Abstract
Acarbose can extend the life span of mice through a process involving the gut microbiota. Several factors affect the life span, including mitochondrial function, cellular senescence, telomere length, immune function, and expression of longevity-related genes. In this review, the effects of acarbose-regulated gut microbiota on the life span-influencing factors have been discussed. In addition, a novel theoretical basis for improving our understanding of the mechanisms by which acarbose extends the life span of mice has been suggested.
Keywords: acarbose, life span, gut microbiota
1. Introduction
Since ancient times, humans have shown considerable interest in extending their life spans, which has inspired many studies on the mechanism underlying the extension of the life span. Recent studies have demonstrated that the human life span is affected by inflammation, mitochondrial function, telomere length, and immune function [1-4]. Diabetes is a common disease among elderly adults, and its complications can lead to organ damage and shortening of the life span. Metformin is a first-line hypoglycemic drug that not only lowers blood glucose levels but also affects the human life span [5]. Metformin can reduce oxidative stress and ameliorate chronic inflammation, which may aid in extending the life span [6].
Acarbose, which was originally discovered to be produced by Actinoplanes sp. SE50/110, is used as a first-line treatment for diabetes [7]. As an inhibitor of α-glucosidase, acarbose delays carbohydrate digestion by competitively inhibiting α-glucosidases and pancreatic α-amylase, thereby reducing blood glucose levels in patients with type 2 diabetes mellitus (T2DM) [8]. In Asia, energy intake is mainly derived from carbohydrates, so acarbose is more commonly used in Asia. After acarbose treatment, the glycated hemoglobin level can be reduced by as much as 0.77%, which is predominantly noted in the Eastern Asian population and those consuming high-carbohydrate diets [9]. Acarbose supplementation can partially offset the age-related dysregulation of glucose and insulin [10,11]. Acarbose can attenuate many risk factors for cardiovascular diseases (CVDs) by reducing the incidence of postprandial hyperglycemia and ameliorating endothelial dysfunction. Acarbose can also improve blood glucose levels and risk factors associated with CVDs when combined with a moderate level of exercise [12]. Furthermore, acarbose-mediated reduction of post-prandial blood glucose levels can indirectly change glucose metabolism, thereby improving insulin sensitivity and affecting the life span [13].
Mechanistically, acarbose has been suggested to influence the human life span by modulating the gut microbiota [14]. Thus, acarbose treatment to maintain blood glucose levels may be a viable approach to maintain health and delay ageing [15].
2. Remodelling the gut microbiota can extend the lifespan
Increasing evidence suggests that the gut microbiota plays a vital role in the occurrence and development of T2DM [8]. Metformin promotes gut microbiota metabolites, such as short-chain fatty acids (SCFAs), which may play a crucial role in extending the life span of Caenorhabditis elegans [16]. Whether metformin affects the life span of worms depends on its sensitivity to Escherichia coliand glucose levels [17]. Similarly, acarbose has been reported to play a role in extending the life span of mice. Poor glucose regulation can accelerate the aging process. Therefore, acarbose can improve health and extend life span by regulating glucose metabolism. Resistant starch can resist degradation by digestive enzymes in the small intestine and reach the large intestine, suggesting that resistant starch modulates gut homeostasis, similar to acarbose [18]. Resistant starch can increase the abundance of SCFAs, especially butyric acid [19,20]. The effect of acarbose on longevity may be mediated by glucose physiology and microbial activity in the intestine. Acarbose can increase the amount of starch that enters the colon, thereby enriching carbohydrate-degrading bacteria and altering the gut microbiota and its fermentation products [21]. Hence, treatment with acarbose can increase the abundance of potential SCFA-producing bacteria [8,14].
Nutritionally, calorie restriction (CR) is considered an effective strategy for extending the life span [22]. Complex interactions between metabolic adaptations and immune and anti-inflammatory responses may contribute to the health-promoting and longevity effects of CR [23,24]. CR can considerably alter the gut microbiota of aging mice [25] and reduce age-related changes in the gut microbiota [26]. Bifidobacterium possesses immune-modulatory and anti-inflammatory effects; therefore, it is believed to be beneficial for colon health [27,28]. In calorie-restricted mice, the decline in the relative abundance of age-related bacteria, such as Bifidobacterium and Akkermansia, could be prevented [26]. Remodeling of the gut microbiota during CR contributes to improved insulin sensitivity and glucose tolerance and decreases fat gain and the formation of beige adipocytes, which in turn extends the life span [29]. Similar to the effect of CR on glucose metabolism and insulin activity in rodents and primates, acarbose supplementation can improve age-related glucose and insulin disorders [15]. The therapeutic effects of acarbose treatment are achieved by the selective inhibition of carbohydrate metabolism for calorie compensation while allowing carbohydrate consumption [30].
Recent studies on CR mimetic (CRM) compounds revealed that acarbose belongs to the upstream type of CRMs and regulates glucose metabolism [31]. CR diet induces homeostasis of the gut microbiota, which is potentially beneficial for health promotion and indicates a close relationship between regulation of the gut microbiota and healthy aging [25,31]. Although CR can extend the life span, implementing long-term CR is quite challenging. Therefore, the use of a CRM compound, such as acarbose, instead of a CR diet, would be more practical and acceptable for patients with diabetes [30,32].
Although direct evidence of the roles of gut microbiota in extending the life span by using acarbose is scarce, the following aspects may be helpful in understanding the underlying mechanism of how acarbose affects the life span as mediated by the gut microbiota. First, how acarbose influences the composition and function of the gut microbiota is discussed. Second, how changes in the gut microbiota influence life span are discussed. Third, the potential gut microbiota-mediated mechanism for the extension of life span by using acarbose is elaborated.
3. Acarbose influence the composition and functions of the gut microbiota
The effect of acarbose on intestinal fermentation products may influence its overall effect on host physiology. SCFAs are the main products of starch fermentation of intestinal bacteria, which are beneficial to health, and a positive correlation has been reported between SCFA levels in mice feces and their survival rates [14]. Acarbose could influence both the composition of the bacterial community and SCFA levels in mice feces. Changes in the butyric and acetic acid levels after acarbose treatment vary in different populations from different countries. The acetic acid and butyric acid levels were found to increase after acarbose treatment in most populations in Germany and the United States [33-35], but no significant difference was observed in the butyric acid level after acarbose treatment in diabetic populations in China [36]. In nondiabetic people in the United States, the butyric acid levels increased, whereas the acetic acid levels did not significantly change after acarbose treatment [37]. In feces of centenarians in China, seven characteristic compounds (i.e., total SCFA; manganese; cobalt; and acetic, propionic, butyric, and valeric acids) were identified. This metabolic pattern, particularly the increase in the levels of total bile acids and SCFAs, may have an important and positive effect on longevity [38]. In another study of centenarians in Sardinia, their intestinal microbiota showed potential health promoting characteristics, which was related to the high capacity of glycolysis and SCFA production, leading to the extension of lifespan[39]. For instance, in mice fed with acarbose, the abundance of the SCFA-producing bacteria Lactobacillus increased [40]. Besides their anti-inflammatory effects, SCFAs can also regulate immunity [41]. In addition to well-known SCFAs, such as acetic, butyric, and propionic acids, other SCFAs have the ability to extend the life span. For example, formate can reduce the generation of reactive oxygen species (ROS), which can also extend the life span. Thus, acarbose affects longevity by influencing the abundance of SCFAs [42].
Remodeling the gut microbiota can also extend the life span, and acarbose is known to regulate the gut microbiota. However, to understand the role of gut microbiota in the extension of the life span by acarbose, it is important to investigate which factors influence the life span based on the gut microbiota and how the gut microbiota affect these influencing factors.
4. Factors that influence lifespan based on the gut microbiota
Aging features include mitochondrial dysfunction, cellular senescence, altered cell-to-cell communication, and loss of protein stabilization. Dysfunctional mitochondria in T cells have been reported to accelerate senescence [43]. With age, cellular stress and damage increase, and correspondingly, the levels of ROS increase in an attempt to increase the chances of survival. Beyond a certain threshold, these changes eventually aggravate age-associated damage [44]. Naturally, telomeres in somatic cells gradually shorten with age, ultimately leading to cellular senescence. Human cells expressing telomerase were shown to regulate telomere length. An increase in telomerase activity results in increased telomere length [45,46]. Immune responses, mitochondrial function, senescence-associated gene expression, and telomeres are the most important factors affecting the life span.
4.1. Immune responses
The innate immune system in mammals induces inflammatory responses, and age-related changes in inflammatory responses are considered to cause aging [47]. The levels of pro-inflammatory factors in the serum of the elderly are higher than those in younger people. The increase in the levels of pro-inflammatory factors is indicative of aging. Furthermore, changes in the activity of the innate immune system are associated with age-related diseases. Studies on C. elegans showed that the innate immune function is regulated by transforming growth factor-β (TGF-β), p38 mitogen-activated protein kinases, and the DAF-2 insulin pathway [48]. Dysregulated signaling in the TGF-β pathway plays a major role in inflammation, fibrogenesis, and immunomodulation [49]. DAF-2 plays an important role in the regulation of the life span partly by controlling immune system-related gene expression [50]. A sustained inflammatory response can damage the innate immune system, activate stress responses, and induce the impairment of cells and molecules [51]. Lower levels of interleukin (IL)-6 were detected in centenarians than older person [47,52]. The pathways of phosphatidylinositol signal transduction, sphingomyelin biosynthesis, and biosynthesis of various N-polysaccharides in the gut microbiota were higher in centenarians, indicating healthy immune functions and a balanced intestinal environment [53].
Accumulating evidence has shown that dysbiosis of the gut microbiota can cause inflammation and that probiotics can regulate the gut microbiota and reduce inflammation. The diversity of the gut microbiota decreases with age. Age-related gut microbiota disorder triggers the innate immune response and low-grade inflammation. This ultimately leads to intestinal dysplasia, which in turn leads to epithelial dysfunction, making the host susceptible to unhealthy aging, infection, and increased mortality [54]. The association between inflammation and the gut microbiota suggests the role of inflammation in the extension of the life span, which is at least partially modulated by the gut microbiota [55]. Intestinal alkaline phosphatase (IAP) is an anti-inflammatory enzyme that helps in clearing many bacteria-derived pro-inflammatory factors, such as LPS. The lack of IAP can promote aging-related inflammation in mice and shorten their life span; on the contrary, IAP supplementation can reduce intestinal permeability, maintain the steady state of the gut microbiota, and inhibit aging-related inflammation, thereby significantly extending the life span [56]. Dietary inulin can increase the levels of beneficial SCFA-producing bacteria, such as Lactobacillus, and inhibit the expression of inflammatory genes from extending the life span [57]. Probiotic-4, a probiotic formula consisting of Bifidobacterium lactis, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus acidophilus, can decrease the expression level of IL-6 [58]. Therefore, the gut microbiota can affect longevity by influencing inflammation and immunity.
4.2. Mitochondrial function
Decreased mitochondrial function plays an important role in promoting aging in humans. Mitochondria may affect the life span mainly through cell aging, chronic inflammation, and stem cell dysfunction [59]. Aging is usually accompanied by reduced mitochondrial-related metabolic activity [44,59,60]. For instance, mutations in mice mitochondrial DNA polymerase lead to mitochondrial DNA deletion, which accelerates aging [61]. During mitochondrial respiration, the generation of ROS and free radicals, such as hydrogen peroxide, result in cumulative damage to DNA, proteins, and lipids, eventually leading to the loss of tissue function and ultimately, death [62]. This could be one of the mechanisms by which mitochondrial dysfunction affects longevity. Recently, it has been reported that the gene expression levels of mitochondrial superoxide dismutase 2 and fission 1 were decreased in aged mutant mice, suggesting that the shorter life span and poor physical condition of this mouse result from mitochondrial dysfunction [63]. Acetic acid and butyric acid produced by the gut microbiota via fermentation have protective effects on oxidative and mitochondrial stress. Thus, they can inhibit Streptozotocinn(STZ)-induced cell apoptosis, mitochondrial dysfunction, and excessive ROS generation [64]. This could further help in understanding the interactions between the gut microbiota and mitochondrial functions that affect the life span.
4.3. Telomeres
One of the main manifestations of aging is telomere shortening [65]. The length of telomeres decreases during a human’s lifetime, limiting cell proliferation, damaging cells, and ultimately shortening the life span. The length of telomeres determines the life span to some extent. Mutations in the gene encoding telomerase can lead to increased telomere shortening, which reduces the regenerative functions of stem cells [66] and activates the p53 pathway [67]. Telomere length and the telomere shortening rate (TSR) are recognized indicators of aging in population studies. Aging is always accompanied by low-grade systemic inflammation, which may be a result of the progression of age and age-related diseases, leading to telomere shortening [68]. SCFAs can regulate the immune response and show anti-inflammatory effects, which can result in the extension of telomere length [69].
4.4. Senescence-associated gene expression
Sirtuin (SIRT) is often referred to as the “longevity gene” because it plays an important role in protein and DNA repairs. SIRT1 and SIRT6 are promising regulators of longevity [70] that belong to the histone deacetylase sirtuin family [71]. Dysbiosis of the gut microbiota can promote neuroinflammation and neurodegeneration. In recent years, there has been increasing evidence that sirtuins play a role in obesity, diabetes, and various age-related neurodegenerative diseases. Although few direct studies have proved that the gut microbiota can influence the longevity gene, including genes belonging to the sirtuin family, metabolites derived from the gut microbiota affect the life span by affecting the longevity gene. SCFAs can promote gut-brain activation by regulating the gut microbiota. SIRT1 plays an important role in maintaining intestinal tissue homeostasis by regulating the gut microbiota1 plays an important role in maintaining intestinal tissue homeostasis by regulating the gut microbiota [72,73]. The butyrate levels in the intestine are positively correlated with the levels of life-prolonging hormone fibroblast growth factor 21, which participates in SIRT1 activation [74]. L. mali (APS1) can increase butyrate levels, activate glucagon like peptide-1(GLP-1) receptors, and enhance the expression of SIRT1 while inhibiting oxidative stress by activating SIRT1 [75]. The increase in serum oxidative status and the subsequent decrease in SIRT6 expression may be caused by the decreased population of Firmicutes/Bacteroidetes and the abundance of Bifidobacterium, suggesting that regulating the expression of the longevity gene by ameliorating gut dysbiosis may be beneficial to life span extension [76].
5. Putative gut microbiota-mediated mechanisms for the extension of the life span by using acarbose
The gut microbiota can regulate the life span by affecting inflammation. However, few studies have assessed the effect of the gut microbiota on oxidative stress or telomere length, and the underlying mechanisms remain unclear. In contrast, acarbose plays a significant role in regulating the gut microbiota, so how does acarbose regulate gut microbiota to affect the life span?
5.1. Anti-inflammatory response
In addition to its anti-hyperglycemic effect, acarbose exerts an anti-inflammatory effect [77]. Increased inflammatory activity is accompanied by brain aging, but acarbose can delay aging in mice by inhibiting the activation of the hypothalamic nuclear factor kappa B (NF-κB) inflammatory pathway [78]. Acarbose has been shown to inhibit interferon-γ inducible protein-10, monocyte chemoattractant protein-1, macrophage-derived chemokine, and TNF-α activation and to downregulate NF-κB-P65 activity in human monocytic THP-1 cells [77]. Probiotics can attenuate the expression of IL-6, which is negatively associated with longevity [52,58]. Furthermore, the levels of IL-6 in patients with diabetes treated with acarbose are also significantly reduced [79]. Adipose tissue inflammation plays an important role in senile diseases, and acarbose is presumed to extend the life span by slowing or preventing adipose tissue inflammation [80]. Acarbose may reduce the expression levels of inflammatory factors by increasing the abundance of beneficial bacteria. For example, acarbose treatment significantly increases the relative abundance of Ruminococcus and Bifidobacterium. Ruminococcus produces acetic and propionic acids, both of which can improve metabolic abnormalities and intestinal inflammation. Many species of Bifidobacterium and Lactobacillus are recognized as SCFA-producing bacteria that exert anti-inflammatory effects [81]. These findings provide strong evidence for the immunosuppressive effect and anti-inflammatory potentials of acarbose.
5.2. Reduced generation of mitochondrial ROS
Hyperglycemia and even a short-term increase in blood glucose levels activate vascular cells and cause endothelial dysfunction. These toxic effects may be a result of the generation of ROS in the vascular systems in diabetic and hyperglycemic states [82,83]. Mitochondrial aconitase is a sensitive marker of oxidative stress in aging-related diseases, and its diminished activity can serve as an indicator of ROS generation in mitochondria [84,85]. The activities of mitochondrial aconitase decreased in sucrose-fed obese Zucker rats with insulin resistance and hyperglycemia, suggesting that mitochondria generate ROS during dysfunctional glucose metabolism. Treatment with acarbose can largely prevent the reduced activity of mitochondrial aconitase, the increase in oxidative stress, and vascular dysfunction caused by hyperglycemia [86].
When the fecal microbiota of patients with depression was transplanted into germ-free rats, mitochondrial damage was observed in intestinal epithelial cells, indicating that intestinal microbial disorders result in mitochondrial damage [87]. As mentioned previously, SCFAs, such as acetic and butyric acids, inhibit mitochondrial dysfunction and ROS overproduction. Furthermore, mopping up ROS or improving mitochondrial dysfunction can delay aging [55]. Based on this, we can speculate that acarbose further extends the life span by increasing the abundance of SCFAs.
5.3. Influence on telomere length
Some dietary components can affect the length of telomeres and lead to aging. For example, excessive sugar consumption can shorten telomeres, whereas increased consumption of plant-based foods rich in antioxidants can maintain telomere length [88]. Because acarbose decreases carbohydrate absorption, it can theoretically extend the length of telomeres. However, acarbose treatment was shown to increase TSR, which accelerates the biological aging of patients. In addition to delaying sucrose and starch digestion to reduce blood glucose levels, acarbose may also disrupt gastrointestinal transport, leading to metabolic disorders that accelerate telomere shortening [89]. Although acarbose was found to increase TSR, in later studies by the same group, this effect was not discovered in all populations but only in certain patients with diabetes. Acarbose was found to show different effects on telomeres in different populations with severe insulin-resistant diabetes (SIRD) and non-SIRD [90].
Acarbose has been widely regarded as increasing the abundance of SCFAs, especially acetic and butyric acids. Using yeast as a model organism, Romano et al. found that acetic acid had the capability of extending the length of telomeres [69]. Butyric acid consumption led to an earlier peak in plasma triglyceride levels and increased plasma total cholesterol levels, which might be responsible for shortening telomere length in postmenopausal women [91]. Treatment with acarbose had various effects on the levels of acetic and butyric acids in different mice strains [14,92,93]. Furthermore, as mentioned previously, the levels of butyric and acetic acids varied in different populations after acarbose treatment, and the levels of butyric acid increased after acarbose treatment in most populations, which may partly explain why treatment with acarbose resulted in shortening of telomere length.
It has been found that the mechanisms by which acarbose affects telomeres differ between patients with and without SIRD [90]. In this article, Huang et al. mentioned that a study they cited had reported that the abundance of Bifidobacterium decreased after acarbose administration and suggested that the reduced abundance of Bifidobacterium, which aggravated inflammation, and the gut microbiota dysbiosis in the SIRD group are characterized by insulin resistance after acarbose treatment. They then presumed that the disordered gut microbiota plays an important role in telomere attrition in patients with SIRD receiving acarbose treatment. However, the study they cited actually showed that the abundance of Bifidobacterium increased after acarbose treatment, which could not account for the role of gut bacteria in shortening telomere length [40]. They did not further examine the levels of butyric and acetic acids. To further demonstrate the role of the gut microbiota in the regulation of telomere length, it is necessary to study the abundance of butyric and acetic acid producers and the levels of SCFA in various populations. In addition, considering that telomere length is not an independent factor affecting the life span, whether acarbose affects life span extension by mainly regulating the inflammatory level and mitochondrial function based on the gut microbiota needs further investigation.
5.4. Influence on longevity genes
Similar to acarbose, galacto-oligosaccharide (GOS) cannot be absorbed in the small intestine, so more carbohydrates reach the large intestine. GOS regulates the gut microbiota and its metabolites through the liver-gut axis. GOS fermentation significantly increases butyrate acid levels, which can activate the hepatic adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK)/SIRT1 signaling pathway, increase SIRT1 expression, restore hepatic antioxidant activity, and alleviate aging [94]. However, there is no direct evidence that acarbose affects the SIRT6 longevity gene.
Acarbose may extend the life span by regulating the gut microbiota and thus, attenuating the inflammatory reaction, which is the risk of the disorder of the mitochondrial function and the telomere attrition. However, there is no direct evidence that acarbose can extend the life span by regulating aging-related genes and telomere length, which is a subject that should be further examined.
Figure 1.
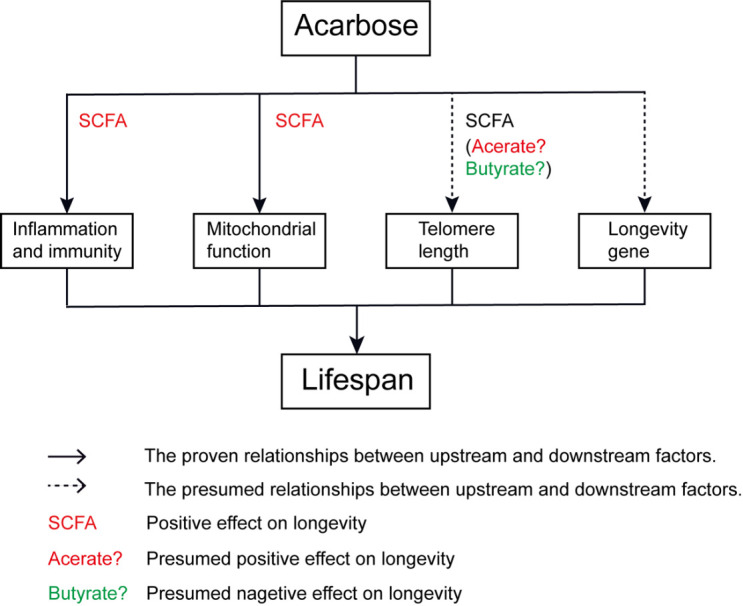
Potential mechanisms by which acarbose extends life span mediated by the gut microbiota.
6. Conclusion
The existing literature provides evidence that acarbose can affect the life span. This review linked inflammation, mitochondria, and telomeres with the gut microbiota, illustrating individual mechanisms involved in acarbose-associated life span extension. Acarbose improves the immune system, inflammatory response, and mitochondrial function by affecting the gut microbiota. Acarbose supplementation is a cost-effective method for delaying aging given its potential health-restorative effects and limited side effects. This offers hope for analyzing the use of acarbose to improve health and reduce the risk of age-related diseases.
Additional experiments should be undertaken to verify our speculations; for instance, which bacteria affect the length of telomeres and mitochondrial function after acarbose intervention needs to be studied. The role of acarbose in affecting telomere length by regulating the gut microbiota should be investigated with a more rigorous scientific approach. The present review describes several mechanisms by which acarbose affects the life span through the gut microbiota by considering different viewpoints and provides a new theoretical basis for the mechanism of acarbose-extended life spans. Hitherto, to the best of our knowledge, no other reviews have explained the mechanisms underlying life span extension by acarbose based on the perspective of gut microbiota. In general, many factors that affect the life span and mechanisms of acarbose that can help extend the life span of humans remain to be studied.
Acknowledgements
This commentary has been funded by Scientific Research Project of Jiangsu Commission of Health (M2021055); Jiangsu Scientific Research Project of Women’s and Children’s Health (F201741); Scientific Research Project of Wuxi Commission of Health (ZZ003); Wuxi scientific and technological development project (N20192024, N20191001 and N2020X007). Translational medicine Research Program of Wuxi Translational Medicine Center (2020ZHYB08).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- [1].Zhang H, Menzies K, Auwerx J (2018). The role of mitochondria in stem cell fate and aging. Development, 145(8):dev143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ogrodnik M, Salmonowicz H, Gladyshev V (2019). Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell, 18:e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prasad K, Wu M, Bondy S (2017). Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech Aging Dev, 164:61-66. [DOI] [PubMed] [Google Scholar]
- [4].Cañadas-Lozano D, Marín-Aguilar F, Castejón-Vega B, Ryffel B, Navarro-Pando JM, Ruiz-Cabello J, et al. (2020). Blockade of the NLRP3 inflammasome improves metabolic health and lifespan in obese mice. GeroScience, 42:715-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Novelle M, Ali A, Diéguez C, Bernier M, de Cabo R (2016). Metformin: A Hopeful Promise in Aging Research. Csh Perspect Med, 6:a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martin-Montalvo A, Mercken E, Mitchell S, Palacios H, Mote P, Scheibye-Knudsen M, et al. (2013). Metformin improves healthspan and lifespan in mice. Nat Commun, 4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolf T, Droste J, Gren T, Ortseifen V, Schneiker-Bekel S, Zemke T, et al. (2017). The MalR type regulator AcrC is a transcriptional repressor of acarbose biosynthetic genes in Actinoplanes sp. SE50/110. BMC Genomics, 18:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, et al. (2017). Effects of Acarbose on the Gut Microbiota of Prediabetic Patients: A Randomized, Double-blind, Controlled Crossover Trial. Diabetes Ther, 8:293-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khwaja N, Arunagirinathan G (2021). Efficacy and Cardiovascular Safety of Alpha Glucosidase Inhibitors. Drug Safety, 16:122-128. [DOI] [PubMed] [Google Scholar]
- [10].Yamamoto M, Otsuki M (2006). Effect of inhibition of alpha-glucosidase on age-related glucose intolerance and pancreatic atrophy in rats. METABOLISM, 55:533-540. [DOI] [PubMed] [Google Scholar]
- [11].Yamamoto M, Jia D, Fukumitsu K, Imoto I, Kihara Y, Hirohata Y, et al. (1999). Metabolic abnormalities in the genetically obese and diabetic Otsuka Long-Evans Tokushima Fatty rat can be prevented and reversed by alpha-glucosidase inhibitor. METABOLISM, 48:347-354. [DOI] [PubMed] [Google Scholar]
- [12].Wagner H, Degerblad M, Thorell A, Nygren J, Ståhle A, Kuhl J, et al. (2006). Combined treatment with exercise training and acarbose improves metabolic control and cardiovascular risk factor profile in subjects with mild type 2 diabetes. Diabetes Care, 29:1471-1477. [DOI] [PubMed] [Google Scholar]
- [13].Dodds S, Parihar M, Javors M, Nie J, Musi N, Dave Sharp Z, et al. (2020). Acarbose improved survival for Apc mice. Aging Cell, 19:e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith B, Miller R, Ericsson A, Harrison D, Strong R, Schmidt T (2019). Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol, 19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brewer R, Gibbs V, Smith D (2016). Targeting glucose metabolism for healthy aging. J Nutr Health Aging, 4:31-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garratt M, Bower B, Garcia G, Miller R (2017). Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell, 16:1256-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cabreiro F, Au C, Leung K, Vergara-Irigaray N, Cochemé H, Noori T, et al. (2013). Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell, 153:228-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wali J, Milner A, Luk A, Pulpitel T, Dodgson T, Facey H, et al. (2021). Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat Metab, 3:810-828. [DOI] [PubMed] [Google Scholar]
- [19].Baxter NT, Lesniak NA, Sinani H, Schloss PD, Koropatkin NM, Marco ML (2019). The Glucoamylase Inhibitor Acarbose Has a Diet-Dependent and Reversible Effect on the Murine Gut Microbiome. Msphere, 4(1):e00528-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liyanage G, Inoue R, Fujitani M, Ishijima T, Shibutani T, Abe K, et al. (2021). Effects of Soy Isoflavones, Resistant Starch and Antibiotics on Polycystic Ovary Syndrome (PCOS)-Like Features in Letrozole-Treated Rats. Nutrients, 13(11):3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wachters-Hagedoorn RE, Priebe MG, Heimweg JA, Heiner AM, Elzinga H, Stellaard F, et al. (2007). Low-dose acarbose does not delay digestion of starch but reduces its bioavailability. Diabetes Med, 24(6):600-6. [DOI] [PubMed] [Google Scholar]
- [22].Fontana L, Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell, 161:106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chung K, Kim D, Park M, Choi Y, Kim N, Lee J, et al. (2013). Recent advances in calorie restriction research on aging. Exp Gerontol, 48:1049-1053. [DOI] [PubMed] [Google Scholar]
- [24].López-Lluch G, Navas P (2016). Calorie restriction as an intervention in ageing. J Physiol, 594:2043-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. (2013). Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun, 4:2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kok D, Rusli F, van der Lugt B, Lute C, Laghi L, Salvioli S, et al. (2018). Lifelong calorie restriction affects indicators of colonic health in aging C57Bl/6J mice. J Nutr Biochem, 56:152-164. [DOI] [PubMed] [Google Scholar]
- [27].Wu B, Yang Y, Xu X, Wang W (2016). Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr, 12:177-182. [DOI] [PubMed] [Google Scholar]
- [28].Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L (2016). Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol, 7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fabbiano S, Suárez-Zamorano N, Chevalier C, Lazarević V, Kieser S, Rigo D, et al. (2018). Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab, 28:907-921.e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gibbs V, Brewer R, Miyasaki N, Patki A, Smith D (2018). Sex-dependent Differences in Liver and Gut Metabolomic Profiles With Acarbose and Calorie Restriction in C57BL/6 Mice. J Gerontol A-biol, 73:157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shintani H, Shintani T, Ashida H, Sato M (2018). Calorie Restriction Mimetics: Upstream-Type Compounds for Modulating Glucose Metabolism. Nutrients, 10(12):1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Smith D, Orlandella R, Allison D, Norian L (2021). Diabetes medications as potential calorie restriction mimetics-a focus on the alpha-glucosidase inhibitor acarbose. GeroScience, 43:1123-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wolever T, Chiasson J (2000). Acarbose raises serum butyrate in human subjects with impaired glucose tolerance. Brit J Nutr, 84:57-61. [PubMed] [Google Scholar]
- [34].Scheppach W, Fabian C, Sachs M, Kasper H (1988). The effect of starch malabsorption on fecal short-chain fatty acid excretion in man. Scand J Gastroentero, 23:755-759. [DOI] [PubMed] [Google Scholar]
- [35].Holt P, Atillasoy E, Lindenbaum J, Ho S, Lupton J, McMahon D, et al. (1996). Effects of acarbose on fecal nutrients, colonic pH, and short-chain fatty acids and rectal proliferative indices. METABOLISM, 45:1179-1187. [DOI] [PubMed] [Google Scholar]
- [36].Zhao L, Zhang F, Ding X, Wu G, Lam Y, Wang X, et al. (2018). Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science, 359:1151-1156. [DOI] [PubMed] [Google Scholar]
- [37].Weaver G, Tangel C, Krause J, Parfitt M, Jenkins P, Rader J, et al. (1997). Acarbose enhances human colonic butyrate production. J Nutr, 127:717-723. [DOI] [PubMed] [Google Scholar]
- [38].Cai D, Zhao S, Li D, Chang F, Tian X, Huang G, et al. (2016). Nutrient Intake Is Associated with Longevity Characterization by Metabolites and Element Profiles of Healthy Centenarians. Nutrients, 8(9):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu L, Zeng T, Zinellu A, Rubino S, Kelvin D, Carru C (2019). A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems, 4(4):e00325-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang M, Feng R, Yang M, Qian C, Wang Z, Liu W, et al. (2019). Effects of metformin, acarbose, and sitagliptin monotherapy on gut microbiota in Zucker diabetic fatty rats. BMJ Open Diab Res Ca, 7:e000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parada Venegas D, De la Fuente M, Landskron G, González M, Quera R, Dijkstra G, et al. (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol, 10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wenzel TJ, Gates EJ, Ranger AL, Klegeris A (2020). Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci, 105:103493. [DOI] [PubMed] [Google Scholar]
- [43].Desdín-Micó G, Soto-Heredero G, Aranda J, Oller J, Carrasco E, Gabandé-Rodríguez E, et al. (2020). T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368: 1371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].López-Otín C, Blasco M, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Awad A, Glousker G, Lamm N, Tawil S, Hourvitz N, Smoom R, et al. (2020). Full length RTEL1 is required for the elongation of the single-stranded telomeric overhang by telomerase. Nucleic Acids Res, 48:7239-7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pickett H, Cesare A, Johnston R, Neumann A, Reddel R (2009). Control of telomere length by a trimming mechanism that involves generation of t-circles. Embo J, 28:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Franceschi C, Bonafè M (2003). Centenarians as a model for healthy aging. Biochem Soc T, 31:457-461. [DOI] [PubMed] [Google Scholar]
- [48].Kurz CL, Tan MW (2004). Regulation of aging and innate immunity in C. elegans. Aging Cell, 3:185-193. [DOI] [PubMed] [Google Scholar]
- [49].Chen J, Gingold J, Su X (2019). Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med, 25:1010-1023. [DOI] [PubMed] [Google Scholar]
- [50].Kaur S, Aballay A (2020). G-Protein-Coupled Receptor SRBC-48 Protects against Dendrite Degeneration and Reduced Longevity Due to Infection. Cell Rep, 31:107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wilson C, Finch C, Cohen H (2002). Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc, 50:2041-2056. [DOI] [PubMed] [Google Scholar]
- [52].Ferrucci L, Harris T, Guralnik J, Tracy R, Corti M, Cohen H, et al. (1999). Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc, 47:639-646. [DOI] [PubMed] [Google Scholar]
- [53].Kim B, Choi C, Shin H, Jin S, Bae J, Han M, et al. (2019). Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J Microbiol Biotechn, 29:429-440. [DOI] [PubMed] [Google Scholar]
- [54].Kim S, Jazwinski S (2018). The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology, 64:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu M, Luo Q, Nie R, Yang X, Tang Z, Chen H (2021). Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit Rev Food Sci, 61:2175-2193. [DOI] [PubMed] [Google Scholar]
- [56].Kühn F, Adiliaghdam F, Cavallaro P, Hamarneh S, Tsurumi A, Hoda R, et al. (2020). Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight, 5(6):e134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dong Y, Sun H, Yang W, Ma S, Du B, Xu H (2019). InRThe Effect of Inulin on Lifespan, Related Gene Expression and Gut Microbiota in /TM3 Mutant : A Preliminary Study. Nutrients, 11(3):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang X, Yu D, Xue L, Li H, Du J (2020). Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B, 10:475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang H, Menzies K, Auwerx J (2018). The role of mitochondria in stem cell fate and aging. Development, 145(8):dev143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang Y, Hekimi S (2015). Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science 350:1204-1207. [DOI] [PubMed] [Google Scholar]
- [61].Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink J, Rovio A, Bruder C, et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature, 429:417-423. [DOI] [PubMed] [Google Scholar]
- [62].Wei Y, Zhang Y, Cai Y, Xu M (2015). The role of mitochondria in mTOR-regulated longevity. Biological reviews of the Cambridge Philosophical Society, 90:167-181. [DOI] [PubMed] [Google Scholar]
- [63].Reichart G, Mayer J, Zehm C, Kirschstein T, Tokay T, Lange F, et al. (2019). Mitochondrial complex IV mutation increases reactive oxygen species production and reduces lifespan in aged mice. Acta Physiol, 225:e13214. [DOI] [PubMed] [Google Scholar]
- [64].Hu S, Kuwabara R, de Haan B, Smink A, de Vos P (2020). Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int J Mol Sci, 21(4):1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Manchia M, Paribello P, Arzedi C, Bocchetta A, Caria P, Cocco C, et al. (2020). A multidisciplinary approach to mental illness: do inflammation, telomere length and microbiota form a loop? A protocol for a cross-sectional study on the complex relationship between inflammation, telomere length, gut microbiota and psychiatric disorders. BMJ Open, 10:e032513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Blasco M (2007). Telomere length, stem cells and aging. Nat Chem Biol, 3:640-649. [DOI] [PubMed] [Google Scholar]
- [67].Begus-Nahrmann Y, Lechel A, Obenauf A, Nalapareddy K, Peit E, Hoffmann E, et al. (2009). p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet, 41:1138-1143. [DOI] [PubMed] [Google Scholar]
- [68].Raj D, Moser J, van der Pol S, van Os R, Holtman I, Brouwer N, et al. (2015). Enhanced microglial pro-inflammatory response to lipopolysaccharide correlates with brain infiltration and blood-brain barrier dysregulation in a mouse model of telomere shortening. Aging Cell, 14:1003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Romano G, Harari Y, Yehuda T, Podhorzer A, Rubinstein L, Shamir R, et al. (2013). Environmental stresses disrupt telomere length homeostasis. Plos Genet, 9:e1003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Xu K, Guo Y, Li Z, Wang Z (2019). Aging Biomarkers and Novel Targets for Anti-Aging Interventions. Adv Exp Med Biol, 1178:39-56. [DOI] [PubMed] [Google Scholar]
- [71].Carraway H, Malkaram S, Cen Y, Shatnawi A, Fan J, Ali H, et al. (2020). Activation of SIRT6 by DNA hypomethylating agents and clinical consequences on combination therapy in leukemia. Sci Rep-UK, 10:10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chandramowlishwaran P, Vijay A, Abraham D, Li G, Mwangi S, Srinivasan S (2020). Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front Neurosci, 14:614331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wellman A, Metukuri M, Kazgan N, Xu X, Xu Q, Ren N, et al. (2017). Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology, 153:772-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kundu P, Lee H, Garcia-Perez I, Tay E, Kim H, Faylon L, et al. (2019). Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med, 11(518):eaau4760. [DOI] [PubMed] [Google Scholar]
- [75].Chen Y, Lin Y, Lin J, Yang N, Chen M (2018). Sugary Kefir Strain Lactobacillus mali APS1 Ameliorated Hepatic Steatosis by Regulation of SIRT-1/Nrf-2 and Gut Microbiota in Rats. Mol Nutr Food Res, 62:e1700903. [DOI] [PubMed] [Google Scholar]
- [76].Li L, Chen B, Zhu R, Li R, Tian Y, Liu C, et al. (2019). Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging, 11:9348-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lin Y, Chen Y, Hsiao H, Kuo C, Chen B, Chen Y, et al. (2019). The effects of acarbose on chemokine and cytokine production in human monocytic THP-1 cells. Hormones, 18:179-187. [DOI] [PubMed] [Google Scholar]
- [78].Sadagurski M, Cady G, Miller RA (2017). Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell, 16(4):652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mo D, Liu S, Ma H, Tian H, Ren Y (2019). Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Dev Ther,Volume 13: 2769-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mau T, O'Brien M, Ghosh A, Miller R, Yung R (2020). Life-span Extension Drug Interventions Affect Adipose Tissue Inflammation in Aging. J Gerontol A-biol, 75:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lê K, Li Y, Xu X, Yang W, Liu T, Zhao X, et al. (2012). Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front Physiol, 3:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schäfer A, Widder J, Eigenthaler M, Bischoff H, Ertl G, Bauersachs J (2004). Increased platelet activation in young Zucker rats with impaired glucose tolerance is improved by acarbose. Thromb Haemostasis, 92:97-103. [DOI] [PubMed] [Google Scholar]
- [83].Du X, Stocklauser-Färber K, Rösen P (1999). Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radical Bio Med, 27:752-763. [DOI] [PubMed] [Google Scholar]
- [84].Yan L, Levine R, Sohal R (1997). Oxidative damage during aging targets mitochondrial aconitase. P Natl Acad Sci USA, 94:11168-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gardner P, Fridovich I (1992). Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem, 267:8757-8763. [PubMed] [Google Scholar]
- [86].Rösen P, Osmers A (2006). Oxidative stress in young Zucker rats with impaired glucose tolerance is diminished by acarbose. Horm Metab Res, 38:575-586. [DOI] [PubMed] [Google Scholar]
- [87].Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F (2020). Gut Microbiota Regulates Depression-Like Behavior in Rats Through the Neuroendocrine-Immune-Mitochondrial Pathway. Neuropsych Dis Treat, 16:859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Freitas-Simoes T, Ros E, Sala-Vila A (2018). Telomere length as a biomarker of accelerated aging: is it influenced by dietary intake? Curr Opin Clin Nutr, 21:430-436. [DOI] [PubMed] [Google Scholar]
- [89].Liu J, Ge Y, Wu S, Ma D, Xu W, Zhang Y, et al. (2019). Association between antidiabetic agents use and leukocyte telomere shortening rates in patients with type 2 diabetes. Aging, 11:741-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Huang J, Peng X, Dong K, Tao J, Yang Y (2021). The Association between Antidiabetic Agents and Leukocyte Telomere Length in the Novel Classification of Type 2 Diabetes Mellitus. Gerontology, 67:60-68. [DOI] [PubMed] [Google Scholar]
- [91].Song Y, You N, Song Y, Kang M, Hou L, Wallace R, et al. (2013). Intake of small-to-medium-chain saturated fatty acids is associated with peripheral leukocyte telomere length in postmenopausal women. J Nutr, 143:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xu G, Cai L, Ni Y, Tian S, Lu Y, Wang L, et al. (2018). Comparisons of Effects on Intestinal Short-Chain Fatty Acid Concentration after Exposure of Two Glycosidase Inhibitors in Mice. Biol Pharm Bull, 41:1024-1033. [DOI] [PubMed] [Google Scholar]
- [93].Harrison D, Strong R, Alavez S, Astle C, DiGiovanni J, Fernandez E, et al. (2019). Acarbose improves health and lifespan in aging HET3 mice. Aging Cell, 18:e12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang W, Liu F, Xu C, Liu Z, Ma J, Gu L, et al. (2021). Lactobacillus plantarum 69-2 Combined with Galacto-Oligosaccharides Alleviates d-Galactose-Induced Aging by Regulating the AMPK/SIRT1 Signaling Pathway and Gut Microbiota in Mice. J Agr Food Chem, 69:2745-2757. [DOI] [PubMed] [Google Scholar]


