Abstract
For decades, traditional in vitro and in vivo models used for the study of Helicobacter pylori infection have relied heavily on the use of gastric cancer cell lines and rodents. Major challenges faced by these methods have been the inability to study cancer initiation in already cancerous cell lines, and the difficulty in translating results obtained in animal models due to genetic differences. These challenges have prevented a thorough understanding of the pathogenesis of disease and slowed the development of cancer therapies and a suitable vaccine against the pathogen. In recent years, the development of gastric organoids has provided great advantages over the traditional in vivo and in vitro models due to their similarities to the human stomach in vivo, their ease of use, and the capacity for long‐term culture. This review discusses the advantages and limitations of existing in vivo and in vitro models of H. pylori infection, and how gastric organoids have been applied to study H. pylori pathogenesis, with a focus on how the pathogen interacts with the gastric epithelium, inflammatory processes, epithelial repair, and cancer initiation. The potential applications of organoids to address more complex questions on the role of hormones, vaccine‐induced immunity are also discussed.
Keywords: Helicobacter pylori, in vitro models, organoid
Significance Statement
Organoid cultures offer advantages over conventional in vitro models and are providing new opportunities for modeling H. pylori disease. This review discusses how gastric organoids have enabled progress in understanding the mechanisms of H. pylori pathogenesis. We focus on recent work on initial interactions with the gastric epithelium, inflammatory processes, epithelial repair, and gastric carcinogenesis. In the next 5 years, the use of gene‐edited organoids and more complex organoid models using co‐culture with immune and other cell populations provide exciting potential for studying inflammation, cancer initiation, and even vaccine‐induced protection against H. pylori.
1. INTRODUCTION
1.1. In vivo and in vitro models of H. pylori infection have both advantages and limitations
Over the years, one of the major challenges in science has been the lack of suitable model systems for infections affecting humans. Host–pathogen research has mainly relied on in vitro and in vivo models in rodents. Recently, the development of model systems such as organoids and lab‐on‐a‐chip systems have opened up more opportunities to increase our understanding of host–pathogen interactions. Helicobacter pylori is a significant cause of chronic gastric diseases, such as ulcers and gastric cancer—the third leading cause of cancer‐related death globally. 1 Increasing resistance to antibiotics and the risk of reinfection have prevented a comprehensive understanding of the host immune response and the development of gastric cancer. 2 , 3 Thus, H. pylori infection studies would benefit from the availability of better model systems that more accurately mimic H. pylori pathogenesis in humans.
In vivo studies on H. pylori pathogenesis have largely relied on the use of mice and gerbils. Ethical and practical constraints have limited the degree to which vaccines and therapies can be tested in primates. Although a challenge model in human volunteers has been established for H. pylori infection, 4 only a limited number of trials have been performed. 5 An interesting outcome of the most recent trial has been that challenge of adults resulted in spontaneous clearance in some volunteers, which has implications for future vaccine trials. Field trials in areas with a high prevalence of H. pylori infections may be the best way forward for clinical testing of vaccines and therapeutics (reviewed in Ref. [6]). Although mouse models have permitted detailed immunological studies, and some models of carcinogenesis exist, there remains the difficulty in translating results obtained in animal models to human patients. The latter has been a particular problem for vaccine development where promising candidates from animal studies have been poorly immunogenic or showed no protective effect in clinical trials. 5 , 6 The difficulty in translating from mouse to human vaccines may be attributed to factors such as the relative genetic uniformity of experimental animals as compared to human populations, regional and population differences in H. pylori strains, differences in bacterial virulence in rodent models, immune response mechanisms, and also to differences in the microbiota of mice and humans. 7 While mouse models have a relatively close genetic similarity to humans and are easily amenable to genetic manipulation, they sometimes produce varying results based on differences in their genetic backgrounds. 8 , 9 There are only a few knockout and transgenic mouse models that develop cancer. Their inability to proceed to the metaplastic stage 10 also limits their application in the study of gastric cancer. Other models such as gerbils (Meriones unguiculatus) can develop tumors; however, it occurs rapidly, they are outbred and the suite of reagents available for immunological studies in mice are not available for gerbils. 11 , 12 , 13
In vitro studies have been chiefly focused on the use of epithelial cells derived from cancerous lesions (e.g., AGS, MKN28) or viral transformation (e.g., GES‐1). These studies have been key to our understanding of the cell signaling events in early H. pylori infection, including the key role of the H. pylori type IV secretion system (TIVSS) in translocating CagA in pathogenesis (reviewed in Ref. [ 14 ]), and the role of other bacterial virulence factors using deletion mutants (reviewed in Ref. [ 15 ]) including the key discovery that the TIVSS actually binds to β1‐ integrin which is located only on the basolateral side in vivo. 16 Continuous cell lines are, however, either transformed in vitro, or originally derived from cancerous cells, are non‐polarized, and it becomes difficult to properly evaluate the host innate immune response at the early stage of H. pylori infection, and the sequence of events that leads to metaplasia. 11 Efforts to use primary cells for these studies have been limited by the short lifespan in vitro, with infected cells not lasting beyond 48 h. 17 , 18 , 19
More recently, in infection biology, the use of stem cell‐derived organoid cultures is being adopted as a suitable model largely due to its ability to closely mimic human physiology. Its potential for genetic manipulation with novel gene‐editing tools such as CRISPRcas9 and siRNA at a faster rate and with less labor compared to animal models also makes it a promising model for infection studies. 2 , 20 , 21 These methods have already facilitated a better understanding of some human diseases. For instance, organoid cultures have been successfully applied to understand the biology of Zika virus and the emergence of microencephaly during brain development. 22 , 23 Organoid systems have also permitted the culturing of Noroviruses 24 and have opened up the possibility of precision medicine for patients with cystic fibrosis. 25 , 26
For H. pylori infection, the use of organoids derived from primary cells for in vitro studies offers advantages over conventional in vivo and in vitro techniques in terms of amenability, accessibility, polarization, and longevity. This potentially eliminates the dependence on neoplastic cells in the investigation of the initiating steps in gastric carcinogenesis and could facilitate an in‐depth understanding of the mechanisms of pathogenesis. There have been a number of reviews on gastric organoids, particularly in the study of gastric cancer and H. pylori pathogenesis, 20 , 27 , 28 , 29 and the generation of human gastric organoids. 30 Recent reviews have discussed advances in techniques, 21 advances and discoveries in the area of cancer initiation, 28 however, the use of organoid models for studies on H. pylori biology, and innate inflammation have not been comprehensively reviewed since 2017. 31 Thus, this review aims to aggregate and discuss recent advances from studies with gastric organoids in terms of how H. pylori interacts with the gastric epithelium, triggers inflammation, and its role in the initiation of gastric cancer. We also propose that the use of more complex organoid models will be beneficial for the study of gastric epithelial repair, the role of gastric hormones, and mechanisms of vaccine‐induced protection.
1.2. Development of mouse and human gastric organoids
The successful development of organoids has hinged on the identification of the necessary growth factors and conditions, and pivotal lineage tracing studies that led to the identification of multiple adult stem cell populations in the gastric gland (reviewed in Ref. [ 32 ]). The initial studies on the development of intestinal organoids from human embryonic and induced pluripotent stem cells (PSC) laid the foundation for the identification of the growth factors required for gastric organoids. 33 , 34 The generation of gastric organoids from PSC involves the use of growth factors which mimic embryonic development and was first described by McCracken et al., 35 Human ES cell lines were first differentiated into definitive endoderm by the addition of activin A. Further treatment with FGF4, Noggin, retinoic acid and WNT3A or its agonist CHIR99021 induces differentiation into posterior foregut spheroids which are then seeded in Matrigel™ to generate gastric organoids which developed a complex three‐dimensional organization and differentiated antral cell types (surface mucous cells, antral gland cells, LGR5+ve cells, endocrine cells), but not parietal or chief cells which are characteristic of the fundus/corpus. 35
Gastric organoids generated from adult stem cells from gastric glands depend on growth factors that mimic tissue regeneration. Studies on gastric homeostasis employing both organoids and animal model studies have also identified the key factors necessary for gastric stem cell proliferation including Wnt3/β‐catenin signaling which is augmented by R‐spondin 36 and differentiation (Notch signaling). 37 A recent study by Wölffling et al. 38 showed that epidermal growth factor (EGF) is a major fate determining factor in the gastric gland, and that gradients of EGF and bone morphogenic factor and Noggin signals are responsible for differentiation and foveolar, chief and acid‐producing parietal cells to generate a gland‐type or a pit‐type phenotype. 38 , 39 , 40 , 41 As a result of these studies, there are now well‐established protocols for gastric organoid cultures, and H. pylori infection 41 , 42 and commercially available media for organoid cultures. 33 , 43 , 44 , 45
The development of gastric organoids from adult gastric glands began with the discovery by Barker and co‐workers 46 that Lgr5+ve stem cells are responsible for the constant renewal of the gastric epithelium and that these stem cells can differentiate into the different cell types in the gastric crypts. These studies also revealed that Wnt signaling is necessary for the expression of Lgr5 and that its agonist R‐spondin further increases this expression. Organoids were generated from the Lgr5+ve stem cells in mouse stomach; however, while the Lgr5+ve stem cells were found in the pyloric region, they were absent in the corpus, suggesting that other stem cells may also be responsible for the renewal of the gastric epithelium. In another study, Stange et al 39 proposed that a subset of chief and parietal cells that express Troy and are located near the base of the corpus glands express Wnt target genes and can act as a "reserve" of stem cells that can renew the corpus epithelium. These stem cells were also shown to act as adult stem cells which could be successfully grown into organoids that differentiated into mucus neck cells and pit cells. Gene expression analysis revealed that Troy+ cells were enriched for expression of Lgr5, and CD44, a marker of epithelial stem cell marker, and a Wnt3A target gene. 39 A later study, using murine fundic glands, generated organoids by co‐culture with immortalized stomach mesenchymal cells as a source of growth factors that were able to differentiate into all fundic cell types including parietal cells. 47
The development of murine organoids has been followed by the successful development of human gastric organoids (Figure 1). Initial studies involved the use of the commercial extracellular matrix mixture ‐ Matrigel™. McCracken et al. 35 first developed human antral spheroids and organoids using induced pluripotent stem cells seeded in Matrigel, while Bartfeld et al. 40 recorded the development of the first human corpus spheroidal organoids using gastric glands seeded in Matrigel. H. pylori infection in these earlier studies was performed by microinjection into the spheroid lumen because the interior formed the luminal edge of the epithelial cells. However, a later study involving the initial generation of antral and corpus spheroids in Matrigel was further developed such that spheroids could be successfully cultured in 2D monolayers on collagen‐coated surface. Although these cultures barely lasted for 24 h, infection did not require microinjection. 11 A later study by the same group described the generation of mucosoid cultures from antral gland using an air–liquid interface (ALI) system. This was made up of collagen‐coated transwell system and permitted simple addition of the bacteria to the luminal side of the culture for infection. 41 Mucosoid cultures can be maintained as ALI for up to a month 41 and the transwell format permits investigation of soluble factors and cells added to different compartments of the ALI culture. Frequent removal of mucus and change of media in the basal compartment are required, 41 H. pylori can be co‐cultured in the apical compartment for several days for experiments.
FIGURE 1.
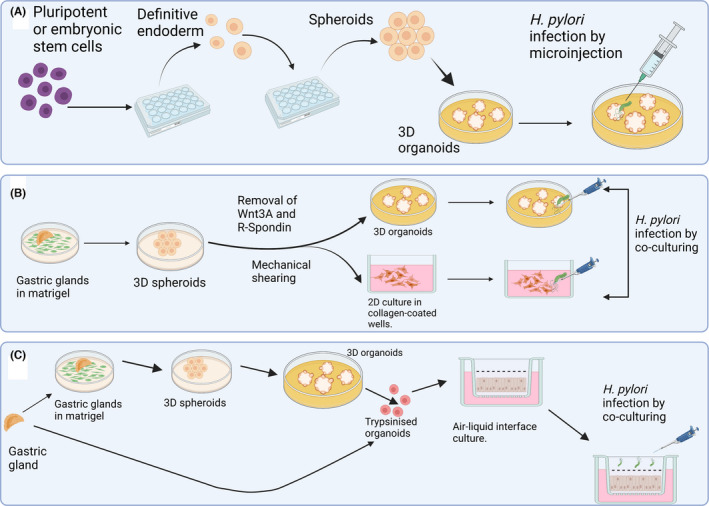
Three major types of gastric organoid culture systems that are currently in use. (A) The generation of 3D gastric organoids with H. pylori infection achieved by microinjection. 35 , 40 By manipulating the growth factors, stem cells were first developed into definitive endoderm, and then into spheroids before eventually being transformed into 3D organoids. (B) 3D spheroids were generated from gastric glands seeded in Matrigel and either used to generate organoids or cultured in a 2D cell culture system. (C) Mucosoid cultures derived either from 3D organoids or directly from gastric glands are cultured in a transwell system. H. pylori infection is achieved by co‐culturing for both (B) and (C)
An interesting feature of the various gastric organoid culture systems is their ability to replicate the characteristic events of a H. pylori infection which has permitted a range of detailed studies on the biology of the interaction of the bacteria with the gastric epithelium which are discussed below.
1.3. Application of organoids to study epithelial interactions and the initiation of inflammation by H. pylori
At the level of initial epithelial interactions, organoid culture models have further increased our understanding of how chemoattraction enables the persistent colonization of the host by H. pylori. Notably, it permitted the identification of TlpB (HP0103) as one of the chemoreceptors responsible for the attraction of H. pylori to the gastric epithelium. Huang et al. 48 reported that H. pylori are attracted to urea secreted by the gastric epithelium and that this is how the pathogen locates the gastric epithelium for colonization. The polarized nature of gastric organoids enabled this discovery. Dulbecco's modified eagle medium (DMEM) was conditioned with epithelial secretions from uninfected human gastric organoids. Free‐swimming wildtype (WT) H. pylori were attracted to the conditioned medium and not attracted to the unconditioned media. Mutant H. pylori lacking just the TlpB gene were not attracted to the conditioned medium, while mutants lacking either TlpA, TlpC, or TlpD were all attracted to the conditioned media, implying that TlpB is responsible for this chemoattraction. This discovery enabled the researchers to further test the effect of TlpB on the ability of H. pylori to colonize persistently in vivo. Mice infected with WT H. pylori had higher colonization six weeks post‐infection compared to mice infected with the mutant lacking TlpB. 48 Additionally, the role of TlpB in sensing urea during infection was investigated in another study where the TlpB chemoreceptor was found to be responsible for the accumulation of H. pylori at damaged epithelial sites. Using murine‐derived gastroids as a restitution model, cellular injury was generated on gastric organoids by photodamage and the accumulation of WT and H. pylori mutants lacking each of the four chemoreceptors TlpA, TlpB, TlpC, and TlpD at the injury sites was monitored using confocal microscopy. Only TlpB mutants were unable to colonize the injury site whereas wildtype and other H. pylori mutants accumulated at the damage sites and also inhibited epithelial restitution. 49 However, unlike the initial study where the TlpB‐mediated response was due to the attraction to urea, the chemoattractant responsible for accumulation at the damage site remains unknown. The microinjection of urea to the lumen of the organoids prevented the accumulation of H. pylori at the damage sites. 49 Thus, implying that the TlpB chemoreceptor may sense different chemicals to attract H. pylori to the epithelium under different conditions.
Gastric organoid cultures appear to mimic the in vivo model more accurately than gastric epithelial cell lines for studying interaction of H. pylori with the apical‐junctional complex. Uotani et al. reported that epithelial morphology and IL‐8 expression were similar to what was found in in vivo model studies. 50 The integrity of the apical‐junctional complex as determined by measurement of transepithelial electrical resistance (TEER) was unaltered after 96‐hour infection with H. pylori. Initial studies with conventional cell lines could only observe TEER for 48 h and had therefore reported a 30%–50% decrease, whereas gastroid monolayers which permitted culturing for up to 96 hours revealed that although there was an initial decrease in TEER after 24 hours of infection, it reverted to normal after 48 hours. It is noteworthy that this study also found that contrary to reports from studies involving gastric cancer cell lines, 51 , 52 IL‐8 expression was independent of H. pylori virulence factors such as CagA. Wildtype H. pylori strain TN2GF4 induced increased expression of IL‐8 in AGS and MKN28 cells compared to CagPAI deletion mutant, but no difference in IL‐8 expression was observed in the gastroid monolayers infected with both strains. Clinical isolates lacking virulence factors such as CagPAI, OipA, VacA, and BabA increased IL‐8 expression in gastroid monolayers, but induced no change in MKN28 cells. Additionally, inhibitors to the JNK, MEK, P38, and IKK pathways reduced IL‐8 expression in gastric cancer cell lines. In the gastroid monolayers, only the inhibitors to P38 and IKK pathways reduced the expression, whereas inhibitors of the JNK and MEK pathways had no effect on IL‐8 expression, implying that different pathways are involved in the production of IL‐8 in gastric organoids (and presumably gastric crypts) and may be more complex than in carcinoma cell lines. While the reports of this study imply that H. pylori may exert different effects on cancer and non‐cancer patients, a limitation of this study is that gastric organoids were derived from a single healthy patient and developed from only the antrum and not the corpus which is mainly affected by gastric cancer. 53 Therefore, further studies with organoids from both stomach regions of cancer and non‐cancer patients, and investigations into the roles of different cell types found in the antrum and corpus are required.
The organoid culture model has also helped in understanding the mechanisms of H. pylori‐induced inflammation in both the innate and adaptive immune systems. Using murine embryo‐derived organoids due to their sterility, Kayisoglu et al. 54 reported that the expression pattern of Toll‐like receptor 4 (TLR4) was independent of an interaction with gut microbiota, and was instead determined by developmental stage. Both the embryo‐derived gastric organoids and gastric organoids derived from adults expressed CXCL2 at a similar level when exposed to lipopolysaccharide (LPS). Morey et al. 55 investigated interferon‐gamma (IFNγ) signalling using gastric cancer cell lines, mouse, and 2D mucosoid primary cell models and reported that H. pylori blocks IFNγ signalling via the JAK/STAT pathway in a cholesterol‐α‐glucosyltransferase (cgt)‐dependent manner in infected cells, while inducing inflammation in the surrounding uninfected cells of the epithelium. MKN45 gastric cancer cell lines, mouse and cells derived from antral gastric organoids infected with H. pylori strain P12 all blocked IFNγ compared to a mutant strain lacking cgt. Confocal microscopy observation of mucosoid cultures infected with H. pylori strain P12 in an air–liquid interface for 3 days and treated with IFNγ for 30 minutes revealed that H. pylori formed an infection foci which had lower levels of phospho‐STAT1 compared to uninfected areas. This implies that H. pylori survives by suppressing the IFNγ signaling while inducing inflammatory responses in the surrounding epithelial cells.
The ability of organoid cultures to replicate increased expression of inflammatory cytokines in response to H. pylori infection has been shown in both mucosoid cultures and 3D spheroid models. 41 , 56 Sebrell et al. reported the upregulation of CXCL1, CXCL2, and CXCL8 in human 3D spheroids infected for 3 hours, and that CXL8 secretion was higher in spheroids infected with CagA+ strains compared to CagA‐deficient strains as previously reported for human gastric epithelial cell lines. 57 Microarray analysis followed by confirmation by qPCR by Boccellato et al. also showed that, in addition to IL‐8, other proinflammatory cytokines and chemokines such as CXCL1, CXCL2, and CXCL3, lymphotoxin B (LTB), IL‐23A and TNF‐α were all upregulated, further consistent with earlier studies that reported the over‐expression of these genes expressed in H. pylori‐infected patients. 58 , 59 , 60
The ability to study epithelial responses to infection without interference by the host immune response in organoids has enabled the assessment of the role of H. pylori infection and NF‐KB on the mechanism of sonic hedgehog (Shh) signaling, which is important for the initiation of gastritis. 61 , 62 These interactions had been impossible to study in vivo due to possible interference by hematopoietic factors. 63 After observing that H. pylori stimulates Shh signaling in the parietal cells of transgenic mouse expressing Shh fused to GFP within 2 days of infection, organoids were generated from both wildtype mice and parietal cell‐specific Shh deletion KO mice and infected with H. pylori to evaluate the role of Shh signaling. Shh signaling was absent in parietal cell‐specific Shh deletion KO mice, but it was observed in organoids derived from control mice. This signaling diminished following one‐hour pre‐treatment of control organoids with NF‐κB inhibitor before infection, implying that Shh signaling in parietal cells is NF‐κB‐dependent.
In a study that employed the use of confocal microscopy and 3D reconstruction of human and animal gastric glands, H. pylori was found to activate and expand the gastric stem cells and gastric progenitor cells when it colonizes the gastric gland. 64 H. pylori mutants lacking ChePep which is required for chemotactic movement 65 were incapable of colonization and did not activate the stem cells. The organoid culture model was used to study the ability of the activated stem cells to form glands ex vivo by growing antral glands from uninfected and two‐month‐infected mice into spherical organoids in Matrigel and measuring their size daily for 6 days. Infected glands were found to be larger in size and grew faster into organoids, suggesting the activation by H. pylori infection. However, due to the short time frame, it is unclear whether this effect can persist for longer periods.
Gastric organoids have also helped in the study of STAT3 signaling, known to be relevant for inflammation‐associated gastric carcinogenesis. 66 , 67 Zhu et al 68 reported that dopamine and cAMP‐regulated phosphoprotein, Mr 32000 (DARPP‐32) induces STAT3 signaling in AGS and MKN45 cells by regulating IGF1R‐SRC signaling. The expression of STAT3, STAT3 target genes, and P‐IGF1R was studied in mouse organoids derived from TFF‐1 KO mouse, DARPP‐32 KO mouse, and TFF‐1/DARPP‐32 double KO mouse. TFF1KO mouse showed higher expression of STAT3 target genes, STAT3 and P‐IGF1R compared to DARPP‐32 KO mouse and TFF‐1/DARPP‐32 double KO mouse. Another study reported that reduction in atrophy, metaplasia, and epithelial cell proliferation in the gastric mucosa of mouse infected with Helicobacter felis for 18 months, correlated with loss of STAT3 signaling. 69 Organoids were generated from wildtype (WT) mouse and STAT3 knockout mice and stimulated with recombinant IL‐6 (rIL‐6) and recombinant IL‐11 (rIL‐11) followed by treatment with JAK inhibitor. Gene expression analysis showed that treatment with rIL‐6 and rIL‐11 increased the expression of intestinal metaplasia‐associated genes‐Intellectin1 (Itln1), and Lysosomal H+ transporting ATPase subunit (ATP6V0d2) but the expression was reduced upon the addition of JAK inhibitor, suggesting that these cytokines initiate intestinal metaplasia in a STAT3‐dependent manner. Although these did not involve infection with H. pylori, dissecting these questions in the context of H. pylori‐induced STAT3 activation may be a viable option.
1.4. Application of organoid cultures to understand gastric cancer initiation and progression
The emergence of organoid cultures has permitted new avenues for enquiries into the initiation of H. pylori‐associated gastric cancer, which has previously been limited by dedifferentiation in cell culture, and limitations of animal models. While organoids may not be able to recapitulate the decades long chronic inflammatory state, a number of the mechanisms involved in cancer progression have been investigated. These studies have been reviewed in detail elsewhere, 20 , 27 , 28 , 29 , 30 , 31 and some major findings that highlight the advantages of organoids are summarized here.
Organoids present the potential to investigate the role of IFNγ in the progression from gastritis to atrophic gastritis and metaplasia, 70 , 71 and the development of spasmolytic polypeptide/Trefoil Factor (TFF) 2‐expressing metaplasia (SPEM) in early‐stage metaplasia. 72 , 73 The latter in a cytotoxic T‐cell co‐culture model that showed that overall, the expression of PD‐L1 on SPEM cells was found to be dependent on Shh signaling, and the interaction of PD‐L1 with PD‐1 on CTLs enables the survival of SPEM cells in the presence of H. pylori infection. 73
DNA damage is a major mechanism in carcinogenesis, and several studies have used organoids to address questions on H. pylori‐induced damage. Bauer et al. reported that the LPS precursor β‐ADP‐heptose was sufficient to cause DNA damage in human‐derived organoids. 74 Expression of Nei‐like DNA glycosylase 2 (Neil 2) which is involved in the repair of DNA damage by removing oxidase species was suppressed in H. pylori‐infected mucosoid cultures. 75 Consistent with this observation, mucosoids generated from Neil2 knockout mice expressed higher levels of inflammatory cytokines. 76 The approach taken in this study provides a mechanistic link between H. pylori infection, inflammation, and initiation of DNA damage.
The combination of organoid culture and gene‐editing techniques provides exciting potential for investigating mechanisms in chronic inflammation and cancer initiation. An example of this is the report by Nanki et al. 77 on establishment of a bank of cancer organoids from cancer tissue with different phenotypes. This bank in itself represents an excellent resource for future studies; the importance of mutations in a panel of genes was confirmed using CRISPR/Cas9 gene‐editing approaches to create gene knockout organoids. The gene‐editing approach offers exciting possibilities for further studies on inflammation and immunity.
Overall, the combination of epithelial cell lines, mouse and human organoids, and complementary studies in WT and transgenic mouse models are helping to piece together a picture of the long‐term processes in carcinogenesis.
1.5. Applications of gastric organoid models for epithelial repair and gastric hormone studies
Gastric organoids have enabled a greater understanding of some of the events that occur during H. pylori infection without involving bacterial infection in the study. To study the signaling cascades involved in epithelial damage repair, Engevik et al 78 used organoids as a reductionist model to address questions that were previously not possible to study in vivo. Epithelial damage was achieved by single‐cell photodamage in gastric organoids derived from TFF2 knockout (KO) mice and transgenic mice expressing a fluorescent Ca2+ reporter, enabling the use of Förster resonance energy transfer (FRET) for the measurement of intracellular Ca2+. It was observed that Ca2+ mobilization was necessary for epithelial repair. Using inhibitors and agonists to epidermal growth factor receptor (EGFR), TFF2 and its receptor‐CXCR4, enabled the identification of these signaling pathways as requirements for epithelial repair. 78 Although this study did not involve infection with H. pylori, gastric mucosal damage is a characteristic feature of H. pylori infection. 49 , 68 , 78 Thus, this model would be ideal to dissect this question in the context of H. pylori‐induced epithelial damage.
Studies examining the role of gastric hormones in H. pylori infection are yet to be conducted with organoid systems. However, the potential for investigations on the role of gastric hormones in inflammation and repair are clear from a number of reports. Ohki et al 79 used mouse intestinal organoids, and reported a high similarity in gene expression between the enteroendocrine cells and the hormones produced by enteric organoids, when compared with that of mouse native tissues. Ghrelin plays a role in growth hormone release and food intake, and has immunomodulatory properties. Ghrelin expression is reduced in H. pylori‐infected patients. 80 , 81 An earlier study reported that mouse intestinal and colonic organoids expressed serotonin, its receptors and the serotonin uptake inhibitor in a similar manner to intact tissue. 82 Gastrin secretion is important for regulation of gastric acid production, and a recent study employing mouse and organoid models showed that gastrin triggers proliferation of enterochromaffin‐like cell (ECL) progenitors. 83 Given the roles played by gastric hormones in H. pylori‐associated disease manifestation and the potential role of other hormones such as leptin in vaccine‐induced immunity to H. pylori, 84 , 85 organoid models present great potential to investigate the contribution of hormones to gastric homeostasis, and in the inflammatory and regulatory immune responses in H. pylori pathogenesis.
1.6. Potential applications of organoids to investigate mechanisms of vaccine‐induced protection
The ability to co‐culture organoids with immune cells has enabled more in‐depth information to be gathered about the roles of both the innate and adaptive immune cells in H. pylori infection. This has been achieved by culturing gastric organoids with immune cells derived from the same host. Co‐culture also offers opportunities to investigate the immune mechanisms underlying vaccine‐induced protection, an area which has proven difficult to dissect using animal models (Figure 2). To date, there have been no specific organoid model studies on vaccination; however, several reports have provided insights into innate interactions with the epithelium that provide support for the usefulness of organoids to investigate complex interactions that involve both innate and adaptive immune responses.
FIGURE 2.
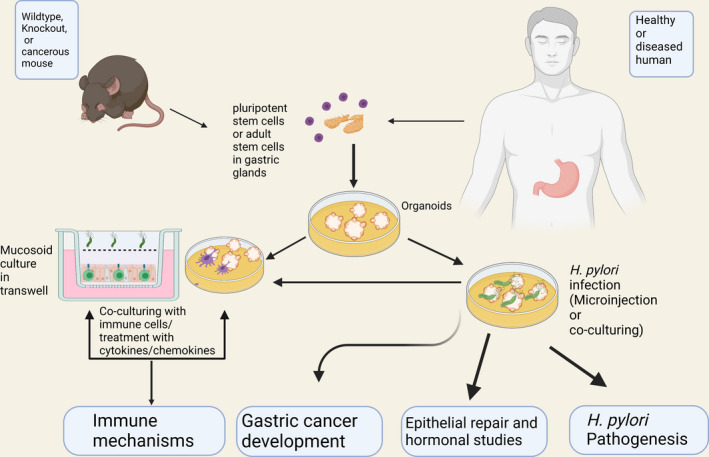
Overview of the potential for use of gastric organoids in the study of H. pylori infection. Gastric organoids generated from mouse and human can be employed to dissect the interactions between H. pylori and the gastric epithelium to study the initiation and progression of gastritis and gastric cancer as well as epithelial repair and hormonal studies. Interactions between the epithelium and immune cell populations can be simulated by addition of chemokines and cytokines or modeled by adding immune cells directly. Organoids generated from transgenic and knockout mouse strains will facilitate the analysis of these interactions. This approach can be used to then study H. pylori pathogenesis, development of gastric cancer and protective mechanisms
Sebrell et al. 56 investigated the chemokine‐dependent recruitment of dendritic cells to the basolateral side of the gastric epithelium during H. pylori infection. In this study, human gastric organoids were co‐cultured with monocyte‐derived dendritic cells and then infected with H. pylori by microinjection. A range of chemokines, including CXCL5, CXCL8, CCL20, and CXCL17, were expressed in response to infection. The recruited dendritic cells eventually ingested H. pylori by phagocytosis when co‐cultured with human gastric organoids, showing the pathogen sampling activities of DC that have previously been reported in vitro. 86 Further, in the study by Suarez et al. discussed above, 87 the effect of H. pylori infection on cytokine production by innate immune cells was assessed by co‐culturing epithelial gastric organoids from both infected WT and Nod1 KO C57BL/6 mice with macrophages from the same mice. Increased cytokine production was observed in Nod1‐deficient samples, especially when both the macrophages and gastroids lacked Nod1, implying that a functional Nod1 suppresses cytokine production. 87
The previously discussed study on the interaction between PD‐L1 on epithelial cells with the PD‐1 on CTLs, a CD8+ T‐cell subset 73 showed that both lymphocyte proliferation and epithelial cell marker interactions can be investigated in organoid models. Similar approaches could be used to investigate interactions with CD4+ T cells which are known to be the main mediators of vaccine‐induced protection (reviewed in Ref. [ 88 ]).
Innate and adaptive immunity also result in changes in expression of mucins and antimicrobial peptides in animal models of H. pylori vaccination. A study by Boccellato et al. 41 in a two‐dimensional mucosoid culture model also provided initial evidence for the bactericidal activity of the mucus and its ability to serve as a physical barrier against H. pylori attachment. The mucosoid organoid model co‐culture offers the potential to investigate the effects of the CD4+ T‐cell subsets (Th1/Th2/Th17/Treg 62 ), and innate lymphoid cells (ILC2 and ILC3) and their cytokines on the antimicrobial activity of the epithelium, and at least in principle to generate “vaccinated” organoids that recapitulate reductions in bacterial numbers seen in vivo. Such a model will be a powerful way to systematically test the roles of specific cytokines and hormones in protective responses, and to dissect the effector mechanisms.
1.7. Progress, challenges, and limitations
The above studies have shown how gastric organoids have been effectively used to extend and complement conventional in vitro and in vivo techniques to understand H. pylori‐induced disease and the resulting host immune response. However, while they have revealed how certain unique features of organoids make them the ideal and perhaps the only viable medium for these studies, it also reveals that there are some limitations. Careful comparison to in vivo models and patient samples will still be required. Development of more complex organoid systems with co‐cultured cells or factors would address some of these issues. As an emerging technique, one important question on using gastric organoids is the limits to which it is possible to mirror in vivo interactions.
The polarized nature of gastric epithelial organoids also provides an advantage over the non‐polarized systems and many signaling events are dependent on cell architecture. A case in point is the mislocalization of scribble, a basolateral polarization marker, by the Cag‐ASPP2 binding which was not observed in AGS cells, but was observed in human antral organoids. 89 Additionally, organoids can contain all cell types of the stomach lineage thereby enabling the study of the specific roles of each cell type in H. pylori pathogenesis. This has helped in the study of the role of acid‐secreting parietal cells in gastric cancer development. 47 , 62 , 73 The gastric corpus is the main region of the stomach affected by H. pylori infection, which is characterized by the loss of parietal cells. 53 , 90 , 91 The absence of parietal cells in the corpus of human gastric organoids had initially limited their study to the use of murine gastric organoids. However, following a modification of the earlier protocol 40 the detection of parietal cells in human corpus organoids and its use for the study of H. pylori‐induced gastric cancer was reported 47 , 73 and may permit earlier studies to be revisited.
Another unique feature of gastric organoid studies is the ability to determine the specific phenotype of generated organoids based on the selection of growth factors and source of stem cells or glands. Thus, enabling the generation of organoids specifically from the antrum/pylorus and corpus/fundus regions for the study of peptic ulcer and gastric cancer, respectively. This enables the understanding of the differential effects of H. pylori infection in these regions of the stomach. 92 Manipulation of growth factors has also allowed the determination of the cell lineages to be generated. Thus, organoids with gland/basal‐type or pit/foveolar‐type phenotypes can be generated and a comparison between the two phenotypes can be made. Studies have reported higher expression of inflammatory cytokines in the gland‐type than in the pit‐type organoids, suggesting that the effect of H. pylori infection in the gland base is higher than in the pits. 41 The ability to co‐culture organoids with stromal cells has also been helpful in this regard. Culturing murine fundic organoids with immortalized stomach mesenchymal cells has been shown to help in the generation and maintenance of organoids expressing markers of epithelial lineages. 63 A 2019 study revealed that the secretion of R‐spondin 3 (Rspo‐3) by the surrounding myofibroblasts of the gland base initiates the basal LGR5+ve cells to secrete antimicrobial factors against H. pylori. 93 This finding raises interesting questions about whether it is the inflammatory cytokine milieu induced by H. pylori infection that drives myofibroblasts to over‐express Rspo‐3, and further why the antimicrobial effect is not successful in clearing H. pylori in the long‐term in vivo?
An important advance in increased complexity of organoids was recently reported where the three primary germ layers derived from pluripotent stem cells were used in an organ assembly approach. 94 Enteric neuroglial, mesenchymal, and epithelial precursors were used to generate human fundic and antral gastric tissue with differentiated glands, surrounding smooth muscle, and functional enteric neurons. After development in vitro, organoids expressed similar levels of gastric hormones and mucous as intact tissue. Organoids transplanted into mouse kidney capsule and grown for up to 12 weeks further developed into epithelia that were morphologically similar to Brunner's glands with expression of mucous and gastric hormones. While the requirement of transplantation for maturation makes this model highly complex, this represents an exciting advance in tissue engineering and for application for the study of H. pylori biology and immune cell interactions.
Organoids replicate the genetic phenotype of the organisms they are generated from. Thus, organoids generated from knockout mice are usually devoid of the genes that were knocked out. Organoids from gastric cancer cell lines also possess the features of the cancerous tissues. 39 , 95 The successful generation of gastric organoids from pediatric biopsies has also been reported, 96 although further studies are required to compare the physiological characteristics with adult organoids. In the long term, this may be a means to identify aspects that differentiate childhood from adult acquisition of H. pylori.
The relative longevity of organoid cultures make gene editing and other genetic manipulation approaches viable and provide a way to study the role of specific genes in H. pylori pathogenesis; this approach is yet to be exploited with regard to H. pylori pathogenesis. Schlaermann et al. provided proof of principle that gastric spheroids could be transfected using a lentiviral GFP construct, 11 and the gene‐editing approaches employed in cancer organoid studies 77 both offer approaches to investigate the role of specific receptors/effectors in infection and pathogenesis. We expect that the use of gene‐edited organoids will expand rapidly in the next few years.
When choosing experimental techniques, labor cost and financial commitments are important factors to consider. The growth factors for organoid cultures are relatively expensive, compared to conventional cell lines. Additionally, maintenance of organoid cultures is usually labor intensive. This is a major challenge for the long‐term culture of organoids. Despite these challenges, gastric organoids are showing good potential to studying the changes that occurs in the gastric epithelium over time, such as the progression from inflammation to gastric cancer, or to investigate vaccination mechanisms. The variation in terms of longevity, labor intensity, and mode of H. pylori infection among the different types of organoid cultures also determines the choice of organoid culture model. Because organoids are relatively complex, in‐depth investigations of protein–protein interactions for example are likely to be better suited to conventional cell line studies. Thus, the choice of techniques should be made in terms of suitability for the study and the specific questions to be addressed.
2. CONCLUSIONS AND FUTURE PERSPECTIVES
The use of gastric organoids has advanced our knowledge in H. pylori infection studies due to their advantages over traditional in vivo and in vitro techniques in terms of polarization, longevity, amenability, and accessibility. As a result, gastric organoids have already led to novel discoveries on chemotaxis, intracellular effects of H. pylori virulence factors, interactions with the apical‐junctional complex; innate immune activation and the initiation of inflammation by H. pylori, that were hitherto impossible in other models. In addition, direct effects of H. pylori on gland stem cells and induction of DNA damage have greatly improved our understanding of the mechanisms of cancer initiation (reviewed in 28 ) resulting in the identification of potential therapeutic targets.
The technique is not a one‐size‐fits‐all approach, however, as certain investigations remain better suited to conventional methods. The development of long‐lasting organoid cultures with reduced cost is highly desirable for the long‐term monitoring of the changes that occurs after infection by the pathogen such as gastritis and the initiation of metaplasia. This would allow for the long‐term monitoring of carcinogenesis, thus reducing the dependence on mice for in vivo carcinogenesis experimentation. Organoid culture models also have the ethical advantage of a reduction in the numbers of experimental animals that are required for host–pathogen studies because glands from a small number of animals can be cultured indefinitely.
Despite numerous studies that have been carried out with gastric organoids in the context of H. pylori infection, the full potential of this technique is yet to be explored. As highlighted in this review, there have been impressive advances in our understanding of gastric homoeostasis. This, coupled with the capability of generating organoids from transgenic and reporter mice, and the amenability of organoids to genetic manipulation opens the door to investigate inflammatory pathways. Further, the roles of gut hormones such as ghrelin, serotonin, and leptin which are known to have both functional and cytokine activity can be addressed. The investigation of vaccination using organoids is more speculative at present, but the foundations have been laid for more complex and detailed studies on acute and chronic inflammation and gastric protective mechanisms.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by RMIT funds to AKW. SI is supported by an RMIT scholarship. Figures were generated using Biorender.com. Open access publishing facilitated by RMIT University, as part of the Wiley ‐ RMIT University agreement via the Council of Australian University Librarians. [Correction added on 25 May 2022, after first online publication: CAUL funding statement has been added.]
Idowu S, Bertrand PP, Walduck AK. Gastric organoids: Advancing the study of H. pylori pathogenesis and inflammation. Helicobacter. 2022;27:e12891. doi: 10.1111/hel.12891
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571‐584. doi: 10.1038/s41580-020-0259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dutta D, Clevers H. Organoid culture systems to study host–pathogen interactions. Curr Opin Immunol. 2017;48:15‐22. doi: 10.1016/j.coi.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham DY, Opekun AR, Osato MS, et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235‐1243. doi: 10.1136/gut.2003.037499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutton P, Boag JM. Status of vaccine research and development for Helicobacter pylori. Vaccine. 2019;37(50):7295‐7299. doi: 10.1016/j.vaccine.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walduck A, Raghavan S. Immunity and vaccine development against Helicobacter pylori. In: Kamiya S, Backert S, eds. Helicobacter pylori in Human Diseases. Advances in Experimental Medicine and Biology. Vol 1149. Springer, Cham; 2019:1‐19. doi: 10.1007/5584_2019_370 [DOI] [PubMed] [Google Scholar]
- 7. Kostic AD, Howitt MR, Garrett WS. Exploring host‐microbiota interactions in animal models and humans. Genes Dev. 2013;27(7):701‐718. doi: 10.1101/gad.212522.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaparakis M, Laurie KL, Wijburg O, et al. CD4+ CD25+ regulatory T cells modulate the T‐cell and antibody responses in Helicobacter‐Infected BALB/c mice. Infect Immun. 2006;74(6):3519‐3529. doi: 10.1128/IAI.01314-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nedrud JG, Czinn SJ, Ding H, et al. Lack of genetic influence on the innate inflammatory response to Helicobacter infection of the gastric mucosa. Front Immunol. 2012;3:181. doi: 10.3389/fimmu.2012.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents helicobacter‐induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology. 2010;138(4):1455‐1467.e4. doi: 10.1053/j.gastro.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 11. Schlaermann P, Toelle B, Berger H, et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65(2):202‐213. doi: 10.1136/gutjnl-2014-307949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rourke JLO, Lee A. Animal models of Helicobacter pylori infection and disease. Microbes Infect. 2003;5(8):741‐748. doi: 10.1016/S1286-4579(03)00123-0 [DOI] [PubMed] [Google Scholar]
- 13. Noto JM, Gaddy JA, Lee JY, et al. Iron deficiency accelerates Helicobacter pylori‐induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479. doi: 10.1172/JCI64373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backert S, Haas R, Gerhard M, Naumann M. The helicobacter pylori type IV secretion system encoded by the cag pathogenicity Island: Architecture, function, and signaling. In: Backert S, Backert E, Grohmann E, eds. Type IV Secretion in Gram‐Negative and Gram‐Positive Bacteria. Current Topics in Microbiology and Immunology. Current Topics in Microbiology and Immunology. Vol 413. Springer, Cham; 2017:187‐220. doi: 10.1007/978-3-319-75241-9_8 [DOI] [PubMed] [Google Scholar]
- 15. Sgouras D, Tegtmeyer N, Wessler S. Activity and functional importance of Helicobacter pylori virulence factors. Adv Exp Med Biol. 2019;1149:35‐56. doi: 10.1007/5584_2019_358 [DOI] [PubMed] [Google Scholar]
- 16. Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862‐866. doi: 10.1038/nature06187 [DOI] [PubMed] [Google Scholar]
- 17. Boxberger HJ, Sessler MJ, Grausam MC, Becker HD, Meyer TF. Isolation and culturing of highly polarized primary epithelial cells from normal human stomach (antrum) as spheroid‐like vesicles. Methods Cell Sci. 1997;19(3):169‐178. doi: 10.1023/A:1009751913391 [DOI] [Google Scholar]
- 18. Richter‐Dahlfors A, Heczko U, Meloche RM, Finlay BB, Buchan AMJ. Helicobacter pylori‐infected human antral primary cell cultures: Effect on gastrin cell function. Am J Physiol ‐ Gastrointest Liver Physiol. 1998;275(3 38‐3):393‐401. doi: 10.1152/ajpgi.1998.275.3.g393 [DOI] [PubMed] [Google Scholar]
- 19. Ootani A, Toda S, Fujimoto K, Sugihara H. An air‐liquid interface promotes the differentiation of gastric surface mucous cells (GSM06) in culture. Biochem Biophys Res Commun. 2000;271(3):741‐746. doi: 10.1006/BBRC.2000.2673 [DOI] [PubMed] [Google Scholar]
- 20. Lau HCH, Kranenburg O, Xiao H, Yu J. Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol. 2020;17(4):203‐222. doi: 10.1038/s41575-019-0255-2 [DOI] [PubMed] [Google Scholar]
- 21. Aguilar C, Alves da Silva M, Saraiva M, et al. Organoids as host models for infection biology – a review of methods. Exp Mol Med. 2021;53:1471‐1482. doi: 10.1038/s12276-021-00629-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dang J, Tiwari SK, Lichinchi G, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19(2):258‐265. doi: 10.1016/j.stem.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816‐818. doi: 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- 24. Ramani S, Atmar RL, Estes MK. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol. 2014;30(1):25‐33. doi: 10.1097/MOG.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dekkers JF, Berkers G, Kruisselbrink E, et al. Characterizing responses to CFTR‐modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016;8(344):344RA84. doi: 10.1126/scitranslmed.aad8278 [DOI] [PubMed] [Google Scholar]
- 26. Berkers G, van Mourik P, Vonk AM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 2019;26(7):1701‐1708.e3. doi: 10.1016/j.celrep.2019.01.068 [DOI] [PubMed] [Google Scholar]
- 27. Alzeeb G, Metges J‐P, Corcos L, Le Jossic‐Corcos C. Three‐dimensional culture systems in gastric cancer research. Cancers (Basel). 2020;12(10):1‐20. doi: 10.3390/cancers12102800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Traulsen J, Zagami C, Daddi AA, Boccellato F. Molecular modelling of the gastric barrier response, from infection to carcinogenesis. Best Pract Res Clin Gastroenterol. 2021;50–51:101737. doi: 10.1016/J.BPG.2021.101737 [DOI] [PubMed] [Google Scholar]
- 29. Idowu S, Bertrand PP, Walduck AK. Homeostasis and cancer initiation: Organoids as models to study the initiation of gastric cancer. Int J Mol Sci. 2022;23(5):2790. doi: 10.3390/ijms23052790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eicher AK, Berns HM, Wells JM. Translating developmental principles to generate human gastric organoids. Cell Mol Gastroenterol Hepatol. 2018;5(3):353‐363. doi: 10.1016/j.jcmgh.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pompaiah M, Bartfeld S. Gastric organoids: An emerging model system to study Helicobacter pylori pathogenesis. In: Tegtmeyer N, Backert S, eds. Molecular Pathogenesis and Signal Transduction by Helicobacter pylori. Current Topics in Microbiology and Immunology. Vol 400. Springer, Cham; 2017:149‐168. doi: 10.1007/978-3-319-50520-6_7 [DOI] [PubMed] [Google Scholar]
- 32. Kurokawa K, Hayakawa Y, Koike K. Plasticity of intestinal epithelium: Stem cell niches and regulatory signals. Int J Mol Sci. 2021;22(1):357. doi: 10.3390/ijms22010357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCracken KW, Howell JC, Spence JR, Wells JM. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6(12):1920‐1928. doi: 10.1038/nprot.2011.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105‐109. doi: 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCracken KW, Catá EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem‐cell‐derived gastric organoids. Nature. 2014;516:400‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sigal M, Logan CY, Kapalczynska M, et al. Stromal R‐spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451‐455. doi: 10.1038/nature23642 [DOI] [PubMed] [Google Scholar]
- 37. Demitrack ES, Gifford GB, Keeley TM, et al. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34(20):2522‐2536. doi: 10.15252/embj.201490583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wölffling S, Daddi AA, Imai‐Matsushima A, et al. EGF and BMPs govern differentiation and patterning in human gastric glands. Gastroenterology. 2021;161(2):623‐636.e16. doi: 10.1053/j.gastro.2021.04.062 [DOI] [PubMed] [Google Scholar]
- 39. Stange D, Koo B‐K, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155(2):357. doi: 10.1016/j.cell.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bartfeld S, Bayram T, Van De Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148(1):126‐136.e6. doi: 10.1053/j.gastro.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boccellato F, Woelffling S, Imai‐Matsushima A, et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut. 2019;68(3):400‐413. doi: 10.1136/gutjnl-2017-314540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartfeld S, Clevers H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter Pylori. J vis Exp. 2015;105:53359. doi: 10.3791/53359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broda TR, McCracken KW, Wells JM. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat Protoc. 2019;14(1):28‐50. doi: 10.1038/s41596-018-0080-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choudhury D, Ashok A, Naing MW. Commercialization of organoids. Trends Mol Med. 2020;26(3):245‐249. doi: 10.1016/j.molmed.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 45. Urbischek M, Rannikmae H, Foets T, Ravn K, Hyvönen M, de la Roche M. Organoid culture media formulated with growth factors of defined cellular activity. Sci Rep. 2019;9(1):6193. doi: 10.1038/s41598-019-42604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barker N, Huch M, Kujala P, et al. Lgr5+ve stem cells drive self‐renewal in the stomach and build long‐lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25‐36. doi: 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 47. Bertaux‐Skeirik N, Feng R, Schumacher MA, et al. CD44 plays a functional role in Helicobacter pylori‐induced epithelial cell proliferation. PLoS Pathog. 2015;11(2):e1004663. doi: 10.1371/journal.ppat.1004663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang J, Sweeney E, Sigal M, et al. Chemodetection and destruction of host urea allows Helicobacter pylori to locate the epithelium. Cell Host Microbe. 2015;18(2):147‐156. doi: 10.1016/j.chom.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanyu H, Engevik KA, Matthis AL, Ottemann KM, Montrose MH, Aihara E. Helicobacter pylori uses the TlpB receptor to sense sites of gastric injury. Infect Immun. 2019;87(9): doi: 10.1128/IAI.00202-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uotani T, Murakami K, Uchida T, et al. Changes of tight junction and interleukin‐8 expression using a human gastroid monolayer model of Helicobacter pylori infection. Helicobacter. 2019;24(3):1‐12. doi: 10.1111/hel.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brandt S, Kwok T, Hartig R, Kö W, Backert S. NF‐B activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102(26):9300‐9305. www.pnas.orgcgidoi10.1073pnas.0409873102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Backert S, Naumann M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18(11):479‐486. doi: 10.1016/j.tim.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 53. Miehlke S, Hackelsberger A, Meining A, et al. Severe expression of corpus gastritis is characteristic in gastric cancer patients infected with Helicobacter pylori. Br J Cancer. 1998;78(2):263‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kayisoglu Ö, Schlegel N, Bartfeld S. Gastrointestinal epithelial innate immunity—regionalization and organoids as new model. J Mol Med. 2021;99(4):517‐530. doi: 10.1007/s00109-021-02043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morey P, Pfannkuch L, Pang E, et al. Helicobacter pylori depletes cholesterol in gastric glands to prevent interferon gamma signaling and escape the inflammatory response. Gastroenterology. 2018;154(5):1391‐1404.e9. doi: 10.1053/J.GASTRO.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 56. Sebrell TA, Hashimi M, Sidar B, et al. A novel gastric spheroid co‐culture model reveals chemokine‐dependent recruitment of human dendritic cells to the gastric epithelium. Cell Mol Gastroenterol Hepatol. 2019;8(1):157‐171.e3. doi: 10.1016/j.jcmgh.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tran CT, Garcia M, Garnier M, Burucoa C, Bodet C. Inflammatory signaling pathways induced by Helicobacter pylori in primary human gastric epithelial cells. Innate Immun. 2017;23(2):165‐174. doi: 10.1177/1753425916681077 [DOI] [PubMed] [Google Scholar]
- 58. Mejías‐Luque R, Zöller J, Anderl F, et al. Lymphotoxin β receptor signalling executes Helicobacter pylori‐driven gastric inflammation in a T4SS‐dependent manner. Gut. 2017;66(8):1369‐1381. doi: 10.1136/gutjnl-2015-310783 [DOI] [PubMed] [Google Scholar]
- 59. Cook KW, Letley DP, Ingram RJM, et al. CCL20/CCR6‐mediated migration of regulatory T cells to the Helicobacter pylori‐infected human gastric mucosa. Gut. 2014;63(10):1550‐1559. doi: 10.1136/gutjnl-2013-306253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hor Y, Voon D‐C, Koo J, et al. A role for RUNX3 in inflammation‐induced expression of IL23A in gastric epithelial cells. Cell Rep. 2014;8(1):50‐58. doi: 10.1016/j.celrep.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schumacher MA, Donnelly JM, Engevik AC, et al. gastric sonic hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142(5):1150‐1159.e6. doi: 10.1053/J.GASTRO.2012.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schumacher M, Feng R, Aihara E, et al. Helicobacter pylori‐induced Sonic Hedgehog expression is regulated by NFκB pathway activation: The use of a novel in vitro model to study epithelial response to infection. Helicobacter. 2015;20(1):19‐28. doi: 10.1111/hel.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schumacher MA, Aihara E, Feng R, et al. The use of murine‐derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593(8):1809‐1827. doi: 10.1113/jphysiol.2014.283028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sigal M, Rothenberg ME, Logan CY, et al. Helicobacter pylori activates and expands Lgr5+ stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148(7):1392‐1404.e21. doi: 10.1053/j.gastro.2015.02.049 [DOI] [PubMed] [Google Scholar]
- 65. Howitt CR. ChePep controls Helicobacter pylori infection of the gastric glands and chemotaxis in the Epsilonproteobacteria. MBio. 2011;2(4):98‐109. doi: 10.1128/mBio.00098-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bronte‐Tinkew DM, Terebiznik M, Franco A, et al. Helicobacter pylori cytotoxin‐associated gene a activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009;69(2):632‐639. doi: 10.1158/0008-5472.CAN-08-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ernst M, Najdovska M, Grail D, et al. STAT3 and STAT1 mediate IL‐11‐dependent and inflammation‐associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118(5):1727‐1738. doi: 10.1172/JCI34944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu S, Soutto M, Chen Z, et al. Activation of IGF1R by DARPP‐32 promotes STAT3 signaling in gastric cancer cells. Oncogene. 2019;38(29):5805‐5816. doi: 10.1038/s41388-019-0843-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ishii Y, Shibata W, Sugimori M, et al. Activation of signal transduction and activator of transcription 3 signaling contributes to helicobacter‐associated gastric epithelial proliferation and inflammation. Gastroenterol Res Pract. 2018;2018:1‐9. doi: 10.1155/2018/9050715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Osaki LH, Bockerstett KA, Wong CF, et al. Interferon‐γ directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol. 2019;247(4):513‐523. doi: 10.1002/path.5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Correa P. A Human model of gastric carcinogenesis. Cancer Res. 1988;48(13):3554‐3560. [PubMed] [Google Scholar]
- 72. Reissfelder C, Stamova S, Gossmann C, et al. Tumor‐specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125(2):739‐751. doi: 10.1172/JCI74894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holokai L, Chakrabarti J, Broda T, et al. Increased programmed death‐ligand 1 is an early epithelial cell response to Helicobacter pylori infection. PLoS Pathog. 2019;15(1):e1007468. doi: 10.1371/journal.ppat.1007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bauer M, Nascakova Z, Mihai A‐I, et al. The ALPK1/TIFA/NF‐κB axis links a bacterial carcinogen to R‐loop‐induced replication stress. Nat Commun. 2020;11(1):5117. doi: 10.1038/s41467-020-18857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sayed IM, Sahan AZ, Venkova T, et al. Helicobacter pylori infection downregulates the DNA glycosylase NEIL2, resulting in increased genome damage and inflammation in gastric epithelial cells. J Biol Chem. 2020;295(32):11082‐11098. doi: 10.1074/jbc.ra119.009981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chakraborty A, Wakamiya M, Venkova‐Canova T, et al. Neil2‐null mice accumulate oxidized DNA bases in the transcriptionally active sequences of the genome and are susceptible to innate inflammation. J Biol Chem. 2015;290(41):24636‐24648. doi: 10.1074/jbc.M115.658146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nanki K, Toshimitsu K, Takano AI, et al. Divergent routes toward Wnt and R‐spondin niche independency during human gastric carcinogenesis. Cell. 2018;174(4):856‐869.e17. doi: 10.1016/j.cell.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 78. Engevik KA, Hanyu H, Matthis AL, et al. Trefoil factor 2 activation of CXCR4 requires calcium mobilization to drive epithelial repair in gastric organoids. J Physiol. 2019;597(10):2673‐2690. doi: 10.1113/JP277259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ohki J, Sakashita A, Aihara E, et al. Comparative analysis of enteroendocrine cells and their hormones between mouse intestinal organoids and native tissues. Biosci Biotechnol Biochem. 2020;84(5):936‐942. doi: 10.1080/09168451.2020.1713043 [DOI] [PubMed] [Google Scholar]
- 80. Alessandro Paoluzi O, Del Vecchio BG, Caruso R, et al. Helicobacter pylori infection associates with a mucosal downregulation of ghrelin, negative regulator of Th1‐cell responses. Helicobacter. 2013;18(6):406‐412. doi: 10.1111/hel.12065 [DOI] [PubMed] [Google Scholar]
- 81. Ichikawa H, Sugimoto M, Sakao Y, et al. Relationship between ghrelin, Helicobacter pylori and gastric mucosal atrophy in hemodialysis patients. World J Gastroenterol. 2016;22(47):10440‐10449. doi: 10.3748/wjg.v22.i47.10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsuruta T, Saito S, Osaki Y, Hamada A, Aoki‐Yoshida A, Sonoyama K. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun. 2016;474(1):161‐167. doi: 10.1016/J.BBRC.2016.03.165 [DOI] [PubMed] [Google Scholar]
- 83. Sheng W, Malagola E, Nienhüser H, et al. Hypergastrinemia expands gastric ECL cells through CCK2R+ progenitor cells via ERK activation. Cell Mol Gastroenterol Hepatol. 2020;10(2):434‐449.e1. doi: 10.1016/j.jcmgh.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walduck AK, Becher D, Cell CDT, Leptin RCDT. CD4 + T reg and the prospects for vaccination against H. pylori infection. Front Immunol. 2012;3(October):1‐8. doi: 10.3389/fimmu.2012.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Becher D, Deutscher ME, Simpfendorfer KR, et al. Local recall responses in the stomach involving reduced regulation and expanded help mediate vaccine‐induced protection against Helicobacter pylori in mice. Eur J Immunol. 2010;40(10):2778‐2790. doi: 10.1002/eji.200940219 [DOI] [PubMed] [Google Scholar]
- 86. afsi N, Voland P, Schwendy S, et al. Human dendritic cells respond to Helicobacter pylori promoting Nk Cell and Th1‐effector responses in vitro. J Immunol. 2004;173(2):1249‐1257. doi: 10.4049/jimmunol.173.2.1249 [DOI] [PubMed] [Google Scholar]
- 87. Suarez G, Romero‐Gallo J, Piazuelo MB, et al. Nod1 imprints inflammatory and carcinogenic responses toward the gastric pathogen Helicobacter pylori. Cancer Res. 2019;79(7):1600‐1611. doi: 10.1158/0008-5472.CAN-18-2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Walduck A, Andersen LP, Raghavan S. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter. 2015;20:17‐25. doi: 10.1111/hel.12252 [DOI] [PubMed] [Google Scholar]
- 89. Buti L, Ruiz‐Puig C, Sangberg D, et al. CagA–ASPP2 complex mediates loss of cell polarity and favors H. Pylori colonization of human gastric organoids. Proc Natl Acad Sci U S A. 2020;117(5):2645‐2655. doi: 10.1073/pnas.1908787117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nam KT, Lee H, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139(6):2028‐2037.e9. doi: 10.1053/j.gastro.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide‐expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146(7):1727‐1738.e8. doi: 10.1053/j.gastro.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Keilberg D, Steele N, Fan S, Yang C, Zavros Y, Ottemann KM. Gastric metabolomics detects Helicobacter pylori correlated loss of numerous metabolites in both the corpus and antrum. Infect Immun. 2021;89(2):e00690‐20. doi: 10.1128/IAI.00690-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sigal M, Reinés MDM, Müllerke S, et al. R‐spondin‐3 induces secretory, antimicrobial Lgr5 + cells in the stomach. Nat Cell Biol. 2019;21:812‐823. doi: 10.1038/s41556-019-0339-9 [DOI] [PubMed] [Google Scholar]
- 94. Eicher AK, Kechele DO, Sundaram N, et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell. 2022;29(1):36‐51.e6. doi: 10.1016/j.stem.2021.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Seidlitz T, Merker SR, Rothe A, et al. Human gastric cancer modelling using organoids. Gut. 2019;68(2):207‐217. doi: 10.1136/gutjnl-2017-314549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jones BC, Calà G, De Coppi P, Giobbe GG. Paediatric gastric organoids as a tool for disease modelling and clinical translation. Pediatr Surg Int. 2021;37:317‐324. doi: 10.1007/s00383-020-04821-x [DOI] [PMC free article] [PubMed] [Google Scholar]


