Abstract
Background
Uric acid nephropathy, also known as hyperuricemia nephropathy or gouty nephropathy, is characterized by uric acid crystal deposition and inflammatory cell infiltration. Herein, we aimed to demonstrate the role of febuxostat on uric acid levels and renal function in patients with chronic kidney disease and hyperuricemia.
Methods
Eight databases included were searched for clinical randomized controlled trials. Meanwhile, the confidence interval (CI) of either relative risk or mean difference was set to 95%. Besides, the heterogeneity of the research results is tested by I2.
Results
Ten studies were ultimately included in this meta-analysis. All of them were considered to be random controlled trials. 10 studies reported the serum uric acid of the test group and the control group, which was significantly lower (SMD: -146.44, 95% Cl: -195.96, -86.93, and P < 0.01) than the control group, EGFR (SMD: 3.21, 95% Cl: 1.17, 5.25, and P < 0.01), serum creatinine (SMD: -15.27, 95% Cl: -20.75, -9.79, and P < 0.01), serum urea nitrogen (SMD: -2.37, 95% Cl: -3.31, -1.61, and P < 0.01), and adverse reactions (OR: 0.74, 95% Cl: 0.32, 1.68, and P = 0.47).
Conclusion
The results of this study suggest that febuxostat may be effective in patients with CKD with HUA, as evidenced by serum uric acid, creatinine, urea nitrogen, and EGFR. However, large sample, multicenter, low risk of bias clinical studies, as well as basic medical research, are needed.
1. Introduction
Hyperuricemia (HUA), commonly defined in the literature as serum uric acid (sUA) levels above 6 mg/dl [1], has a prevalence of 21% in the United States [2]. It is associated with a higher risk of high blood pressure, coronary artery disease, and stroke [3]. HUA is associated with the pathophysiology of kidney disease, making clinical research an active research interest [4, 5].
Previous meta-analyses have shown that HUA is a risk factor for the development of chronic kidney disease (CKD) and incidental kidney disease, including end-stage renal disease, albuminuria, or elevated serum creatinine [6]. Rodenbach et al. [7] conducted a 5-year cross-sectional study, and the results showed that the HUA group of CKD children had a worse prognosis than the normal serum uric acid group. It was reported that more than 70% of HUA patients suffered from different degrees of HUA. 36.3% of patients tend to have renal failure [8]. Another study showed that nearly 0.6% to 1.0% of patients with renal failure were caused by gout. A New Zealand report showed that the patients who died due to gouty nephropathy can account for 16% to 27% of the patients who died due to gout complications [9].
Febuxostat is a novel xanthine oxidase-selective inhibitor that inhibits both xanthine oxidase and xanthine dehydrogenase activities and reduces blood urate concentrations [10]. Febuxostat is mainly metabolized by the liver into inactive metabolites, which can be completely excreted from the body. Therefore, the metabolic process is less affected by renal function, and its renal toxicity is also mild. To a certain extent, it can improve renal function and reduce serum creatinine [11, 12]. Thus, we conducted a meta-analysis to examine the efficacy and safety of febuxostat in patients with CKD with HUA.
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Study Design
All randomized controlled trials (RCTs) investigating febuxostat combined with other therapies in the treatment of CKD with HUA were not limited by language or publication status.
2.1.2. Research Object
This includes patients with CKD with HUA, with no recent history of drug therapy such as febuxostat and allopurinol.
2.1.3. Intervention Measures
The experimental group was treated with febuxostat or febuxostat in combination with other therapies for intervention, and the control group was treated with nonfebuxostat, including allopurinol or placebo therapy. In addition, the following exclusion criteria were applied: ① nonrandomized controlled trial research literature, ② literature that did not report febuxostat as an intervention measure, ③ literature without original data or incomplete research data, ④ inconsistent outcome indicators or statistical methods, and ⑤ literature review or animal experiment research.
2.1.4. Outcome Indicators
Through the review of clinical trials published in major databases and academic journals to evaluate CKD with HUA, we found that the commonly used evaluation indicators include the following: ① serum uric acid, ② EGFR, ③ serum creatinine, ④ serum urea nitrogen, and ⑤ adverse reactions.
2.2. Search Strategy
We searched PubMed, Embase, Cochrane Library, Web of Science, Wan-Fang database, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals Database (VIP), and CBM Libraries for relevant randomized controlled trials in each database from March 2012 up to March 2022. For English databases, we used free text terms such as “febuxostat” or “chronic kidney disease” or “hyperuricemia.” For the Chinese databases, it is suggested the search terms could be presented using the Chinese phonetic alphabet.
2.3. Literature Screening and Data Extraction
Two researchers conducted literature screening independently in strict accordance with inclusion and exclusion criteria. Then, they managed and identified the retrieved literature by the NoteExpress software (v.2.7.1). After picking, researchers read the topic and abstract for preliminary screening and then further read the full text for rescreening to determine whether to include and extract valid data, respectively, to establish Excel effective data extraction table. In case of disagreement, a third researcher shall be invited to solve the disagreement through consultation.
2.4. Statistical Analysis
The Stata 15.1 software was used to perform the meta-analysis. If for the binary classification variables using relative risk (RR), said the confidence interval (CI) is set to 95%. Continuity variables were represented by mean difference (MD), and confidence interval (CI) was set at 95%. Heterogeneity of research results was tested by I2. If I2 ≤ 50%, outcome data of fixed effects model (FE) were selected for analysis; if I2 > 50%, outcome data of random effects model (RE) were selected for reference analysis. At the same time, sensitivity analysis was used to observe heterogeneous sources and evaluate the stability of meta-analysis results.
3. Results
3.1. Search Results
Based on the search strategy, 631 references were identified. After excluding duplicate studies, 306 studies were scanned based on abstract and title. Then, 12 articles were evaluated in full text. After full text evaluation, 2 records were excluded for the following reasons: data mismatch (n = 1) and missing data (n = 1). Ultimately, 10 studies [13–16] were included in this meta-analysis (Table 1). The PRISMA statement flow chart shows this process (Figure 1).
Table 1.
Baseline characteristics.
| Study (ref.) | Sample size (T/C) | Man/woman | Age (years) (mean ± SD) (T/C) | T | C | Outcomes |
|---|---|---|---|---|---|---|
| Huang 2018 [14] | 42/41 | 27/14 | 61.97 ± 3.12 | Febuxostat | Placebo | ①③④⑤ |
| Sircar 2015 [15] | 45/48 | 66/27 | 56.22 ± 9.83/61.83 ± 12.00 | Febuxostat | Placebo | ②⑤ |
| Zhang 2021 (1) [16] | 36/30 | 41/25 | 80.13 ± 9.67 | Febuxostat | Placebo | ①②③④⑤ |
| Zhao 2021 [17] | 41/41 | 47/35 | 68.45 ± 5.20/68.56 ± 5.29 | Febuxostat | Placebo | ①③④⑤ |
| Sezai 2015 [18] | 56/53 | 85/24 | 69.4 ± 10.0/69.1 ± 9.2 | Febuxostat | Allopurinol | ② |
| Tanaka 2015 [19] | 21/19 | 35/5 | 70.1 ± 9.5/66.1 ± 7.0 | Febuxostat | Allopurinol | ② |
| Zhang 2021 (2) [20] | 27/27 | 31/23 | 51.58 ± 1.45/51.56 ± 1.44 | Febuxostat | Allopurinol | ①③④⑤ |
| Zhu 2018 [21] | 32/32 | NA | NA | Febuxostat | Allopurinol | ①③④⑤ |
| Zhang 2021 (3) [22] | 67/67 | 66/68 | 55.1 ± 9.4/54.2 ± 8.4 | Febuxostat | Allopurinol | ①②③④⑤ |
| Wang 2022 [23] | 69/69 | 96/42 | 81.35 ± 2.52/81.40 ± 2.49 | Febuxostat | Allopurinol | ①②④⑤ |
①: serum uric acid; ②: EGFR; ③: serum creatinine; ④: serum urea nitrogen; ⑤: adverse reactions.
Figure 1.
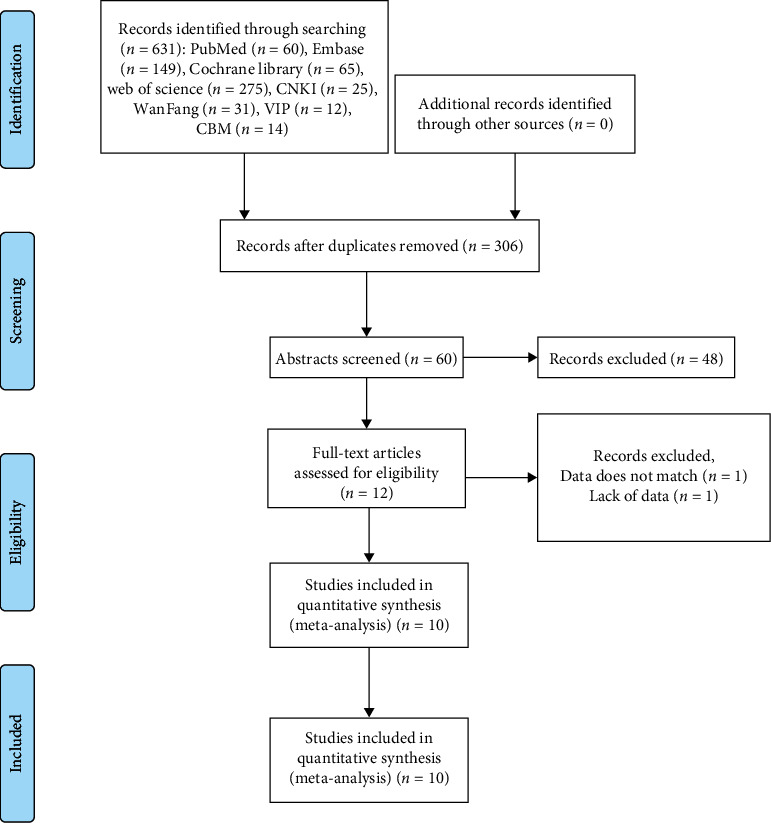
Flow chart.
3.2. Serum Uric Acid
Six studies reported the serum uric acid of the test group and the control group. Meta-analysis showed that the serum uric acid of the test group was significantly lower (SMD: -146.44, 95% Cl: -195.96, -86.93, and P < 0.01, Figure 2) than the control group. The results of all these trials showed high heterogeneity, and thus, a sensitivity analysis was conducted (Figure 3). Compared with the control group, febuxostat significantly reduces the level of uric acid in patients with CKD.
Figure 2.
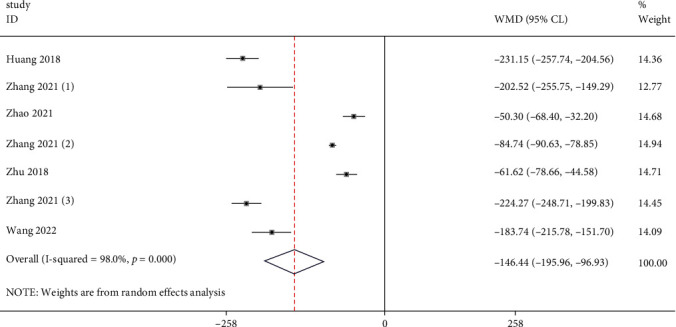
Forest illustration of the serum uric acid.
Figure 3.
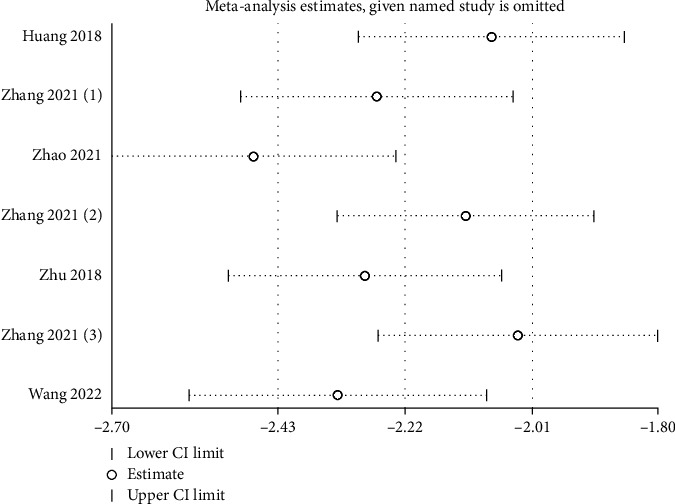
Sensitivity analysis of the serum uric acid.
3.3. EGFR
Six studies reported the EGFR of the test group and the control group. Meta-analysis showed that the EGFR of the test group was significantly higher (SMD: 3.21, 95% Cl: 1.17, 5.25, and P < 0.01, Figure 4) than the control group. Compared with the control group, febuxostat significantly improves the EGFR level in patients with CKD and HUA.
Figure 4.
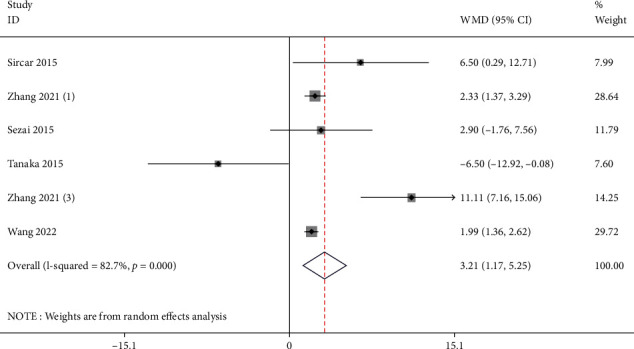
Forest illustration of the EGFR.
3.4. Serum Creatinine
Six studies reported the serum creatinine of the test group and the control group. Meta-analysis showed that the serum creatinine of the test group was significantly lower (SMD: -15.27, 95% Cl: -20.75, -9.79, and P < 0.01, Figure 5) than the control group.
Figure 5.
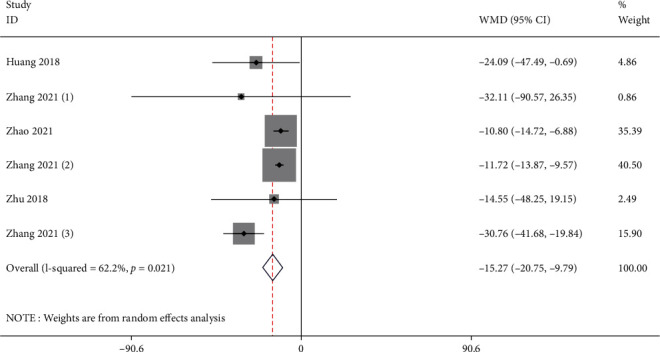
Forest illustration of the serum creatinine.
3.5. Serum Urea Nitrogen
Seven studies reported the serum urea nitrogen of the test group and the control group. Meta-analysis showed that the serum urea nitrogen of the test group was significantly lower (SMD: -2.37, 95% Cl: -3.31, -1.61, and P < 0.01, Figure 6) than the control group.
Figure 6.
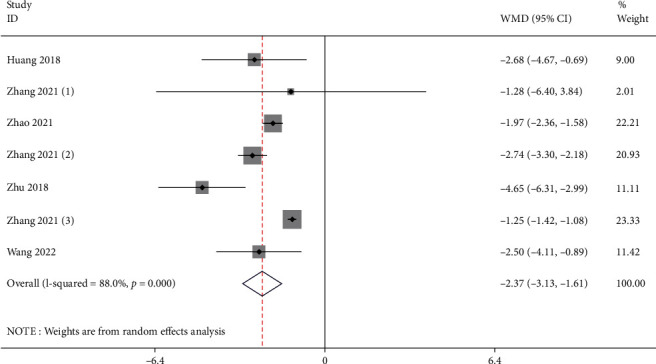
Forest illustration of the serum urea nitrogen.
3.6. Adverse Reactions
Seven studies reported the adverse reactions of the test group and the control group. Meta-analysis showed that there was no significant difference in the adverse reactions between the test group and the control group (OR: 0.74, 95% Cl: 0.32, 1.68, and P = 0.47, Figure 7).
Figure 7.
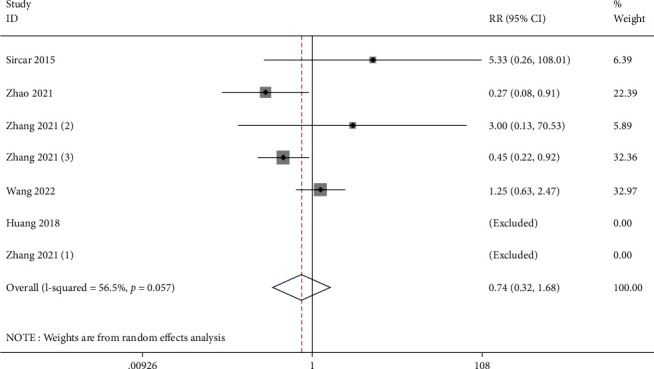
Forest illustration of the adverse reactions.
4. Discussion
Uric acid nephropathy is also known as hyperuricemia nephropathy or gouty nephropathy, and the main pathological changes are the renal pathological changes caused by uric acid crystal deposition and inflammatory cell infiltration are mainly manifested as interstitial nephritis and impaired renal tubular function and structure [17]. According to its pathological changes and clinical manifestations, it is mainly divided into 3 types: chronic uric acid nephropathy, acute uric acid nephropathy, and uric acid nephrolithiasis.
Uric acid is produced by the metabolism of DNA and/or RNA in the body and serves as the final product of purine metabolism. The product is excreted. Since humans lack urate oxidase and cannot decompose it into soluble allantoin, they are prone to hyperuricemia. Uric acid mainly comes from two ways: internal and external, of which endogenous accounts for 80%, which is synthesized by nucleic acid decomposition and amino acid, phosphoribosyl, etc. Nucleic acid protein food is decomposed. Uric acid is mainly excreted by the kidneys and intestines, of which 75% is excreted through the kidneys, and the rest is excreted after being degraded by microorganisms in the intestines [15, 18–20].
A total of 10 literatures were included in this study, including 436 patients in the experimental group and 427 in the control group. Meta-analysis showed that patients with CKD with HUA who received febuxostat had lower serum uric acid compared with controls. Meta-analysis showed satisfactory serum uric acid level for the experimental group (SMD: -146.44, 95% Cl: -195.96, -86.9, and P < 0.01). Based on the results of the meta-analysis of EGFR, compared with the control group, febuxostat significantly improves the EGFR level in patients with CKD with HUA (SMD: 3.21, 95% Cl: 1.17, 5.25, and P < 0.01). For the results of the meta-analysis of urine creatinine and urea nitrogen, compared with the control group, febuxostat significantly reduces the level of urine creatinine and urea nitrogen in patients with CKD with HUA (SMD: -15.27, 95% Cl: -20.75, -9.79, and P < 0.01 and SMD: -2.37, 95% Cl: -3.31, -1.61, and P < 0.01). There was no statistical difference in adverse reactions between the control group and the observation group after treatment (OR: 0.74, 95% Cl: 0.32, 1.68, and P = 0.47).
This study also has certain limitations. First, 10 RCTs included 863 patients. The overall sample size is not very large. All RCTs were single-center. The lack of multicenter studies may affect the representativeness of the conclusions to some extent. Second, the small sample size is not sufficient to fully assess the safety of febuxostat or other drugs.
5. Conclusion
The results of this study suggest that febuxostat may be effective in patients with CKD with HUA, as evidenced by serum uric acid, creatinine, urea nitrogen, and EGFR. However, large sample, multicenter, low risk of bias clinical studies, as well as basic medical research, are needed.
Data Availability
The data could be obtained by contacting the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bardin T., Richette P. Definition of hyperuricemia and gouty conditions. Current Opinion in Rheumatology . 2014;26(2):186–191. doi: 10.1097/BOR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y., Pandya B. J., Choi H. K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. The American journal of medicine . 2012;125(7):679–687.e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y., Pandya B. J., Choi H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis and Rheumatism . 2011;63(10):3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Lozada L. G., Tapia E., Avila-Casado C., et al. Mild hyperuricemia induces glomerular hypertension in normal rats. American Journal of Physiology. Renal Physiology . 2002;283(5):F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Lozada L. G., Tapia E., Soto V., et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrology, Dialysis, Transplantation . 2007;23(4):1179–1185. doi: 10.1093/ndt/gfm783. [DOI] [PubMed] [Google Scholar]
- 6.James M. T., Levey A. S., Tonelli M., et al. Incidence and prognosis of acute kidney diseases and disorders using an integrated approach to laboratory measurements in a universal health care system. JAMA Network Open . 2019;2(4):p. e191795. doi: 10.1001/jamanetworkopen.2019.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodenbach K. E., Schneider M. F., Furth S. L., et al. Hyperuricemia and progression of CKD in children and adolescents: the chronic kidney disease in children (CKiD) cohort study. American Journal of Kidney Diseases . 2015;66(6):984–992. doi: 10.1053/j.ajkd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G. H., Sun X. P., Li J., Liu R. K., Yang Z., Hua Q. Association between serum uric acid level and endothelial dysfunction in elderly individuals with untreated mild hypertension. Journal of Geriatric Cardiology . 2020;17(5):264–269. doi: 10.11909/j.issn.1671-5411.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J., Wang Z., Wang Y. P. Identification of common key genes associated with Crohn's disease and IgA nephropathy. European Review for Medical and Pharmacological Sciences . 2022;26(10):3607–3620. doi: 10.26355/eurrev_202205_28857. [DOI] [PubMed] [Google Scholar]
- 10.Quilisadio J. E. C., Salido E. O., Penserga E. G. Achievement of the target serum urate level among patients with gout treated with allopurinol or febuxostat in an arthritis clinic in the Philippines. Modern Rheumatology . 2021;31(3):755–761. doi: 10.1080/14397595.2020.1800557. [DOI] [PubMed] [Google Scholar]
- 11.Kojima S., Uchiyama K., Yokota N., et al. C-reactive protein levels and cardiovascular outcomes after febuxostat treatment in patients with asymptomatic hyperuricemia: post-hoc analysis of a randomized controlled study. Cardiovascular Drugs and Therapy . 2022 doi: 10.1007/s10557-022-07347-7. [DOI] [PubMed] [Google Scholar]
- 12.Pavelcova K., Bohata J., Pavlikova M., Bubenikova E., Pavelka K., Stiburkova B. Evaluation of the influence of genetic variants of SLC2A9 (GLUT9) and SLC22A12 (URAT1) on the development of hyperuricemia and gout. Journal of Clinical Medicine . 2020;9(8):p. 2510. doi: 10.3390/jcm9082510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuruta Y., Mochizuki T., Moriyama T., et al. Switching from allopurinol to febuxostat for the treatment of hyperuricemia and renal function in patients with chronic kidney disease. Clinical Rheumatology . 2014;33(11):1643–1648. doi: 10.1007/s10067-014-2745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sezai A., Soma M., Nakata K. I., et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD) Journal of Cardiology . 2015;66(4):298–303. doi: 10.1016/j.jjcc.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Hu A. M., Brown J. N. Comparative effect of allopurinol and febuxostat on long-term renal outcomes in patients with hyperuricemia and chronic kidney disease: a systematic review. Clinical Rheumatology . 2020;39(11):3287–3294. doi: 10.1007/s10067-020-05079-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Wan D., Yang G., Peng Q., Wang X. Febuxostat is superior to allopurinol in delaying the progression of renal impairment in patients with chronic kidney disease and hyperuricemia. International Urology and Nephrology . 2019;51(12):2273–2283. doi: 10.1007/s11255-019-02318-8. [DOI] [PubMed] [Google Scholar]
- 17.Di Stolfo G., Mastroianno S., Potenza D. R., et al. Serum uric acid as a prognostic marker in the setting of advanced vascular disease: a prospective study in the elderly. Journal of Geriatric Cardiology . 2015;12(5):515–520. doi: 10.11909/j.issn.1671-5411.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. International Journal of Cardiology . 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 19.Hassan W., Shrestha P., Sumida K., et al. Association of uric acid-lowering therapy with incident chronic kidney disease. JAMA Network Open . 2022;5(6):p. e2215878. doi: 10.1001/jamanetworkopen.2022.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao X., Guo D., Yuan X., Xu B. The predictive value of cystatin C combined with lactic acid and uric acid in the occurrence of acute kidney injury in sepsis. Clinical Nephrology . 2022;97(1):60–62. doi: 10.5414/CN110707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data could be obtained by contacting the corresponding author.


