Abstract
Osteoporotic fractures (OF) are a global public health problem currently. Many risk prediction models for OF have been developed, but their performance and methodological quality are unclear. We conducted this systematic review to summarize and critically appraise the OF risk prediction models. Three databases were searched until April 2021. Studies developing or validating multivariable models for OF risk prediction were considered eligible. Used the prediction model risk of bias assessment tool to appraise the risk of bias and applicability of included models. All results were narratively summarized and described. A total of 68 studies describing 70 newly developed prediction models and 138 external validations were included. Most models were explicitly developed (n=31, 44%) and validated (n=76, 55%) only for female. Only 22 developed models (31%) were externally validated. The most validated tool was Fracture Risk Assessment Tool. Overall, only a few models showed outstanding (n=3, 1%) or excellent (n=32, 15%) prediction discrimination. Calibration of developed models (n=25, 36%) or external validation models (n=33, 24%) were rarely assessed. No model was rated as low risk of bias, mostly because of an insufficient number of cases and inappropriate assessment of calibration. There are a certain number of OF risk prediction models. However, few models have been thoroughly internally validated or externally validated (with calibration being unassessed for most of the models), and all models showed methodological shortcomings. Instead of developing completely new models, future research is suggested to validate, improve, and analyze the impact of existing models.
Keywords: osteoporotic fractures, prediction model, systematic review, critical appraisal
Osteoporotic fractures (OF) are fractures that occur during minor trauma or daily activities, which are a serious consequence of osteoporosis [1]. The common fracture sites are vertebral, hip, distal radius, proximal humerus, and pelvis [2]. Osteoporosis causes more than nine million new fractures worldwide every year, it is estimated that an OF occurs every three seconds [3], and one-third of women and one-fifth of men will suffer an OF in their lifetime [4]. OF can cause pain, severe disability and mortality, as well as burdens on families and society. It seriously impairs the quality of life of patients [5].
Prevention of OF requires early and accurate identification of individuals at risk and taking effective preventive interventions in time [6]. Bone mineral density (BMD) test is the gold standard for diagnosing osteoporosis. It is often used to identify patients with osteoporosis or low BMD. Nevertheless, studies have shown that the BMD test alone does not reliably predict whether individuals will develop a fracture [7]. In addition, high cost, ionizing radiation, and low mobility of the BMD test limit its clinical application [8]. Therefore, in many clinical guidelines, it is now recommended to use prediction models integrating several risk factors to identify individuals at high risk of OF [9].
At present, numerous prediction tools for OF have been developed, including but not limited to the World Health Organization (WHO) Fracture Risk Assessment Tool (FRAX) algorithm [10], Qfracture algorithm [11], and Garvan Fracture Risk Calculator (Garvan) [12]. Some of them have been recommended in clinical guidelines for treatment management [13,14] and more and more advocated by health policymakers. Although there are some systematic reviews on OF prediction models [15-17], they are outdated with the latest literature search being performed in 2017 [16]. Further limitations include restriction to a few specific tools [17] or a certain population like women [15], or no critical appraisal of the included models with standardized criteria [16,17]. Hence, an updated systematic review of prediction models for OF is needed.
We conducted this systematic review and critical appraisal to summarize the characteristics of the development and validation of OF risk prediction model, assess its methodological quality and reporting quality, and provide up-to-date evidence for clinical implementation and future research.
METHODS
This systematic review was reported by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18]. The protocol of this systematic review has been registered in PROSPERO (registration number: CRD42020199196).
Search strategy
We systematically searched PubMed, Embase, and PsycINFO from inception to April 3, 2021. In addition, the reference lists of included studies were manually reviewed. The search strategy included the key concepts of i) osteoporotic fractures and osteoporosis and ii) risk prediction and related terms. The detailed search strategies are presented in Supplementary table 1.
Eligible criteria
Cohort studies that develop or validate risk prediction models for OF in the general population were considered eligible. Studies were excluded if i) the prediction model consisted of only one predictor; ii) they targeted secondary OF or focused on specific patient groups for the treatment of OF or related conditions; iii) the performance of the model was not reported; iv) they were reviews, conference abstracts, letters or protocols. In addition, if the development article was not available, the corresponding externally verified articles were excluded.
Literature selection
Two reviewers (HW and JS) independently selected the studies, determined eligibility, and resolved the discrepancies by consensus. When the difference is not resolved, the third reviewer (LQ) was invited to make a consensus decision.
Data extraction
Two reviewers (XS and YC) independently extracted the data with a pre-developed data extraction form, which was developed by following the guidance of the critical appraisal and data extraction for systematic reviews of prediction modelling studies (CHARMS) checklist [19]. Extracted the following information from each included study: i) characteristics of the study (e.g., study design, data source); ii) data related to participants (e.g., country or region of participants, age, gender, events per variable (EPV)); iii) details about model development and validation (e.g., type of prediction model, predictors included in the model, modelling method) and model performance.
Multiple different models were included in a study, for example, separate models for men and women, separate models for different outcomes (e.g., hip fracture, major osteoporotic fractures (MOF), were included separately. When multiple versions (e.g., with different risk factors) of a model for the same population and outcome were included in a study, the model with the best performance was selected for data extraction. When an article validated multiple models, separate data extraction was performed for each model.
Model performance was assessed by discrimination and calibration. Discrimination is often quantified by the C index or area under the receiver operating characteristic curve (AUC). A C index or AUC less than 0.5 suggests no discrimination, 0.5 to 0.7 is poor, 0.7 to 0.8 is acceptable, 0.8 to 0.9 is excellent, and higher than 0.9 is outstanding [20]. Calibration can be visualized by a calibration plot and is usually quantified using the calibration intercept and the calibration slope, with a slope close to 1 and an intercept close to 0 indicating good calibration [21]. The indexes mentioned above were extracted from the publications when available. Sensitivity and specificity were extracted as well if available. Additionally, EPV was calculated to measure model overfitting. An EPV less than 20 was considered as overfitting for model development while less than 100 for model validation [22].
Risk of bias and applicability assessment
The risk of bias and applicability of each included study was independently assessed by two reviewers (ZZ and XS) using the prediction model risk of bias assessment tool (PROBAST) [23,24]. Discrepancies were resolved by consensus between the two reviewers, and a third author (YG) was invited for consensus adjudication in need. For risk of bias assessment, it contains four domains: participants, predictors, outcome, and analysis. Each domain was judged as low, high, or unclear risk of bias. The overall risk of bias was summarized according to the following rules: when all the four domains were judged as “low” risk of bias, the overall risk of bias was “low”; otherwise, “high” or “unclear” risk of bias was graded accordingly [23,24]. For applicability assessment, it contains three domains: participants, predictors, and outcome. It has similar assessment rules and procedures to the risk of bias assessment.
Statistical Analysis
All results were narratively summarized and described without any quantitative synthesis due to variation in predictors and characteristics of participants among the included prediction models.
RESULTS
Study selection
The literature search identified 2852 records, of which 784 were removed due to duplication, and 1882 were excluded based on title and abstract. A total of 186 full texts were assessed, of which 68 articles met the eligibility criteria were included in this review (Fig. 1). In total, 38 articles focused on one or more development of OF risk prediction models, and 44 articles described one or more external validation of OF risk prediction models. Articles frequently concern combinations of development and external validation, leading to the total number of articles does not sum up to 68.
Figure 1.
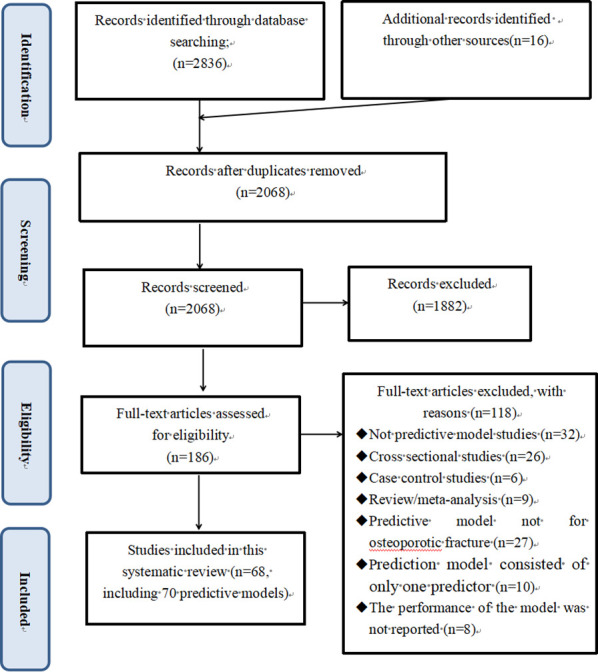
PRISMA flow diagram for literature search and selection.
Studies focused on the development of OF prediction models
Populations and outcomes
Thirty-eight [10-12,25-59] articles represented the development of 70 different models in total. Most of the participants were from the UK (n=17, 24%), China (n=17, 24%) or the US (n=12, 17%), the remaining (n=24, 34%) were from other countries in Oceania, Western Europe, or East Asia, while there were no models developed using data from Africa, South America, and the Middle East. The average age of participants ranged from 56.7 to 80.5 years. The follow-up duration ranged from 1 to 13 years, with 30 (43%) models equal to or more than 10 years. The outcomes covered MOF (n=35, 50%), hip fracture (n=25, 36%), any fractures (n=6, 9%), and other fractures (n=4, 6%). Diagnosis of fracture was mostly through medical records (n=39, 56%) or self-reported (n=18, 26%), 11 (16%) models were radiographic reports, and the remaining two (3%) models were self-reported and confirmed by medical records (Table 1).
Table 1.
Basic characteristics of included studies.
| First author, year | Model; No.a |
Study design | Data source; country or region of participants |
Age (SD) (years) | Female (%) | Follow up duration (SD) (year) | Outcome | Measurement of fracture | Incidence of fracture (%) | Sample size |
|---|---|---|---|---|---|---|---|---|---|---|
| Model developmentb | ||||||||||
| Dargent- Molina 2002[25] | NR; 1 |
R | EPIDemiologie de l'OSteoporose study; France | 80.5(3.7) | 100 | 3.7(0.8) | Hip fracture | Self-reported | 4.0 | 6933 |
| Colón- Emeric 2002l [26] | NR; 4 |
R | Established population for epidemiologic studies of the elderly; US | M: 73.4(6.7) F: 74.5(6.6) |
65.0 | 3.0(NR) | Any fracturesc | Self-reported | Hip: 3.8 Any: 11.0 |
7654 |
| McGrother 2002[27] | NR; 1 |
P | A large general practice; UK | 77.9(6.1) | 100 | 3.0(NR) | Hip fracture | Medical records | 2.0 | 1289 |
| Albertsson 2007[28] | FRAMO; 1 |
R | Three rural primary health care; Sweden | 78.8(6.5) | 100 | 2.0(NR) | Hip fracture | Radiographic reports | 1.2 | 1248 |
| Robbins 2007[29] | WHI; 1 |
P | Female’s Health Initiative 40 clinical centers; US | NR | 100 | 8.0(1.7) | Hip fracture | Self-reported and confirmed by medical records | 0.1 | 93676 |
| Nguyen 2008[12] | Garvan; 4 |
P | Dubbo osteoporosis epidemiology study; Australia | M: 70.0(6.0) F: 71.0(8.0) |
61.3 | M: 12.0(NR) F: 13.0(NR) |
Any fractures | Radiographic reports | M: 17.4 F: 31.4 |
M: 858 F: 1358 |
| Kanis 2008l [10] | FRAX; 8 |
P | Nine population-based cohort studiesd; UK | 65.0 | 68.0 | 10.0(NR) | MOFe | Self-reported or confirmed by medical records | Hip: 1.8 MOF: 7.2 |
273826 |
| Hippisley- Cox 2009[11] | QFracture; 4 |
P | Version 20 of the QResearch; UK | NR | NR | 10.0(NR) | MOF | Medical records | Hip: 0.4(M) 1.2(F) MOF: 1.0(M) 3.1(F) |
M: 1807996 F: 1825816 |
| Tanaka 2010l [30] | FRISC; 2 |
R | Three population-based cohort studies; Japan | 63.4(11.1) | 100 | 5.3(NR) | Any fracturesf | Medical records | 21.4 | 2187 |
| Yun 2010k [31] | NR, FRAX; 4 |
R | Medicare current beneficiary survey; UK | NR | NR | 2.0(NR) | MOF | Medical records | NR | 12337 |
| Sambrook 2011[32] | NR; 1 |
R | The global longitudinal Osteoporosis studyg; UK | NR | 100 | 2.0(NR) | Hip fracture | Self-reported | 4.5 | 19586 |
| Bow 2011[33] | NR; 1 |
P | Mr. and Ms. Os study; China | 68.0(10.3) | 0 | 3.5(2.9) | MOF | Self-reported and confirmed by medical records | 2.0 | 1,810 |
| Henry 2011k [34] | FRISK, FRAX, Garvan; 4 |
P | Geelong osteoporosis study; Australia | NR | 100 | 9.6(NR) | MOF | Radiographic reports | 20.8 | 600 |
| Tamaki 2011k [35] | NR, FRAX; 6 |
R | Population-based cohort study; Japan | 56.7(9.6) | 100 | 10.0(NR) | MOF | Radiographic reports | MOF: 5.3 Hip: 0.5 |
815 |
| Hippisley- Cox 2012[36] | Updated QFracture; 4 |
P | Version 32 of the QResearch; UK | NR | NR | 10.0(NR) | MOF | Medical records | Hip: 0.3(M) 0.9(F) MOF: 0.9(M) 2.8(F) |
4726046 |
| LaFleur 2012[37] | NR; 2 |
P | Veterans health administration system; US | 66.9(10.3) | 0 | 2.8(NR) | MOF | Medical records | Hip: 0.3 MOF: 1.2 |
84763 |
| Schousboe 2014[38] | NR; 1 |
P | Study of osteoporotic Fractures; US | 75.0 | 100 | NR | Vertebral fractures | Radiographic reports | 20.4 | 5560 |
| Yu 2014k [39] | FRAX+S, FRAX; 16 |
P | Population-based cohort study; China | 72.5(5.2) | 50.0 | 10.2 | MOF | Medical records | Hip: 3.3 MOF: 14.1 |
4000 |
| Iki 2015k [40] | FRAX +TBS, FRAX; 2 |
P | Study of Fujiwara-kyo Osteoporosis Risk in male; Japan | 73.0(5.1) | 0 | 4.5(NR) | MOF | Radiographic reports | 1.2 | 1872 |
| Jang 2016[41] | NR; 2 |
P | Health and genome study; Korea | M: 61.3(7.1) F: 61.1(7.1) |
52.7 | 7.0(NR) | MOF | Self-reported | M:9.9 F: 12.3 |
M: 363 F: 405 |
| Kim 2016[42] | KFRS; 2 |
P | National Health Insurance Service; Korea | M: 59.8(7.9) F: 60.6(8.3) |
48.5 | 7.0(NR) | MOF | Medical records | M: 1.3 F: 4.3 |
M: 370225 F: 348253 |
| Francesco 2017l [43] | FRA-HS; 2 |
P | IMS health longitudinal study; Italy | 60.1(12.8) | 55.0 | 10.0(NR) | MOF | Medical records | 5.9 | 490013 |
| Kruse 2017[44] | NR; 2 |
R | Health database; Denmark | NR | 86.1 | 5.0(NR) | Hip, femoral fractures | Medical records | 6.6(M/F) | M: 717 F: 4722 |
| Li 2017[45] | NR; 1 |
P | Global longitudinal study of osteoporosis in female 3-year cohort; Canada | 69.4(8.9) | 100 | 3.0(NR) | MOF | Self-reported | 4.0 | 3985 |
| Su 2017[46] | NR; 2 |
P | Mr. and Ms. Os study; China | M: 72.4(NR) F: 72.6(NR) |
50.3 | M: 9.9(2.8) F: 8.8(1.5) |
MOF | Medical records | M: 6.6 F: 11.0 |
M: 1923 F: 1950 |
| Weycker 2017[47] | NR; 2 |
R | Study of osteoporotic fractures; US | NR | 100 | 1.0(NR) | Any fracturesh | Self-reported | Hip: 2.2 Non vertebral: 6.6 |
2,499 |
| Sundh 2017k [48] | FRAX+MS, FRAX; 2 |
P | Population-based cohort study; Sweden | NR | 100 | 10.0(NR) | MOF | Medical records | 16.3 | 412 |
| Biver 2018k [50] | NR, FRAX; 2 |
P | Geneva retirees cohort study; Switzerland | 65.0(1.4) | 100 | 5.0(1.8) | MOF | Self-reported | 19.1 | 740 |
| Reber 2018[49] | NR; 1 |
R | Social insurance for agriculture, forestry and horticulture; Germany | 75.4(6.3) | 48.8 | 2.0(NR) | MOF | Medical records | 2.6 | 298530 |
| Su 2018k [52] | FRAX+Fall, FRAX; 4 |
P | Mr. and Ms. Os study; China | M: 72.4(NR) F: 72.6(NR) |
50.0 | M: 9.9(2.8) F: 8.8(1.5) |
MOF | Medical records | M: 7.0 F: 11.8 |
M: 2000 F: 2000 |
| Rubin 2018[51] | FREM; 2 |
P | National registers data; Denmark | NR | 51.9 | 10.0(NR) | MOF | Medical records | M: 0.6 F: 1.4 |
M: 12011143 F: 1294206 |
| Su 2019(1)k [53] | NR, FRAX; 3 |
P | Osteoporotic fractures in men; China | 73.6(5.9) | 0 | 8.6(2.5) | Hip fracture | Self-reported or confirmed by radiographic reports | 2.9 | 5977 |
| Engels 2020[54] | NR; 1 |
R | Administrative claims data; Germany | 75.7(6.20) | 48.8 | 4.0(NR) | Hip fracture | Medical records | 0.6 | 78074 |
| Kong 2020[55] | NR; 1 |
P | Health and genome Study; Korea | 61.2(8.7) | 56.4 | 7.5(1.6) | MOF | Self-reported or confirmed by radiographic reports | 25.6 | 2227 |
| Sheer 2020[56] | NR; 1 |
R | Humana research; US | 74.3(NR) | 56.0 | 1.0(NR) | MOF | Medical records or self-reported | 6.6 | 1287354 |
| Wu 2020[57] | NR; 1 |
P | Osteoporotic fractures in men Study; US | NR | 0 | NR | MOF | Radiographic reports | 8.8 | 5130 |
| Lu 2021m [58] | GSOS, FRAX; 6 |
R | Five population-based cohort studiesi; UK, US, Sweden, China | NR | 54.0 | NR | MOF | Medical records or radiographic reports | Hip: 2.5 MOF: 6.0 |
431621 |
| de Vries 2021[59] | NR; 1 |
R | Population-based cohort study; Netherlands | 68.0(NR) | 74.0 | 5.0(NR) | MOF | Medical records | 11.0 | 7578 |
| Model validationn | ||||||||||
| Ensrud 2009[60] | FRAX; 4 |
P | Study of osteoporotic fractures; US | 71.3(5.1) | 100 | 9.2(1.8) | MOF | Self-reported and confirmed by radiographic reports | Hip: 6.2 MOF: 16.6 |
6252 |
| Hundrup 2010[61] | WHI; 1 |
P | Danish Nurse Cohort Study; Denmark | 61.0(6.9) | 100 | 5.0(NR) | Hip fracture | Medical records | 0.9 | 13353 |
| Leslie 2010[62] | FRAX; 4 |
R | Manitoba bone density program; Canada | M: 68.2(10.1) F: 65.7(9.8) |
92.7 | 10.0(NR) | MOF | Medical records | Hip: 1.4 MOF: 6.4 |
39603 |
| Sornay- Rendu 2010[63] | FRAX; 2 |
P | Os des femmes de Lyon cohort; France | 58.8(10.3) | 100 | 10.0(NR) | MOF | Self-reported and confirmed by radiographic reports | MOF: 13.4 | 867 |
| Trémollieres 2010[64] | FRAX; 1 |
P | Menopause et Os cohort study; US | 54.0(4.0) | 100 | 13.4(1.4) | MOF | Self-reported and confirmed by radiographic reports | 6.6 | 2196 |
| Bolland 2011[65] | FRAX, Garvan; 6 |
P | Population-based cohort study; New Zealand | 74.2(4.2) | 100 | 8.8(2.4) | Any fracturesj | Self-reported | Hip: 4.0 FRAX: 16.1 Garvan: 19.6 |
1422 |
| Langsetmo 2011[66] | Garvan; 4 |
P | Osteoporosis epidemiology study; Canada | M: 67.6(7.6) F: 67.7(7.6) |
72.1 | M: 8.3(NR) F: 8.6(NR) |
MOF | Self-reported | Hip: NR(M/F) MOF: 7.2(M) 14.0(F) |
M: 1606 F: 4152 |
| Pressman 2011[67] | FRAX; 2 |
R | Population-based cohort study; US | NR | 100 | 6.6(NR) | Hip fracture | Medical records | 1.7 | 94489 |
| Tanaka 2011[68] | FRISC; 1 |
R | Population-based cohort study; Japan | 63.3(10.8) | 100 | 10.0(NR) | MOF | Radiographic reports | 18.4 | 765 |
| Collins 2011[69] | QFracture; 4 |
P | Health improvement network database; UK | M: 47.0(NR) F: 48.0(NR) |
50.6 | 10.0(NR) | MOF | Medical records | MOF: 0.1(M) 0.3(F) Hip: 0.1(M/F) |
M: 1108219 F: 1136417 |
| Fraser 2011[70] | FRAX; 4 |
P | Multi-centre osteoporosis study; Canada | M: 65.3(9.1) F: 65.8(8.8) |
40.2 | 10.0(NR) | MOF | Self-reported and confirmed by a doctor | MOF: 6.4(M) 12.0(F) Hip: 2.4(M) 2.7(F) |
6697 |
| Azagra 2012[71] | FRAX; 4 |
P | Fracture risk factors and bone densitometry type central dual X-ray cohort; Spain | 56.8(8.0) | 100 | 10.0(NR) | MOF | Self-reported and confirmed by medical records | MOF: 8.4 Hip: 2.2 |
770 |
| Cheung 2012[72] | FRAX; 4 |
P | Mr. and Ms. Os study; China | 62.1(8.5) | 100 | 4.5(2.8) | MOF | Self-reported and confirmed by medical records | MOF: 4.7 Hip: 0.9 |
2266 |
| González- Macías 2012[73] | FRAX; 2 |
P | Ecografía Oseaen Atención Primaria cohort study; Italy | 72.3(5.3) | 100 | 3.0(NR) | MOF | Radiographic reports | Hip: 1.0 MOF: 3.8 |
5201 |
| Briot 2013[74] | FRAX; 2 |
P | Osteoporosis and ultrasound study; Germany | 74.2(NR) | 100 | 6.0(NR) | MOF | Self-reported and confirmed by radiographic reports | MOF: 4.9 | 1748 |
| Czerwiński 2013[75] | FRAX; 1 |
R | Cra cow Medical Centre data; Poland | 63.8(6.7) | 100 | 11.0(NR) | MOF | Self-reported | 22.1 | 5092 |
| Cordomí 2013[76] | FRAX; 1 |
R | Centre for technical studies with radioactive isotopes; Spain | 56.8(7.8) | 100 | 11.0(NR) | MOF | Self-reported | 18.1 | 1231 |
| Ettinger 2013[77] | FRAX; 4 |
R | Osteoporotic fractures in men study; US | 73.5(5.8) | 0 | 8.4(2.3) | MOF | Medical records | Hip: 2.7 MOF: 6.4 |
5891 |
| Rubin 2013[78] | FRAX; 1 |
P | Population-based cohort study; Denmark | 64.0(13.0) | 100 | 3.0(NR) | MOF | Medical records | 4.0 | 3614 |
| Ahmed 2014[79] | Garvan; 4 |
R | Tromsø study; Australia | NR | 54.7 | M: 7.1(NR) F: 6.9(NR) |
MOF | Medical records | M: 1.2 F: 3.2 |
2992 |
| Friis- Holmberg 2014[80] | FRAX; 4 |
P | Health examination survey; Denmark | M: 58.3(10.6) F: 56.8(10.2) |
59.2 | 4.3(NR) | MOF | Medical records | Hip: 0.4 MOF: 3.1 |
12758 |
| Van Geel 2014[81] | FRAX, Garvan; 7 |
P | Ten general practice centers cohort study; Netherlands | 67.8(5.8) | 100 | 5.0(NR) | MOF | Self-reported and confirmed by radiographic reports | Hip: 1.2 MOF: 9.5 |
506 |
| Klop 2016[82] | FRAX; 2 |
R | Clinical practice research Datalink cohort study; UK | 62.9(11.4) | 67.8 | 9.0(NR) | MOF | Medical records | Hip: 1.4 MOF: 5.0 |
38755 |
| Orwoll 2017[83] | FRAX; 6 |
R | Osteoporotic fractures in men study; Sweden, US, China | 75.0(3.0)o 74.0(6.0)p 72.0(5.0)q |
0 | 10.6(NR)o 8.6(NR)p 9.8(NR)q |
MOF | Medical records or radiographic reports | Hip: 6.8o, 3.2p, 3.1q MOF: 16.4o, 7.2p, 3.1q |
2542o 1469p 1476q |
| Dagan 2017[84] | QFracture, FRAX, Garvan; 6 |
R | Electronic health record; Israel | NR | 54.6 | 4.7(NR) | MOF | Medical records | MOF: 7.7 Hip: 2.7 |
1054815 |
| Holloway 2018[85] | FRAX; 2 |
P | Geelong osteoporosis study; Australia | 70.0(NR) | 0 | 9.5(NR) | MOF | Radiographic reports | Hip: 2.4 MOF: 8.5 |
591 |
| Crandall 2019[86] | FRAX, Garvan; 4 |
P | Women’s Health Initiative observational study; US | 57.9(4.1) | 100 | 10.0(NR) | MOF | Medical records or self-reported | Hip: 0.7 MOF: 8.4 |
Hip: 62723 MOF: 63621 |
| Holloway- Kew 2019[87] | FRAX, Garvan; 8 |
P | Geelong osteoporosis Study; Australia | M: 69.0(NR) F: 71.0(NR) |
49.6 | 10.0(NR) | MOF | Radiographic reports | M: 8.9 F: 14.2 |
M: 821 F: 809 |
| Su 2019(2)[88] | FRAX+TBS, FRAX; 4 |
P | Mr. and Ms. Os study; China | M: 72.3(4.9) F: 72.5(5.3) |
50.3 | M: 9.9(2.8) F: 8.8(1.5) |
MOF | Medical records or self-reported | M: 6.6 F: 11.0 |
M: 1923 F: 1950 |
| Tamaki 2019[89] | FRAX+TBS, FRAX; 4 |
P | Population-based cohort study; Japan | 58.1(10.6) | 100 | 10.0(NR) | MOF | Radiographic reports | 4.3 | 1541 |
F: female; FRA-HS: fracture health search; FRAMO: fracture and mortality index; FRAX: fracture risk assessment tool; FREM: fracture risk evaluation model; FRISC: fracture and immobilization score; FRISK: fracture risk; Garvan: Garvan Fracture Risk Calculator; gSOS: genomic speed of sound; KFRS: Korean fracture risk score; M: male; NR: not reported; MOF: major osteoporotic fracture; MST: mandibular sparse trabeculation; P: prospective cohort study; R: retrospective cohort study; S: sarcopenia; TBS: trabecular bone score; WHI: women's health initiative;
Naming of models or tools, and No. refers to the number of models that were developed or the number of times models was externally validated in the article.
Development of new model;
Included hip, vertebrae (symptomatic), wrist, meta-carpal, humerus, scapula, clavicle, distal femur, proximal tibia, patella, pelvis and sternum;
Included the Rotterdam Study, The European Vertebral Osteoporosis Study (later the European Prospective Osteoporosis Study), The Canadian Multicentre Oosteoporosis Study (CaMos), Rochester, Sheffield, Dubbo, a cohort from Hiroshima and two cohorts from Gothenburg;
Included hip, wrist, vertebral, forearm or humerus fractures;
Included hip fracture, surgical neck fracture of the humerus, distal forearm fracture, or clinical vertebral fracture;
Included Australia, Belgium, Canada, France, Germany, Italy, The Netherlands, Spain, the United Kingdom, and the United States;
Included ankle, clavicle, elbow, face, foot, finger, hand, heel, hip, humerus, knee, lower leg, pelvis, rib, toe, upper leg, or wrist fractures;
Included the UK Biobank, the United States-based Osteoporotic Fractures in Men Study, the Sweden-based Osteoporotic Fractures in Men Study, the Study of Osteoporotic Fractures, and the China Kadoorie Biobank;
FRAX-defined osteoporotic fractures were fractures of the shoulder, hip, or forearm and clinical vertebral fractures; Garvan-defined osteoporotic fractures were fractures of the hip, vertebrae (symptomatic), forearm, metacarpal, humerus, scapula, clavicle, distal femur, proximal tibia, patella, pelvis, or sternum
The study not only developed new models, but also externally verified the existing models.
The study developed and externally verified new models.
The study not only developed and externally verified new models, but also externally verified the existing models.
External validation of existing model;
Sweden.
US.
China.
Sample size
The sample size of included models ranged from 405 to 12,011,134, and the incidence of fracture ranged from 0.1% to 31.4%. The EPV ranged from 0.1 to 6,613.3. Of the 70 models, 30 (43%) had an EPV less than 20, indicating the existence of over-model fitting (Table 1 andTable 2).
Table 2.
Information related to predictive model of included studies.
| Author | Type of predictive model | EPV | No. of included predictors | Modeling method | Type of validation | Performancea (95% CI, if reported) | |||
|---|---|---|---|---|---|---|---|---|---|
| AUC/C index | Sensitivity | Specificity | Calibration | ||||||
| WHI (women's health initiative) | |||||||||
| Robbins 2007[29] | Development and internal validationb | 27.3 | 10 | Cox’s proportional hazards | Cross validation | 0.80(0.77 to 0.82) | NR | NR | P=0.20h |
| Hundrup 2010[61] | External validationc | 12.2 | 10 | Logistic regression | Geographical validation | 0.82 | 0.69 | 0.80 | 1.08i |
| FRAMO (fracture and mortality index) | |||||||||
| Albertsson 2007[28] | Development only | 1.4 | 4 | Cox’s proportional hazards | NA | 0.72(0.64 to 0.81) | 0.81 | 0.64 | NR |
| Garvan (Garvan Fracture Risk Calculator) | |||||||||
| Nguyen 2008[12] | Development and internal validation | M: 11.5 F: 32.8 |
4 | Cox’s proportional hazards | Bootstrapping | Model 1: 0.75(M/F) Model 2: 0.74(M), 0.72(F) |
NR | NR | 0.01 to 0.02j |
| Bolland 2011[65] | External validation | Hip: 11.4 MOF: 55.8 |
5 | NR | Geographical validation | Hip: 0.67 (0.60-0.75)k MOF: 0.64 (0.60-0.67)k |
NR | NR | P<0.01h |
| Henry 2011[34] | External validation | 25.0 | 5 | NR | Geographical validation | 0.70(0.65 to 0.75) | NR | NR | NR |
| Langsetmo 2011[66] | External validation | Hip: NR(M/F) MOF: 29.0(M) 145.8(F) |
4 | Cox’s proportional hazards | Geographical validation | Hip: 0.85(M), 0.80(F) MOF: 0.69(M), 0.70(F) |
NR | NR | NR |
| Van Geel 2014[81] | External validation | Hip: 1.5 MOF: 12.0 |
5 | NR | Geographical validation | Model 1: 0.70(hip), 0.70(MOF) Model 2: NR(hip), 0.65(MOF) |
NR | NR | NR |
| Ahmed 2014[79] | External validation | 71.2 | 5 | NR | Geographical validation | Model 1: 0.61(M), 0.62(F) Model 2: 0.57(M), 0.58(F) |
NR | NR | NR |
| Dagan 2017[84] | External validation | Hip: 5618.2 MOF: 16312.8 |
5 | NR | Geographical validation | Hip: 0.78k MOF: NRk |
Hip: 0.57 MOF: NR |
Hip: 0.81 MOF: NR |
0.68i |
| Crandall 2019[86] | External validation | Hip: 87.8 MOF: 1068.8 |
4 | Logistic regression | Geographical validation | Hip: 0.57(0.55 to 0.60) MOF: 0.57(0.57 to 0.58) |
Hip: 0.81 MOF: 0.16 |
Hip: 0.31 MOF: 0.94 |
NR |
| Holloway- Kew 2019[87] | External validation | M: 3.4 F: 8.4 |
5 | Logistic regression | Geographical validation | Model 1: 0.68(0.63 to 0.73)(M) 0.70(0.65 to 0.74)(F) Model 2: 0.67(0.62 to 0.72)(M) 0.67(0.62 to 0.71)(F) |
NR | NR | NR |
| FRAX (fracture risk assessment tool) | |||||||||
| Kanis 2008[10] | Development and external validationd | Hip: 77.3 MOF: 301.6 |
11 | Poisson regression | Geographical validation | Hip: 0.66n, 0.74o MOF: 0.60n, 0.62o |
NR | NR | NR |
| Ensrud 2009[60] | External validation | Hip: 35.4 MOF: 94.3 |
11 | Logistic regression | Geographical validation | Hip: 0.71n, 0.75o MOF: 0.61n, 0.68o |
NR | NR | NR |
| Leslie 2010[62] | External validation | Hip: 49.9 MOF: 231.2 |
11 | Cox’s proportional hazards | Geographical validation | Hip: 0.79(0.78 to 0.81)n 0.83(0.82 to 0.85)o MOF: 0.66(0.65 to 0.67)n 0.69(0.68 to 0.71)o |
NR | NR | Hip: 0.92(M) 1.03(F)i MOF: 1.24)(M) 1.13(F)i |
| Sornay- Rendu 2010[63] | External validation | MOF: 1.5 | 11 | NR | Geographical validation | 0.75(0.71 to 0.79)n 0.78(0.72 to 0.82)o |
NR | NR | NR |
| Trémollieres 2010[64] | External validation | 13.2 | 11 | Cox’s proportional hazards | Geographical validation | 0.63(0.56 to 0.69)o | NR | NR | NR |
| Yun 2010[31] | External validation | Hip: 17.0 MOF: 39.1 |
11 | Logistic regression | Geographical validation | Hip: 0.64(0.60 to 0.68)o MOF: 0.55(0.53 to 0.58)o |
NR | NR | NR |
| Bolland 2011[65] | External validation | Hip: 5.2 MOF: 20.8 |
11 | NR | Geographical validation | Hip: 0.69 (0.63 to 0.76)n, 0.70 (0.64 to 0.77)o, MOF: 0.62 (0.58 to 0.66)n 0.64 (0.60 to 0.68)o |
NR | NR | Hip: P=0.18h,n P<0.01h,o MOF: P<0.01h |
| Pressman 2011[67] | External validation | 143.5 | 11 | Logistic regression | Geographical validation | 0.83(0.82 to 0.84)n 0.84(0.83 to 0.85)o |
NR | NR | NR |
| Henry 2011[34] | External validation | 11.4 | 11 | NR | Geographical validation | 0.66(0.61 to 0.71)n 0.68(0.63 to 0.73)o |
NR | NR | NR |
| Tamaki 2011[35] | External validation | Hip: 3.9 MOF: 0.4 |
11 | Logistic regression | Geographical validation | Hip: 0.86(0.68 to 1.00)n 0.88(0.73 to 1.00)o MOF: 0.67(0.59 to 0.75)n 0.69(0.61 to 0.76)o |
NR | NR | NR |
| Fraser 2011[70] | External validation | Hip: 15.9 MOF: 63.2 |
11 | Cox’s proportional hazards | Geographical validation | Hip: 0.77(0.73 to 0.80)n 0.80(0.77 to 0.83)o MOF: 0.66(0.63 to 0.68)n 0.69(0.67 to 0.7)o |
NR | NR | Hip: 1.83(M) 0.93(F)i MOF: 1.26(M) 1.07(F)i |
| Azagra 2012[71] | External validation | Hip: 1.5 MOF: 5.9 |
11 | NR | Geographical validation | Hip: 0.89n, 0.85o MOF: 0.69n, 0.72o |
NR | NR | P>0.05h |
| Cheung 2012[72] | External validation | Hip: 1.9 MOF: 9.6 |
11 | Cox’s proportional hazards | Geographical validation | Hip: 0.90(0.83 to 0.97)n 0.88(0.82 to 0.94)o MOF: 0.71(0.66 to 0.76)n 0.73(0.68 to 0.80)o |
NR | NR | NR |
| González- Macías 2012[73] | External validation | Hip: 5.0 MOF: 18.3 |
11 | NR | Geographical validation | Hip: 0.64o MOF: 0.62o |
NR | NR | NR |
| Briot 2013[74] | External validation | 7.7 | 11 | Logistic regression | Geographical validation | 0.62(0.56 to 0.68)n 0.66(0.60 to 0.73)o |
NR | NR | NR |
| Czerwiński 2013[75] | External validation | 29.5 | 11 | NR | Geographical validation | 0.59(0.54 to 0.64)o | NR | NR | NR |
| Cordomí 2013[76] | External validation | MOF: 20.2 | 11 | NR | Geographical validation | 0.61(0.57 to 0.65)o | NR | NR | NR |
| Ettinger 2013[77] | External validation | Hip: 14.6 MOF: 34.0 |
11 | Logistic regression | Geographical validation | Hip: 0.69n, 0.77o MOF: 0.63n, 0.67o |
NR | NR | NR |
| Rubin 2013[78] | External validation | 15.6 | 10o | Cox’s proportional hazards | Geographical validation | 0.72(0.69, 0.76)n | NR | NR | NR |
| Friis- Holmberg 2014[80] | External validation | Hip: 4.9 MOF: 35.9 |
11 | Cox’s proportional hazards | Geographical validation | MOF: 0.67(0.61 to 0.73)o(M) 0.72(0.69 to 0.75)o(F) Hip: 0.72(0.60 to 0.84)o(M) 0.86(0.81 to 0.92)o(F) |
NR | NR | NR |
| Van Geel 2014[81] | External validation | Hip: 0.5 MOF: 4.4 |
11 | NR | Geographical validation | Hip: 0.70o MOF: 0.65n, 0.69o |
NR | NR | NR |
| Yu 2014[39] | External validation | Hip: 12.0 MOF: 51.3 |
11 | Cox’s proportional hazards | Geographical validation | Hip: 0.70n(M), 0.76o(M) 0.73n(F), 0.76o(F) MOF: 0.61n(M), 0.64o(M) 0.60n(F), 0.62o(F) |
NR | NR | NR |
| Iki 2015[40] | External validation | 2.8 | 11 | Logistic regression | Geographical validation | 0.68(0.59 to 0.78)o | NR | NR | NR |
| Klop 2016[82] | External validation | Hip: 48.7 MOF: 175.0 |
10o | Logistic regression | Geographical validation | Hip: 0.83n MOF: 0.71n |
NR | NR | 1.02i |
| Orwoll 2017[83] | External validation | NR | 11 | Logistic regression | Geographical validation | Hip: 0.72p, 0.78q, 0.74r MOF: 0.65p, 0.65q, 0.69r |
NR | NR | NR |
| Sundh 2017[48] | External validation | 7.1 | 10o | NR | Geographical validation | 0.75(0.70 to 0.81)n | NR | NR | NR |
| Dagan 2017[84] | External validation | Hip: 2553.7 MOF: 7414.9 |
11 | NR | Geographical validation | Hip: 0.82o MOF: 0.71o |
Hip: 0.66 MOF: 0.47 |
Hip: 0.81 MOF: 0.82 |
0.94i |
| Biver 2018[50] | External validation | 12.8 | 11 | Cox’s proportional hazards | Geographical validation | 0.71o | NR | NR | NR |
| Su 2018[52] | External validation | M: 12.6 F: 21.5 |
11 | Cox’s proportional hazards | Geographical validation | M: 0.69(0.64 to 0.73)o F: 0.61(0.58 to 0.65)o |
NR | NR | NR |
| Holloway 2018[85] | External validation | Hip: 1.3 MOF: 4.5 |
11 | NR | Geographical validation | Hip: 0.74o MOF: 0.85o |
Hip: 0.57 MOF: 0.02 |
Hip: 0.84 MOF: 0.99 |
NR |
| Crandall 2019[86] | External validation | Hip: 39.9 MOF: 485.8 |
10o | Logistic regression | Geographical validation | Hip: 0.64(0.61 to 0.66)n MOF: 0.58(0.57 to 0.59)n |
Hip: 0.81 MOF: 0.59 |
Hip: 0.81 MOF: 0.68 |
NR |
| Holloway- Kew 2019[87] | External validation | M: 7.3 F: 11.5 |
10o | Logistic regression | Geographical validation | M: 0.70(0.65 to 0.76)n 0.72(0.67 to 0.78)o F: 0.74(0.69 to 0.78)n 0.75(0.71 to 0.80)o |
NR | NR | NR |
| Su 2019(1)[53] | External validation | 17.3 | 10o | Cox proportional hazard | Geographical validation | 0.70(0.67 to 0.74)n | 0.62 | 0.78 | NR |
| Su 2019(2)[88] | External validation | M: 11.5 F: 19.5 |
11 | Cox proportional hazard | Geographical validation | M: 0.68(0.63 to 0.73)o F: 0.63(0.59 to 0.67)o |
NR | NR | NR |
| Tamaki 2019[89] | External validation | 6.1 | 11 | Logistic regression | Geographical validation | 0.67(0.61 to 0.73)n 0.68(0.62 to 0.74)o |
NR | NR | NR |
| Lu 2021[58] | External validation | Hip: 776.0 MOF: 1862.4 |
11 | Cox’s proportional hazards | Geographical validation | MOF: 0.76(0.75 to 0.76)o Hip: 0.81(0.80 to 0.81)o |
NR | NR | NR |
| FRAX+S (fracture risk assessment tool and sarcopenia) | |||||||||
| Yu 2014[39] | Development onlye | Hip: 11.0 MOF: 47.1 |
12 | Cox’s proportional hazards | NA | Hip: 0.73n, 0.78o(M) 0.73n, 0.75o(F) MOF: 0.62n, 0.66o(M) 0.60n, 0.62o(F) |
NR | NR | NR |
| FRAX+TBS (fracture risk assessment tool and trabecular bone score) | |||||||||
| Iki 2015[40] | Development only | 0.1 | 12 | Logistic regression | NA | 0.68(0.57 to 0.80)o | NR | NR | NR |
| Su 2019(2)[88] | External validation | M: 10.6 F: 17.8 |
12 | Cox proportional hazard | Geographical validation | M: 0.69(0.65 to 0.74)o F: 0.63(0.59 to 0.67)o |
NR | NR | NR |
| Tamaki 2019[89] | External validation | 5.6 | 12 | Logistic regression | Geographical validation | 0.68(0.62 to 0.74)n 0.68(0.62 to 0.74)o |
NR | NR | NR |
| FRAX+MST (fracture risk assessment tool and mandibular sparse trabeculation) | |||||||||
| Sundh 2017[48] | Development only | 5.9 | 11o | NR | NA | 0.75(0.70 to 0.81)n | NR | NR | NR |
| FRAX+FALL (fracture risk assessment tool and history of falls) | |||||||||
| Su 2018[52] | Development only | M: 11.6 F: 19.7 |
12 | Cox’s proportional hazards | NA | M: 0.69(0.65 to 0.74)o F: 0.61(0.58 to 0.65)o |
NR | NR | NR |
| QFracture | |||||||||
| Hippisley- Cox 2009[11] | Development and internal validation | Hip: 161.4(M) 489.6(F) MOF: 417.6(M) 1281.6(F) |
M: 12 F: 17 |
Cox’s proportional hazards | Training test split | Hip: 0.86(0.85 to 0.86)(M) 0.89(0.89 to 0.89)(F) MOF: 0.69(0.68 to 0.69)(M) 0.79(0.79 to 0.79)(F) |
NR | NR | 0.99i |
| Collins 2011[69] | External validation | Hip: 274.8(M) 833.2(F) MOF: 559.4(M) 1732.3(F) |
M: 12 F: 17 |
NR | Geographical validation | Hip: 0.86(M), 0.89(F) MOF: 0.74(M), 0.82(F) |
NR | NR | Hip: 0.01(M) 0.01(F)j MOF: 0.01(M) 0.03(F)j |
| Updated QFracture | |||||||||
| Hippisley- Cox 2012[36] | Development and internal validation | Hip: 166.6(M) 479.5(F) MOF: 461.2(M) 1467.0(F) |
M: 26 F: 25 |
Cox’s proportional hazards | Training test split | Hip: 0.88(0.87 to 0.88 )(M) 0.89(0.89 to 0.90) (F) MOF: 0.71(0.70 to 0.72) (M) 0.79(0.79 to 0.79) (F) |
Hip: 0.64(M) 0.60(F) MOF: 0.37(M) 0.35(F) |
NR | P>0.05h |
| Dagan 2017[84] | External validation | Hip: 906.2 MOF: 2631.1 |
31 | NR | Geographical validation | Hip: 0.88 MOF: 0.71 |
Hip: 0.70 MOF: 0.46 | Hip: 0.81 MOF: 0.82 |
0.60i |
| FRISC (fracture and immobilization score) | |||||||||
| Tanaka 2010[30] | Development and external validation | 23.9 | 5 | Poisson regression | Geographical validation | 0.73(0.66 to 0.79) | NR | NR | P=0.17h |
| Tanaka 2011[68] | External validation | 28.2 | 5 | Cox’s proportional hazards | Geographical validation | 0.73(0.69 to 0.78) | NR | NR | NR |
| FRISK (fracture risk) | |||||||||
| Henry 2011[34] | Development only | 25.0 | 5 | NR | NA | 0.66(0.60 to 0.71) | 59.2 | 0.65 | NR |
| KFRS (Korean fracture risk score) | |||||||||
| Kim 2016[42] | Development and internal validation | M: 543.2 F: 1661.2 |
9 | Cox’s proportional hazards | Training test split | M: 0.68, F: 0.65 | NR | NR | 1.00i |
| FRA-HS (fracture health search) | |||||||||
| Francesco 2017[43] | Development and external validation | 6613.3 | 9 | Cox’s proportional hazards | Geographical validation | 0.85 | NR | NR | 1.00(0.83 to 1.18)i |
| FREM (fracture risk evaluation model) | |||||||||
| Rubin 2018[51] | Development and internal validation | M: 2.3 F: 5.7 |
M: 44 F: 39 |
Logistic regression | Training test split | M: 0.75(0.74 to 0.76) F: 0.75(0.74 to 0.80) |
NR | NR | 0.01j |
| GSOS (genomic speed of sound) | |||||||||
| Lu 2021[58] | Development, internal and external validationf | MOF: <0.1 Hip: <0.1 |
21717 | Cox’s proportional hazards | Training test split, geographical validation | MOF: 0.73(0.73 to 0.74) Hip: 0.80(0.79 to 0.81) |
NR | NR | NR |
| Models without a specific name | |||||||||
| Dargent- Molina 2002[25] | Development only | NR | 5 | Cox’s proportional hazards | NA | NR | 0.37 | 0.85 | NR |
| Colón- Emeric 2002[26] | Development and external validation | Hip: 11.7 Any: 33.7 |
Hip: 7 Any: 6 |
Logistic regression | Geographical validationg | Hip: 0.75 Any: 0.57 |
NR | NR | NR |
| McGrother 2002[27] | Development and internal validation | 1.4 | 6 | Cox’s proportional hazards | Cross validation | 0.82 | 0.67(0.54 to 0.80) | 0.68(0.65 to 0.72) | NR |
| Yun 2010[31] | Development only | NR | NR | Logistic regression | NA | Hip: 0.74(0.70 to 0.77) MOF: 0.71(0.69 to 0.73) |
NR | NR | NR |
| Sambrook 2011[32] | Development only | NR | 2 | Cox’s proportional hazards | NA | 0.78 | NR | NR | NR |
| Bow 2011[33] | Development only | 1.1 | 7 | Cox’s proportional hazards | NA | 0.82 | NR | NR | NR |
| Tamaki 2011[35] | Development only | Hip: 0.4 MOF: 3.9 |
3 | Logistic regression | NA | Hip: 0.90(0.77 to 1.00) MOF: 0.71(0.63 to 0.79) |
NR | NR | NR |
| LaFleur 2012[37] | Development and internal validation | NR | Hip: 10 MOF: 12 |
Cox’s proportional hazards | Bootstrapping | Hip: 0.81 MOF: 0.74 |
0.84 | 0.75 | NR |
| Schousboe 2014[38] | Development and internal validation | 172.1 | 7 | Logistic regression | Bootstrapping | 0.69 | NR | NR | P>0.05h |
| Jang 2016[41] | Development only | M: 4.0 F: 5.6 |
M: 5 F: 7 |
Logistic regression | NA | M: 0.74, F: 0.73 | NR | NR | P>0.05h |
| Kruse 2017[44] | Development and internal validation | M: <0.1 F: 0.2 |
M: 9 F: 11 |
Machine learning | Bootstrapping | M: 0.89(0.82 to 0.95) F: 0.91(0.88 to 0.93) |
M: 0.69 F: 0.88 |
M: 0.69 F: 0.81 |
NR |
| Li 2017[45] | Development only | 11.5 | 5 | Cox’s proportional hazards | NA | 0.71 | NR | NR | NR |
| Su 2017[46] | Development only | M: 21.0 F: 35.8 |
2 | Poisson regression | NA | M: 0.67(0.62 to 0.71) F: 0.58(0.55 to 0.62) |
M: 0.64 F: 0.69 |
M: 0.74 F: 0.42 |
NR |
| Weycker 2017[47] | Development only | NR | Hip: 5 Non vertebral: 7 |
Cox’s proportional hazards | NA | Hip: 0.71(0.67 to 0.76) Non vertebral: 0.62(0.59 to 0.65) |
NR | NR | P=0.41h |
| Biver 2018[50] | Development only | 8.3 | 12 | Cox’s proportional hazards | NA | 0.76 | NR | NR | NR |
| Reber 2018[49] | Development and internal validation | 436.9 | 3 | Cox’s proportional hazards | Training test split | 0.70(0.69 to 0.71) | NR | NR | NR |
| Su 2019(1)[53] | Development and internal validation | Model 1: 57.3 Model 2: 13.2 |
Model 1: 3 Model 2: 13 |
Machine learning | Cross validation | Model 1: 0.71(0.68 to 0.75) Model 2: 0.73(0.69 to 0.76) |
NR | NR | NR |
| Engels 2020[54] | Development and internal validation | 80.6 | 23 | Machine learning | Training test split | 0.70(0.68 to 0.71) | NR | NR | 0.03j |
| Kong 2020[55] | Development and internal validation | 19.9 | 21 | Machine Learning | Cross validation | 0.69 | NR | NR | NR |
| Sheer 2020[56] | Development and internal validation | 1896.5 | 6 | Cox’s proportional hazards | Training test split | 0.71 | NR | NR | NR |
| Wu 2020[57] | Development and internal validation | 0.4 | 1115 | Machine learning | Cross validation | 0.71 | NR | NR | NR |
| de Vries 2021[59] | Development and internal validation | 18.3 | 8 | Cox’s proportional hazards | Cross validation | 0.70(0.66 to 0.73) | NR | NR | NR |
AUC: area under receiver operating characteristic curve; EPV: events per variable; M: male; MOF: major osteoporotic fracture; NA: not applicable; NR: not reported;
Performance is given for the strongest form of validation reported;
Development and internal validation refers to the study developed and internally validated the new model;
External validation refers to the study only externally validated the existing model;
Development and external validation refers to the study developed and externally validated the new model;
Development only refers to the study only developed the new model;
Development, internal and external validation refers to the study developed, internally and externally validated the new model;
External validation in different population only;
Pvalue refers to the results of Hosmer-Lemeshow test;
Refers to value of calibration slope;
Refers to value of calibration intercept;
The type of model used is not reported;
Without bone mineral density;
With bone mineral density;
Sweden;
US;
China;
Predictors
The number of predictors included in development models ranged from 2 to 21,717 (2 models did not report related information). Most models contained less than 15 predictors (n=55, 79%), while three (4%) models included more than 100 predictors (Table 2). Most models (n=31, 44%) contained some similar predictors, including age, prior fractures, and body mass index (BMI). Other commonly selected predictors were smoking status (n=35, 50%), BMD (n=31, 44%), alcohol use (n=30, 43%), rheumatoid arthritis (n=28, 40%). Sex was included in 25 (36%) models. However, most models were sex-specific, with 23 (33%) models for males only while 31 (44%) for females only. All three models with more than 100 predictors included single nucleotide polymorphisms (SNPs) as predictors (Supplementary table 2).
Modelling
Most prediction models (n=42, 60%) were developed using Cox proportional hazards regression, followed with Logistic regression (n=12, 17%), machine learning (n=7, 10%), and Poisson regression (n=7, 10%), while the remaining two (3%) did not report related information.
Performance
Sixty-nine (99%) models reported information about discrimination, with AUC or C index ranging from 0.60 to 0.91. To be specific, two (3%) models showed outstanding discrimination, nine (13%) showed excellent discrimination, 39 (57%) showed acceptable discrimination, and 20 (57%) showed poor discrimination. Calibration was reported among 25 (36%) models, with all of them being judged as good fitness. Calibration was assessed using Hosmer-Lemeshow test (n=11, 16%), the calibration slope (n=7, 10%), and the calibration intercept (n=7, 10%). Thirty-three (47%) models were internally validated using training test split (n=17), bootstrapping (n=9), and cross validation (n=7). It is worth noting that only four (6%) models used suitable methods for both internal validation (using bootstrapping or cross validation) and calibration calculation (using calibration slope or calibration intercept) (Table 2).
Model presentation
Only 39 (56%) models provided model presentation as a web calculator, nomogram, or risk score of each predictor to allow practical use, while the remaining 31 (44%) models did not offer related information.
Risk of bias and applicability
All 70 models were judged as high overall risk of bias. Respectively 31 (44%) and 10 (14%) models had an unclear and high risk of bias in the outcome domain. Mainly because it is unclear whether a prespecified or standard outcome definition or subjective outcome measures (e.g., self-reported) had been used. All models (n=70, 100%) were at high risk of bias for the analysis domain, which is commonly due to the risk of overfitting caused by an insufficient number of cases, or categorization of continuous predictors. In addition, the calibration of many models was not assessed or was not assessed correctly (e.g., using Hosmer-Lemeshow test). In terms of applicability, 44 (63%) models had a low concern while the remaining 26 (37%) had a high concern. The most common concern about applicability was the outcome domain, which focused on hip fracture. The models focused on predicting hip fracture may not accurately predict all osteoporosis fractures. Details on the risk of bias and applicability assessments are presented inFigure 2 and Supplementary table 3.
Figure 2.

Summary results on risk of bias and applicability assessment (using PROBAST) of development of osteoporotic fracture prediction models.
Studies focus on external validation of OF prediction model
In 44 articles [10,26,30,31,34,35,39,40,43,48,50,52,53,58,60-89], 138 external validations were performed. However, most (n=48, 69%) of the 70 developed models has never been externally validated. Out of the 22 (31%) models externally validated, 15 (21%) were validated once, and five (7%) were validated more than five times (range: 5 to 37). The most commonly validated models were FRAX with BMD (for MOF) (n=37, 27%) and FRAX with BMD (for hip fracture) (n=23, 17%) (Table 1 andTable 2).
Study populations and outcomes
All the external validations were conducted in a different geographical area from the development study. Most of the participants were from China (n=29, 21%), US (n=27, 20%) or UK (n=16, 12%), with the remaining (n=66, 48%) from countries in Oceania, Western Europe or East Asia. It is worth noting that no external validation was conducted among participants from Africa, South America, and the Middle East. Most models (n=109, 79%) were sex-specific, with 76 (55%) being validated for female, and 33 (24%) for male. The average age of participants ranged from 54 to 75 years. The outcomes included MOF (n=84, 61%), hip fracture (n=50, 36%) and any fractures (n=4, 9%). Diagnosis of fracture was mostly through medical records (n=57, 41%), following with self-reported (n=28, 20%), self-reported with another confirmation method (n=28, 20%) and radiograph reports (n=25, 18%) (Table 1).
Sample size
The sample size ranged from 412 to 1,136,417, and the incidence of fracture ranged from 0.1% to 22.1%. The EPV ranged from 0.1 to 16,312.8, and 114 (83%) models were less than 100, indicating the existence of over model fitting (Table 1 andTable 2).
Performance
The discrimination of 136 (99%) models was reported as an AUC or C index (range: 0.55 to 0.90). Among them, one (1%) showed outstanding discrimination, 23 (15%) showed excellent discrimination, 45 (38%) showed acceptable discrimination, and 67 (38%) showed poor discrimination. Calibration measurements were reported for 33 (24%) models, with 31 (22%) models showing good fitness. Calibration was assessed with calibration slope (n=18, 13%), the Hosmer-Lemeshow test (n=11, 8%), and the calibration intercept (n=4, 3%). Only 22 (16%) models used suitable methods (calibration slope or calibration intercept) for calibration calculation (Table 2).
The discrimination of the four most frequently validated models, including FRAX with BMD (for MOF), FRAX with BMD (for hip fracture), FRAX without BMD (for MOF), and FRAX without BMD (for hip fracture), varied among the studies, with AUC/C index ranged from 0.55 to 0.85, 0.64 to 0.88, 0.58 to 0.75, 0.64 to 0.90, respectively. Other commonly validated models, including the Garvan Model 1 and Garvan Model 2 in females, showed AUC/C index between 0.57 to 0.80, 0.58 to 0.78, respectively.
There were some FRAX extension models based on FRAX predictors and other predictors, such as FRAX plus sarcopenia [39], FRAX plus history of falls [52], FRAX plus trabecular bone score (TBS) [40,88,89]. The model performance of the extension models (AUC/C index: 0.60 to 0.78) was slightly improved compared with FRAX alone (AUC/C index: 0.60 to 0.74). However, most of them had not been externally validated yet.
The AUC/C indexes of the models using the machine learning modelling method were between 0.69 and 0.91, indicating relatively good discrimination. Some models only included two or three predictors, such as Sambrook 2011 (age, prior fractures) [32], Su 2017 (TBS, femoral neck BMD) [46], Tamaki 2011 (age, weight, femoral neck BMD) [35], with AUC/C indexes being 0.78, 0.67, and 0.90, respectively. Wu 2020 [57], gSOS (for MOF) [58], and gSOS (for hip fracture) [58] included SNPs as predictors, all contained more than 1000 predictors, with AUC/C indexes being 0.71, 0.73 and 0.80, respectively.
Risk of bias and applicability
Most models (n=126, 91%) were judged as high overall risk of bias, while the remaining 12 (9%) were unclear risk of bias, and no low risk of bias model was identified. The most common issues were seen in the analysis domain, in which 126 (91%) models were rated as high risk of bias. The most common reason was the insufficient number of cases or the incorrect assessment of calibration. Several models have an unclear risk (n=58, 42%) or high risk (n=15, 11%) of bias in outcome domain. It is mainly because of the unclarity of whether a prespecified or standard outcome definition or subjective outcome measures (e.g., self-reported) had been used. In applicability section, 88 (64%) models had a low concern, and the remaining 50 (36%) models had a high concern, because they focused on hip fracture in the outcome domain. Details on risk of bias and applicability assessments are presented inFigure 3 and Supplementary table 4.
Figure 3.

Summary results on risk of bias and applicability assessment (using PROBAST) of external validation of osteoporotic fracture prediction model.
Model comparison
FRAX, QFracture, and Garvan were the three most used tools in clinical practice. In addition, there were also some tools with a potential clinical value that had been externally verified with good performance (e.g., FRA-HS, WHI). The details of these models that have been externally validated as well as their advantages and disadvantages were summarized inTable 3.
Table 3.
Predictors, advantages and disadvantages of externally validated models.
| Author | Model | Details of the predictors included in the model | Advantages | Disadvantages |
|---|---|---|---|---|
| Colón- Emeric 2002[26] | Colón-Emeric- Any | Gender, ethnicity, BMI, activity of daily living difficulty, antiepileptic use, Rosow-Breslau impairmenta | • Relatively easy to measure • Contains few predictors |
• Performance is poor • Rarely externally verified • Dose-response is not included |
| Colón-Emeric- Hip | Age, gender, ethnicity, BMI, stroke history, cognitive impairment, Rosow-Breslau impairmenta | • Relatively easy to measure • Contains few predictors |
• Performance is acceptable • Rarely externally verified • Dose-response is not included |
|
| Robbins 2007[29] | WHI | Age, general health, BMI, prior fractures, ethnicity, physical activity, smoking status, family history of fractures, corticosteroid use, treated diabetes | • Easy to measure • Performance is excellent • Includes dose-response for general health and physical activity |
• Rarely externally verified • Not applicable to male |
| Nguyen 2008[12] | Garvan-Model 1 | Age, femoral neck BMD, prior fractures, history of falls | • Contains few predictors • Includes dose-response for number of prior fractures and falls • Commonly used in clinical practice |
• Performances range from poor to acceptable • Need to measure BMD |
| Garvan-Model 2 | Age, weight, prior fractures, history of falls | • Easy to measure • Contains few predictors • Includes dose-response for number of prior fractures and falls • Commonly used in clinical practice |
• Performances range from poor to acceptable | |
| Kanis 2008[10] | FRAX-with BMD | Age, gender, BMI, prior fractures, family history of fractures, glucocorticoid use, smoking status, alcohol use, RA, secondary osteoporosis, femoral neck BMD | • Had been externally verified many times • Widely used in clinical practice |
• Performances range from poor to acceptable • Need to measure BMD • Dose-response is not included |
| FRAX-without BMD | Age, gender, BMI, prior fractures, family history of fractures, glucocorticoid use, smoking status, alcohol use, RA, secondary osteoporosis | • Had been externally verified many times • Widely used in clinical practice • Relatively easy to measure |
• Performances range from poor to acceptable. • Dose-response is not included |
|
| Hippisley- Cox 2009[11] | QFracture-M | Age, BMI, smoking status, alcohol use, RA, cardiovascular disease, type 2 diabetes, asthma, tricyclic antidepressants use, corticosteroids use, history of falls, liver disease | • Performances range from acceptable to excellent • Includes dose-response for smoking, alcohol use, type of diabetes • Commonly used in clinical practice • Relatively easy to measure |
• Contains many predictors |
| QFracture-F | Hormone replacement therapy use, age, BMI, smoking status, alcohol use, parental history of osteoporosis, RA, cardiovascular disease, type 2 diabetes, asthma, tricyclic antidepressants, corticosteroids use, history of falls, menopausal symptoms, chronic liver disease, gastrointestinal malabsorption, other endocrine disorders | • Performance is excellent • Includes dose-response for smoking, alcohol use, type of diabetes • Commonly used in clinical practice • Relatively easy to measure |
• Contains many predictors | |
| Tanaka 2010[30] | FRISC | Age, weight, prior fractures, back pain, lumbar BMD | • Contains few predictors | • Performance is acceptable • Need to measure BMD • Not applicable to male • Dose-response is not included |
| Hippisley- Cox 2012[36] | Updated QFracture-F | Age, BMI, ethnicity, alcohol use, smoking status, chronic obstructive pulmonary disease or asthma, any cancer, cardiovascular disease, dementia, epilepsy, history of falls, chronic liver disease, Parkinson’s disease, RA or systemic lupus erythematosus, chronic renal disease, type 1 diabetes, type 2 diabetes, prior fractures, endocrine disorders, gastrointestinal malabsorption, antidepressants, corticosteroids use, unopposed hormone replacement therapy, parental history of osteoporosis | • Performances range from acceptable to excellent • Includes dose-response for smoking, alcohol use, type of diabetes • Commonly used in clinical practice • Relatively easy to measure |
• Contains many predictors |
| Updated QFracture-M | Age, BMI, ethnicity, alcohol use, smoking status, chronic obstructive pulmonary disease or asthma, any cancer, cardiovascular disease, dementia, epilepsy, history of falls, chronic liver disease, Parkinson’s disease, RA or systemic lupus erythematosus, chronic renal disease, type 1 diabetes, type 2 diabetes, prior fractures, endocrine disorders, gastrointestinal malabsorption, antidepressants, corticosteroids use, unopposed hormone replacement therapy, parental history of osteoporosis, care home residence | • Performances range from acceptable to excellent • Includes dose-response for smoking, alcohol use, type of diabetes • Commonly used in clinical practice • Relatively easy to measure |
• Contains many predictors | |
| Iki 2015[40] | FRAX+TBS | Age, gender, BMI, prior fractures, family history of fractures, glucocorticoid use, smoking status, alcohol use, RA, secondary osteoporosis, femoral neck BMD, trabecular bone score | • It is an extended model of FRAX-with BMD, with its performance better than that of FRAX-with BMD | • Need to measure BMD • Rarely externally verified • Dose-response is not included |
| Francesco 2017[43] | FRA-HS | Age, gender, prior fractures, secondary osteoporosis, corticosteroids use, RA, BMI, smoking status, alcohol abuse disorder | • Relatively easy to measure • Performance is excellent |
• Rarely externally verified • Dose-response is not included |
| Lu 2021[58] | GSOS | 21,717 SNP | • Performances range from acceptable to excellent | • Contains many predictors • Predictors are difficult to measure |
BMD: bone mineral density; BMI: body mass index; F: female; FRA-HS: Fracture health search; FRAX: fracture risk assessment tool; FRISC: fracture and immobilization score; GSOS: Genomic speed of sound; M: male; RA: rheumatoid arthritis; SNP: Single Nucleotide Polymorphisms; TBS: trabecular bone score; WHI: women's health initiative;
Rosow-Breslau impairment is defined as difficulty doing heavy work, walking upstairs, or unable to walk a mile.
DISCUSSION
This systematic review summarized and critically appraised 68 studies focused on OF risk prediction models in the general population, with 70 developed models and 138 external validations. Only a few models showed outstanding (n=3, 1%) or excellent (n=32, 15%) prediction discrimination. There was a paucity (n=22, 31%) of external validation models among these developed models. Notwithstanding there were a few notable exceptions, such as FRAX with BMD (for MOF) and FRAX with BMD (for hip fracture)). Calibration of developed models (n=25, 36%) or external validation models (n=33, 24%) were rarely assessed. Moreover, no model was appraised as having a low risk of bias.
We found much variability in the geographical location of both model development and model validation. However, the majority of models were developed and validated in the UK, the US, or China. Although studies have shown that osteoporosis fractures in low or middle-income countries are also prevalent [90], no model has been developed or validated among the population from Africa, South America, and the Middle East. Tailored models for populations in these countries are important because it is well known that predictor-outcome associations vary among ethnic groups [91]. In the future, more external validation studies among the aforementioned uncovered populations are needed to improve the generalizability of existing models, which is also a cost-effective choice than investing extra research funding in developing new models [92].
Although postmenopausal females are at high risk of OF, with the increase of age, the incidence of OF in males will increase significantly. Furthermore, the mortality and disability of OF in males are higher than that in females [93]. Therefore, osteoporosis is an underestimated bone condition among the male population [94]. Although research progress has been made on OF in male [37,57], we found that most models were developed (n=31, 44%) and validated (n=76, 55%) specifically for female, with relatively less models being specifically developed (n=23, 33%) or validated (n=33, 24%) for male. Future studies are suggested to pay attention to risk prediction models specific to the male population.
It is worth noting that some models only included a few numbers of predictors (e.g., two or three predictors) [32,35,46], or easily measured predictors [29] also showed promising model performance when compared to those models [57] that used multiple complex predictors like SNPs. Moreover, due to a large number of predictors and resources demanding for measurement, the practical application of these complex models (including a large number of SNPs) is limited. On the other hand, as the gold standard for the diagnosis of osteoporosis, BMD has been included in several prediction models [34,35,39,40,46,48]. This review found that many studies showed Garvan and FRAX with BMD had higher discrimination than Garvan and FRAX without BMD [39]. However, we also observed similar or even better model performance in models without BMD, such as QFracture [84], and WHI [29], indicating that BMD may not be an essential predictor for future fracture. Hence, an increasing number of predictors or including complex predictors may not necessarily improve model performance. Complex predictors (e.g., BMD, SNPs) could be replaced by other easily measurable predictors (e.g., age, prior fractures, history of falls) for future studies under the circumstances when it is unavailable, difficult to obtain, or showed no evidence of improving model performance.
FRAX, QFracture, and Garvan are the top three commonly used models for OF prediction. FRAX (10 or 11 predictors) is a model recommended by the WHO to evaluate the risk of OF [10]. It has strong applicability and operability and has been used worldwide [17]. In this systematic review, we found that FRAX with BMD (for MOF) (n=37, 27%) was the most externally validated model, but its model performance was not particularly good; Compared with FRAX alone, the model performance of its extended model was slightly improved, but most of them had not been externally verified. The Garvan (4 predictors) contained the least predictors that are easy to measure as well [12]. That facilitates its practical use. However, the model performance of the Garvan was relatively poor [16]. The QFracture was developed through electronic medical records and showed the best model performance among the three models. Nevertheless, the larger number of predictors (26 predictors for males and 25 predictors for females) limits its practical application to a certain extent [11]. Moreover, there were some models (e.g., FRA-HS) with potentially clinical value and good performance [43], had neither been externally verified in different populations nor were rarely used in clinical practice. As a result, there is no one fit for all models being recommended in this review. The model performance, applicability, and characteristics should be considered for selecting OF prediction model [16].
Modeling methods include classical regression methods (e.g., Cox proportional hazards regression, Logistic regression) and artificial intelligence methods (e.g., machine learning). Generally, classical regression methods have the defect of lower prediction performance [57]. Compared with classical regression methods, artificial intelligence methods have a powerful ability for data analysis and exploration. Models developed through artificial intelligence methods showed the advantages of accuracy, sensitivity, and efficiency [59,95]. In this systematic review, 7 (10%) models that adopted machine learning methods indicated relatively good discrimination. However, artificial intelligence modeling requires huge and high-quality data. In addition, the model is prone to overfitting [59]. Nonetheless, with the coming of the big data era, artificial intelligence methods have more applications in the medical field and could be considered as a flexible alternative for risk prediction in large datasets.
This systematic review did not consider model impact studies, which will quantify the benefits, harms, and costs of introducing a new prediction risk model through comparative design, it is also the final crucial step to identify whether the model can be applied to the clinic [96,97]. A recent related systematic review only identified three model impact studies on OF [98]. Results from this systematic review showed that population screening could effectively reduce OF and hip fractures, however, the information on the costs and screening interval was still unclear [98]. More rigorous impact studies are needed to determine whether OF risk prediction models should be implemented in clinical practice.
Recommendations and implications
Accurate OF risk evaluation can allow clinicians and individuals in understanding the risk of OF and guide them to make decisions to mitigate the risks [99]. When choosing a model for the prediction of OF risk, its accuracy, applicability, convenience, data availability, and cost should be considered. When developing models, simple models with less number or easily measured predictors should be considered as a priority choice to improve the clinical feasibility and practicality of the models. Given a large number of existing models, priority for the future studies should recalibrate and extend the existing OF prediction models to improve prediction performance, and conduct external verification and analysis of model impact, instead of developing new models from scratch [92].
Strengths and limitations
The strengths of this review include systematic literature search, rigorous study selection, and detailed data extraction on the main characteristics of OF prediction models. Furthermore, we evaluated the risk of bias and applicability of all the identified models to suggest where improvements are needed in future OF prediction model studies. However, this review also has some limitations. Firstly, due to the varied heterogeneity across studies, the results were not quantitatively synthesized, which limited the comparability of models. Secondly, although we conducted an exhaustive literature search, some relevant citations may be missed due to no attempt of grey literature search. This may underestimate the number of development and validation models,
Conclusion
In conclusion, our systematic review found that although there were a certain number of OF risk prediction models, most of the developed models had not been thoroughly internally validated or externally validated (with calibration being unassessed for most of the models). Most of the models showed poor performance as well. Moreover, all models suffered from methodological shortcomings. Given the availability of large and combined datasets, more rigorous studies are suggested to validate, improve and analyze the impact of existing OF risk prediction models in the general population rather than developing completely new models. Rigorous studies on OF prediction models are needed to target to males and the population in low or middle-income countries.
Supplementary Materials
The Supplementary data can be found online at: www.aginganddisease.org/EN/10.14336/AD.2021.01206
Acknowledgments
The research was supported by the Special Funding for the Construction of Innovative Provinces in Hunan (No.2019SK2141), National Key R&D Program of China (No. 2020YFC2008600), the China Oceanwide Holding Group Project Fund (No. 143010100) and the High-level Talents Introduction Plan from Central South University (No.502045003).
Footnotes
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- [1].Xia B, Zhang Z, Lin H, Jin X, Yu W, Fu Q (2019). Guidelines for the diagnosis and management of primary osteoporosis (2017). Chin J Osteoporos, 25:281-309. [Google Scholar]
- [2].Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, et al. (2014). The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int, 25:1439-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, et al. (2013). Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int, 24:2135-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooper C, S Ferrar S, editors. IOF Compendium of Osteoporosis. International Osteoporosis Foundation; 2020. [Google Scholar]
- [5].Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, et al. (2016). The current economic burden of illness of osteoporosis in Canada. Osteoporos Int, 27:3023-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Parreira PCS, Maher CG, Megale RZ, March L, Ferreira ML (2017). An overview of clinical guidelines for the management of vertebral compression fracture: a systematic review. Spine J, 17:1932-1938. [DOI] [PubMed] [Google Scholar]
- [7].Aspray TJ (2015). Fragility fracture: recent developments in risk assessment. Ther Adv Musculoskelet Dis, 7:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen SJ, Chen YJ, Cheng CH, Hwang HF, Chen CY, Lin MR (2016). Comparisons of Different Screening Tools for Identifying Fracture/Osteoporosis Risk Among Community-Dwelling Older People. Medicine (Baltimore), 95:e3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. (2010). 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ, 182:1864-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008). FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int, 19(4):385-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hippisley-Cox J, Coupland C (2009). Predicting risk of osteoporotic fracture in men and women in England and Wales: Prospective derivation and validation of QFractureScores. BMJ (Online), 339:1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV (2008). Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int, 19:1431-1444. [DOI] [PubMed] [Google Scholar]
- [13].Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, et al. (2009). Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas, 62:105-108. [DOI] [PubMed] [Google Scholar]
- [14].Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, et al. (2013). Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas, 75:392-396. [DOI] [PubMed] [Google Scholar]
- [15].Rubin KH, Friis-Holmberg T, Hermann AP, Abrahamsen B, Brixen K (2013). Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res, 28:1701-1717. [DOI] [PubMed] [Google Scholar]
- [16].Beaudoin C, Moore L, Gagné M, Bessette L, Ste-Marie LG, Brown JP, et al. (2019). Performance of predictive tools to identify individuals at risk of non-traumatic fracture: a systematic review, meta-analysis, and meta-regression. Osteoporos Int, 30:721-740. [DOI] [PubMed] [Google Scholar]
- [17].El-Hajj Fuleihan G, Chakhtoura M, Cauley JA, Chamoun N (2017). Worldwide Fracture Prediction. J Clin Densitom, 20:397-424. [DOI] [PubMed] [Google Scholar]
- [18].(2021) PRISMA 2020. J Clin Epidemiol, 134:A5-A6. [DOI] [PubMed] [Google Scholar]
- [19].Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. (2014). Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med, 11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mandrekar JN (2010). Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol, 5:1315-1316. [DOI] [PubMed] [Google Scholar]
- [21].Miller ME, Hui SL, Tierney WM (1991). Validation techniques for logistic regression models. Stat Med, 10:1213-1226. [DOI] [PubMed] [Google Scholar]
- [22].Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. (2015). Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med, 162:W1-W73. [DOI] [PubMed] [Google Scholar]
- [23].Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. (2019). PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann Intern Med, 170:51-58. [DOI] [PubMed] [Google Scholar]
- [24].Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. (2019). PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann Intern Med, 170:W1-W33. [DOI] [PubMed] [Google Scholar]
- [25].Dargent-Molina P, Douchin MN, Cormier C, Meunier PJ, Bréart G (2002). Use of clinical risk factors in elderly women with low bone mineral density to identify women at higher risk of hip fracture: The EPIDOS prospective study. Osteoporos Int, 13:593-599. [DOI] [PubMed] [Google Scholar]
- [26].Colón-Emeric CS, Pieper CF, Artz MB (2002). Can historical and functional risk factors be used to predict fractures in community-dwelling older adults? development and validation of a clinical tool. Osteoporos Int, 13:955-961. [DOI] [PubMed] [Google Scholar]
- [27].McGrother CW, Donaldson MM, Clayton D, Abrams KR, Clarke M (2002). Evaluation of a hip fracture risk score for assessing elderly women: the Melton Osteoporotic Fracture (MOF) study. Osteoporos Int, 13:89-96. [DOI] [PubMed] [Google Scholar]
- [28].Albertsson DM, Mellstrom D, Petersson C, Eggertsen R (2007). Validation of a 4-item score predicting hip fracture and mortality risk among elderly women. Annals of Family Medicine, 5:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, et al. (2007). Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA, 298:2389-2398. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka S, Yoshimura N, Kuroda T, Hosoi T, Saito M, Shiraki M (2010). The Fracture and Immobilization Score (FRISC) for risk assessment of osteoporotic fracture and immobilization in postmenopausal women-A joint analysis of the Nagano, Miyama, and Taiji Cohorts. Bone, 47:1064-1070. [DOI] [PubMed] [Google Scholar]
- [31].Yun H, Delzell E, Ensrud KE, Kilgore ML, Becker D, Morrisey MA, et al. (2010). Predicting hip and major osteoporotic fractures using administrative data. Arch Intern Med, 170:1940-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sambrook PN, Flahive J, Hooven FH, Boonen S, Chapurlat R, Lindsay R, et al. (2011). Predicting fractures in an international cohort using risk factor algorithms without BMD. J Bone Miner Res, 26:2770-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bow CH, Tsang SW, Loong CH, Soong CS, Yeung SC, Kung AW (2011). Bone mineral density enhances use of clinical risk factors in predicting ten-year risk of osteoporotic fractures in Chinese men: the Hong Kong Osteoporosis Study. Osteoporos Int, 22:2799-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Henry MJ, Pasco JA, Merriman EN, Zhang Y, Sanders KM, Kotowicz MA, et al. (2011). Fracture risk score and absolute risk of fracture. Radiology, 259:495-501. [DOI] [PubMed] [Google Scholar]
- [35].Tamaki J, Iki M, Kadowaki E, Sato Y, Kajita E, Kagamimori S, et al. (2011), Fracture risk prediction using FRAX: A 10-year follow-up survey of the Japanese Population-Based Osteoporosis (JPOS) Cohort Study. Osteoporosis Int, 22:3037-3045. [DOI] [PubMed] [Google Scholar]
- [36].Hippisley-Cox J, Coupland C (2012). Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: Prospective open cohort study. BMJ, 345:e3427. [DOI] [PubMed] [Google Scholar]
- [37].LaFleur J, Nelson RE, Yao Y, Adler RA, Nebeker JR (2012). Validated risk rule using computerized data to identify males at high risk for fracture. Osteoporos Int, 23:1017-1027. [DOI] [PubMed] [Google Scholar]
- [38].Schousboe JT, Rosen HR, Vokes TJ, Cauley JA, Cummings SR, Nevitt M, et al. (2014). Prediction models of prevalent radiographic vertebral fractures among older women. J Clin Densitom, 17:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yu R, Leung J, Woo J (2014). Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc, 15:918-923. [DOI] [PubMed] [Google Scholar]
- [40].Iki M, Fujita Y, Tamaki J, Kouda K, Yura A, Sato Y, et al. (2015). Trabecular bone score may improve FRAX prediction accuracy for major osteoporotic fractures in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Osteoporosis Int, 26:1841-1848. [DOI] [PubMed] [Google Scholar]
- [41].Jang EJ, Lee YK, Choi HJ, Ha YC, Jang S, Shin CS, et al. (2016). Osteoporotic Fracture Risk Assessment Using Bone Mineral Density in Korean: A Community-based Cohort Study. J Bone Metab, 23:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kim HY, Jang EJ, Park B, Kim TY, Shin SA, Ha YC, et al. (2016). Development of a Korean Fracture Risk Score (KFRS) for predicting osteoporotic fracture risk: Analysis of data from the Korean National Health Insurance Service. PloS one, 11:e0158918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Francesco L, Elisa B, Raffaella M, Alessandro P, Iacopo C, Giampiero M, et al. (2017). Assessing Risk of Osteoporotic Fractures in Primary Care: Development and Validation of the FRA-HS Algorithm. Calcif Tissue Int, 100:537-549. [DOI] [PubMed] [Google Scholar]
- [44].Kruse C, Eiken P, Vestergaard P (2017). Machine Learning Principles Can Improve Hip Fracture Prediction. Calcif Tissue Int, 100:348-360. [DOI] [PubMed] [Google Scholar]
- [45].Li G, Papaioannou A, Thabane L, Levine MAH, Ioannidis G, Wong AKO, et al. (2017). Modifying the Phenotypic Frailty Model in Predicting Risk of Major Osteoporotic Fracture in the Elderly. J Am Med Dir Assoc, 18:414-419. [DOI] [PubMed] [Google Scholar]
- [46].Su Y, Leung J, Hans D, Aubry-Rozier B, Kwok T (2017). Added clinical use of trabecular bone score to BMD for major osteoporotic fracture prediction in older Chinese people: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporosis Int, 28:151-160. [DOI] [PubMed] [Google Scholar]
- [47].Weycker D, Edelsberg J, Barron R, Atwood M, Oster G, Crittenden DB, et al. (2017). Predictors of near-term fracture in osteoporotic women aged ≥65 years, based on data from the study of osteoporotic fractures. Osteoporos Int, 28:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sundh V, Hange D, Ahlqwist M, Hakeberg M, Lissner L, Jonasson G (2017). FRAX and mandibular sparse trabeculation as fracture predictors: a longitudinal study from 1980 to 2002. Eur J Oral Sci, 125:135-140. [DOI] [PubMed] [Google Scholar]
- [49].Reber KC, König HH, Becker C, Rapp K, Büchele G, Mächler S, Lindlbauer I (2018). Development of a risk assessment tool for osteoporotic fracture prevention: A claims data approach. Bone, 110:170-176. [DOI] [PubMed] [Google Scholar]
- [50].Biver E, Durosier-Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S (2018). Evaluation of Radius Microstructure and Areal Bone Mineral Density Improves Fracture Prediction in Postmenopausal Women. J Bone Miner Res, 33:328-337. [DOI] [PubMed] [Google Scholar]
- [51].Rubin KH, Möller S, Holmberg T, Bliddal M, Søndergaard J, Abrahamsen B (2018). A New Fracture Risk Assessment Tool (FREM) Based on Public Health Registries. J Bone Miner Res, 33:1967-1979. [DOI] [PubMed] [Google Scholar]
- [52].Su Y, Leung J, Kwok T (2018). The role of previous falls in major osteoporotic fracture prediction in conjunction with FRAX in older Chinese men and women: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int, 29:355-363. [DOI] [PubMed] [Google Scholar]
- [53].Su Y, Kwok TCY, Cummings SR, Yip BHK, Cawthon PM (2019). Can Classification and Regression Tree Analysis Help Identify Clinically Meaningful Risk Groups for Hip Fracture Prediction in Older American Men (The MrOS Cohort Study)? JBMR Plus, 3:e10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Engels A, Reber KC, Lindlbauer I, Rapp K, Buchele G, Klenk J, et al. (2020). Osteoporotic hip fracture prediction from risk factors available in administrative claims data-A machine learning approach. PLoS One, 15:e0232969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kong SH, Ahn D, Kim B, Srinivasan K, Ram S, Kim H, et al. (2020). A Novel Fracture Prediction Model Using Machine Learning in a Community-Based Cohort. JBMR Plus, 4:e10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sheer RL, Barron RL, Sudharshan L, Pasquale MK (2020). Validated prediction of imminent risk of fracture for older adults. Am J Manag Care, 26:e91-e97. [DOI] [PubMed] [Google Scholar]
- [57].Wu Q, Nasoz F, Jung J, Bhattarai B, Han MV (2020). Machine Learning Approaches for Fracture Risk Assessment: A Comparative Analysis of Genomic and Phenotypic Data in 5130 Older Men. Calcif Tissue Int, 107:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lu T, Forgetta V, Keller-Baruch J, Nethander M, Bennett D, Forest M, et al. (2021). Improved prediction of fracture risk leveraging a genome-wide polygenic risk score. Genome Med, 13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].de Vries BCS, Hegeman JH, Nijmeijer W, Geerdink J, Seifert C, Groothuis-Oudshoorn CGM (2021). Comparing three machine learning approaches to design a risk assessment tool for future fractures: predicting a subsequent major osteoporotic fracture in fracture patients with osteopenia and osteoporosis. Osteoporos Int, 32:437-449. [DOI] [PubMed] [Google Scholar]
- [60].Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG, Fink HA, et al. (2009). A comparison of prediction models for fractures in older women: Is more better? Arch Intern Med, 169:2087-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hundrup YA, Jacobsen RK, Andreasen AH, Davidsen M, Obel EB, Abrahamsen B (2010). Validation of a 5-year risk score of hip fracture in postmenopausal women. The Danish Nurse Cohort Study. Osteoporos Int, 21:2135-2142. [DOI] [PubMed] [Google Scholar]
- [62].Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2010). Independent clinical validation of a Canadian FRAX tool: Fracture prediction and model calibration. J Bone Miner Res, 25:2350-2358. [DOI] [PubMed] [Google Scholar]
- [63].Sornay-Rendu E, Munoz F, Delmas PD, Chapurlat RD (2010). The FRAX tool in French women: How well does it describe the real incidence of fracture in the OFELY cohort? J Bone Miner Res, 25:2101-2107. [DOI] [PubMed] [Google Scholar]
- [64].Trémollieres FA, Pouillès JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P (2010). Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Miner Res, 25:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, et al. (2011). Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res, 26:420-427. [DOI] [PubMed] [Google Scholar]
- [66].Langsetmo L, Nguyen TV, Nguyen ND, Kovacs CS, Prior JC, Center JR, et al. (2011). Independent external validation of nomograms for predicting risk of low-trauma fracture and hip fracture. CMAJ, 183:E107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pressman AR, Lo JC, Chandra M, Ettinger B (2011). Methods for assessing fracture risk prediction models: experience with FRAX in a large integrated health care delivery system. J Clin Densitom, 14:407-415. [DOI] [PubMed] [Google Scholar]
- [68].Tanaka S, Kuroda T, Saito M, Shiraki M (2011). Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. J Bone Miner Res, 26:2778-2784. [DOI] [PubMed] [Google Scholar]
- [69].Collins GS, Mallett S, Altman DG (2011). Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ, 342:d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. (2011). Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int, 22:829-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Azagra R, Roca G, Encabo G, Aguye A, Zwart M, Guell S, et al. (2012). FRAX tool, the WHO algorithm to predict osteoporotic fractures: The first analysis of its discriminative and predictive ability in the Spanish FRIDEX cohort. BMC Musculoskelet Disord, 13:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cheung EY, Bow CH, Cheung CL, Soong C, Yeung S, Loong C, et al. (2012). Discriminative value of FRAX for fracture prediction in a cohort of Chinese postmenopausal women. Osteoporos Int, 23:871-878. [DOI] [PubMed] [Google Scholar]
- [73].González-Macías J, Marin F, Vila J, Díez-Pérez A (2012). Probability of fractures predicted by FRAX® and observed incidence in the Spanish ECOSAP Study cohort. Bone, 50:373-377. [DOI] [PubMed] [Google Scholar]
- [74].Briot K, Paternotte S, Kolta S, Eastell R, Felsenberg D, Reid DM, et al. (2013). FRAX®: prediction of major osteoporotic fractures in women from the general population: the OPUS study. PLoS One, 8:e83436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Czerwiński E, Borowy P, Kumorek A, Amarowicz J, Górkiewicz M, Milert A (2013). Fracture risk prediction in outpatients from Krakow Region using FRAX tool versus fracture risk in 11-year follow-up. Ortop Traumatol Rehabi, 15:617-628. [DOI] [PubMed] [Google Scholar]
- [76].Tebé Cordomí C, Del Río LM, Di Gregorio S, Casas L, Estrada MD, Kotzeva A, et al. (2013). Validation of the FRAX predictive model for major osteoporotic fracture in a historical cohort of Spanish women. J Clin Densitom, 16:231-237. [DOI] [PubMed] [Google Scholar]
- [77].Ettinger B, Ensrud KE, Blackwell T, Curtis JR, Lapidus JA, Orwoll ES (2013). Performance of FRAX in a cohort of community-dwelling, ambulatory older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int, 24:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rubin KH, Abrahamsen B, Friis-Holmberg T, Hjelmborg JVB, Bech M, Hermann AP, et al. (2013). Comparison of different screening tools (FRAX, OST, ORAI, OSIRIS, SCORE and age alone) to identify women with increased risk of fracture. A population-based prospective study. Bone, 56:16-22. [DOI] [PubMed] [Google Scholar]
- [79].Ahmed LA, Nguyen ND, Bjørnerem Å, Joakimsen RM, Jørgensen L, Størmer J, et al. (2014). External validation of the Garvan nomograms for predicting absolute fracture risk: the Tromsø study. PloS One, 9:e107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Friis-Holmberg T, Rubin KH, Brixen K, Tolstrup JS, Bech M (2014). Fracture risk prediction using phalangeal bone mineral density or FRAX(®)?-A Danish cohort study on men and women. J Clin Densitom, 17:7-15. [DOI] [PubMed] [Google Scholar]
- [81].Van Geel TACM, Eisman JA, Geusens PP, Van Den Bergh JPW, Center JR, Dinant GJ (2014). The utility of absolute risk prediction using FRAX and Garvan Fracture Risk Calculator in daily practice. Maturitas, 77:174-179. [DOI] [PubMed] [Google Scholar]
- [82].Klop C, de Vries F, Bijlsma JW, Leufkens HG, Welsing PM (2016). Predicting the 10-year risk of hip and major osteoporotic fracture in rheumatoid arthritis and in the general population: an independent validation and update of UK FRAX without bone mineral density. Ann Rheum Dis, 75:2095-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Orwoll ES, Lapidus J, Wang PY, Vandenput L, Hoffman A, Fink HA, et al. (2017). The Limited Clinical Utility of Testosterone, Estradiol, and Sex Hormone Binding Globulin Measurements in the Prediction of Fracture Risk and Bone Loss in Older Men. J Bone Miner Res, 32:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dagan N, Cohen-Stavi C, Leventer-Roberts M, Balicer RD (2017). External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: Retrospective cohort study. BMJ, 356:i6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Holloway KL, Mohebbi M, Betson AG, Hans D, Hyde NK, Brennan-Olsen SL, et al. (2018). Prediction of major osteoporotic and hip fractures in Australian men using FRAX scores adjusted with trabecular bone score. Osteoporos Int, 29:101-108. [DOI] [PubMed] [Google Scholar]
- [86].Crandall CJ, Larson J, LaCroix A, Cauley JA, LeBoff MS, Li W, et al. (2019). Predicting Fracture Risk in Younger Postmenopausal Women: Comparison of the Garvan and FRAX Risk Calculators in the Women's Health Initiative Study. J Gen Intern Med, 34:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Holloway-Kew KL, Zhang Y, Betson AG, Anderson KB, Hans D, Hyde NK, et al. (2019). How well do the FRAX (Australia) and Garvan calculators predict incident fractures? Data from the Geelong Osteoporosis Study. Osteoporos Int, 30:2129-2139. [DOI] [PubMed] [Google Scholar]
- [88].Su Y, Woo JW, Kwok TCY (2019). The Added Value of SARC-F to Prescreening Using FRAX for Hip Fracture Prevention in Older Community Adults. J Am Med Dir Assoc, 20:83-89. [DOI] [PubMed] [Google Scholar]
- [89].Tamaki J, Iki M, Sato Y, Winzenrieth R, Kajita E, Kagamimori S (2019). Does Trabecular Bone Score (TBS) improve the predictive ability of FRAX® for major osteoporotic fractures according to the Japanese Population-Based Osteoporosis (JPOS) cohort study? J Bone Miner Metab, 37:161-170. [DOI] [PubMed] [Google Scholar]
- [90].Maalouf G, Gannagé-Yared MH, Ezzedine J, Larijani B, Badawi S, Rached A, et al. (2007). Middle East and North Africa consensus on osteoporosis. J Musculoskelet Neuronal Interact, 7:131-143. [PubMed] [Google Scholar]
- [91].Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJ, Asselbergs FW, Anderson TJ, et al. (2015). Race/Ethnic Differences in the Associations of the Framingham Risk Factors with Carotid IMT and Cardiovascular Events. PLoS One, 10:e0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Damen JA, Hooft L, Schuit E, Debray TP, Collins GS, Tzoulaki I, et al. (2016). Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ, 353:i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. (2010). Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med, 152:380-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rinonapoli G, Ruggiero C, Meccariello L, Bisaccia M, Ceccarini P, Caraffa A (2021). Osteoporosis in Men: A Review of an Underestimated Bone Condition. Int J Mol Sci, 22:2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].DeGregory KW, Kuiper P, DeSilvio T, Pleuss JD, Miller R, Roginski JW, et al. (2018). A Review of Machine Learning in Obesity. Obes Rev, 19:668-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Au EH, Francis A, Bernier-Jean A, Teixeira-Pinto A (2020). Prediction modeling-part 1: regression modeling. Kidney Int, 97:877-884. [DOI] [PubMed] [Google Scholar]
- [97].Moons KG, Altman DG, Vergouwe Y, Royston P (2009). Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ, 338:b606. [DOI] [PubMed] [Google Scholar]
- [98].Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM (2020). Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int, 31:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Leslie WD, Lix LM (2014). Comparison between various fracture risk assessment tools. Osteoporos Int, 25:1-21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


