Abstract
Aging is a major global challenge, and there is growing demand for new strategies to address the burden of aging. The intensive search for antiaging agents has led to the discovery of a variety of drugs that promote the extension of healthspan and/or life. Metformin is a safe, effective, and globally affordable antihyperglycemic agent that has gained much attention in recent years as a potential antiaging treatment. Metformin has been shown to significantly delay the onset of age-related diseases and increase lifespan in several model organisms. In this paper, we reviewed aging hallmarks and the role of metformin in countering these hallmarks. We examined the beneficial effects of metformin on several age-related diseases and the feasibility of metformin as an agent to extend lifespan and healthspan. Finally, we discussed new research directions to better understand the translational potential of metformin in humans.
Keywords: metformin, aging hallmarks, antiaging, age-related diseases, healthspan
It is expected that by 2050, the number of people aged 60 years and older worldwide will reach 2 billion [1]. An increase in the number of elderly individuals is associated with an increase in disability and illness, which impose heavy social and economic burdens on individuals, families and countries. Therefore, there is growing demand for new strategies that address the burden of the aging process. Aging is a progressive, accumulative and natural phenomenon that leads to functional declines in living organisms [2]. Aging is often referred to as a risk factor for age-related diseases, including osteoporosis, type 2 diabetes, atherosclerosis, cardiovascular diseases (CVDs), cancer, liver and kidney failure and immune system dysfunctions, as well as neurodegenerative disorders [3,4].Therefore, aging is sometimes described as the “sum of age-related diseases” [5]. To extend healthspan, efforts are underway to develop antiaging drugs that could slow aging and delay age-related diseases. Multiple clinical trials have suggested the positive effects of metformin on age-related diseases, and the “Targeting Aging with Metformin” (TAME) trial was developed to develop an aging paradigm that can be used to delay aging.
Metformin, which is a Food and Drug Administration (FDA)-approved first-line drug for treating type 2 diabetes, has been used clinically for over 60 years for its effectiveness, safety, and low cost [6]. Numerous studies have shown that metformin treatment enhances insulin sensitivity, induces glycolysis, improves peripheral glucose uptake and suppresses hepatic gluconeogenesis [7,8]. Beyond its antihyperglycemic effects, metformin can exert multiple antiaging effects at the cellular and organism levels [9], which are closely associated with improvements in aging hallmarks, such as inflammation [10], autophagy [11] and cellular senescence [12]. It has been shown that metformin significantly delays the onset of age-associated diseases and effectively reduces the risk of many age-related diseases in humans, including CVD, neurodegenerative disease, cancer, and chronic inflammation [13]. Moreover, the effects of metformin on extending lifespan and healthspan have been demonstrated in several experimental models, including C. elegans [14] and mice [15]. Given its excellent effects on age-related diseases and longevity, metformin may be of interest as a potential antiaging agent in humans.
This article aimed to review the role of metformin as a possible antiaging drug. We summarize the hallmarks of aging and the role that metformin may have in countering these hallmarks, highlighting the known and putative mechanisms behind its antiaging properties. In addition, we analyzed the beneficial effects of metformin on several age-related diseases and the feasibility of metformin for extending lifespan and healthspan. Finally, we discuss new research directions to better understand the translational potential of metformin in humans.
Effects of Metformin on the Hallmarks of Aging
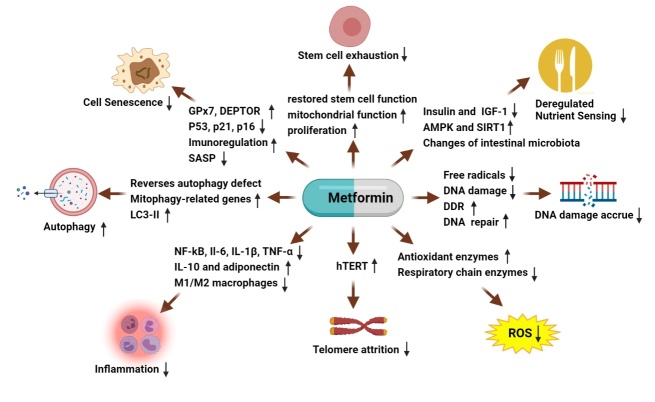
The aging process is a progressive multifactorial phenomenon that exhibits different characteristics at the molecular, cellular, and organismal levels. Deregulated nutrient sensing, DNA damage, the accumulation of reactive oxygen species (ROS), telomere attrition, inflammation, autophagy decline, cellular senescence and stem cell depletion have been identified in aging and play crucial roles during the aging process [16]. Each of the hallmarks of aging is connected to undesirable alterations in cellular, molecular, and physiological functionality, which may represent common features of aging in different organisms. Metformin has important effects on all of these features of aging (Table 1).
Table 1.
The Hallmarks of Aging and the Effects of Metformin.
| Aging Hallmarks | Observations | Effects of Metformin | References |
|---|---|---|---|
| Deregulated nutrient sensing | nutrient sensing signaling pathways (mTORC, IIS, AMPK and sirtuins) deregulated during aging; activation of AMPK and sirtuins or suppression of mTOR and IIS can delay aging and promote healthyspan. | decrease insulin and IGF-1 levels and improving insulin sensitivity; restored glucose-sensing pathway by induced changes in the upper small intestinal microbiota; activated AMPK and SIRT1, mediated longevity extension. | [19,26,32-37]. |
| DNA damage | DNA damage accrues with age; aberrant DDR and deficient DNA repair are closely associated with aging and premature aging syndromes. | scavenges free radicals, prevents insulin/UV-induced DNA damage and TBI-induced DSBs; stimulates DDR, significantly increases XPC and XRCC1, activates AMPK, promotes DNA damage repair. | [18-24] |
| ROS | high ROS levels contribute to aging onset and progression; oxidant production is increased, and antioxidant enzymes are decreased with aging; the overexpression of catalase increases the lifespan of mice; the accumulation of ROS can reduce lifespan. | decreases ROS by act on mitochondria respiratory complex I and activates AMPK; inhibits TBI-induced oxidative stress by upregulation of SOD, CAT, and GPX1 expression; enhances the activities of antioxidant enzymes in mice treated with CCl4, cisplatin, and doxorubicin. | [22,25-32] |
| Telomere attrition | telomeres shorten with cell division; telomerase absence causes age-related pathologies and premature aging phenotypes and is reversed by telomerase overexpression/reactivation. | prevents telomere attrition in the male offspring of mothers with gestational diabetes; increases hTERT expression and activity to delay endothelial cell senescence and protect organism against age-associated atherosclerosis. | [33-36] |
| Inflammation | contributes to most diseases that are typical of old age; proinflammatory cytokines increase with aging; NF-κB is activated in mouse models of progeria and inhibiting NF-κB signaling prevents age-associated dysfunction. | significantly reduces biomarkers of inflammation; decreases the expression of proinflammatory cytokines and enhances anti-inflammatory cytokine levels; suppresses NF-κB; improves macrophage polarization from the proinflammatory M1 to the anti-inflammatory M2 phenotype. | [37-45] |
| Autophagy | aging is associated with reduced autophagic activity; antiaging is related to autophagy induction; autophagy loss in aged MuSCs and CD4+T lymphocytes leads to declines in function, and these effects are reversed by autophagy re-establishment; reductions in autophagy regulators accelerate aging. | inhibits nucleus pulposus cell and hPDLC senescence by inducing autophagy; reverses autophagy defectsinCD4+T cells from older individuals; enhances mitophagy by upregulating mitophagy-related genes; reduces α-synuclein accumulation by enhancing LC3-II-mediated autophagy in dopaminergic neurons. | [41,46-50] |
| Cellular Senescence | senescent cells accumulate with aging; transplanting senescent cells into young mice drives aging; eliminating senescent cells slows the aging process in progeroid mice and attenuates the aging phenotype. | prevents CSE-induced hBEC senescence; inhibits hyperglycemia-induced endothelial cells senescence; attenuates ceramide-induced and stress-induced cellular senescence; delays senescence in human fibroblasts and MSCs; clears senescent cells and inhibits the expression of senescence-related proteins and the abundance of SASP. | [16,52-59] |
| Stem cell depletion | senescent satellite cells reduce the regeneration of muscle tissues; the number of NSCs and MuSCs decreases and the function and activity of HSCs and ISCs decline with age, which is implicated in tissues degeneration and homeostasis declines, driving aging and age-related diseases. | prevents BMSC senescence and enhances their function in the context of injury; promotes the proliferation and differentiation of NSCs; targets satellite cells to prevent their dysregulation after burn and increases their total numbers; improves mitochondrial function in aged OPCs; restores differentiation and rejuvenation capacities. | [43,47,60-65] |
Abbreviations: TBI, total-body irradiation; XPC, xeroderma pigmentosum C; UV, ultraviolet; DSBs, DNA double-strand breaks; DDR, DNA damage response; XRCC1, X-ray repair cross complementing 1; ROS, reactive oxygen species; AMPK, AMP-activated protein kinase; HSCs, haemopoietic stem cells; SOD, Superoxide dismutase; CAT, Catalase; GPX1, glutathione peroxidase; hTERT, human telomerase reverse transcriptase; NF-κB, nuclear factor-kappa B; MuSCs, muscle stem cells; hPDLCs, human periodontal ligament cells; SASP, senescence associated secretory phenotype; CSE, cigarette smoke extract; hBEC, human bronchial epithelial cells ; MSCs, mesenchymal stem cells; ISCs, intestinal stem cells; HSCs, hematopoietic stem cells; NSCs, neural stem cells; BMSCs, Bone marrow stem cells; OPCs, oligodendrocyte precursor cells; T2DM, type II diabetics mellitus; APP, amyloid precursor protein; HFD, high-fat-diet.
Deregulated nutrient sensing
Aging causes multiple alterations in biological processes; among them, nutrient-sensing signaling pathways have captured much interest because they are deregulated during aging [17]. The mechanistic target of rapamycin complex (mTORC) and insulin and the insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway have prominent aging-modulating effects that act as the major nutrient-sensitive regulator in response to changes in nutrients or growth factors [18]. Evidence has accumulated that inhibition of the mTOR or IIS pathway extends the median and maximal lifespans in yeast [19], C. elegans[20], D. melanogaster [21], and mice [22-24]. The most efficient measure to extend lifespan, calorie restriction (CR), has been successfully tested in diverse eukaryotic species and has been demonstrated to be associated with reduced IIS and TOR activity [25]. Other nutrient sensors, such as AMP-activated protein kinase (AMPK) and sirtuin signaling, tend to be deregulated with aging. AMPK, a key regulator of cellular energy, is activated in C. elegans during nutrient-deprivationor low-energetic conditions and extends the lifespan [26,27], Drosophila [28] and mammals [29] by inhibiting TORC1 activity, activating autophagy, and improving cellular stress resistance. Sirtuins, a class of histone deacetylases involved in lifespan regulation, protect against the aging process by enhancing cellular stress resistance [30]. NAD+-dependent deacetylase sirtuin 1 (SIRT1) could also induce autophagy by directly deacetylating AuTophaGy (ATG) proteins, alleviating many age-related diseases and improving the healthspan [31].
Studies have shown that metformin interacts with several known nutrient-sensing pathways associated with aging, with effects resembling those of CR, including postponing aging, extending lifespan and decreasing the incidence and progression of age-associated diseases [32]. Metformin has been shown to decrease insulin and IGF-1 levels and improve insulin sensitivity [33]. Metformin has been the preferred glucose-lowering and insulin-sensitizing pharmacological agent treatment for T2DM. Metformin also induced changes in the upper small intestinal microbiota, increased sodium glucose cotransporter-1 (SGLT1) expression and glucagon-like peptide 1 (GLP-1) secretion, restored the glucose-sensing pathway, and thus reversed glucose-sensing defects induced by a high-fatdiet (HFD) in the upper small intestine [34]. As an established AMPK activator, metformin inhibits complex I of the electron transport chain, leading to an increased ADP/ATP ratio and activated AMPK, which mediates longevity extension in C. elegans[35]. SIRT1 can be activated directly by metformin by increasing cellular NAD+ levels [36]. Metformin can also exert antiaging effects by inhibiting mTOR in AMPK-dependent and -independent manners [37,38]. Evidence is mounting that metformin can effectively improve nutrient-sensing pathways that postpone aging and decrease the risk for age-associated diseases.
DNA damage
Among the characteristics of aging, DNA damage has been considered a key factor contributing to aging. DNA damage can be caused by a variety of exogenous or endogenous agents, such as ultraviolet (UV) light, ionizing radiation (IR), genotoxic chemicals, and products of cellular metabolism. Multiple forms of DNA damage can accrue with age in humans, and persistent DNA damage can block transcription and replication, thus promoting cellular senescence and apoptosis, which are cellular phenotypes associated with aging [39]. The DNA repair system and DNA damage responses (DDRs) are essential for preventing and monitoring DNA damage. Emerging evidence suggests that aberrant DDRs and deficient DNA repair are closely associated with aging[40]. Aging and age-related diseases are caused in part by the accumulation of DNA damage, which is probably due to declines in DNA repair capacity, as well as ongoing DNA damage induction [41]. Several premature aging syndromes have underlying genetic DNA repair defects, such as Werner syndrome, in which patients have defects in base excision repair (BER) [40], and Cockayne syndrome and Xeroderma pigmentosum, in which patients have deficiencies in nucleotide excision repair (NER) [42]. Moreover, insufficient DNA repair also plays a role in Alzheimer’s disease (AD) and Parkinson’s disease (PD) [43]. This evidence strongly supports the connection between aging and DNA damage.
Metformin has shown effectiveness in preventing DNA damage and promoting DNA damage repair. Metformin scavenges free radicals, prevents damage to nuclear and mitochondrial DNA, and enhances DNA repair. The pretreatment of cells with metformin followed by culture in the presence of metformin and insulin prevented insulin-induced DNA damage [44]. In a total-body irradiation (TBI) mouse model, metformin significantly inhibited TBI-induced increases in ROS production and DNA double-strand breaks (DSBs) in bone marrow hematopoietic stem cells (HSCs) [45]. Metformin treatment reduced DNA damage and ameliorated spontaneous chromosome breakage in human Fanconi anemia patient-derived cells [46]. Su et al.validated the effectiveness of metformin in reducing the risk of aging by protecting against UV-induced DNA damage [47]. Moreover, metformin may stimulate DNA damage responses, facilitating DNA damage repair and reducing genomic instability. X-ray repair cross complementing 1 (XRCC1) is the major protein in the DNA BER system that is significantly reduced in patients with diabetes, whereas XRCC1 is significantly increased in diabetic patients treated with metformin [48]. Metformin-treated diabetic patients may have higher BER activity than others. Metformin increased the expression of xeroderma pigmentosum C (XPC), a key protein required for NER, and promoted UVB-induced DNA damage repair [49]. In addition, metformin inhibits mitochondrial complex I-mediated oxidative phosphorylation, decreasing cellular ATP levels and thereby activating AMPK, which then activates p53-mediated DNA repair [48]. These results suggest that metformin can prevent DNA damage and promote DNA damage repair, which provides a novel mechanism to explain the antiaging effects of metformin.
Reactive oxygen species (ROS)
The free radical theory of aging proposes that ROS are a major determinant of aging [50]. ROS accumulation can reduce lifespan by harming cellular organelles and macromolecules [51]. High levels of mitochondrial ROS contribute to aging in genetically modified animals; for example, superoxide dismutase-deficient animals showed typical characteristics of premature aging [52,53]. Increased oxidative stress has been implicated in a variety of age-related pathologies. Several studies have shown that oxidative stress is primarily responsible for the loss of self-renewal and premature exhaustion of HSCs in mice with mutations associated with ataxia telangiectasia; however, prolonged treatment of these mice with an antioxidant could rescue this defect [54]. Under normal circumstances, ROS production and clearance are in a delicate dynamic balance, and the antioxidant system plays an important role in this balance [55]. In aging, oxidant production is increased, while antioxidant enzymes are decreased. Studies have shown that mice overexpressing mitochondrial catalase counteract oxidative damage and live longer [56]. Low ROS levels improve defense mechanisms, which contribute to stress resistance and longevity, while high ROS levels may contribute to aging onset and progression [57]. Therefore, interventions that reduce higher concentrations of ROS under pathological conditions by both scavenging free radicals and enhancing antioxidant defenses are proposed as antiaging strategies.
Metformin decreases ROS production by directly acting on mitochondrial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and class 1 respiratory chain enzymes and can be considered an antioxidant agent [58]. Metformin interferes with mitochondria and reduces ATP production, leading to the activation of AMPK [59], which has been shown to reduce ROS levels, increase the expression of the antioxidant thioredoxin and induce the antioxidant status of vascular endothelial cells [60]. Studies have also shown that metformin attenuates paraquat-induced elevations in ROS in an AMPK-independent manner [61]. In addition, metformin may also reduce ROS levels by increasing the activities of antioxidant enzymes. The administration of metformin to rats before cisplatin, CCl4or doxorubicin treatment restored the activities of GSH, CAT, and SOD [62-64]; metformin inhibited TBI-induced chronic oxidative stress in HSCs by upregulating the expression of the antioxidant enzymes SOD,CAT, and GPX1 [45]. These studies show that metformin reduces ROS levels by inhibiting mitochondrial respiratory complex I or enhancing antioxidant defenses, which may suppress aging onset and progression.
Telomere attrition
Telomeres act as caps at the ends of eukaryotic chromosomes to protect genetic information from damage caused by the inability of DNA polymerase to completely replicate chromosome ends [65]. Telomere length is an important predictor of species lifespans [66]. Longer telomeres predict less early disease and longer lifespans [67]. Telomerase adds repetitive telomeric DNA sequences at the ends of chromosomes to repair telomeres after cell division [68]. In humans, a lack of telomerase causes a variety of age-related pathologies, including liver disease, pulmonary fibrosis, premature gray hair, and aplastic anemia [69]. In mice, telomerase deficiency is associated with a shorter lifespan and early onset of aging phenotypes, and telomerase overexpression has been demonstrated to increase healthspan and longevity and reverse age-related pathology [70]. These results indicate that the recovery of telomeres is crucial to improving health and even increasing longevity.
Metformin treatment has a beneficial effect against telomere attrition. In mothers with gestational diabetes, metformin treatment prevented telomere attrition in their male offspring [71]. Chronic metformin administration delays endothelial cell senescence by increasing hTERT (telomerase reverse transcriptase, the rate-limiting factor in the formation of functional telomerase) expression and activity, thereby attenuating vascular aging and protecting organisms against age-associated atherosclerosis [72]. HTERT protein and its activity were significantly increased, and all senescence markers were reduced in human aortic endothelial cells (HAECs) that were grown in the presence of metformin compared to untreated cells [72]. However, some studies have reported that treatment with metformin inhibited telomerase activity by decreasing hTERT mRNA levels in endometrial cancer cell lines compared to the control [73]. Further studies are needed to understand the effects of metformin on telomeres in aging and cancer.
Inflammation
Chronic low-grade inflammation (also termed inflammaging) has been shown to contribute to most diseases that are typical of old age, such as AD, atherosclerosis, cancer, sarcopenia and diabetes [74]. Healthy aging in centenarians correlates with low levels of inflammatory markers [75]. Proinflammatory cytokines typically increase with aging; for example, IL-6 is a hallmark of many chronic inflammatory states in humans and is associated with slower walking speed, impaired muscle strength and sarcopenia [76,77]. In mouse models of progeria, the activation of nuclear factor-kappa B (NF-κB) upregulates the expression of proinflammatory cytokines, while the inhibition of NF-κB prevents age-associated dysfunction [78]. In the fly intestine, preventing activation of proinflammatory NF-κB signaling enhances regenerative homeostasis and extends lifespan [79,80]. These studies suggest that inflammaging is a possible mechanism underlying the aging process [81].
Accumulating evidence suggests that metformin can increase healthspan and lifespan through anti-inflammatory effects [9,82]. Metformin reduces the risk and progression of inflammaging by significantly reducing biomarkers of inflammation. In human studies, metformin was shown to reduce inflammation by decreasing the expression of multiple proinflammatory cytokines (Il-6, IL-1β, TNF-α, MMP-2, MMP-8, AGEs, NF-κB, STAT3, Saa1 and Saa2) and increasing the levels of IL-10 and adiponectin [83,84]. Metformin significantly reduced proinflammatory markers (IL-6, TNF-α, CRP) in the serum of type 2 diabetic patients and suppressed NF-κB levels in the context of diabetes and intestinal inflammation through AMPK-independent and -dependent processes [85,86]. Some findings from experimental models also demonstrated that metformin exerts an anti-inflammatory effect. Metformin administration ameliorates the inflammatory response associated with diabetic nephropathy in type 2 diabetic mice by reducing the increases in NF-κB and serum levels of proinflammatory cytokines (IL-1β and TNF-α) [87]. In HFD-fed rats, metformin decreased the levels of TNF-α, MCP-1, IL-1β and leptin in the intestine (colon), adipose tissue and serum [88]. The anti-inflammatory effect of metformin has also been observed in models of systemic lupus erythematosus (SLE), experimental autoimmune arthritis (EAE), colitis and obesity [89]. A recent paper by Bharath et al. revealed that metformin could reduce proinflammatory cytokines by reducing M1 macrophages and improving macrophage polarization to an anti-inflammatory M2 phenotype [90]. This evidence suggests a promising role of metformin in targeting age-related chronic inflammation via its pleiotropic effects on inflammatory responses.
Autophagy
Autophagy is a catabolic recycling system that degrades and recycles dysfunctional or unnecessary cellular organelles and molecules to sustain cellular functions. Autophagy and aging have an interconnected relationship. Rubinsztein et al. suggested a causal link between aging and autophagy involving a vicious cycle in which aging causes a decline in autophagy that then exacerbates aging [91]. Studies have also shown that autophagy is essential for longevity and healthspan, and the suppression of autophagy accelerates aging and shortens lifespan [92]. Another type of autophagy that specifically clears damaged mitochondria called mitophagy is also critical for the prevention of aging [93]. Bharath et al. reported that CD4+T lymphocytes from elderly individuals exhibit defective mitophagy, which may contribute to aging-associated chronic inflammation, and enhancing mitophagy may ameliorate inflammaging to prolong the health span [90]. The loss of autophagy in aged muscle stem cells (MuSCs) leads to a decline in their function and number, and MuSC aging can be reversed by the re-establishment of autophagy [94]. Animal experiments (yeast, C. elegans, Drosophila and mice) demonstrated that the inactivation of or reductions in key autophagy regulators such as Atg1, Atg7, Atg8, Atg11, or Beclin1 accelerated aging and reduced lifespan, while the overexpression of Atg5 or Atg12 delayed aging and extended lifespan [95].
The proautophagic activity of metformin is mainly mediated by the activation of AMPK, which is inactivated in senescent cells and activated by metformin, significantly preventing the development of senescence [95]. Furthermore, metformin alleviated oxidative stress-induced senescence in human periodontal ligament cells (hPDLCs)by inducing autophagy, reversed autophagy defects in CD4+ T cells from older subjects and rejuvenated cell functions [90,96]. In a Clk1-mutant PD mouse model, metformin treatment reduced α-synuclein accumulation by enhancing LC3-II-mediated autophagy in dopaminergic neurons and the midbrain [97]. In addition, metformin enhanced mitophagy by upregulating the transcriptional expression of mitophagy-related genes, resulting in the rapid degradation of damaged mitochondria, thereby delaying aging, and improving cardiovascular function in aging [98]. Metformin’s potential in the treatment of neurodegenerative and other aging-related diseases has also been recognized. The neuroprotective effects of metformin mainly involve activating autophagy, which seems to be defective in neurodegenerative disorders [99]. In conclusion, these results indicate that autophagy is a novel therapeutic target for metformin, which may delay the progression of disease, extend lifespan and improve healthspan.
Cellular senescence
Cellular senescence is considered an aging hallmark based on two factors: (1) senescent cells accumulate in organismal tissues, which parallel age advancement; and (2) senescent cells accelerate the age-related decrease in tissue regeneration [16]. The accumulation of senescent cells in various tissues is thought to be a major factor contributing to aging and age-related diseases [100]. Senescent cells impact neighboring cells and drive systemic aging through the senescence-associated secretory phenotype (SASP) [101]. Transplanting senescent cells into young mice is sufficient to cause physical dysfunction and spread cellular senescence in mouse tissues [102]. Even the functional decline that occurs during normal aging could be slowed by eliminating senescent cells, such as through the use of Bcl-XL inhibitors that can selectively eliminate senescent cells and slow the aging process in the organism [103]. The role of senescent cells in driving aging was also demonstrated by the clearance of senescent cells from progeroid mice carrying a p16-INK-ATTAC transgene in which a suicide gene was expressed specifically in p16INK4a-positive senescent cells [104]. These results suggested that inhibiting/eliminating senescent cells or suppressing the SASP could reverse aging.
Metformin prevented cigarette smoke extract (CSE)-induced human bronchial epithelial cell senescence by decreasing DEP domain-containing mTOR-interacting protein (DEPTOR) expression [105]. Pretreatment of myoblasts with metformin could attenuate ceramide-induced cellular senescence by limiting increases in p53 and p21 protein levels [106]. Low-dose metformin treatment delayed senescence in human diploid fibroblasts and mesenchymal stem cells by upregulating endoplasmic reticulum-localized glutathione peroxidase 7 (GPx7) [107]. Studies have also shown that metformin attenuates stress-induced senescence in C2C12 myoblasts[106], endothelial cells [108], periodontal ligament cells (PDLCs) [96], nucleus pulposus cells[109] and mouse olfactory ensheathing cells [110]. In addition, metformin could trigger immune-mediated clearance of senescent cells and inhibit the expression of genes coding for multiple inflammatory cytokines during cellular senescence [111]. Metformin treatment could reduce the expression of senescence-related proteins, including p21 and p16, and the abundance of inflammatory cytokines, chemokines and oncogenes that are hallmarks of SASP [112]. The tissue sweeper function of metformin may enhance the clearance of senescent cells and halt the progression of aging.
Stem cell depletion/exhaustion
Stem cell decline is a prominent, well-established causative factor for aging. The lifelong persistence of stem cells in tissues leads to cellular damage, which may lead to cellular senescence, decreased self-renewal and regenerative capacity, and eventually functional depletion. Moreover, a loss of stem cell numbers and/or function is closely associated with tissue degeneration and homeostasis declines and ultimately drives aging and age-related diseases [113]. During aging, tissues exhibit decreases in the numbers of stem cells, as is commonly seen in skeletal muscle [94] and the nervous system [114]. However, hematopoietic stem cells (HSCs) and intestinal stem cells (ISCs) can maintain their populations or even increase in number with aging but are concomitant with a decline in function and activity [115,116]. The decline in stem cell activity and number with aging results in muscle weakness, hair graying or loss, slower wound healing and decreased immunity [117]. Given the importance of stem cells during the organismal lifetime, the suppression of senescence or restoration of stem cell functions may be a promising therapeutic strategy for geriatric diseases.
There is evidence to suggest that metformin has a beneficial effect on stem cells. Metformin administration decreased stem cell senescence in bone marrow [118] and enhanced stem cell function in the context of injury [86]. Metformin promoted the proliferation of endogenous neural stem cells, increased the total number of neural precursor cells, and improved sensory-motor function after brain injury in mice [119]. Similarly, metformin could target satellite cells to prevent their dysregulation after severe burn injury and increase the proliferation and total number of satellite cells in skeletal muscle[86]. Importantly, metformin restored the function of senescent stem cells. Metformin improved mitochondrial function in aged oligodendrocyte precursor cells (OPCs), contributing to the restoration of differentiation capacity and functional rejuvenation [120]. The effect of metformin on stem cells is complicated, and different types of stem cells may respond differently to metformin, which requires further study.
The aging process is complex, and the interplay of the above hallmarks of aging leads to aging on an organism level. Although the contribution of each of these hallmarks to the progression of biological aging is not fully elucidated, targets that can ameliorate several of these hallmarks can protect against age-related functional declines. Metformin ameliorates the hallmarks of aging through multiple pathways (Fig. 1).Table 1 shows that metformin has a positive impact on the hallmarks of aging. Our understanding of the hallmarks of biological aging and metformin's effects on each hallmark of aging will be helpful for preventing or delaying the progression of aging.
Figure 1.
Metformin counters aging hallmarksthrough different pathways.
Effects of metformin on age-related diseases
Aging is an irreversible biological process that is the driving force behind all age-related diseases. Thus, delaying aging slows the onset and development of various age-related diseases and extends lifespan. Metformin exerts antiaging effects by intervening in nutrient sensing, DNA damage, the accumulation of ROS, telomere attrition, inflammation, cellular senescence, stem cell depletion and autophagy and can then be used to prevent aging-related diseases. Over the years, metformin has been shown to exert positive pleiotropic effects on human health. In addition to its ability to improve hyperglycemia, metformin treatment may reduce the risk of many other aging-related diseases, such as cancer, CVD and neurodegeneration [121].
Metformin and cancer
Metformin initially entered the spotlight as an anticancer agent due to its effects of reducing cancer risk and mortality in patients with diabetes [122]. Since then, several studies have also shown that metformin has utility in cancer prevention and/or treatment [123,124]. The anticancer effects of metformin are realized in several ways. First, metformin reduces insulin and IGF-1 levels [125], both of which can promote tumorigenesis by stimulating the proliferation of epithelial cells. Second, it is widely known that metformin activates AMPK and represses mTOR signaling pathways, leading to cell cycle arrest and apoptosis and inhibiting cancer cell growth and proliferation [126-128]. Third, metformin treatment can impede tumor cell escape from immunosurveillance and enhance the clearance of premalignant/tumor senescent cells (including those with the ability to initiate tumors), thus significantly decreasing the accumulation and malignant/metastatic progression of dysfunctional/ premalignant cells [129]. Fourth, metformin may have a role in inhibiting the self-renewal, proliferation and metastatic ability of cancer stem cells (CSCs)by targeting specific pathways and/or regulatory genes [130]. Metformin can inhibit the proliferation, invasion, and survival and induce apoptosis of ovarian, pancreatic, breast, and colonic CSCs and prevent cancer recurrence and spread [131-134]. Fifth, metformin has been reported to inhibit the activity of the transcription factor NF-κB, leading to reduced secretion of proinflammatory cytokinesthat may have tumorigenic effects [135].
However, some studies have reported inconsistent or conflicting data on metformin's anticancer effects. These studies found that metformin treatment had no association with reduced cancer risk. The reasons for this may be due to diverse analysis methodologies, heterogeneity of studies, different types of cancer or dosage and age at onset of treatment with metformin [136,137]. Although these studies reported inconsistent data, the vast majority of the data support the potential of metformin in decreasing the risk of cancers. We await the results over the coming years to establish a beneficial role of metformin in cancer development.
Metformin and neurodegenerative diseases
Aging is the greatest risk factor for neurodegenerative disease, and therefore, delayed aging may be an effective way to reduce the occurrence and progression of neurodegenerative disease. The main hallmarks of aging in one way or another are associated with the pathogenesis of neurodegenerative diseases.
Neuroinflammation is considered a major driving force in the progression of neurodegenerative diseases. Substantial evidence suggests that neuroinflammation mediated by microglia plays a pivotal role in PD pathogenesis [138]. Neuroinflammation also contributes to AD pathophysiology, manifested as glial activation and proinflammatory cytokinesecretion, which may lead to neuronal damage [139]. Metformin has certain anti-inflammatory and immunological inhibitory effects. In APP/PS1 mice, metformin exerts its neuroprotective effects by attenuating microglia and astrocyte activation, suppressing inflammatory cytokine secretion and activation and decreasing the expression of proinflammatory factors from activated glia [140]. Additionally, metformin increased microglial phagocytosis capacity and skewed the microglia toward the M2 phenotype [141]. In PD mouse models, metformin reduced the levels of microglia and the proinflammatory cytokines IL-6, TNFα, Il-1β, and iNOS in the substantia nigra pars compacta [97].
Failure of autophagy leads to the accumulation of misfolded/aggregated proteins and damaged mitochondria, which are associated with the pathogenesis and progression of PD and AD [142-144]. The defining pathological hallmarks of AD are the accumulation of neurofibrillary tangles (NFTs, composed of hyperphosphorylated tau protein) and amyloid plaques (composed of extracellular aggregates of amyloid-β) in the brain [145]. SNCA aggregation and mitochondrial abnormalities are key features of PD progression and are associated with impaired degradation mechanisms attributed to deficient autophagy [146]. Several studies have shown that the neuroprotective effect of metformin is associated with autophagic induction. Metformin has been shown to prevent SNCA phosphorylation and aggregation and promote damaged mitochondrial clearance through enhanced mitophagy via AMPK activation [147]. In MTPP/p-induced experimental models of PD, metformin prevented substantia nigra (SN) dopaminergic neuronal death by AMPK-dependent autophagy induction [97]. Similar neuroprotective effects have also been reported in experimental models of AD. In APP/PS1 mice, metformin treatment activates AMPK and reduces the activity of mTOR signaling, thereby facilitating autophagy and promoting lysosomal degradation of Aβ [148], eventually significantly reducing Aβ levels in the brain.
Neuronal death is considered to be the main contributor to cognitive decline in AD [149]. PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta [150]. Recent evidence shows that AMPK activation by metformin contributes to the prevention of substantia nigra dopaminergic neuronal death [97]. In animal models of PD, metformin prevents MPTP-induced dopaminergic neuron degeneration and death via autophagy induction and ROS clearance [97]. In APP/PS1 mice, metformin promoted the repair of damaged neurons and decreased neuronal death and amyloid plaque load [151]. Similarly, cell studies have demonstrated that metformin protects PC12 cells and hippocampal neurons from H2O2-induced oxidative damage by activating the AMPK pathway and attenuates Cd-induced PC12 cells, SH-SY5Y cells and primary neuron death by suppressing intracellular abnormal ROS accumulation [152].
These results suggest that metformin can exert a significant neuroprotective role by targeting the hallmarks of aging. Metformin may be a promising therapeutic strategy against neurodegenerative diseases.
Metformin and cardiovascular disease
CVD is the leading cause of death in the elderly. The rise in the incidence of age-related CVDs poses a serious threat to human health and longevity. Aging often serves as an independent risk factor for CVD, leading to progressive structural and functional deterioration of the heart and vasculature mainly manifested as endothelial defects, cardiac and vascular remodeling and loss of vascular compliance [153], increasing the susceptibility of individuals to atherosclerosis, stroke, coronary artery disease, hypertension, heart failure, and atrial fibrillation.
The potential cardiovascular protective effects of metformin are also an active area of research, which reduces the incidence and all-cause mortality of cardiovascular events [154]. Metformin may reduce the risk of CVD by reducing cholesterol levels, blood pressure, body weight, and the progression of atherosclerosis [155]. Animal studies have shown that metformin delays vascular aging and protects against age-associated atherosclerosis in ApoE-/- mice [72]. Metformin has strong protective effects against CVD by improving lipometabolism, reducing the level of LDL cholesterol, and attenuating cardiomyocyte contractile defects by activating AMPK [156]. Metformin similarly reduces the risk of atherosclerosis in diabetic and nondiabetic individuals by improving endothelial cell function and reducing the inflammatory response, which participates in the formation of atherosclerotic thrombi [157]. Metformin exerts a direct vascular anti-inflammatory effect by inhibiting NF-kB, which activates multiple pro-inflammatory factors, including the pro-atherogenic cytokines IL-6 and IL-8 [158]. Metformin alleviates hyperglycemia-induced endothelial impairment and suppresses myocardial apoptosis and inflammation by downregulating autophagy [159,160]. However, other studies report paradoxical data about the regulation of autophagy by metformin. These studies found that metformin provides cardioprotection in δ-sarcoglycan deficiency-induced dilated cardiomyopathy by enhancing autophagy [161]. Therefore, it is still necessary to clarify additional mechanisms of the cardiovascular protective effects mediated by autophagy. The overall evidence from both clinical trials and experimental animal studies supports the benefit of metformin in CVD.
Effects of metformin on healthspan and lifespan
Worldwide, the proportion of older people is rapidly increasing. Modern medicine has proven that aging is the main risk factor for adult diseases [162]. Therefore, the health issues of older individuals are increasingly important. Standard medical treatments are initiated when overt diseases are diagnosed, which extends the “unhealthy” phase of life by preventing death from these diseases (From Fig. 2A to 2B). This strategy led us to the awkward position of longevity with poor health. A significant number of individuals will rely on drugs through the latter half of life. Therefore, extending the healthspan has been gaining attention in recent years. To date, aging is unavoidable, but the outlook on delaying the aging process and disease onset to extend healthspan is optimistic. Given the effects of metformin in attenuating the hallmarks of aging and preventing age-related disease, metformin has shown much promise as a potential antiaging drug. Diet with metformin at the stage of prediseases not only extends lifespan but also the ‘healthspan’, which is the period in which people remain disease-free and vigorous [13,163] (From Fig. 2B to 2C).
Figure 2.
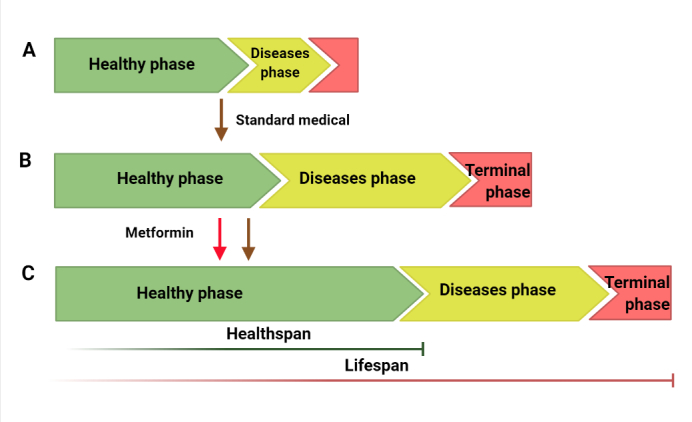
Effects of metformin on healthspan and lifespan. Standard medicineextends the “unhealthy” phase of life (from A to B). Diet with metformin at thepreddisease stage extends lifespan and healthspan (From A to C). Healthy phase, before overt diseases become apparent; disease phase, characterized by symptoms of overt diseases; terminal phase, characterized by organ damage, failure and loss of functions.
A number of studies support the role of metformin in extending healthspan and lifespan in different animal models, such as worms and rodents [164]. In rodents, metformin treatment reduces chronic inflammation and the accumulation of oxidative damage, induces autophagy, delays the onset of carcinoma, inhibits the growth of tumors, and improves cardiovascular health, all of which can contribute to delaying aging and extending healthspan [165,166]. In nematodes, metformin treatment extended the mean lifespan, reduced the accumulation of lipofuscin and prolonged the healthspan through AMPK [35]. In addition, the effects of metformin on lifespan and health have been demonstrated in humans. T2DM patients taking metformin had significantly lower all-cause mortality than nondiabetic individuals. A meta-analysis by Campbell et al. included 53 studies showing that T2DM patients taking metformin have less CVD and cancer than other diabetic patients and lower mortality than nondiabetic patients as well as other diabetic patients receiving nonmetformin therapies, such as insulin and sulfonylurea [167]. A recent randomized comparative study showed that combined with metformin, both liraglutide and sitagliptin, reduced body weight, intrahepatic lipids, and visceral adipose tissue in addition to improving glycemic control and thus significantly reduced the risks of nonalcoholic fatty liver disease and cardiovascular events in patients with T2DM [168]. Metformin administration has also been shown to be beneficial in improving lipid metabolism, including decreasing plasma triglyceride (TG) and low-density lipoprotein (LDL) cholesterol levels, and reduces mortality and morbidity in diabetic patients with heart failure and atherothrombosis compared to sulfonylurea [169]. However, metformin’s effects are not consistent. First, different initiation times of metformin treatment have different effects on lifespan. Diet with metformin that begins early in life leads to a healthier and longer lifespan in mice, but initiation at older ages shows reduced efficacy [15]. The effect of metformin on lifespan and health was attenuated with increased age at initiation. Second, different doses of metformin can produce different results. Metformin exhibited a typical dose response in some animal studies. For example, treatment with a low dose of metformin starting at middle age extended the healthspan and lifespan of mice and nematodes, while a high dose caused toxic effects and significantly shortened the lifespan [15]. Third, the effect of metformin varied according to sex and species. Metformin extended the mean lifespan of male silkworms without a significant effect on females [170] and prolonged the lifespan of female mice but not males[171]. Moreover, not all studies have shown similar effects of metformin on lifespan or healthspan. Some studies have shown that metformin treatment at a low dose did not increase lifespan in flies, and there was a significant decrease in survival in response to a high dose [172]. In conclusion, the effects of metformin on healthspan and lifespan vary depending on the species and sex of the animals, as well as dose and initiation time.
The mechanisms by which metformin extends healthspan and lifespan have not yet been fully elucidated. One well-accepted mechanism is that metformin can mimic the effects of dietary restriction by stimulating AMPK to reduce energy-consuming processes [107]. Recent studies also indicated that metformin indirectly extends lifespan by altering the gut microbiome, especially by changing microbial folate and methionine metabolism [173,174]. To date, there has been no evidence of such effects in humans. Further work is needed to determine the mechanism by which metformin prolongs healthspan and lifespan and whether metformin leads to improvements in healthspan and lifespan in humans.
Conclusions and perspectives
The present review demonstrates that metformin ameliorates some of the hallmarks of aging, delays or prevents age-associated diseases, and extends lifespan and healthspan. Considering these promising findings and the safety and long-term use of metformin in humans, metformin is currently considered a hopeful candidate drug against aging and age-associated diseases. However, the application of metformin as an antiaging drug in nondiabetic individuals is still at a very early stage, and several questions have to be clarified. 1) We need to develop a standard score for aging. This score could be used by researchers to assess the effect of interventions that modulate human aging. 2) Metformin’s antiaging efficacy in nondiabetic patients needs to be verified. The ongoing metformin trials are almost all based on observations of potential benefits in diabetic populations. It remains unknown whether nondiabetic individuals taking metformin will live longer and healthier without side effects. 3)The doses and initiation time of metformin treatment for antiaging in humans need to be determined. 4) The mechanisms underlying metformin's protective effects against aging remain to be explained. It is unclear whether metformin protects against aging by targeting multiple aging pathways downstream or by direct effects on multiple aging regulators. 5) It is necessary to highlight the potential side effects of long-term metformin use, such as lactic acidosis and vitamin B12 deficiency, although these side effects are very rare. Therefore, further studies are still required to firmly establish a beneficial role of metformin in healthy individuals. Two large clinical trials, ‘TAME’ and ‘Metformin in Longevity Study (MILES)’, have been initiated in the United States and aim to assess the usefulness of metformin in nondiabetic patients to determine whether metformin can delay aging and improve human health span. Metformin can be used to delay aging and extend human healthspan and lifespan, and the future is promising.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (81800792, 81671619, 81771226, U1804186) and the Key Scientific Research Projects of Institutions of Higher Learning in Henan Province (19B180009).
Footnotes
Conflicts of interest
The authors disclose no potential conflicts of interest.
References
- [1].Rudnicka E, Napierala P, Podfigurna A, Meczekalski B, Smolarczyk R, Grymowicz M (2020). The World Health Organization (WHO) approach to healthy ageing. Maturitas, 139:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. (2010). Aging, frailty and age-related diseases. Biogerontology, 11:547-563. [DOI] [PubMed] [Google Scholar]
- [3].Niccoli T, Partridge L (2012). Ageing as a risk factor for disease. Curr Biol, 22:R741-752. [DOI] [PubMed] [Google Scholar]
- [4].de Yebenes VG, Briones AM, Martos-Folgado I, Mur SM, Oller J, Bilal F, et al. (2020). Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler Thromb Vasc Biol, 40:2408-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li Z, Zhang Z, Ren Y, Wang Y, Fang J, Yue H, et al. (2021). Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology, 22:165-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marshall SM (2017). 60 years of metformin use: a glance at the past and a look to the future. Diabetologia, 60:1561-1565. [DOI] [PubMed] [Google Scholar]
- [7].Adak T, Samadi A, Unal AZ, Sabuncuoglu S (2018). A reappraisal on metformin. Regul Toxicol Pharmacol, 92:324-332. [DOI] [PubMed] [Google Scholar]
- [8].Song R (2016). Mechanism of Metformin: A Tale of Two Sites. Diabetes Care, 39:187-189. [DOI] [PubMed] [Google Scholar]
- [9].Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016). Metformin as a Tool to Target Aging. Cell Metab, 23:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo Y, Shi J, Wang Q, Hong L, Chen M, Liu S, et al. (2021). Metformin alleviates allergic airway inflammation and increases Treg cells in obese asthma. J Cell Mol Med, 25:2279-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C (2021). The effects of metformin on autophagy. Biomed Pharmacother, 137:111286. [DOI] [PubMed] [Google Scholar]
- [12].Sunjaya AP, Sunjaya AF (2021). Targeting ageing and preventing organ degeneration with metformin. Diabetes Metab, 47:101203. [DOI] [PubMed] [Google Scholar]
- [13].Blitzer AL, Ham SA, Colby KA, Skondra D (2021). Association of Metformin Use With Age-Related Macular Degeneration: A Case-Control Study. JAMA Ophthalmol, 139:302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, et al. (2016). An Ancient, Unified Mechanism for Metformin Growth Inhibition in C. elegans and Cancer. Cell, 167:1705-1718 e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. (2013). Metformin improves healthspan and lifespan in mice. Nat Commun, 4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Efeyan A, Comb WC, Sabatini DM (2015). Nutrient-sensing mechanisms and pathways. Nature, 517:302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim J, Guan KL (2019). mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol, 21:63-71. [DOI] [PubMed] [Google Scholar]
- [19].Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. (2005). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science, 310:1193-1196. [DOI] [PubMed] [Google Scholar]
- [20].Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F (2003). Genetics: influence of TOR kinase on lifespan in C. elegans. Nature, 426:620. [DOI] [PubMed] [Google Scholar]
- [21].Chang K, Kang P, Liu Y, Huang K, Miao T, Sagona AP, et al. (2020). TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy, 16:1807-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kapahi P, Kaeberlein M, Hansen M (2017). Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res Rev, 39:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S (2021). The role of curcumin in aging and senescence: Molecular mechanisms. Biomed Pharmacother, 134:111119. [DOI] [PubMed] [Google Scholar]
- [24].Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460:392-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fontana L, Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell, 161:106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu H, Ding J, Kohnlein K, Urban N, Ori A, Villavicencio-Lorini P, et al. (2020). The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy, 16:1618-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heintz C, Doktor TK, Lanjuin A, Escoubas C, Zhang Y, Weir HJ, et al. (2017). Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature, 541:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fan SZ, Lin CS, Wei YW, Yeh SR, Tsai YH, Lee AC, et al. (2021). Dietary citrate supplementation enhances longevity, metabolic health, and memory performance through promoting ketogenesis. Aging Cell, 10.1111/acel.1351010.1111/acel.13510:e13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dorigatti JD, Thyne KM, Ginsburg BC, Salmon AB (2021). Beta-guanidinopropionic acid has age-specific effects on markers of health and function in mice. Geroscience, 43:1497-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen C, Zhou M, Ge Y, Wang X (2020). SIRT1 and aging related signaling pathways. Mech Ageing Dev, 187:111215. [DOI] [PubMed] [Google Scholar]
- [31].Martinez-Chacon G, Paredes-Barquero M, Yakhine-Diop SMS, Uribe-Carretero E, Bargiela A, Sabater-Arcis M, et al. (2021). Neuroprotective properties of queen bee acid by autophagy induction. Cell Biol Toxicol, 10.1007/s10565-021-09625-w10.1007/s10565-021-09625-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kulkarni AS, Gubbi S, Barzilai N (2020). Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab, 32:15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xiao H, Huang X, Wang S, Liu Z, Dong R, Song D, et al. (2020). Metformin ameliorates bleomycin-induced pulmonary fibrosis in mice by suppressing IGF-1. Am J Transl Res, 12:940-949. [PMC free article] [PubMed] [Google Scholar]
- [34].Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, et al. (2018). Metformin Alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab, 27:101-117 e105. [DOI] [PubMed] [Google Scholar]
- [35].Onken B, Driscoll M (2010). Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One, 5:e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458:1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guo L, Cui J, Wang H, Medina R, Zhang S, Zhang X, et al. (2021). Metformin enhances anti-cancer effects of cisplatin in meningioma through AMPK-mTOR signaling pathways. Mol Ther Oncolytics, 20:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, et al. (2010). Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab, 11:390-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ou HL, Schumacher B (2018). DNA damage responses and p53 in the aging process. Blood, 131:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pan MR, Li K, Lin SY, Hung WC (2016). Connecting the Dots: From DNA Damage and Repair to Aging. Int J Mol Sci, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang H, Lautrup S, Caponio D, Zhang J, Fang EF (2021). DNA Damage-Induced Neurodegeneration in Accelerated Ageing and Alzheimer's Disease. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kajitani GS, Nascimento LLS, Neves MRC, Leandro GDS, Garcia CCM, Menck CFM (2021). Transcription blockage by DNA damage in nucleotide excision repair-related neurological dysfunctions. Semin Cell Dev Biol, 114:20-35. [DOI] [PubMed] [Google Scholar]
- [43].Setayesh T, Misik M, Langie SAS, Godschalk R, Waldherr M, Bauer T, et al. (2019). Impact of Weight Loss Strategies on Obesity-Induced DNA Damage. Mol Nutr Food Res, 63:e1900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Othman EM, Oli RG, Arias-Loza PA, Kreissl MC, Stopper H (2016). Metformin Protects Kidney Cells From Insulin-Mediated Genotoxicity In Vitro and in Male Zucker Diabetic Fatty Rats. Endocrinology, 157:548-559. [DOI] [PubMed] [Google Scholar]
- [45].Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y, et al. (2015). Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med, 87:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang QS, Tang W, Deater M, Phan N, Marcogliese AN, Li H, et al. (2016). Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood, 128:2774-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Su WH, Chan CET, Lian T, Biju M, Miura A, Alkhafaji SA, et al. (2021). Protection of nuclear DNA by lifespan-extending compounds in the yeast Saccharomyces cerevisiae. Mutat Res, 822:111738. [DOI] [PubMed] [Google Scholar]
- [48].Dogan Turacli I, Candar T, Yuksel EB, Kalay S, Oguz AK, Demirtas S (2018). Potential effects of metformin in DNA BER system based on oxidative status in type 2 diabetes. Biochimie, 154:62-68. [DOI] [PubMed] [Google Scholar]
- [49].Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY (2013). Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene, 32:2682-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gough DR, Cotter TG (2011). Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis, 2:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mohammadzadeh A, Mirza-Aghazadeh-Attari M, Hallaj S, Saei AA, Alivand MR, Valizadeh A, et al. (2019). Crosstalk between P53 and DNA damage response in ageing. DNA Repair (Amst), 80:8-15. [DOI] [PubMed] [Google Scholar]
- [52].Kwon MJ, Lee KY, Lee HW, Kim JH, Kim TY (2015). SOD3 Variant, R213G, Altered SOD3 Function, Leading to ROS-Mediated Inflammation and Damage in Multiple Organs of Premature Aging Mice. Antioxid Redox Signal, 23:985-999. [DOI] [PubMed] [Google Scholar]
- [53].Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, et al. (2009). Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci, 64:1212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sillar JR, Germon ZP, DeIuliis GN, Dun MD (2019). The Role of Reactive Oxygen Species in Acute Myeloid Leukaemia. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Obrador E, Liu-Smith F, Dellinger RW, Salvador R, Meyskens FL, Estrela JM (2019). Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol Chem, 400:589-612. [DOI] [PubMed] [Google Scholar]
- [56].Dogar I, Dixon S, Gill R, Young A, Mallay S, Oldford C, et al. (2020). C57BL/6J mice upregulate catalase to maintain the hydrogen peroxide buffering capacity of liver mitochondria. Free Radic Biol Med, 146:59-69. [DOI] [PubMed] [Google Scholar]
- [57].Yan LJ (2014). Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol, 2:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ribeiro FM, Ratti BA, Dos Santos Rando F, Fernandez MA, Ueda-Nakamura T, de Oliveira Silva Lautenschlager S, et al. (2020). Metformin effect on driving cell survival pathway through inhibition of UVB-induced ROS formation in human keratinocytes. Mech Ageing Dev, 192:111387. [DOI] [PubMed] [Google Scholar]
- [59].Chauhan AS, Zhuang L, Gan B (2020). Spatial control of AMPK signaling at subcellular compartments. Crit Rev Biochem Mol Biol, 55:17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hwang HJ, Jung TW, Kim JW, Kim JA, Lee YB, Hong SH, et al. (2019). Protectin DX prevents H2O2-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell Signal, 53:14-21. [DOI] [PubMed] [Google Scholar]
- [61].Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. (2012). Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila), 5:536-543. [DOI] [PubMed] [Google Scholar]
- [62].Asensio-Lopez MC, Lax A, Pascual-Figal DA, Valdes M Sanchez-Mas J (2011). Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic Biol Med, 51:1861-1871. [DOI] [PubMed] [Google Scholar]
- [63].Dai J, Liu M, Ai Q, Lin L, Wu K, Deng X, et al. (2014). Involvement of catalase in the protective benefits of metformin in mice with oxidative liver injury. Chem Biol Interact, 216:34-42. [DOI] [PubMed] [Google Scholar]
- [64].Sahu BD, Kuncha M, Putcha UK, Sistla R (2013). Effect of metformin against cisplatin induced acute renal injury in rats: a biochemical and histoarchitectural evaluation. Exp Toxicol Pathol, 65:933-940. [DOI] [PubMed] [Google Scholar]
- [65].Smith EM, Pendlebury DF, Nandakumar J (2020). Structural biology of telomeres and telomerase. Cell Mol Life Sci, 77:61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Whittemore K, Vera E, Martinez-Nevado E, Sanpera C, Blasco MA (2019). Telomere shortening rate predicts species life span. Proc Natl Acad Sci U S A, 116:15122-15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Deems NP, Leuner B (2020). Pregnancy, postpartum and parity: Resilience and vulnerability in brain health and disease. Front Neuroendocrinol, 57:100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Blackburn EH (2001). Switching and signaling at the telomere. Cell, 106:661-673. [DOI] [PubMed] [Google Scholar]
- [69].Armanios M, Blackburn EH (2012). The telomere syndromes. Nat Rev Genet, 13:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hong J, Yun CO (2019). Telomere Gene Therapy: Polarizing Therapeutic Goals for Treatment of Various Diseases. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Garcia-Martin I, Penketh RJA, Janssen AB, Jones RE, Grimstead J, Baird DM, et al. (2018). Metformin and insulin treatment prevent placental telomere attrition in boys exposed to maternal diabetes. PLoS One, 13:e0208533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Karnewar S, Neeli PK, Panuganti D, Kotagiri S, Mallappa S, Jain N, et al. (2018). Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim Biophys Acta Mol Basis Dis, 1864:1115-1128. [DOI] [PubMed] [Google Scholar]
- [73].Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA Bae-Jump VL (2010). Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol, 116:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. (2003). Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation, 108:2317-2322. [DOI] [PubMed] [Google Scholar]
- [75].Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, et al. (2015). Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine, 2:1549-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Thomas R, Wang W, Su DM (2020). Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun Ageing, 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Troesch B, Eggersdorfer M, Laviano A, Rolland Y, Smith AD, Warnke I, et al. (2020). Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Soria-Valles C, Osorio FG, Gutierrez-Fernandez A, De Los Angeles A, Bueno C, Menendez P, et al. (2015). NF-kappaB activation impairs somatic cell reprogramming in ageing. Nat Cell Biol, 17:1004-1013. [DOI] [PubMed] [Google Scholar]
- [79].Guo L, Karpac J, Tran SL, Jasper H (2014). PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell, 156:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li H, Qi Y, Jasper H (2016). Preventing Age-Related Decline of Gut Compartmentalization Limits Microbiota Dysbiosis and Extends Lifespan. Cell Host Microbe, 19:240-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH (2020). Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford), 59:1426-1438. [DOI] [PubMed] [Google Scholar]
- [82].Hahad O, Frenis K, Kuntic M, Daiber A, Munzel T (2021). Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Han J, Li Y, Liu X, Zhou T, Sun H, Edwards P, et al. (2018). Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS One, 13:e0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sharma AK, Raikwar SK, Kurmi MK, Srinivasan BP (2013). Gemfibrozil and its combination with metformin on pleiotropic effect on IL-10 and adiponectin and anti-atherogenic treatment in insulin resistant type 2 diabetes mellitus rats. Inflammopharmacology, 21:137-145. [DOI] [PubMed] [Google Scholar]
- [85].Chen W, Liu X, Ye S (2016). Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J Inflamm (Lond), 13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yousuf Y, Datu A, Barnes B, Amini-Nik S, Jeschke MG (2020). Metformin alleviates muscle wasting post-thermal injury by increasing Pax7-positive muscle progenitor cells. Stem Cell Res Ther, 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Louro TM, Matafome PN, Nunes EC, da Cunha FX, Seica RM (2011). Insulin and metformin may prevent renal injury in young type 2 diabetic Goto-Kakizaki rats. Eur J Pharmacol, 653:89-94. [DOI] [PubMed] [Google Scholar]
- [88].Lehtonen S (2020). Metformin Protects against Podocyte Injury in Diabetic Kidney Disease. Pharmaceuticals (Basel), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Palsson-McDermott EM O'Neill LAJ (2020). Targeting immunometabolism as an anti-inflammatory strategy. Cell Res, 30:300-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, et al. (2020). Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab, 32:44-55.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rubinsztein DC, Marino G, Kroemer G (2011). Autophagy and aging. Cell, 146:682-695. [DOI] [PubMed] [Google Scholar]
- [92].Hansen M, Rubinsztein DC, Walker DW (2018). Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol, 19:579-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K (2021). Molecular mechanisms and physiological functions of mitophagy. EMBO J, 40:e104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S (2020). Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Margulis B, Tsimokha A, Zubova S, Guzhova I (2020). Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis In Aging Cells. Cells, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M, et al. (2020). Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology, 21:13-27. [DOI] [PubMed] [Google Scholar]
- [97].Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G (2016). Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson's Disease via Autophagy and Mitochondrial ROS Clearance. Int J Neuropsychopharmacol, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ma WQ, Sun XJ, Wang Y, Zhu Y, Han XQ, Liu NF (2019). Restoring mitochondrial biogenesis with metformin attenuates beta-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol Cell Endocrinol, 479:39-53. [DOI] [PubMed] [Google Scholar]
- [99].Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. (2008). Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci, 28:6926-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P (2019). Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput Struct Biotechnol J, 17:721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Amaya-Montoya M, Perez-Londono A, Guatibonza-Garcia V, Vargas-Villanueva A, Mendivil CO (2020). Cellular Senescence as a Therapeutic Target for Age-Related Diseases: A Review. Adv Ther, 37:1407-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. (2018). Senolytics improve physical function and increase lifespan in old age. Nat Med, 24:1246-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, et al. (2016). Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun, 7:11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL (2020). Reducing Senescent Cell Burden in Aging and Disease. Trends Mol Med, 26:630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Saito N, Araya J, Ito S, Tsubouchi K, Minagawa S, Hara H, et al. (2019). Involvement of Lamin B1 Reduction in Accelerated Cellular Senescence during Chronic Obstructive Pulmonary Disease Pathogenesis. J Immunol, 202:1428-1440. [DOI] [PubMed] [Google Scholar]
- [106].Jadhav KS, Dungan CM, Williamson DL (2013). Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech Ageing Dev, 134:548-559. [DOI] [PubMed] [Google Scholar]
- [107].Fang J, Yang J, Wu X, Zhang G, Li T, Wang X, et al. (2018). Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell, 17:e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hunt NJ, Lockwood GP, Kang SWS, Pulpitel T, Clark X, Mao H, et al. (2020). The Effects of Metformin on Age-Related Changes in the Liver Sinusoidal Endothelial Cell. J Gerontol A Biol Sci Med Sci, 75:278-285. [DOI] [PubMed] [Google Scholar]
- [109].Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y, et al. (2016). Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis, 7:e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Smieszek A, Strek Z, Kornicka K, Grzesiak J, Weiss C, Marycz K (2017). Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels-An Ex Vivo Study. Int J Mol Sci, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. (2013). Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell, 12:489-498. [DOI] [PubMed] [Google Scholar]
- [112].Menendez JA (2020). Metformin: Sentinel of the Epigenetic Landscapes That Underlie Cell Fate and Identity. Biomolecules, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhao J, Zhang L, Lu A, Han Y, Colangelo D, Bukata C, et al. (2020). ATM is a key driver of NF-kappaB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging (Albany NY), 12:4688-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nicaise AM, Willis CM, Crocker SJ, Pluchino S (2020). Stem Cells of the Aging Brain. Front Aging Neurosci, 12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Espinosa-Parrilla Y, Gonzalez-Billault C, Fuentes E, Palomo I, Alarcon M (2019). Decoding the Role of Platelets and Related MicroRNAs in Aging and Neurodegenerative Disorders. Front Aging Neurosci, 11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, et al. (2019). NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell, 18:e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Schultz MB, Sinclair DA (2016). When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development, 143:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu W, Zhang L, Xuan K, Hu C, Liu S, Liao L, et al. (2018). Alpl prevents bone ageing sensitivity by specifically regulating senescence and differentiation in mesenchymal stem cells. Bone Res, 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, et al. (2015). Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Reports, 5:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, et al. (2019). Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell, 25:473-485.e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Soukas AA, Hao H, Wu L (2019). Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol Metab, 30:745-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005). Metformin and reduced risk of cancer in diabetic patients. BMJ, 330:1304-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kang J, Jeong SM, Shin DW, Cho M, Cho JH, Kim J (2021). The Associations of Aspirin, Statins, and Metformin With Lung Cancer Risk and Related Mortality: A Time-Dependent Analysis of Population-Based Nationally Representative Data. J Thorac Oncol, 16:76-88. [DOI] [PubMed] [Google Scholar]
- [124].Wang C, Zhang T, Liao Q, Dai M, Guo J, Yang X, et al. (2021). Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell, 12:128-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Pollack MN (2007). Insulin, insulin-like growth factors, insulin resistance, and neoplasia. Am J Clin Nutr, 86:s820-822. [DOI] [PubMed] [Google Scholar]
- [126].Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, et al. (2018). Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res, 37:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Fatehi Hassanabad A, MacQueen KT (2021). Molecular mechanisms underlining the role of metformin as a therapeutic agent in lung cancer. Cell Oncol (Dordr), 44:1-18. [DOI] [PubMed] [Google Scholar]
- [128].Ahn HK, Lee YH, Koo KC (2020). Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Menendez JA, Cufi S, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Vellon L, et al. (2011). Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging (Albany NY), 3:1063-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cioce M, Pulito C, Strano S, Blandino G, Fazio VM (2020). Metformin: Metabolic Rewiring Faces Tumor Heterogeneity. Cells, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Brown JR, Chan DK, Shank JJ, Griffith KA, Fan H, Szulawski R, et al. (2020). Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mayer MJ, Klotz LH, Venkateswaran V (2015). Metformin and prostate cancer stem cells: a novel therapeutic target. Prostate Cancer Prostatic Dis, 18:303-309. [DOI] [PubMed] [Google Scholar]
- [133].Saini N, Yang X (2018). Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai), 50:133-143. [DOI] [PubMed] [Google Scholar]
- [134].Samuel SM, Varghese E, Koklesova L, Liskova A, Kubatka P, Busselberg D (2020). Counteracting Chemoresistance with Metformin in Breast Cancers: Targeting Cancer Stem Cells. Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wu X, Qian S, Zhang J, Feng J, Luo K, Sun L, et al. (2021). Lipopolysaccharide promotes metastasis via acceleration of glycolysis by the nuclear factor-kappaB/snail/hexokinase3 signaling axis in colorectal cancer. Cancer Metab, 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG (2017). Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia, 60:1639-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, et al. (2012). Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia, 55:2593-2603. [DOI] [PubMed] [Google Scholar]
- [138].Zhang YN, Fan JK, Gu L, Yang HM, Zhan SQ, Zhang H (2021). Metabotropic glutamate receptor 5 inhibits alpha-synuclein-induced microglia inflammation to protect from neurotoxicity in Parkinson's disease. J Neuroinflammation, 18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. (2015). Neuroinflammation in Alzheimer's disease. Lancet Neurol, 14:388-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lu XY, Huang S, Chen QB, Zhang D, Li W, Ao R, et al. (2020). Metformin Ameliorates Abeta Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer's Disease. Oxid Med Cell Longev, 2020: 2315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, et al. (2014). Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun, 40:131-142. [DOI] [PubMed] [Google Scholar]
- [142].Menzies FM, Fleming A, Rubinsztein DC (2015). Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci, 16:345-357. [DOI] [PubMed] [Google Scholar]
- [143].Mizushima N, Levine B (2020). Autophagy in Human Diseases. N Engl J Med, 383:1564-1576. [DOI] [PubMed] [Google Scholar]
- [144].Li W, He P, Huang Y, Li YF, Lu J, Li M, et al. (2021). Selective autophagy of intracellular organelles: recent research advances. Theranostics, 11:222-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gibbons GS, Lee VMY, Trojanowski JQ (2019). Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol, 76:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003). Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem, 278:25009-25013. [DOI] [PubMed] [Google Scholar]
- [147].Thellung S, Corsaro A, Nizzari M, Barbieri F, Florio T (2019). Autophagy Activator Drugs: A New Opportunity in Neuroprotection from Misfolded Protein Toxicity. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Pei JJ, Hugon J (2008). mTOR-dependent signalling in Alzheimer's disease. J Cell Mol Med, 12:2525-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rui Y, Zheng JQ (2016). Amyloid beta oligomers elicit mitochondrial transport defects and fragmentation in a time-dependent and pathway-specific manner. Mol Brain, 9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, et al. (2017). Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548:592-596. [DOI] [PubMed] [Google Scholar]
- [151].Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, et al. (2018). Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun, 69:351-363. [DOI] [PubMed] [Google Scholar]
- [152].Chen X, Wu W, Gong B, Hou L, Dong X, Xu C, et al. (2020). Metformin attenuates cadmium-induced neuronal apoptosis in vitro via blocking ROS-dependent PP5/AMPK-JNK signaling pathway. Neuropharmacology, 175:108065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jakovljevic DG (2018). Physical activity and cardiovascular aging: Physiological and molecular insights. Exp Gerontol, 109:67-74. [DOI] [PubMed] [Google Scholar]
- [154].Charytan DM, Solomon SD, Ivanovich P, Remuzzi G, Cooper ME, McGill JB, et al. (2019). Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab, 21:1199-1208. [DOI] [PubMed] [Google Scholar]
- [155].Petrie JR, Chaturvedi N, Ford I, Hramiak I, Hughes AD, Jenkins AJ, et al. (2017). Metformin in adults with type 1 diabetes: Design and methods of REducing with MetfOrmin Vascular Adverse Lesions (REMOVAL): An international multicentre trial. Diabetes Obes Metab, 19:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Xu T, Brandmaier S, Messias AC, Herder C, Draisma HH, Demirkan A, et al. (2015). Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care, 38:1858-1867. [DOI] [PubMed] [Google Scholar]
- [157].Jenkins AJ, Welsh P, Petrie JR (2018). Metformin, lipids and atherosclerosis prevention. Curr Opin Lipidol, 29:346-353. [DOI] [PubMed] [Google Scholar]
- [158].Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. (2006). Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol, 26:611-617. [DOI] [PubMed] [Google Scholar]
- [159].Huang KY, Que JQ, Hu ZS, Yu YW, Zhou YY, Wang L, et al. (2020). Metformin suppresses inflammation and apoptosis of myocardiocytes by inhibiting autophagy in a model of ischemia-reperfusion injury. Int J Biol Sci, 16:2559-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Niu C, Chen Z, Kim KT, Sun J, Xue M, Chen G, et al. (2019). Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy, 15:843-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kanamori H, Naruse G, Yoshida A, Minatoguchi S, Watanabe T, Kawaguchi T, et al. (2019). Metformin Enhances Autophagy and Provides Cardioprotection in delta-Sarcoglycan Deficiency-Induced Dilated Cardiomyopathy. Circ Heart Fail, 12:e005418. [DOI] [PubMed] [Google Scholar]
- [162].Decker C, Sadhu S, Fredman G (2021). Pro-Resolving Ligands Orchestrate Phagocytosis. Front Immunol, 12:660865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Justice JN, Gubbi S, Kulkarni AS, Bartley JM, Kuchel GA, Barzilai N (2021). A geroscience perspective on immune resilience and infectious diseases: a potential case for metformin. Geroscience, 43:1093-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Rottenberg H, Hoek JB (2021). The Mitochondrial Permeability Transition: Nexus of Aging, Disease and Longevity. Cells, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Cordoba-Lanus E, Cazorla-Rivero S, Garcia-Bello MA, Mayato D, Gonzalvo F, Ayra-Plasencia J, et al. (2021). Telomere length dynamics over 10-years and related outcomes in patients with COPD. Respir Res, 22:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM, Lushchak O (2019). Metformin as a geroprotector: experimental and clinical evidence. Biogerontology, 20:33-48. [DOI] [PubMed] [Google Scholar]
- [167].Campbell JM, Bellman SM, Stephenson MD, Lisy K (2017). Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev, 40:31-44. [DOI] [PubMed] [Google Scholar]
- [168].Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, et al. (2019). Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology, 69:2414-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Markowicz-Piasecka M, Huttunen KM, Mateusiak L, Mikiciuk-Olasik E, Sikora J (2017). Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr Pharm Des, 23:2532-2550. [DOI] [PubMed] [Google Scholar]
- [170].Song J, Jiang G, Zhang J, Guo J, Li Z, Hao K, et al. (2019). Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori. Aging (Albany NY), 11:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, et al. (2010). Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY), 2:945-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Slack C, Foley A, Partridge L (2012). Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One, 7:e47699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, et al. (2013). Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell, 153:228-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Prattichizzo F, Giuliani A, Mensa E, Sabbatinelli J, De Nigris V, Rippo MR, et al. (2018). Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev, 48:87-98. [DOI] [PubMed] [Google Scholar]