Abstract
With the rapid aging in the global population, delay of aging has become a hot research topic. Lipid rafts (LRs) are microdomains in the plasma membrane that contain sphingolipids and cholesterol. Emerging evidence indicates an interesting interplay between LRs and aging. LRs and their components are altered with aging. Further, the aging process is strongly influenced by LRs. In recent years, LRs and their component signaling molecules have been recognized to affect aging by interfering with its hallmarks. Therefore, targeting LRs is a promising strategy to delay aging.
Keywords: aging, senescence, lifespan, aging hallmarks, lipid raft, membrane raft
1. Introduction
The aging global population poses an increasing burden on public healthcare. In 2020, there were 722 million individuals aged over 65 years (accounting for 9.318 percent of the total population), and the proportion of senior people continues to rise (data from the World Bank: population ages 65 and above, total (accessed December 10, 2021) https://databank.shihang.org/home.aspx). The incidence of cardiovascular and cerebrovascular diseases, neurodegenerative diseases, metabolic diseases, and cancers with aging is also increasing each year.
Aging is generally considered a complicated process that cannot be avoided. However, it does have common denominators, such as 1) loss of proteostasis 2) altered intercellular communication 3) genomic instability 4) mitochondrial dysfunction 5) epigenetic alterations 6) deregulated nutrient-sensing 7) cellular senescence 8) telomere attrition 9) stem cell exhaustion [1]. Lipid rafts (LRs) are key functional microdomains involved in signal transduction and membrane trafficking. Because signal transduction is essential for aging processes, the relationship between LRs and aging has attracted increasing attention. Furthermore, recend studies indicate that LRs are promising targets to attenuate or delay human aging, which is signifcant for regulating aging procedures and achieving therapeutic effects.
2. Structure and functions of LRs
The LR hypothesis was proposed in 1997 [2]. LRs are microdomains (10-200 nm) with a short life, and their components, such as sphingolipids, cholesterol, and proteins, are assembled to function and disassemble quickly afterward. The components and sizes of LRs are not constant, and they can merge with each other to become larger when necessary [3]. As hubs for signal transduction, LRs contain various signal proteins, including glycosylphosphatidylinositol (GPI)-anchored proteins, Src family kinases (SFKs), and Epidermal growth factor receptor (EGFR) [4]. LRs are mainly composed of sphingolipids, which tend to display longer and more saturated hydrocarbon chains and contribute to thickening LRs. Moreover, sphingolipids are rich in oxygen-containing groups that can form hydrogen bonds, making LRs more tightly packed than the surrounding areas. Known as liquid-ordered domains (Lo), these tightly packed regions are surrounded by a sea of liquid-disordered (Ld) phospholipids that lack cholesterol [5]. Because sphingolipids are prone to forming hydrogen bonds, cholesterol has a slightly stronger affinity for sphingolipids. Specifically, cholesterol serves as a spacer and dynamic glue between hydrocarbon chains to assemble the LRs and maintain their integrity [6,7].
Caveolae are stereoscopic-type LRs that are invaginated into the plasma membrane; their proteins differ from those found in LRs and include the specific caveolin and cavin family members [8]. Among the extensively studied caveolin family proteins, caveolin-1 is a critical regulator of cell senescence [9-11].
Owing to the complexity of LR composition and their “small, heterogeneous, and highly dynamic” characteristics, their separation and visualization progress remain hindered to a certain extent. Despite this controversy, detergents such as Triton X-100 are still used to purify LRs [12]. Sucrose gradient centrifugation is the most used method for further fractionation of LRs [13]. Next, markers of LRs, such as sphingolipid/cholesterol [13], ganglioside M1(GM1) [14], CD36 [15], flotillin-1, and caveolin-2 [16], are identified by chromatography-mass spectroscopy or Western blotting to determine the LR fractions. Usually, LRs cannot be observed directly in living cells, but fluorescent probes are available for their imaging. For example, Alexa Flour 488/555/594-conjugated cholera toxin B (CtxB) can label GM1 in green/orange/red fluorescence to visualize LRs indirectly [17]; Laurdan staining, which emits blue fluorescence, can also be used to observe LRs [18,19]. In recent years, new fluorescent sphingomyelin analogs and fluorescent ganglioside analogs have been discovered, which facilitate LR tracking in living cells [20,21]; this has led to dramatic progress in the field.
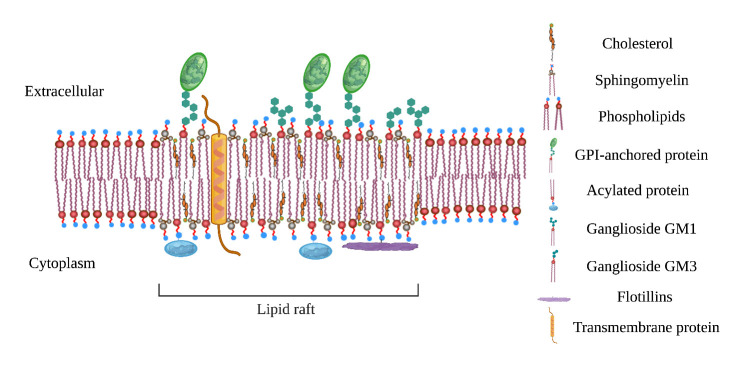
Figure 1.
Plane structure diagram of lipid rafts. Constitutive LR residents include GM1, GM3, and GPI-anchored proteins on the outside of the plasma membrane, acylated proteins and flotillins inside the plasma membrane, and transmembrane proteins embedded in the plasma membrane.
3. Alterations of LRs during aging
The functions and composition of LRs are altered with aging. Such alterations occur in T-cells, neutrophils, fibroblasts, erythrocytes, and nerve cells. For instance, T cells from elder subjects have higher cholesterol and GM1 (a marker of LRs) levels in their LRs and lower LR fluidity [22,23]. In addition, the distribution of LRs is disorganized, whereas they are homogeneous in T-cells from young individuals [24]. This disorganization of the LR can reduce its aggregation, which may alter cellular signal transduction and communication. In aging human fibroblasts, LRs, cholesterol, and flotillin (an LR marker) are reduced [25], whereas polymerase I and transcript release factor (PTRF), a member of the cavin family in caveolae, is increased [26]. Further, signaling competent caveolae are lost, and the caveolae fraction contains lower levels of caveolin 1 and 2, resulting in impaired signal transduction [27]. In human red blood cells, the LR protein marker flotillin-2 also decreases during aging [28]. Likewise, in human frontal cortex nerve cells, LR structures are substantially altered when the brain cortex ages, which is termed “LR aging” [29]. Regarding LR functions, these microdomains are known to be involved in the initial signal complex formation during T cell activation [30]. With increasing age, LRs recruit less lymphocyte-specific protein tyrosine kinase (Lck) and linker of activated T cells (LAT) (T cell regulators [31-33]) causing compromised T cell function in seniors [22]. In neutrophils, the LR-relevant Toll-like receptors 4 (TLR4) signaling pathway is altered with aging. Under lipopolysaccharide (LPS, a TLR4 ligand) stimulation, there is no recruitment of the TLR4 downstream signal molecule IL-1 receptor-associated kinase-1 (IRAK-1) to LRs in elderly donor neutrophils, which results in impaired TLR4-driven signaling events [34]. Changes in the role of LRs with aging could thus be a cause of decreased neutrophil function. Owing to the dysfunction of T cells and neutrophils during aging, the immune response is reduced, contributing to immunosenescence. Notably, a study published in Nature has indicated that immunosenescence drives the aging of other organs, ultimately promoting organism aging [35].
Similar results have also been reported in animals. In wild-type mice, LRs undergo age-related changes, such as decreased cholesterol content and increased sphingomyelin levels [36]. In mouse CD8+ T cells, GM1 levels in LRs increase with aging [37]. However, there are no apparent age-dependent disparities in the GM1 levels of rat brain synaptic LRs. In addition, the content and activity of Ca2+-ATPase (an intracellular free Ca2+ precise regulator) in the LR domain is downregulated with increasing age, and importantly, Ca2+ homeostasis dysregulation is associated with brain aging [38].
Together, these data emphasize that major changes occur in LR structure and function with age, suggesting that these changes may contribute to cell signal transduction failure.
Table 1.
Alterations in lipid rafts and their composition with aging.
| Species | Notes | Age-related alteration in LRs | Age-related alteration in components of LRs | References |
|---|---|---|---|---|
| Human | T cells | The fluidity of LRs ↓ | Cholesterol ↑ | [22] |
| The distribution of LRs is disorganized | GM1 ganglioside ↑ | [22,23] | ||
| Fibroblasts | The signaling competent caveolae ↓ | Cholesterol ↓ | [25,27] | |
| Flotillin ↓ | [25] | |||
| PTRF ↑ | [26] | |||
| Neutrophils | The LR-dependent TLR4 signal ↓ | TLR4 ↑ | [34] | |
| Red blood cells | Flotillin-2 ↓ | [28] | ||
| Nerve cells | The lipid structure (phospholipid-bound fatty acids and specific lipid classes) of LRs is altered | [29] | ||
| Mouse | Frontal lobe | Cholesterol ↓ | [36] | |
| Sterol ester ↓ | [36] | |||
| Sphingomyelin ↑ | [36] | |||
| Saturated fatty acid ↑ | [36] | |||
| Phospholipids/cholesterol ratio ↑ | [36] | |||
| Cortical(3xTgAD) | LRs density ↑ | [39] | ||
| CD8 + T cell | GM1 ganglioside ↑ | [37] | ||
| Rat | Cerebral synaptic cells | GM2 ganglioside - | [38] | |
| Ca2+ -ATPase protein ↓ | [38] | |||
| Rhesus macaque | Frontal lobe | GM3 ↑ | [40] | |
| Sphingomyelin ↑ | [40] |
-: invariant; ↓: decrease; ↑: increase; 3xTgAD: a triple-transgenic model of Alzheimer's disease
4. LR and genomic instability
Accumulation of genetic damage is a generally recognized cause of genomic instability [41], which includes direct lesions in DNA (nuclear DNA damage, mitochondrial DNA damage, telomere attrition) as well as defects in nuclear architecture [1]. Usually, organisms can repair themselves after DNA damage, but severe DNA damage or a lack of DNA repair exacerbates the aging process [1,42,43].
LRs can affect DNA integrity. For example, LR-mediated signaling can modulate reactive oxygen species (ROS) to influence DNA damage and repair responses, and LR disruption can suppress DNA repair responses [44]. Meanwhile, deficiency of CD59 (a GPI-anchored protein on LR) can exacerbate DNA damage and induce cellular senescence [45]. The level of caveolin-1 in LRs is often upregulated after DNA damage and this activates DNA repair [46].
These results indicate that genomic stability can be affected by LRs and the relevant signals to ameliorate aging.
5. LR and loss of proteostasis
Proteostasis can control non-native proteins accumulate through molecular chaperones, cochaperones, and proteolytic systems [47]. However, proteostasis diminishes with age [48], enhancing the risk of protein misfolding and aggregation, a hallmark of aging [1], which may be deleterious to cells [47,49]. Notably, LRs are integral to proteostasis.
Molecular chaperones, heat shock protein (HSPs), assist protein refolding [50]. According to previous reports, distinct reorganization of LRs is required to generate and transmit stress signals for stimulating HSP genes, thereby upregulating HSP expression [51]. Further studies have revealed that this reorganization is induced by Ras-related C3 botulinum toxin substrate 1 (Rac1)-mediated actin polymerization [52,53]. Meanwhile, the level of heat-induced HSP expression is impaired if LRs are disrupted [54]. These results indicate that the signal for HSP gene activation is transmitted through LRs. Although the role of LRs remains unclear, evidence suggests that remodeling plasma LRs can activate stress signal transduction pathways [55]. Notably, lifespan is positively determined by HSPs, and HSP expression has been shown to extend the lifespan of Drosophila [56]. HSP induction during aging may thus preserve protein homeostasis and lifespan by refolding damaged proteins that accumulate throughout aging.
If these folding attempts are futile, abnormal proteins are degraded by two central proteolytic systems (ubiquitin/proteasome and autophagic/lysosomal systems), which also decay with age [57]. Epidermal growth factor (EGF) signaling can affect C. elegans longevity by stimulating the ubiquitin/proteasome system [58]. Meanwhile, the EGF receptor is localized to LRs [59], which means that ubiquitin/proteasome system activity can be enhanced by activating EGFR on LRs to help maintain protein homeostasis and prolong lifespan. Further, the autophagic/lysosomal system is linked with mammalian target of rapamycin (mTOR) activation; specifically, autophagy can be stimulated by mTOR downregulation [60]. LRs appear to be essential for regulating the mTOR pathway by promoting phosphoinositide 3-kinase (PI3K) recruitment and V-akt murine thymoma viral oncogene homolog (Akt) activation [61,62]. Moreover, phosphatase and tensin homologue protein (PTEN) can suppress the PTEN/Akt/mTORC1 pathway, thereby activating autophagy by mobilizing the LR domain [63,64]. As an integral component of LRs, cholesterol is another factor that affects autophagy. Cholesterol accumulation reduces autophagic activity by suppressing the fusion of lysosomes with autophagic vacuoles [65,66]. Notably, autophagy has a beneficial systemic effect on lifespan [67].
As previously discussed, LR remodeling favors the activation of HSPs, thereby refolding deleterious proteins. Further, excitation of the ubiquitin/proteasome system and autophagic recovery of protein homeostasis provide exciting possibilities for extending longevity.
6. LR and deregulated nutrient-sensing
Deregulated nutrient sensing is considered a hallmark of aging. It is closely affiliated with several nutrient-sensing systems, such as the insulin/insulin-like signaling pathway (IIS) pathway, which is involved in glucose sensing; mTOR, which participates in the detection of elevated amounts of amino acids [1,68]. Nutrient-sensing system alterations affect lifespan as increased nutrient signaling speeds up aging whereas reduced nutrient signaling prolongs lifespan, that is , reduces the functions of growth hormone (GH), insulin-like growth factor 1 receptor (IGF-1R), or downstream biological factors such as Akt and mTOR, and increases the activity of AMPK, Sirt1, PTEN, and Forkhead box class O (FOXO) [1,69]. Signal transduction and activation of the nutrient-sensing systems mentioned above require the participation of LRs.
The IIS pathway is regulated by LRs in multifaceted ways. First, activation of the IIS signal requires ligands to bind to the central regulator IGF-1R (located in LRs) [70]. Second, LRs are indispensable for IGF-1R downstream signals [70]. The binding of IGF-1 and IGF-1R activates the PI3K/Akt pathway, and the phosphorylation of Akt requires LRs. If LRs are destroyed, Akt phosphorylation is blocked [71], possibly because Akt activation requires PI3K recruitment to LRs [62]. Akt has many downstream targets including mTOR and FOXO. Among these, FOXOs represent a well-conserved group of transcription factors; however, when phosphorylated, they lose their ability to function as transcriptional activators [72,73]. Inhibition of LR clustering has been reported to impede the PI3K/ Akt/ FOXO pathway [74]; in particular, FOXO phosphorylation is attenuated by LR disruption [75], suggesting that LRs are involved in FOXO signal transduction. Additionally, FOXO is a core longevity-promoting transcription factor involved in the IIS pathway [76,77]. Upregulating FOXO activity through the regulation of LRs is thus a promising way to delay aging [76].
CD24, located in the LR, can recruit PTEN to the LR and modulate the downstream pathway [63]. Upon PTEN inhibition, the PI3K/Akt signaling pathway is activated and upregulates mTOR1 [78], thereby interfering with protein and lipid synthesis as well as energy metabolism [79].
In summary, LR can mobilize and activate IIS pathway signaling molecules, which in turn regulate the signal transduction of mTOR and FOXO. Thus, prolonging health span by controlling LR is theoretically feasible.
7. LR and mitochondrial dysfunction
Mitochondrial dysfunction leads to accelerated aging in mammals [1,80-82]. Recently, LRs and their residents have been reported to modulate mitochondrial function. Data from Yu’s laboratory revealed that caveolin-1 deficiency limits the expression of cardiolipin biosynthetic enzymes that decrease cardiolipin content (an essential lipid for mitochondrial respiration), thereby reducing mitochondrial respiration, culminating in mitochondrial dysfunction and premature senescence [83]. Asterholm and colleagues found caveolin-1-null mouse embryonic fibroblasts displayed altered mitochondrial metabolism and higher mitochondrial membrane potential [73]. Furthermore, caveolin-1 deficiency leads to mitochondrial dysfunction by reducing membrane fluidity and mitochondrial respiratory chain efficiency, consequently causing ROS buildup [84]. Actually, the LR has more than one molecule involved in mitochondrial function. Src kinases, one of LR residents, are also important regulators of mitochondrial function, and have emerged as key players in mitochondrial tyrosine phosphorylation events [85]. Inhibition of SFKs ameliorates mitochondrial dysfunction [86]. Hunterour et al. discovered that c-Src, one of the most prevalent SFKs undermines mitochondrial energy metabolism by weakening the mitochondrial oxidative phosphorylation complexes [87]. Cholesterol also affects mitochondrial function as oxidized cholesterol derivatives (7-ketocholesterol) impede mitochondrial metabolism by lowering membrane potential [88,89]. In particular, 7-ketocholesterol can modify cytoplasmic mitochondrial distribution and clusters [90].
The free radical theory of aging suggests that excessive ROS production by mitochondria damages the mitochondrial genome and proteins, causing deterioration of mitochondrial function as well as further organism dysfunction and shortens lifespan [91]. Aggregation of LR with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes facilitates ROS production in intestinal epithelial cells [92]. Moreover, 7-ketocholesterol has been proved to interacts with NADPH-oxidase to trigger ROS overproduction [93-95]. Nuclear factor erythroid-2-related factor-2 (Nrf2), a leucine zipper transcription factor, can protect against harmful ROS and mediate the translation of antioxidant enzymes [96,97]. Here, caveolin-1 can restrict antioxidant enzyme expression by inhibiting Nrf2 endogenously [98] and accelerating premature senescence [99]. Thioredoxin reductase 1 (TrxR1) is a small oxidoreductase that contributes to the regulation of cellular redox homeostasis [100]. As it is also a caveolae resident protein, the combination of caveolin-1 and TrxR1 can inhibit TrxR activity, thereby accelerating stress-induced premature senescence [101].
Overall, Src kinases and caveolin-1 are key regulators of mitochondrial function in LRs and derepressing the abnormal activity of Src kinases or upregulating caveolin-1 is a promising strategy to ameliorate aging.
8. LR and cellular senescence
Generally, aging begins at the cellular level. Senescent cells accumulate as people grow older, resulting in aging and the promotion of age-related pathologies. However, mounting evidence has shown that LRs and their molecular composition are crucial for cellular senescence.
Stable cell cycle arrest is an important hallmark of cellular senescence [102]. Caveolin-1 can mediate cell cycle arrest, implying that caveolin-1 causes cellular senescence [103]. Furthermore, experimental results from Volonte et al. indicate that increased caveolin-1 expression causes senescence in murine fibroblasts, and that restoring caveolin-1 can reverse this condition [104].
With an in-depth molecular mechanism study based on phenotype, caveolin-1 has been shown to modulate senescence and organismal aging by inhibiting the effects of mouse double minute 2 homolog (Mdm2), protein phosphatase 2A-C subunit (PP2A-C), Sirt1, TrxR1, Nrf2, and EGFR on P53 [105]. First, caveolin-1 inhibits P53 degradation by binding Mdm2, followed by p53/p21 upregulation and induction of premature senescence [106]. Second, caveolin-1 triggers Ataxia telangiectasia-mutated (ATM) (P53 activator [107]) by isolating PP2A-C (ATM negative regulator) into caveolae domains, sequentially stimulating the p53/p21 pathway, leading to lung fibroblast senescence [108]. Third, caveolin-1 is a new Sirt1 blocker. The combination of Sirt1 and caveolin-1 caused by oxidants suppresses Sirt1 activity, which promotes p53 acetylation and induces premature senescence [109]. Serving as a TrxR1 antagonist, caveolin-1 suppresses TrxR activity, inhibiting the p53/p21 pathway, thus promoting premature senescence [101]. Furthermore, caveolin-1 can directly bind Nrf2 and prevent oxidant-induced Nrf2-related signaling, thereby accelerating aging [110]. Finally, caveolin-1 can directly combine with EGFR and limit its activation [111,112], thus attenuating EGF signaling in senescent cells [113]. Reducing caveolin-1 levels can restore the downstream signaling cascades of EGF cell cycle progression and reverse senescent phenotypes [114]. Growth factor responsiveness decreases because of the upregulated caveolin levels in senescent cells. Moreover, PTRF is necessary for caveolae to form and function [115,116] and is upregulated in senescent cells. Upregulated PTRF interacts with caveolin-1, leading to cellular senescence via the p53/p21 pathways [26,117].
In addition, 7-ketocholesterol was found to induce senescence in mouse endothelial progenitor cells via the Notch pathway [118]. 7-ketocholesterol has potential as an aging biomarker, as its accumulation is directly linked with various aging-related diseases [119,120].
Taken together, suppression of caveolin-1 or PTRF can clearly decelerate cellular senescence. On one hand, inhibiting caveolin-1 reverses the senescence phenotype [114,121]; on the other hand, reducing PTRF expression extends the cellular replicative lifespan [26]. However, cellular senescence is a terminal cell fate that prevents cells from proliferating indefinitely and can thus suppress tumorigenesis [122]. Therefore, determining the break-even point of cellular senescence is also a challenge in the future.
9. LR and stem cell exhaustion
Stem cells have been shown to replenish cells and regulate lifespan [123]. Stem cell exhaustion results in a decline in tissue regeneration, which is a significant hallmark of aging [1]. Stem cell surface molecules or secreted molecules from stem cells trigger hibernation or cell cycle entry [124,125]. Previous studies have shown that stem cell signaling pathways require LR to accurately modulate signal intensity [126].
LRs regulate stem cells in several ways. More specifically, LRs are indispensable for hematopoietic stem and progenitor cell (HSPC) retention in bone marrow niches and are hampered when LRs are disrupted [127]. The clustering of LRs in hematopoietic stem cells (HSCs) can augment downstream signaling pathways and deliver signals to cells, thereby inducing HSCs to re-enter the cell cycle. In contrast, the inhibition of LR aggregation disturbs PI3K/Akt/FOXO, resulting in the accumulation of FOXO transcription factors and expression of p57 cyclin-dependent kinase inhibitors, which cause HSC hibernation [74]. Additionally, LRs are required for the aforementioned pathway to be effectively activated, for recruiting the proliferation mediator Kit [128]. The mTOR signal is also mediated by LR via the PI3K/Akt pathway, and mTOR activity increases in HSCs with aging [129]. Using the mTOR inhibitor rapamycin to reduce mTOR activity can restore HSC function and increase lifespan [129]. However, the hematological toxicity of rapamycin cannot be ignored [130].
Intriguingly, LRs also function through LR-related proteins. Incorporation of CXC chemokine receptor 4 (CXCR4) into LRs activates Rac1 (a small GTPase involved in HSPC migration) and enables a more effective response to the stromal-derived factor-1 (SDF-1) gradient, which primes homing-related responses [131]. Further, the raft-resident protein Lyn (a tyrosine kinase belonging to the Src family) contributes to stem cell regulation [132]. In addition, Prion protein (PrP), a GPI-anchored protein mainly located in LRs [133], can stimulate mesenchymal stem cells [134] as well as HSCs [135] to proliferate and self-renew. LR-associated ADAM12 plays a pivotal role in esenchymal stem cell differentiation into smooth muscle cells [136].
Overall, LR clusters and LR-associated proteins have been implicated in stem cell regulation and the control of cell fate decisions. Treatments targeting LRs may thus be a new approach for stem cell rejuvenation.
10. LR and altered intercellular communication: inflammaging
Communication between cells is necessary for optimal collaboration; however, aging alters intercellular communication, including neuroendocrine dysfunction, inflammation, immunosenescence, and bystander effects [1]. Inflammation is a prevalent age-related alteration in intercellular communication [137]. The nuclear factor kappa-B (NF-κB) signaling pathway is a prominent inflammatory signaling pathway that regulates cellular inflammatory responses [138]. As the “ears and mouth” of cells, LRs are responsible for cellular signal transduction, especially inflammatory signaling. NF-κB signaling can be triggered by TLR and tumor necrosis factor alpha (TNF-α) receptors through binding to their corresponding ligands [139,140].
TLRs are transmembrane proteins of the pattern recognition receptor family. They can activate the pro-inflammatory NF-κB signaling pathway by binding to endogenous ligands [139]. TLR activation occurs in LRs. One study demonstrated that increased LRs recruit more myeloid differential protein-88 (MyD88)-dependent TLRs to LRs, boosting downstream signal transduction and promoting inflammation [141]. Moreover, TLR signal transduction requires the cooperation of raft protein [142]. CD14, a GPI-anchored protein localized in LRs [143], facilitates the transfer of LPS to TLR4 and subsequently activates the NF-κB pathway [144].
TNF-α, a proinflammatory cytokine, can activate NF-κB signaling and initiate an inflammatory response [145]. After the binding of TNF receptor 1 and TNFα, TNF receptor 1 translocates to LRs and initiates the transcription factor NF-κB by forming a receptor-induced signaling complex that binds several signaling proteins [140]. During this process, LRs act as a platform for TNF-α to mediate signal transduction. When LRs increase, TNF-α secretion is accelerated [146]. In senescent endothelial cells, caveolae and caveolin-1 are increased, whereas NF-κB activation induced by TNFα is decreased. Interestingly, this phenomenon can be reversed when caveolin-1 is knocked down. In other words, increased caveolae and caveolin-1 may inhibit the NF-κB pathway and prevent inflammation in senescent cells [147]. Meanwhile, 7-ketocholesterol induces TNF-α expression in human monocytes [148], indicating that it can affect cell communication.
Overall, LRs affect inflammation by regulating NF-κB expression. Numerous studies have focused on LRs to control the inflammatory status. For example, allicin inhibits mastitis by diminishing the LR form and inhibiting signals downstream of TLR2 and TLR6 [149]. Selenium is also used to alleviate lipopolysaccharide-induced endometritis by attenuating LR levels and impeding the recruitment of TLR4 into LRs [150]. Meanwhile, inhibiting NF-κB signaling is reported to delay senescence and aging in mice [151]. Hence, selectively blocking LR-dependent inflammatory processes may be a suitable strategy to delay aging.
11. Perspectives
The pursuit of longevity is the ultimate goal of humans, and aging remains a considerable challenge. Despite the continuous advances in our understanding of LRs over the last two decades, some questions remain unanswered. However, aging is apparently regulated by LR. Aging drastically affects the components and functions of LRs. Further, considering the evidence discussed here, the influences of LRs on the hallmarks of aging are apparent (Fig. 2). Many of these hallmarks contribute to the development of sustained inflammatory stage and aging [152]. Hence, attempts to “cure” aging should involve amelioration of inflammaging (chronic, sterile, low-grade inflammation during aging) [153], which can be achieved by regulating LRs.
Figure 2.
Interplay between lipid rafts and aging hallmarks. This figure shows the signaling pathways and molecules related to LR and the seven aging hallmarks described in this review.
Modulation of cholesterol is one way to regulate LRs, as cholesterol is a critical constituent of LRs. Most cellular cholesterol exists in the membrane and is enriched in LRs [154]. Depleting cholesterol can disrupt the form of LRs and reduce the content of LRs [149,155], suggesting that cholesterol-lowering drugs such as statins, can alleviate inflammaging to anti-aging by inhibiting the formation of LRs. As expected, clinical results have demonstrated that new statin use is associated with a decreased death rate among American veterans (75 years and older) [156]. However, one of the frequently reported adverse reactions of statins is memory impairment and cognitive decline [157,158]. Coincidentally, Alzheimer's disease, which is characterized by cognitive and memory deterioration, is associated with reduced levels of cholesterol and LRs in the frontal cortex [36,159]. Based on these results, we speculate that the adverse effects of statins on memory and cognitive alterations may partly be due to their cholesterol-lowering effects and hindered formation of LRs. Therefore, when using statins to delay aging, it is recommended to adopt some pharmaceutical modifications to increase the polarity of the statins or to choose hydrophilic statins instead of lipophilic statins for making them selective and inaccessible to the central nervous system, thus reducing their side effects.
Overall, aging has been proven modifiable, and some drugs for slow aging have been discovered. For example, rapamycin inhibits mTOR activation to delay aging; senolytics can target and eliminate senescent cells; sirtuin activators, which enhance sirtuin activity; Nicotinamide adenine dinucleotide (NAD) precursors that can supply cellular NAD levels; antidiabetic drugs such as metformin and acarbose; and non-steroidal anti-inflammatory drugs, can also be used [152,160]. However, none of drugs target LRs to delay aging, making it a future objective. Overall, targeting LRs will be a novel strategy for prolonging life, and statins might be promising candidates for new anti-aging agents.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 81973668, 81774130), the National Science Fund of Hunan Province for Distinguished Young Scholars (No. 2018JJ1018), the Key Project of the Educational Department of Hunan Province (No. 20A375), the Pharmaceutical Open Fund of Domestic First-class Disciplines (cultivation) of Hunan Province (No. 2021YX07), the Scientific Research Project of Changsha Science and Technology Bureau (No. kq2004060), Key Project of Hunan Provincial Health Commission (202213055529), and First-Class Discipline of Pharmaceutical Science of Hunan.
Footnotes
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
References
- [1].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simons K, Ikonen E (1997). Functional rafts in cell membranes. Nature, 387:569-572. [DOI] [PubMed] [Google Scholar]
- [3].Pike LJ. (2006). Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res, 47:1597-1598. [DOI] [PubMed] [Google Scholar]
- [4].Simons K, Ehehalt R (2002). Cholesterol, lipid rafts, and disease. J Clin Invest, 110:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McMullen TPW, Lewis RNAH, McElhaney RN (2004). Cholesterol-phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr Opin Colloid Interface Sci, 8:459-468. [Google Scholar]
- [6].Lingwood D, Simons K (2010). Lipid rafts as a membrane-organizing principle. Science, 327:46-50. [DOI] [PubMed] [Google Scholar]
- [7].Simons K, Toomre D (2000). Lipid rafts and signal transduction. Nat Rev Mol Cell Biol, 1:31-39. [DOI] [PubMed] [Google Scholar]
- [8].Parton RG, Tillu VA, Collins BM (2018). Caveolae. Curr Biol, 28:R402-R405. [DOI] [PubMed] [Google Scholar]
- [9].Dasari A, Bartholomew JN, Volonte D, Galbiati F (2006). Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res, 66:10805-10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Volonte D, Galbiati F (2020). Caveolin-1, a master regulator of cellular senescence. Cancer Metastasis Rev, 39:397-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nguyen KCT, Cho KA (2017). Versatile Functions of Caveolin-1 in Aging-related Diseases. Chonnam Med J, 53:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grassi S, Giussani P, Mauri L, Prioni S, Prinetti A (2021). Isolation and Analysis of Lipid Rafts from Neural Cells and Tissues. Methods Mol Biol, 2187:1-25. [DOI] [PubMed] [Google Scholar]
- [13].Royer M, Lemaire-Ewing S, Desrumaux C, Monier S, Pais de Barros J, Athias A, et al. (2009). 7-ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E: a specific role for alpha-tocopherol with consequences on cell death. J Biol Chem, 284:15826-15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meng G, Liu Y, Lou C, Yang H (2010). Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol, 161:1628-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grazide S, Maestre N, Veldman RJ, Bezombes C, Maddens S, Levade T, et al. (2002). Ara-C- and daunorubicin-induced recruitment of Lyn in sphingomyelinase-enriched membrane rafts. FASEB J, 16:1685-1687. [DOI] [PubMed] [Google Scholar]
- [16].Ragot K, Mackrill JJ, Zarrouk A, Nury T, Aires V, Jacquin A, et al. (2013). Absence of correlation between oxysterol accumulation in lipid raft microdomains, calcium increase, and apoptosis induction on 158N murine oligodendrocytes. Biochem Pharmacol, 86:67-79. [DOI] [PubMed] [Google Scholar]
- [17].Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J, et al. (2019). Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics, 9:265-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kahn E, Baarine M, Dauphin A, Ragot K, Tissot N, Seguin A, et al. (2011). Impact of 7-ketocholesterol and very long chain fatty acids on oligodendrocyte lipid membrane organization: evaluation via LAURDAN and FAMIS spectral image analysis. Cytometry A, 79:293-305. [DOI] [PubMed] [Google Scholar]
- [19].Kaiser H, Lingwood D, Levental I, Sampaio JL, Kalvodova L, Rajendran L, et al. (2009). Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A, 106:16645-16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kinoshita M, Suzuki KGN, Matsumori N, Takada M, Ano H, Morigaki K, et al. (2017). Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J Cell Biol, 216:1183-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Suzuki KGN, Ando H, Komura N, Konishi M, Imamura A, Ishida H, et al. (2018). Revealing the Raft Domain Organization in the Plasma Membrane by Single-Molecule Imaging of Fluorescent Ganglioside Analogs. Methods Enzymol, 598:267-282. [DOI] [PubMed] [Google Scholar]
- [22].Larbi A, Douziech N, Dupuis G, Khalil A, Pelletier H, Guerard K, et al. (2004). Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukoc Biol, 75:373-381. [DOI] [PubMed] [Google Scholar]
- [23].Larbi A, Dupuis G, Khalil A, Douziech N, Fortin C, Fülöp TJ (2006). Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal, 18, 1017-1030. [DOI] [PubMed] [Google Scholar]
- [24].Larbi A, Dupuis G, Douziech N, Khalil A, Fülöp TJ (2004). Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Ann N Y Acad Sci, 1030:125-133. [DOI] [PubMed] [Google Scholar]
- [25].Nakamura M, Kondo H, Shimada Y, Waheed AA, Ohno-Iwashita Y (2003). Cellular aging-dependent decrease in cholesterol in membrane microdomains of human diploid fibroblasts. Exp Cell Res, 290:381-390. [DOI] [PubMed] [Google Scholar]
- [26].Bai L, Deng X, Li J, Wang M, Li Q, An W, et al. (2011). Regulation of cellular senescence by the essential caveolar component PTRF/Cavin-1. Cell Res, 21:1088-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wheaton K, Sampsel K, Boisvert FM, Davy A, Robbins S, Riabowol K (2001). Loss of functional caveolae during senescence of human fibroblasts. J Cell Physiol, 187:226-235. [DOI] [PubMed] [Google Scholar]
- [28].Ciana A, Achilli C, Gaur A, Minetti G (2017). Membrane Remodelling and Vesicle Formation During Ageing of Human Red Blood Cells. Cell Physiol Biochem, 42:1127-1138. [DOI] [PubMed] [Google Scholar]
- [29].Díaz M, Fabelo N, Ferrer I, Marín R (2018). "Lipid raft aging" in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer's disease. Neurobiol Aging, 67:42-52. [DOI] [PubMed] [Google Scholar]
- [30].Alonso MA, Millán J (2001). The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J Cell Sci, 114:3957-3965. [DOI] [PubMed] [Google Scholar]
- [31].Fuller DM, Zhang W (2009). Regulation of lymphocyte development and activation by the LAT family of adapter proteins. Immunol Rev, 232:72-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palacios EH, Weiss A (2004). Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene, 23:7990-8000. [DOI] [PubMed] [Google Scholar]
- [33].Kästle M, Merten C, Hartig R, Kaehne T, Liaunardy-Jopeace A, Woessner NM, et al. (2020). Tyrosine 192 within the SH2 domain of the Src-protein tyrosine kinase p56(Lck) regulates T-cell activation independently of Lck/CD45 interactions. Cell Commun Signal: CCS, 18:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fulop T, Larbi A, Douziech N, Fortin C, Guérard K, Lesur O, et al. (2004). Signal transduction and functional changes in neutrophils with aging. Aging Cell, 3:217-226. [DOI] [PubMed] [Google Scholar]
- [35].Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. (2021). An aged immune system drives senescence and ageing of solid organs. Nature, 594:100-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fabelo N, Martín V, Marín R, Santpere G, Aso E, Ferrer I, et al. (2012). Evidence for premature lipid raft aging in APP/PS1 double-transgenic mice, a model of familial Alzheimer disease. J Neuropathol Exp Neurol, 71:868-881. [DOI] [PubMed] [Google Scholar]
- [37].de Mello CV, Nguyen D, Giri B, Bunbury A, Schaffer E, Taub DD (2004). Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol, 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiang L, Bechtel MD, Galeva NA, Williams TD, Michaelis EK, Michaelis ML (2012). Decreases in plasma membrane Ca²+-ATPase in brain synaptic membrane rafts from aged rats. J Neurochem, 123:689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chadwick W, Brenneman R, Martin B, Maudsley S (2010). Complex and multidimensional lipid raft alterations in a murine model of Alzheimer's disease. Int J Alzheimers Dis, 2010: 604792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lam SM, Chua GH, Li X, Su B, Shui G (2016). Biological relevance of fatty acyl heterogeneity to the neural membrane dynamics of rhesus macaques during normative aging. Oncotarget, 7:55970-55989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kubben N, Misteli T (2017). Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nature Reviews. Nat Rev Mol Cell Biol, 18:595-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Best BP (2009). Nuclear DNA damage as a direct cause of aging. Rejuvenation Res, 12:199-208. [DOI] [PubMed] [Google Scholar]
- [43].Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, et al. (2013). The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev, 12:661-684. [DOI] [PubMed] [Google Scholar]
- [44].Huang H, Weaver A, Wu E, Li Y, Gao H, Fan W, et al. (2013). Lipid-based signaling modulates DNA repair response and survival against Klebsiella pneumoniae infection in host cells and in mice. Am J Respir Cell Mol Biol, 49:798-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou Y, Chu L, Wang Q, Dai W, Zhang X, Chen J, et al. (2018). CD59 is a potential biomarker of esophageal squamous cell carcinoma radioresistance by affecting DNA repair. Cell Death Dis, 9:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhu H, Yue J, Pan Z, Wu H, Cheng Y, Lu H, et al. (2010). Involvement of Caveolin-1 in repair of DNA damage through both homologous recombination and non-homologous end joining. PLoS One, 5:e12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Labbadia J, Morimoto RI (2015). The biology of proteostasis in aging and disease. Annu Rev Biochem, 84:435-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sabath N, Levy-Adam F, Younis A, Rozales K, Meller A, Hadar S, et al. (2020). Cellular proteostasis decline in human senescence. Commun Biol, 117:31902-31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009). Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem, 78:959-991. [DOI] [PubMed] [Google Scholar]
- [50].Lindquist S (1986). The heat-shock response. Annu Rev Biochem, 55; 1151-1191. [DOI] [PubMed] [Google Scholar]
- [51].Nagy E, Balogi Z, Gombos I, Akerfelt M, Björkbom A, Balogh G, et al. (2007). Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci U S A, 104:7945-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Navarro-Lérida I, Sánchez-Perales S, Calvo M, Rentero C, Zheng Y, Enrich C, et al. (2012). A palmitoylation switch mechanism regulates Rac1 function and membrane organization. EMBO J, 31:534-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gungor B, Gombos I, Crul T, Ayaydin F, Szabó L, Török Z, et al. (2014). Rac1 participates in thermally induced alterations of the cytoskeleton, cell morphology and lipid rafts, and regulates the expression of heat shock proteins in B16F10 melanoma cells. PloS One, 9:e89136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Crul T, Csoboz B, Gombos I, Marton A, Peter M, Balogh G, et al. (2020). Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells. Cells, 9:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gombos I, Crul T, Piotto S, Güngör B, Török Z, Balogh G, et al. (2011). Membrane-lipid therapy in operation: the HSP co-inducer BGP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PloS One, 6:e28818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, et al. (2005). Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol, 208:697-705. [DOI] [PubMed] [Google Scholar]
- [57].Koga H, Kaushik S, Cuervo AM (2011). Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev, 10:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu G, Rogers J, Murphy CT, Rongo C (2011). EGF signalling activates the ubiquitin proteasome system to modulate C. elegans lifespan. EMBO J, 30:2990-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zajchowski LD, Robbins SM (2002). Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem, 269:737-752. [DOI] [PubMed] [Google Scholar]
- [60].He C, Klionsky DJ (2009). Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet, 43:67-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, et al. (2011). PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A, 108:14509-14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sural-Fehr T, Singh H, Cantuti-Catelvetri L, Zhu H, Marshall MS, Rebiai R, et al. (2019). Inhibition of the IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal vulnerability in a genetic lysosomal glycosphingolipidosis. Dis Model Mech, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sun J, Feng D, Xi H, Luo J, Zhou Z, Liu Q, et al. (2020). CD24 blunts the sensitivity of retinoblastoma to vincristine by modulating autophagy. Mol Oncol, 14:1740-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, et al. (2001). The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem, 276:35243-35246. [DOI] [PubMed] [Google Scholar]
- [65].Fraldi A, Annunziata F, Lombardi A, Kaiser H, Medina DL, Spampanato C, et al. (2010). Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J, 29:3607-3620. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [66].Nury T, Yammine A, Ghzaiel I, Sassi K, Zarrouk A, Brahmi F, et al. (2021). Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing Res Rev, 68:101324. [DOI] [PubMed] [Google Scholar]
- [67].Hansen M, Rubinsztein DC, Walker DW (2018). Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol, 19:579-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Houtkooper RH, Williams RW, Auwerx J (2010). Metabolic networks of longevity. Cell, 142:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fontana L, Partridge L, Longo VD (2010). Extending healthy life span--from yeast to humans. Science , 328:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K (2003). Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem, 278:11561-11569. [DOI] [PubMed] [Google Scholar]
- [71].Romanelli RJ, Mahajan KR, Fulmer CG, Wood TL (2009). Insulin-like growth factor-I-stimulated Akt phosphorylation and oligodendrocyte progenitor cell survival require cholesterol-enriched membranes. J Neurosci Res, 87:3369-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96:857-868. [DOI] [PubMed] [Google Scholar]
- [73].Zhang X, Tang N, Hadden TJ, Rishi AK (2011). Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta, 1813:1978-1986. [DOI] [PubMed] [Google Scholar]
- [74].Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, et al. (2006). Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J, 25:3515-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Katoh S, Kamimoto T, Yamakawa D, Takakura N (2009). Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells. Exp Cell Res, 315:2818-2823. [DOI] [PubMed] [Google Scholar]
- [76].Martins R, Lithgow GJ, Link W (2016). Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell, 15:196-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Salih DAM, Brunet A (2008). FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol, 20:126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Keyes KT, Xu J, Long B, Zhang C, Hu Z, Ye Y (2010). Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. Am J Physiol Heart Circ Physiol, 298:H1198-H1208. [DOI] [PubMed] [Google Scholar]
- [79].Laplante M, Sabatini DM (2012). mTOR signaling in growth control and disease. Cell, 149:274-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science, 309:481-484. [DOI] [PubMed] [Google Scholar]
- [81].Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature, 429:417-423. [DOI] [PubMed] [Google Scholar]
- [82].Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, et al. (2008). DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet, 40:392-394. [DOI] [PubMed] [Google Scholar]
- [83].Yu D, Jung SH, An H, Lee S, Hong J, Park JS, et al. (2017). Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell, 16:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bosch M, Marí M, Herms A, Fernández A, Fajardo A, Kassan A, et al. (2011). Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr Biol : CB, 21:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hebert-Chatelain E (2013). Src kinases are important regulators of mitochondrial functions. Int J Biochem Cell Biol, 45:90-98. [DOI] [PubMed] [Google Scholar]
- [86].Pak ES, Uddin MJ, Ha H (2020). Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice. Int J Mol Sci, 21:8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hunter CA, Koc H, Koc EC (2020). c-Src kinase impairs the expression of mitochondrial OXPHOS complexes in liver cancer. Cell Signal, 72:109651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Massey JB, Pownall HJ (2005) . The polar nature of 7-ketocholesterol determines its location within membrane domains and the kinetics of membrane microsolubilization by apolipoprotein A-I. Biochemistry: 44, 10423-10433. [DOI] [PubMed] [Google Scholar]
- [89].Vejux A, Abed-Vieillard D, Hajji K, Zarrouk A, Mackrill JJ, Ghosh S, et al. (2020). 7-Ketocholesterol and 7β-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem Pharmacol, 173:113648. [DOI] [PubMed] [Google Scholar]
- [90].Nury T, Sghaier R, Zarrouk A, Ménétrier F, Uzun T, Leoni V, et al. (2018). Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: Attenuation by α-tocopherol. Biochimie, 153:181-202. [DOI] [PubMed] [Google Scholar]
- [91].HARMAN D (1965). The free radical theory of aging: effect of age on serum copper levels. J Gerontol, 20:151-153. [DOI] [PubMed] [Google Scholar]
- [92].Lee S, Jung YH, Oh SY, Song EJ, Choi SH, Han HJ (2015). Vibrio vulnificus VvhA induces NF-κB-dependent mitochondrial cell death via lipid raft-mediated ROS production in intestinal epithelial cells. Cell Death Dis, 6:1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Leonarduzzi G, Vizio B, Sottero B, Verde V, Gamba P, Mascia C, et al. (2006). Early involvement of ROS overproduction in apoptosis induced by 7-ketocholesterol. Antioxid Redox Signal, 8:375-380. [DOI] [PubMed] [Google Scholar]
- [94].Brahmi F, Vejux A, Sghaier R, Zarrouk A, Nury T, Meddeb W, et al. (2019). Prevention of 7-ketocholesterol-induced side effects by natural compounds. Crit Rev Food Sci Nutr, 59:3179-3198. [DOI] [PubMed] [Google Scholar]
- [95].Vejux A, Abed-Vieillard D, Hajji K, Zarrouk A, Mackrill JJ, Ghosh S, et al. (2020). 7-Ketocholesterol and 7β-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem Pharmacol, 173:113648. [DOI] [PubMed] [Google Scholar]
- [96].Baird L, Dinkova-Kostova AT (2011). The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol, 85:241-272. [DOI] [PubMed] [Google Scholar]
- [97].Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010). When NRF2 talks, who's listening? Antioxid Redox Signal, 13:1649-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Li W, Liu H, Zhou J, Cao J, Zhou X, Choi AMK, et al. (2012). Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2). J Biol Chem, 287:20922-20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di Y, et al. (2013). Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell, 24:1852-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Behne D, Kyriakopoulos A (2001). Mammalian selenium-containing proteins. Annu Rev Nutr, 21:453-473. [DOI] [PubMed] [Google Scholar]
- [101].Volonte D, Galbiati F (2009). Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep, 10:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ben-Porath I, Weinberg RA (2005). The signals and pathways activating cellular senescence. Int J Biochem Cell Biol, 37:961-976. [DOI] [PubMed] [Google Scholar]
- [103].Galbiati F, Volonté D, Liu J, Capozza F, Frank PG, Zhu L, et al. (2001). Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell, 12:2229-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Volonte D, Zhang K, Lisanti MP, Galbiati F (2002). Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell, 13:2502-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rufini A, Tucci P, Celardo I, Melino G (2013). Senescence and aging: the critical roles of p53. Oncogene, 32:5129-5143. [DOI] [PubMed] [Google Scholar]
- [106].Bartholomew JN, Volonte D, Galbiati F (2009). Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res, 69:2878-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cheng Q, Chen L, Li Z, Lane WS, Chen J (2009). ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J, 28:3857-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Volonte D, Kahkonen B, Shapiro S, Di Y, Galbiati F (2009). Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. J Biol Chem, 284:5462-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Volonte D, Zou H, Bartholomew JN, Liu Z, Morel PA, Galbiati F (2015). Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J Biol Chem, 290:4202-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di Y, et al. (2013). Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell, 24:1852-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Couet J, Sargiacomo M, Lisanti MP (1997). Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem, 272:30429-30438. [DOI] [PubMed] [Google Scholar]
- [112].Park WY, Cho KA, Park JS, Kim DI, Park SC (2001). Attenuation of EGF signaling in senescent cells by caveolin. Ann N Y Acad Sci, 928:79-84. [DOI] [PubMed] [Google Scholar]
- [113].Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, et al. (2000). Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem, 275:20847-20852. [DOI] [PubMed] [Google Scholar]
- [114].Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, et al. (2003). Senescent phenotype can be reversed by reduction of caveolin status. J Biol Chem, 278:27789-27795. [DOI] [PubMed] [Google Scholar]
- [115].Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, et al. (2008). PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell, 132:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liu L, Pilch PF (2008). A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem, 283:4314-4322. [DOI] [PubMed] [Google Scholar]
- [117].Volonte D, Galbiati F (2011). Polymerase I and transcript release factor (PTRF)/cavin-1 is a novel regulator of stress-induced premature senescence. J Biol Chem, 286:28657-28661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liang J, Ke X, Yang R, Wang X, Du Z, Hu C (2020). Notch pathway activation mediated the senescence of endothelial progenitor cells in hypercholesterolemic mice. J Bioenerg Biomembr, 52:431-440. [DOI] [PubMed] [Google Scholar]
- [119].Anderson A, Campo A, Fulton E, Corwin A, Jerome WGR, O'Connor MS (2020). 7-Ketocholesterol in disease and aging. 7-Ketocholesterol in disease and aging, 29:101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mahalakshmi K, Parimalanandhini D, Sangeetha R, Livya Catherene M, Beulaja M, Thiagarajan R, et al. (2021). Influential role of 7-Ketocholesterol in the progression of Alzheimer's disease. Prostaglandins Other Lipid Mediat, 156:106582. [DOI] [PubMed] [Google Scholar]
- [121].Cho KA, Park SC (2005). Caveolin-1 as a prime modulator of aging: a new modality for phenotypic restoration? Mech Ageing Dev, 126:105-110. [DOI] [PubMed] [Google Scholar]
- [122].Volonte D, Vyas AR, Chen C, Dacic S, Stabile LP, Kurland BF, et al. (2018). Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J Biol Chem, 293:1794-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sharpless NE, DePinho RA (2007). How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol, 8:703-713. [DOI] [PubMed] [Google Scholar]
- [124].Fuchs E, Tumbar T, Guasch G (2004). Socializing with the neighbors: stem cells and their niche. Cell, 116:769-778. [DOI] [PubMed] [Google Scholar]
- [125].Wilson A, Trumpp A (2006). Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol, 6:93-106. [DOI] [PubMed] [Google Scholar]
- [126].Altrock E, Muth CA, Klein G, Spatz JP, Lee-Thedieck C (2012). The significance of integrin ligand nanopatterning on lipid raft clustering in hematopoietic stem cells. Biomaterials, 33:3107-3118. [DOI] [PubMed] [Google Scholar]
- [127].Adamiak M, Poniewierska-Baran A, Borkowska S, Schneider G, Abdelbaset-Ismail A, Suszynska M, et al. (2016). Evidence that a lipolytic enzyme--hematopoietic-specific phospholipase C-β2--promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes. Leukemia, 30:919-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K (2007). Lipid rafts are required for Kit survival and proliferation signals. Blood, 110:1739-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chen C, Liu Y, Liu Y, Zheng P (2009). mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal, 2:a75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ramalingam P, Poulos MG, Gutkin MC, Katsnelson L, Freire AG, Lazzari E, et al. (2020). Endothelial mTOR maintains hematopoiesis during aging. J Exp Med, 217:e20191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. (2005). Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood, 105:40-48. [DOI] [PubMed] [Google Scholar]
- [132].O'Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa, DeBerry C, Metcalfe DD, et al. (2001). Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood, 98:343-350. [DOI] [PubMed] [Google Scholar]
- [133].Taylor DR, Hooper NM (2006). The prion protein and lipid rafts. Mol Membr Biol, 23:89-99. [DOI] [PubMed] [Google Scholar]
- [134].Mohanty ST, Cairney CJ, Chantry AD, Madan S, Fernandes JA, Howe SJ, et al. (2012). A small molecule modulator of prion protein increases human mesenchymal stem cell lifespan, ex vivo expansion, and engraftment to bone marrow in NOD/SCID mice. Stem Cells, 30:1134-1143. [DOI] [PubMed] [Google Scholar]
- [135].Zhang CC, Steele AD, Lindquist S, Lodish HF (2006). Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci U S A, 103:2184-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kim YM, Kim J, Heo SC, Shin SH, Do EK, Suh D, et al. (2012). Proteomic identification of ADAM12 as a regulator for TGF-β1-induced differentiation of human mesenchymal stem cells to smooth muscle cells. PloS One, 7:e40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Coppé J, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol: 6, 2853-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, et al. (2012). NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest, 122:2601-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Akira S, Takeda K, Kaisho T (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol, 2:675-680. [DOI] [PubMed] [Google Scholar]
- [140].Legler DF, Micheau O, Doucey M, Tschopp J, Bron C (2003). Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity, 18:655-664. [DOI] [PubMed] [Google Scholar]
- [141].Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. (2010). Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res, 51:3196-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K (2015). Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci, 72:557-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Simmons DL, Tan S, Tenen DG, Nicholson-Weller A, Seed B (1989). Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood, 73:284-289. [PubMed] [Google Scholar]
- [144].Ciesielska A, Matyjek M, Kwiatkowska K (2021). TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci, 78:1233-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Locksley RM, Killeen N, Lenardo MJ (2001). The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell, 104:487-501. [DOI] [PubMed] [Google Scholar]
- [146].Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, et al. (2007). Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res, 48:299-306. [DOI] [PubMed] [Google Scholar]
- [147].Powter EE, Coleman PR, Tran MH, Lay AJ, Bertolino P, Parton RG, et al. (2015). Caveolae control the anti-inflammatory phenotype of senescent endothelial cells. Aging Cell, 14:102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Prunet C, Montange T, Véjux A, Laubriet A, Rohmer J, Riedinger J, et al. (2006). Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A, 69:359-373. [DOI] [PubMed] [Google Scholar]
- [149].Chen Y, Wang Y, Yang M, Guo M (2019). Allicin Inhibited Staphylococcus aureus -Induced Mastitis by Reducing Lipid Raft Stability via LxRα in Mice. J Agric Food Chem, 67:10863-10870. [DOI] [PubMed] [Google Scholar]
- [150].Chen Y, Zhao Y, Yang J, Jing H, Liang W, Chen M, et al. (2020). Selenium alleviates lipopolysaccharide-induced endometritis via regulating the recruitment of TLR4 into lipid rafts in mice. Food Funct, 11:200-210. [DOI] [PubMed] [Google Scholar]
- [151].Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, et al. (2012). NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest, 122:2601-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature, 571:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018). Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol, 14:576-590. [DOI] [PubMed] [Google Scholar]
- [154].Luo J, Yang H, Song B (2020). Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol, 21:225-245. [DOI] [PubMed] [Google Scholar]
- [155].Meng G, Liu Y, Lou C, Yang H (2010). Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol, 161:1628-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Orkaby AR, Driver JA, Ho Y, Lu B, Costa L, Honerlaw J, et al. (2020). Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA, 324:68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Golomb BA, McGraw JJ, Evans MA, Dimsdale JE (2007). Physician response to patient reports of adverse drug effects: implications for patient-targeted adverse effect surveillance. Drug Saf, 30:669-675. [DOI] [PubMed] [Google Scholar]
- [158].Samaras K, Makkar SR, Crawford JD, Kochan NA, Slavin MJ, Wen W, et al. (2019). Effects of Statins on Memory, Cognition, and Brain Volume in the Elderly. J Am Coll Cardiol, 74:2554-2568. [DOI] [PubMed] [Google Scholar]
- [159].Santos G, Díaz M, Torres NV (2016). Lipid Raft Size and Lipid Mobility in Non-raft Domains Increase during Aging and Are Exacerbated in APP/PS1 Mice Model of Alzheimer's Disease. Front Physiol, 7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Partridge L, Fuentealba M, Kennedy BK (2020). The quest to slow ageing through drug discovery. Nat Rev Drug Discov, 19:513-532. [DOI] [PubMed] [Google Scholar]