Abstract
Oysters are permanently exposed to various microbes, and their defense system is continuously solicited to prevent accumulation of invading and pathogenic organisms. Therefore, impairment of the animal's defense system usually results in mass mortalities in cultured oyster stocks or increased bacterial loads in food products intended for human consumption. In the present study, experiments were conducted to examine the effects of stress on the juvenile oyster's resistance to the oyster pathogen Vibrio splendidus. Oysters (Crassostrea gigas) were challenged with a low dose of a pathogenic V. splendidus strain and subjected to a mechanical stress 3 days later. Both mortality and V. splendidus loads increased in stressed oysters, whereas they remained low in unstressed animals. Injection of noradrenaline or adrenocorticotropic hormone, two key components of the oyster neuroendocrine stress response system, also caused higher mortality and increased accumulation of V. splendidus in challenged oysters. These results suggest that the physiological changes imposed by stress, or stress hormones, influenced host-pathogen interactions in oysters and increased juvenile C. gigas vulnerability to Vibrio splendidus.
As with many filter-feeding benthic invertebrates, oysters are constantly exposed to invasive and pathogenic microorganisms. Efficient humoral and cellular defense mechanisms normally help to reduce infection by microbes (2, 9, 10). Recent studies have demonstrated that certain environmental conditions or the presence of oyster parasites such as the protozoan Perkinsus marinus may suppress the bactericidal activity of oyster immune cells, thus allowing the accumulation of bacteria in oyster tissues (7, 24, 25). Further work is needed to better understand how environmental conditions may influence host-pathogen interactions in oysters and lead to the presence and persistence of certain bacteria in these animals.
The significance of such studies is multifaceted. Indeed, outbreaks of disease in oysters have both ecological consequences in marine ecosystems and economic implications for the oyster farming industry. Moreover, pathogenic bacteria that evade killing by the oyster defense system and accumulate in tissues have been implicated in several food-borne human diseases (7, 18, 19, 24, 26).
Several authors have suggested that stress may be associated with high bacterial loads and disease outbreaks in mollusks (1, 15). Recent studies have shown that in oysters, stress induces neuroendocrine responses involving the release of catecholamines (CA), such as noradrenaline (NA) and dopamine, in the hemolymph. Neuropeptides such as adrenocorticotropic hormone (ACTH) control this catecholaminergic response. Indeed, previous experiments showed that following an injection of 1 μM ACTH/g into oysters, the concentration of circulating NA increased up to 18 to 20 ng/ml, which is equivalent to NA concentrations measured in oysters after a 15-min shaking stress (13). Although these hormonal responses are thought to favor the survival of healthy animals in stressful situations, their consequences for mollusk-pathogen interactions require further clarification.
In the present study, we investigated the effects of stress and stress-induced neuroendocrine changes on the vulnerability of juvenile oysters (Crassostrea gigas) to a pathogenic vibrio strain. This strain was isolated from juvenile oysters affected by recurrent summer mortalities in France and was shown to cause high mortality rates in juvenile oysters when injected at concentrations of 104 CFU/oyster and above. Genotypic characterization identified this pathogen as Vibrio splendidus (12).
MATERIALS AND METHODS
Bacterial cultures.
The pathogenic vibrio strain used in this study was isolated from juvenile oysters affected by summer mortalities during the 2000 epizootic. Previous 16S ribosomal DNA analysis permitted identification of this pathogen as V. splendidus (12). Bacterial cultures were maintained in Zobell medium or on thiosulfate-citrate-bile salts-sucrose (TCBS) medium plates (Gibco).
Oyster treatments.
Juvenile C. gigas oysters (500 to 600 mg [dry weight]) were maintained in polyethylene tanks (60 to 70 oysters per tank) containing 110 liters of aerated and continuously flowing (50 liters/h) natural seawater at 14 to 15°C. Animals were notched on the valve margin to allow injections and then left undisturbed for a 10-day acclimation period. Oysters were subjected to an injection of either 10 μl of sterile Zobell medium or 10 μl of a bacterial suspension (100 bacteria/oyster) in Zobell medium. Three days after challenge, oysters were subjected to a physical stress (consisting of shaking animals in a 20-liter plastic container rotating at 100 rpm; this treatment did not cause any damage to oyster shells or tissues) or to an injection of either 10 μl of filtered seawater (FSW) alone or 10 μl of FSW containing NA or ACTH (Sigma). Final NA concentrations were either 5 or 30 ng/g (wet weight) of oyster. Final ACTH concentrations were either 0.2 or 1 μM/g (wet weight) of oyster. All injections were performed in the adductor muscle.
CA measurements.
Immediately after stress or 10 min after injection of drugs, circulating CA levels in oysters were measured as follows. Hemolymph was sampled from the pericardial cavity using 1-ml syringes and 26-gauge by 1/2-in. needles. Pools of 0.5 to 1 ml were centrifuged at 600 × g for 10 min to remove the cells from the hemolymph, and cell-free supernatants were used to quantify circulating CA levels. Fifty microliters of 3,4-dihydroxybenzylamine (10 pg/μl) was added. CA were then extracted by absorption on alumina, and CA levels were determined by liquid chromatography with electrochemical detection (8, 13). The elution peaks from samples were spiked with NA, adrenaline, and dopamine external standards (Sigma) for confirmation of their identity. Only NA results are reported here. All treatments were repeated at least three times using batches of 20 oysters each.
Mortality and bacterial analyses.
Numbers of dead oysters were recorded daily over a 10-day period. Three animals were removed daily for bacteriological analyses. Oysters were removed from their shells, and preweighted samples were minced in 5 ml of sterile seawater. The resulting solution was vigorously vortexed for 1 min and centrifuged at 200 × g for 5 min at 4°C. The pelleted tissues were discarded, and the supernatant was serially diluted (1/102 to 1/105), spread onto TCBS agar, and cultured at 17 to 18°C for 24 to 48 h. V. splendidus forms typical spreading yellow colonies on TCBS. These colonies were counted, and data were expressed as log CFU per oyster. When a more accurate identification was needed, standard physiological and biochemical tests were performed using the API 20E system (bioMérieux, Marcy l'Etoile, France), as previously described (12).
Statistical analyses.
All data are presented as means and standard errors from at least three experiments, unless otherwise indicated. For comparison of two means, paired or unpaired Student t tests were used where appropriate. For multiple comparisons, the data were analyzed by one-way analysis of variance.
RESULTS
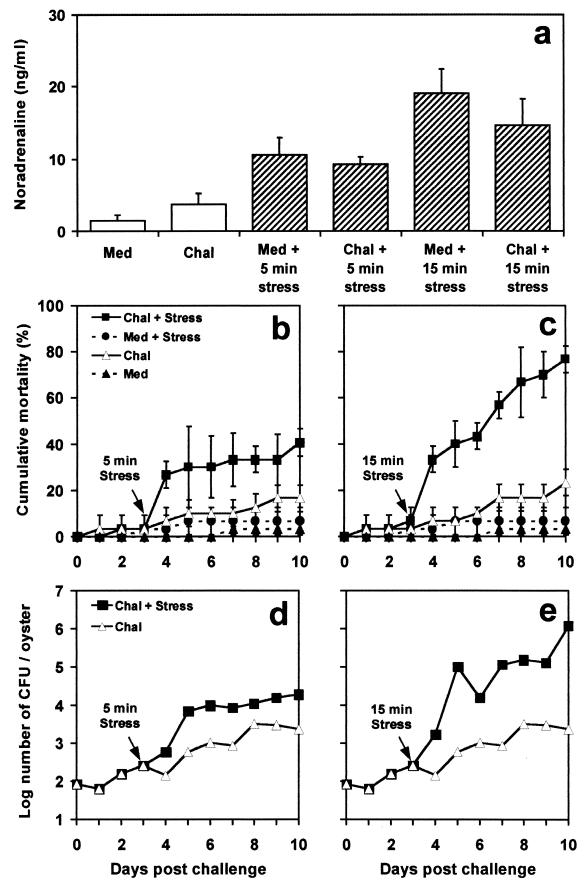
The results in Fig. 1a show that the circulating NA concentration was 1.52 ± 0.71 ng/ml in juvenile oysters injected with Zobell medium. The NA concentration increased significantly (P < 0.01) after the injection of a low dose (100 bacteria/oyster) of V. splendidus. In unchallenged oysters, a 5- or 15-min mechanical stress induced a significant (P < 0.01) 2.5- or 10-fold NA increase, respectively. The mean stress-induced NA increase was lower in oysters challenged with V. splendidus; however, the NA responses to stress were not significantly different (P < 0.05) in challenged and unchallenged oysters. Mortality remained below 10% in unchallenged oysters and below 25% in challenged, unstressed oysters (Fig. 1b and c). In oysters subjected to both bacterial challenge and a 5-min shaking stress (Fig. 1b), mortality tended to increase. However, this increase was not significant (P < 0.01) compared to mortalities in challenged, unstressed oysters. In challenged oysters subjected to a 15-min shaking stress (Fig. 1c), mortality increased significantly (P < 0.01) 24 h after application of the stress and reached 76.67% ± 5.77% at the end of the experiments. Furthermore, V. splendidus loads remained at <3 × 103 CFU/oyster in challenged, unstressed oysters, whereas they tended to increase rapidly in challenged animals subjected to a mechanical stress (Fig. 1d and e).
FIG. 1.
Effect of a 5- or 15-min mechanical stress on circulating NA concentrations (a), cumulative mortality (b and c), and V. splendidus loads (d and e) in juvenile oysters challenged (Chal) with a low dose of V. splendidus (100 bacteria/oyster) or injected with sterile culture medium (Med). Data in panels a to c are means and standard errors from three replicate experiments. For panels d and e, bacterial counts were performed five times on solid-TCBS cultures obtained from a single experiment.
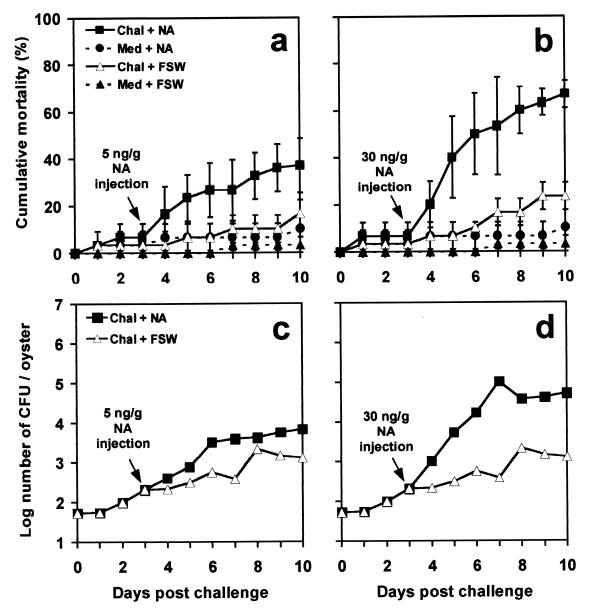
To determine whether neuroendocrine changes induced by stress may affect the capacity of juvenile oysters to face an infection by V. splendidus, challenged and unchallenged oysters were subjected to an injection of NA. Concentrations of 5 and 30 ng of NA/g were chosen because they are equivalent to NA concentrations measured in oysters after a weak or intense mechanical stress, respectively (13). In oysters subjected to both bacterial challenge and a 5-ng/g NA injection (Fig. 2a), mortality tended to increase. However, this increase was not significant (P < 0.01) compared to mortalities in challenged oysters injected with FSW. An injection of 30 ng of NA/g caused mortality to increase significantly (P < 0.01) in challenged oysters (Fig. 2b). V. splendidus loads increased (Fig. 2c and d) and reached a maximum of 5 × 105 CFU/oyster in oysters injected with 30 ng of NA/g.
FIG. 2.
Effect of an injection of 5 or 30 ng of NA/g or FSW on cumulative mortality (a and b) and V. splendidus loads (c and d) in juvenile oysters challenged (Chal) with a low dose of V. splendidus (100 bacteria/oyster) or injected with sterile culture medium (Med). Data in panels a and b are means and standard errors from three replicate experiments. For panels c and d, bacterial counts were performed five times on solid-TCBS cultures obtained from a single experiment.
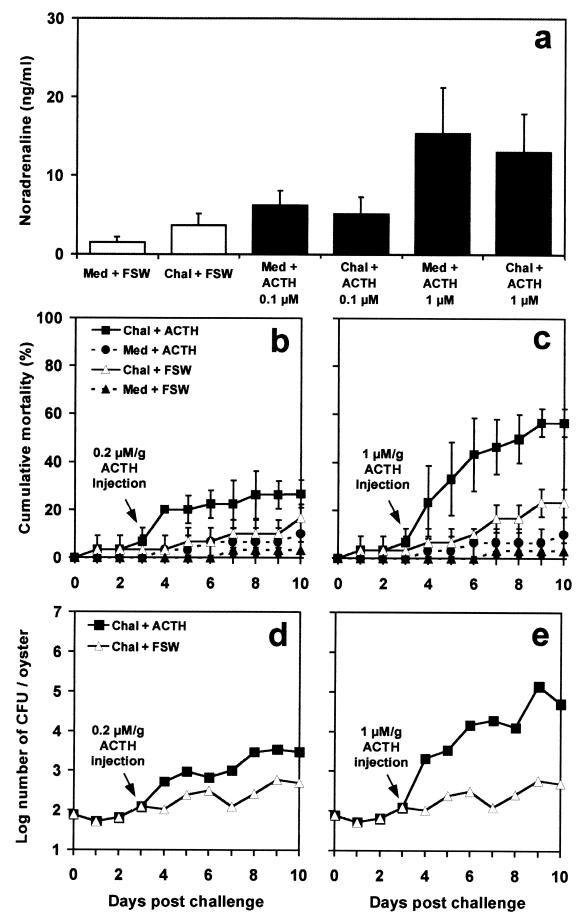
Previous studies have shown that ACTH controls the catecholaminergic response to stress in mollusks (13). To determine the effects of this neuropeptide on the capacity of juvenile oysters to face an infection with V. splendidus, animals were subjected to an injection of 0.2 or 1 μM ACTH/g, two concentrations that elicit NA responses comparable to those measured in oysters after a weak or intense stress, respectively (13). The results (Fig. 3a) show that, under the experimental conditions used in this study, NA increases caused by an injection of 0.2 or 1 μM ACTH/g were comparable (P < 0.01) to those measured in juvenile oysters subjected to a 5- or 15-min mechanical stress (Fig. 1a), respectively. In oysters subjected to both bacterial challenge and an injection of 0.2 μM ACTH/g (Fig. 3b), mortality tended to increase. However, this increase was not significant (P < 0.01) compared to mortalities in challenged oysters injected with FSW. Following an injection of 1 μM ACTH/g (Fig. 3c), mortality increased significantly (P < 0.01) in challenged oysters, and it reached 56.66% ± 5.77% at the end of the experiments. Simultaneously, V. splendidus loads increased (Fig. 3d and e) and reached a maximum of 5.27 × 104 CFU/oyster 10 days after challenge in oysters injected with 1 μM ACTH/g.
FIG. 3.
Effect of an injection of 0.2 or 1 μM ACTH/g or FSW on circulating NA concentrations (a), cumulative mortality (b and c), and V. splendidus loads (d and e) in juvenile oysters challenged (Chal) with a low dose of V. splendidus (100 bacteria/oyster) or injected with sterile culture medium (Med). Data in panels a to c are means and standard errors from three replicate experiments. For panels d and e, bacterial counts were performed five times on solid-TCBS cultures obtained from a single experiment.
DISCUSSION
Being highly dynamic, the marine environment provides a wide array of stress sources that impinge on oysters throughout their life cycle. Temperature and salinity changes, quantitative and qualitative variations of food, the presence of predators or competitors, and the appearance of toxic algae or pollutants are known to be deleterious to bivalves (11). Aquaculture practices such as handling, sorting, grading, and transport impose additional stress (4) that may compromise physiological functions in oysters.
As a primary response to stress, oysters develop a neuroendocrine reaction in which neuropeptides such as ACTH induce and control the release of CA (13). CA play essential roles in several physiological processes in mollusks, including feeding (27), locomotion (22), respiration (23), and reproduction (16). Thus, the stress-induced neuroendocrine changes are thought to divert the organism's energy resources away from physiological functions such as reproduction, growth, and certain immune processes to allow metabolic and behavioral adaptations that may help the animal to overcome the threat and survive (17, 20). However, under certain circumstances, redirecting internal energy to specific physiological functions may weaken the animal's defenses against a preexisting threat such as the presence of pathogenic or invading microbes. This concept is well known in vertebrates (3, 17, 23) but requires further clarification for invertebrates.
In the present study, we have shown that a mechanical stress favors the occurrence of mortality in juvenile oysters previously challenged with low doses of the oyster pathogen V. splendidus (12). This result is consistent with previous studies suggesting that stress and certain diseases are linked in bivalves (1, 15). Our results also show that stress led to higher V. splendidus loads in oysters, confirming that, in the present experiments, mortality was due to the accumulation of the bacterial pathogen in oyster tissues (Fig. 1e, 2d, and 3e).
A 30-ng/g injection of NA, the major CA released in oyster hemolymph in response to stress (13), increased both mortality and V. splendidus accumulation in oyster tissues. This result suggests that the negative effect of stress on the survival of challenged oysters is at least partially due to NA-mediated physiological changes. Interestingly, this result is consistent with previous in vitro studies showing that NA tends to inhibit immunological functions in oysters (14). Thus, it is possible that the redirection of energy imposed by stress or the stress-induced NA release occurred to the detriment of immune functions. In the present study, this situation may have provided a favorable environment for preexisting host-pathogen interactions to turn to the advantage of the pathogen.
The use of phenylephrine, an α-adrenoceptor agonist, and isoproterenol, a β-adrenoceptor agonist, showed that the suppressive effects of NA on oyster hemocyte functions involves β-adrenoceptor-mediated cellular signaling pathways (14). However, in the present study, injection of these analogs into both challenged and unchallenged oysters (data not shown) led to highly variable results and failed to clearly demonstrate the involvement of α- or β-adrenergic receptors in the NA-induced vulnerability of oysters to V. splendidus. This may be due to a lack of stability of these drugs in oysters in vivo.
Interestingly, a recent study has suggested that NA may play a role in the pathophysiology of infectious diseases through its capacity to induce the release of iron from iron-chelating serum proteins (5). Iron is essential for the growth of several microbes (28), including the oyster parasite P. marinus (6), and for the pathogenicity of certain vibrios (21). Thus, the stress-induced secretion of NA may lead to a release of iron from oyster iron-chelating proteins and promote the replication and/or pathogenicity of V. splendidus in infected oysters.
The neuropeptide ACTH (1 μM/g) also led to higher mortality and increased accumulation of V. splendidus in juvenile oysters. Some of the effects of ACTH are probably due to the stimulatory role played by this neuropeptide in NA secretion in oysters (13, 20). However, several studies have suggested that ACTH may also control the release of other stress hormones in invertebrates (20). Therefore, the possibility that other hormones are involved in oyster stress-induced vulnerability to V. splendidus cannot be excluded.
The present study provides new data on the influence of stress and stress-associated neuroendocrine changes on the persistence and pathogenicity of a bacterial agent in shellfish. Considering that other vibrios, including species that are pathogenic to humans, are known to be transiently or permanently present in oysters (18, 19, 26), this new information may reach beyond the field of invertebrate pathology and help in the understanding of how human bacterial pathogens persist and accumulate in shellfish intended for human consumption.
ACKNOWLEDGMENTS
This work was supported by grants from the “Conseil Régional de Bretagne,” “Département du Finistère, Côtes d'Armor et Ille-et-Vilaine,” and the “Section Régionale Conchylicole de Bretagne Nord.”
REFERENCES
- 1.Bricelj V M, Ford S E, Borrero F J, Perkins F O, Rivara G, Hillman R E, Elston R A, Chang J. Unexplained mortalities of hatchery-reared, juvenile oysters, Crassostrea virginica (Gmelin) J Shellfish Res. 1992;11:331–347. [Google Scholar]
- 2.Cheng T C. Hemocytes: forms and functions. In: Kennedy V S, Newell R I E, Eble F, editors. The eastern oyster Crassostrea virginica. College Park: Maryland Sea Grant College; 1996. pp. 299–333. [Google Scholar]
- 3.Chrousos G P, Gold P W. The concepts of stress and stress system disorders. Overview of physical and behavioural homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 4.Colombo L, Pickering A D, Belvedere P, Shreck C B. Stress inducing factors and stress reaction in aquaculture. Spec Publ Eur Aquacult Soc. 1990;12:93–121. [Google Scholar]
- 5.Freestone P P E, Lyte M, Neal C P, Maggs A F, Haigh R D, William P H. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol. 2000;182:6091–6098. doi: 10.1128/jb.182.21.6091-6098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier J D, Vasta G R. Inhibition of in vitro replication of the oyster parasite Perkinsus marinus by the natural iron chelators transferrin, lactoferrin, and desferrioxamine. Dev Comp Immunol. 1994;18:277–286. doi: 10.1016/s0145-305x(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 7.Genthner F J, Volety A K, Oliver L M, Fisher W S. Factors influencing in vitro killing of bacteria by hemocytes of the eastern oyster (Crassostrea virginica) Appl Environ Microbiol. 1999;65:3015–3020. doi: 10.1128/aem.65.7.3015-3020.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein D S, Feuerstein C, Izzo J L, Kopin I J, Keiser H R. Validity and reliability of liquid chromatography with electrochemical detection for measuring plasma levels of norepinephrine and epinephrine in man. Life Sci. 1981;28:467–475. doi: 10.1016/0024-3205(81)90139-9. [DOI] [PubMed] [Google Scholar]
- 9.Harris-Young L, Tamplin M L, Fisher W S, Mason J W. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl Environ Microbiol. 1993;59:1012–1017. doi: 10.1128/aem.59.4.1012-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris-Young L, Tamplin M L, Mason J W, Aldrich H C, Jackson J K. Viability of Vibrio vulnificus in association with hemocytes of the American oyster (Crassostrea virginica) Appl Environ Microbiol. 1995;61:52–57. doi: 10.1128/aem.61.1.52-57.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy V S, Newell R I E, Eble F, editors. The eastern oyster Crassostrea virginica. College Park: Maryland Sea Grant College; 1996. [Google Scholar]
- 12.Lacoste, A., F. Jalabert, S. K. Malham, A. Cueff, F. Gélébart, C. Cordevant, M. Lange, and S. A. Poulet. A Vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the Bay of Morlaix (North Brittany, France). Dis. Aquat. Org., in press. [DOI] [PubMed]
- 13.Lacoste A, Malham S K, Cueff A, Jalabert F, Gélébart F, Poulet S A. Evidence for a form of adrenergic response to stress in the oyster Crassostrea gigas. J Exp Biol. 2001;204:1247–1255. doi: 10.1242/jeb.204.7.1247. [DOI] [PubMed] [Google Scholar]
- 14.Lacoste A, Malham S K, Cueff A, Poulet S A. Noradrenaline modulates hemocyte reactive oxygen species production via β-adrenergic receptors in the oyster Crassostrea gigas. Dev Comp Immunol. 2001;25:285–289. doi: 10.1016/s0145-305x(00)00067-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Taylor G T, Bricelj M, Ford S E, Zahn S. Evaluation of Vibrio spp. and microplankton blooms as causative agents of juvenile oyster disease in Crassostrea virginica (Gmelin) J Shellfish Res. 1996;15:319–329. [Google Scholar]
- 16.Martínez G, Rivera A. Role of monoamines in the reproductive process of Argopecten purpuratus. Invertebr Reprod Dev. 1994;25:167–174. [Google Scholar]
- 17.Maule A G, Vanderkooi S P. Stress-induced immune-endocrine interaction. In: Balm P H M, editor. Stress physiology in animals. Sheffield, England: Sheffield Academic Press Ltd.; 1999. pp. 205–245. [Google Scholar]
- 18.Motes M L, DePaola A, Cook D W, Veazey J E, Hunsucker J C, Garthright W E, Blodgett R J, Chirtel S J. Influence of water temperature and salinity on Vibrio vulnificus in northern Gulf and Atlantic coast oysters (Crassostrea virginica) Appl Environ Microbiol. 1998;64:1459–1465. doi: 10.1128/aem.64.4.1459-1465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Academy of Science. Seafood safety report. Microbiological and parasitic exposures and health effects. Washington, D.C.: National Academy Press; 1991. pp. 33–94. [Google Scholar]
- 20.Ottaviani E, Franceschi C. The neuroendocrinology of stress from invertebrates to man. Prog Neurobiol. 1996;48:421–440. doi: 10.1016/0301-0082(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 21.Patel M, Isaacson M. The effect of iron on the toxigenicity of Vibrio cholerae. Am J Trop Med Hyg. 1999;60:392–396. doi: 10.4269/ajtmh.1999.60.392. [DOI] [PubMed] [Google Scholar]
- 22.Sakharov D A, Salànski J. Effects of dopamine antagonists on snail locomotion. Experientia. 1982;38:1090–1091. [Google Scholar]
- 23.Syed N I, Winlow W. Respiratory behavior in the pond snail Lymnaea stagnalis. II. Neural elements of the central pattern generator. J Comp Physiol. 1991;169A:557–568. [Google Scholar]
- 24.Tall B D, La Peyre J F, Bier J W, Miliotis M D, Hanes D E, Kothary H M, Shah D B, Faisal M. Perkinsus marinus extracellular protease modulates survival of Vibrio vulnificus in Eastern oyster (Crassostrea virginica) hemocytes. Appl Environ Microbiol. 1999;65:4261–4263. doi: 10.1128/aem.65.9.4261-4263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamplin M L, Capers G. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol. 1992;58:1506–1510. doi: 10.1128/aem.58.5.1506-1510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamplin M L. The ecology of Vibrio vulnificus. In: Watkins W, McCarthy S, editors. Proceedings of the 1994 Workshop. Washington, D.C.: Office of Seafood; 1995. pp. 75–86. [Google Scholar]
- 27.Teyke T, Rosen S C, Weiss K R, Kupfermann I. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 1993;630:226–237. doi: 10.1016/0006-8993(93)90661-6. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg E D. Microbial pathogens with impaired ability to acquire host iron. Biometals. 2000;13:85–89. doi: 10.1023/a:1009293500209. [DOI] [PubMed] [Google Scholar]