Abstract
Hepatic ischemia/reperfusion injury (IRI) is mainly characterized by high activation of immune inflammatory responses and metabolic responses. Understanding the molecular and metabolic mechanisms underlying development of hepatic IRI is critical for developing effective therapies for hepatic IRI. Recent advances in research have improved our understanding of the pathogenesis of IRI. During IRI, hepatocyte injury and inflammatory responses are mediated by crosstalk between the immune cells and metabolic components. This crosstalk can be targeted to treat or reverse hepatic IRI. Thus, a deep understanding of hepatic microenvironment, especially the immune and metabolic responses, can reveal new therapeutic opportunities for hepatic IRI. In this review, we describe important cells in the liver microenvironment (especially non-parenchymal cells) that regulate immune inflammatory responses. The role of metabolic components in the diagnosis and prevention of hepatic IRI are discussed. Furthermore, recent updated therapeutic strategies based on the hepatic microenvironment, including immune cells and metabolic components, are highlighted.
Keywords: hepatic microenvironment, ischemia/reperfusion injury, immune cell, metabolic compartment, inflammatory response, therapeutic strategies
1. Introduction
Ischemia/reperfusion injury (IRI), resulting from ischemic insult and subsequent blood reperfusion, occurs in all aerobic cells that require mitochondrial oxidative phosphorylation for energy provision [1]. IRI is caused by trauma, shock, ischemic stroke, thrombolysis, coronary disease intervention and major surgeries [2-4]. Two major types of liver IRI have been reported, including warm injury and cold injury. Warm injury occurs during shock, trauma, respiratory failure, bleeding, heart failure, and prolonged surgical liver resection due to impaired blood perfusion [5,6]. Cold injury mainly occurs during liver transplantation due to ex vivo cold-preservation of the donor organ, and the subsequent warm reperfusion to the implanted organ [7].
The degree of hepatic IRI is dependent on duration and type of ischemia, as well as the condition of the liver [8,9]. In a clinical trial, cirrhotic or chronic liver injury showed worse tolerance to IR insult [10]. Evidence from preclinical experiments also support the standpoint that the background of hepatic microenvironment significantly determines the tolerance of IR [11].
The development of hepatic IRI is a complex process involving several factors such as metabolic disorders and inflammatory responses [12]. Considerable studies have investigated the molecular mechanisms of hepatic IRI, especially roles of Kupffer cells (KCs) in the generation of reactive oxygen species (ROS) and regulation of inducible nitric oxide synthase [13]. However, few studies have explored the impact of metabolic components on the development and progression of IRI. Furthermore, the key role of immune and metabolic compartments in hepatic microenvironment has not been systematically reported yet. In this review, the pathological changes associated with hepatic IRI are stated as well as the role of hepatic microenvironment (especially non-parenchymal cells). The immune responses and metabolic compartments involved in the process of IRI are discussed. Also, current therapeutic strategies for IRI are presented. Finally, the clinical translation potential of the findings from basic research are reviewed.
2. Pathophysiological alterations of hepatic IRI
Originally, IRI was first described by Jennings in 1960 as a phenomenon caused by hypoxia and accentuated by restoration of oxygen into tissues [14]. Development of hepatic IRI has two interrelated phases: ischemic insult and inflammation-mediated reperfusion injury (Fig. 1).
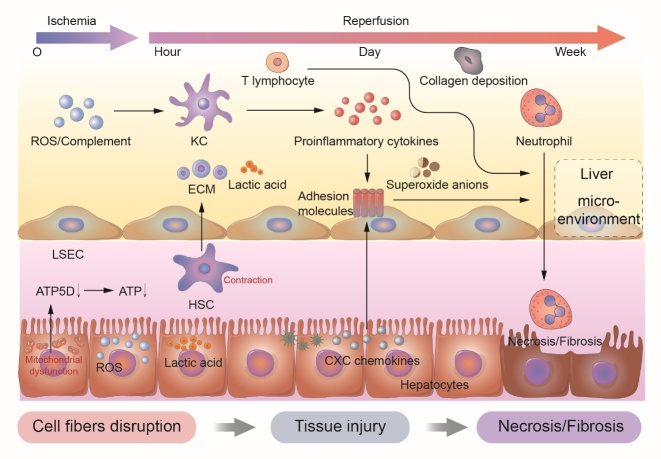
Figure 1.
The regulation of liver microenvironment during different periods of hepatic ischemia/reperfusion injury. A summary of the immune components of liver microenvironment during the continuous phase of ischemia/reperfusion injury is manifested. LSEC, liver sinusoidal endothelial cell; HSC, hepatic stellate cell; KC, kupffer cell; ROS, reactive oxygen species; ECM, extracellular matrix.
During liver transplantation, early allograft dysfunction (EAD) and primary non-function (PNF) have been associated with high rate of mortality and often due to perioperative IRI [15,16]. Numerous studies have demonstrated that IRI is one of the most important factors leading to EAD which has an incidence up to 43.7% in patients with IRI [17,18]. Hepatic EAD and failure of remnant liver increase the shortage of liver donors [19]. More importantly, the two factors may complicate post-transplant patient care, leading to poor liver transplantation (LT) outcomes. To improve outcomes, the specific mechanisms leading to hepatic IRI, and more targeted therapies should be explored.
The ischemia phase, which initiates reperfusion injury, is characterized by various factors involved in the inflammatory reactions. During this phase, vascular closure or obstruction decrease the expression of adenosis triphosphate synthase subunit delta (ATP5D) of the respiratory chain in mitochondria, thereby compromising ATP synthesis [20-22]. This is also accompanied by aggregation of lactic acid and ketone bodies in hepatic cells, causing metabolic acidosis [23].
The process of reperfusion has two phases. During the initial phase, KCs (liver-resident macrophages) are activated to induce oxidative stress. In the later period, 6-24 hours after reperfusion, numerous hepatic non-parenchymal cells are activated or accumulated to release inflammatory mediators, cytokines and complements [24,25]. Hypoxanthine oxidase catalyzes the breakdown of hypoxanthine to form water and oxygen, thus releasing ROS [26]. ROS generated by O2 reintroduction into ischemic tissues leads to severe liver damage. The mitochondria are important sources of ROS generated in the liver cells [27]. So, the maintenance of mitochondrial viability is important to the treatment of IRI. More importantly, it has been reported that AMP-activated protein kinase (AMPK) and protein kinase C (PKC) are activated by excessive AMP, resulting in the translocation of reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH) subunits p67 and p47 from the cytosol to membrane, where they activate membrane subunit p91 and NADPH oxidase in turn [28]. Activation of NADPH oxidase leads to the production of large amounts of superoxide anions, which aggravate hepatic damage, leading to organ failure.
3. Role of age in hepatic IRI
In recent years, the percentage of liver grafts obtained from aged donors (over 70 years old) has been on the increase. This has led to the development of strategies to prevent IRI in aged individuals receiving liver transplantation. Accumulating experimental evidence has indicated that the aging process of liver has 3 dominant processes: enhancement of inflammatory response, impairment of intracellular energy metabolism and alteration of autophagy [29].
It has been reported that enhancing intercellular adenosine triphosphate (ATP) levels through glucose administration has effectively mitigated liver IRI [30]. In addition, application of pentoxifylline to inhibit the activation of TNF-α and melatonin to increase nitric oxide (NO) formation alleviated liver damage in aged individuals [31]. As for the autophagy, aging contributes to autophagy impairment, which renders aged livers susceptible to IRI. Lithium, as an autophagy inducer, has been shown to be effective to restore the reduced tolerance to liver IRI [32].
4. Hepatic microenvironment
The hepatic microenvironment comprises hepatic parenchymal cells, hepatic non-parenchymal cells (hepatic stellate cells, macrophages, sinusoidal endothelial cells, neutrophils, and lymphocytes), extracellular matrix, and nervous systems. Both parenchymal and non-parenchymal cells in the microenvironment modulate IRI progression, especially non-parenchymal cells [33-35]. Therefore, the characteristics of non-parenchymal cells (Fig. 2) will be discussed in more details in the ensuing sections.
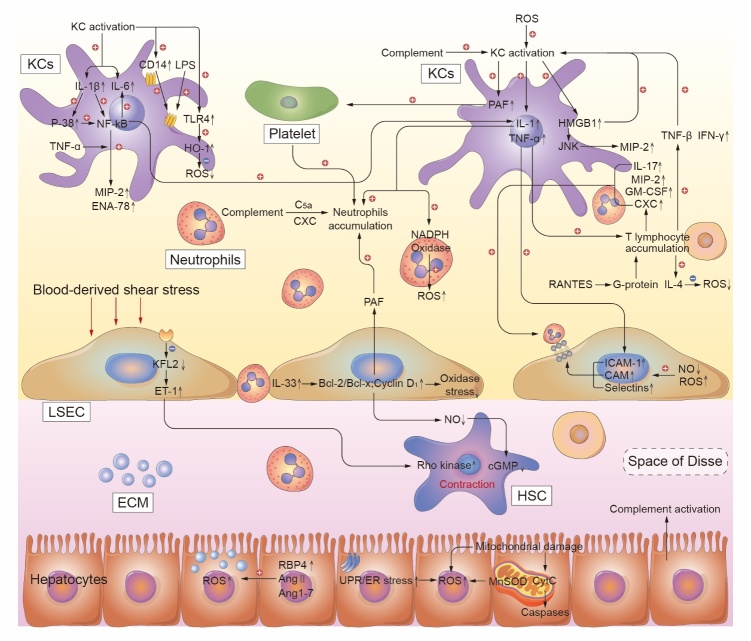
Figure 2.
The regulation of liver microenvironment components, including hepatic parenchymal cells, hepatic non-parenchymal cells (hepatic stellate cells, Kupffer cells, sinusoidal endothelial cells, neutrophils and lymphocytes), and extracellular matrix, during hepatic ischemia/reperfusion injury. A summary of the specific molecular mechanisms regulating hepatocytes and interactions in the liver microenvironment are shown. LSEC, liver sinusoidal endothelial cell; HSC, hepatic stellate cell; KC, Kupffer cell; ROS, reactive oxygen species; ECM, extracellular matrix; IL-1, interleukin 1; IL-6, interleukin 6; IL-17, interleukin 17; IL-33, interleukin 33; IL-1β, interleukin 1β; HMGB1, high-mobility group box 1; IAC, inflammation associated cytokine; TNF-α, tumor necrosis factor α; PAF, platelet activating factor; MIP-2, macrophage inflammatory protein 2; ENA-78, epithelial neutrophil activating protein 78; NF-κB, nuclear factor κB; TLR4, Toll like receptor 4; LPS, lipopolysaccharide; HO-1, heme oxygenase-1; RANTES, regulated upon activation normal T cell expressed and secreted factor; VEGF, vascular endothelial growth factor; IFN-γ, interferon γ; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICAM-1, intercellular adhesion molecule-1; Bcl-2/Bcl-x, B cell lymphoma 2/x; TXA2, thromboxane; KFL2, kruppel like transcription factor 2; ET-1, endothelin 1; JNK, N terminal kinase; CAM, cell adhesion molecule; cGMP, cyclic guanosine monophosphate; RBP4, retinol binding protein; Ang 1-7, angiotensin 1-7; Ang Ⅱ, angiotensin Ⅱ; MnSOD, manganese containing superoxide dismutase; CytC, cytochrome C.
4.1. Hepatic stellate cells
Hepatic stellate cells (HSCs), as the major components of the non-parenchymal cells in the liver, have important physiological and pathological roles on intrahepatic Disse space, which is an area between hepatocytes and sinusoids. HSCs can participate in the regulation of blood flow with extending cytoplasmic processes around hepatic sinusoids. Once activated, HSCs transform into myofibroblast-like cells, which express proteins like myosin and α-smooth muscle actin (α-SMA), allowing them to contract [36,37].
Endothelin (ET) has been reported to regulate hepatic sinus blood flow through HSCs [38]. Recent clinical trials have shown that HSCs are the effector of sevoflurane, which decrease ROS and hydrogen peroxide (H2O2) production, and hepatocytic apoptosis [39]. These effects are produced by suppressing the expression of BCL2-associated X (Bax) and elevating B cell lymphoma 2 (Bcl-2) levels[40]. In mouse model of IRI, Xu et al. showed that sevoflurane can inhibit the expression of high mobility group box 1 (HMGB1) to up-regulate microRNA (miR)-142 [41]. Adoptive HSCs confer protection against IRI by inducing newborn inducible regulatory T cells (iTregs) and increasing the stability of natural regulatory T cells (nTregs) [42]. Also, FGF10, belongs to the FGF subfamily, shown to be predominantly secreted by HSCs in vitro experiments [43]. In the early phase of IRI, overexpression of FGF10 alleviated liver dysfunction through the activation of phosphatidy-linositol-3-kinase (PI3K)/AKT/nuclear factor-erythroid 2-related factor 2 (NRF2) pathways. This protective role was abolished by NRF2 knockout in mice. Elsewhere, it was found that FGF10 overexpression also increased hepatocyte proliferation in the late phase of IRI [44,45].
The proliferation of HSCs at the boundaries of necrotic liver regions improves hepatic repair and regeneration [46]. Classical theory stipulates that those fibrotic livers are at a higher risk of developing IRI. However, fibrotic livers have an enhanced repair capacity compared with normal liver [47]. HSCs also can regulate the activity and viability of progenitor cells, adjacent hepatocytes, and Ly6Clo macrophages, to promote hepatic recovery after IRI [48,49].
4.2. Macrophages
Liver injury provokes the activation of Kupffer cells (KCs), infiltration of circulating macrophages, and employment of peritoneal macrophages. Different subpopulations of macrophages have varying roles in the development of liver IRI [50]. During liver injury, majority of KCs are rapidly activated by ROS, releasing HMGB1 and inflammation-associated cytokines (IAC) like interleukin 1 (IL-1), interleukin 6 (IL-6), interleukin 1β (IL-1β), TNF-α and platelet-activating factor (PAF) [51,52]. In turn, ROS can cause mitochondrial damage, resulting in the leakage of mitochondrial deoxyribo-nucleic acid (mtDNA) into the cytosol [53]. Then the mtDNA can be recognized by DNA sensor cyclic GMP-AMP synthase (cGAS), and can activate stimulator of interferon genes (STING), leading to impaired innate immune response [54]. A previous animal study showed that increased mtDNA induced STING activation in macrophages, which triggered a more severe immune response accompanied by upregulating of IL-6, IL-18, IL-1β, and TNF-α levels via the STING-NLRP3 pathway [55].
A proportion of KCs are activated by IFN-γ, which is produced by CD4+ T-cells and natural killer T-cells [56]. Indeed, TNF-α and IL-1 are among the most important cytokines contributing to the development of hepatic IRI. IL-1 can stimulate the release of ROS from neutrophils, thereby amplifying TNF-α production [57]. TNF-α also provokes the expression of P-selectin in liver sinusoidal endothelial cells (LSECs), hence contribute to neutrophil recruitment [58]. In addition, TNF-α has been reported to enhance the release of other factors like macrophage inflammatory protein-2 (MIP-2), epithelial neutrophil activating protein-78 (ENA-78), cytokine-induced neutrophil chemoattractant-1 (CINC), and various CXC motif chemokines [59], which significantly enhance neutrophils infiltration.
Recent studies have demonstrated that the role of nuclear factor κB (NF-κB) varies across different cell types [51]. In the liver, NF-κB mainly promotes the expression of TNF-α and IL-6, contributing to inflammatory responses [60]. Resting-state KCs express a small amount of CD14 receptor and Toll-like receptor 4 (TLR4). However, it has been shown that CD14 receptor and TLR4 are upregulated in response to hepatic IRI. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein, activates TLR4 upon binding to LPS, which leads to inflammatory responses and oxidative stress [61,62]. Moreover, the expression of transmembrane G protein-coupled bile acid receptor (TGR5) is highly observed in KCs. Yang et al. [63] found that TGR5 mitigated the increase in TLR4-NF-κB and reduced caspase 8 activation after IRI. In addition, TGR5 has been revealed to attenuate liver damage following IRI through the Keap 1-NRF2 pathway, as evidence by serum ALT and AST tests and cytokines expression [64]. Jin et al. [65] found that the activation of farnesoid X receptor (FXR) led to upregulation of small heterodimer partner (SHP) in KCs, which decreased the proinflammation injury but increased expression levels of anti-inflammatory gene expression following TLR stimulation.
The activation status of KCs correlates with the aggregation of platelets [66] and over 50% of platelets adhere to the activated KCs in the early-phase of IRI. This adherence to KCs disturbs hepatic microcirculation and aggravates hepatic IRI. These effects are mediated by the PAF released from activated KCs [67]. Furthermore, deletion of the serum complement attenuates KC-induced oxidative stress. Complement-depleted animals have decreased the accumulation of neutrophil [68-70]. Interestingly, it was found that macrophage extracellular trap was increased in co-cultured experiment subjected to IRI. Moreover, macrophage extracellular trap aggravated ferroptosis, thereby causing an increasing post-ischemia liver damage [71].
However, the effects of KCs in other studies are opposite. In most studies, the role of KCs was investigated at the tissue level but not at cell type-specific level. Tissue resident macrophages, derived from yolk sac, play an important role in the maintenance of tissue homeostasis [72,73]. Previously, it was found that the activation of immune response by IR was associated with the necrotic depletion of KCs. During post-ischemia, KCs conferred anti-inflammatory and anti-lethality effect, indicating that KCs can be a novel mechanism against liver IRI, such as RIP-1-dependent necrosis [74]. In addition, fully mature F4/80hi GATA6+ peritoneal cavity macrophages were reported to be recruited to the injury site via the mesothelium following liver injury. These cells acquired an activated phenotype and dismantle nuclei of necrotic cells to promote full revascularization of the injury site [75].
Heme oxygenase-1 (HO-1), a rate-limiting enzyme mainly released by KCs, emerges the anti-oxidative and anti-inflammatory functions. It has been reported that treatment with HO-1 inducer, Copp, decreased the levels of many inflammatory factors [76]. HO-1-SIRT1-p53 complex downregulated KCs recruitment thereby alleviated IRI [77]. In addition, a recent study has found that the activation of the NRF2/HO-1 pathway suppressed the NLRP3 inflammasome via enhancing KC autophagy, hence alleviated hepatic IRI [78]. Although KCs can polarize to M1 or M2, they usually polarize to M1 type after hepatic IRI, which aggravates hepatic IRI [79]. Activation of M2 macrophages has been reported to counteract the pro-inflammatory effects of M1 when activated, which inhibits pro-inflammatory signaling [80]. A previous study has demonstrated that SS-31, a mitochondrial-targeted antioxidant peptide, directly decreases ROS production and regulates signal transducer and activator of transcription 1/3 (STAT1/STAT3) signaling in macrophages, causing M2 polarization phenotype. The peptide also decreased the production of proinflammation cytokines, thereby mitigating inflammatory response in the liver [81].
4.3. T lymphocyte
T lymphocytes are derived from bone marrow pluripotent stem cells, which are a major component of lymphocytes. Mature T-lymphocytes are located in thymus-dependent areas of peripheral immune organs and involved in cellular immunity and immune regulation. In 1997, it was reported for the first time that T-lymphocytes are increased rapidly in post-ischemic liver, and it is the CD4+ T-cells but not CD8+ T-cells that accumulate in the liver 1 h after IRI [82].
An antigen-independent mechanism of T-lymphocyte activation following RANTES stimulation has been found to initiate the gathering of T-lymphocyte directly via a G-protein-coupled pathway [83]. Additionally, the CD154-CD40 T-cells co-stimulation pathway has been identified as an effective driver of T-lymphocyte accumulation and activation [84]. IL-17, released by T-lymphocytes, is associated with the recruitment of neutrophils. IL-17 also contributes to CXC chemokine secretion through other cells, including endothelial cells, fibroblasts, epithelial cells, and osteoblasts. Mice with CD4-konckout and treatment with anti-IL-17 antibodies showed reduced expression of macrophage inflammatory protein 2 (MIP-2) [85,86]. About 30% of recruited CD4+ T-cells are located in hepatic sinusoids, where they enhance platelet-adherence and neutrophil-recruitment, causing micro-vascular injury and hepatocyte cell death [87,88].
As early as in 1995, lymphocytes were reported to exert pro-inflammatory or anti-inflammatory effects by producing IFN-γ or IL-4, respectively [89]. IFN-γ triggers early inflammatory responses and KCs activation, but IL-4 suppresses the inflammatory response. Additionally, T-cell immunoglobulin mucin (TIM) family members may downregulate IR-triggered hepatic injury and cytokine/chemokine programs [90]. Indeed, interaction between TIM-3 and its galectin-9 (Gal-9) ligand inhibits Th1-mediated auto/allo-immune responses. Specifically, it can promote peripheral immune tolerance by readjusting macrophage activation and supporting hepatic homeostasis [91,92].
4.4. Neutrophils
Neutrophils, as innate immune cells, can identify inflammatory sites and eliminate microorganisms or damaging cells. It is inflammatory response overreaction that causing liver IRI. Some studies suggest that neutrophils have a pivotal role mainly in the late period of liver IRI [93,94].
Although hepatocytes generate ROS during the initial period of hepatic injury, it is ROS released by neutrophils that causes the most severe injury to hepatocytes, leading to mitochondrial permeability transition or mitochondrial dysfunction with calcium accumulation [95]. Once recruited to liver, neutrophils can express cell-surface adhesion molecules, such as P-selectin, L-selectin, and β2-integrins (CD11b/CD18) which bind to hepatocytes through the intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) on LSECs [96,97]. It has been suggested that high expression of adhesion molecules, especially P-selectin, increases neutrophils production and subsequent liver damage [98]. In one study, recombinant P-selection glycoprotein ligand immunoglobulin (rPSGL-Ig), as a glycoprotein that binds P-selectin, was found to inhibit neutrophil adhesion. Moreover, desferriexochelin 772SM (D-Exo) enhanced the capacity of rPSGL-Ig to better protect against liver IRI [99]. Degranulation of activated neutrophils may release numerous proteases, including cathepsin G, elastase, heparinase, and hydrolytic enzymes [100]. It has been found that treatments with protease inhibitors can attenuate hepatic damage, thus the ONO-5046 may become an effective target for liver IRI prevention [101].
4.5. Liver sinusoidal endothelial cell
Liver sinusoidal endothelial cells (LSECs) account for up to 70% of hepatic non-parenchymal cells. LSECs form the vascular wall of hepatic sinusoid but do not have an organized basal membrane. The cytoplasm of these flattened cells contains clusters called sieve plates, which make the hepatic microvascular endothelium discontinuous. Reports have suggested that LSECs confer protection against inflammation and regulate vascular homeostasis, vascular tone, and toxicant clearance [102]. On the other hand, hepatic sinusoid has been found to be susceptible to IRI. Injury to LSECs is more serious compared to injury to hepatocytes [103]. LSECs are the main source of IL-33 in normal liver, although necrotic cells also release IL-33 which signals tissue damage. IL-33 can activate cyclin D1, p38, MAPK and Bcl-2, thereby protect against hepatic injury and suppress inflammatory responses [104-106].
However, LSECs have been shown to exert detrimental effects. For example, the elevation of ICAM-1 in LSECs was reported to aggravate hepatic IRI, leading to the aggregation and adherence of neutrophils and platelets to LSEC, which stagnate the hepatic sinus microcirculation [107]. Other studies have shown that LSECs release NO and ET, which help to balance of hepatic microcirculation. Increased levels of ET and thromboxane (TXA2) after IR can trigger contraction of the sinusoidal lumen and HSCs, hence exacerbate the injury [108].
5. Metabolic compartment
The metabolic compartments are also generally thought to influence the degree of hepatic IRI [109]. Assessment of metabolic variation over time can reveal the pathophysiological state of hepatic IRI and is important to the optimal choice of treatments (Fig. 3).
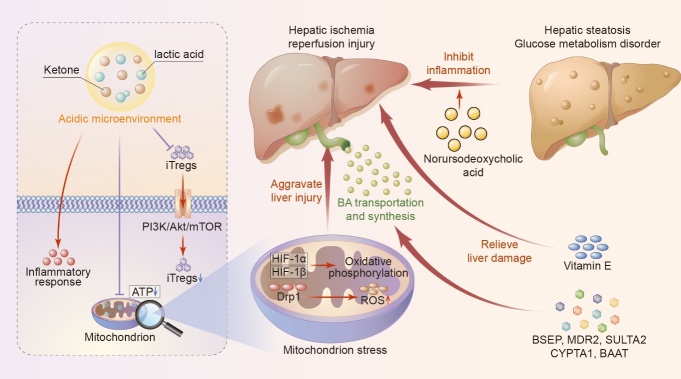
Figure 3.
Metabolic compartments regulate the process of hepatic ischemia/reperfusion injury. A summary of metabolic components of the liver microenvironment during phase of ischemia/reperfusion injury is manifested. iTregs, inducible regulatory T cells; ATP, adenosine triphosphate; BA, bile acid; Drp1, dynamin-related protein 1; HIF-1α, hypoxia-inducible factor-1.
5.1. Metabolic acidosis
Acidic microenvironment, as a result of accumulation of acidic substances such as ketone bodies and lactic acid, promotes hepatocyte injury associated with IRI. High levels of lactate act as Damage-Associated Molecular Patterns (DAMPs) thus promoting inflammatory response at the reperfusion phase [110]. Furthermore, intracellular acidosis causes imbalance of protein turnover leading to enzymatic inhibition and vital protein destruction as well as blocking ATP reserve reconstitution after reperfusion [111,112].
Notably, iTregs are different from naive T-cells in the peripheral environment in that they promote the recovery of liver function after IRI. Acidic microenvironment inhibits the generation and function of CD4+CD25+Foxp3+ iTregs through PI3K/AKT/mTOR signaling [113,114]. Several studies have explored the role of acid-base homeostasis in maintaining normal cellular response and immune system homeostasis. Furthermore, acidic microenvironment triggers upregulation of NO synthase in macrophages, accumulation of neutrophils, deactivation of the cytoplasmic- and membrane-associated enzyme, as well as downregulates synthesis of cAMP, proteins, and DNA [115,116]. Furthermore, Golse et al.reported that arterial lactate concentration at the end of LT (LCEOT) ≥5 mmol/L is an effective predictor of early graft outcomes [117]. Therefore, acidic microenvironment plays an important role in the progression of liver IRI, and lactate clearance can be used to alleviate liver IRI.
5.2. Glucose and fatty acid metabolism
One study have demonstrated that the availability of glycolytic substrates maintains ATP and promotes functional recovery during reperfusion phase [118]. Given this, the low pool of glycogen in IR causes more rapid ATP depletion, as well as alterations in tissue antioxidant defenses and dysregulation of mitochondrial function [119]. However, a different study reported that low pool of glycogen can reduce KCs phagocytosis and the generation of TNF-α thus improving organ viability and survival during long periods of ischemia [120]. Therefore, a beneficial effect of high glycogen content is mainly observed during short ischemia, whereas low metabolic reserves are preferentially needed in the process of long ischemia [102].
Glycogen synthase kinase 3 (GSK3) is a ubiquitous serine/threonine kinase, involved in regulation of glycogen synthesis [121]. Notably, inhibition of GSK3 in liver IRI models ameliorates liver damage via an IL-10-mediated immune regulatory mechanism resulting in reduced serum ALT levels and complete lobular architecture. Inactivation of GSK3 in liver IR is a self-regulatory mechanism in liver homeostasis that occurs through activation of PI3K [122,123]. In addition, Zhou et al. [124] demonstrated that GSK3β promotes activation of macrophage inflammation by suppressing the AMP-activation protein kinase and inducing the novel innate immune negative regulator SHP. Moreover, TGR5 is implicated in the regulation of energy homeostasis and glucose metabolism [125]. Although their roles in modulation of innate immune activation in liver IRI have been widely explored, their roles in regulating liver metabolism to influence hepatic IRI have not been fully elucidated.
Selzner et al. [126] reported that steatotic livers are vulnerable to hepatocyte damage and may be associated with poor tolerance to IRI. Steatotic livers produce less ATP compared with non-steatotic livers during the hepatic IRI, due to upregulation of the mitochondrial uncoupling protein 2 expression [127]. Additionally, hepatocytes with fatty infiltration have been found to develop massive necrosis after IRI, whereas apoptosis is mainly observed in non-steatotic livers after IRI [128]. In addition, steatotic livers exhibit lower expression of inositol-requiring enzyme 1 and PKR-like endoplasmic reticulum kinase, which may increase the risk of steatotic livers to IRI [129]. Elke et al. [130] revealed that steatosis exacerbates early IRI by enhancing effector immune cell infiltration, including higher mRNA expression of CXCL-1 and CD3.
5.3. Metabolic dysregulation of mitochondria
Mitochondria is a major target in ischemia injury, and dysregulation of mitochondria homeostasis and cellular energetics aggravate liver damage following ischemia injury. ROS released by the mitochondria during ischemia injury beyond antioxidant capacities promotes cellular DNA damage, calcium overload and mitochondrial lipid peroxidation. This leads to the release of cytochrome c and cellular damage [131,132].
Intracellular trafficking of mitochondria plays an important role in meeting local metabolic demands as well as self-renewal of the organ. Tunneling nanotubes (TNTs) mediate intercellular transfer of mitochondria. Notably, inhibitors of TNT were found to decrease mitochondrial intercellular transfer, thus alleviating the ischemia injury [133]. Given these results, use of stem or progenitor cells as a vehicle for normal mitochondrial diversion to cells with damaged mitochondria as a result of ischemia injury presents high therapeutic potential [134].
Hypoxia-inducible factor-1 (HIF-1α) significantly accumulated during ischemia injury, it binds to the β submit and is translocated to the nucleus to promote transcription of other genes. Most of these genes could be implicated in the glycolytic pathway and causes a switch in the production of energy from oxidative phosphorylation to glycolysis, thus exacerbating hypoxia in organs [135]. Moreover, dynamin-related protein 1 (Drp1) modulates the morphology of mitochondria and inhibits protective mitophagy by upregulating expression of mito-Clec16a. Moreover, Drp1 mediates metabolic disorders and decreases the levels of mitochondrial glutathione thus impairing free radical scavenging, resulting in further increase in ROS levels [136].
5.4. Role of metabolites in liver IRI
Several metabolites have been reported to associated with adverse liver-related events, as well as liver IRI. Some studies reported that genes associated with bile acid (BA) transportation and synthesis (i.e., BSEP, MDR2, SULTA2, CYP7A1 and BAAT), as well as nuclear factors implicated in regulation hepatic metabolism (i.e., SREBF1 and FXR), are tied with progression of hepatic IRI [137-139]. BA is an important signaling molecules with pleiotropic effects on liver physiology. High serum levels of taurochenodeoxycholate, which is a complex formed from conjugation of the BA chenodeoxycholate with taurine, is associated with a rapid liver-related adverse events. On the contrary, norursodeoxycholic acid, as a secondary bile acid, exhibits positive effects on the liver histology [140]. These findings indicate that targeting different bile acids can alleviate liver IRI.
A previous retrospective single-center cohort study, which included 187 participants, reported that vitamin E, primary bile acid and serotonin are associated with occurrence of future liver-related events [141]. A double blind randomized, and placebo-controlled trial was conducted previously whereby patients received three infusions containing vitamin E and the results showed significant improvement for ALT, AST and lactate dehydrogenase (LDH) levels after surgery [142].
Serotonin can be produced by cholangiocytes and stellate cells in the liver. Moreover, it also can be produced in the gut then it is metabolically transformed to 5-hydroxyindoleacetic (5-HIAA) in the liver [143]. Serotonin significantly enhances human megakaryocytes (MKs) growth through 5-HT2BR with subsequent activation of p-Erk1/2, which induces cytoskeleton reorganization and subsequent proplatelet formation [144]. Platelets can induce opposite effects by causing ischemia liver injury as well as promote subsequent tissue repair process. Nocito et al. [145] reported that platelets promote tissue repair and liver regeneration after normothermic hepatic ischemia in mice. Meanwhile, platelet-derived serotonin stimulates the proliferation of liver.
6. Remedies of hepatic IRI
Despite the tremendous efforts to develop therapies for IRI, the available treatments for IRI are not effective in all subsets of patients. Numerous studies have explored therapeutic efficacy of amino acid drugs (such as glycine and N-acetylcysteine), oxygen radical reducing drugs (including antioxidants, nitric oxide and carbon monoxide) and anti-inflammatory drugs (prostacyclin, atropine, and glucocorticoids) in treatment of IRI. Several molecular factors and genes have also been investigated as potential treatment targets, such as interferon regulatory factors, IL-10, programmed death factor, and bcl-6 [146]. A summary of these pharmacological and gene-based therapeutic targets is provided inTable 1.
Table 1.
Pharmacological/Gene therapy.
| Time | Strategies and Description | Species | Ischemic time | Effect | Result |
|---|---|---|---|---|---|
|
2008 [170] |
PPAR-α agonists and adiponectin siRNA | Rat | 60 min | MAPK expression and adiponectin accumulation↓ | Oxidative and hepatic injury↓ |
|
2008 [171] |
Allopurinol and apocynin (inhibitor of XOD and NADPH oxidase) | Mice | 30 min | Generation of superoxide anions↓ | Hepatic injury↓ |
|
2008 [172] |
Ascorbate (scavenger of ROS) | Rat | 30 min | Apoptosis of KCs↓ | Hepatic injury↓ |
|
2008 [173] |
Captopril (Ang 2 blockers) | Rat | 60 min | BK generation and PPAR-γ ↑ |
Hepatic injury↓ |
|
2008 [174] |
Tetrandine (scavenge ROS and inhibit lipid peroxidation) | Mice | 90 min | Neutrophil accumulation, TNF-α and MDA↓ SOD↑ |
Liver edema and hepatic injury↓ |
|
2009 [175] [176] |
Mutation of TLR4 or TLR4 knockout | Mice | 60 min | Release of pro-inflammatory cytokines and neutrophil infiltration↓ | Hepatic injury and damage of LSECs↓ |
|
2010 [177] |
Carbon monoxide-releasing molecule-2 (CORM-2) | Rat | 60 min | Neutrophil infiltration, TNF-α, IL-6, ICAM-1↓ Bcl-2↑ |
Hepatic injury and levels of apoptosis↓ |
|
2010 [178] |
Metron factor-1 (MF-1) | Rat | 90 min | Oxygen free radicals↓ NO synthesis and survival↑ |
Hepatic injury and oxidative stress↓ |
|
2010 [179] |
Sirolimus (immunossupressant drug) |
Rat | 60 min | Tissue myeloperoxidase and neutrophil infiltration↓ | Hepatic injury and liver cell apoptosis↓ |
|
2011 [180] |
Atorvastatin (HMG-CoA reductase inhibitor) | Mice | 60 min | STAR overexpression and mGSH depletion↓ | Hepatic injury and oxidative stress↓ |
|
2011 [181] |
n-3 PUFA (polyunsaturated fatty acid) | Rat | 60 min | NF-κB, TNF-α and IL-1β↓ | Hepatic injury and oxidative stress↓ |
|
2011 [182] |
rPSGL-Ig (selectin antagonist) | Human | 60min | IL-10↑ | Hepatic injury and oxidative stress↓ |
|
2012 [183] |
ABC294640 (selective inhibitor of sphingosine kinase-2) | Mice | 60 min | S1P, neutrophil infiltration, NO synthase, NF-κB and TNF-α↓ | Hepatocyte death and hepatic injury↓ |
|
2012 [184] |
Fasudil (a Rho-kinase inhibitor) | Rat | 30 min | HSC activation, endothelin 1 and portal perfusion pressure↓ | Hepatic injury and hepatic susceptibility↓ |
|
2012 [185] |
Nilotinib (tyrosine kinase inhibitor and against JNK and p38 in vitro) | Mice | 60 min | Recruitment of inflammatory monocytes, IL-1β, IL-6, MCP-1, MIP-2, JNK and p38 MAPK↓ | Hepatocyte apoptosis and hepatic injury↓ |
|
2012 [186] |
Deletion of FGL2/Fibroleukin (transgenic) | Mice | 60 min | Hepatocyte and LSEC protection | Hepatic injury and IRI cascade↓ |
|
2013 [187] |
RMT1-10 (TIM-1 blocker) | Mice | 20 h | Neutrophil and macrophage infiltration/activation, NF-κB and IFN-γ↓ IL-10, IL-22, Bcl-2↑ |
Hepatic injury and oxidative stress↓ |
|
2013 [188] |
Simvastatin (immunossupressant drug) | Mice | 16 h | Autophagy induction, LSEC injury↓ NO↑ |
Liver damage, oxidative stress and endothelial dysfunction↓ |
|
2014 [189] |
rMnSOD (antioxidant) | Mice | 20 min | Accumulation of superoxide anion and inflammation↓ NO↑ |
Hepatic injury and oxidative stress↓ |
|
2015 [190] [191] |
Knockout of IRF9 | Mice | 60 min | Serum ALT/AST, immune cell infiltration and levels of inflammatory cytokines ↓ | Hepatic injury and hepatocyte apoptosis↓ |
|
2016 [192] |
Total flavonoids (TFs) | Rat | 60 min | MPO, LDH, MDA, IL-6, TNF-α and IL-β↓ SOD and GSH-Px↑ |
Improve liver histopathology and ultrastructure |
|
2016 [193] |
Overactivation of Nrf2-ARE | Mice | 60 min | IL-6, IL-1β and levels of 8-isoprostanes↓ | Hepatocellular damage, necrosis, apoptosis and oxidative stress↓ |
|
2017 [194] |
Extracellular vesicles from mesenchymal stem cell (MSC-EV) | Murine | 90 min | NF-κB and IL-6↓ Expression of NACHT, LLR and PYD domains-containing protein 12↑ |
Hepatic caspase 3-positive and apoptotic cells↓ |
|
2017 [195] |
Inhibition of RAP1/KC/NLRP3 inflammasomes | Mice and human | 45 min | Activation of NLRP3 and levels of ALT and ALT↓ | Hepatic protection |
|
2018 [196] |
Knockout of CARD6 | Mice | 60 min | NF-κB, JNK, p38 and inflammatory chemokines↓ | Hepatic injury and liver cell death↓ |
|
2019 [197] |
Salicylate acetyl-3-aminoethyl salicylic acid (ac3AESA) | Murine | 60 min | Activation of KC, IL-6, TNF-α, IL-β, CXCL2 and CXCL8↓ | Hepatic injury and allograft damage↓ |
|
2019 [198] |
Omeprazole (buffer the acid microenvironment) | Mice and human | 60 min | Function of CD4CD25Foxp3 iTregs↑ | Hepatic injury↓ |
|
2020 [199] |
PINK1 (mediate mitophagy) | Mice | 60 min | ROS production, NLRP3, and KC-mediated inflammation↓ | Hepatic injury and mitochondrial dysfunction↓ |
|
2020 [200] |
Inhibition of miR-450b-5p | Mice | 60 min | CRYAB and M2 polarization↑ NF-κB↓ |
Hepatic protection |
|
2021 [201] |
Overexpression of miR122 | Mice and human | 60 min | PHD1↓ HIF1α expression↑ |
Hepatic ischemia tolerance↑ |
|
2021 [202] |
Inject rhMANF | Mice and human | 90 min | Activated ATF4/CHOP and JNK/c-JUN/CHOP pathways↓ | UPR injury and hepatocellular damage↓ |
PPAR-α, peroxisome proliferators-activated receptor-α; PPAR-γ, peroxisome proliferators-activated receptor-γ; TLR4, Toll like receptor 4; TNF-α, tumor necrosis factor α; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; IL-1β, interleukin 1β; HMGB1, high-mobility group box 1; IAC, inflammation associated cytokine; IL-1, interleukin 1; IL-6, interleukin 6; IL-10, interleukin 10; IL-22, interleukin 22; NF-κB, nuclear factor κB; ICAM-1, intercellular adhesion molecule-1; Bcl-2/Bcl-x, B cell lymphoma 2/x; MIP-2, macrophage inflammatory protein 2; JNK, N terminal kinase; IFN-γ, interferon γ; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein; LDH, Lactate dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, se-dependent enzyme glutathione peroxidase.
Further clinical trials should be conducted to develop and test effective interventions for hepatic IRI. Evidence from animal experiments have shown that curcumin treatment alleviates the negative effects of IRI in different organs including brain, heart, kidney, intestine, ovary, testis and liver [147-154]. Therapeutic effects of curcumin are mediated by mechanisms such as anti-oxidative stress, anti-inflammation and reduction of adhesion molecules thus ameliorating hepatic IRI. Curcumin has been reported to suppress oxidative stress by upregulating expression of antioxidant enzymes and inhibiting production of ROS [155]. Notably, curcumin has a strong intrinsic activity, thus curcumin is applied in treatment of various diseases. However, some studies also have reported limitations associated with bioavailability of curcumin such as limited tissue distribution, low serum levels, short half-life and apparent rapid metabolism [156]. Adjuvants which can bypass the metabolic pathways of curcumin, are revealed to be one of the major therapies used to improve its bioavailability. For instance, liposomes, micelles, nanoparticles and phospholipid complexes are used to improve the bioavailability of curcumin [157,158]. In addition, various extracts or secretions from traditional Chinese medicinal herbs may potentially inhibit oxidative stress and inflammation. For example, resveratrol and pterostilbene exhibit anti-cell proliferation, anti-oxidative stress and anti-inflammation effects [159]. However, further animal and clinical studies are needed to further verify these findings.
Mesenchymal stem cells (MSCs) have unique immunomodulatory properties. MSCs are invaluable cell types used for repair of tissue or organ damage. MSCs have been reported to suppress the infiltration of inflammatory cytokines and promote expression of anti-inflammatory cytokines [160]. Kharaziha et al. [161] and Mohamadnejad et al. [162] carried out successful trials which indicated that transplantation of autologous MSCs significantly improves liver function in IRI patients. In addition, MSCs are effective delivery vehicles characterized by injury tropism [163]. A previous study reported that engineered human induced pluripotent stem cells (hiPSC-MSCs) delivering GPx3 significantly suppresses senescence of liver and then alleviates hepatic IRI [164]. Therefore, therapeutic properties of MSCs on IRI have high potential for treatment of hepatic IRI.
Gene therapy is novel strategy for treatment of patients by modulating gene expression through approaches such as knockout, knockdown, correction or knock-in, thus it has high potential therapy for treatment of various diseases [165]. Knockout of CARD6 exhibits beneficial effects against myocardial IRI, by modulating apoptosis signal-regulating kinase 1 (ASK1) and several other signaling pathways. This implies that inhibition of ASK1 is an effective strategy for the treatment of hepatic IRI. Viral-based methods present an effective choice for the delivery of genes in gene therapies, with adeno-associated virus (AAV) being the most promising viral vector [166]. However, limitations such as packaging capacity (<4.7 kb), safety concern correlated to immunogenicity, and high cost associated with AAV restrain application of AAVs in gene therapy [167]. In light of this, studies are exploring chemical-based methods, such as polymer-based vectors as alternatives, owing to their low cost, high tunability and immune-compatibility [168]. For example, Reineke et al. [169] designed a new class of carbohydrate-based polymers and referred them as poly(glycoamidoamine)s (PGAAs), which are effective and biocompatible transfection reagents.
7. Conclusion and prospect
Numerous clinical and animal experiments have been conducted to explore molecular mechanisms associated with hepatic IRI and the findings show high potential in development of therapies for hepatic IRI. However, the complex interactions between hepatic microenvironment and IRI have not been fully elucidated thus limiting design of effective regimens for hepatic IRI patients. Possible mechanisms of liver IRI, including the interaction of various immune cells, effects of metabolites on IRI progression and the role of mitochondrial in liver IRI have been summarized in the present study.
Findings from pre-clinical studies indicate that several therapies are effective, however, results from clinical trials present low efficacy. Mechanisms of hepatic IRI vary with experimental conditions, such as period of ischemia (minutes to weeks), extension of ischemia (partial or complete), type of ischemia (cold or warm) and targeted immune cells. Therefore, findings from animal experiments cannot be directly translated to the clinical management of IRI. Furthermore, potential application of basic research knowledge should be taken into consideration owing to the extended-criteria or even increasingly complex situations of exact conditions of patients.
Furthermore, the possible cell therapy and gene therapy which are promising strategies for IRI treatment are explored from a clinical/translational perspective in the current review. However, the cross-talk between the hepatic microenvironment and the processes of IRI have not been fully explored, thus further studies should be conducted to fully elucidate this cross-talk. Studies should explore the pathogenesis of hepatic IRI to provide a basis for designing therapeutic strategies to ameliorate hepatic IRI, or even cure the disease.
Acknowledgements
This work was supported by the National Natural Science Funds for Distinguished Young Scholars of China (81625003); Youth Program of National Natural Science Foundation of China (82003248); National Science and Technology Key Program (81930016); Key R&D Project of Zhejiang Province (2019C03050); and ; National Key Research and Development Program of China (2021YFA 1100500).
Footnotes
Conflicts of Interest
The authors declare no competing interests.
References
- [1].de Groot H, Rauen U (2007). Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc, 39:481-484. [DOI] [PubMed] [Google Scholar]
- [2].Binder A, Ali A, Chawla R, Aziz HA, Abbate A, Jovin IS (2015). Myocardial protection from ischemia-reperfusion injury post coronary revascularization. Expert Rev Cardiovasc Ther, 13:1045-1057. [DOI] [PubMed] [Google Scholar]
- [3].Ginsberg MD (2016). Expanding the concept of neuroprotection for acute ischemic stroke: The pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol, 145-146:46-77. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez LM, Moeser AJ, Blikslager AT (2015). Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol, 308:G63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhai Y, Busuttil RW, Kupiec-Weglinski JW (2011). Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant, 11:1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nickkholgh A, Barro-Bejarano M, Liang R, Zorn M, Mehrabi A, Gebhard MM, et al. (2008). Signs of reperfusion injury following CO2 pneumoperitoneum: an in vivo microscopy study. Surg Endosc, 22:122-128. [DOI] [PubMed] [Google Scholar]
- [7].Liang R, Bruns H, Kincius M, Lin T, Ludwig J, Dei-Anane G, et al. (2009). Danshen protects liver grafts from ischemia/reperfusion injury in experimental liver transplantation in rats. Transpl Int, 22:1100-1109. [DOI] [PubMed] [Google Scholar]
- [8].Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, et al. (2007). Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol, 292:G1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR (2009). Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev: CD007632. [DOI] [PubMed] [Google Scholar]
- [10].Manekeller S, Sioutis M, Hirner A, Minor T (2008). [Influence of neoadjuvant chemotherapy on liver integrity and ischemic tolerance]. Z Gastroenterol, 46:17-21. [DOI] [PubMed] [Google Scholar]
- [11].Jang JH, Kang KJ, Kang Y, Lee IS, Graf R, Clavien PA (2008). Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transpl, 14:980-988. [DOI] [PubMed] [Google Scholar]
- [12].Yang Y, Zhang S, Fan C, Yi W, Jiang S, Di S, et al. (2016). Protective role of silent information regulator 1 against hepatic ischemia: effects on oxidative stress injury, inflammatory response, and MAPKs. Expert Opin Ther Targets, 20:519-531. [DOI] [PubMed] [Google Scholar]
- [13].Wasim Dar, Elise Sullivan, John Bynon, et al. (2019). Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int, 39(5):788-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM (2005). The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl, 11:1031-1047. [DOI] [PubMed] [Google Scholar]
- [15].Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. (2010). Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl, 16:943-949. [DOI] [PubMed] [Google Scholar]
- [16].Hudcova J, Scopa C, Rashid J, Waqas A, Ruthazer R, Schumann R (2017). Effect of early allograft dysfunction on outcomes following liver transplantation. Clin Transplant, 31(2). [DOI] [PubMed] [Google Scholar]
- [17].Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. (2012). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol, 57:675-688. [DOI] [PubMed] [Google Scholar]
- [18].Lee DD, Croome KP, Shalev JA, Musto KR, Sharma M, Keaveny AP, et al. (2016). Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol, 15:53-60. [DOI] [PubMed] [Google Scholar]
- [19].Chen XB, Xu MQ (2014). Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int, 13:125-137. [DOI] [PubMed] [Google Scholar]
- [20].He K, Yan L, Pan CS, Liu YY, Cui YC, Hu BH, et al. (2014). ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol, 307:H1764-1776. [DOI] [PubMed] [Google Scholar]
- [21].Li C, Li Q, Liu YY, Wang MX, Pan CS, Yan L, et al. (2014). Protective effects of Notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol, 306:G111-122. [DOI] [PubMed] [Google Scholar]
- [22].Tu L, Pan CS, Wei XH, Yan L, Liu YY, Fan JY, et al. (2013). Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation, 20:736-747. [DOI] [PubMed] [Google Scholar]
- [23].Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA (2008). Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res, 147:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Teoh NC (2011). Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection-the good, bad and deadly. J Gastroenterol Hepatol, 26Suppl 1:180-187. [DOI] [PubMed] [Google Scholar]
- [25].Qin X, Gao B (2006). The complement system in liver diseases. Cell Mol Immunol, 3:333-340. [PubMed] [Google Scholar]
- [26].Meneshian A, Bulkley GB (2002). The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation, 9:161-175. [DOI] [PubMed] [Google Scholar]
- [27].Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, et al. (2005). The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res, 124:160-168. [DOI] [PubMed] [Google Scholar]
- [28].Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R (2005). Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation, 111:1448-1454. [DOI] [PubMed] [Google Scholar]
- [29].Bertuzzo VR, Cescon M, Odaldi F, Di Laudo M, Cucchetti A, Ravaioli M, et al. (2017). Actual Risk of Using Very Aged Donors for Unselected Liver Transplant Candidates: A European Single-center Experience in the MELD Era. Ann Surg, 265:388-396. [DOI] [PubMed] [Google Scholar]
- [30].Selzner M, Selzner N, Jochum W, Graf R, Clavien PA (2007). Increased ischemic injury in old mouse liver: an ATP-dependent mechanism. Liver Transpl, 13:382-390. [DOI] [PubMed] [Google Scholar]
- [31].Kireev RA, Cuesta S, Ibarrola C, Bela T, Moreno Gonzalez E, Vara E, et al. (2012). Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. J Surg Res, 178:922-934. [DOI] [PubMed] [Google Scholar]
- [32].Rubinsztein DC, Marino G, Kroemer G (2011). Autophagy and aging. Cell, 146:682-695. [DOI] [PubMed] [Google Scholar]
- [33].DeLeve LD (2015). Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology, 61:1740-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kitano M, Bloomston PM (2016). Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis. J Clin Med, 5(3):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sorensen KK, Simon-Santamaria J, Mc-Cuskey RS, Smedsrod B (2015). Liver Sinusoidal Endothelial Cells. Compr Physiol, 5:1751-1774. [DOI] [PubMed] [Google Scholar]
- [36].Tsuchida T, Friedman SL (2017). Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol, 14:397-411. [DOI] [PubMed] [Google Scholar]
- [37].de Oliveira da Silva B, Ramos LF, Moraes KCM (2017). Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol Int, 41:946-959. [DOI] [PubMed] [Google Scholar]
- [38].Rubbia-Brandt L, Mentha G, Desmouliere A, Alto Costa AM, Giostra E, Molas G, et al. (1997). Hepatic stellate cells reversibly express alpha-smooth muscle actin during acute hepatic ischemia. Transplant Proc, 29:2390-2395. [DOI] [PubMed] [Google Scholar]
- [39].Beck-Schimmer B, Roth-Z'graggen B, Booy C, Koppel S, Spahn DR, Schlapfer M, et al. (2018). Sevoflurane Protects Hepatocytes From Ischemic Injury by Reducing Reactive Oxygen Species Signaling of Hepatic Stellate Cells: Translational Findings Based on a Clinical Trial. Anesth Analg, 127:1058-1065. [DOI] [PubMed] [Google Scholar]
- [40].Wu Y, Gu C, Huang X (2016). Sevoflurane protects against hepatic ischemia/reperfusion injury by modulating microRNA-200c regulation in mice. Biomed Pharmacother, 84:1126-1136. [DOI] [PubMed] [Google Scholar]
- [41].Xu L, Ge F, Hu Y, Yu Y, Guo K, Miao C (2021). Sevoflurane Postconditioning Attenuates Hepatic Ischemia-Reperfusion Injury by Limiting HMGB1/TLR4/NF-kappaB Pathway via Modulating microRNA-142 in vivo and in vitro. Front Pharmacol, 12:646307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Feng M, Wang Q, Wang H, Wang M, Guan W, Lu L (2014). Adoptive transfer of hepatic stellate cells ameliorates liver ischemia reperfusion injury through enriching regulatory T cells. Int Immunopharmacol, 19:267-274. [DOI] [PubMed] [Google Scholar]
- [43].Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, et al. (2007). Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology, 46:1187-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Utley S, James D, Mavila N, Nguyen MV, Vendryes C, Salisbury SM, et al. (2014). Fibroblast growth factor signaling regulates the expansion of A6-expressing hepatocytes in association with AKT-dependent beta-catenin activation. J Hepatol, 60:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li S, Zhu Z, Xue M, Pan X, Tong G, Yi X, et al. (2021). The protective effects of fibroblast growth factor 10 against hepatic ischemia-reperfusion injury in mice. Redox Biol, 40:101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Konishi T, Schuster RM, Lentsch AB (2018). Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol, 314:G471-G482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cheng X, Yang YL, Li WH, Liu M, Zhang SS, Wang YH, et al. (2020). Dynamic Alterations of Brain Injury, Functional Recovery, and Metabolites Profile after Cerebral Ischemia/Reperfusion in Rats Contributes to Potential Biomarkers. J Mol Neurosci, 70:667-676. [DOI] [PubMed] [Google Scholar]
- [48].Mochizuki A, Pace A, Rockwell CE, Roth KJ, Chow A, O'Brien KM, et al. (2014). Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1alpha-dependent manner by modulating macrophage phenotype in mice. J Immunol, 192:3847-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE (2010). Hepatic stellate cells' involvement in progenitor-mediated liver regeneration. Lab Invest, 90:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. (2005). Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest, 115:56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Konishi T, Lentsch AB (2017). Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr, 17:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cheng Z, He W, Zhou X, Lv Q, Xu X, Yang S, et al. (2011). Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol, 664:20-28. [DOI] [PubMed] [Google Scholar]
- [53].Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. (2019). Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep, 29:1261-1273 e1266. [DOI] [PubMed] [Google Scholar]
- [54].West AP, Shadel GS (2017). Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol, 17:363-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhong W, Rao Z, Rao J, Han G, Wang P, Jiang T, et al. (2020). Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell, 19:e13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Caldwell CC, Tschoep J, Lentsch AB (2007). Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol, 82:457-464. [DOI] [PubMed] [Google Scholar]
- [57].Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LH (2011). Tumor necrosis factor-alpha in liver ischemia/reperfusion injury. J Invest Surg, 24:178-188. [DOI] [PubMed] [Google Scholar]
- [58].Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, et al. (2001). Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology, 33:100-113. [DOI] [PubMed] [Google Scholar]
- [59].Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E (2001). Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res, 99:201-210. [DOI] [PubMed] [Google Scholar]
- [60].Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun, 9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peng Y, Liu ZJ, Gong JP, Liu HZ, Gan L, Li SB (2005). [Expression of CD14 and Toll-like receptor 4 on Kupffer cells and its role in ischemia-reperfusion injury on rat liver graft]. Zhonghua Wai Ke Za Zhi, 43:274-276. [PubMed] [Google Scholar]
- [62].Luan X, Liu Y, Li M (2012). The role of CD14 and Toll-like receptor 4 of Kupffer cells in hepatic ischemia-reperfusion injury in rats. Transplant Proc, 44:937-941. [DOI] [PubMed] [Google Scholar]
- [63].Yang H, Zhou H, Zhuang L, Auwerx J, Schoonjans K, Wang X, et al. (2017). Plasma membrane-bound G protein-coupled bile acid receptor attenuates liver ischemia/reperfusion injury via the inhibition of toll-like receptor 4 signaling in mice. Liver Transpl, 23:63-74. [DOI] [PubMed] [Google Scholar]
- [64].Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K (2011). The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol, 54:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jin D, Lu T, Ni M, Wang H, Zhang J, Zhong C, et al. (2020). Farnesoid X Receptor Activation Protects Liver From Ischemia/Reperfusion Injury by Up-Regulating Small Heterodimer Partner in Kupffer Cells. Hepatol Commun, 4:540-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ogawa K, Kondo T, Tamura T, Matsumura H, Fukunaga K, Oda T, et al. (2013). Influence of Kupffer cells and platelets on ischemia-reperfusion injury in mild steatotic liver. World J Gastroenterol, 19:1396-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tamura T, Kondo T, Pak S, Nakano Y, Murata S, Fukunaga K, et al. (2012). Interaction between Kupffer cells and platelets in the early period of hepatic ischemia-reperfusion injury--an in vivo study. J Surg Res, 178:443-451. [DOI] [PubMed] [Google Scholar]
- [68].Straatsburg IH, Boermeester MA, Wolbink GJ, van Gulik TM, Gouma DJ, Frederiks WM, et al. (2000). Complement activation induced by ischemia-reperfusion in humans: a study in patients undergoing partial hepatectomy. J Hepatol, 32:783-791. [DOI] [PubMed] [Google Scholar]
- [69].Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ (1993). Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol, 264:G801-809. [DOI] [PubMed] [Google Scholar]
- [70].He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S (2009). A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest, 119:2304-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wu S, Yang J, Sun G, Hu J, Zhang Q, Cai J, et al. (2021). Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol, 178(18):3783-3796 [DOI] [PubMed] [Google Scholar]
- [72].Gomez-Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature, 518:547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Davies LC, Taylor PR (2015). Tissue-resident macrophages: then and now. Immunology, 144:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yue S, Zhou H, Wang X, Busuttil RW, Kupiec-Weglinski JW, Zhai Y (2017). Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J Immunol, 198:3588-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang J, Kubes P (2016). A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell, 165:668-678. [DOI] [PubMed] [Google Scholar]
- [76].Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ (2010). Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol, 16:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ye L, He S, Mao X, Zhang Y, Cai Y, Li S (2020). Effect of Hepatic Macrophage Polarization and Apoptosis on Liver Ischemia and Reperfusion Injury During Liver Transplantation. Front Immunol, 11:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Xue R, Qiu J, Wei S, Liu M, Wang Q, Wang P, et al. (2021). Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells. Ann Transl Med, 9:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nagy LE (2015). The Role of Innate Immunity in Alcoholic Liver Disease. Alcohol Res, 37:237-250. [PMC free article] [PubMed] [Google Scholar]
- [80].Sica A, Mantovani A (2012). Macrophage plasticity and polarization: in vivo veritas. J Clin Invest, 122:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shang L, Ren H, Wang S, Liu H, Hu A, Gou P, et al. (2021). SS-31 Protects Liver from Ischemia-Reperfusion Injury via Modulating Macrophage Polarization. Oxid Med Cell Longev, 2021: 6662156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF (1997). CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest, 100:279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB (2005). Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Ajp Gastrointestinal & Liver Physiology, 289:G969-G976. [DOI] [PubMed] [Google Scholar]
- [84].Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, et al. (2002). CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation, 74:315-319. [DOI] [PubMed] [Google Scholar]
- [85].Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. (2001). IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol, 108:430-438. [DOI] [PubMed] [Google Scholar]
- [86].Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL (2004). Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol, 76:135-144. [DOI] [PubMed] [Google Scholar]
- [87].Nowak P, Wachowicz B (2002). Peroxynitrite-mediated modification of fibrinogen affects platelet aggregation and adhesion. Platelets, 13:293-299. [DOI] [PubMed] [Google Scholar]
- [88].Khandoga A, Hanschen M, Kessler JS, Krombach F (2006). CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology, 43:306-315. [DOI] [PubMed] [Google Scholar]
- [89].Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H (1995). Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature, 373:255-257. [DOI] [PubMed] [Google Scholar]
- [90].Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. (2010). T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology, 139:2195-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. (2002). Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature, 415:536-541. [DOI] [PubMed] [Google Scholar]
- [92].Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. (2003). Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol, 4:1093-1101. [DOI] [PubMed] [Google Scholar]
- [93].Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG (2004). Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol, 172:45-53. [DOI] [PubMed] [Google Scholar]
- [94].Liu ZX, Han D, Gunawan B, Kaplowitz N (2006). Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology, 43:1220-1230. [DOI] [PubMed] [Google Scholar]
- [95].Jaeschke H, Woolbright BL (2012). Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando), 26:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jaeschke H (2006). Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol, 290:G1083-1088. [DOI] [PubMed] [Google Scholar]
- [97].Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A (2010). Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl, 16:1016-1032. [DOI] [PubMed] [Google Scholar]
- [98].Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G (2002). Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med, 33:1200-1208. [DOI] [PubMed] [Google Scholar]
- [99].Amersi F, Dulkanchainun T, Nelson SK, Farmer DG, Kato H, Zaky J, et al. (2001). A novel iron chelator in combination with a P-selectin antagonist prevents ischemia/reperfusion injury in a rat liver model. Transplantation, 71:112-118. [DOI] [PubMed] [Google Scholar]
- [100].Kushimoto S, Okajima K, Uchiba M, Murakami K, Harada N, Okabe H, et al. (1996). Role of granulocyte elastase in ischemia/reperfusion injury of rat liver. Crit Care Med, 24:1908-1912. [DOI] [PubMed] [Google Scholar]
- [101].Li XK, Matin AF, Suzuki H, Uno T, Yamaguchi T, Harada Y (1993). Effect of protease inhibitor on ischemia/reperfusion injury of the rat liver. Transplantation, 56:1331-1336. [DOI] [PubMed] [Google Scholar]
- [102].Peralta C, Jimenez-Castro MB, Gracia-Sancho J (2013). Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol, 59:1094-1106. [DOI] [PubMed] [Google Scholar]
- [103].Banga NR, Prasad KR, Burn JL, Homer-Vanniasinkam S, Graham A (2012). An in vitro model of warm hypoxia-reoxygenation injury in human liver endothelial cells. J Surg Res, 178:e35-41. [DOI] [PubMed] [Google Scholar]
- [104].Marvie P, Lisbonne M, L'Helgoualc'h A, Rauch M, Turlin B, Preisser L, et al. (2010). Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med, 14:1726-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J (2015). The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron, 85:703-709. [DOI] [PubMed] [Google Scholar]
- [106].Liew FY, Girard JP, Turnquist HR (2016). Interleukin-33 in health and disease. Nat Rev Immunol, 16:676-689. [DOI] [PubMed] [Google Scholar]
- [107].Xue Q, Yuan Z, Chen Z, Hao R, Liu C, Tu B (2012). Protective role of nitric oxide induced by ischemic preconditioning on cold ischemic-reperfusion injury of rat liver graft. Transplant Proc, 44:948-951. [DOI] [PubMed] [Google Scholar]
- [108].Farmer DG, Kaldas F, Anselmo D, Katori M, Shen XD, Lassman C, et al. (2008). Tezosentan, a novel endothelin receptor antagonist, markedly reduces rat hepatic ischemia and reperfusion injury in three different models. Liver Transpl, 14:1737-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nordstrom A, Lewensohn R (2010). Metabolomics: moving to the clinic. J Neuroimmune Pharmacol, 5:4-17. [DOI] [PubMed] [Google Scholar]
- [110].Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A (2016). Transplantation and Damage-Associated Molecular Patterns (DAMPs). Am J Transplant, 16:3338-3361. [DOI] [PubMed] [Google Scholar]
- [111].Jaeschke H (2003). Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol, 284:G15-26. [DOI] [PubMed] [Google Scholar]
- [112].Defamie V, Cursio R, Le-Brigand K, Moreilhon C, Saint-Paul MC, Laurens M, et al. (2008). Gene expression profiling of human liver transplants identifies an early transcriptional signature associated with initial poor graft function. Am J Transplant, 8:1221-1236. [DOI] [PubMed] [Google Scholar]
- [113].Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC (2018). Regulatory T cells in the treatment of disease. Nat Rev Drug Discov, 17:823-844. [DOI] [PubMed] [Google Scholar]
- [114].Rafiee P, Theriot ME, Nelson VM, Heidemann J, Kanaa Y, Horowitz SA, et al. (2006). Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol, 291:C931-945. [DOI] [PubMed] [Google Scholar]
- [115].Lardner A (2001). The effects of extracellular pH on immune function. J Leukoc Biol, 69:522-530. [PubMed] [Google Scholar]
- [116].Martinez D, Vermeulen M, Trevani A, Ceballos A, Sabatte J, Gamberale R, et al. (2006). Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol, 176:1163-1171. [DOI] [PubMed] [Google Scholar]
- [117].Golse N, Guglielmo N, El Metni A, Frosio F, Cosse C, Naili S, et al. (2019). Arterial Lactate Concentration at the End of Liver Transplantation Is an Early Predictor of Primary Graft Dysfunction. Ann Surg, 270:131-138. [DOI] [PubMed] [Google Scholar]
- [118].Jaeschke H (1996). Preservation injury: mechanisms, prevention and consequences. J Hepatol, 25:774-780. [DOI] [PubMed] [Google Scholar]
- [119].Stadler M, Nuyens V, Seidel L, Albert A, Boogaerts JG (2005). Effect of nutritional status on oxidative stress in an ex vivo perfused rat liver. Anesthesiology, 103:978-986. [DOI] [PubMed] [Google Scholar]
- [120].Sankary HN, Chong A, Foster P, Brown E, Shen J, Kimura R, et al. (1995). Inactivation of Kupffer cells after prolonged donor fasting improves viability of transplanted hepatic allografts. Hepatology, 22:1236-1242. [DOI] [PubMed] [Google Scholar]
- [121].Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A (2009). Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol, 156:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Martin M, Rehani K, Jope RS, Michalek SM (2005). Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol, 6:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, et al. (2011). Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology, 54:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhou H, Wang H, Ni M, Yue S, Xia Y, Busuttil RW, et al. (2018). Glycogen synthase kinase 3beta promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J Hepatol, 69:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang YD, Chen WD, Yu D, Forman BM, Huang W (2011). The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology, 54:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA (2000). Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology, 32:1280-1288. [DOI] [PubMed] [Google Scholar]
- [127].Evans ZP, Palanisamy AP, Sutter AG, Ellett JD, Ramshesh VK, Attaway H, et al. (2012). Mitochondrial uncoupling protein-2 deficiency protects steatotic mouse hepatocytes from hypoxia/reoxygenation. Am J Physiol Gastrointest Liver Physiol, 302:G336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fernandez L, Carrasco-Chaumel E, Serafin A, Xaus C, Grande L, Rimola A, et al. (2004). Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am J Transplant, 4:888-899. [DOI] [PubMed] [Google Scholar]
- [129].Ben-Mosbah I, Alfany-Fernandez I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, et al. (2010). Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis, 1:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Eggenhofer E, Groell A, Junger H, Kasi A, Kroemer A, Geissler EK, et al. (2021). Steatotic Livers Are More Susceptible to Ischemia Reperfusion Damage after Transplantation and Show Increased gammadelta T Cell Infiltration. Int J Mol Sci, 22(4):2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL (2009). Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta, 1787:1395-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wu CC, Bratton SB (2013). Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal, 19:546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. (2014). Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol, 51:455-465. [DOI] [PubMed] [Google Scholar]
- [134].Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. (2014). Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J, 33:994-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Semenza GL (2011). Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta, 1813:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Duan C, Kuang L, Xiang X, Zhang J, Zhu Y, Wu Y, et al. (2020). Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-, BAX-, and GSH- pathways. Cell Death Dis, 11:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Navarro-Sabate A, Peralta C, Calvo MN, Manzano A, Massip-Salcedo M, Rosello-Catafau J, et al. (2006). Mediators of rat ischemic hepatic preconditioning after cold preservation identified by microarray analysis. Liver Transpl, 12:1615-1625. [DOI] [PubMed] [Google Scholar]
- [138].Raza A, Dikdan G, Desai KK, Shareef A, Fernandes H, Aris V, et al. (2010). Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transpl, 16:588-599. [DOI] [PubMed] [Google Scholar]
- [139].Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, et al. (2007). Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol, 293:G25-35. [DOI] [PubMed] [Google Scholar]
- [140].Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, et al. (2019). Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol, 4:781-793. [DOI] [PubMed] [Google Scholar]
- [141].Wegermann K, Howe C, Henao R, Wang Y, Guy CD, Abdelmalek MF, et al. (2021). Serum Bile Acid, Vitamin E, and Serotonin Metabolites Are Associated With Future Liver-Related Events in Nonalcoholic Fatty Liver Disease. Hepatol Commun, 5:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bartels M, Biesalski HK, Engelhart K, Sendlhofer G, Rehak P, Nagel E (2004). Pilot study on the effect of parenteral vitamin E on ischemia and reperfusion induced liver injury: a double blind, randomized, placebo-controlled trial. Clin Nutr, 23:1360-1370. [DOI] [PubMed] [Google Scholar]
- [143].Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, et al. (2011). Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol, 300:G303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ye JY, Liang EY, Cheng YS, Chan GC, Ding Y, Meng F, et al. (2014). Serotonin enhances megakaryopoiesis and proplatelet formation via p-Erk1/2 and F-actin reorganization. Stem Cells, 32:2973-2982. [DOI] [PubMed] [Google Scholar]
- [145].Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, et al. (2007). Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology, 45:369-376. [DOI] [PubMed] [Google Scholar]
- [146].Akhtar MZ, Henderson T, Sutherland A, Vogel T, Friend PJ (2013). Novel Approaches to Preventing Ischemia-Reperfusion Injury During Liver Transplantation. Transplantation Proceedings, 45:2083-2092. [DOI] [PubMed] [Google Scholar]
- [147].Sak ME, Soydinc HE, Sak S, Evsen MS, Alabalik U, Akdemir F, et al. (2013). The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int J Surg, 11:967-970. [DOI] [PubMed] [Google Scholar]
- [148].Behroozi-Lak T, Ebrahimpour M, Zarei L, Pourjabali M, Farhad N, Mohaddesi H (2018). Systemic administration of curcumin nanoparticles protects ischemia-reperfusion injury in ovaries: An animal model study. Rev Assoc Med Bras (1992), 64:22-31. [DOI] [PubMed] [Google Scholar]
- [149].Aydin MS, Caliskan A, Kocarslan A, Kocarslan S, Yildiz A, Gunay S, et al. (2014). Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int J Surg, 12:601-605. [DOI] [PubMed] [Google Scholar]
- [150].Shahed AR, Jones E, Shoskes D (2001). Quercetin and curcumin up-regulate antioxidant gene expression in rat kidney after ureteral obstruction or ischemia/reperfusion injury. Transplant Proc, 33:2988. [DOI] [PubMed] [Google Scholar]
- [151].Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, et al. (2005). Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res, 82:138-148. [DOI] [PubMed] [Google Scholar]
- [152].Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK (2003). Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock, 19:150-156. [DOI] [PubMed] [Google Scholar]
- [153].Eser A, Hizli D, Haltas H, Namuslu M, Kosus A, Kosus N, et al. (2015). Effects of curcumin on ovarian ischemia-reperfusion injury in a rat model. Biomed Rep, 3:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wei SM, Yan ZZ, Zhou J (2009). Curcumin attenuates ischemia-reperfusion injury in rat testis. Fertil Steril, 91:271-277. [DOI] [PubMed] [Google Scholar]
- [155].Bavarsad K, Riahi MM, Saadat S, Barreto G, Atkin SL, Sahebkar A (2019). Protective effects of curcumin against ischemia-reperfusion injury in the liver. Pharmacol Res, 141:53-62. [DOI] [PubMed] [Google Scholar]
- [156].Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007). Bioavailability of curcumin: problems and promises. Mol Pharm, 4:807-818. [DOI] [PubMed] [Google Scholar]
- [157].Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, et al. (2007). Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): a novel strategy for human cancer therapy. J Nanobiotechnology, 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Letchford K, Liggins R, Burt H (2008). Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: theoretical and experimental data and correlations. J Pharm Sci, 97:1179-1190. [DOI] [PubMed] [Google Scholar]
- [159].Zhang H, Wang C, Li X, Zhang Y (2016). Effects of pterostilbene on treating hyperprolactinemia and related mechanisms. Am J Transl Res, 8:3049-3055. [PMC free article] [PubMed] [Google Scholar]
- [160].Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. (2016). Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis, 7:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, et al. (2007). Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med, 10:459-466. [PubMed] [Google Scholar]
- [162].Kharaziha P, Hellstrom PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. (2009). Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol, 21:1199-1205. [DOI] [PubMed] [Google Scholar]
- [163].Zhang Y, Liao S, Yang M, Liang X, Poon MW, Wong CY, et al. (2012). Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transplant, 21:2225-2239. [DOI] [PubMed] [Google Scholar]
- [164].Qi X, Ng KT, Lian Q, Li CX, Geng W, Ling CC, et al. (2018). Glutathione Peroxidase 3 Delivered by hiPSC-MSCs Ameliorated Hepatic IR Injury via Inhibition of Hepatic Senescence. Theranostics, 8:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Van-Bruggen C, Hexum JK, Tan Z, Dalal RJ, Reineke TM (2019). Nonviral Gene Delivery with Cationic Glycopolymers. Acc Chem Res, 52:1347-1358. [DOI] [PubMed] [Google Scholar]
- [166].Yang P, Chou SJ, Li J, Hui W, Liu W, Sun N, et al. (2020). Supramolecular nanosubstrate-mediated delivery system enables CRISPR-Cas9 knockin of hemoglobin beta gene for hemoglobinopathies. Sci Adv, 6(43):eabb7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu Z, Yang H, Colosi P (2010). Effect of genome size on AAV vector packaging. Mol Ther, 18:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mintzer MA, Simanek EE (2009). Nonviral vectors for gene delivery. Chem Rev, 109:259-302. [DOI] [PubMed] [Google Scholar]
- [169].Liu Y, Reineke TM (2010). Degradation of poly(glycoamidoamine) DNA delivery vehicles: polyamide hydrolysis at physiological conditions promotes DNA release. Biomacromolecules, 11:316-325. [DOI] [PubMed] [Google Scholar]
- [170].Massip-Salcedo M, Zaouali MA, Padrissa-Altes S, Casillas-Ramirez A, Rodes J, Rosello-Catafau J, et al. (2008). Activation of peroxisome proliferator-activated receptor-alpha inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia-reperfusion. Hepatology, 47:461-472. [DOI] [PubMed] [Google Scholar]
- [171].Liu PG, He SQ, Zhang YH, Wu J (2008). Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol, 14:2832-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ichiki A, Miyazaki T, Nodera M, Suzuki H, Yanagisawa H (2008). Ascorbate inhibits apoptosis of Kupffer cells during warm ischemia/reperfusion injury. Hepatogastroenterology, 55:338-344. [PubMed] [Google Scholar]
- [173].Casillas-Ramirez A, Amine-Zaouali M, Massip-Salcedo M, Padrissa-Altes S, Bintanel-Morcillo M, Ramalho F, et al. (2008). Inhibition of angiotensin II action protects rat steatotic livers against ischemia-reperfusion injury. Crit Care Med, 36:1256-1266. [DOI] [PubMed] [Google Scholar]
- [174].Cheng F, Li Y, Feng L, Li S (2008). Effects of tetrandrine on ischemia/reperfusion injury in mouse liver. Transplant Proc, 40:2163-2166. [DOI] [PubMed] [Google Scholar]
- [175].Hui W, Jinxiang Z, Heshui W, Zhuoya L, Qichang Z (2009). Bone marrow and non-bone marrow TLR4 regulates hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun, 389:328-332. [DOI] [PubMed] [Google Scholar]
- [176].Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD (2009). Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl, 15:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wei Y, Chen P, de Bruyn M, Zhang W, Bremer E, Helfrich W (2010). Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats. BMC Gastroenterol, 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Xue F, Zhang JJ, Xu LM, Zhang C, Xia Q (2010). Protective effects of HGF-MSP chimer (metron factor-1) on liver ischemia-reperfusion injury in rat model. J Dig Dis, 11:299-305. [DOI] [PubMed] [Google Scholar]
- [179].Liu YX, Jin LM, Zhou L, Xie HY, Jiang GP, Chen H, et al. (2010). Sirolimus attenuates reduced-size liver ischemia-reperfusion injury but impairs liver regeneration in rats. Dig Dis Sci, 55:2255-2262. [DOI] [PubMed] [Google Scholar]
- [180].Llacuna L, Fernandez A, Montfort CV, Matias N, Martinez L, Caballero F, et al. (2011). Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury. J Hepatol, 54:1002-1010. [DOI] [PubMed] [Google Scholar]
- [181].Zuniga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, et al. (2011). N-3 PUFA supplementation triggers PPAR-alpha activation and PPAR-alpha/NF-kappaB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS One, 6:e28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, Ponthieux S, Gjertson DW, Cheadle C, et al. (2011). rPSGL-Ig for improvement of early liver allograft function: a double-blind, placebo-controlled, single-center phase II study. Am J Transplant, 11:786-797. [DOI] [PubMed] [Google Scholar]
- [183].Shi Y, Rehman H, Ramshesh VK, Schwartz J, Liu Q, Krishnasamy Y, et al. (2012). Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. J Hepatol, 56:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Kuroda S, Tashiro H, Igarashi Y, Tanimoto Y, Nambu J, Oshita A, et al. (2012). Rho inhibitor prevents ischemia-reperfusion injury in rat steatotic liver. J Hepatol, 56:146-152. [DOI] [PubMed] [Google Scholar]
- [185].Ocuin LM, Zeng S, Cavnar MJ, Sorenson EC, Bamboat ZM, Greer JB, et al. (2012). Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol, 57:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Selzner N, Liu H, Boehnert MU, Adeyi OA, Shalev I, Bartczak AM, et al. (2012). FGL2/fibroleukin mediates hepatic reperfusion injury by induction of sinusoidal endothelial cell and hepatocyte apoptosis in mice. J Hepatol, 56:153-159. [DOI] [PubMed] [Google Scholar]
- [187].Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, et al. (2013). Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant, 13:56-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gracia-Sancho J, Garcia-Caldero H, Hide D, Marrone G, Guixe-Muntet S, Peralta C, et al. (2013). Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J Hepatol, 58:1140-1146. [DOI] [PubMed] [Google Scholar]
- [189].Hide D, Ortega-Ribera M, Fernandez-Iglesias A, Fondevila C, Salvado MJ, Arola L, et al. (2014). A novel form of the human manganese superoxide dismutase protects rat and human livers undergoing ischaemia and reperfusion injury. Clin Sci (Lond), 127:527-537. [DOI] [PubMed] [Google Scholar]
- [190].Wang PX, Zhang R, Huang L, Zhu LH, Jiang DS, Chen HZ, et al. (2015). Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. J Hepatol, 62:111-120. [DOI] [PubMed] [Google Scholar]
- [191].Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD (2003). Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol, 38:59-66. [DOI] [PubMed] [Google Scholar]
- [192].Tao X, Sun X, Xu L, Yin L, Han X, Qi Y, et al. (2016). Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients, 8(7):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Lee LY, Harberg C, Matkowskyj KA, Cook S, Roenneburg D, Werner S, et al. (2016). Cell-specific overactivation of nuclear erythroid 2 p45-related factor 2-mediated gene expression in myeloid cells decreases hepatic ischemia/reperfusion injury. Liver Transpl, 22:1115-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, et al. (2017). Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl, 23:791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Liu H, Lo CM, Yeung OWH, Li CX, Liu XB, Qi X, et al. (2017). NLRP3 inflammasome induced liver graft injury through activation of telomere-independent RAP1/KC axis. J Pathol, 242:284-296. [DOI] [PubMed] [Google Scholar]
- [196].Qin JJ, Mao W, Wang X, Sun P, Cheng D, Tian S, et al. (2018). Caspase recruitment domain 6 protects against hepatic ischemia/reperfusion injury by suppressing ASK1. J Hepatol, 69:1110-1122. [DOI] [PubMed] [Google Scholar]
- [197].Lai X, Gong J, Wang W, Cao D, Wang M, Liu Y, et al. (2019). Acetyl-3-Aminoethyl Salicylate Ameliorates Hepatic Ischemia/Reperfusion Injury and Liver Graft Survival Through a High-Mobility Group Box 1/Toll-Like Receptor 4-Dependent Mechanism. Liver Transpl, 25:1220-1232. [DOI] [PubMed] [Google Scholar]
- [198].Gan X, Zhang R, Gu J, Ju Z, Wu X, Wang Q, et al. (2019). Acidic Microenvironment Regulates the Severity of Hepatic Ischemia/Reperfusion Injury by Modulating the Generation and Function of Tregs via the PI3K-mTOR Pathway. Front Immunol, 10:2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Xu Y, Tang Y, Lu J, Zhang W, Zhu Y, Zhang S, et al. (2020). PINK1-mediated mitophagy protects against hepatic ischemia/reperfusion injury by restraining NLRP3 inflammasome activation. Free Radic Biol Med, 160:871-886. [DOI] [PubMed] [Google Scholar]
- [200].Huang Z, Mou T, Luo Y, Pu X, Pu J, Wan L, et al. (2020). Inhibition of miR-450b-5p ameliorates hepatic ischemia/reperfusion injury via targeting CRYAB. Cell Death Dis, 11:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ju C, Wang M, Tak E, Kim B, Emontzpohl C, Yang Y, et al. (2021). Hypoxia-inducible factor-1alpha-dependent induction of miR122 enhances hepatic ischemia tolerance. J Clin Invest, 131(7):e140300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Yang Y, Wang P, Zhang C, Huang F, Pang G, Wei C, et al. (2021). Hepatocyte-derived MANF alleviates hepatic ischaemia-reperfusion injury via regulating endoplasmic reticulum stress-induced apoptosis in mice. Liver Int, 41:623-639. [DOI] [PubMed] [Google Scholar]