Abstract
Objective
To evaluate the effects of He's Yangchao Recipe (HSYC) on ameliorating ovarian oxidative stress of aging mice under consecutive superovulation.
Methods
An 8-month-old C57BL/6 female mouse was chosen to establish an aging model under ovarian hyperstimulation. Mice were randomly separated into four groups: R1 as the control group, R4 as the model group, NR4 with N-acetyl-L-cysteine (NAC) administration, and TR4 with HSYC administration. Oocyte collection, in vitro fertilization, and embryo culture were performed. The serum hormone levels were measured by enzyme-linked immunosorbent assays (ELISA); the reactive oxygen species (ROS) level of oocytes, the number of growing follicles, corpus luteum, ovulated oocytes, and developing embryos at each stage, along with the proportions of fragmented oocytes and abnormal mitochondria in granulosa cells (GCs) and the apoptosis rate of GCs were calculated; the mRNA and protein levels of JNK, P53, BAX were detected by real-time PCR and the Simple Western System.
Results
HSYC enhanced estradiol, progesterone, and inhibin-B levels and increased growing follicle and corpus luteum and ovulated egg counts compared to the R4 group (P < 0.05), whereas it decreased the proportions of fragmented oocytes (P < 0.01); Meanwhile, embryos from mice subjected to four superovulation cycles with HSYC treated had a higher hatching potential. The ROS level of oocytes is downregulated by HSYC (P < 0.01) and the percentage of abnormal mitochondrial in ovaries of the TR4 group was also significantly declined compared to the R4 group (P < 0.05); the most TUNEL-positive cells proportion was detected in the R4 group; nevertheless, HSYC effectively attenuated this detrimental effect (P < 0.05). The mRNA and protein expressions of JNK and P53 in ovary tissues were reduced in the TR4 group while these genes were upregulated by repeated superovulation (P < 0.05).
Conclusions
HSYC exerted promising effects on promoting the diminished ovarian reserve and decreased oocyte quality induced by both aging and consecutive ovarian superovulation, potentially via the ROS/JNK/p53 pathway.
1. Introduction
Aging, a hotspot arising intense discussion, is considered the main trigger that causes gradual depletion of ovarian reserve and a reduced ability to produce oocytes competent for fertilization in females. Altogether with a progressive reduction of the follicles, woman aging also involves a compromised competence of the embryos [1]. A large retrospective study showed that females over the age of 42 had a live-born baby rate of only 1%, but those under 35 had a rate of 26% [2]. The aging phenomena can be attributed to increased reactive oxygen species (ROS) levels and cumulative oxidative stress in somatic cells [3, 4]. Moreover, mitochondria are the most remarkable targets of ROS, and the dysfunction of mitochondria would induce nondisjunction of the chromosomes, gestation failure, and decreased embryonic viability [5, 6].
Procedures for superovulation and assisted reproductive technologies (ART) are highly successfully and widely used as clinical approaches to treating couples with infertility issues. Though repeated superovulation is considered safe, previous studies have demonstrated that it has some detrimental effects on female reproduction. Several studies showed that ovarian hyperstimulation can lead to pregnancy loss and delayed puberty in female offspring, decreased serum hormone levels, impaired mitochondrial function in ovarian cells, and reduced the oocyte and embryo quality [7–10].
Nowadays, the number of elderly women seeking ART help is gradually increasing. One research studying the intrinsic fertility of the human oocyte showed that natural cycles have higher intrinsic fertility per oocyte than hyperstimulated cycles [2]. It's also been reported that in in vitro fertilization (IVF), maternal age is among the strongest predictors of success [11].
It has been demonstrated that traditional Chinese medicine (TCM) is a promising alternative form of treatment for gynecological endocrinology dysfunctions [12–14], with significant efficacy and reduced side effects through various herbal combinations [15]. He's Yangchao Recipe (HSYC, with China Patent Application number of 201710902472X) is a special herbal prescription that originates from the ancestral experience of He's School Doctrine of Gynecology, which is one of the first studios of inheritance of academic schools of traditional Chinese medicine approved by the National Administration of TCM, and it is also the intangible cultural heritage of Hangzhou [16]. Results from previous studies of our research group have already shown that HSYC effectively increased the anti-Müllerian hormone (AMH) level and the growing follicle counts, as well as reducing serum FSH and improving ovarian reserve in patients with diminished ovarian reserve (DOR) [17, 18]. Animal experiment shows that high-dosage HSYC could notably improve ovarian reserve and alleviate the further development of DOR in mice [19].
To elucidate the potential mechanism of the therapeutic effect of HSYC, an 8-month-old C57BL/6 female mouse (represents humans at ages 38–47 according to The Jackson Laboratory) undergoing consecutive superovulation was chosen as a model to explore the regulatory effect of HSYC on protecting ovarian functions and oocytes/embryos quality after repeated superovulation. Figure 1 shows the workflow of the study. Our results demonstrated that consecutive superovulation can compromise oocyte quality and embryo development competence, increase oxidative stress and granular cell apoptosis along with damaging mitochondrial functions, but these detrimental effects from the oxidative insult were attenuated by HSYC, probably involving ROS/JNK/P53 signaling pathway.
Figure 1.
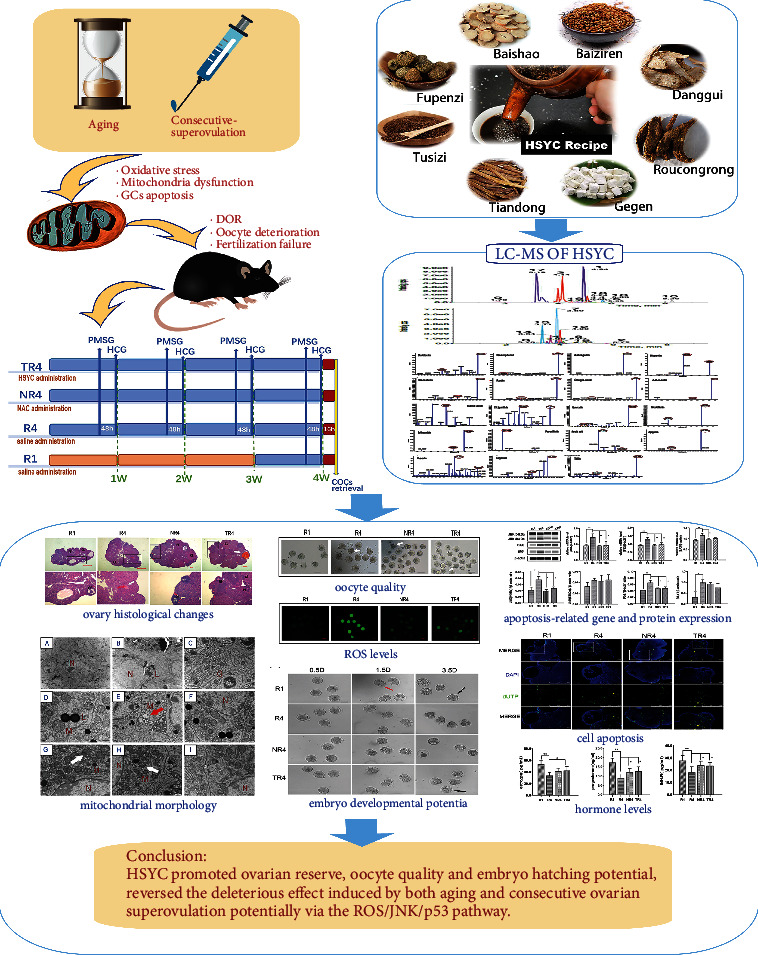
A workflow of this study.
2. Materials and Methods
2.1. He's Yangchao Recipe
The HSYC recipe used in this study was provided by the Department of Pharmacy, Hangzhou Hospital of Traditional Chinese Medicine, and authenticated by Zhejiang University of Traditional Chinese Medicine. After the material herbs of HSYC being soaked for 30 min, HSYC was decocted with distilled water and heated for 1 h after boiling, and the mixture was then filtered. HSYC was concentrated with rotary evaporators, boiled into a thick slurry, and then was sealed and refrigerated at −20°C for later use. HSYC consists of eight traditional Chinese herbs (Table 1) as follows: 10.3% Paeonia lactiflora Pall; 15.5% Cuscuta chinensis Lam; 15.5% Cistanche salsa (C.A.Mey.) Beck; 10.3% Angelica sinensis (Oliv.) Diels; 15.5% Rubus chingii Hu; 12.4% Pueraria lobata (Willd.) Ohwi; 10.3% Asparagus cochinchinensis (Lour.) Merr; and 10.3% Platycladus orientalis (Linn.) Franco.
Table 1.
Composition of HSYC.
| Chinese name | English name | Family | Plant part | Crude herb (g) |
|---|---|---|---|---|
| Baishao | Paeonia lactiflora pall | Paeoniaceae | Root | 10 |
| Tusizi | Cuscuta chinensis lam | Convolvulaceae | Seeds | 15 |
| Roucongrong | Cistanche salsa (C.A.Mey.) beck | Orobanchaceae | Stem | 15 |
| Danggui | Angelica sinensis (Oliv.) diels | Apiaceae | Root | 10 |
| Fupenzi | Rubus chingii Hu | Rosaceae | Fruit | 15 |
| Gegen | Pueraria lobata (Willd.) Ohwi | Leguminosae | Root | 12 |
| Tiandong | Asparagus cochinchinensis (Lour.) Merr. | Asparagaceae | Root | 10 |
| Baiziren | Platycladus orientalis (Linn.) Franco | Cupressaceae | Seed | 10 |
2.2. UPLC-ESI-MS/MS
Mix the samples with vortex after thawing and 1000 ul of them was centrifuged (12000 r/min, 4°C) for 10 min, and then filtered and stored in a sample flask. UPLC-ESI-MS/MS implement conditions are as follows: the column involved in UPLC was Agilent SB-C18 (1.8 μm, 2.1 mm ∗ 100∗mm); pure water and 0.1% formic acid mobile phase was used for solvent A, and acetonitrile with 0.1% formic acid for solvent B; A gradient program starts with conditions under 95% A, and 5% B and lasted for 9 min, and subsequently, a linear gradient was programmed to 5% A, 95% B and lasted for 1 min. The ratio of the B phase is reduced to 5% again in 10.00–11.10 min and remained for 14 min, and then the effluent was connected to an ESI-Q TRAP-MS; AB4500 Q TRAP UPLC/MS/MS system was used to acquire triple quadrupole (QQQ) scans; Analyst 1.6.3. software (AB Sciex) was used to analyze the results.
2.3. Animals and Ethical Approval
Sixty 8-month-old female C57BL/6 mice (28–33 g), together with five nine-week-old male mice (18–20 g) for in vitro fertilization, were purchased from Beijing Charles River Laboratory Animal Company (Certificate No. SCXK 2019-0009, Beijing, China). Mice were housed in a barrier facility at the Animal Experimental Research Center of Zhejiang Chinese Medical University with constant temperatures, humidity, and daylight (12 hours light/12 hours darkness). All of the experimental procedures were supervised by the Institutional Animal Care and Use Committee of the Zhejiang Chinese Medical University (Approval number: IACUC-20201123-01), and the research was conducted in accordance with ARRIVE guidelines [20].
2.4. Repeated Superovulation Procedures
The 8‐month‐old female mice were randomly separated into four groups. R4 mice were the ones that underwent four superovulatory cycles. In addition, R4 mice taking N-acetyl-L-cysteine (NAC) (Sigma-Aldrich, St. Louis, USA) were termed as NR4 mice, and the remained ones taking traditional Chinese herbal prescription HSYC were termed as TR4 mice. The selection for dosage of NAC and HSYC was based on the dosage used in clinical trials and conversion of human to mouse dose. NAC dosage was determined to be 870 mg/kg [21] and 29.1 g/kg for HSYC [19] via intragastric administration daily. A dose of 10 IU of pregnant mare serum gonadotropin (PMSG; Nanjing Aibei Biotechnology Company, Nanjing, China) was administered to the female mice for repeated superovulation, followed 48 h later by 10 IU of human chorionic gonadotropin (HCG; Nanjing Aibei Biotechnology Company, Nanjing, China) every other week for 4 weeks. Mice injected with PMSG only once were set to be R1 mice. Figure 2 shows the superovulation injection protocols in the present study.
Figure 2.
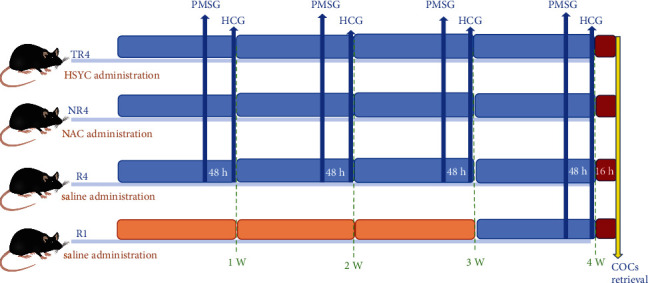
The superovulation treatment protocol in the present study.
2.5. Oocyte Collection, In Vitro Fertilization, and Embryo Culture
After the female mice were sacrificed by CO2 inhalation, the ampulla of the oviduct was obtained and placed in a test tube containing Hepes buffer, and the bulge of the ampulla of the oviduct was cut through with the needle to allow the cumulus-oocyte complexes (COCs) to slide out. The COCs from 9 mice per group were placed into the prepared Hepes containing hyaluronidase, and the number and morphology of oocytes were observed under the Stereo Microscope after 5–10 minutes of digestion. These oocytes were then collected for the subsequent experiments. The COCs from another 6 mice per group were placed in HTF fertilized drop in an incubator. The eggs were picked up and washed after 6 hours, moved to the M2 medium with mineral oil, and put into an incubator containing 5% CO2, whose temperature was set to 37°C, and continue to culture. Observe and record the condition of embryos at 0.5 d, 1.5 d, and 3.5 d, respectively. In addition, ovaries were collected in cryopreservation tubes, placed into liquid nitrogen, and then transferred to −80°C for future analysis.
2.6. ROS Assessment
Intracellular ROS levels were evaluated by the dichlorodihydrofluorescein diacetate (DHFC-DA) probe method. Oocytes were incubated with 10 mM DHFC-DA (Beyotime Biotechnology, Shanghai, China) in PBS for 20 minutes at 37°C in a dark interior. Images were captured by an inverted fluorescent microscope (Zeiss Axio Observer.A1).
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of serum estrogen, progesterone, and INH-B were detected using the ELISA kits (Vankew, Shanghai, China), as instructed by the manufacturer.
2.8. Histopathology and Electron Microscopy
The ovary tissues were fixed in 4% paraformaldehyde after mice were sacrificed and then went through paraffin embedding and serial sections. 4 μm thick ovary sections were stained with hematoxylin and eosin (H & E) on a glass slide. Images of the ovaries' structure and follicles counts were taken by microscope (Motic AE2000). Immediately after dissection, the ovary tissue was fixed with glutaraldehyde and osmic acid, dehydrated, and transferred to a resin mixture. 100 nm thick ovary sections were stained with uranyl acetate and lead citrate. Images were photographed by transmission electron microscope (HITACHI-H7650).
2.9. TUNEL Staining of Ovarian Cells
Apoptosis of ovarian granulosa cells was observed by a One-Step TUNEL Apoptosis Assay Kit (Beyotime Biotechnology, Shanghai, China). The paraffin sections of ovaries were digested with proteinase K for 30 min and then incubated in a TUNEL test reagent composed of enzyme terminal deoxynucleotidyl transferase (TdT) and fluorescein-dUTP for 1 h in a dark interior at 37°C, and mounted in fluorescence decay-resistant medium containing DAPI. The digital images were recorded using a fluorescence microscope (Leica DM2500B).
2.10. Protein Expression Analysis
To extract the total protein from the mice's ovarian tissues, we used a total protein extraction reagent (KeyGen Biotech, Jiangsu, China) according to the manufacturer's protocol. The protein concentration was assayed using the BCA Protein Assay kit (KeyGen Biotech, Jiangsu, China). The Simple Western (WesProteinSimple, San Jose, CA, USA) system was used for protein quantification (JNK, Bax, and p53). The antibodies used in the above protein determination are all from Cell Signaling Technology (CST, Danvers, MA, USA). Then, the signal intensities were quantified and analyzed using Compass Software (ProteinSimple).
2.11. Real-Time PCR
RNA samples were prepared using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Shiga, Japan), and a reverse transcriptase reaction was performed using PrimeScript RT Master Mix (TaKaRa, Shiga, Japan). SYBR Premix Ex TaqTM II (Perfect Real Time) (TaKaRa, Shiga, Japan) was used for the detection of the specified genes. The conditions for the reverse transcription reaction are as follows: 37°C 15 min⟶85°C 5 s⟶4°C. The reverse transcription reaction system was added to tubes used for fluorescence quantitative reaction, and the amplification reaction conditions are as follows: predenaturation: 95°C 3 min; 40 cycles (95°C 10 s, 60°C 30 s); dissolution curve: starting from 55°C, increasing by 0.5°C each 30 s until 95°C, and circulate once. All reaction information is collected by ABI StepOnePlus™ Real-Time PCR System, and normalized expression was calculated as relative fold change using the formula 2−ΔΔCT. Table 2 shows the primer sequences used in the quantitative real-time PCR experiment, and β-actin was considered as the internal reference gene.
Table 2.
Primer sequences of the target genes.
| Gene | Primer | Sequences(5′ to 3′) |
|---|---|---|
| JNK | Forward primer | AGTGACAGTAAAAGCGATGGTC |
| Reverse primer | AGCACAAACAATTCCTTGGGC | |
|
| ||
| p53 | Forward primer | CCCCTGTCATCTTTTGTCCCT |
| Reverse primer | AGCTGGCAGAATAGCTTATTGAG | |
|
| ||
| Bax | Forward primer | TGAAGACAGGGGCCTTTTTG |
| Reverse primer | AATTCGCCGGAGACACTCG | |
|
| ||
| β-Actin | Forward primer | CATCCGTAAAGACCTCTATGCCAAC |
| Reverse primer | ATGGAGCCACCGATCCACA | |
2.12. Statistical Analysis
The experimental data were analyzed by Prism GraphPad software, and all results were indicated by mean ± SD. One-way analysis of variance (ANOVA) was used to analyze differences among the groups. P values < 0.05 were commonly considered to be statistically significant, and the number of asterisks indicates the following levels of statistical significance: ∗∗P < 0.01, ∗P < 0.05.
3. Results
3.1. The Main Bioactive Components in HSYC by LC-QQQ-MS
LC-MS was implemented to identify the main bioactive components in HSYC, and qualitative analysis was performed to elucidate the components of the HSYC based on secondary spectrum information and the local database MWDB (Metware database). As shown in Table 3, Figures 3 and 4, the main active substances are daidzein, kaempferol, astragalin, hyperin, genistein, rutin, ellagic acid, acteoside, gallic acid, Z-ligustilide, quercetin, nicotiflorin, echinacoside, paeoniflorin, ferulic acid, apigenin, puerarin, calycosin, and trillin.
Table 3.
The RT, MW, MS-MS fragment ions, DP and CE of HSYC.
| Component | Classification | Precursor ion (m/z) | Product ion (m/z) | MW (Da) | Ionization model | DP (V) | CE (eV) | RT (min) |
|---|---|---|---|---|---|---|---|---|
| Daidzein | Isoflavones | 255.07 | 137.00 | 254.06 | [M + H]+ | 40 | 40 | 4.94 |
| Kaempferol | Flavonols | 285.04 | 151.00 | 286.05 | [M − H]− | -40 | -30 | 5.72 |
| Astragalin | Flavonols | 449.11 | 287.06 | 448.10 | [M + H]+ | 50 | 30 | 4.14 |
| Hyperin | Flavonols | 463.09 | 300.00 | 464.10 | [M − H]− | -60 | -40 | 3.88 |
| Genistein | Isoflavones | 271.06 | 215.07 | 270.05 | [M + H]+ | 20 | 40 | 5.65 |
| Rutin | Flavonols | 609.15 | 301.00 | 610.15 | [M − H]− | -40 | -40 | 3.75 |
| Ellagic acid | Tannin | 301.00 | 185.02 | 302.01 | [M − H]− | -60 | -40 | 3.89 |
| Acteoside | Phenolic acids | 623.20 | 461.17 | 624.21 | [M − H]− | -50 | -30 | 4.03 |
| Gallic acid | Tannin | 171.03 | 107.01 | 170.02 | [M + H]+ | 50 | 30 | 1.79 |
| Z-Ligustilide | Others | 191.11 | 117.07 | 190.10 | [M + H]+ | 20 | 20 | 8.26 |
| Quercetin | Flavonols | 303.05 | 137.02 | 302.04 | [M + H]+ | 50 | 50 | 5.08 |
| Nicotiflorin | Flavonoid | 593.15 | 285.04 | 594.16 | [M − H]− | -50 | -30 | 4.00 |
| Echinacoside | Phenolic acids | 785.25 | 623.20 | 786.26 | [M − H]− | -50 | -50 | 3.33 |
| Paeoniflorin | Monoterpenoids | 479.16 | 121.03 | 480.16 | [M − H]− | -50 | -30 | 3.64 |
| Ferulic acid | Phenolic acids | 193.05 | 134.01 | 194.06 | [M − H]− | -20 | -20 | 4.12 |
| Apigenin | Flavonoid | 271.06 | 153.01 | 270.05 | [M + H]+ | 50 | 30 | 5.63 |
| Puerarin | Isoflavones | 417.12 | 297.07 | 416.11 | [M + H]+ | 50 | 30 | 3.19 |
| Calycosin | Flavonoid | 285.08 | 225.06 | 284.07 | [M + H]+ | 50 | 30 | 5.24 |
| Trillin | Steroidalsaponins | 577.37 | 271.21 | 576.36 | [M + H]+ | 50 | 30 | 4.75 |
MW: molecular weight, DP: declustering potential, CE: collision energy, and RT: retention time.
Figure 3.
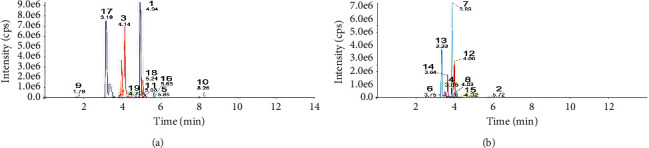
MRM chromatograms in (a) negative and (b) positive modes. Identification: 1, daidzein; 2, kaempferol; 3, astragalin; 4, hyperin; 5, genistein; 6, rutin; 7, ellagic acid; 8, acteoside; 9, gallic acid; 10, Z-ligustilide; 11, quercetin; 12, nicotiflorin; 13, echinacoside; 14, paeoniflorin; 15, ferulic acid; 16, apigenin; 17, puerarin; 18, calycosin and 19, trillin.
Figure 4.
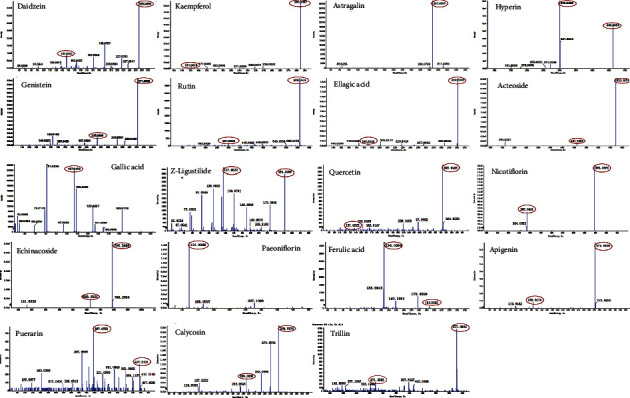
The product ion scan spectra of 19 bioactive compounds in HSYC.
3.2. HSYC Improves Reproductive Endocrinology Dysfunction in Mice
Superovulation following decreased ovarian function is usually accompanied by decreased levels of estrogen, progesterone, and INH-B, as is shown by the difference between groups R1 and R4 in Figure 5. However, HSYC regulated hormone levels, with progesterone, estrogen, and INH-B levels increased (P < 0.05).
Figure 5.
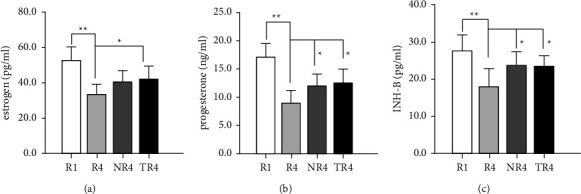
The effect of HSYC on improving reproductive endocrinology dysfunction. (a–c) The serum content of estrogen, progesterone, and INH-B level of mice in different groups, respectively.
3.3. HSYC Reduces ROS Levels in Oocytes of Repeated Superovulation Mice
We examined oocytes' ROS levels retrieved from the oviduct and observed that R4 oocytes had dramatically higher ROS levels than R1 oocytes (P < 0.01), while ROS levels were significantly reduced in HSYC-treated oocytes and NAC-treated oocytes (P < 0.01; Figures 6(d) and 6(e)). The above results suggest that consecutive superovulation increased ROS levels while HSYC administration could resist oxidative stress.
Figure 6.
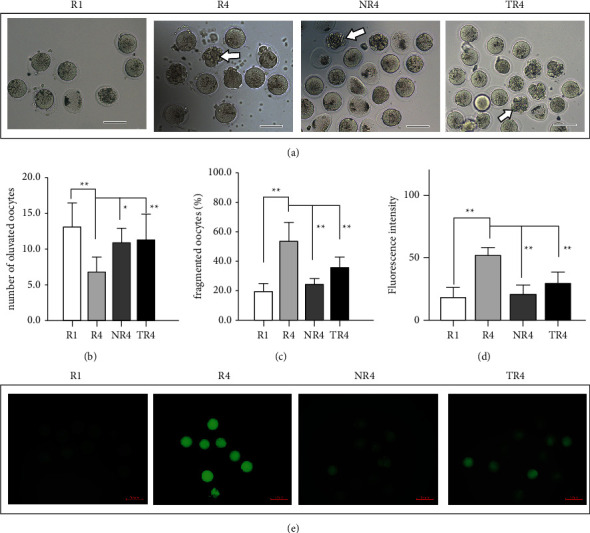
Effect on the quantity and quality of oocytes ovulated into the oviducts of HSYC (a–c) and different ROS levels in oocytes from mice undergoing repeated superovulation with or without intervention (d, e). (a) Representative images of morphologically normal and fragmented oocytes. White arrow: oocytes with obvious cytoplasmic fragments. (e) Representative fluorescence images of ROS; scale bars are 50 μm. (b) Graph showing the proportion of morphologically abnormal and fragmented oocytes in different groups. (c) Graph showing the average number of oocytes ovulated per mouse (n = 9 per group, 18 oviducts). (d) Quantitative fluorescence intensities of ROS staining in oocytes from the R1 (n = 32), R4 (n = 36), NR4 (n = 38), and TR4 (n = 42) groups, and n denotes the number of oocytes for each group. ROS: reactive oxygen species.
3.4. HSYC Increases the Quantity and Quality of Oocytes
We observed the oocytes retrieved from oviducts under the microscope to determine whether HSYC could influence the quantity and quality of oocytes (Figure 6(a)). As shown in Figure 6(b), fewer eggs per oviduct from superovulated mice were retrieved compared to the R1 group (P < 0.01), but HSYC could increase the egg counts (P < 0.01). We also evaluated the effects of HSYC treatment on oocyte quality in mice after application of HSYC for one month, and the proportions of fragmented oocytes were markedly decreased in HSYC-treated mice and NAC-treated mice compared to the R4 group (P < 0.01, Figure 6(c)).
3.5. HSYC Treatment Ameliorated the Histological Changes in Ovaries
We observed pathologic changes in the ovary to elucidate the mechanism by which the number of ovulated oocytes increased by HSYC after superovulation. Contrary to the R4 group, significantly increased numbers of developing follicles were observed in the HSYC group as well as the total follicles (Figure 7(d)). While the number of the corpus luteum increased in all three groups after the fourth cycle of ovulation compared to the R1 mice, it showed a more significant incline in the TR4 group (P < 0.01) than in the NR4 group (P < 0.05) (Figure 7(b)). Hemorrhagic corpus luteum, characterized by corpus luteum infiltrated with erythrocyte, was occasionally observed in ovaries in the present study, presumably indicating recent ovulation (Figure 7(a)). However, there was no difference in the percentage of Hemorrhagic CLs in the prevalence of this phenotype among the four groups (Figure 7(c)). Administration of HSYC had a considerable effect on the histological changes in the ovary during repeated ovulation.
Figure 7.
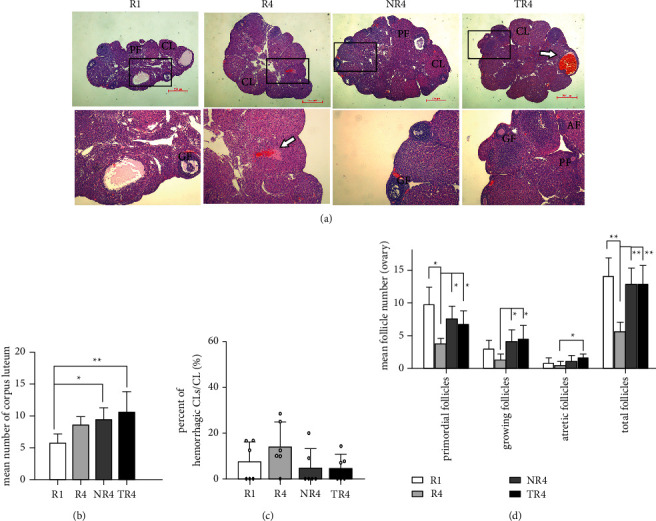
Effect on follicles after 28 days of HSYC administration. (a) Follicles observed after H & E staining. PF: primary follicles; GF: growing follicles; AF: atretic follicles.CL: corpus luteum. White arrow: hemorrhagic CLs. Scale bars, 500 μm and 200 μm. (b, c) The number of CLs and the percent of hemorrhagic CLs in each group. (d) The numbers of follicles at different developmental stages of maturation.
3.6. HSYC Improved the Mitochondrial Morphology in Ovary
We use electron microscopy to view ultrastructural changes in the ovaries (Figures 8(a)–8(i)). What we observed is that there were marked differences among four groups in mitochondrial morphology. In the R1 group, most of the mitochondria were normal, the structures of which were regular, round, oval, or rod-shaped, with clear and complete crista, nevertheless mitochondria of granulosa cells in ovaries suffering from superovulation showed abnormal and damaged structures, being swollen and ruptured with aggregation, extrusion, and fusion, with cristae being vacuolated and barely visible. The percentage of abnormal mitochondrial (mitochondria with vague cristae and vacuolated mitochondria) of the TR4 group is significantly declined than the R4 group (P < 0.05, Figures 8(j) and 8(k)).
Figure 8.
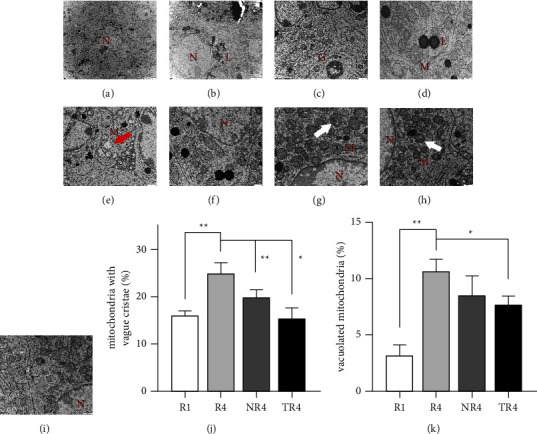
Effect on ultrastructural changes of the ovarian granulosa cells of HSYC. (a–e) Normal ultrastructure in control group mice ovaries. (f–i) Representative images of mitochondria around the granulosa cells in each group, (f) R1 group, (g) R4 group, (h) NR4 group, and (i) TR4 group. (G: golgi complex; L: lipid; M: mitochondrial; N: nucleus of granulosa cells; white arrow: abnormal mitochondria; red arrow: vacuolated mitochondria). (j, k) The percentage of mitochondria with vague cristae and vacuolated mitochondria in all mitochondria.
3.7. A Positive Effect of HSYC on Reducing Cell Apoptosis
The percentage of apoptotic granulosa cells in the antral follicles of ovaries after consecutive superovulation was estimated in the four groups (Figure 9(a)). TUNEL-positive cells occurred more in the R4 group than in the R1 group, suggesting the aggravation of apoptosis due to ovarian hyperstimulation, whereas HSYC significantly decreased the percentage of TUNEL-positive cells (P < 0.05, Figure 9(b)). HSYC showed a positive effect on the reduction of ovarian cell apoptosis of HSYC.
Figure 9.
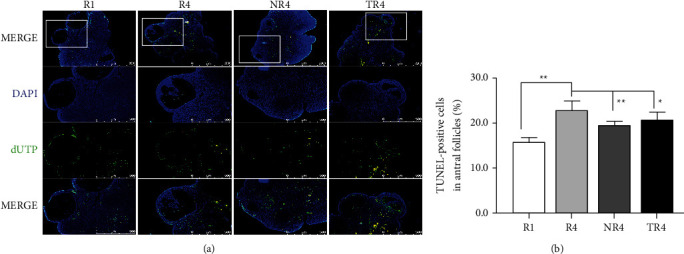
Effect of HSYC on ovarian cell apoptosis. (a) Apoptotic nuclei and total nuclei show green and blue fluorescence respectively. Scale bar: 500 μm. (b) The percentage of TUNEL-positive granulosa cells in the antral follicles.
3.8. HSYC Inhibited JNK and p53 mRNA Expression
The JNK and p53 mRNA increased significantly in the R4 group versus that in the R1 group, while HSYC could reduce the JNK and p53 mRNA expression, as well as NAC, did (P < 0.05, Figures 10(b) and 10(c)). HSYC did not show a capacity in affecting the BAX mRNA expression while NAC did (P > 0.05, Figure 10(d)).
Figure 10.
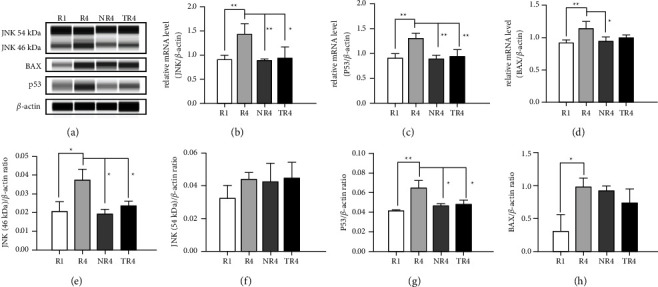
The effects of HSYC on superovulation-induced changes of protein JNK, p53, BAX, and mRNA levels of JNK, p53, and BAX. (a) Representative band images of the three proteins, JNK, P53, and BAX. (e–h) Relative protein levels of JNK, P53, and BAX. (b–d) Relative mRNA expression levels of JNK, P53, and BAX. The data represent at least three independent experiments.
3.9. HSYC Inhibited JNK and p53 Protein Expression
Consecutive superovulation in the R4 model group significantly increased JNK (46 kDa) and p53 expression in the ovary compared to the R1 group. However, HSYC significantly decreased JNK (46 kDa) and p53 protein expression compared with the R4 group, as presented in Figures 10(f) and 10(g) (P < 0.05). The results above measured by WES are consistent with the mRNA expression measured by RT-PCR. Nevertheless, the JNK (54 kDa) and BAX protein expressions did not show significant statistical differences among the four groups (Figures 10(e) and 10(h)). Representative band images of the target proteins are shown in Figure 10(a).
3.10. HSYC Improved on Developmental Potential of Embryos
As shown in Figure 11, repeated superovulation resulted in a significant decline in embryos quantity obtained after four superovulation cycles, and the number of zygotes obtained from mice COCs after in vitro fertilization was markedly higher in TR4 than that in the R4 group (P < 0.05), whereas NR4 group has a more significant higher zygote count than in the R4 group (P < 0.01). The number of 2-cell stage embryos retrieved from the TR4 mice is as well higher than that in the R4 group (P < 0.05). The difference between the morula counts of the TR4 group and the R4 group showed statistical significance (P < 0.05). Embryos from mice subjected to four superovulation cycles with HSYC treated had a higher hatching potential compared with all other groups without HSYC administration.
Figure 11.
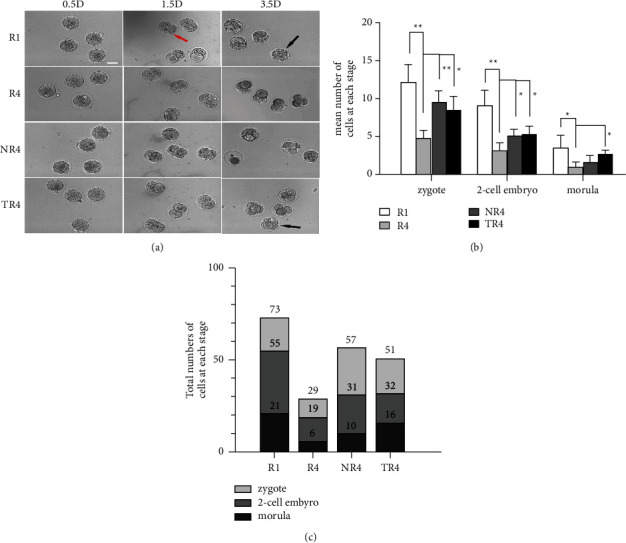
Effect on the quality and quantity of embryos of HSYC. (a) Representative images of embryos, were acquired on 0.5 D, 1.5 D, and 3.5 D in vitro embryo culture. The red arrow indicates two-cell stage embryos, and the black arrowhead refers to morula. Scale bars = 50 μm. (b) The total amount and the average number of embryos at different stages in each group.
4. Discussion
The aging phenomena can be attributed to increased ROS levels and accumulated oxidative damage in somatic cells and the affection of oxidative stress to oocytes has been well-demonstrated [5, 22, 23]. ROS are not only constantly generated but also eliminated in the mitochondria thus maintaining redox balance and homeostasis, and when the redox balance is broken due to aging, ROS are accumulated [24]. Mitochondria, which is crucial for controlling cell survival and death, is the most remarkable target of ROS. The accumulation of spontaneous mitochondria damage was due to increased ROS in oocytes, and mitochondria dysfunction would induce chromosomal nondisjunction, pregnancy loss, or decreased embryo viability [5, 6].
Previous studies have suggested that consecutive superovulation can harm fertility and fecundity in mice, whose AMH expressions, along with the concentrations of estrogen and progesterone significantly decreased [25]; Results of recent studies also suggest that multiple superovulations affect mitochondrial function in cumulus cells, inducing apoptosis and mitochondrial DNA (mtDNA) damage as well as altering histone modifications in early embryos, so as to decrease ovarian functions, reduce the oocyte and embryo quality and delay embryonic development [8–10]. Increased cytoplasmic fragmentation, abnormal mitochondrial distribution, and spindle damage were also observed in oocytes ovulated from mice that underwent superovulation [26]. And to be more precise, it seems that repeated superovulation induces strong oxidative stress and damage to all reproductive organs of female mice, which results in subsequent negative effects mentioned above [27, 28]. Women of late childbearing age ovulate fewer eggs beyond a loss of ovarian reserve [29]. Meanwhile, there are often difficulties for these women to respond to ovarian stimulation when undergoing IVF [30], and when pregnancy does occur to them, they will have a higher incidence of aneuploid blastocysts and unexplained recurrent abortion [31].
In this present study, we can see that as previously reported, consecutive superovulation in aged mice caused the declined serum estrogen, progesterone, and INH-B expressions, followed by reduced growing follicles, indicating impaired ovarian functions. Aging together with superovulation, contributes to the oxidative stress and ROS accumulation in the ovary, leading to the dysfunction of mitochondria of granulosa cells and the accelerated apoptosis in ovarian cells. Increased oocyte cytoplasmic fragmentation has also been observed. As is demonstrated by some research, the average number of ovulated oocytes of 4-week-old female C57BL/6 mice undergoing superovulation could reach to 42.7, while the mice aged 8 months (30 weeks) could only ovulate 16.2 oocytes [32]. Although the number of follicles after super-stimulation in 8-month-old mice is less than that of mice at their young age, the condition of continuous super-stimulation reduces ovulation simultaneously.
In contrast to the model group without drug intervention, the results of the present study revealed the efficacy of HSYC in improving ovarian function, fertility, and embryonic development. Under oxidative stress, HSYC lessened the apoptotic rate of granulosa cells (GCs) and the ROS Level of oocytes, along with an escalation in serum hormone expressions and growing follicles in ovaries. The normal morphological rate of mitochondria also increased, followed by the enhanced embryonic development potential. The Corpus luteum of the three groups after 4 times super-stimulation increased greatly, resulting in an augment in ovarian volume, while the amount of corpus luteum in the TR4 group was significantly larger than that in the R4 group, which may be due to the exhausted ovarian reserve after R4 group experienced 4 hyperstimulation without in time intervention, and we can draw this conclusion from the different ovulated follicle counts between R4 group and TR4 group.
The c-jun N-terminal kinase (JNK) has a necessarily close connection with oxidative damage close [33]. It has been well-demonstrated that ROS are potent inducers of JNK, and there are some studies suggesting that the proapoptotic effect of JNK activation in ROS-mediated apoptosis is closely related to the mitochondrial pathway and the p53 pathway. As is shown in the present study, the increased JNK and P53 mRNA along with corresponding proteins expression induced by ovarian super-stimulation and aging are all attenuated by HSYC. Meanwhile, JNK could mediate mitochondrial translocation of the proapoptotic gene BAX and thus activate proapoptotic Bcl-2 family members [34]; nevertheless, the change of BAX expression among R4, NR4, and TR4 groups did not show statistical significance in this present experiment.
The main components of HSYC have a long history of application in the historical process of traditional medicine. Cuscuta chinensis Lam is used for kidney tonifying and could prevent habitual abortion and improve excessive cold in female reproductive organs [35]. Pueraria lobata (Willd.) Ohwi exhibits phytoestrogen-like activities, regulates the endocrine system, and has beneficial effects on menopausal metabolic dysfunction due to the high levels of isoflavones it contains [36]. Danggui, the name of which in English is Angelica sinensis (Oliv.) Diels, is used to invigorate the blood circulation in menstrual disorders [37], while Baishao (Paeonia lactiflora Pall) is used to nourish blood and regulate menstruation according to the basic theory of TCM. Danggui–Baishao, which is recognized as an herb pair, is used to nourish blood and can significantly enhance the proliferation of granulosa cells in rat ovaries [38]. And research shows that TCM treatment involving kidney tonifying and blood activating methods has been proven effective in patients with primary ovarian insufficiency (POI) [39].
Bioactive compounds in traditional Chinese medications are rich in antioxidants, mainly including flavonoids, phenols, and polysaccharides. Flavonoids are potent antioxidants protecting plants from unfavorable environmental conditions [40]. Furthermore, HSYC is a great source of flavonoids, which act as antioxidants and regulate ROS homeostasis [41]. The main component are as follows: daidzein, kaempferol, astragalin, hyperin, genistein, rutin, ellagic acid, acteoside, gallic acid, Z-ligustilide, quercetin, nicotiflorin, echinacoside, paeoniflorin, ferulic acid, apigenin, puerarin, calycosin, and trillin.
Daidzein, Astragalin, Hyperin, and Genistein could lift the estrogen and progesterone levels of rats with impaired ovarian function. Moreover, Daidzein could elevate total antioxidant capacity in rats, attenuate ROS-induced toxicity by antioxidant action in ovarian cells [42, 43]; Kaempferol could maintain follicular survival, increase active mitochondria levels, prevent the H2O2-induced compromise of mitochondrial membrane potential (MMP) and ROS generation [44, 45]. Astragalin could enhance ovarian reserve and reduce ovarian GCs apoptosis in aged female rats via the Bcl-2 relative pathway [46]. Hyperin could increase proliferation and cell viability in H2O2 stimulated GCs, reverse the increased MDA level and decrease SOD, GSH-Px, and CAT, thus frequently used Chinese herbs like Cuscuta Chinensis Lam which contain Hyperin could improve ovarian functions through these effects [47, 48]. Genistein has antioxidant activity against radiation-mediated oxidative stress and Cyclophosphamide-induced ovarian toxicity through improving ovarian histology and immunostaining of ovarian iNOS, thus reversing ovarian apoptosis [49, 50], and meanwhile, it could elevate MMP of GCs, followed by a decline in the levels of intracellular mitochondrial superoxide and the apoptotic rate [51]. Ellagic acid could revoke ROS by manipulating oxidative biomarkers within the ovarian cells and exhibits a significant antioxidant capacity in aging and could protect the embryo DNA and development from the oxidative insult [52, 53]. The addition of acteoside during in vitro maturation could improve the rate of blastocyst formation Improve and mitochondrial morphology with decreased ROS level [54, 55]. Acteoside could also attenuate the drop of the MMP in the Chinese hamster ovary cell line (CHO) treated with H2O2 [56]. Rutin treatment before cisplatin could reduce apoptosis to preserve the normal follicles, decrease ROS levels, increase GSH levels and enhance mitochondria functions in ovaries. Rutin could also ameliorate the ischemia-reperfusion (I/R)-induced ovarian injury in rats via its possible antioxidative effects [57, 58]. Gallic acid could restrain granulosa cells apoptosis by inhibiting the expression of proapoptotic genes in mouse ovaries [59].
The relative comparative advantage of TCM compared to modern medicines is that TCM stresses compatibility, and the bioactive components in TCM act via multiple targets, while the mode of modern medicines is “one drug for one target” [60]. HSYC can not only resist oxidative stress and improve ovarian function but also regulate hormone levels. From the perspective of TCM theory, HSYC could tonify the liver and kidney, nourish essence and blood, soften the liver and benefit heart Qi, regulating body functions with a holistic concept. While resisting oxidative stress, it also improves symptoms such as insomnia, lassitude, nervousness, hot flashes, and night sweats. Herein, HSYC takes effect through multiple targets and multiple links, highlighting the unique advantages of Chinese traditional medicine.
5. Conclusions
The fertility of aging mice decreases and repeated superovulation could cause oxidative stress to damage ovarian function, and further reduce the number and quality of eggs. The LC-MS results indicate that HSYC contains ample and certain antioxidant bioactive compounds. The present study revealed that the TCM formula, HSYC, has exerted promising effects in promoting ovarian reserve, oocyte quality, and embryo hatching potential, and could reverse the deleterious effect induced by both aging and consecutive ovarian superovulation, potentially via the ROS/JNK/p53 pathway.
Acknowledgments
This work was financially supported through grants from the Nature Science Foundation Project of Zhejiang Province (grant number LQ19H270008), Zhejiang Traditional Chinese Medicine Modernization Special Project (grant number 2021ZX012), Zhejiang Basic Public Welfare Research Program (LGF22H27009), and Key Medical Discipline Project of Hangzhou (grant number 2020SJZDXK02).
Contributor Information
Qin Zhang, Email: zhaqin01@163.com.
Jing Ma, Email: 2021b085@zcmu.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Ying Zhao and Yun Chen contributed equally to this work.
References
- 1.Capalbo A., Hoffmann E. R., Cimadomo D., Maria Ubaldi F., Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Human Reproduction Update . 2017;23(6):706–722. doi: 10.1093/humupd/dmx026. [DOI] [PubMed] [Google Scholar]
- 2.Silber S. J., Kato K., Aoyama N., et al. Intrinsic fertility of human oocytes. Fertility and Sterility . 2017;107(5):1232–1237. doi: 10.1016/j.fertnstert.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature . 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi H., Takahashi T., Nagase S. Oocyte aging underlies female reproductive aging: biological mechanisms and therapeutic strategies. Reproductive Medicine and Biology . 2015;14(4):159–169. doi: 10.1007/s12522-015-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki H., Hamatani T., Kamijo S., et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Frontiers in Endocrinology . 2019;10:p. 811. doi: 10.3389/fendo.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion . 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Ding J., Tan X., Song K., Ma W., Xiao J., Zhang M. Effect of controlled ovarian hyperstimulation on puberty and estrus in mice offspring. Reproduction . 2017;154(4):433–444. doi: 10.1530/rep-16-0572. [DOI] [PubMed] [Google Scholar]
- 8.Tang S. B., Yang L. L., Zhang T. T., et al. Multiple superovulations alter histone modifications in mouse early embryos. Reproduction . 2019;157(6):511–523. doi: 10.1530/rep-18-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J. K., Wang Q., Zhang T. T., Yin S., Zhang C. L., Ge Z. J. Repeated superovulation may affect mitochondrial functions of cumulus cells in mice. Scientific Reports . 2016;6(1) doi: 10.1038/srep31368.31368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie X., Dai Y., Zheng Y., et al. Establishment of a mouse model of premature ovarian failure using consecutive superovulation. Cellular Physiology and Biochemistry . 2018;51(5):2341–2358. doi: 10.1159/000495895. [DOI] [PubMed] [Google Scholar]
- 11.Nelson S. M., Lawlor D. A. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144, 018 treatment cycles. PLoS Medicine . 2011;8(1) doi: 10.1371/journal.pmed.1000386.e1000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Qin F., Liu A., et al. RETRACTED: kuntai capsule attenuates premature ovarian failure through the PI3K/AKT/mTOR pathway. Journal of Ethnopharmacology . 2019;239 doi: 10.1016/j.jep.2019.111885.111885 [DOI] [PubMed] [Google Scholar]
- 13.Gao L., Zhang Y., Xu H., Zhao F., Wang W. Therapeutic effects of modified gengnianchun formula on stress-induced diminished ovarian reserve based on experimental approaches and network pharmacology. Drug Design, Development and Therapy . 2020;14:4975–4992. doi: 10.2147/dddt.s279553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Song K., Ma W., Ding J., Chen Z., Zhang M. Immunomodulatory mechanism of bushen huoxue recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model. Journal of Ethnopharmacology . 2017;208:44–56. doi: 10.1016/j.jep.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W., Huai Y., Miao Z., Qian A., Wang Y. Systems pharmacology for investigation of the mechanisms of action of traditional Chinese medicine in drug discovery. Frontiers in Pharmacology . 2019;10:p. 743. doi: 10.3389/fphar.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L., Cheng S., Chen B., Gao W., Weijiang C. Research on the construction status and suggestions of the first batch of “national academic school of traditional Chinese medicine inheritance studio construction units” in Zhejiang Province. Journal of Zhejiang University of Traditional Chinese Medicine . 2020;44 [Google Scholar]
- 17.Zhang Q., Yang L., Chen M., Xu R. The clinical observation on diminished ovarian reserve of kidney deficiency syndrome treated with HE’s Yangchao granule. Journal of Zhejiang Chinese Medical University . 2020;44 [Google Scholar]
- 18.Chen Y., Wang L., Zhang Q. Qin Zhang used “He’s Yangchao recipe” to treat 3 cases of infertility with diminished ovarian reserve. Jiangsu Journal of Traditional Chinese Medicine . 2020;52:62–64. [Google Scholar]
- 19.Zhao Y., Zhang Q. Effect of He’s Yangchao recipe on ovarian reserve function in DOR mice. Zhejiang Clinical Medical Journal . 2019;21:1479–1481. [Google Scholar]
- 20.McGrath J. C., Drummond G. B., McLachlan E. M., Kilkenny C., Wainwright C. L. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. British Journal of Pharmacology . 2010;160(7):1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes K., Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Medicine . 2017;47(8):1619–1636. doi: 10.1007/s40279-017-0677-3. [DOI] [PubMed] [Google Scholar]
- 22.Becatti M., Fucci R., Mannucci A., et al. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive Technology procedures. International Journal of Molecular Sciences . 2018;19(2):p. 592. doi: 10.3390/ijms19020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Okuka M., McLean M., Keefe D. L., Liu L. Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro. Free Radical Biology and Medicine . 2010;48(12):1663–1676. doi: 10.1016/j.freeradbiomed.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Kauppila T. E. S., Kauppila J. H. K., Larsson N. G. Mammalian mitochondria and aging: an update. Cell Metabolism . 2017;25(1):57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Lai Z., Shi L., et al. Correction: repeated superovulation increases the risk of osteoporosis and cardiovascular diseases by accelerating ovarian aging in mice. Aging (Albany NY) . 2018;10(9) doi: 10.18632/aging.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalthur G., Salian S. R., Nair R., et al. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reproduction, Fertility and Development . 2016;28(12):p. 2027. doi: 10.1071/rd15184. [DOI] [PubMed] [Google Scholar]
- 27.Chao H. T., Lee S. Y., Lee H. M., Liao T. L., Wei Y. H., Kao S. H. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Annals of the New York Academy of Sciences . 2005;1042(1):148–156. doi: 10.1196/annals.1338.016. [DOI] [PubMed] [Google Scholar]
- 28.Park S. J., Kim T. S., Kim J. M., Chang K. T., Lee H. S., Lee D. S. Repeated superovulation via PMSG/hCG administration induces 2-cys peroxiredoxins expression and overoxidation in the reproductive tracts of female mice. Molecular Cell . 2015;38(12):1071–1078. doi: 10.14348/molcells.2015.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mara J. N., Zhou L. T., Larmore M., et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging (Albany NY) . 2020;12(10):9686–9713. doi: 10.18632/aging.103237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shestakova I. G., Radzinsky V. E., Khamoshina M. B. Occult form of premature ovarian insufficiency. Gynecological Endocrinology . 2016;32(sup2):30–32. doi: 10.1080/09513590.2016.1232676. [DOI] [PubMed] [Google Scholar]
- 31.Shahine L. K., Marshall L., Lamb J. D., Hickok L. R. Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertility and Sterility . 2016;106(5):1124–1128. doi: 10.1016/j.fertnstert.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Takeo T., Mukunoki A., Nakagata N. Ovulation of juvenile, mature, and aged female C57BL/6 mice following coadministration of inhibin antiserum and equine chorionic gonadotropin. Theriogenology . 2019;135:1–6. doi: 10.1016/j.theriogenology.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Ren X., Wang S., Zhang C., et al. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicology and Environmental Safety . 2020;192 doi: 10.1016/j.ecoenv.2020.110266.110266 [DOI] [PubMed] [Google Scholar]
- 34.Shen H. M., Liu Z. G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radical Biology and Medicine . 2006;40(6):928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 35.Donnapee S., Li J., Yang X., et al. Cuscuta chinensis Lam.: a systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. Journal of Ethnopharmacology . 2014;157:292–308. doi: 10.1016/j.jep.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Lee M. R., Kim B., Lee Y., et al. Ameliorative effects of pueraria lobata extract on postmenopausal symptoms through promoting estrogenic activity and bone markers in ovariectomized rats. Evidence-Based Complementary and Alternative Medicine . 2021;2021:8. doi: 10.1155/2021/7924400.7924400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Liu J., Nie G., Li Y., Yang H. Danggui buxue tang rescues folliculogenesis and ovarian cell apoptosis in rats with premature ovarian insufficiency. Evidence-Based Complementary and Alternative Medicine . 2021;2021:11. doi: 10.1155/2021/6614302.6614302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Y., Qu C., Tang Y., et al. Herb pairs containing Angelicae sinensis radix (Danggui): a review of bio-active constituents and compatibility effects. Journal of Ethnopharmacology . 2016;181:158–171. doi: 10.1016/j.jep.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Li H. F., Shen Q. H., Chen W. J., Chen W. M., Feng Z. F., Yu L. Y. Efficacy of traditional Chinese medicine tonifying kidney (bushen) and activating blood (huoxue) prescription for premature ovarian insufficiency: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine . 2020;2020:13. doi: 10.1155/2020/1789304.1789304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabavi S. M., Samec D., Tomczyk M., et al. Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnology Advances . 2020;38 doi: 10.1016/j.biotechadv.2018.11.005.107316 [DOI] [PubMed] [Google Scholar]
- 41.Liang B., Zhu Y. C., Lu J., Gu N. Effects of traditional Chinese medication-based bioactive compounds on cellular and molecular mechanisms of oxidative stress. Oxidative Medicine and Cellular Longevity . 2021;2021:9. doi: 10.1155/2021/3617498.3617498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q., Chen D., Yu B., et al. Effects of dietary daidzein supplementation on reproductive performance, serum hormones, and reproductive-related genes in rats. Nutrients . 2018;10(6):p. 766. doi: 10.3390/nu10060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H., Zhang C., Zeng W. Estrogenic and antioxidant effects of a phytoestrogen daidzein on ovarian germ cells in embryonic chickens. Domestic Animal Endocrinology . 2006;31(3):258–268. doi: 10.1016/j.domaniend.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Santos J. M. S., Monte A. P. O., Lins T., et al. Kaempferol can be used as the single antioxidant in the in vitro culture medium, stimulating sheep secondary follicle development through the phosphatidylinositol 3-kinase signaling pathway. Theriogenology . 2019;136:86–94. doi: 10.1016/j.theriogenology.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 45.Yao X., Jiang H., NanXu Y., Piao X., Gao Q., Kim N.-H. Kaempferol attenuates mitochondrial dysfunction and oxidative stress induced by H2O2 during porcine embryonic development. Theriogenology . 2019;135:174–180. doi: 10.1016/j.theriogenology.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Wei M., Mahady G., Liu D., Zheng Z., Lu Y. Astragalin, a flavonoid from morus alba (mulberry) increases endogenous estrogen and progesterone by inhibiting ovarian granulosa cell apoptosis in an aged rat model of menopause. Molecules . 2016;21(5):p. 675. doi: 10.3390/molecules21050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie X., Sheng W., Hou D., Liu Q., Wang R., Tan Y. Effect of Hyperin and Icariin on steroid hormone secretion in rat ovarian granulosa cells. Clinica Chimica Acta . 2019;495:646–651. doi: 10.1016/j.cca.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Fan G., Wei F., Bu Y., Huang W. Hyperoside protects rat ovarian granulosa cells against hydrogen peroxide-induced injury by sonic hedgehog signaling pathway. Chemico-Biological Interactions . 2019;310 doi: 10.1016/j.cbi.2019.108759.108759 [DOI] [PubMed] [Google Scholar]
- 49.Haddad Y. H., Said R. S., Kamel R., Morsy E. M. E., El-Demerdash E. Phytoestrogen genistein hinders ovarian oxidative damage and apoptotic cell death-induced by ionizing radiation: co-operative role of ER-beta, TGF-beta, and FOXL-2. Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-70309-2.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh D. O., Mansour D. F. Ovario-protective effects of genistein against cyclophosphamide toxicity in rats: role of anti-mullerian hormone and oestradiol. European Journal of Pharmacology . 2016;789:163–171. doi: 10.1016/j.ejphar.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Luo M., Yang Z. Q., Huang J. C., Wang Y. S., Guo B., Yue Z. P. Genistein protects ovarian granulosa cells from oxidative stress via cAMP-PKA signaling. Cell Biology International . 2020;44(2):433–445. doi: 10.1002/cbin.11244. [DOI] [PubMed] [Google Scholar]
- 52.Mottola F., Scudiero N., Iovine C., Santonastaso M., Rocco L. Protective activity of ellagic acid in counteract oxidative stress damage in zebrafish embryonic development. Ecotoxicology and Environmental Safety . 2020;197 doi: 10.1016/j.ecoenv.2020.110642.110642 [DOI] [PubMed] [Google Scholar]
- 53.Baeeri M., Momtaz S., Navaei-Nigjeh M., et al. Molecular evidence on the protective effect of ellagic acid on phosalone-induced senescence in rat embryonic fibroblast cells. Food and Chemical Toxicology . 2017;100:8–23. doi: 10.1016/j.fct.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Kim K. J., Chun J. L., Lee K. B., et al. Effect of acteoside on the re-localization and abnormal morphology of mitochondria in porcine oocytes during in vitro maturation. Journal of Assisted Reproduction and Genetics . 2016;33(7):939–948. doi: 10.1007/s10815-016-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J. H., Chun J. L., Kim K. J., et al. Effect of acteoside as a cell protector to produce a cloned dog. PLoS One . 2016;11(7) doi: 10.1371/journal.pone.0159330.e0159330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q., Pan J., Zhao C., Wang Y., Jia Z., Zheng R. Non-enzymatic fast repair of DNA oxidative damage might also exist in cells. Cell Biology International . 2008;32(6):654–662. doi: 10.1016/j.cellbi.2008.01.291. [DOI] [PubMed] [Google Scholar]
- 57.Lins T. L. B. G., Gouveia B. B., Barberino R. S., et al. Rutin prevents cisplatin-induced ovarian damage via antioxidant activity and regulation of PTEN and FOXO3a phosphorylation in mouse model. Reproductive Toxicology . 2020;98:209–217. doi: 10.1016/j.reprotox.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Nayki C., Nayki U., Keskin Cimen F., et al. The effect of rutin on ovarian ischemia-reperfusion injury in a rat model. Gynecological Endocrinology . 2018;34(9):809–814. doi: 10.1080/09513590.2018.1450378. [DOI] [PubMed] [Google Scholar]
- 59.Li B., Weng Q., Liu Z., et al. Selection of antioxidants against ovarian oxidative stress in mouse model. Journal of Biochemical and Molecular Toxicology . 2017;31(12) doi: 10.1002/jbt.21997.e21997 [DOI] [PubMed] [Google Scholar]
- 60.Chen Y. H., Bi J. H., Xie M., et al. Classification-based strategies to simplify complex traditional Chinese medicine (TCM) researches through liquid chromatography-mass spectrometry in the last decade (2011–2020): theory, technical route and difficulty. Journal of Chromatography A . 2021;1651 doi: 10.1016/j.chroma.2021.462307.462307 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


