and other mechanisms can regulate neuroinflammation in neurodegenerative diseases by activating multiple molecular and cellular pathways. In this review, we detail the physiological basis of the KD, its functions in regulating neuroinflammation, and its protective role in normal brain aging and neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD). We aimed to elucidate the underlying neuroinflammatory mechanisms of KD therapies in neurodegenerative diseases and provide novel insights into their application for neurodegenerative disease prevention and treatment.
Keywords: ketogenic diet, ketone bodies, neuroinflammation, neurodegenerative diseases, experimental and clinical evidence
The ketogenic diet (KD) is a high-fat, low-carbohydrate, and moderate protein dietary intervention [1]. As one of the forms of fasting, KD can make the organism substitute fat for carbohydrates as an energy source. During the KD, fatty acids are converted to ketone bodies (KBs) by liver metabolism and then enter the bloodstream to induce nutritional ketosis and participate in subsequent physiological or pathological reactions [2,3]. KD was initially established as an alternative therapy for refractory epilepsy in the 1920s [4]. Adapted versions of the KD are currently widely applied to treat obesity [5], diabetes [6], and age-associated diseases [7]. Considering the safety and accessibility of KD, exogenous ketone supplements (KS) have been developed to mimic the physiological effects of KD on various diseases [8,9].
Neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD), are characterized by progressive and chronic loss of the structure and functions of neuronal materials [10,11]. The etiologies and pathogenesis of neurodegenerative diseases are complicated and varied, and include protein aggregations, genetic mutations, and infections. The discovery of chronic activation of the immune response in the central nervous system (CNS) and elevated levels of inflammatory factors in patients and animal models suggest that neuroinflammation plays a remarkable role in the complex pathogenesis of neurodegenerative diseases [12]. The key pathological hallmarks of neuro-inflammation are activation and proliferation of the major CNS immune cells, microglia and astrocytes, which are accompanied by the regulation and release of inflammatory mediators [13,14]. As there is currently neither a cure nor an effective disease-modifying therapy for most neurodegenerative diseases, developing effective strategies for therapies is crucial. Recently, emerging evidence has underlined both pathophysiological and clinical benefits of KD in neurodegenerative diseases, indicating that KD is a possible treatment option for neurological illnesses [15]. KD can exert protective effects by modulating multiple neuroinflammatory pathways [16,17]. In this review article, we aimed to detail the physiological basis of KD and to summarize the current data regarding the possible neuroinflammatory mechanisms of KD in various neurodegenerative disorders. We also provide an overview of the current experimental and clinical evidence supporting the therapeutic potential of KD for normal brain aging and neurodegenerative disorders specifically targeting neuroinflammation.
Biochemistry and physiological function of KD
Brief history of KD
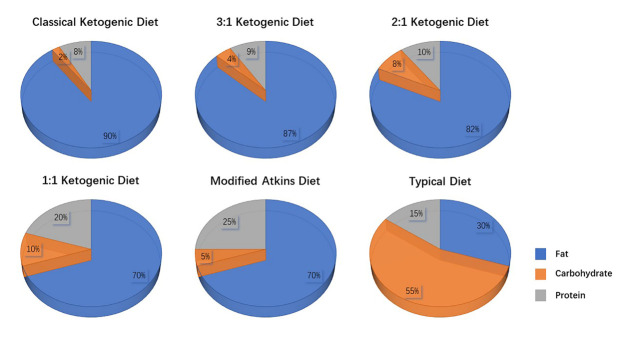
In 1921, Woodyatt et al. found that the levels of KBs, beta-hydroxybutyric acid (βHB), and acetone were elevated in healthy subjects who followed fasting or a low carbohydrate and high-fat diet. Concurrently, Wilder et al. confirmed that a special high-fat and low carbohydrate-based diet contributed to improved seizure control in drug-resistant epilepsy patients. These significant findings might reveal the origin of the classical KD [18]. The prominent features of the classical KD are a high fat content and a low carbohydrate content, with a macronutrient ratio of fat to protein and carbohydrate standing at 4:1. The total daily carbohydrate intake is limited to 20-50 g, which can provide 5%-10% of the total energy intake per day. The carbohydrate restriction inhibits the increase in insulin levels, which in turn promotes the conversion of fatty acids to KBs (βHB, acetoacetate, and acetone) and provides approximately 90% of the dietary energy in the classical KD [1]. The classical KD has been modified to enhance its palatability and flexibility, such as the modified Atkins diet (MAD) [19] and lower ratio KD (3:1, 2:1, and 1:1) [20].Figure 1 shows the macronutrient composition of the classical KD and its common modifications. The ultimate goal of the KD is the production of KBs, and subjects can test the levels of KBs through blood or urine samples to ensure the adequacy of their dietary intervention [21].
Figure 1.
Characteristics of the classical ketogenic diet and its common modifications.
Ketogenesis in the liver
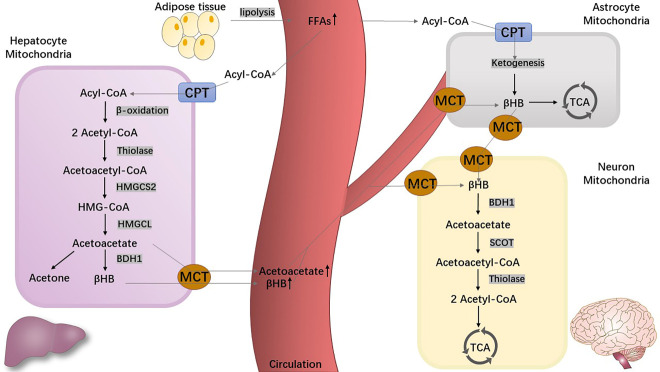
Under normal conditions, humans obtain energy through glycolysis, the conversion of glucose to pyruvate. However, under conditions of nutrient deprivation, such as dietary carbohydrate restriction, fasting, KD, and prolonged physical exercise, the flexible human metabolism triggers ketogenesis and produces KBs as a substitute for glucose (the main metabolic fuel). This metabolic process occurs primarily in the hepatic mitochondrial matrix [22] as well as in extra-hepatic mitochondria (astrocytes and kidneys), albeit to a lesser degree [23]. This metabolic process is depicted inFigure 2. Increasing glucagon and epinephrine levels and decreasing insulin levels induce lipolysis from the adipose tissue and release fatty acids into the bloodstream by enhancing the key enzyme activities in these processes (hormone-sensitive lipase, adipose lipase enzymes, and adipose triglyceride lipase). The free fatty acids (FFAs) are enzymatically converted into fatty acyl-coenzyme A (acyl-CoA) and further transported into hepatocellular mitochondria through the carnitine palmitoyl transferase (CPT) [24,25]. The increasing acetyl-CoA level via β-oxidation in the hepatic mitochondrial matrix is far beyond the metabolic ability of the Krebs cycle, also named the tricarboxylic acid (TCA) cycle, which subsequently initiates ketogenesis [26]. With a chain of biochemical reactions, acetyl-CoA is broken down into acetoacetate by thiolase, 3-hydroxymethylglutaryl-CoA (HMG-CoA) synthase 2 (HMGCS2), and HMG-CoA lyase (HMGCL) [27]. Acetoacetate is reduced to βHB by β-hydroxybutyrate dehydrogenase 1 (BHD1), and finally, both βHB and acetoacetate are delivered to the bloodstream or target organs through monocarboxylate transporters (MCTs) [28]. Additionally, acetone is produced from a small part of acetoacetate through spontaneous decarboxylation and then metabolized in the liver. Under normal physiological conditions, both βHB and acetoacetate are excreted by the kidneys, whereas acetone is excreted by the lungs [29,30].
Figure 2.
The metabolism of ketone bodies. Abbreviations: acetoacetyl-CoA, acetoacetyl-coenzyme A; acetyl-CoA, acetyl-coenzyme A; acyl-CoA, acyl-coenzyme A; βHB, beta-hydroxybutyric acid; BHD1, β-hydroxybutyrate dehydrogenase 1; CPT, carnitine palmitoyl transferase; FFAs, free fatty acids; HMG-CoA, 3-hydroxymethylglutaryl-CoA; HMGCL, 3-hydroxymethylglutaryl-CoA lyase; HMGCS2, 3-hydroxymethylglutaryl-CoA synthase 2; MCTs, monocarboxylate transporters; SCOT, succinyl-CoA-3- ketoacid CoA transferase; TCA, tricarboxylic acid.
Due to the lack of critical enzymes for ketolysis in the liver, βHB and acetoacetate are released into the circulatory system to be metabolized in non-hepatic oxidative tissues, including the brain, skeletal muscle, heart, and kidney. Under healthy conditions, the plasma βHB level is quite low (less than 0.3 mmol/L), whereas the glucose level is high (approximately 4 mmol/L) [7]. Under a state of carbohydrate limitation, a human liver can deliver 150-300 g of KBs into the circulatory system per day [2]. The ratio of βHB and acetoacetate levels in plasma is approximately 5:1, which can fluctuate according to the mitochondrial redox ratio in the liver [31,32]. After a 3-4 day fast or starvation, the βHB level in plasma rises to 5-6 mmol/L, which can meet ≥ 50% of the brain energy requirements [33]. After dietary intervention or diabetic ketosis, the βHB level may surge to > 25 mmol/L [34].
Ketolysis in the brain
At the start of dietary carbohydrate or energy restriction, the glucose stored in the brain can supply adenosine triphosphate (ATP) for only a few minutes [35]. Once the KBs achieve concentrations > 4 mmol/L, a switch from glucose to KBs occur in the brain energy source [36]. KBs can be taken up into the brain tissue via MCTs on micro-vascular endothelial cells and astrocytes, and the transport process depends on the concentrations of KBs in the circulation [15,37]. MCTs are a family of five proton-linked transporters that passively transport metabolic substrates, such as lactate, pyruvate, and KBs. The family contains four isoforms in mammals, including MCT1 (SLC16A1), MCT2 (SLC16A7), MCT3 (SLC16A8), and MCT4 (SLC16A3), each of which has characteristic substrates and affinities [28]. As the exclusive known transporters of KBs, MCTs are ubiquitously expressed in the brain. MCT expression in the brain is significantly upregulated following the administration of KD in rats [38,39]. MCT1 possesses a relatively low affinity for βHB, principally located in endotheliocytes and astrocytes of the blood-brain barrier (BBB) [40]. MCT2 has a high affinity for βHB and is almost exclusively located in the post synaptic density of neurons [41]. Additionally, low-affinity MCT2 is mainly located in astrocytes [42]. Interestingly, a recent study found that FFAs can cross the BBB and participate in ketogenesis in astrocytes, mediated by ventromedial hypothalamic (VMH) neurons [43]. Further, the endogenous KBs produced from astrocytes transfer to nearby neurons for energy supply, which the process is similar to the astrocyte neuron lactate shuttle (ANLS) [23]. Generally, neurons might be capable of βHB uptake, while glia cells might be capable of release or uptake of βHB.
The reverse reactions of ketogenesis, known as ketolysis, occur in the mitochondrial matrix. First, βHB is oxidized into acetoacetate by BHD1 following its arrival in mitochondria. Then, acetoacetate is catalyzed and catabolized to two molecules of acetyl-CoA to participate in the TCA cycle by the action of succinyl-CoA-3- ketoacid CoA transferase (SCOT) and thiolase [44]. Of note, the metabolism of KBs does not require ATP but can generate more energy than glucose [45,46]. Furthermore, studies on rodents have reported that the rate at which neurons, astrocytes, and oligodendrocytes can metabolize KBs to supply energy is higher than the rate at which they metabolize glucose. Compared to astrocytes, neurons and oligodendrocytes might achieve higher efficiency of KB oxidative metabolism [15,47]. The synthesis and catabolism of KBs involved in the brain are illustrated inFigure 2.
Neuroprotective effects of KD
KD plays neuroprotective roles in the CNS through inhibition of glycolysis and the production of KBs. First, as an essential feature of dietary restriction, inhibition of glycolysis regulates insulin secretion and improves insulin sensitivity and glucose tolerance, which consequently delays the onset of age-related conditions and prolongs the lifespan of various species [17,48]. Second, KBs compensate for mitochondrial dysfunction in neurons and glial cells by improving mitochondrial respiration and increasing mitochondrial ATP production [49]. A previous study suggested that βHB catabolism regulates nicotinamide adenine dinucleotide (decreasing the NAD+/NADH ratio), while coenzyme Q (increasing the Q/QH2 ratio) couples the balance in βHB-perfusion of rat hearts. The vast difference in the redox potentials of these two couples facilitates the electron transfer process from NADH to Q and ATP production during oxidative phosphorylation [50]. Furthermore, the interaction between α-synuclein aggregation and mitochondrial function may involve blockade of complex I (CI) in the pathomechanism of PD [51]. Production of succinate, an oxidative substrate of complex II (CII), is regarded as the rate limiting step of βHB catabolism. Studies have shown that βHB metabolism can circumvent pathological CI inhibition by increasing flux through CII by generating succinate in dopaminergic neurons [52,53]. Third, a KD alleviates the production of reactive oxygen species (ROS) and abates inflammatory responses through various signaling pathways. Fourth, a KD may be able to prevent neuronal apoptosis via the sirtuin (SIRT)-1 signaling pathway. A KD reduces the SIRT-1-mediated hyperacetylation of the transcription factor p53 and consequently down-regulates the proapoptotic protein (BAX) and up-regulates the anti-apoptotic proteins (Bcl-2 and Bcl-xL) in the Cockayne syndrome animal model [54].
Strategies for nutritional ketosis induction
Ketosis is a physiopathologic process that is achieved by disturbances in the carbohydrate metabolism, owing to KD or dietary carbohydrate restrictions. In contrast to diabetic ketoacidosis, nutritional ketosis is a safe and sustained physiological state with plasma βHB concentrations ranging from 0.5 to 5 mmol/L [55,56]. The physiological characteristics of nutritional ketosis are normal blood gas, elevated KBs, and low and stable glucose. Besides KD, fasting, medium-chain fatty acids (MCFAs), and exogenous ketones can also induce nutritional ketosis. With the development of medical science, prolonged fasting has been modified into alternating and intermittent variations of fasting to achieve nutritional ketosis safely [57]. Another alternative to KD is the intake of exogenous MCFAs, which are absorbed rapidly into the portal vein and preferentially enter β-oxidation and ketogenesis in the liver [58]. MCFAs are composed of caprylic acid (C8) and capric acid (C10), which are found chiefly in coconut and palm kernel oil [6]. Dietary supplements of exogenous ketones, such as ketone salts and esters in the form of dissolvable powders or liquids, also induce ketosis [59]. Exogenous ketones directly increase the circulatory pool of KBs and accurately manipulate the KB levels with no decline in insulin or glucose levels [60,61].
Limitations of KD
Despite the widely recognized protective role of a KD, its suitability and safety need to be evaluated in further research. A major limitation of the application of the KD is the use of KBs in targeted organs, especially in the brain. The kinetic profile of KBs appears to be strongly affected by the formulation and dose of KD therapies. A clinical study showed that the maximum plasma levels of βHB and acetoacetate were achieved within 15 min in healthy subjects after the injection of DL-β-hydro-xybutyrate or acetoacetate. The fractional rate constant of disappearance (K), volume of distribution (V), and metabolic clearance (MCR) of of βHB and acetoacetate are 66 and 52 × 10-3 min-1, 28.3 and 30.6 L, and 1.59 and 1.58 L/min, respectively [62]. In another similar study, 54 healthy subjects received (R)-3-hydroxybutyl (R)-3-hydroxybutyrate orally to evaluate the kinetic parameters of this burgeoning βHB monoester [8]. The βHB monoester at 357 and 714 mg/kg allowed maximum plasma levels of KBs to be achieved safely in 1-2 h, which was similar to those observed following fasting for several days. It is recognized that the use of KBs in an organism is determined by the concentration of KBs in the bloodstream. Although there is a linear relationship between the usage rate and blood concentration at lower concentrations of KBs, there is a plateau when the usage rate reaches the maximum at higher concentrations of KBs [63]. KBs, especially βHB, are readily transported through the BBB via MCTs to participate in brain energy metabolism [64]. Brain uptake of βHB and acetoacetate increases frequently during KD therapies or short-term starvation [65], which seems to be related to the alternation of BBB permeability [66], transporters [65], and capillary density [67]. However, the KB concentrations in the brain and cerebrospinal fluid were much lower than those in the bloodstream during starvation or KD therapies [63]. Thus, a new ketogenic therapy that is more easily absorbed and used by the brain deserves further research and development.
An additional concern of the applicability of the KD is patient compliance with particular dietary recommendations [7]. Although patients are initially determined and motivated to comply with the KD, they are prone to abandon dietary treatment due to poor tolerance to a large number of fat-rich foods in the later stage. Therefore, we should consider whether intermittent KD therapy may offer beneficial effects analogous to those observed with the standard KD schedule. This speculation has been recently evaluated, and the results confirmed that intermittent KD weekly prevents obesity and improves midlife survival and memory in aging mice [68].
As multiple methods of nutritional ketosis induction have been increasingly used for neurologic disorders, concern and research into potential adverse effects have gradually increased. A clinical study of patients with epilepsy treated with KD reports the adverse effects including dyslipidemia, gastrointestinal effects (nausea, vomiting, constipation and diarrhea) and weight loss [20]. Moreover, KD may lead to dehydration, hypoglycemia, hyponatremia, atherosclerosis, impaired hepatic functions, and deficiencies of multiple nutrient elements [69]. Of note, anorexia and acute pancreatitis are considered a contraindication of KD therapies. Approaches are recommended to prevent and control the side effects of KD, such as having small, frequent meals (SFMs) throughout the day, increasing intake of dietary fiber, vitamins, minerals, and water, and performing more exercise [20,70]. Taken together, a standardized treatment protocol for KD that details the dose and duration of dietary intervention, accurately monitors the KB level, and prevents potential adverse effects remains to be established.
Possible anti-inflammatory mechanisms of KD
Inflammation is a comprehensive array of biological processes that occur in response to foreign organisms and injury suffered by cells or tissues. A rapid and effective inflammatory response eliminates invading pathogens, promotes wound healing and angiogenesis, and ultimately repairs damaged tissues. Neuroinflammation is defined as an inflammatory process that occurs in the brain or spinal cord and is caused by diverse pathological lesions, such as trauma, infection, ischemia, and degeneration [71]. Resident CNS glial cells (microglia and astrocytes) and peripheral infiltrating immune and endothelial cells mediate neuroinflammation by regulating the production of inflammatory cytokines, ROS, chemokines, and small-molecule messengers. According to the course of the inflammatory response, neuroinflammation can be divided into acute and chronic. Acute neuroinflammation, an immediate response to damaging stimuli, induces beneficial effects resulting from the removal of possible destructive factors, phagocytosis of cell debris, and maintenance of homeostasis. However, uncontrolled neuroinflammation induces a chronic inflammatory response, which is characterized by microglia overactivation, production of neurotoxic factors, and destruction of neurons and glial cells, which together accelerate the deterioration of the condition [72]. The context, course, and duration of the main stimulus or injury affect the degree of these neuroinflammatory responses to a large extent.
Microglia and astrocytes are the innate immune cells primarily involved in neuroinflammation [73,74]. Microglia are the resident macrophages in the brain and are key players in neuroinflammation [75]. Microglia have pro-inflammatory or neuroprotective roles in the CNS depending on their phenotype (proinflammatory M1 phenotype and anti-inflammatory M2 phenotype) and activation pathways. The M1-type microglia are activated via nuclear factor-κB (NF-κB) and signal transducers and activators of transcription 3 (STAT3) signaling pathways to generate pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-12, IL-23, cyclooxygenase (COX)-1, COX-2, ROS, and nitric oxide (NO). In contrast, the M2-type microglia are activated to release neurotrophic factors, such as IL-4, IL-10, IL-13, and transforming growth factor-β (TGF-β). Astrocytes are the most common type of glial cells and can regulate cerebral blood flow, maintain neurotransmitter homeostasis, control synapse formation, and form distinctive perivascular channels to eliminate potentially neurotoxic agents. Similar to microglia, activated astrocytes have dual phenotypes (A1 phenotype and A2 phenotype) to respond to pathological insults and participate in the neuroinflammatory process. The proinflammatory A1-type astrocytes are induced by inflammatory insult and produce pro-inflammatory cytokines (such as IL-1β, TNF-α, IL-6, and NO) via the NF-κB signaling pathway. The anti-inflammatory A2-type astrocytes are induced to release anti-inflammatory cytokines (such as IL-4, IL-10, IL-13, and TGF-β) via the STAT3 pathway.
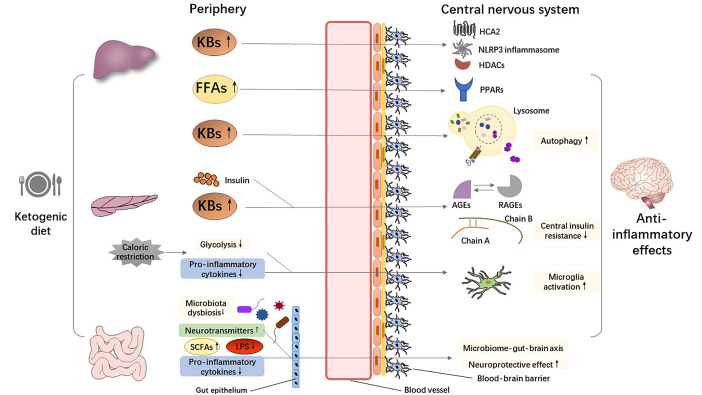
It has been implicated that the neuroinflammatory process contributes to the pathology of several neurodegenerative diseases [76]. As resident neuroimmune cells of the CNS, microglia and astrocytes modulate the inflammatory response and release pro-inflammatory or anti-inflammatory cytokines in response to abnormal accumulation of pathogenic proteins (such as tau-containing neurofibrillary tangles in AD and α-synuclein aggregation in PD) and invasion of pathogens [77]. It is accepted that both central (such as pathological protein deposition and brain injury) and systemic (such as infections, dietary patterns, and lifestyles) effector specificity mechanisms can result in CNS neuroinflammation. There is mounting evidence to suggest that a KD plays neuro-protective and disease-modifying roles in neurodegenerative diseases by regulating both central and peripheral inflammatory mechanisms [20]. We expound the multi-dimensional potential mechanisms involved in the anti-inflammatory properties of a KD inFigure 3.
Figure 3.
Putative anti-inflammatory mechanisms mediated by the ketogenic diet. Abbreviations: AGEs, advanced glycation end products; FFAs, free fatty acids; HCA2, hydrocarboxylic acid receptor 2; HDACs, histone deacetylases; KBs, ketone bodies; LPS, lipopolysaccharide; NLRP3, nucleotide-binding domain-like receptor protein 3; PPARs, peroxisome proliferator-activated receptors; RAGEs, receptors for AGEs; SCFA, short chain fatty acid.
KBs as signal mediators
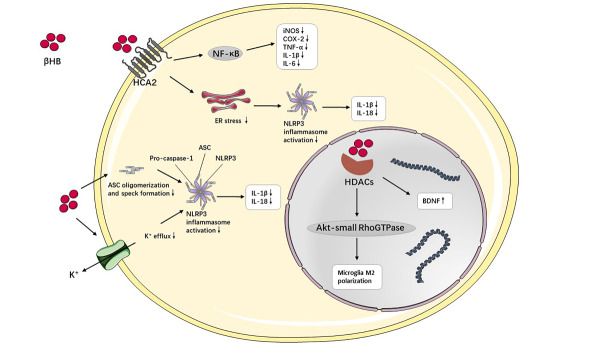
Besides being energy substrates, KBs are also active as intracellular signaling mediators, which participate in intracellular signaling cascades and regulate neuroinflammation directly or indirectly, especially βHB [78].Figure 4 shows the putative anti-inflammatory mechanism of βHB mediated by cell-signaling pathways. Hydrocarboxylic acid receptor 2 (HCA2), also named G-Coupled Protein Receptor (GPR) 109A, is a G protein-coupled receptor that is expressed abundantly in microglia, macrophages, and dendritic cells. The activation of HCA2 triggers the neuroprotective subset of macrophages dependent on COX-1 generating prostaglandin D2 (PGD2), which serve to relieve neuroinflammation [70]. As a specific endogenous ligand of HCA2, βHB is capable of directly binding to HCA2 and activating it with a half maximal effective concentration (EC50) of approximately 0.7 mmol/L, which is similar to that in human nutritional ketosis [79,80]. βHB can bind to HCA2 to further inhibit the production of pro-inflammatory cytokines and enzymes via the NF-κB pathway in activated primary microglia pretreated with βHB and stimulated with lipopolysaccharide (LPS) [81]. However, the activation of HCA2 by βHB also eases the inflammatory response through partly reducing endoplasmic reticulum (ER) stress, nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome activity, and IL-1β and IL-18 levels [82].
Figure 4.
The intracellular molecular mechanism of neuroinflammation mediated by beta-hydroxybutyric acid (βHB). βHB interacts with hydrocarboxylic acid receptor 2 (HCA2), nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome and histone deacetylases (HDACs) directly or indirectly to exert anti-inflammatory effects. Abbreviations: ASC, apoptosis-associated speck-like protein with a caspase recruitment domain; BDNF, brain-derived neurotrophic factor; βHB, beta-hydroxybutyric acid; COX, cyclooxygenase; ER, endoplasmic reticulum; HCA2, hydrocarboxylic acid receptor 2; HDACs, histone deacetylases; IL, interleukin; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor-κB; NLRP3, nucleotide-binding domain-like receptor protein 3; TNF-α, tumor necrosis factor-α.
Additionally, the innate immune sensor, the NLRP3 inflammasome, is widely expressed in the cytoplasm of immune cells to modulate neuroinflammation and can be activated by the alternation of cytoplasmic K+ levels. βHB inhibits the activation of the NLRP3 inflammasome and subsequently IL-1β and IL-18 generation by blocking K+ efflux and decreasing oligomerization and speck formation of apoptosis-associated speck-like protein with caspase-recruitment domain (ASC). Of note, the interaction between βHB and the NLRP3 inflammasome is independent of the oxidation in the TCA cycle, HCA2, SIRT-2, and uncoupling protein-2 (UCP-2) [83].
Data suggest that βHB can engage with histone deacetylases (HDACs) to alter the expression of detoxifying genes at the epigenetic level. βHB inhibits class I HDACs (1, 2, 3, and 8) and increases the expression of brain-derived neurotrophic factor (BDNF) [45,84]. It is well recognized that BDNF induces the activation of microglia and astrocytes, thereby contributing to neuroinflammation through various signaling pathways [85]. The inhibition of HDAC activity by βHB may have other downstream actions. For example, βHB triggers the M2 microglia phenotype, which is reversed by HDAC suppression, potentially via the Akt (protein kinase B)-small RhoGTPase (Rac1, Cdc42) axis [86].
Peroxisome proliferator-activated receptors (PPARs) activation
PPARs, including PPARα, PPARβ, and PPARγ, are nuclear receptors that act as ligand-inducible transcription factors to modulate lipid and glucose metabolism. PPARα expression is constitutively found in the liver, heart, and kidney, while PPARβ is mainly expressed in the brain, skin, and adipose tissue. PPARγ is also expressed in the adipose tissue, brain, muscle, and various tissues [87,88]. PPARs are highly upregulated in the brains of study animals under a ketotic state, which may result from the activation of PPARγ co-activator 1α (PGC-1α) and SIRT-1 or KBs [89,90]. Moreover, PPARα and PPARγ play a leading role in the regulation of ketogenesis and the ketolysis process, both of which can be activated by KD-induced elevated polyunsaturated fatty acids in the periphery and brain [68,91,92]. Previous studies have suggested that MCFAs, as a component of certain KDs, are direct agonists of PPARγ [93,94]. Although the mechanisms need to be explored in more depth, current evidence suggests that a KD can play an anti-inflammatory role by activating PPARs. Indeed, PPARα activated by FFAs has been shown to inhibit the NF-κB signal transduction pathway, leading to downregulation of inducible nitric oxide synthase (iNOS) and COX-2 to modulate the inflammatory response [95]. There is some evidence to confirm that the activation of PPARγ induced by a KD has a marked suppressive effect on neuroinflammation in the hippocampus of a mouse model of seizures [96]. Moreover, the activation of PPARs reduces the inflammation response in the periphery and brain, which seems to result from the restraint of NF-κB, nuclear factor of activated T-cells (NFAT), and signal transduction and transcription-1 (STAT-1) [97,98]. The elevated PPARγ level blocks STAT-1 and p38 mitogen-activated protein kinase (MAPK) signaling and impedes the appearance of a reparative or pro-inflammatory microglia phenotype, thereby facilitating the transition of microglia to anti-inflammatory phenotype [99,100]. Interestingly, the activation of PPARγ seems to exert more prominent effects in facilitating the anti-inflammatory microglia phenotype under conditions of increased FFA levels, which might be involved in the mammalian target of rapamycin (mTOR)-PPARγ signaling pathway [101].
Autophagy regulation
Autophagy is a tightly orchestrated cellular process mediated by lysosomes, which degrades and eliminates intracellular endogenous components (misfolded or aggregated proteins and damaged organelles) and exogenous stimuli (bacteria, virus, and parasites). Autophagy has been subdivided into three major types according to their distinct patterns of cargo delivery to the lysosome: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) [102,103]. A growing body of evidence suggests that autophagy is closely linked to aging and to the onset and progression of neurodegenerative diseases [104-106]. Recent studies have demonstrated that autophagy limits inflammatory pathologies and alleviates the burden of infectious agents to exert anti-inflammatory and neuroprotective effects [107,108].
The KD, the βHB component to be exact, might ease inflammatory reactions mediated by autophagy in neurodegenerative diseases. During glucose deprivation, elevated βHB stimulates autophagy and improves cortical neuronal survival [109]. Nutritional ketosis can up-regulate macroautophagy in the brain through inhibition of the mechanistic target of rapamycin complex 1 (mTORC1) and activation of sirtuin-1 (Sirt1) and hypoxia-inducible factor-1 (HIF-1) [110,111]. βHB promotes CMA activation directly by increasing protein oxidation, which ultimately leads to the clearance of oxidation-damaged proteins [112]. Additionally, βHB attenuates autophagic degradation and improves neuronal damage induced by N-methyl-D-aspartate (NMDA) in the striatum of rat [113]. Compared to the cerebral cortex, more autophagosome formation occurs in the hippocampus in response to a KD [114]. Interestingly, HMGCS2, the control enzyme in ketogenesis, induces autophagic clearance of the β-amyloid precursor protein (APP), which is mediated by acetoacetate metabolism and the mTOR signaling pathway; this suggests that HMGCS2 inhibits the subsequent production and deposition of amyloid β (Aβ) [115].
Insulin sensitivity improvement
Insulin resistance and hyperglycemia are associated with brain aging and neurodegenerative diseases and can aggravate the pathological process through a complex network of feedback mechanisms, especially in AD and PD [116]. Insulin resistance may be induced by diabetes, hypertriglyceridemia, obesity, and systemic chronic inflammation [117]. Moreover, the reduction of glucose transporters and disturbance of insulin signaling pathways contribute to insulin resistance at the molecular level [118]. Insulin resistance and impaired glucose metabolism accelerate the formation of advanced glycation end products (AGEs), which can bind to receptors for AGEs (RAGEs) on neurons and glia cells to exert harmful effects. The activation of RAGEs facilitates the expression of pro-inflammatory cytokines, such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α), along with ROS production, which further results in neuroinflammation, oxidative stress, and neuronal degeneration [119,120]. Recent experimental and clinical studies have demonstrated that a KD can improve insulin sensitivity as well as glucose homeostasis [121-123]. Further studies have shown that βHB, the main KBs in the brain, restrains insulin glycation, AGE accumulation [124,125], and liposomal lipid peroxidation to ultimately prevent microglial apoptosis [126]. Thus, we can speculate that the anti-inflammatory effects of KD could hinder the development of neurodegenerative diseases through increasing insulin sensitivity.
Caloric restriction
The implementation of KD has shown some weight loss effects by reducing food intake and increasing energy expenditure [127]. Although controversial, KD can be linked to caloric restriction in some cases [7]. As a basic characteristic of calorie restriction, suppression of glycolysis might alter microglial phenotypes and exert anti-inflammatory action by regulating the NAD+/NADH ratio and the transcriptional inhibitor C-terminal-binding protein (CtBP) [92,128]. Caloric restriction can also modulate the expression of inflammatory regulators, such as NF-κB inhibitor, PPARs, and tissue inhibitor of metalloproteinases-3 (Timp3) [129,130], and down-regulate the levels of TNF-α, IL-2, IL-4, IL-6, COX-2, and iNOS [131]. Additionally, the anti-inflammatory effects of calorie restriction are likely to be caused by various epigenetic regulations, including microRNA [132]. The upregulation of miR-16-5p, miR-196b-5p, and miR-218-5p has been reported in the serum of calorie-restricted mice, and notably, miR-16-5p significantly reduced TNF-α, IL-1β, and IL-6 levels after LPS stimulation [133]. Some reviews have elaborated on the possible mechanisms of the calorie restriction anti-inflammatory properties of KD [17,134].
Gut microbiota modulation
Considering that gut microbes have an influence on central behavior and pathology, the concept of the microbiome-gut-brain axis has been proposed recently. The gut microbiota involves nervous, immune, and endocrine mechanisms and is termed the “second brain” [135,136]. The gut microbiota influences the host CNS via various neurotransmitters (such as norepinephrine, dopamine, and γ-aminobutyric acid) in the gut. Dietary features, both total calorie and individual nutrients (such as carbohydrate, fat, protein, and vitamins), are considered to have a major impact on microbiota composition. While diet-induced alterations occur over time, diet-induced alterations of the gut microbiota may happen rapidly if shifting diets are sufficiently dramatic [137]. It has recently been suggested that KD-induced gut microbiota profile changes also mediate neuro-inflammation [16].
There is accumulating evidence to support that KD alters the gut bacterial profile in both animal models and human studies. Indeed, after 16 weeks of KD treatment, the neurovascular functions, including BBB function and cerebral blood flow, were shown to be sufficiently improved to accelerate Aβ clearance in young healthy mice. This KD intervention also altered the composition of the gut microbiota, with enrichment of beneficial microbes (such as Lactobacillus and Akkermansia muciniphila) and a reduction of pro-inflammatory microbes (such as Turicibacter and Desulfovibrio) [138]. A previous clinical study demonstrated that patients with mild cognitive impairment (MCI) have a relatively high abundance of Enterobacteriaceae and Erysipelotriaceae but a relatively low abundance of Lachnobacterium and Bifidobacterium after a modified Mediterranean-ketogenic diet (MMKD). The MMKD also reduces acetate and lactate in feces and increases butyrate, which is a beneficial short-chain fatty acid (SCFA), to exert neuroprotective actions [139]. In previous in vivo and in vitro experiments, compared to the high-fat diet, KD resulted in the suppression of bifidobacterial growth through the host producing βHB. The gut microbiota induced by KD down-regulate pro-inflammatory Th17 cells and, consequently, the Th17-associated immune response in the intestine, which might explain the potential protective mechanism of KD in metabolic syndrome and neurological diseases [140]. The abnormally altered gut microbiota, known as gut microbiome dysbiosis, is associated with neuro-degenerative diseases. Gut microbiome dysbiosis decreases the levels of neurotransmitters and SCFAs and increases the level of LPS, ultimately adjusting the interplay between the CNS and gut [141]. Moreover, the gut microbiome dysbiosis contributes to intestinal inflammation and intestinal barrier dysfunction, further leading to elevated levels of inflammatory cytokines and LPS in circulation [142]. KD promotes the production of SCFAs and restrains the production of γ-glutamyl amino acid through altering particular microbes, such as Lactobacillus and Akkermansia muciniphila [136,141]. Considering the lack of studies of KD on gut microbiota, more research is needed to confirm the anti-inflammatory role of KD in the CNS.
Effects of KD on normal brain aging and neurodegenerative diseases
The majority of previous studies have examined the effects of KD on normal brain aging and neurodegenerative diseases. KD is increasingly regarded as an alternative therapy for a range of neurodegenerative diseases. In this section, we summarize the main published experimental and clinical evidence related to the role of KD in normal brain aging and neurodegenerative diseases (presented inTable 1), especially the involvement in neuroinflammatory mechanisms.
Table 1.
Preclinical and clinical studies on ketogenic diet in normal brain aging and neurodegenerative diseases.
| Models | Intervention | KD effects on inflammatory or other brain pathology markers | KD effects on clinical features | Study 1st author (ref) |
|---|---|---|---|---|
| Normal brain aging | ||||
| Animal models | KD; medium chain triglyceride | Modulates the synaptic stability and synaptic plasticity | Improves cognitive function | Newman JC [68]; Pan Y [144]; Wang D [146] |
| KD | Alters expression transporters for different energy substrates and neurotransmitters | Enhances motor performance | Hernandez AR [145] | |
| AD | ||||
| Animal models | KD; ketone ester | Reduces Aβ and hyperphosphorylated tau deposition | Relieves anxiety, improves cognitive function | Van der Auwera I [153]; Kashiwaya Y [154] |
| βHB; triheptanoin | Inhibits NLRP3 inflammasome activation, microgliosis and reduces plaque formation; inhibits astrogliosis and pro-inflammatory cytokines production, improve mitochondrial status; reduces APP and increases NEP mediated by GPR109A | Improves cognitive function | Shippy DC [155]; Aso E [156]; Wu Y [157] | |
| Ketone ester; 3-Hydroxybutyrate methyl ester | Promotes TCA cycle metabolites and decreases mitochondrial redox potential; reduces Aβ deposition, protects mitochondrial functionality and corrects the intracellular redox state, inhibits cell apoptosis | Improves the spatial learning and working memory; | Pawlosky RJ [158]; Zhang J [159] | |
| βHB | Inhibits cell apoptosis with the reduction of p53, caspase-3, caspase-9, caspase-12 levels and the Bax/Bcl-2 ratio | Xie G [160] | ||
| KD, ketone ester | Elevates the level of n-acetyl-aspartate | Improves the motor performance, does not improve cognitive performance; improves the abnormal behaviour | Brownlow ML [161]; Pawlosky RJ [162] | |
| Human | ||||
| Medium chain triglyceride; MMKD | Increases cerebrospinal fluid Aβ42 and decreases tau protein mediated by the alteration of gut mycobiome, gut bacteria and SCFAs, increases cerebral perfusion and cerebral KBs uptake | Improves memory in subjects with MCI | Rebello CJ [164]; Neth BJ [163]; Nagpal R [165]; Nagpal R [139] | |
| KD; medium chain triglyceride; MAD | Improves the quality of life and daily function; Ameliorates cognitive impairment, especially in the APOE ɛ4 negative patients | Phillips MCL [166]; Ota M [167]; Brandt J [168]; Henderson ST [169]; Reger MA [170]; Ohnuma T [171] | ||
| Caprylic triglyceride | Dose not improve functional ability or cognitive impairment | Henderson ST [172] | ||
| Medium chain triglyceride | Enhances brain ketone uptake and energy supply; enhances rCBF in specific brain regions | Croteau E [173]; Torosyan N [174] | ||
| PD | ||||
| Animal models | βHB; C8 | Attenuates the loss of dopamine, improves mitochondrial respiration and ATP production, up-regulates the expression of PGC-1α and PEPCK |
Improves the motor deficits | Tieu K [52]; Joniec-Maciejak I [182] |
| KD | Increases Nissl and tyrosine hydroxylase-positive neurons, increase dopamine and dihydroxyphenylacetic acid and glutathione | Cheng B [183] | ||
| KD; βHB | Protects dopaminergic neurons, inhibits GPR109A, NF-κB signal pathway, microglial activation and downstream pro-inflammatory cytokines production | Improves the motor dysfunction | Yang X [184]; Fu SP [81] | |
| KD | Dose not protect dopaminergic neurons | Kuter KZ [185] | ||
| Human | KD | Improve the motor and non-motor symptoms, especially cognitive function; improve the voice quality | Vanitallie TB [186]; Phillips MCL [188]; Koyuncu H [187] | |
| ALS | ||||
| Animal models | KD | Protects motor neurons, promotes ATP production and mitochondrial respiration | Enhances the motor performance | Zhao Z [191] |
| Triheptanoin; Caprylic triglyceride | Increases mitochondrial oxygen consumption | Improves the motor functions, delays the onset of motor symptoms and increases the survival | Tefera TW [192]; Zhao W [193] | |
| Deanna protocol | Enhances the motor performance and increases the survival | Ari C [194] | ||
| HD | ||||
| Animal models | KD | Attenuates motor deficits and slow down the loss of weight | Chen JY [196] | |
| βHB | Attenuates striatal lesions and microgliosis, inhibits histone deacetylation | Ameliorates motor dysfunction, extends the lifespans | Lim Soyeon [197] |
Abbreviations: Aβ, amyloid β; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; APOE ɛ4, epsilon 4 allele of the apolipoprotein E gene; APP, β-amyloid precursor protein; ATP, adenosine triphosphate; βHB, beta-hydroxybutyric acid; C8, caprylic acid; GPR, G-Coupled Protein Receptor; HD, Huntington’s disease; KBs, ketone bodys; KD, ketogenic diet; MAD, modified Atkins diet; MCI, mild cognitive impairment; MMKD, modified Mediterranean-ketogenic diet; NEP, neprilysin; NF-κB, nuclear factor-κB; NLRP3, nucleotide-binding domain-like receptor protein 3; PD, Parkinson’s disease; PEPCK, phosphoenolpyruvate carboxylase; PGC-1α, peroxisome proliferator-activated receptor γ co-activator-1α; rCBF, regional cerebral blood flow; SCFA, short chain fatty acid; TCA, tricarboxylic acid.
KD and normal brain aging
Brain aging is relevant to cognitive decline and is considered a dominant risk factor for neurodegenerative diseases, including AD, PD, and vascular dementia [143]. Several studies have explored the effects of KD on cognitive function and synaptic plasticity in aged animals [68,144-146]. Indeed, some KD methods, including traditional KD and exogenous KS, have demonstrated a positive effect on cognitive function in aged animals. Moreover, medium chain triglyceride diets have been shown to increase Ube3a protein expression and down-regulate arc, plk3, junb, egr2, and nr4a1genes to modulate synaptic stability and synaptic plasticity in aged rats [146]. Additionally, KD was found to contribute to the alteration of transport protein expression, including glucose, monocarboxylates, vesicular glutamate, and γ-aminobutyric acid transporters, in the hippocampus of aged rats, which might correct age-related abnormal energy metabolism, synaptic transmission, and neurotransmitter systems [145].
KD and AD
AD is the most common neurodegenerative disease and the most common cause of dementia in elderly people and is manifested by memory decline and subsequent mental and behavioral impairment, with insidious onset and slow progression [147]. The core histopathological hallmarks of AD are extracellular deposition of Aβ plaques, intracellular hyperphosphorylation, and abnormal aggregation of tau protein. There are more than 25 million people with dementia worldwide, most of whom have AD. The prevalence of dementia has notably increased, leading to onerous burdens on individual patients, caregivers, and society in both developed and developing countries [148]. MCI is regarded as a transitional stage between normal aging and dementia; therefore, it is crucial to find effective prevention therapies to delay the onset of MCI and slow cognitive decline [149].
Several studies have confirmed that βHB inhibits Aβ-induced neurotoxicity [150] mediated by facilitating Aβ efflux across the BBB [151], restraining HDAC1/3, and up-regulating the level of tyrosine kinase receptor A (TrkA) [152]. Several studies have shown that various types of KD can lessen Aβ, hyperphosphorylate tau deposition, and improve cognitive function in transgenic mouse models of AD [153,154]. First, βHB improved cognitive performance and attenuated related pathology by targeting diverse inflammatory mechanisms in AD models. βHB inhibited NLRP3 inflammasome activation, microgliosis, astrogliosis, and pro-inflammatory cytokine production [155-157]. βHB modulated β-amyloid precursor protein (APP) and neprilysin (NEP) expression mediated by GPR109A to suppress the amyloid plaque formation in 5xFAD (familial AD) mice [157]. Second, ketone ester significantly contributed to supplying energy by KB metabolism, protecting mitochondrial functionality, and correcting the intracellular redox state in transgenic AD mice [156,158,159]. Third, βHB directly inhibited the apoptotic signaling pathway with the reduction of p53, caspase-3, caspase-9, caspase-12 levels, and the Bax/Bcl-2 ratio [159,160]. Tg4510 (a model of tau deposition) and APP/PS1 (a model of amyloid deposition) mice fed a KD for 3 months performed significantly better on the motor tests, compared to those fed a normal diet, although this was not the case in cognitive tests [161]. A KD was also shown to play a protective role in the improvement of abnormal behavior, including avoidance-related behavior and exploratory activity, by elevating the level of n-acetyl-aspartate in the hippocampus of 3xTgAd mice [162].
Several studies have found relationships between the KD and cognitive decline in humans. Indeed, medium chain triglyceride supplementation for 24 weeks or the MMKD for 6 weeks appeared to improve memory in subjects with MCI in two randomized controlled trials [163,164]. The MMKD could regulate the biomarker levels of AD with increasing Aβ42 and decreasing tau protein in cerebrospinal fluid, which might be mediated by the alteration of gut mycobiome, gut bacteria, and SCFAs [139,165]. Likewise, clinical trials suggested that different types of KDs significantly improved the quality of life and daily function [166] and eased AD-related cognitive impairment [167,168], especially in subjects without the epsilon 4 allele of the apolipoprotein E gene (APOE ɛ4), suggesting that KB metabolism is related to APOE ɛ4 status [169-171]. However, a multicenter clinical trial found that the AC-1204 formulation of caprylic triglyceride had no significant effect on improving functional ability or cognition among 413 patients with AD and without the APOE ɛ4 allele [172]. Some studies could explain the mechanisms underlying the protective effect of KD on AD. Medium chain triglyceride supplements triggered brain ketone uptake and energy supply to compensate for the brain energy deficit caused by hypometabolism among patients with AD [173]. However, caprylidene, a medium chain triglyceride, enhanced regional cerebral blood flow (rCBF) in specific brain regions, including the temporal cortex, cerebellum, and hypothalamus, among patients with AD and without the APOE ɛ4 allele [174].
KD and PD
PD is the second most common progressive neurodegenerative disorder characterized by motor disturbances (such as resting tremors, bradykinesia, muscle rigidity, and slowness of movement) and non-motor disturbances (such as sleep dysfunction, autonomic dysfunction, hyposmia, cognitive, and psychiatric symptoms) [175]. The core neuropathological hallmarks of PD are substantial loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), along with the accumulation of α-synuclein, known as Lewy neurites or Lewy bodies [176]. As the fastest-growing neurological disorder, the number of people with PD is projected to increase to more than 12 million in the coming decades, driven primarily by the aging population [177].
Several experimental studies have shown that βHB has a neuroprotective effect in several cell models of PD through various mechanisms, including attenuation of mitochondrial dysfunction [150] via modulating CI or CII [178] and diminishing synphilin-1 aggregation [179], inhibition of apoptosis via increasing the ratio of Bcl-2/Bax mRNA [180], and regulation of neuroinflammation via inhibition of NLRP3 inflammasome activation [181]. βHB administered subcutaneously conferred protection partly against motor disturbance and reduction of striatal dopamine in mice induced by 1-methyl-4-phenol-1,2,5,6-tetrahydropyridine (MPTP), which might be related to a CII-dependent mechanism and easing of the mitochondrial respiration chain and ATP production [52]. Similarly, another study revealed that C8, one of the highest producers of KBs, significantly prevented dopaminergic neurodegeneration in the striatum of the MPTP mouse model of PD. Further study demonstrated that this appeared to be mediated by the up-regulation of PGC-1α and phosphoenolpyruvate carboxylase (PEPCK), which are regarded as markers of mitochondrial activity [182]. KD has also been shown to up-regulate glutathione of the striatum, thereby reversing the decrease in dopamine and dihydroxyphenylacetic acid in a 6-hydroxydopamine- (6-OHDA) induced rat model of PD [183]. KD was also shown to exhibit neuroprotective and anti-inflammatory effects via restraining microglial activation and reducing pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in the MPTP mouse model [184]. Moreover, βHB interacted with GPR109A and suppressed NF-κB activation and downstream pro-inflammatory cytokine production, such as iNOS, COX-2, TNF-α, IL-1β, and IL-6, in LPS-treated in vivo and vitro PD models [81]. However, according to recent research, a long-term KD lasting for 7 weeks did not prevent 6-OHDA-induced damage to dopaminergic nigral neurons, indicating that the KD seemed to affect astrocytic energy support but not oxidative stress [185].
Few trials of KD in patients with PD have been implemented. A pilot study indicated that the Unified Parkinson’s Disease Rating Scale (UPDRS) scores were improved after five patients with PD adhered to a KD for 28 days [186]. Additionally, a 3-month KD increased the voice quality of patients, as evaluated by the voice handicap index of patients with PD [187]. In another pilot randomized controlled trial, 47 patients with PD were randomly assigned to a KD group and a low-fat group over 8 weeks, and the primary outcomes of motor and non-motor symptoms were assessed by the International Parkinson and Movement Disorder Society-sponsored UPDRS (MDS-UPDRS). The findings suggested that both diets could significantly improve the motor and non-motor symptoms, with the KD showing a greater effect on less L-dopa-responsive non-motor symptoms. Meanwhile, the randomized controlled trial confirmed that a low-fat diet or KD for patients with PD over 8 weeks was safe and reasonable, with excessive hunger being the most common adverse effect during the overall process [188]. Of note, a study discovered that KD might not disturb the pharmacokinetics of levodopa, as detected using the high-performance liquid chromatography method using UV detection (HPLC-UV) in patients with PD [189].
KD and ALS
ALS is the most common motor neuron disease, characterized by the progressive loss of upper and lower motor neurons in the brain and spinal cord. The weakness or paralysis of the skeletal muscle results in motor dysfunction in both limbs and the bulbar region. Ultimately, death due to respiratory paralysis usually occurs within 2-5 years of the onset of the above-mentioned symptoms [190]. The first published research in 2006 showed that a KD significantly enhanced motor performance in SOD1-G93A transgenic ALS mice, which might be related to the preservation of motor neurons and promotion of ATP production and mitochondrial respiration [191]. Some triglycerides, including caprylic triglyceride and triheptanoin, could improve motor functions via the protection of motor neurons [192,193]. Specifically, caprylic triglyceride facilitated mitochondrial oxygen consumption [193], and triheptanoin delayed the related symptoms and improved the survival [192] in SOD1-G93A mice. Moreover, better motor performance and an increase in survival were observed in ALS mice under the Deanna protocol, a metabolic therapy derived from the KD [194].
KD and HD
HD is a common autosomal dominantly inherited disorder caused by an abnormally expanded CAG trinucleotide repeat in the huntingtin gene on chromosome 4. HD is characterized by progressive uncontrollable dance-like movements and cognitive and psychiatric disturbance [195]. An investigation showed that symptomatic R6/2 mice raised on a KD lost weight more slowly, achieved higher scores in behavior tests, and had fewer stereotypes compared to R6/2 mice raised on a normal diet [196]. Additionally, when βHB was administered subcutaneously to the 3-nitropropionic acid (3-NP) toxic model of HD, the motor dysfunction, striatal lesions, and microgliosis were attenuated. Similarly, βHB extended the lifespan, alleviated motor dysfunction, and inhibited histone deacetylation of the striatum in R6/2 transgenic mice treated in the same way. Further research proved that βHB inhibited histone deacetylation through an HDAC-independent mechanism in PC12 cells with inducible expression of human mutant huntingtin [197]. Thus, additional investigations of KD effects in animal models and patients with HD are necessary.
Conclusions and perspectives
The prevalence of neurodegenerative disorders is increasing owing to lifespan extension and unhealthy aging. Given that there is currently no cure nor a disease-modifying therapy available for neurodegenerative disorders, identifying preventive interventions and therapeutic strategies is crucial to delay the onset of neurodegenerative disease and slow progression. From the recognition that KD is not merely a traditional treatment of epilepsy but that is also plays a neuroprotective role in neurodegenerative disorders, it has been suggested that KD might be a promising way to protect against the multiple symptoms of neuro-degenerative disorders. Numerous studies have shown that KD contributes to energy metabolism, oxidative stress, neuroinflammation, and apoptosis in neurodegenerative diseases through pleiotropic mechanisms. In this review, we summarize the beneficial effect of KD in regulating neuroinflammation and provide a comprehensive overview of experimental and clinical evidence of KD in normal brain aging and neurodegenerative diseases.
Although the present findings suggest that KD offers a promising therapeutic approach for neurodegenerative disorders, further preclinical studies aimed at identifying the characteristic effects of KD on the pathophysiology of neurodegenerative disorders are needed. Additionally, further large-scale and long-term clinical research on neurodegenerative disorders, especially randomized control trials, is warranted to evaluate the efficacy, sustainability, and the adverse effects of KD. Choosing optimal approaches to inducing nutritional ketosis for individuals should be considered carefully in future clinical practice. Compared to the classical KD, exogenous KS or a lower ratio KD might be a relatively accessible way to achieve nutritional ketosis in many aspects. While there is still a long way to go, the prominent effects of KD suggest its use as a new therapeutic target and strategy for neurodegenerative disorders.
Acknowlegements
This review was supported by the Special Fund for Military Health Committee (Grant No. 21BJZ18, 18BJZ30 and 17BJZ36) and the Capital Clinical Characteristic Application Research Project (Grant No. Z151100004015206).
Footnotes
Competing interests
The authors have no conflicts of interest to declare.
References
- [1].Kossoff EH, Hartman AL (2012). Ketogenic diets: new advances for metabolism-based therapies. Curr Opin Neurol, 25:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Puchalska P, Crawford PA (2017). Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab, 25:262-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nyenwe EA, Kitabchi AE (2016). The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism, 65:507-521. [DOI] [PubMed] [Google Scholar]
- [4].Huttenlocher PR (1976). Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res, May:536-540. [DOI] [PubMed] [Google Scholar]
- [5].Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. (2020). Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev Endocr Metab Disord, 21:5-16. [DOI] [PubMed] [Google Scholar]
- [6].Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. (2018). Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol, 17:84-93. [DOI] [PubMed] [Google Scholar]
- [7].Wlodarek D (2019). Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer's Disease and Parkinson's Disease). Nutrients, 11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, et al. (2012). Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol, 63:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stubbs BJ, Cox PJ, Kirk T, Evans RD, Clarke K (2019). Gastrointestinal Effects of Exogenous Ketone Drinks are Infrequent, Mild, and Vary According to Ketone Compound and Dose. Int J Sport Nutr Exerc Metab, 29:596-603. [DOI] [PubMed] [Google Scholar]
- [10].Soto C, Pritzkow S (2018). Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci, 21:1332-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dugger BN, Dickson DW (2017). Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol, 9:a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ransohoff RM (2016). How neuroinflammation contributes to neurodegeneration. Science, 353:777-783. [DOI] [PubMed] [Google Scholar]
- [13].Tang Y, Le W (2016). Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol, 53:1181-1194. [DOI] [PubMed] [Google Scholar]
- [14].Subhramanyam CS, Wang C, Hu Q, Dheen ST (2019). Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol, 94:112-120. [DOI] [PubMed] [Google Scholar]
- [15].Jensen NJ, Wodschow HZ, Nilsson M, Rungby J (2020). Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int J Mol Sci, 21:8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koh S, Dupuis N, Auvin S (2020). Ketogenic diet and Neuroinflammation. Epilepsy Res, 167:106454. [DOI] [PubMed] [Google Scholar]
- [17].Fontana L, Ghezzi L, Cross AH, Piccio L (2021). Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J Exp Med, 218:e20190086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wheless JW (2008). History of the ketogenic diet. Epilepsia, 49Suppl 8:3-5. [DOI] [PubMed] [Google Scholar]
- [19].Cervenka MC, Terao NN, Bosarge JL, Henry BJ, Klees AA, Morrison PF, et al. (2012). E-mail management of the modified Atkins Diet for adults with epilepsy is feasible and effective. Epilepsia, 53:728-732. [DOI] [PubMed] [Google Scholar]
- [20].McDonald TJW, Cervenka MC (2018). Ketogenic Diets for Adult Neurological Disorders. Neurotherapeutics, 15:1018-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Davis JJ, Fournakis N, Ellison J (2021). Ketogenic Diet for the Treatment and Prevention of Dementia: A Review. J Geriatr Psychiatry Neurol, 34:3-10. [DOI] [PubMed] [Google Scholar]
- [22].Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, et al. (2020). Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev, 21:e13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guzmán M, Blázquez C (2001). Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab, 12:169-173. [DOI] [PubMed] [Google Scholar]
- [24].Vazquez-Vela ME, Torres N, Tovar AR (2008). White adipose tissue as endocrine organ and its role in obesity. Arch Med Res, 39:715-728. [DOI] [PubMed] [Google Scholar]
- [25].Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, et al. (2015). Mitochondria: The ketogenic diet-A metabolism-based therapy. Int J Biochem Cell Biol, 63:55-59. [DOI] [PubMed] [Google Scholar]
- [26].Hegardt FG (1999). Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J., 1999; 338(Pt 3):569-582. [PMC free article] [PubMed] [Google Scholar]
- [27].Newman JC, Verdin E (2014). beta-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract, 106:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Halestrap AP (2012). The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life, 64:1-9. [DOI] [PubMed] [Google Scholar]
- [29].Balasse EO, Féry F (1989). Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev, 5:247-270. [DOI] [PubMed] [Google Scholar]
- [30].Green A, Bishop RE (2019). Ketoacidosis - Where Do the Protons Come From? Trends Biochem Sci, 44:484-489. [DOI] [PubMed] [Google Scholar]
- [31].Abdul Kadir A, Clarke K, Evans RD (2020). Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis, 1866:165739. [DOI] [PubMed] [Google Scholar]
- [32].Cahill GF Jr. (2006). Fuel metabolism in starvation. Annu Rev Nutr, 26:1-22. [DOI] [PubMed] [Google Scholar]
- [33].Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, et al. (2020). Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov, 19:609-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, et al. (1995). Medical aspects of ketone body metabolism. Clin Invest Med, 18:193-216. [PubMed] [Google Scholar]
- [35].Waitt AE, Reed L, Ransom BR, Brown AM (2017). Emerging Roles for Glycogen in the CNS. Front Mol Neurosci, 10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Veech RL (2004). The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids, 70:309-319. [DOI] [PubMed] [Google Scholar]
- [37].Morris G, Maes M, Berk M, Carvalho AF, Puri BK (2020). Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur Psychiatry, 63:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR (2001). Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int, 38:519-527. [DOI] [PubMed] [Google Scholar]
- [39].Hargrave SL, Davidson TL, Lee TJ, Kinzig KP (2015). Brain and behavioral perturbations in rats following Western diet access. Appetite, 93:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W, et al. (2019). Brain Endothelial Cells Maintain Lactate Homeostasis and Control Adult Hippocampal Neurogenesis. Cell Stem Cell, 25:754-767.e9. [DOI] [PubMed] [Google Scholar]
- [41].Pierre K, Magistretti PJ, Pellerin L (2002). MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab, 22:586-595. [DOI] [PubMed] [Google Scholar]
- [42].Achanta LB, Rae CD (2017). beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res, 42:35-49. [DOI] [PubMed] [Google Scholar]
- [43].Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE (2014). Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes, 63:1259-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zarnowska IM (2020). Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients, 12:2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Koppel SJ, Swerdlow RH (2018). Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int, 117:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF Jr (2001). Ketone bodies, potential therapeutic uses. IUBMB Life, 51:241-247. [DOI] [PubMed] [Google Scholar]
- [47].Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987). Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res, 18:551-561. [DOI] [PubMed] [Google Scholar]
- [48].de Cabo R, Mattson MP (2019). Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med, 381:2541-2551. [DOI] [PubMed] [Google Scholar]
- [49].Norwitz NG, Hu MT, Clarke K (2019). The Mechanisms by Which the Ketone Body D-beta-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson's Disease. Front Nutr, 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, et al. (1995). Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J., 9:651-658. [DOI] [PubMed] [Google Scholar]
- [51].Thomas HE, Zhang Y, Stefely JA, Veiga SR, Thomas G, Kozma SC, et al. (2018). Mitochondrial Complex I Activity Is Required for Maximal Autophagy. Cell Rep, 24:2404-2417 e2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, et al. (2003). D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest, 112:892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008). Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem, 283:9089-9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, et al. (2014). A high-fat diet and NAD (+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab, 20:840-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Poff AM, Koutnik AP, Egan B (2020). Nutritional Ketosis with Ketogenic Diets or Exogenous Ketones: Features, Convergence, and Divergence. Curr Sports Med Rep, Jul; 19(7):251-259. [DOI] [PubMed] [Google Scholar]
- [56].Miller VJ, Villamena FA, Volek JS (2018). Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J Nutr Metab, 2018: 5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Patterson RE, Sears DD (2017). Metabolic Effects of Intermittent Fasting. Annu Rev Nutr, 37:371-393. [DOI] [PubMed] [Google Scholar]
- [58].Page KA, Williamson A, Yu N, McNay EC, Dzuira J, McCrimmon RJ, et al. (2009). Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes, 58:1237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wood TR, Stubbs BJ, Juul SE (2018). Exogenous Ketone Bodies as Promising Neuroprotective Agents for Developmental Brain Injury. Dev Neurosci, 40:451-462. [DOI] [PubMed] [Google Scholar]
- [60].Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. (2017). On the Metabolism of Exogenous Ketones in Humans. Front Physiol, 8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Soto-Mota A, Vansant H, Evans RD, Clarke K (2019). Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol, 109:104506. [DOI] [PubMed] [Google Scholar]
- [62].Bradley JA SR, Hill GL, Morgan DB (1981). Ketone kinetics in man. Horm Metab Res, 13:131-134. [DOI] [PubMed] [Google Scholar]
- [63].Robinson AM, Williamson DH (1980). Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev, Jan;60:143-187. [DOI] [PubMed] [Google Scholar]
- [64].Pardridge WM (1991). Blood-brain barrier transport of glucose, free fatty acids, and ketone bodies. Adv Exp Med Biol, 291:43-53. [DOI] [PubMed] [Google Scholar]
- [65].Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R, et al. (2011). Mild experimental ketosis increases brain uptake of 11C-acetoacetate and 18F-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr Neurosci, 14:51-58. [DOI] [PubMed] [Google Scholar]
- [66].Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB (1995). Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am J Physiol 268(6 Pt 1):E1161-E1166. [DOI] [PubMed] [Google Scholar]
- [67].Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC (2007). Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab, 292:E1607-1615. [DOI] [PubMed] [Google Scholar]
- [68].Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. (2017). Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab, 26:547-557 e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ulamek-Koziol M, Pluta R, Bogucka-Kocka A, Czuczwar SJ (2016). To treat or not to treat drug-refractory epilepsy by the ketogenic diet? That is the question. Ann Agric Environ Med, 23:533-536. [DOI] [PubMed] [Google Scholar]
- [70].Rusek M, Pluta R, Ulamek-Koziol M, Czuczwar SJ (2019). Ketogenic Diet in Alzheimer's Disease. Int J Mol Sci, 20:3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].DiSabato DJ, Quan N, Godbout JP (2016). Neuroinflammation: the devil is in the details. J Neurochem, 139 Suppl 2:136-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chen HL, Liao F, Lin TN, Liu FT (2014). Galectins and neuroinflammation. Adv Neurobiol, 9:517-542. [DOI] [PubMed] [Google Scholar]
- [73].Kwon HS, Koh SH (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener, 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2017). Neuroinflammation pathways: a general review. Int J Neurosci, 127:624-633. [DOI] [PubMed] [Google Scholar]
- [75].Leng F, Edison P (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol, 17:157-172. [DOI] [PubMed] [Google Scholar]
- [76].Chen WW, Zhang X, Huang WJ (2016). Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep, 13:3391-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schain M, Kreisl WC (2017). Neuroinflammation in Neurodegenerative Disorders-a Review. Curr Neurol Neurosci Rep, 17:25. [DOI] [PubMed] [Google Scholar]
- [78].Newman JC, Verdin E (2017). beta-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr, 37:51-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (2005). (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem, 280:26649-26652. [DOI] [PubMed] [Google Scholar]
- [80].Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Muller-Fielitz H, et al. (2014). The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun, 5:3944. [DOI] [PubMed] [Google Scholar]
- [81].Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, et al. (2015). Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation, 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Trotta MC, Maisto R, Guida F, Boccella S, Luongo L, Balta C, et al. (2019). The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS One, 14:e0211005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. (2015). The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med, 21:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhao M, Huang X, Cheng X, Lin X, Zhao T, Wu L, et al. (2017). Ketogenic diet improves the spatial memory impairment caused by exposure to hypobaric hypoxia through increased acetylation of histones in rats. PLoS One, 12:e0174477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ (2019). Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol Neurobiol, 56:3295-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Huang C, Wang P, Xu X, Zhang Y, Gong Y, Hu W, et al. (2018). The ketone body metabolite beta-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia, 66:256-278. [DOI] [PubMed] [Google Scholar]
- [87].Christofides A, Konstantinidou E, Jani C, Boussiotis VA (2021). The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism, 114:154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mirza AZ, Althagafi II, Shamshad H (2019). Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem, 166:502-513. [DOI] [PubMed] [Google Scholar]
- [89].Grabacka M, Pierzchalska M, Dean M, Reiss K (2016). Regulation of Ketone Body Metabolism and the Role of PPARalpha. Int J Mol Sci, 17:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fujita Y, Yamashita T (2018). Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front Neurosci, 12:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bentourkia M, Tremblay S, Pifferi F, Rousseau J, Lecomte R, Cunnane S (2009). PET study of 11C-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am J Physiol Endocrinol Metab, 296:E796-801. [DOI] [PubMed] [Google Scholar]
- [92].Morris G, Puri BK, Maes M, Olive L, Berk M, Carvalho AF (2020). The role of microglia in neuroprogressive disorders: mechanisms and possible neurotherapeutic effects of induced ketosis. Prog Neuropsychopharmacol Biol Psychiatry, 99:109858. [DOI] [PubMed] [Google Scholar]
- [93].Ulamek-Koziol M, Czuczwar SJ, Januszewski S, Pluta R (2019). Ketogenic Diet and Epilepsy. Nutrients, 11:2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liberato MV, Nascimento AS, Ayers SD, Lin JZ, Cvoro A, Silveira RL, et al. (2012). Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) gamma activators and pan-PPAR partial agonists. PLoS One, 7:e36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cullingford TE (2004). The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fatty Acids, 70:253-264. [DOI] [PubMed] [Google Scholar]
- [96].Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, et al. (2011). Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol, 232:195-202. [DOI] [PubMed] [Google Scholar]
- [97].Yang XY, Wang LH, Farrar WL (2008). A Role for PPARgamma in the Regulation of Cytokines in Immune Cells and Cancer. PPAR Res, 2008: 961753. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [98].Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (2011). The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res, 2:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ji H, Wang H, Zhang F, Li X, Xiang L, Aiguo S (2010). PPARgamma agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogen-activated protein kinase signaling pathways. Inflamm Res, 59:921-929. [DOI] [PubMed] [Google Scholar]
- [100].Xing B, Xin T, Hunter RL, Bing G (2008). Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J Neuroinflammation, 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fumagalli M, Lombardi M, Gressens P, Verderio C (2018). How to reprogram microglia toward beneficial functions. Glia, 66:2531-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Glick D, Barth S, Macleod KF (2010). Autophagy: cellular and molecular mechanisms. J Pathol, 221:3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bourdenx M, Martin-Segura A, Scrivo A, Rodriguez-Navarro JA, Kaushik S, Tasset I, et al. (2021). Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell, 184:2696-2714 e2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, et al. (2021). Autophagy in healthy aging and disease. Nature Aging, 1:634-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Scrivo A, Bourdenx M, Pampliega O, Cuervo AM (2018). Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol, 17:802-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. (2019). Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci, 22:401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Levine B, Mizushima N, Virgin HW (2011). Autophagy in immunity and inflammation. Nature, 469:323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Matsuzawa-Ishimoto Y, Hwang S, Cadwell K (2018). Autophagy and Inflammation. Annu Rev Immunol, 36:73-101. [DOI] [PubMed] [Google Scholar]
- [109].Camberos-Luna L, Geronimo-Olvera C, Montiel T, Rincon-Heredia R, Massieu L (2016). The Ketone Body, beta-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem Res, 41:600-609. [DOI] [PubMed] [Google Scholar]
- [110].McCarty MF, DiNicolantonio JJ, O'Keefe JH (2015). Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med Hypotheses, 85:631-639. [DOI] [PubMed] [Google Scholar]
- [111].Loos B, Klionsky DJ, Wong E (2017). Augmenting brain metabolism to increase macro- and chaperone-mediated autophagy for decreasing neuronal proteotoxicity and aging. Prog Neurobiol, 156:90-106. [DOI] [PubMed] [Google Scholar]
- [112].Finn PF, Dice JF (2005). Ketone bodies stimulate chaperone-mediated autophagy. J Biol Chem, 280:25864-25870. [DOI] [PubMed] [Google Scholar]
- [113].Montiel T, Montes-Ortega LA, Flores-Yanez S, Massieu L (2020). Treatment with the Ketone Body D-beta-hydroxybutyrate Attenuates Autophagy Activated by NMDA and Reduces Excitotoxic Neuronal Damage in the Rat Striatum In Vivo. Curr Pharm Des, 26:1377-1387. [DOI] [PubMed] [Google Scholar]
- [114].Liskiewicz D, Liskiewicz A, Nowacka-Chmielewska MM, Grabowski M, Pondel N, Grabowska K, et al. (2021). Differential Response of Hippocampal and Cerebrocortical Autophagy and Ketone Body Metabolism to the Ketogenic Diet. Front Cell Neurosci, 15:733607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hu LT, Zhu BL, Lai YJ, Long Y, Zha JS, Hu XT, et al. (2017). HMGCS2 promotes autophagic degradation of the amyloid-beta precursor protein through ketone body-mediated mechanisms. Biochem Biophys Res Commun, 486:492-498. [DOI] [PubMed] [Google Scholar]
- [116].Kellar D, Craft S (2020). Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol, 19:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Saltiel AR, Olefsky JM (2017). Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest, 127:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Akhtar A, Sah SP (2020). Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer's disease. Neurochem Int, 135:104707. [DOI] [PubMed] [Google Scholar]
- [119].Pugazhenthi S, Qin L, Reddy PH (2017). Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis, 1863:1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Juranek J, Ray R, Banach M, Rai V (2015). Receptor for advanced glycation end-products in neurodegenerative diseases. Rev Neurosci, 26:691-698. [DOI] [PubMed] [Google Scholar]
- [121].Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. (2020). Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes, 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mohamed HE, El-Swefy SE, Rashed LA, Abd El-Latif SK (2010). Biochemical effect of a ketogenic diet on the brains of obese adult rats. J Clin Neurosci, 17:899-904. [DOI] [PubMed] [Google Scholar]
- [123].Campbell I, Campbell H (2020). Mechanisms of insulin resistance, mitochondrial dysfunction and the action of the ketogenic diet in bipolar disorder. Focus on the PI3K/AKT/HIF1-a pathway. Med Hypotheses, 145:110299. [DOI] [PubMed] [Google Scholar]
- [124].Gharib R, Khatibi A, Khodarahmi R, Haidari M, Husseinzadeh S (2020). Study of glycation process of human carbonic anhydrase II as well as investigation concerning inhibitory influence of 3-beta-hydroxybutyrate on it. Int J Biol Macromol, 149:443-449. [DOI] [PubMed] [Google Scholar]
- [125].Bohlooli M, Moosavi-Movahedi AA, Taghavi F, Saboury AA, Maghami P, Seyedarabi A, et al. (2014). Inhibition of fluorescent advanced glycation end products (AGEs) of human serum albumin upon incubation with 3-beta-hydroxybutyrate. Mol Biol Rep, 41:3705-3713. [DOI] [PubMed] [Google Scholar]
- [126].Sabokdast M, Habibi-Rezaei M, Moosavi-Movahedi AA, Ferdousi M, Azimzadeh-Irani E, Poursasan N (2015). Protection by beta-Hydroxybutyric acid against insulin glycation, lipid peroxidation and microglial cell apoptosis. Daru, 23:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Asrih M, Altirriba J, Rohner-Jeanrenaud F, Jornayvaz FR (2015). Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue. PLoS One, 10:e0126364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ghosh S, Castillo E, Frias ES, Swanson RA (2018). Bioenergetic regulation of microglia. Glia, 66:1200-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sung B, Park S, Yu BP, Chung HY (2004). Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci, 59:997-1006. [DOI] [PubMed] [Google Scholar]
- [130].Swindell WR (2009). Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics, 10:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Maalouf M, Rho JM, Mattson MP (2009). The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev, 59:293-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hernandez-Saavedra D, Moody L, Xu GB, Chen H, Pan YX (2019). Epigenetic Regulation of Metabolism and Inflammation by Calorie Restriction. Adv Nutr, 10:520-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yamada K, Takizawa S, Ohgaku Y, Asami T, Furuya K, Yamamoto K, et al. (2020). MicroRNA 16-5p is upregulated in calorie-restricted mice and modulates inflammatory cytokines of macrophages. Gene, 725:144191. [DOI] [PubMed] [Google Scholar]
- [134].Gabande-Rodriguez E, Gomez de Las Heras MM, Mittelbrunn M (2019). Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells, 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Martin CR, Osadchiy V, Kalani A, Mayer EA (2018). The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol, 6:133-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Rawat K, Singh N, Kumari P, Saha L (2021). A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev Neurosci, 32:143-157. [DOI] [PubMed] [Google Scholar]
- [137].Quigley EMM (2017). Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep, 17:94. [DOI] [PubMed] [Google Scholar]
- [138].Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, et al. (2018). Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep, 8:6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Nagpal R, Neth BJ, Wang S, Craft S, Yadav H (2019). Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine, 47:529-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. (2020). Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell, 181:1263-1275 e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gubert C, Kong G, Renoir T, Hannan AJ (2020). Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol Dis, 134:104621. [DOI] [PubMed] [Google Scholar]
- [142].Morkl S, Lackner S, Meinitzer A, Mangge H, Lehofer M, Halwachs B, et al. (2018). Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr, 57:2985-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Isaev NK, Stelmashook EV, Genrikhs EE (2019). Neurogenesis and brain aging. Rev Neurosci, 30:573-580. [DOI] [PubMed] [Google Scholar]
- [144].Pan Y, Larson B, Araujo JA, Lau W, de Rivera C, Santana R, et al. (2010). Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br J Nutr, 103:1746-1754. [DOI] [PubMed] [Google Scholar]
- [145].Hernandez AR, Hernandez CM, Campos KT, Truckenbrod LM, Sakarya Y, McQuail JA, et al. (2018). The Antiepileptic Ketogenic Diet Alters Hippocampal Transporter Levels and Reduces Adiposity in Aged Rats. J Gerontol A Biol Sci Med Sci, 73:450-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wang D, Mitchell ES (2016). Cognition and Synaptic-Plasticity Related Changes in Aged Rats Supplemented with 8- and 10-Carbon Medium Chain Triglycerides. PLoS One, 11:e0160159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lane CA, Hardy J, Schott JM (2018). Alzheimer's disease. Eur J Neurol, 25:59-70. [DOI] [PubMed] [Google Scholar]
- [148].Eratne D, Loi SM, Farrand S, Kelso W, Velakoulis D, Looi JC (2018). Alzheimer's disease: clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry, 26:347-357. [DOI] [PubMed] [Google Scholar]
- [149].Anderson ND (2019). State of the science on mild cognitive impairment (MCI). CNS Spectr, 24:78-87. [DOI] [PubMed] [Google Scholar]
- [150].Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000). D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A, 97:5440-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Versele R, Corsi M, Fuso A, Sevin E, Businaro R, Gosselet F, et al. (2020). Ketone Bodies Promote Amyloid-beta1-40 Clearance in a Human in Vitro Blood-Brain Barrier Model. Int J Mol Sci, 21:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Li X, Zhan Z, Zhang J, Zhou F, An L (2020). beta-Hydroxybutyrate Ameliorates Abeta-Induced Downregulation of TrkA Expression by Inhibiting HDAC1/3 in SH-SY5Y Cells. Am J Alzheimers Dis Other Demen, 35:1533317519883496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Van der Auwera I, Wera S, Van Leuven F, Henderson ST (2005). A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer's disease. Nutr Metab (Lond), 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, et al. (2013). A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging, 34:1530-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Shippy DC, Wilhelm C, Viharkumar PA, Raife TJ, Ulland TK (2020). beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer's disease pathology. J Neuroinflammation, 17:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Aso E, Semakova J, Joda L, Semak V, Halbaut L, Calpena A, et al. (2013). Triheptanoin supplementation to ketogenic diet curbs cognitive impairment in APP/PS1 mice used as a model of familial Alzheimer's disease. Curr Alzheimer Res, Mar; 10:290-297. [DOI] [PubMed] [Google Scholar]
- [157].Wu Y, Gong Y, Luan Y, Li Y, Liu J, Yue Z, et al. (2020). BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer's disease. FASEB J, 34:1412-1429. [DOI] [PubMed] [Google Scholar]
- [158].Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, Veech RL (2017). Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J Neurochem, 141:195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan JS, et al. (2013). 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer's disease via mitochondria protection mechanism. Biomaterials, 34:7552-7562. [DOI] [PubMed] [Google Scholar]
- [160].Xie G, Tian W, Wei T, Liu F (2015). The neuroprotective effects of β-hydroxybutyrate on Aβ-injected rat hippocampus in vivo and in Aβ-treated PC-12 cells in vitro. Free Radic Res, 49:139-150. [DOI] [PubMed] [Google Scholar]
- [161].Brownlow ML, Benner L, D'Agostino D, Gordon MN, Morgan D (2013). Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer's pathology. PLoS One, 8:e75713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pawlosky RJ, Kashiwaya Y, King MT, Veech RL (2020). A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer's Disease. Int J Mol Sci, 21:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Neth BJ, Mintz A, Whitlow C, Jung Y, Solingapuram Sai K, Register TC, et al. (2020). Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study. Neurobiol Aging, 86:54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL (2015). Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin, 3:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Nagpal R, Neth BJ, Wang S, Mishra SP, Craft S, Yadav H (2020). Gut mycobiome and its interaction with diet, gut bacteria and alzheimer's disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine, 59:102950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Phillips MCL, Deprez LM, Mortimer GMN, Murtagh DKJ, McCoy S, Mylchreest R, et al. (2021). Randomized crossover trial of a modified ketogenic diet in Alzheimer's disease. Alzheimers Res Ther, 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, et al. (2019). Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett, 690:232-236. [DOI] [PubMed] [Google Scholar]
- [168].Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC (2019). Preliminary Report on the Feasibility and Efficacy of the Modified Atkins Diet for Treatment of Mild Cognitive Impairment and Early Alzheimer's Disease. J Alzheimers Dis, 68:969-981. [DOI] [PubMed] [Google Scholar]
- [169].Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC (2009). Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond), 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. (2004). Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiology of Aging, 25:311-314. [DOI] [PubMed] [Google Scholar]
- [171].Ohnuma T, Toda A, Kimoto A, Takebayashi Y, Higashiyama R, Tagata Y, et al. (2016). Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer's disease: a prospective, open-label pilot study. Clin Interv Aging, 11:29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Henderson ST, Morimoto BH, Cummings JL, Farlow MR, Walker J (2020). A Placebo-Controlled, Parallel-Group, Randomized Clinical Trial of AC-1204 in Mild-to-Moderate Alzheimer's Disease. J Alzheimers Dis, 75:547-557. [DOI] [PubMed] [Google Scholar]
- [173].Croteau E, Castellano CA, Richard MA, Fortier M, Nugent S, Lepage M, et al. (2018). Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer's Disease. J Alzheimers Dis, 64:551-561. [DOI] [PubMed] [Google Scholar]
- [174].Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, et al. (2018). Changes in regional cerebral blood flow associated with a 45day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer's disease: Results of a randomized, double-blinded, pilot study. Exp Gerontol, 111:118-121. [DOI] [PubMed] [Google Scholar]
- [175].Raza C, Anjum R, Shakeel NUA (2019). Parkinson's disease: Mechanisms, translational models and management strategies. Life Sci, 226:77-90. [DOI] [PubMed] [Google Scholar]
- [176].Hayes MT (2019). Parkinson's Disease and Parkinsonism. Am J Med, 132:802-807. [DOI] [PubMed] [Google Scholar]
- [177].Dorsey ER, Sherer T, Okun MS, Bloem BR (2018). The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis, 8:S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Imamura K, Takeshima T, Kashiwaya Y, Nakaso K, Nakashima K (2006). D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson's disease. J Neurosci Res, 84:1376-1384. [DOI] [PubMed] [Google Scholar]
- [179].Kabiraj P, Pal R, Varela-Ramirez A, Miranda M, Narayan M (2012). Nitrosative stress mediated misfolded protein aggregation mitigated by Na-D-beta-hydroxybutyrate intervention. Biochem Biophys Res Commun, 426:438-444. [DOI] [PubMed] [Google Scholar]
- [180].Cheng B, Yang X, Hou Z, Lin X, Meng H, Li Z, et al. (2007). D-beta-hydroxybutyrate inhibits the apoptosis of PC12 cells induced by 6-OHDA in relation to up-regulating the ratio of Bcl-2/Bax mRNA. Auton Neurosci, 134:38-44. [DOI] [PubMed] [Google Scholar]
- [181].Deora V, Albornoz EA, Zhu K, Woodruff TM, Gordon R (2017). The Ketone Body beta-Hydroxybutyrate Does Not Inhibit Synuclein Mediated Inflammasome Activation in Microglia. J Neuroimmune Pharmacol, 12:568-574. [DOI] [PubMed] [Google Scholar]
- [182].Joniec-Maciejak I, Wawer A, Turzyńska D, Sobolewska A, Maciejak P, Szyndler J, et al. (2018). Octanoic acid prevents reduction of striatal dopamine in the MPTP mouse model of Parkinson’s disease. Pharmacological Reports, 70:988-992. [DOI] [PubMed] [Google Scholar]
- [183].Cheng B, Yang X, An L, Gao B, Liu X, Liu S (2009). Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson's disease. Brain Res, 1286:25-31. [DOI] [PubMed] [Google Scholar]
- [184].Yang X, Cheng B (2010). Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci, 42:145-153. [DOI] [PubMed] [Google Scholar]
- [185].Kuter KZ, Olech Ł, Głowacka U, Paleczna M (2021). Increased Beta-Hydroxybutyrate Level Is Not Sufficient for the Neuroprotective Effect of Long-Term Ketogenic Diet in an Animal Model of Early Parkinson's Disease. Exploration of Brain and Liver Energy Metabolism Markers. Int J Mol Sci, 22(14):7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB (2005). Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology, 64:728-730. [DOI] [PubMed] [Google Scholar]
- [187].Koyuncu H, Fidan V, Toktas H, Binay O, Celik H (2021). Effect of ketogenic diet versus regular diet on voice quality of patients with Parkinson's disease. Acta Neurol Belg, 121:1729-1732. [DOI] [PubMed] [Google Scholar]
- [188].Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP (2018). Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov Disord, 33:1306-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Elbarbry F, Nguyen V, Mirka A, Zwickey H, Rosenbaum R (2019). A new validated HPLC method for the determination of levodopa: Application to study the impact of ketogenic diet on the pharmacokinetics of levodopa in Parkinson's participants. Biomed Chromatogr, 33:e4382. [DOI] [PubMed] [Google Scholar]
- [190].Brown RH, Al-Chalabi A (2017). Amyotrophic Lateral Sclerosis. N Engl J Med, 377:162-172. [DOI] [PubMed] [Google Scholar]
- [191].Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. (2006). A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci, 7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Tefera TW, Wong Y, Barkl-Luke ME, Ngo ST, Thomas NK, McDonald TS, et al. (2016). Triheptanoin Protects Motor Neurons and Delays the Onset of Motor Symptoms in a Mouse Model of Amyotrophic Lateral Sclerosis. PLoS One, 11:e0161816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zhao W, Varghese M, Vempati P, Dzhun A, Cheng A, Wang J, et al. (2012). Caprylic triglyceride as a novel therapeutic approach to effectively improve the performance and attenuate the symptoms due to the motor neuron loss in ALS disease. PLoS One, 7:e49191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ari C, Poff AM, Held HE, Landon CS, Goldhagen CR, Mavromates N, et al. (2014). Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS One, 9:e103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].McColgan P, Tabrizi SJ (2018). Huntington's disease: a clinical review. Eur J Neurol, 25:24-34. [DOI] [PubMed] [Google Scholar]
- [196].Chen JY, Tran C, Hwang L, Deng G, Jung ME, Faull KF, et al. (2016). Partial Amelioration of Peripheral and Central Symptoms of Huntington's Disease via Modulation of Lipid Metabolism. J Huntingtons Dis, 5:65-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lim S, Chesser AS, Grima JC, Rappold PM, Blum D, Przedborski S, et al. (2011). D-beta-hydroxybutyrate is protective in mouse models of Huntington's disease. PLoS One, 6:e24620. [DOI] [PMC free article] [PubMed] [Google Scholar]