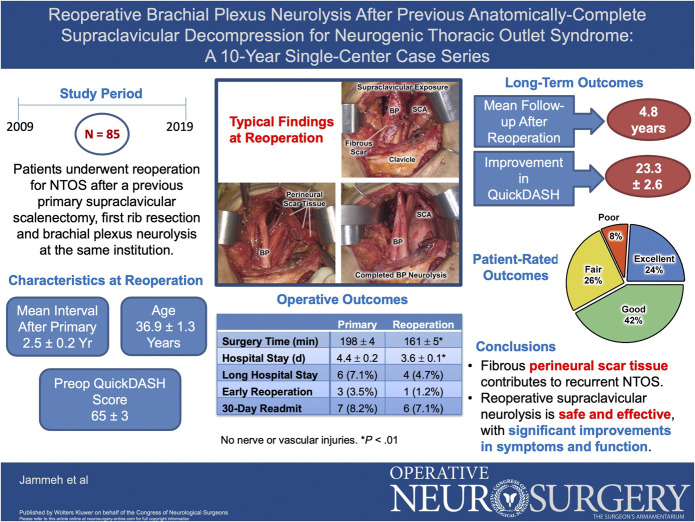
Abstract
BACKGROUND:
Optimal management of recurrent neurogenic thoracic outlet syndrome (NTOS) remains a considerable challenge.
OBJECTIVE:
To assess the safety and effectiveness of reoperative brachial plexus neurolysis in patients with recurrent NTOS.
METHODS:
From 2009 to 2019, 85 patients underwent reoperative supraclavicular brachial plexus neurolysis for recurrent NTOS after a previous anatomically complete supraclavicular decompression. Data from a prospectively maintained database were analyzed retrospectively.
RESULTS:
The mean patient age at reoperation was 36.9 ± 1.3 (range 15-64) years, 75% were female, and the interval after previous primary operation was 2.5 ± 0.2 years. Intervening injury had precipitated recurrent NTOS in 14 patients (16%), and the mean Disability of the Arm, Shoulder, and Hand (QuickDASH) score before reoperation was 65.2 ± 2.6, reflecting substantial disability. Operative findings consisted of dense fibrous scar tissue surrounding/encasing the brachial plexus. Compared with the previous primary operations, reoperations had a shorter operative time (198 ± 4 vs 161 ± 5 minutes, P < .01) and hospital stay (4.4 ± 0.2 vs 3.6 ± 0.1 days, P < .01), but there were no significant differences in the frequency of prolonged hospitalization (7.1% vs 4.7%), early reoperation (3.5% vs 1.2%), or 30-day hospital readmission (8.2% vs 7.1%). During a median follow-up of 4.8 years, QuickDASH scores improved by 23.3 ± 2.6 (34.2% ± 3.6%; P < .01) and patient-rated outcomes were excellent in 24%, good in 42%, fair in 26%, and poor in 8%.
CONCLUSION:
Reoperative supraclavicular brachial plexus neurolysis is technically challenging but safe and effective treatment for recurrent NTOS, with significant improvements in symptoms and function. Diminishing perineural scar tissue development and avoiding secondary injury would likely decrease the need for reoperations.
KEY WORDS: Brachial plexus, Case series, Compression neuropathy, Nerve wrapping, Patient-reported outcomes measures, Reoperation, Thoracic outlet syndrome
ABBREVIATIONS:
- ATOS
arterial thoracic outlet syndrome
- BP
brachial plexus
- EAST
elevated arm stress test
- F/U
follow-up
- NTOS
neurogenic thoracic outlet syndrome
- QuickDASH
11-item disability of the arm, shoulder, and hand survey instrument
- SC
supraclavicular
- SVS
Society for Vascular Surgery
- TOS
thoracic outlet syndrome
- VTOS
venous thoracic outlet syndrome.
Neurogenic thoracic outlet syndrome (NTOS) is an uncommon condition caused by dynamic compression of the brachial plexus.1-8 NTOS arises because of predisposing variations in anatomy and neck or upper extremity injury, with diagnosis requiring exclusion of other conditions and specific clinical criteria.9-12 Physical therapy and pain management can improve symptoms in many patients with NTOS, but surgical treatment is recommended for those with disabling symptoms and failure to improve with conservative measures.11-14 Surgical decompression can be safely conducted through transaxillary, supraclavicular, or posterior approaches, with excellent early results and sustained outcomes.15-25
Despite effective surgical treatment for NTOS, approximately 5% to 30% of patients experience minimal improvement or symptom recurrence during long-term follow-up.26-40 Incomplete operations and perineural scar tissue have been implicated in the development of recurrent NTOS.26-40 Reoperations involving resection of residual scalene muscles and first rib remnants can be safe and effective, as demonstrated in a recent study from our medical center.40 However, it is less clear how to optimally address patients who have previously undergone an anatomically complete decompression in which the scalene muscles and first rib have already been removed. The purpose of this study was to assess clinical characteristics and long-term results in a more homogeneous population of patients who underwent reoperative brachial plexus neurolysis after a previous anatomically complete supraclavicular decompression for NTOS.
METHODS
Derivation of the Study Population
The study population was derived from patients treated at our institution between June 2009 and April 2019. Patients with arterial or venous TOS were excluded, as were patients with neurogenic symptoms combined with either of the vascular forms of TOS, in accord with Society for Vascular Surgery (SVS) reporting standards.9-12
During the study interval, 1561 patients underwent surgical treatment for NTOS, of which 238 (15%) were reoperative procedures (Figure 1). Reoperative procedures conducted after previous operations at other institutions (n = 90) were excluded as the subjects of a recently published study.40 There were 106 supraclavicular reoperations performed after a previous anatomically complete primary operation conducted at our institution, with 21 representing multiple or other reoperations, leaving 85 patients to constitute the study population for this report. All these patients had undergone a previous anatomically complete primary operation consisting of supraclavicular anterior and middle scalenectomy, first rib resection, and complete brachial plexus neurolysis, with or without adjunctive pectoralis minor tenotomy.
FIGURE 1.
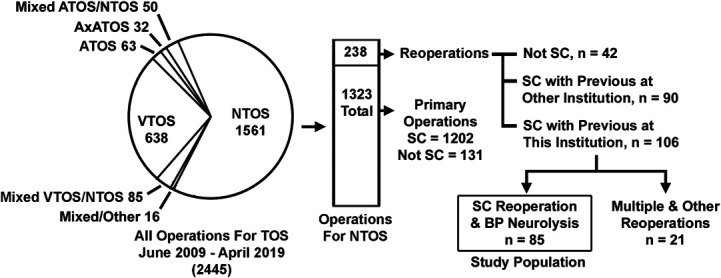
Derivation of the study population. Pie chart showing the proportion of patients undergoing surgical treatment for NTOS, ATOS, and VTOS during the study period, with the vertical bar illustrating the number of primary and reoperative procedures for NTOS. The study population for this investigation was composed of 85 patients having SC reoperations with BP neurolysis for recurrent NTOS, after a previous anatomically complete primary operation at our institution. ATOS, arterial thoracic outlet syndrome; BP, brachial plexus; NTOS, neurogenic thoracic outlet syndrome; SC, supraclavicular; VTOS, venous thoracic outlet syndrome. Adapted with permission by STM agreement from Jammeh ML, Ohman JW, Vemuri C et al, “Anatomically complete supraclavicular reoperation for recurrent neurogenic thoracic outlet syndrome: clinical characteristics, operative findings, and long-term outcomes,” Hand (NY), published online ahead of print: January 27, 2021, doi: 10.1177/1558944720988079.40
Detailed information regarding each patient was obtained from a prospectively maintained database and summarized from office notes, hospital charts, imaging studies, operative findings, and records from treating physicians and therapists. This study was approved by the Institutional Review Board at our medical center, with all patients providing written informed consent to study participation. This case series has been reported in line with the Preferred Reporting of Case Series in Surgery Guidelines.41
Clinical Diagnosis, Disability, and Initial Treatment
Each patient met clinical diagnostic criteria for NTOS as developed by the Consortium for Outcomes Research and Education on Thoracic Outlet Syndrome and the SVS reporting standards.9-12 Presenting symptoms typically consisted of pain, numbness, and paresthesia affecting the neck and upper extremity, with characteristic physical examination findings of localizing tenderness and reproduction of upper extremity symptoms on palpation over the supraclavicular space and during provocative maneuvers. The level of functional disability was assessed using the 11-item version of the Disabilities of the Arm, Shoulder, and Hand (QuickDASH) survey instrument, which has been validated for various upper extremity disorders including NTOS.11,14,40 All patients underwent initial conservative treatment with NTOS-specific physical therapy and pain management approaches.14,42
Surgical Treatment
Reoperative surgical treatment was offered to patients with a sound clinical diagnosis of recurrent NTOS, significant functional disability, and insufficient symptom improvement with physical therapy and pain management.14,19,24,40 Throughout the study period, this consisted of supraclavicular re-exploration by a single senior surgeon, with complete external neurolysis of all 5 nerve roots and 3 trunks of the brachial plexus.24 In many patients, adjunctive subcoracoid re-exploration with brachial plexus neurolysis was also performed through a separate deltopectoral groove incision, including pectoralis minor tenotomy if not performed previously.25 These operations thereby differed from the reoperations performed in our previously published study in which the procedures involved resection of residual scalene muscles and first rib remnants.40 At the end of the procedure, the supraclavicular brachial plexus was wrapped with the same absorbable polylactide film used in primary operations (off-label use of SurgiWrap Bioresorbable Sheet, MAST Biosurgery USA, Inc) to promote nerve mobility and diminish the potential for perineural adhesions. Postoperative complications, hospital stay, and readmissions were all recorded in the prospective database.
Follow-up and Outcomes Measures
As previously described, patients resumed physical therapy 3 to 4 weeks after reoperation and were seen for office visits at least every 3 to 4 months after the initial recovery from surgery.40 At each visit, patients completed the QuickDASH survey and were asked to rate their outcome of treatment on a simple scale using one of the following descriptors: “excellent” (relief of almost all major symptoms with only some mild residual symptoms that do not significantly limit enjoyment of life), “good” (relief of most major symptoms with some mild residual symptoms that significantly limit enjoyment of life), “fair” (partial relief of some symptoms, whereas other major symptoms persist), or “poor” (not enough relief in symptoms to have made the operation worthwhile).
Statistical Analysis
The principal outcome measure was the percent improvement in the QuickDASH score between initial preoperative evaluation and the longest interval of follow-up. Descriptive data are presented as mean ± SE, median and range, or the frequency (percent incidence). For two-group comparisons, Fisher's exact test or the unpaired student t-test with two-tailed distribution was used to determine statistical significance. All statistical tests were performed using Prism version 6.0h (GraphPad Software, Inc), with P values <.05 considered significant.
RESULTS
Presenting Characteristics
The study population consisted of 64 women (75%) and 21 men (25%) with a mean age of 36.9 ± 1.3 (median 37, range 15-64) years at the time of reoperation (Table 1). The age distribution included 9 patients (11%) younger than age 21 years and 76 (89%) older than age 21 years (Figure 2A). The mean interval between the previous primary operation and reoperation was 30.0 ± 2.6 months (median 22.2 months, range 4-135 months), with 59% developing recurrent NTOS within 2 years and 89% within 5 years (Figure 2B). Of the 85 patients undergoing reoperation, 67 underwent the previous primary operation during the period encompassed by this study (18 had the previous primary operation before June 2009). During the same time interval, there were 1202 patients who underwent an anatomically complete supraclavicular primary operation. The overall long-term recurrence rate for primary operations was thereby estimated to be 5.6%.
TABLE 1.
Presenting Characteristics of the Study Population
| Demographics | |
| Mean age at primary operation (y) | 34.4 ± 1.3 |
| Mean age at reoperation (y) | 36.9 ± 1.3 |
| Mean interval to reoperation (mo) | 30.0 ± 2.6 |
| Female sex | 64 (75.3%) |
| Right side affected | 46 (54.1%) |
| Local metropolitan referral | 21 (24.7%) |
| Regional referral (<200 miles) | 38 (44.7%) |
| Distant referral (>200 miles) | 26 (30.1%) |
| Examination findings | |
| 3-min EAST (s) | 73.9 ± 5.6 |
| QuickDASH score | 65.2 ± 2.60 |
| Preoperative work status | |
| Full time | 25 (29.4%) |
| Part time or restricted | 6 (7.1%) |
| Disabled or unemployed | 36 (42.3%) |
| Student | 17 (20.0%) |
| Retired | 1 (1.2%) |
EAST, elevated arm stress test; QuickDASH, 11-item version of the disabilities of the arm, shoulder, and hand.
Data shown indicate the number of patients (%) for categorical variables or the mean ± SE for continuous measures.
FIGURE 2.
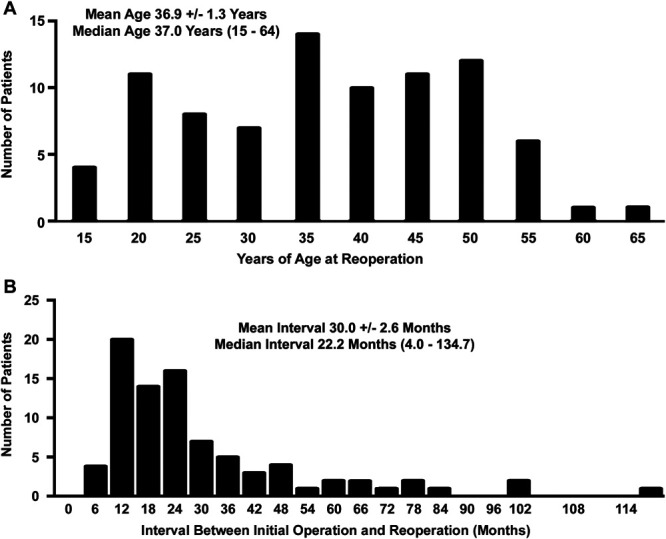
Distribution of the study population. A, Age distribution of patients in the study population. B, Distribution of patients according to the interval between the previous operation and reoperation.
Each patient met the Consortium for Outcomes Research and Education on Thoracic Outlet Syndrome and SVS criteria for a diagnosis of recurrent NTOS.9-12 The mean duration of the 3-minute Elevated Arm Stress Test was 73.9 ± 5.6 seconds, and the mean QuickDASH score before reoperation was 65.2 ± 2.6, reflecting a substantial level of disability.11 Only 25 patients (29%) were working full time at presentation, with 6 (7%) working part time under restrictions and 36 (42%) being disabled or unemployed. There was a history of injury in 29 patients (34%) before the previous primary operation and an intervening injury that had precipitated recurrent NTOS in 14 patients (16.5%), at a mean interval of 1.5 ± 0.3 years after the primary operation (Table 2).
TABLE 2.
Prevalence of Previous and Intervening Injuries in the Study Population
| History of previous injury before primary operation | |
| None | 56 (65.9%) |
| Motor vehicle collision | 9 (10.6%) |
| Work-related | 4 (4.7%) |
| Sports-related | 5 (5.9%) |
| Fall on the arm | 11 (12.9%) |
| All types | 29 (34.1%) |
| History of intervening injury after primary operation | |
| None | 71 (83.5%) |
| Motor vehicle collision | 4 (4.7%) |
| Work-related | 0 (0%) |
| Sports-related | 5 (5.9%) |
| Fall on the arm | 5 (5.9%) |
| All types | 14 (16.5%) |
Data shown indicate the number of patients (%).
Surgical Treatment
All patients underwent supraclavicular re-exploration and complete external neurolysis for fibrous perineural scar tissue surrounding/encasing the brachial plexus, typically with adherence to the scalene fat pad, extrapleural fascia, and bed of the previously resected first rib. No patient was found to have residual scalene muscle or a first rib remnant. Most of the operations were conducted solely with a supraclavicular approach, whereas 24 (28%) included a reoperative subcoracoid exploration with brachial plexus neurolysis and 10 (12%) included a primary pectoralis minor tenotomy based on localizing tenderness at preoperative examination. There were no intraoperative complications in the study population, including nerve injury. The overall duration of supraclavicular reoperation was 161 ± 5 minutes, which compared favorably with the duration of the previous primary operation (198 ± 4 minutes; P < .01) (Table 3). The hospital length of stay after reoperations was 3.6 ± 0.1 days, which was also less than that after the previous primary operation (4.4 ± 0.2 days; P = .01). There were no significant differences between reoperations and the previous primary operations regarding other measures of perioperative care, including the incidence of prolonged hospital stay, early reoperations, or readmission to the hospital within 30 days of operation (Table 3).
TABLE 3.
Perioperative Care, Previous Primary Operations vs Reoperations
| Outcome Measures | Previous primary operation | Reoperation | P value |
|---|---|---|---|
| Duration of operation (min) | 198 ± 4 | 161 ± 5 | <.01a |
| Hospital length of stay (d) | 4.4 ± 0.2 | 3.6 ± 0.1 | .01a |
| Prolonged hospital stay (>6 d) | 6 (7.1%)b | 4 (4.7%)c | .75d |
| Early reoperation | 3 (3.5%)e | 1 (1.2%)f | .62d |
| 30-day readmission | 7 (8.2%)g | 6 (7.1%)h | >.99d |
Unpaired t-test.
Lymph leak (n = 5) and pain control (n = 1).
Pain control (n = 2), wound hematoma (n = 1), and prolonged nausea/vomiting (n = 1).
Fisher's exact test.
Operative control of lymph leak (n = 3).
Wound re-exploration for hematoma (n = 1).
Pain control (n = 3), dehydration (n = 1), pancreatitis (n = 1), lymph leak (n = 1), and wound infection and pleural effusion (n = 1).
Pain control (n = 5) and wound infection (n = 1).
Data shown indicate the mean ± SE for continuous measures or the number and percent of patients for categorical variables.
Follow-up and Outcomes
The mean duration of clinical follow-up after reoperations was 5.5 ± 0.3 years (Table 4). The mean QuickDASH score at follow-up was 37.6 ± 2.8, and for individual patients, the mean decline in the QuickDASH score was 23.3 ± 2.6 (34.2% ± 3.6%), reflecting a significant improvement compared with preoperative QuickDASH scores (P < .01). There were no differences between reoperations and the previous primary operations regarding preoperative QuickDASH scores, postoperative follow-up QuickDASH scores, or the extent of decline in QuickDASH scores although the percent improvement in QuickDASH scores was somewhat less after reoperations when compared with the previous primary operations (34.2% ± 3.6% vs 45.1% ± 3.1%; P = .02).
TABLE 4.
Comparison of Outcomes, Previous Primary Operations vs Reoperations
| Outcome Measures | Previous primary operation | Reoperation | P value |
|---|---|---|---|
| Initial QuickDASH score | 60.0 ± 2.0 | 65.2 ± 2.0 | .06a |
| F/U QuickDASH score | 33.6 ± 2.1 | 37.6 ± 2.8 | .26a |
| Initial vs F/U QuickDASH score | P < .01a | P < .01a | |
| F/U decline in QuickDASH score | 27.6 ± 2.0 | 23.3 ± 2.6 | .19a |
| F/U %decline in QuickDASH score | 45.1 ± 3.1 | 34.2 ± 3.6 | .02a |
F/U, follow-up; QuickDASH, 11-item disability of the arm, shoulder, and hand survey instrument.
Paired t-test.
The mean duration of F/U after reoperations was 5.5 ± 0.3 years (median, 4.8 years; range 1.3-11.1 years). Data shown indicate the mean ± SE.
Patient-rated outcomes corresponded with the outcomes measured by changes in QuickDASH scores, after both primary operations and reoperations (Figure 3A and 3B). The proportion of patients with outcomes rated as excellent was less after reoperations than after the previous primary operations, the proportion with outcomes rated as good was equivalent, and the proportion with outcomes rated as fair was greater after reoperations (Figure 3C). During long-term follow-up after reoperation, 11 patients (12.9%) later underwent a third supraclavicular operation for re-recurrent symptoms, a recurrence rate higher than the 5.6% observed for primary operations (P = .02).
FIGURE 3.
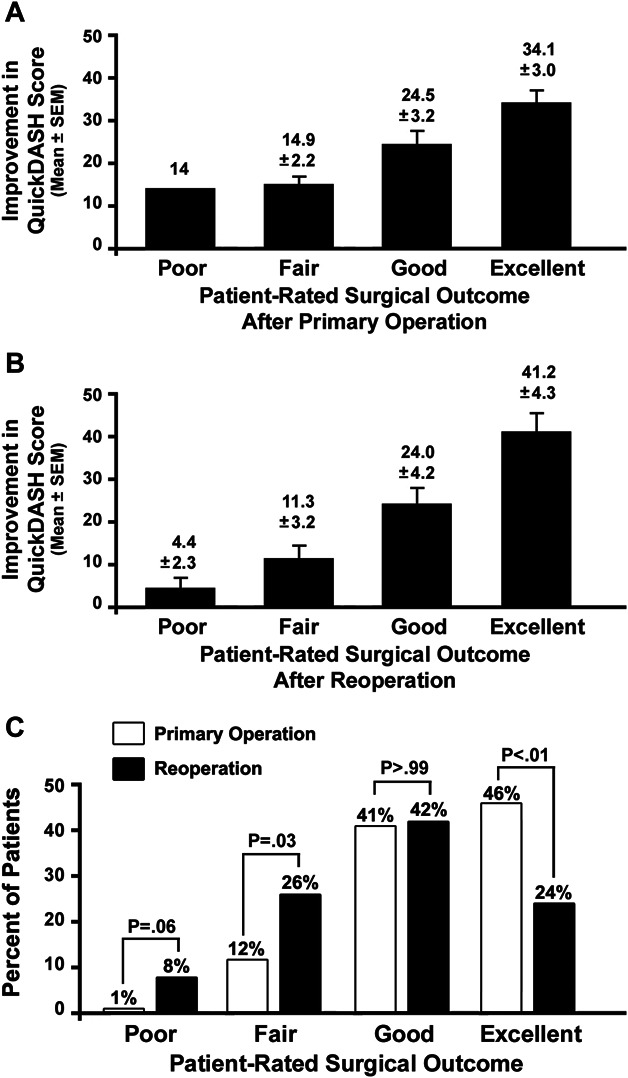
Outcomes measures for patients undergoing supraclavicular reoperation for NTOS. A, Bar graph comparing the percent improvement in QuickDASH scores for different patient-reported outcomes for patients undergoing previous primary operation for NTOS. B, Bar graph comparing the percent improvement in QuickDASH scores for different patient-reported outcomes for patients undergoing supraclavicular reoperation for recurrent NTOS. C, Bar graphs illustrating the proportion of patients in each patient-reported outcomes category after previous primary operations and reoperations for NTOS. Paired comparisons were made using Fisher's exact test. NTOS, neurogenic thoracic outlet syndrome; QuickDASH, 11-item Disability of the arm, shoulder, and hand survey instrument.
DISCUSSION
Key Results in the Context of Relevant Literature
Although reported outcomes are excellent for contemporary surgical treatment of NTOS, up to 30% of patients can be expected to have persistent symptoms or to develop later recurrence.26-40 In a previously published study, we reported excellent outcomes for reoperations that were performed for recurrent NTOS after procedures performed at other institutions, in which the operative findings frequently included residual scalene muscle and first rib remnants.40 In this study, we assessed the separate and more homogeneous population of patients with recurrent NTOS who had previously undergone anatomically complete supraclavicular decompression at our institution, in which the reoperative procedure consisted solely of brachial plexus neurolysis.
The initial success of the previous primary operations was reflected by a mean improvement in QuickDASH scores of 27.6 ± 2.0 (45.1% ± 3.1%) with 74 of 85 patients (87%) rating their outcomes as good or excellent. We estimated the recurrence rate after previous primary operations to be 5.6%, which compares favorably with other studies.26-40 We also found that the mean time interval from the previous primary operation to reoperation for recurrence was approximately 2.5 years. These observations reinforce that long-term follow-up and careful assessment of recurrent symptoms are needed in all patients who have undergone surgical treatment for NTOS. It is notable that the incidence of recurrent NTOS is often not described in studies focused on short-term and mid-term results of surgery and those solely using patient-reported outcomes measures and that defining recurrence rates requires follow-up for at least several years.32,33,38,40 The low recurrence rate for primary operations defined in this study thereby serves as a useful benchmark for future investigations.
This study differs from previous reports because the reoperations described here did not involve structural anatomic factors responsible for recurrent nerve compression, removal of residual (reattached) scalene muscle, or resection of any retained/residual first rib.40 Rather, the cause of recurrent NTOS in the patients evaluated in this study was solely attributable to fibrous perineural scar tissue that had developed during follow-up. Reoperative supraclavicular exploration and brachial plexus neurolysis require meticulous careful dissection to avoid nerve and vascular injury, but the perioperative outcomes in this series, including low early complication rates, did not seem to be influenced by the previous operation. Both the average operative time and hospital stay were also lower after reoperations than primary operations. These findings demonstrate that supraclavicular reoperation is a viable option for patients with disabling recurrent symptoms, even when no specific structural deficit is apparent relative to the previous procedure(s).
This study highlights that prevention of recurrent NTOS is an elusive goal, even after anatomically complete thoracic outlet decompression. We found an intervening secondary injury to be documented in 16% of the patients in this series, but this is likely an underappreciated factor in stimulating perineural fibrosis. In many patients, progressive increases in activity over time may also result in exacerbations of symptoms that may subsequently progress into full recurrence. Early suspicion of recurrence is valuable toward instigating conservative treatment measures that may forestall the need for reoperation.
Another factor that could limit the potential for recurrence is the use of external nerve wrapping as a physical barrier to prevent perineural scar that might otherwise compress, tether, and irritate the brachial plexus. There are precedents for this strategy in the treatment of recurrent ulnar neuropathy and carpal tunnel syndrome and in spine operations, as well as in NTOS, using a spectrum of different biocompatible materials (eg, polymeric films, carbohydrate gels, collagen matrix, amniotic membrane, and autologous vein).43-46 Throughout the course of this study, it was our regular practice to wrap the supraclavicular brachial plexus with a bioabsorbable polylactide film expected to dissolve within 3 to 4 months. It is possible that after primary operations, patients might have become more susceptible to development and accumulation of perineural scar after absorption of this barrier, especially in the context of increasing activity or secondary injury. Anecdotal use of the human amniotic membrane to wrap the brachial plexus suggests that this material might have advantages as a more durable biological extracellular matrix to suppress perineural fibrosis, but this is yet to be thoroughly evaluated.47
Long-term outcomes in this study, as assessed by clinical improvement in symptoms and changes in QuickDASH scores, were satisfactory in nearly 90% of patients. Although the results in this patient population were comparable with those undergoing primary supraclavicular decompression for NTOS, the extent of improvement after reoperations (measured by QuickDASH scores) was not as great as that observed after primary operations. The overall rate of re-recurrence after reoperation for NTOS in our population was 12.9%, which is higher than the 5.6% recurrence rate for patients undergoing primary surgical decompression. This underscores the need for long-term follow-up, ongoing clinical assessment, and prompt consideration for reoperation when appropriate.
Strengths, Limitations, and Directions for Future Research
The main strengths of this study are that all patients underwent a standardized treatment strategy with favorable and sustained outcomes and that all patients were followed with quantitative outcomes measures to allow more accurate comparisons between groups and over time. One of the limitations of this study is that we are unable to be certain if, during long-term follow-up after reoperation, some patients might have had recurrent symptoms or secondary operations elsewhere after our last recorded office visit. We feel that this is unlikely given that most of the patients in this series continued long-term follow-up with our center. Another limitation is that we do not have more detailed data on the time course and trajectory of recurrent symptoms after primary operations, which would be a valuable direction for future research. Because the basis for this study was the identified population of patients who had undergone reoperations for recurrent NTOS, we were unable to more comprehensively analyze factors that might have contributed to recurrence after primary operations. We were also unable to assess nonoperative methods of treatment for recurrent symptoms that might have been successful in avoiding the need for surgery. These important questions would have to be addressed more specifically in a prospectively designed study. Finally, it remains important to reiterate that reoperations for NTOS are technically challenging and likely to carry greater risks for nerve and/or vascular injury than primary operations, because of the extensive scarring encountered and distortions in anatomy. These procedures should therefore be undertaken only by surgeons with considerable experience with brachial plexus surgery and operations for NTOS.
CONCLUSION
For carefully selected patients with recurrent NTOS despite a previous anatomically complete decompression, reoperative supraclavicular brachial plexus neurolysis is technically challenging but safe and effective. Long-term outcomes show that supraclavicular reoperations can achieve significant symptom reduction and functional improvement for approximately 90% of patients with recurrent NTOS. Diminishing the development of perineural scar tissue and avoiding secondary injury during follow-up would likely decrease the need for reoperative interventions.
Footnotes
Presented in part at the Young Neurosurgeons Research Forum session at the 89th Annual Scientific Meeting of the American Association of Neurological Surgeons (AANS), Orlando, FL, August 21, 2021.
Operative Neurosurgery Speaks! Audio abstracts available for this article at operativeneurosurgery-online.com. Audio abstracts can be located under “Associated Multimedia.”
Funding
This work was supported in part by the Thoracic Outlet Syndrome Research and Education Fund of the Foundation for Barnes Jewish Hospital, BJC Healthcare, St. Louis, Missouri. The Foundation for Barnes Jewish Hospital had no involvement in the study design, collection, analysis, and interpretation of the data; manuscript writing; or the decision to submit the manuscript for publication.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
In virtually all articles on treating TOS, there are patients included who are revisions of previously performed TOS procedures. It is usually the authors' opinion that various technical factors that differ from the authors' preferred method of treatment are the cause of the failure. These include failing to resect the rib or enough of it, failure to resect muscle or enough, etc. Although the authors' primary premise is that revision can be safe and effective, by far, the most important premise and finding is that recurrences will occur in the face of complete resection of everything touching the nerves. This has been known and understood by peripheral nerve surgeons and perhaps will now be understood by those performing TOS procedures, which after all, are decompression of peripheral nerves. This should be the primary goal of the procedure regardless of the subspecialty of the surgeon. Decompression of arteries and veins is largely secondary, and much more effort needs to be put into preventing recurrent scarring of the nerves.
Richard Meyer
Birmingham, Alabama, USA
REFERENCES
- 1.Sheth RN, Belzberg AJ. Diagnosis and treatment of thoracic outlet syndrome. Neurosurg Clin N Am. 2001;12(2):295-309. [PubMed] [Google Scholar]
- 2.Mackinnon SE, Novak CB. Thoracic outlet syndrome. Curr Probl Surg. 2002;39(11):1070-1145. [DOI] [PubMed] [Google Scholar]
- 3.Toussaint CP, Zager EL. Thoracic outlet syndrome. In: Youmans JR, Winn HR, eds. Youmans Neurological Surgery. 6th ed. Elsevier; 2011:2440-2446. [Google Scholar]
- 4.Kuhn JE, Lebus V GF, Bible JE. Thoracic outlet syndrome. J Am Acad Orthop Surg. 2015;23(4):222-232. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RW. Thoracic outlet syndrome: neurogenic. In: Sidawy AN, Perler BA, eds. Rutherford’s Vascular Surgery and Endovascular Therapy. 9th ed. Elsevier; 2018:1619-1638. [Google Scholar]
- 6.Jones MR, Prabhakar A, Viswanath O, et al. Thoracic outlet syndrome: a comprehensive review of pathophysiology, diagnosis, and treatment. Pain Ther. 2019;8(1):5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Dierks G, Vervaeke HE, et al. Thoracic outlet syndrome: a narrative review. J Clin Med. 2021;10(5):962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiLosa KL, Humphries MD. Epidemiology of thoracic outlet syndrome. Semin Vasc Surg. 2021;34(1):65-70. [DOI] [PubMed] [Google Scholar]
- 9.Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg. 2016;64(3):e23-e35. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RW. Diagnosis of neurogenic thoracic outlet syndrome: 2016 consensus guidelines and other strategies. In: Illig KA, Thompson RW, Freischlag JA, et al., eds. Thoracic Outlet Syndrome. 2nd ed. Springer Nature; 2021:67-97. [Google Scholar]
- 11.Balderman J, Holzem K, Field BJ, et al. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2017;66(2):533.e2-544.e2. [DOI] [PubMed] [Google Scholar]
- 12.Pesser N, Goeteyn J, van der Sanden L, et al. Feasibility and outcomes of a multidisciplinary care pathway for neurogenic thoracic outlet syndrome: a prospective observational cohort study. Eur J Vasc Endovasc Surg. 2021;61(6):1017-1024. [DOI] [PubMed] [Google Scholar]
- 13.Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218. [DOI] [PubMed] [Google Scholar]
- 14.Balderman J, Abuirqeba AA, Eichaker L, et al. Physical therapy management, surgical treatment, and patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2019;70(3):832-841. [DOI] [PubMed] [Google Scholar]
- 15.Dubuisson AS, Kline DG, Weinshel SS. Posterior subscapular approach to the brachial plexus. Report of 102 patients. J Neurosurg. 1993;79(3):319-330. [DOI] [PubMed] [Google Scholar]
- 16.Urschel HC, Jr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg. 1998;228(4):609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheth RN, Campbell JN. Surgical treatment of thoracic outlet syndrome: a randomized trial comparing two operations. J Neurosurg Spine. 2005;3(5):355-363. [DOI] [PubMed] [Google Scholar]
- 18.Tender GC, Kline DG. Anterior supraclavicular approach to the brachial plexus. Neurosurgery. 2006;58(4 suppl 2):ONS-360-ONS-364. [DOI] [PubMed] [Google Scholar]
- 19.Caputo FJ, Wittenberg AM, Vemuri C, et al. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg. 2013;57(1):149-157. [DOI] [PubMed] [Google Scholar]
- 20.Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg. 2015;220(5):934-939. [DOI] [PubMed] [Google Scholar]
- 21.Hong J, Pisapia JM, Ali ZS, et al. Long-term outcomes after surgical treatment of pediatric neurogenic thoracic outlet syndrome. J Neurosurg Pediatr. 2018;21(1):54-64. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraj A, Duncan AA, Kalra M, Bower TC, Gloviczki P. Outcomes of transaxillary approach to cervical and first-rib resection for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2018;51:147-149. [DOI] [PubMed] [Google Scholar]
- 23.Ransom EF, Minton HL, Young BL, et al. Intermediate and long-term outcomes following surgical decompression of neurogenic thoracic outlet syndrome in an adolescent patient population. Hand (NY). 2022;17(1):43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson RW, Ohman JW. Surgical techniques: operative decompression using the supraclavicular approach for neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al., eds. Thoracic Outlet Syndrome. 2nd ed. Springer Nature; 2021:265-285. [Google Scholar]
- 25.Vemuri C, Thompson RW. Surgical techniques: pectoralis minor tenotomy for neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al., eds. Thoracic Outlet Syndrome. 2nd ed. Springer Nature; 2021:295-301. [Google Scholar]
- 26.Urschel HC, Jr, Razzuk MA, Albers JE, et al. Reoperation for recurrent thoracic outlet syndrome. Ann Thorac Surg. 1976;21(1):19-25. [DOI] [PubMed] [Google Scholar]
- 27.Roos DB. Recurrent thoracic outlet syndrome after first rib resection. Acta Chir Belg. 1980;79(5):363-372. [PubMed] [Google Scholar]
- 28.Sessions RT. Reoperation for thoracic outlet syndrome. J Cardiovasc Surg (Torino). 1989;30(3):434-444. [PubMed] [Google Scholar]
- 29.Sanders RJ, Haug CE, Pearce WH. Recurrent thoracic outlet syndrome. J Vasc Surg. 1990;12(4):390-400. [PubMed] [Google Scholar]
- 30.Lindgren KA, Leino E, Lepäntalo M, Paukku P. Recurrent thoracic outlet syndrome after first rib resection. Arch Phys Med Rehabil. 1991;72(3):208-210. [PubMed] [Google Scholar]
- 31.Cheng SW, Stoney RJ. Supraclavicular reoperation for neurogenic thoracic outlet syndrome. J Vasc Surg. 1994;19(4):565-572. [DOI] [PubMed] [Google Scholar]
- 32.Ambrad-Chalela E, Thomas GI, Johansen JH. Recurrent neurogenic thoracic outlet syndrome. Am J Surg. 2004;187(4):505-510. [DOI] [PubMed] [Google Scholar]
- 33.Altobelli GG, Kudo T, Haas BT, Chandra FA, Moy JL, Ahn SS. Thoracic outlet syndrome: pattern of clinical success after operative decompression. J Vasc Surg. 2005;42(1):122-128. [DOI] [PubMed] [Google Scholar]
- 34.Sanders RJ. Recurrent neurogenic thoracic outlet syndrome stressing the importance of pectoralis minor syndrome. Vasc Endovascular Surg. 2011;45(1):33-38. [DOI] [PubMed] [Google Scholar]
- 35.Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945. [DOI] [PubMed] [Google Scholar]
- 36.Gelabert HA, Jabori S, Barleben A, et al. Regrown first rib in patients with recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):933-938. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg JI, Alix K, Nehler MR, Johnston RJ, Brantigan CO. Computed tomography-guided reoperation for neurogenic thoracic outlet syndrome. J Vasc Surg. 2015;61(2):469-474. [DOI] [PubMed] [Google Scholar]
- 38.Annest SJ, Melendez BA, Sanders RJ. Recurrent and residual neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al., eds. Thoracic Outlet Syndrome. 2nd ed. Springer Nature; 2021:333-340. [Google Scholar]
- 39.Phillips WW, Donahue DM. Reoperation for persistent or recurrent neurogenic thoracic outlet syndrome. Thorac Surg Clin. 2021;31(1):89-96. [DOI] [PubMed] [Google Scholar]
- 40.Jammeh ML, Ohman JW, Vemuri C, et al. Anatomically complete supraclavicular reoperation for recurrent neurogenic thoracic outlet syndrome: clinical characteristics, operative findings, and long-term outcomes. Hand (NY). Published online ahead of print January 27, 2021. doi: 10.1177/1558944720988079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O'Neill N; for the PROCESS Group. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting of CasESeries in Surgery (PROCESS) guidelines. Int J Surg. 2020;84:231-235. [DOI] [PubMed] [Google Scholar]
- 42.Hisamoto J. Physical therapy as primary treatment for neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al., eds. Thoracic Outlet Syndrome, 2nd ed. Springer Nature; 2021:211-228. [Google Scholar]
- 43.Thakker A, Sharma SC, Hussain NM, Devani P, Lahiri A. Nerve wrapping for recurrent compression neuropathy: a systematic review. J Plast Reconstr Aesthet Surg. 2021;74(3):549-559. [DOI] [PubMed] [Google Scholar]
- 44.Dy CJ, Aunins B, Brogan DM. Barriers to epineural scarring: role in treatment of traumatic nerve injury and chronic compressive neuropathy. J Hand Surg Am. 2018;43(4):360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders RJ, Hammond SL, Rao NM. Observations on the use of Seprafilm on the brachial plexus in 249 operations for neurogenic thoracic outlet syndrome. Hand (NY). 2007;2:179-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCall TD, Grant GA, Britz GW, Goodkin R, Kliot M. Treatment of recurrent peripheral nerve entrapment problems: role of scar formation and its possible treatment. Neurosurg Clin N Am. 2001;12(2):329-339. [PubMed] [Google Scholar]
- 47.Sanders RJ, Annest SJ. Amnion membrane improves results in treating neurogenic thoracic outlet syndrome. J Vasc Surg Cases Innov Tech. 2018;4(2):163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]