Abstract
Background:
There are limited data on the association of material deprivation with clinical care and outcomes after atrial fibrillation (AF) diagnosis in jurisdictions with universal health care.
Methods:
This was a population-based cohort study of individuals ≥66 years of age with first diagnosis of AF between April 1, 2007, and March 31, 2019, in the Canadian province of Ontario, which provides public funding and prohibits private payment for medically necessary physician and hospital services. Prescription medications are subsidized for residents >65 years of age. The primary exposure was neighborhood material deprivation, a metric derived from Canadian census data to estimate inability to attain basic material needs. Neighborhoods were categorized by quintile from Q1 (least deprived) to Q5 (most deprived). Cause-specific hazards regression was used to study the association of material deprivation quintile with time to AF-related adverse events (death or hospitalization for stroke, heart failure, or bleeding), clinical services (physician visits, cardiac diagnostics), and interventions (anticoagulation, cardioversion, ablation) while adjusting for individual characteristics and regional cardiologist supply.
Results:
Among 347 632 individuals with AF (median age 79 years, 48.9% female), individuals in the most deprived neighborhoods (Q5) had higher prevalence of cardiovascular disease, risk factors, and noncardiovascular comorbidity relative to residents of the least deprived neighborhoods (Q1). After adjustment, Q5 residents had higher hazards of death (hazard ratio [HR], 1.16 [95% CI, 1.13–1.20]) and hospitalization for stroke (HR, 1.16 [95% CI, 1.07–1.27]), heart failure (HR, 1.14 [95% CI, 1.11–1.18]), or bleeding (HR, 1.16 [95% CI, 1.07–1.25]) relative to Q1. There were small differences across quintiles in primary care physician visits (HR, Q5 versus Q1, 0.91 [95% CI, 0.89–0.92]), echocardiography (HR, Q5 versus Q1, 0.97 [95% CI, 0.96–0.99]), and dispensation of anticoagulation (HR, Q5 versus Q1, 0.97 [95% CI, 0.95–0.98]). There were more prominent disparities for Q5 versus Q1 in cardiologist visits (HR, 0.84 [95% CI, 0.82–0.86]), cardioversion (HR, 0.80 [95% CI, 0.76–0.84]), and ablation (HR, 0.45 [95% CI, 0.30–0.67]).
Conclusions:
Despite universal health care and prescription medication coverage, residents of more deprived neighborhoods were less likely to visit cardiologists or receive rhythm control interventions after AF diagnosis, even though they exhibited higher cardiovascular disease burden and higher risk of adverse outcomes.
Keywords: anticoagulants, atrial fibrillation, cardiologists, delivery of health care, electric countershock, social class
Clinical Perspective.
What is New
This was a population-based study of individuals ≥66 years of age newly diagnosed with atrial fibrillation (AF) in the Canadian province of Ontario, which provides universal health care and prescription drug coverage to residents and prohibits private payment for covered services.
Residents of more deprived neighborhoods had higher rates of death, stroke, heart failure, and bleeding after AF diagnosis but were less likely to visit cardiologists or receive rhythm control interventions, despite adjustment for comorbidities.
Residents of more materially deprived neighborhoods had minimal differences in primary care access, anticoagulation, and echocardiography after AF diagnosis relative to residents of less deprived neighborhoods.
What Are the Clinical Implications?
It may be possible to ameliorate disparities in some care processes, such as anticoagulation for AF, by reducing financial barriers to health care access.
Universal health care is less likely to eliminate disparities in care processes that are complex, time-intensive, and dependent on specialists without addressing other barriers to accessing health care.
Universal health care systems can have substantial disparities in accessing scarce health care resources (such as ablation for AF in this study), highlighting the need for additional measures to promote health equity.
Editorial, see p 172
Atrial fibrillation (AF) is the most common sustained arrhythmia1 and portends high risk of stroke, heart failure (HF), and death.2–4 As with most cardiovascular diseases (CVDs), individuals with AF who reside in neighborhoods with lower measures of socioeconomic status (SES) have been consistently reported to have higher risks of adverse outcomes.5,6 Many (but not all) studies also report that residents of neighborhoods with lower SES are less likely to receive optimal care for AF.7–11 This association of SES with outcomes among people with AF may be partly mediated through material deprivation and reduced access to health insurance.
Stroke prevention is a central focus of care for individuals with AF, which lends itself to a concrete intervention (anticoagulation of higher-risk patients) that is strongly recommended by guidelines.12 Beyond anticoagulation, optimal care for individuals with AF also requires a multifaceted approach to address risk factors for common comorbidities, such as HF and atherosclerotic CVD, because they contribute a greater burden of mortality and morbidity than stroke.2–4 Rhythm control can also improve quality of life for people with AF. Furthermore, recent data suggest better outcomes for selected patients who receive early rhythm control, particularly ablation, soon after AF diagnosis.13,14 These aspects of care for individuals with AF place greater demands on their time and require more specialized expertise that is often centralized in tertiary care settings. Thus, there may be varying gradients by material deprivation for different care processes, with greater disparities observed for processes of care that are complex, time-intensive, and dependent on specialists.
Most studies on the association of SES with care for individuals with AF come from health care systems that allow for private payment to access health care, making it difficult to disentangle inability to pay for health care from other challenges faced by residents of lower SES neighborhoods. The Canadian province of Ontario provides public funding for medically necessary physician and in-hospital care through the Ontario Health Insurance Plan, while prohibiting private payment for these services. Prescription medications are also provided through the Ontario Drug Benefit program to individuals ≥65 years of age with copayments of $2 to $6.11 per prescription after a $100 annual deductible. There is no limit on the number of prescriptions covered by the Ontario Drug Benefit, as long as they are part of the provincial formulary. These policies provide the opportunity to study the association of material deprivation with care for individuals with AF when there are few barriers related to the direct costs of prescriptions and physician and hospital services.
We conducted a population-based cohort study to evaluate the association between neighborhood-level material deprivation with processes of care and outcomes after AF diagnosis in older adults in Ontario, Canada. The stated hypotheses were as follows: (1) residents of neighborhoods with greater material deprivation would receive worse AF care despite being at greater risk for adverse outcomes; (2) health care disparities would be less prominent for anticoagulation, which is strongly emphasized in guidelines, funded for elderly individuals, and can be provided by general practitioners; (3) larger disparities would be observed for rhythm control interventions, particularly ablation, because they are emphasized less strongly within guidelines and require access to specialized care, with concomitant patient/caregiver burdens of time and resources required for travel, appointments, and potential procedures.
Methods
Cohort Creation
This population-based cohort study was conducted using administrative databases that were linked using unique encoded patient identifiers and analyzed at ICES (previously the Institute for Clinical Evaluative Sciences).15 The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. Data sharing agreements prohibit ICES from making the data set publicly available, but access may be granted to those who meet prespecified criteria for confidential access (available at www.ices.on.ca/DAS).
The Canadian Institute for Health Information Discharge Abstract Database records data on hospitalized patients and the National Ambulatory Care Reporting System collects data on emergency department visits. Physician billing claims are recorded in the Ontario Health Insurance Plan database and drug dispensation records in the Ontario Drug Benefit database. The Ontario Laboratories Information System database contains information on laboratory test results, including creatinine levels. The Registered Persons Database maintains vital statistics, including date of death. The Immigration, Refugees and Citizenship Canada Permanent Resident database was used to identify individuals who immigrated to Canada after 1984 (henceforth referred to as recent immigrants). Invasive cardiac procedures, including AF ablation, are tracked by CorHealth, a nonprofit agency funded by the Ministry of Health.
These linked datasets were used to create a cohort of community-dwelling adults who were documented to have AF between April 1, 2007, and March 31, 2019. The algorithm requires 1 record of AF in hospital discharge records or the emergency department or 4 physician billing claims for International Classification of Diseases, 9th revision, code 427 in 365 days (physician billing claims to Ontario Health Insurance Plan use International Classification of Diseases, 9th revision, diagnostic codes). The algorithm was validated to have specificity of 99.1% (95% CI, 98.9%–99.3%) for AF.16 The index date was that of the earliest AF hospital, emergency department, or physician billing record. For patients identified through physician billing codes, this approach may introduce an immortal time bias but was adopted for the primary analyses because patients can receive interventions and be hospitalized between the first and fourth physician visits. In a sensitivity analysis, the index date was set to the date of the fourth physician billing code. We excluded individuals with missing key data (age, sex, Ontario Health Insurance Plan number, material deprivation score), AF diagnoses in the preceding 5 years, or age <66 or >105 years; those who were not residents of Ontario; and those who were long-term care residents.
The key exposure was neighborhood material deprivation, which is 1 of the 4 dimensions of the 2016 Ontario Marginalization Index.17,18 This measure aims to estimate neighborhood residents’ inability to attain basic material needs by incorporating indicators of household income, housing quality, educational attainment, and the family structure of neighborhood residents. The specific indicators used to determine material deprivation are summarized in Table S1. These indicators were obtained from 2016 Canadian census–derived data on 20 640 dissemination areas and 2376 census tracts.19 The dissemination area, which is the smallest standard geographic area for which all census data are disseminated, consists of 1 or more adjacent blocks with a mean population of 400 to 700 people. The census tract is a small, relatively stable geographic unit of <10 000 people located in census metropolitan areas and census agglomerations having an urban core population of ≥50 000 people. The immigration and visible minority dimension of the Ontario Marginalization Index17,18 was also determined; this estimates neighborhood concentrations of people who self-identify as a visible minority or who immigrated to Canada within the previous 5 years.
Ontario neighborhoods were categorized by quintile of material deprivation from the lowest quintile (Q1; least deprived) to the highest quintile (Q5; most deprived) because the principal component scores can be skewed.17,18 The ICES physician database was used to estimate the per capita cardiologist supply in each of the 76 local health integration network planning areas (subregions) within the province.20 Other covariates of interest were determined from administrative data sets using validated algorithms wherever possible. The lookback period21 for identification of baseline data in administrative datasets was lifetime for chronic diseases, 5 years for episodic diagnoses (eg, myocardial infarction), 1 year for physician visits, diagnostic tests, and laboratory measurements, and 6 months for drug dispensation records.
Three categories of outcomes were studied over 365 days after the index date. The first category encompassed adverse events relevant to individuals with AF: all-cause mortality and hospitalization with most responsible diagnosis of stroke, HF, or bleeding (in separate analyses). The second category encompassed clinical services: visiting a primary care physician, visiting a cardiologist, or receipt of echocardiography. We also studied ambulatory electrocardiographic monitoring (Holter or loop monitors) as a surrogate for testing intensity or patient availability for determination of rate control or symptom–rhythm correlation. The third category encompassed AF-related interventions: anticoagulation with warfarin or direct oral anticoagulant, antiarrhythmic drug therapy (amiodarone, sotalol, flecainide, or propafenone [dronedarone and dofetilide are not covered by the Ontario Drug Benefit]), cardioversion, and AF ablation. For individuals who were dispensed at least 1 prescription for anticoagulation, drug dispensation records were analyzed to determine nonpersistence over 1 year. Nonpersistence was defined as noncontinuous use of an anticoagulant after first dispensation during the study period with an allowable gap of up to 30 days between prescription refills. Analyses focused on AF ablation and direct oral anticoagulants were conducted for the subset of individuals with an index date after 2012 given limited availability of these interventions before 2013.
Statistical Analyses
Individuals were categorized by quintile of neighborhood material deprivation. Baseline characteristics were described using the median (interquartile range) for continuous variables. Statistical significance was tested using the Kruskal-Wallis test. Categorical data were summarized using percentages (with counts) with the χ2 test for determination of statistical significance. The complement of the Kaplan-Meier function was used to estimate 1-year risk of death and cumulative incidence functions were used to estimate the incidence of other outcomes.
Cause-specific hazards regression was used to study the association of deprivation quintile with the aforementioned outcomes. We accounted for clustering at the level of the dissemination area in our analytic models by estimating the maximum partial likelihood using a robust sandwich covariance matrix to account for intracluster dependence when estimating regression measures. Death was treated as a competing risk and individuals were censored if they were alive and event-free for the outcome of interest at 365 days. The models adjusted for age, sex, source of AF diagnosis (hospitalization, emergency department, outpatient), year of diagnosis, rural residence, recent immigration, HF, hypertension, diabetes, stroke or transient ischemic attack, vascular disease, previous bleeding, liver dysfunction, chronic obstructive pulmonary disease, chronic kidney disease, cancer, excessive alcohol use, recreational drug use, hospital frailty score,22 and the per capita supply of cardiologists in the local health integration network subregion (see Table S2 for variable definitions).
Two sets of post hoc analyses were conducted after review of these results. First, the regression analyses were repeated, with inclusion of cardiologist visits after the index date as a time-varying covariate. Second, residents of neighborhoods in the lowest and highest quintiles of material deprivation were compared after 1:1 propensity score matching using a caliper width of 0.1. The logistic regression model used to estimate the propensity score incorporated the same variables used in the cause-specific hazard models. Outcomes were then compared between the matched sets, while accounting for the matched nature of the sample.
All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc). Given the large sample size, P values were not reported so as to direct attention to effect size and 95% CIs.
Results
Baseline Characteristics
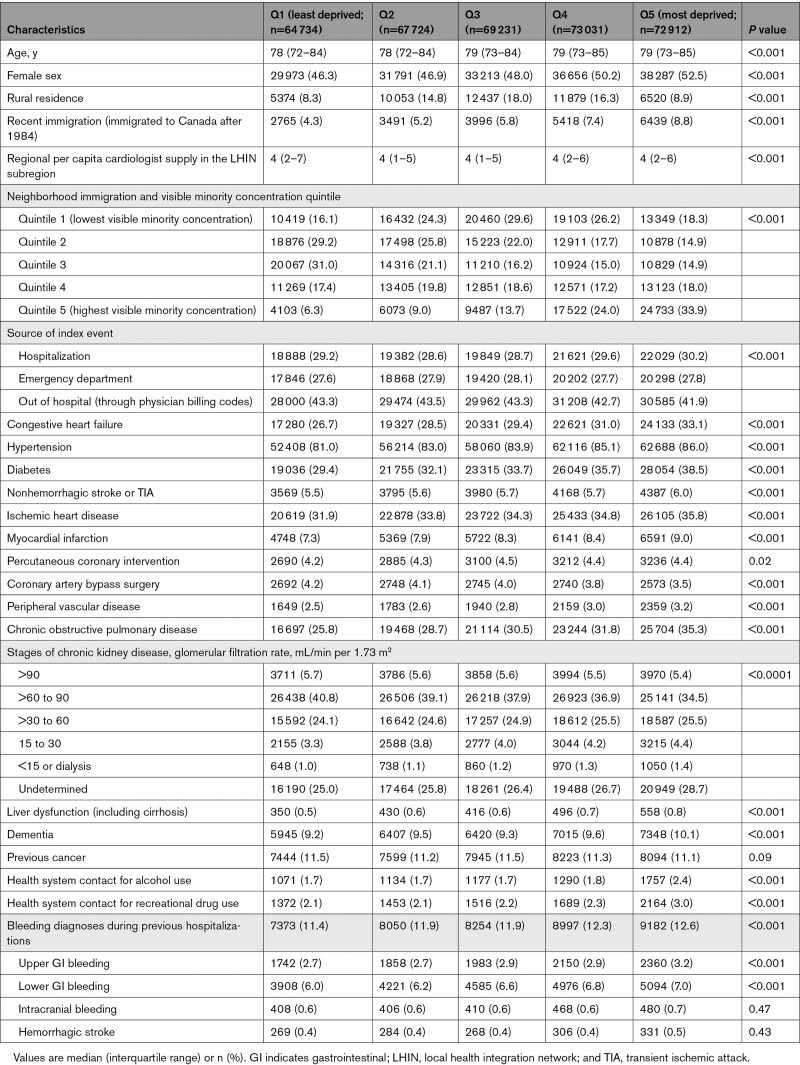
A total of 347 632 individuals met study criteria (see Figure S1). Median age was 79 years (interquartile range, 72–84) and 177 712 (51.1%) participants were men. Baseline characteristics are summarized in the Table. There was greater representation of individuals from neighborhoods with higher deprivation scores in the AF cohort, with 64 734 (18.6%) from the least deprived quintile (Q1) and 72 912 (21.0%) from the most deprived quintile (Q5). Neighborhood material deprivation was positively correlated with immigration and visible minority quintile. Residents of more deprived neighborhoods had greater representation of women (46.3% women in Q1 versus 52.5% in Q5) and recent immigrants (4.3% in Q1 versus 8.8% in Q5). Individuals with AF from more deprived neighborhoods were more likely to have preexisting cardiovascular disease and potential complications of AF, such as stroke, HF, myocardial infarction, and dementia. They were also more likely to have hypertension, diabetes, previous bleeding, and noncardiovascular comorbidity.
Table.
Baseline Characteristics by Quintile of Material Deprivation of Individuals’ Residence
Outcomes and Clinical Care After AF Diagnosis
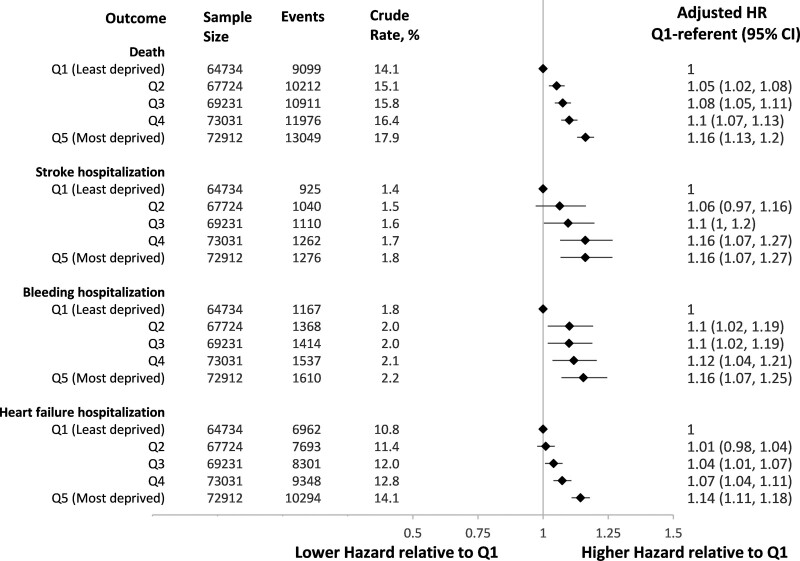
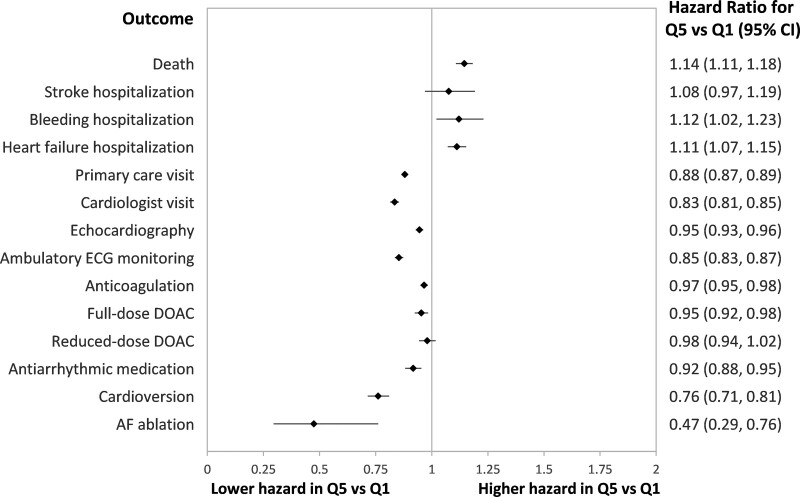
Adverse outcomes occurred with a gradient of increasing risk from less to more deprived neighborhoods. The crude risk of death at 1 year was 17.9% (95% CI, 17.6%–18.2%) in Q5 and 14.1% (95% CI, 13.8%–14.3%) in Q1 residents. The 1-year risk of hospitalization was 1.8% (95% CI, 1.7%–1.8%) in Q5 versus 1.4% (1.3%–1.5%) in Q1 residents for stroke, 2.2% (95% CI, 2.1%–2.3%) in Q5 versus 1.8% (95% CI, 1.7%–1.9%) in Q1 residents for bleeding, and 14.1% (95% CI, 13.9%–14.4%) in Q5 versus 10.8% (95% CI, 10.5%–11.0%) in Q1 residents for HF. After adjustment for baseline characteristics (Figure 1), individuals residing in the most deprived quintile (Q5) had a higher hazard of death (hazard ratio [HR], 1.16 [95% CI, 1.13–1.20]) and hospitalization for stroke (HR, 1.16 [95% CI, 1.07–1.27]), HF (HR, 1.14 [95% CI, 1.11–1.18]), or bleeding (HR, 1.16 [95% CI, 1.07–1.25]) than residents of the least deprived quintile (Q1).
Figure 1.
Adverse outcomes within 1 year of first atrial fibrillation documentation by material deprivation of individuals’ neighborhood .
Hazard ratios are relative to individuals living in neighborhoods in the first quintile (least deprived) and are adjusted for age, sex, source of AF diagnosis (hospitalization, emergency department, outpatient), year of diagnosis, rural residence, immigration status, heart failure, hypertension, diabetes, stroke or transient ischemic attack, vascular disease, previous bleeding, liver dysfunction, chronic obstructive pulmonary disease, chronic kidney disease, cancer, excessive alcohol use, recreational drug use, hospital frailty score, and per capita cardiologist supply.
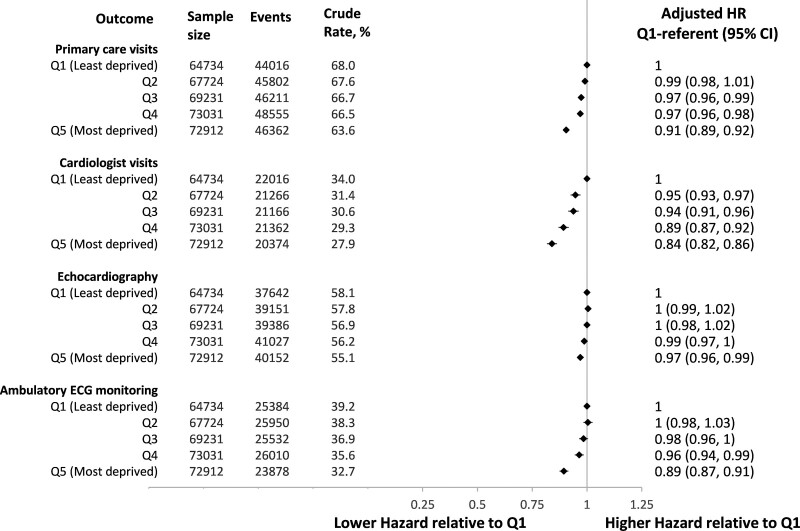
Despite having worse baseline health and higher rates of adverse outcomes, individuals with AF living in the most deprived neighborhoods were less likely to receive physician visits or cardiac diagnostics (Figure 2). The 1-year incidence of primary care physician visits was 63.6% (95% CI, 63.2%–63.9%) for Q5 versus 68.0% (95% CI, 67.6%–68.4%) for Q1 residents; the 1-year incidence of cardiologist visits was 27.9% (95% CI, 27.6%–28.3%) for Q5 residents versus 34.0% (95% CI, 33.6%–34.4%) for Q1 residents. After adjusting for baseline characteristics, residents of the most deprived neighborhoods were less likely to see a primary care physician (HR, 0.91 [95% CI, 0.89–0.92], Q5 relative to Q1) and even less likely to see a cardiologist (HR, 0.84 [95% CI, 0.82–0.86], Q5 versus Q1). They were less likely to receive an echocardiogram (HR, 0.97 [95% CI, 0.96–0.99], Q5 versus Q1) or ambulatory electrocardiographic monitoring (HR, 0.89 [95% CI, 0.87–0.91], Q5 versus Q1) relative to individuals living in less deprived neighborhoods.
Figure 2.
Atrial fibrillation–related clinical services within 1 year of first atrial fibrillation documentation by material deprivation of individuals’ neighborhood. Hazard ratios are relative to individuals living in neighborhoods in the first quintile (least deprived) and are adjusted for age, sex, source of atrial fibrillation diagnosis (hospitalization, emergency department, outpatient), year of diagnosis, rural residence, immigration status, heart failure, hypertension, diabetes, stroke or transient ischemic attack, vascular disease, previous bleeding, liver dysfunction, chronic obstructive pulmonary disease, chronic kidney disease, cancer, excessive alcohol use, recreational drug use, hospital frailty score, and per capita cardiologist supply.
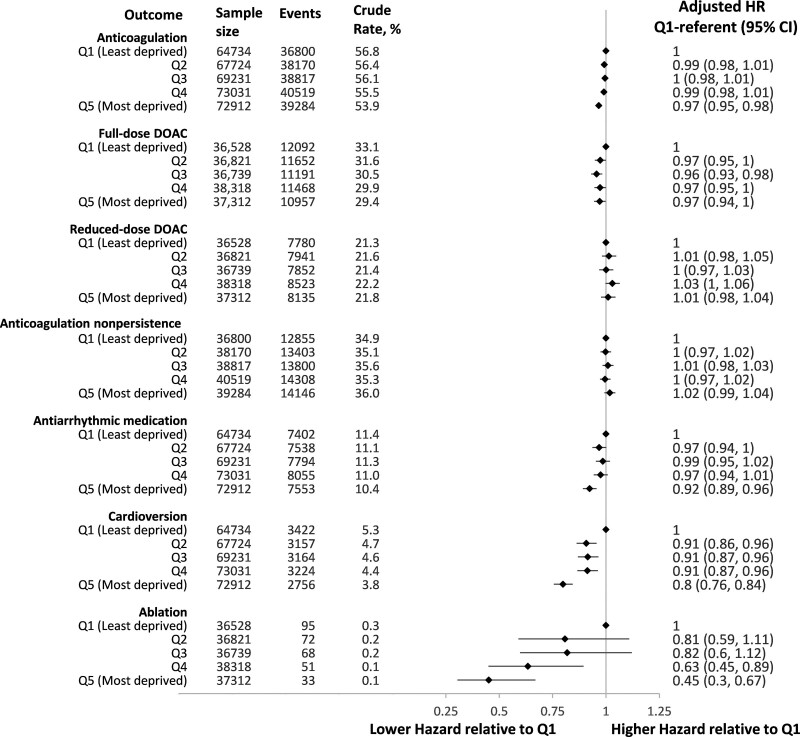
Figure 3 demonstrates gradients in the receipt of AF-related interventions by neighborhood quintile. The crude incidence of anticoagulation at 1 year was 53.9% (95% CI, 53.5%–54.2%) for Q5 residents and 56.8% (56.5%–57.2%) for Q1 residents. After adjustment, there was a lower hazard of dispensing anticoagulation for individuals living in the most deprived neighborhoods (HR, 0.97 [95% CI, 0.95–0.98], Q5 versus Q1). When analyzing individuals diagnosed after 2012, there was less dispensation of full-dose direct oral anticoagulants (HR, 0.97 [95% CI, 0.94–1.00], Q5 versus Q1) and comparable dispensation of reduced-dose direct oral anticoagulants (HR, 1.01 [95% CI, 0.98–1.04], Q5 versus Q1). Among 193 590 individuals who were dispensed an anticoagulant, 68 512 (35.4%) met the definition for nonpersistence, without a significant difference across neighborhood quintiles (HR for nonpersistence in Q5 versus Q1, 1.02 [95% CI, 0.99–1.04]).
Figure 3.
Atrial fibrillation–related interventions within 1 year of first atrial fibrillation documentation by material deprivation of individuals’ neighborhood. Hazard ratios are relative to individuals living in neighborhoods in the first quintile (least deprived) and are adjusted for age, sex, source of atrial fibrillation diagnosis (hospitalization, emergency department, outpatient), year of diagnosis, rural residence, immigration status, heart failure, hypertension, diabetes, stroke or transient ischemic attack, vascular disease, previous bleeding, liver dysfunction, chronic obstructive pulmonary disease, chronic kidney disease, cancer, excessive alcohol use, recreational drug use, hospital frailty score, and per capita cardiologist supply. DOAC indicates direct oral anticoagulant.
Rhythm control was attempted in a minority of individuals; the 1-year crude incidence of antiarrhythmic dispensation was 10.4% (95% CI, 10.1%–10.6%) in Q5 versus 11.4% (95% CI, 11.2%–11.7%) in Q1 residents and the incidence of cardioversion was 3.8% (95% CI, 3.6%–3.9%) in Q5 residents versus 5.3% (95% CI, 5.1%–5.5%) in Q1 residents. These disparities in rhythm control persisted after adjusting for comorbidities that may decrease eligibility for these interventions. Relative to residents of the least deprived neighborhoods (Q1), those residing in the most deprived quintile (Q5) had an HR of 0.92 (95% CI, 0.89–0.96) for antiarrhythmic medication dispensation and 0.80 (95% CI, 0.76–0.84) for cardioversion.
A total of 319 individuals among 185 718 people diagnosed after 2012 underwent AF ablation within 1 year of AF diagnosis (see Table S3). The median time to ablation was 238 days (interquartile range, 160–311). People undergoing ablation were younger (median age 70 years [interquartile range, 68–73]) than those who did not receive the intervention, with no significant differences by sex, immigration status, rurality, or neighborhood immigration and visible minority concentration quintile. People undergoing ablation were generally healthier with lower prevalence of most comorbidities. Despite constituting 20.1% of the cohort diagnosed after 2012, only 10.3% of people who underwent ablation were residents of Q5 neighborhoods. In contrast, 29.8% of people who underwent ablation were from Q1 neighborhoods, despite constituting 19.7% of the cohort diagnosed after 2012. After adjustment, there was a significantly lower hazard of ablation for individuals living in the most deprived neighborhoods (adjusted HR relative to Q1, 0.45 [95% CI, 0.30–0.67]).
Sensitivity and Post Hoc Analyses
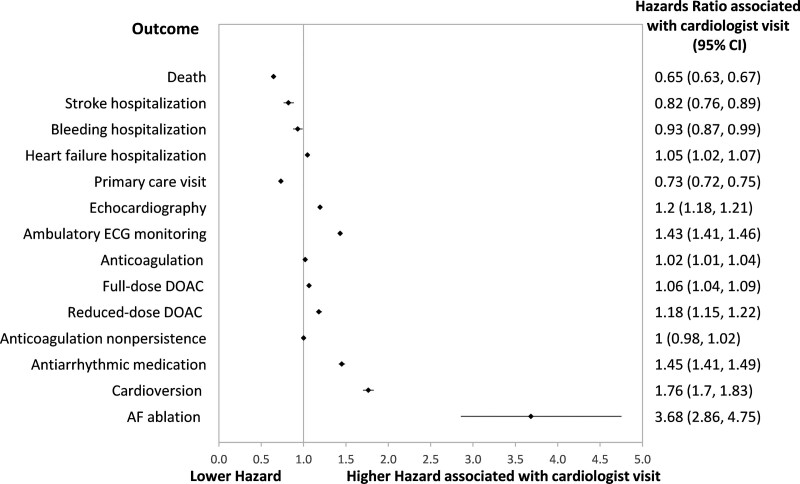
There were comparable results in the sensitivity analyses that set the index date as that of the fourth outpatient physician billing for people diagnosed with AF out of hospital (see Figures S2–S4). When visiting a cardiologist after the index date was incorporated into the cause-specific hazard regression analyses as a time-varying covariate, residence in Q5 continued to be associated with higher hazard of adverse outcomes but lower hazard of receiving most processes of care (illustrated in Figures S5–S7). Seeing a cardiologist was associated with a lower hazard of death, stroke, or bleeding hospitalizations, but higher hazard of HF hospitalization. Visiting a cardiologist was positively associated with receipt of most processes of care but was associated with lower hazard of a primary care physician visit and no difference in anticoagulation nonpersistence (see Figure 4).
Figure 4.
Hazard ratios associated with visiting a cardiologist after the index date. Cardiologist visits were modeled as a time-varying covariate in cause-specific regression models modeling time to each of the outcomes listed in the y axis. The models were adjusted for age, sex, source of atrial fibrillation (AF) diagnosis (hospitalization, emergency department, outpatient), year of diagnosis, rural residence, immigration status, heart failure, hypertension, diabetes, stroke or transient ischemic attack, vascular disease, previous bleeding, liver dysfunction, chronic obstructive pulmonary disease, chronic kidney disease, cancer, excessive alcohol use, recreational drug use, hospital frailty score, and per capita cardiologist supply. DOAC indicates direct oral anticoagulant; and ECG, electrocardiogram.
The propensity score–matched analysis yielded 44 186 pairs of matched individuals from Q1 and Q5 neighborhoods, with standardized differences ≤0.01 in all measured baseline characteristics included in the model (see Table S4). The association of outcomes with neighborhood quintile was comparable with what was observed in the regression analysis, with higher risk of most adverse outcomes but lower likelihood of receiving most processes of care for residents in Q5 relative to Q1 neighborhoods. The single exception was that there was no significant difference in the hazard of stroke within matched participants from Q5 relative to Q1 neighborhoods (HR, 1.08 [95% CI, 0.97–1.19]). The HRs are summarized in Figure 5.
Figure 5.
Hazard ratios from cause-specific regression models comparing time to each of the outcomes listed among individuals with atrial fibrillation residing in neighborhoods with the highest quintile of material deprivation (Q5) relative to matched individuals residing in neighborhoods with the lowest quintile of material deprivation (Q1). Individuals were matched using propensity score methods as described in the text. DOAC indicates direct oral anticoagulant; and ECG, electrocardiogram.
Discussion
This population-based cohort study described the association of neighborhood-level material deprivation with processes of care and outcomes for people with AF in a single-payer health system that minimizes direct medical care costs. Compared with people with AF residing in the least deprived neighborhoods, individuals residing in the most deprived neighborhoods had ≈15% higher hazard of death or hospitalization for stroke, HF, or bleeding at 1 year. Despite this, there was a notable risk–treatment mismatch, with less receipt of most care processes by residents of more deprived neighborhoods.
This cohort of people with incident AF had greater representation from residents of more deprived neighborhoods, mirroring observations from other jurisdictions with universal health care.23 There was notable heterogeneity in the associations of neighborhood material deprivation with different aspects of care for individuals with AF. There were minimal differences in anticoagulation dispensation rates and no detectible differences in persistence after initiation of anticoagulation. There were small differences in the likelihood of undergoing an echocardiogram or accessing primary care. The absence of substantial differences in these care processes may reflect the strong emphasis on these measures in AF guidelines, relative ease of implementation, or fewer barriers to access in a single-payer system. These measures also typically impose less burden on patients and caregivers in terms of time and other resources. Previous studies from other health care systems have been inconsistent as to whether SES is associated with differential use of antithrombotic therapy in AF.10,11 The findings of this study and previous literature suggest that inequities in anticoagulation related to material deprivation can be reduced if barriers to payment and primary care access are reduced.24
The observation of worse outcomes in more deprived neighborhoods despite minimal differences in anticoagulation (the only intervention consistently shown to reduce mortality in AF) suggests that the worse prognosis may be driven by the higher baseline comorbidity burden. Despite adjustment for traditional risk factors and comorbidities that can be identified from administrative data, other factors such as AF burden or lifestyle factors could not be accounted for. There was greater representation from women, recent immigrants, and visible minorities in more deprived neighborhoods, highlighting the potential contribution of intersecting social factors. The worse outcomes may also reflect less frequent or less systematic follow-up after starting anticoagulation (not captured in a time-to-first-event analysis) and undertreatment of cardiovascular risk factors such as hypertension or dyslipidemia. Indeed, previous studies have reported that lower measures of SES are associated with less AF awareness and less health care contact for AF.5,25,26
An important observation was that residents of more deprived neighborhoods were less likely to visit a cardiologist, notwithstanding their higher burden of baseline cardiovascular disease, risk factors, and higher risk for adverse outcomes. Visiting a cardiologist was associated with better outcomes despite adjusting for baseline characteristics, but this does not necessarily imply causation, because cardiologist access may reflect better access to health care or healthier behaviors. Residents of the most deprived neighborhoods were also less likely to receive rhythm control interventions, with increasing gradients from antiarrhythmic medications to cardioversion to ablation. The administrative datasets do not contain quality of life measures to determine whether these differences in rhythm control are appropriate.
Previous studies have consistently demonstrated that patients with lower SES are less likely to access specialized AF interventions such as ablation7,8 despite generally having worse symptomatic status.27,28 The current study shows that these disparities persist in a health care system where it is prohibited to pay out of pocket to access specialized AF care. This may reflect a lower propensity to refer patients with greater noncardiovascular comorbidity burden.29 It may also indicate that there is less attentiveness by providers to quality of life concerns for residents of more marginalized neighborhoods and to care measures that are less strongly emphasized by guidelines.30 This can be compounded by less self-advocacy by residents of deprived neighborhoods to access scare resources for the purposes of improving quality of life.31–35 Patients from deprived neighborhoods (and their family members) may have less ability to participate in treatment plans that require larger time commitments, more frequent physician visits, or more complex care. For example, the need for time off work, arranging care from other family members, transportation costs, and hospital parking fees can create substantial burdens. These collectively add up to create important barriers to accessing health care that are comparable to or greater than the direct costs of health care.36–38 This can create considerable impediments to accessing “universal” health care for residents of more deprived neighborhoods. The differences were most stark for early access to ablation; this was the least accessible intervention studied, thus requiring greater patient advocacy and persistence in navigating the health system compared with other treatments for AF.
These observations highlight persistent disparities in care for individuals with AF and their outcomes even in the setting of single-payer health care, where direct financial barriers to health care access are expected to be minimized. These residual barriers need to be identified through future research so that appropriate interventions can be designed and implemented. For example, nurse-led AF clinics offer a potential approach to decentralize care for individuals with AF and improve access for residents from higher-risk neighborhoods.39–41 The lessons learned from COVID-19 vaccine distribution42 can inform the locations of AF clinics to match regional risk estimates so that care is provided in an equitable (rather than equal) manner. AF clinics can also be leveraged to enhance patients’ health literacy and self-management skills25,28,33,34,43 and the incorporation of navigation services may improve access to specialized care for eligible patients.32,43 The implementation of protocolized care pathways for people with newly diagnosed AF may also allow for community-based AF care while increasing the likelihood of considering all potentially beneficial interventions with less bias.44,45 The emergence of telehealth and novel technologies may also allow for provision of requisite care for individuals with AF while minimizing in-person visits,46,47 although this needs to be implemented thoughtfully to avoid exacerbating SES-based disparities.48
Limitations
In this observational study, the potential for residual confounding cannot be eliminated despite adjusting for an extensive list of covariates. The higher baseline prevalence of potential AF complications in neighborhoods with greater material deprivation may have led to differential rates of AF diagnosis across material deprivation quintiles. Important clinical variables including AF type (paroxysmal, persistent, or permanent), AF burden, symptom status, echocardiographic measures, and lifestyle factors including weight, smoking, diet, and physical activity were unavailable in the administrative datasets used. If differences in baseline comorbidity were incompletely accounted for, that may explain disparities in treatments and outcomes independent of material deprivation status. The unit of exposure was at the neighborhood level and the association between outcomes and neighborhood material deprivation may be weaker than at the individual level. Some aspects of intersectionality could not be studied in the absence of individual-level data on race and gender; this may be relevant because women and recent immigrants were overrepresented in more deprived neighborhoods. Entry into the study cohort required health system contact for AF, particularly for outpatients, who needed 4 physician visits within 1 year. Thus, people with undiagnosed AF and those with inconsistent health system contact were excluded. This may lead to underestimation of the association between material deprivation and care measures.
Conclusions
In a universal health care system with comprehensive provider, procedure, and prescription drug coverage, older individuals with AF living in more materially deprived neighborhoods were less likely to access specialty cardiology services and rhythm control interventions despite being at higher risk of adverse outcomes and having greater CVD burden at baseline. Disparities were smaller for processes that are strongly emphasized by guidelines and can be facilitated by primary care, such as anticoagulation. These data can inform discussions about which aspects of care for individuals with AF will require interventions beyond addressing barriers to payment to reduce disparities resulting from material deprivation.
Article Information
Acknowledgments
The authors thank Dr Jack V. Tu (deceased May 30, 2018) for securing grant funding that enabled this research and whose ideas and vision catalyzed its development. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES, the Ministry of Health, or Ministry of Long-Term Care is intended or should be inferred. Parts of this material are based on data or information compiled and provided by Immigration, Refugees and Citizenship Canada current to May 2017, the Canadian Institute for Health Information, and Cancer Care Ontario. However, the analyses, conclusions, opinions, and statements expressed in this material are those of the authors, and not necessarily those of Immigration, Refugees and Citizenship Canada, Canadian Institute for Health Information, or Cancer Care Ontario. The clinical registry data used in this publication are from participating hospitals through CorHealth Ontario, which serves as an advisory body to the Minister of Health, is funded by the Ministry of Health, and is dedicated to improving the quality, efficiency, access, and equity in the delivery of the continuum of adult cardiac, vascular, and stroke services in Ontario, Canada. The authors thank IQVIA Solutions Canada Inc for use of its Drug Information File.
Sources of Funding
This study was funded by an Institute for Circulatory and Respiratory Health/Canadian Institutes of Health Research Team Grant to the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) from the Canadian Institutes of Health Research (CIHR Foundation grant FDN 143313). Dr Abdel-Qadir is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. Drs Austin, Kapral, and Atzema were supported by Mid-Career Investigator Awards from the Heart and Stroke Foundation of Ontario. Dr Atzema was also supported by ICES, the Practice Plan of the Department of Emergency Services at Sunnybrook Health Sciences, and the Sunnybrook Research Institute. Dr Kapral holds the Lillian Love Chair in Women’s Health from the University Health Network, Canada. Dr McNaughton was supported by ICES, the Practice Plan of the Department of Emergency Services at Sunnybrook Health Sciences, the Sunnybrook Research Institute, the National Institutes of Health (R21HL140381), VA (IIR-19-134), and Pfizer. Dr Tu is supported by a Research Scholar Award from the Department of Family and Community Medicine at the University of Toronto. Dr Wijeysundera was supported by a Phase 2 Clinician Scientist Award from the Heart and Stroke Foundation of Canada, Ontario office. Dr Ko is supported by the Jack Tu Chair in Cardiovascular Outcomes Research. Dr Lee is supported by the Ted Rogers Chair in Heart Function Outcomes. The funding sources were not involved in the design or conduct of the study, data management or analysis, or manuscript preparation, review, or authorization for submission.
Disclosures
Dr Ha reports receiving fees for giving lectures and serving on advisory boards from Bayer, Bristol Myers Squibb, Pfizer, and Servier. In the past 3 years, Harlan Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, F-Prime, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, and Martin/Baughman Law Firm. He is a co-founder of Refactor Health and HugoHealth, and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Johnson & Johnson. The other authors report no conflicts.
Supplemental Material
Figures S1–S7
Tables S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- CVD
- cardiovascular disease
- HF
- heart failure
- HR
- hazard ratio
- SES
- socioeconomic status
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.058949.
For Sources of Funding and Disclosures, see page 169.
Contributor Information
Leo E. Akioyamen, Email: sele.akioyamen@mail.utoronto.ca.
Jiming Fang, Email: jiming.fang@ices.on.ca.
Andrea Pang, Email: andreajessie.pang@gmail.com.
Andrew C.T. Ha, Email: Andrew.Ha@uhn.ca.
Cynthia A. Jackevicius, Email: cjackevicius@westernu.edu.
David A. Alter, Email: david.alter@ices.on.ca.
Peter C. Austin, Email: peter.austin@ices.on.ca.
Clare L. Atzema, Email: clare.atzema@ices.on.ca.
R. Sacha Bhatia, Email: sacha.bhatia@wchospital.ca.
Gillian L. Booth, Email: Gillian.Booth@unityhealth.to.
Sharon Johnston, Email: sharonjohnston@montfort.on.ca.
Irfan Dhalla, Email: irfan.dhalla@unityhealth.to.
Moira K. Kapral, Email: Moira.Kapral@uhn.ca.
Harlan M. Krumholz, Email: harlan.krumholz@yale.edu.
Candace D. McNaughton, Email: candace.mcnaughton@utoronto.ca.
Idan Roifman, Email: idan.roifman@sunnybrook.ca.
Karen Tu, Email: k.tu@utoronto.ca.
Jacob A. Udell, Email: jay.udell@utoronto.ca.
Harindra C. Wijeysundera, Email: harindra.wijeysundera@sunnybrook.ca.
Dennis T. Ko, Email: dennis.ko@ices.on.ca.
Michael J. Schull, Email: michael.schull@ices.on.ca.
Douglas S. Lee, Email: douglas.lee@ices.on.ca.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 3.Singh SM, Abdel-Qadir H, Pang A, Fang J, Koh M, Dorian P, Wijeysundera HC, Ko DT. Population trends in all-cause mortality and cause-specific death with incident atrial fibrillation. J Am Heart Assoc. 2020;9:e016810. doi: 10.1161/JAHA.120.016810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 5.Hagengaard L, Andersen MP, Polcwiartek C, Larsen JM, Larsen ML, Skals RK, Hansen SM, Riahi S, Gislason G, Torp-Pedersen C, et al. Socioeconomic differences in outcomes after hospital admission for atrial fibrillation or flutter. Eur Heart J Qual Care Clin Outcomes. 2021;7:295–303. doi: 10.1093/ehjqcco/qcz053 [DOI] [PubMed] [Google Scholar]
- 6.Kargoli F, Shulman E, Aagaard P, Briceno DF, Hoch E, Di Biase L, Fisher JD, Gross J, Kim SG, Krumerman A, et al. Socioeconomic status as a predictor of mortality in patients admitted with atrial fibrillation. Am J Cardiol. 2017;119:1378–1381. doi: 10.1016/j.amjcard.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 7.Al-Hijji MA, Deshmukh AJ, Yao X, Mwangi R, Sangaralingham LR, Friedman PA, Asirvatham SJ, Packer DL, Shah ND, Noseworthy PA. Trends and predictors of repeat catheter ablation for atrial fibrillation. Am Heart J. 2016;171:48–55. doi: 10.1016/j.ahj.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 8.Eberly LA, Garg L, Yang L, Markman TM, Nathan AS, Eneanya ND, Dixit S, Marchlinski FE, Groeneveld PW, Frankel DS. Racial/ethnic and socioeconomic disparities in management of incident paroxysmal atrial fibrillation. JAMA Netw Open. 2021;4:e210247. doi: 10.1001/jamanetworkopen.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kummer BR, Bhave PD, Merkler AE, Gialdini G, Okin PM, Kamel H. Demographic differences in catheter ablation after hospital presentation with symptomatic atrial fibrillation. J Am Heart Assoc. 2015;4:e002097. doi: 10.1161/JAHA.115.002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sholzberg M, Gomes T, Juurlink DN, Yao Z, Mamdani MM, Laupacis A. The influence of socioeconomic status on selection of anticoagulation for atrial fibrillation. PLoS One. 2016;11:e0149142. doi: 10.1371/journal.pone.0149142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Hylek E, Kowey PR, Mahaffey KW, O’Brien EC, et al. ; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am Heart J. 2017;189:40–47. doi: 10.1016/j.ahj.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 13.Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, Niebauer M, Makati K, Halperin B, Gauri A, et al. ; STOP AF First Trial Investigators. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 14.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. ; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 15.Schull MJ, Azimaee M, Marra M, Cartagena RG, Vermeulen MJ, Ho M, Guttmann A. ICES: data, discovery, better health. Int J Popul Data Sci. 2019;4:1135. doi: 10.23889/ijpds.v4i2.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu K, Nieuwlaat R, Cheng SY, Wing L, Ivers N, Atzema CL, Healey JS, Dorian P. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32:1561–1565. doi: 10.1016/j.cjca.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Flora M, Trevor VI. 2016 Ontario Marginalization Index User Guide. St; Michael’s Hospital/Public Health Ontario: 2018. https://www.publichealthontario.ca/-/media/documents/o/2017/on-marg-userguide.pdf [Google Scholar]
- 18.Matheson FI, Dunn JR, Smith KL, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health. 2012;103(8 Suppl 2):S12–S16. doi: 10.1007/BF03403823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics Canada. Dictionary, Census of Population, 2016 Accessed February 7, 2022. https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/index-eng.cfm
- 20.Land Information Ontario. Local Health Integration Network (LHIN) sub-region boundaries Accessed November 27, 2021. https://www.arcgis.com/home/item.html?id=b33cedfd7b7648749045b5c4b1e7cea7
- 21.Czwikla J, Jobski K, Schink T. The impact of the lookback period and definition of confirmatory events on the identification of incident cancer cases in administrative data. BMC Med Res Methodol. 2017;17:122. doi: 10.1186/s12874-017-0407-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wodschow K, Bihrmann K, Larsen ML, Gislason G, Ersbøll AK. Geographical variation and clustering are found in atrial fibrillation beyond socioeconomic differences: a Danish cohort study, 1987-2015. Int J Health Geogr. 2021;20:11. doi: 10.1186/s12942-021-00264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essien UR, Dusetzina SB, Gellad WF. A policy prescription for reducing health disparities-achieving pharmacoequity. JAMA. 2021;326:1793–1794. doi: 10.1001/jama.2021.17764 [DOI] [PubMed] [Google Scholar]
- 25.Reading SR, Go AS, Fang MC, Singer DE, Liu IA, Black MH, Udaltsova N, Reynolds K; Anticoagulation and Risk Factors in Atrial Fibrillation–Cardiovascular Research Network (ATRIA-CVRN) Investigators. Health literacy and awareness of atrial fibrillation. J Am Heart Assoc. 2017;6:e005128. doi: 10.1161/JAHA.116.005128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández Madrid A, Potpara TS, Dagres N, Chen J, Larsen TB, Estner H, Todd D, Bongiorni MG, Sciaraffia E, Proclemer A, et al. Differences in attitude, education, and knowledge about oral anticoagulation therapy among patients with atrial fibrillation in Europe: result of a self-assessment patient survey conducted by the European Heart Rhythm Association. Europace. 2016;18:463–467. doi: 10.1093/europace/euv448 [DOI] [PubMed] [Google Scholar]
- 27.Golwala H, Jackson LR, Simon DN, Piccini JP, Gersh B, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas L, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am Heart J. 2016;174:29–36. doi: 10.1016/j.ahj.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 28.Goli NM, Thompson T, Sears SF, Mounsey JP, Chung E, Schwartz J, Wood K, Walker J, Guise K, Gehi AK. Educational attainment is associated with atrial fibrillation symptom severity. Pacing Clin Electrophysiol. 2012;35:1090–1096. doi: 10.1111/j.1540-8159.2012.03482.x [DOI] [PubMed] [Google Scholar]
- 29.Lee DS, Tu JV, Juurlink DN, Alter DA, Ko DT, Austin PC, Chong A, Stukel TA, Levy D, Laupacis A. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA. 2005;294:1240–1247. doi: 10.1001/jama.294.10.1240 [DOI] [PubMed] [Google Scholar]
- 30.Macle L, Andrade JG, Atzema C, Bell A, Cairns JA, Connolly S, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al. Management of atrial fibrillation: complete guidelines listing: 2010 to 2016 Accessed November 29, 2021. https://ccs.ca/app/uploads/2020/12/2016-AF-Update_Supplement_Final_PDF.pdf
- 31.Wiltshire J, Cronin K, Sarto GE, Brown R. Self-advocacy during the medical encounter: use of health information and racial/ethnic differences. Med Care. 2006;44:100–109. doi: 10.1097/01.mlr.0000196975.52557.b7 [DOI] [PubMed] [Google Scholar]
- 32.Manderson B, McMurray J, Piraino E, Stolee P. Navigation roles support chronically ill older adults through healthcare transitions: a systematic review of the literature. Health Soc Care Community. 2012;20:113–127. doi: 10.1111/j.1365-2524.2011.01032.x [DOI] [PubMed] [Google Scholar]
- 33.Eneanya ND, Winter M, Cabral H, Waite K, Henault L, Bickmore T, Hanchate A, Wolf M, Paasche-Orlow MK. Health literacy and education as mediators of racial disparities in patient activation within an elderly patient cohort. J Health Care Poor Underserved. 2016;27:1427–1440. doi: 10.1353/hpu.2016.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhaliwal KK, King-Shier K, Manns BJ, Hemmelgarn BR, Stone JA, Campbell DJ. Exploring the impact of financial barriers on secondary prevention of heart disease. BMC Cardiovasc Disord. 2017;17:61. doi: 10.1186/s12872-017-0495-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell DJT, Manns BJ, Leblanc P, Hemmelgarn BR, Sanmartin C, King-Shier K. Finding resiliency in the face of financial barriers: development of a conceptual framework for people with cardiovascular-related chronic disease. Medicine (Baltimore). 2016;95:e5561. doi: 10.1097/MD.0000000000005561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath P. Three dollars for medicine, but eight dollars to park and get it: the cost of parking in a public hospital system. Just Policy. 2002;28:59–61. [Google Scholar]
- 37.Lee A, Shah K, Chino F. Assessment of parking fees at national cancer institute-designated cancer treatment centers. JAMA Oncol. 2020;6:1295–1297. doi: 10.1001/jamaoncol.2020.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitch MI, Longo CJ, Chan RJ. Cancer patients’ perspectives on financial burden in a universal healthcare system: analysis of qualitative data from participants from 20 provincial cancer centers in Canada. Patient Educ Couns. 2021;104:903–910. doi: 10.1016/j.pec.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 39.Hendriks J, Tomini F, van Asselt T, Crijns H, Vrijhoef H. Cost-effectiveness of a specialized atrial fibrillation clinic vs. usual care in patients with atrial fibrillation. Europace. 2013;15:1128–1135. doi: 10.1093/europace/eut055 [DOI] [PubMed] [Google Scholar]
- 40.Rivera-Caravaca JM, Gil-Perez P, Lopez-García C, Veliz-Martínez A, Quintana-Giner M, Romero-Aniorte AI, Fernandez-Redondo C, Muñoz L, Quero E, Esteve-Pastor MA, et al. A nurse-led atrial fibrillation clinic: impact on anticoagulation therapy and clinical outcomes. Int J Clin Pract. 2020;74:e13634. doi: 10.1111/ijcp.13634 [DOI] [PubMed] [Google Scholar]
- 41.Ariyarathna N, Hastie C, McCallum C, McManus M, Moosavi V, Yang Q, Nkoane-Kelaeng B, Olivia C, Farshid A. Nurse-led protocol-based atrial fibrillation clinic associated with high quality care for patients. Heart Lung Circ. 2019;28:S222. doi: 10.1016/j.hlc.2019.06.207 [Google Scholar]
- 42.Wrigley-Field E, Kiang MV, Riley AR, Barbieri M, Chen YH, Duchowny KA, Matthay EC, Van Riper D, Jegathesan K, Bibbins-Domingo K, et al. Geographically targeted COVID-19 vaccination is more equitable and averts more deaths than age-based thresholds alone. Sci Adv. 2021;7:eabj2099. doi: 10.1126/sciadv.abj2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin LT, Schonlau M, Haas A, Derose KP, Rosenfeld L, Buka SL, Rudd R. Patient activation and advocacy: which literacy skills matter most? J Health Commun. 2011;16(Suppl 3):177–190. doi: 10.1080/10810730.2011.604705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw RJ, McDuffie JR, Hendrix CC, Edie A, Lindsey-Davis L, Nagi A, Kosinski AS, Williams JW, Jr. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113–121. doi: 10.7326/M13-2567 [DOI] [PubMed] [Google Scholar]
- 45.Pamboukian SV, Costanzo MR, Meyer P, Bartlett L, McLeod M, Heroux A. Influence of race in heart failure and cardiac transplantation: mortality differences are eliminated by specialized, comprehensive care. J Card Fail. 2003;9:80–86. doi: 10.1054/jcaf.2003.11 [DOI] [PubMed] [Google Scholar]
- 46.Varma N, Marrouche NF, Aguinaga L, Albert CM, Arbelo E, Choi JI, Chung MK, Conte G, Dagher L, Epstein LM, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Europace. 2021;23:313. doi: 10.1093/europace/euaa187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pluymaekers NAHA, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S, Vernooy K, Crijns HJGM, Hendriks JM, Linz D. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace. 2021;23:345–352. doi: 10.1093/europace/euaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, Snider C, Chokshi NP, Eneanya ND, Takvorian SU, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic. JAMA Netw Open. 2020;3:e2031640. doi: 10.1001/jamanetworkopen.2020.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.