Abstract
Background:
Heart transplantation volumes have increased in recent years, yet less than a third of donors are typically accepted for transplantation. Whether donor sex, donor drug use, or perception of increased risk affects utilization for transplantation is unclear.
Methods:
The United Network for Organ Sharing database was queried for donors from January 1, 2007, to December 31, 2017. Donor toxicology was collected when available. Multivariate analysis was conducted to examine correlations with donor utilization.
Results:
Between January 1, 2007, and December 31, 2017, there were 87 816 heart donors aged ≥15 years. The mean age was 42.7±15.8 years, and 24 831 donors (28.3%) were utilized for heart transplantation. Subsequent analyses focused on donors between 15 and 39 years old.
The strongest associations with donor acceptance were for male donor sex, blood type, hepatitis C antibody, donor age, left ventricular hypertrophy, and history of donor drug use. After removing hepatitis C, Public Health Service Increased Risk was identified as a strong negative predictor. Most positive drug toxicology results were associated with donor nonuse except for donors between 15 and 19 years of age. Exceptions included alcohol, marijuana, and cocaine. Opiates were associated with less utilization at all donor ages. The Public Health Service Increased Risk status was associated with significantly less utilization in all age groups except 15- to 19-year-old donors.
Conclusions:
While male donors were preferentially utilized, donors with drug use or those deemed Public Health Service Increased Risk were significantly less utilized for heart transplantation. Further consideration of such donors would be appropriate particularly as the demand for transplantation continues to increase.
Keywords: cannabis, heart transplantation, hepatitis C, multivariate analysis, tissue donors
What Is New?
Heart donor acceptance has increased from 26.4% in 2007 to 30.1% in 2017 (P<0.0001), with most donors declined. The current study is the first to examine this issue by donor age, focusing on donors aged 15 to 39 years. The undue influence of a history of drug use, positive toxicology for drug use, and identification as Public Health Service Increased Risk have contributed to the current situation where the majority of donor hearts are discarded. Increasing use of existing donors is as important as efforts to increase the frequency of consent for organ donation as the chronic organ shortage continues.
What Are The Clinical Implications?
By identifying the most important correlates of donor utilization, this work will help focus efforts to increase heart transplant volume. This work can be coupled with other research which suggests the safety of donors with various risk factors. Since each transplant program individually is small, the analysis of large data sets is valuable to highlight opportunities for improved use of donor organs.
After a significant period of stagnant growth, heart transplantation volumes have increased worldwide, especially in the United States.1,2 Some of this growth is due to the opioid epidemic, which has increased the number of donors dying of anoxia following overdoses.3 Previous work has shown that only a fraction of offered heart donors are ultimately accepted for transplantation, and this has not materially changed in recent years.4–9 The impact of specific issues such as donor sex, drug use, or perceived risk has not been explored in depth.
Previous reports on drug use in donors and the effect on outcomes have all been based on history.10,11 This information is recorded via several yes/no variables in the United Network for Organ Sharing (UNOS) registry database. Recently, data utilizing the donor toxicology results and historical variables were published, showing that survival was similar regardless of drug use (historical or toxicological evidence).12 However, this analysis could not comment on the donors who were not utilized for transplantation. Using the same UNOS data set,12 the current analysis endeavors to examine donors in the recent period, looking at the correlates of donor acceptance for heart transplantation, as well as factors associated with nonuse including the impact of donor drug use and increased risk behaviors.
Methods
UNOS, under contract from the Organ Procurement and Transplant Network, has maintained a registry of data on all solid organ transplants performed in the United States since 1987. Over time, the fields of data collected have changed with additions and deletions. In late 2006, a free-text field was added to capture the results of donor toxicology (typically urine toxicology). This field is not structured, and Organ Procurement Organization local coordinators enter the results of any testing, but this is subject to misspellings, typographic errors, and data entry errors because of no structure imposed on data entry. This field is not mandatory and can be left blank.
The UNOS Standard Transplant Analysis and Research file was requested with the addition of the donor toxicology field, linked by donor identification number. These data cannot be forwarded by the authors based on the Data Use Agreement but may be requested from UNOS. Since the data request involved a free-text field, the local Institutional Review Board (Eastern Virginia Medical School) granted an approval with a waiver of informed consent. A total of 51 205 toxicology records were parsed, but not all donors were accepted for transplantation. This study examined transplants between January 1, 2007, and December 31, 2017. Various attempts were made to parse the free-text field automatically, though this task was challenging due to the wide variations in spelling of drugs, as well as abbreviations. Therefore, a custom program was written in Microsoft Access (Microsoft, Redmond, Washington) to display the drug toxicology field along with checkboxes on a computer screen so the authors could manually parse the field and discern which drug results were positive. Specific fields were added for the following categories to cover numerous possible drugs, which might be noted in the text field.
The following fields were recorded: cocaine, opiates, marijuana (tetrahydrocannabinol), alcohol, benzodiazepines, barbiturates, amphetamine, methamphetamine, phencyclidine, buprenorphine, ecstasy (3,4-methylenedioxymethamphetamine), methadone, oxycodone, propoxyphene, synthetic opioid, acetaminophen, ethylene glycol, isopropanol, lithium, methanol, methaqualone, salicylates, and tricyclic antidepressants. Logic was built into the Microsoft Access program to compare subsequent records and automatically parse fields for records with the exact same words as ones interpreted by the human operator.
The authors defined a Measured Toxicology Score (MTS) as the sum of the fields with 1 point for each positive field. This gave a range of 0 to 23 and allowed comparison of donors with multiple positive toxicology results. An unknown category was created for donors with a blank toxicology field.
The UNOS Standard Transplant Analysis and Research file includes 5 fields relating to drug and alcohol use during the period studied, which are detailed in Table S1. Each of these fields contains a true/false response, and the UNOS Toxicology Score (UTS) was defined by the authors as the sum of the fields, with a possible range of 0 to 5.
Statistical Methods
Descriptive statistics were used where appropriate with means and SDs for normally distributed variables and medians with interquartile ranges (IQRs) for non-normally distributed variables. One-way ANOVA was used to compare the means of normally distributed variables across categories. Pearson χ2 was used with categorical variables, which were analyzed in 2×2 tables. Univariate and multivariate logistic regression analysis was used to examine correlates of donor use for heart transplantation. Statistical analysis was performed with JMP 16.0 (SAS Institute, Cary, NC).
Results
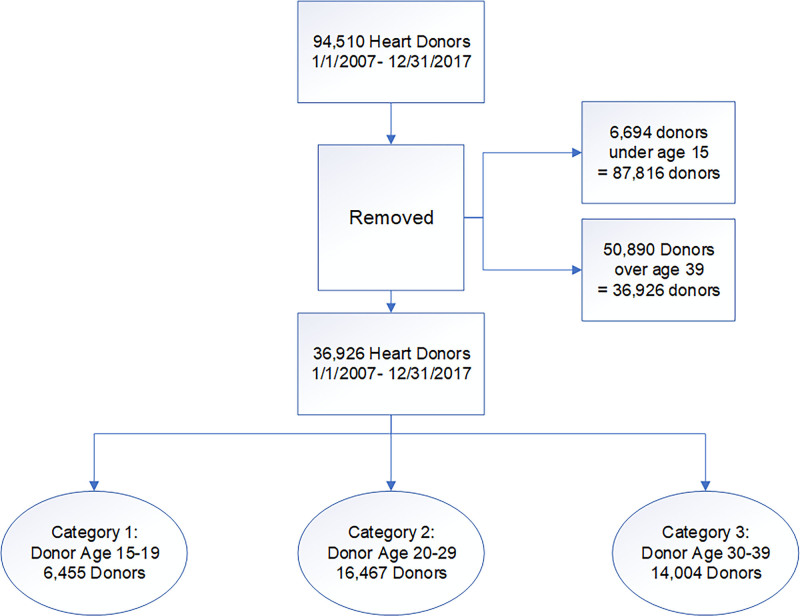
The flow of patients is illustrated in Figure 1. Between January 1, 2007, and December 31, 2017, there were 94 510 heart donors offered for transplantation. Of these, 6694 were <15 years of age and were excluded, leaving 87 816 donors. The mean age was 42.7±15.8 years with a median age of 44 (IQR, 29–55) years and a bimodal distribution (peaks at young and older ages). A total of 24 831 donors (28.3%) were utilized for heart transplantation. The median age of donors accepted was 29 (IQR, 22–40) years for heart transplantation and 49 (IQR, 36–58) years for discarded donors (P<0.0001; Kruskal-Wallis rank-sum).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of flow of donors in the study.
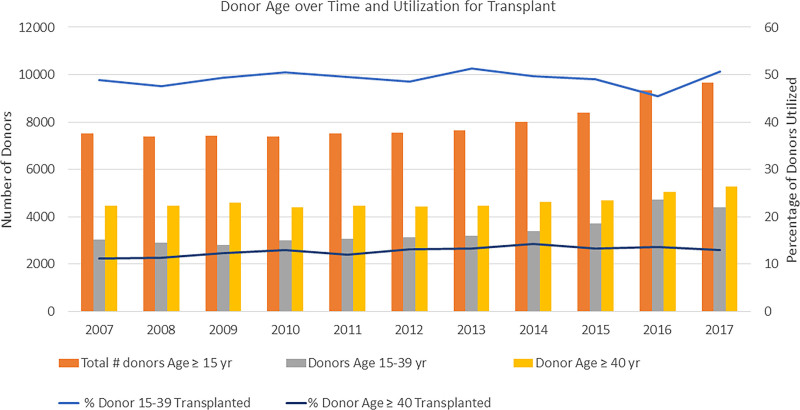
As shown in Figure 2, the number of donors began to increase in 2014 and has increased >25 % by 2017. In 2007, there were 7504 donors offered, increasing by 28.9% to 9669 donors in 2017. The median donor age dropped from 45 (IQR, 28–55) years in 2007 to 42 (IQR, 29–54) years in 2017. Heart donor acceptance increased marginally from 26.4% in 2007 to 30.1% in 2017 (P<0.0001 across years). As illustrated on the graph, the largest component of the increase in donors per year has been among donors between the ages of 15 and 39 years. The rate of acceptance among donors in this younger age group has fluctuated but has stayed fairly close to 50% (48.8%–50.7%; P=0.13) while the acceptance rate for donors beyond the age of 40 years has remained below 15% over time (11.2%–13%; P<0.0001).
Figure 2.
The number of donors over time has increased sharply in the last 3 years of the cohort. However, <15% of donors over the age of 40 years are utilized, and approximately half of the younger donors are utilized overall. Over time, the use of older donors did rise statistically significantly (11.2%–13%; P<0.0001), but the percentage utilization of younger donors did not change significantly (48.8%–50.7%; P=0.13).
Correlates of Donor Acceptance in Younger Donors
A total of 50 890 donors were above the age of 39 years and were excluded from further analysis to examine correlates of acceptance and nonacceptance in a population of younger donors, leaving 36 926 donors between ages 15 and 39 years.
Factors were entered into a multivariate logistic fit to evaluate variables associated with the donor being utilized for transplantation including donor age, sex, blood type, body mass index, identification as Public Health Service Increased Risk (PHS-IR) status, presence of hepatitis C antibody, presence of left ventricular hypertrophy, as well as if the UTS or MTS was ≥1. The strongest associations were for male donor sex, blood type, presence of hepatitis C antibody, donor age, left ventricular hypertrophy, and UTS ≥1. Donor body mass index, PHS-IR status, and MTS ≥1 were not independent predictors.
Male donors were highly favored for acceptance with an odds ratio of 2.68 ([95% CI, 1.95–3.7] P<0.0001). The odds of accepting a blood type O donor were 2.39× higher than a blood type B donor ([95% CI, 1.4–4.08] P=0.0014). The odds of accepting a hepatitis C donor were 0.06 ([95% CI, 0.009–0.47] P=0.007).
The odds of accepting a donor declined 0.97 per year of donor age ([95% CI, 0.95–0.99] P=0.009). The odds of accepting a donor with left ventricular hypertrophy (as defined by UNOS yes/no field, no specific echo data) was 0.58 ([95% CI, 0.37–0.91] P=0.02). The odds of accepting a donor with at least 1 positive category in the UTS was 0.69 ([95% CI, 0.51–0.94] P=0.02).
Removing the hepatitis C antibody from the logistic fit (since use was so rare during the time period studied) resulted in PHS-IR being a highly significant predictor of donor nonuse. In the revised model, PHS-IR was associated with an odds ratio of 0.62 ([95% CI, 0.44–0.87] P=0.007).
Acceptance of Donors Based on Donor Age Category
The following analyses examine whether the use of donors was similar across donor ages or whether age modified the utilization of donors. Donor age was split into categories 1, 2, or 3 representing ages 15 to 19, 20 to 29, or 30 to 39 years with 6455, 16 467, and 14 004 donors for each respective age category. These analyses present unadjusted associations between identified risk factors and use for transplantation.
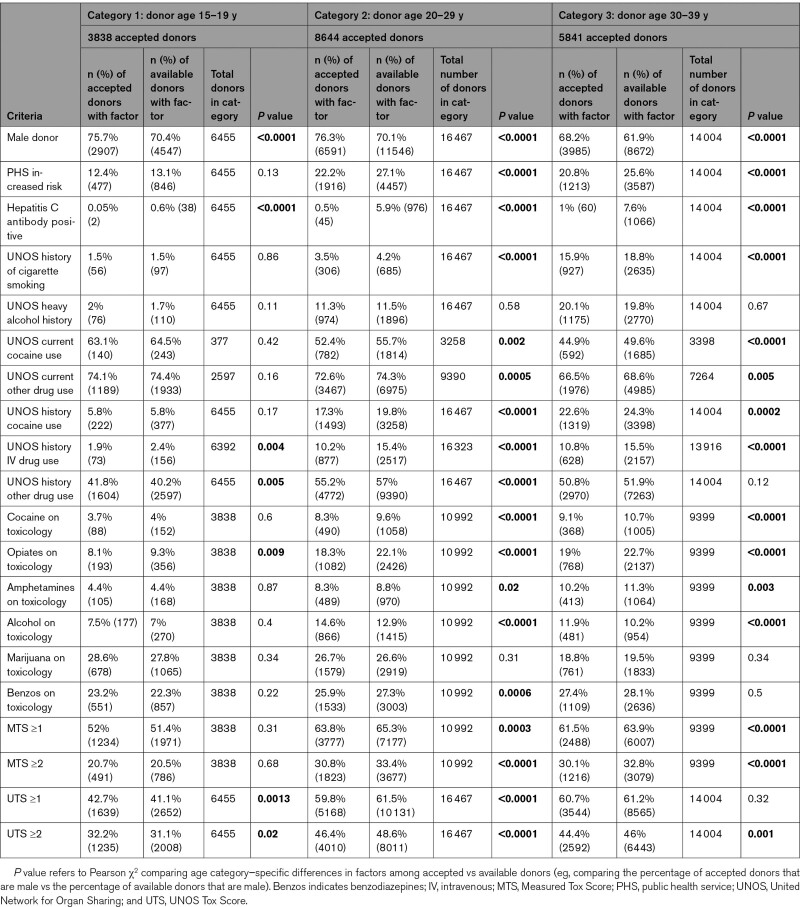
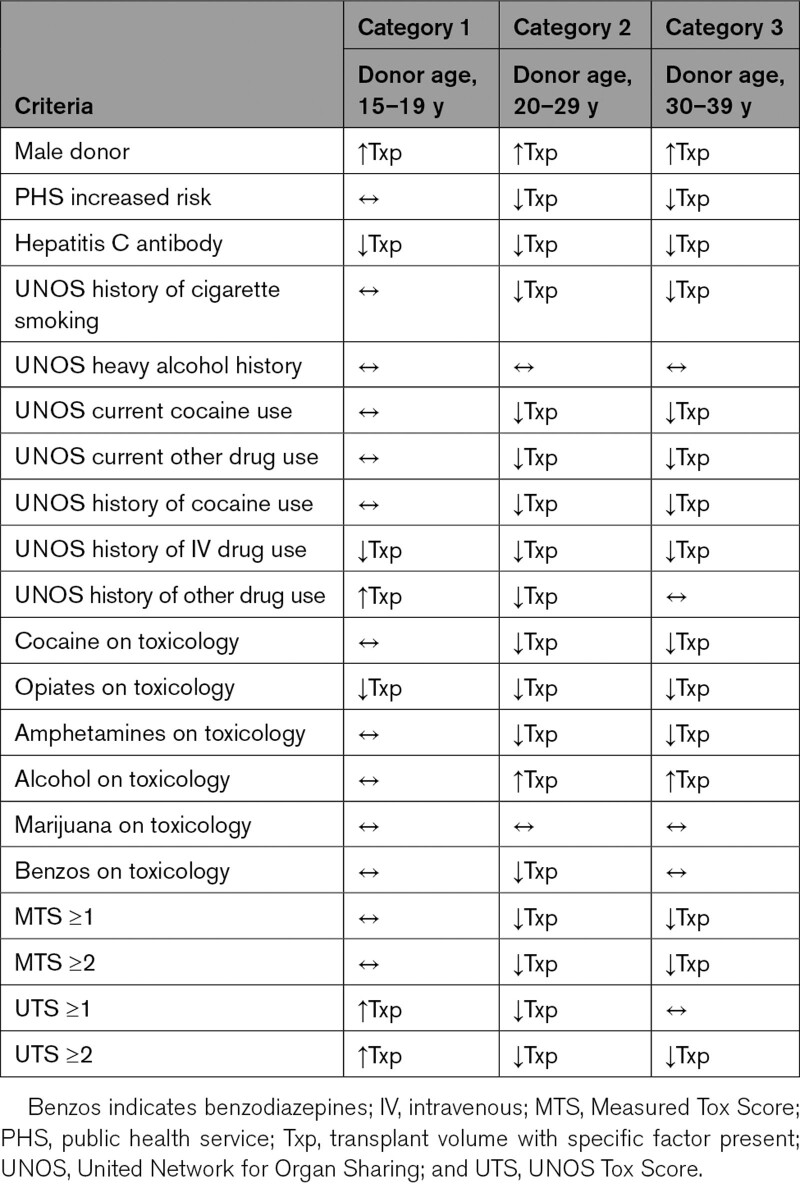
Two-by-2 tables comparing each risk factor and utilization for transplant were prepared, with 1 table for each donor age category, utilizing the Pearson χ2 to determine whether the utilization of available donors for transplant was different based on various factors. Table 1 shows the various factors including PHS-IR, male sex, as well as various drug-related factors. Table 2 summarizes the information in a graphical format with arrows depicting higher or lower frequency of donor utilization for each donor factor.
Table 1.
Utilization of Offered Donors According to Donor Age Category and Donor Demographics (Unadjusted Comparisons)
Table 2.
Summary of Effect of Donor Characteristics on Utilization Across 3 Categories of Donor Age (Unadjusted Comparisons)
Donor Age
The mean age of donors who were accepted versus rejected for transplant was not different for category 1 donors but was different for all the other categories. For category 1, the mean donor ages were similar (mean age: rejected donors, 17.37±1.3 years versus accepted donors, 17.4±1.3 years; P=0.24). In category 2, the mean age of rejected donors was 24.5±2.8 years versus 24.1±2.9 years of accepted donors (P<0.0001). In category 3, the mean age of rejected donors was 34.7±2.9 years versus 34.3±2.9 years of accepted donors (P<0.0001).
Male Donor Sex
Male donors were utilized more frequently across all age groups. For categories 1, 2, and 3, 70.4%, 70.1%, and 61.9% of offered donors were men, but 75.7%, 76.3%, and 68.2% of transplant recipients received male donors (all comparisons, P<0.0001).
PHS Increased Risk
There was no difference in utilization of donors of age 15 to 19 (category 1) years with PHS-IR (13.1% of donors were deemed PHS-IR and 12.4% of transplants utilized PHS-IR donors, P=0.13, χ2). However, donors in categories 2 and 3 had a significantly lower use of donors with the PHS-IR designation (category 2: 27.1% versus 22.2%, P<0.0001; category 3: 25.6% versus 20.8%, P<0.0001). The specific risk factor(s) that led to PHS-IR designation were not captured in the UNOS registry.
Hepatitis C Antibody
Hepatitis C antibody positivity was associated with significantly lower utilization across all age categories. For categories 1, 2, and 3, 0.6%, 5.9%, and 7.6% of offered donors were hepatitis C antibody positive, but such donors were only utilized for 0.05%, 0.5%, and 1% of transplant recipients (all comparisons P<0.0001).
Cigarette and Alcohol Use
The most robust UNOS data exist for the history of cigarettes variable. A history of tobacco use did not affect organ utilization with category 1 donors (1.5% of donors and recipients had such a history, P=0.86), but with categories 2 and 3, there were significant differences. Of the donors, 4.2% and 18.8% had a history of tobacco use, but 3.5% and 15.9% of transplants utilized such donors (P<0.0001 for both comparisons). History of heavy alcohol use recorded by UNOS (defined as ≥2 standard drinks a day) was not associated with differences across the age categories. For categories 1, 2, and 3, 1.7%, 11.5%, and 19.8% of donors had this history, and 2%, 11.3%, and 20.1% of transplants utilized donors with a heavy alcohol history (all P=NS).
UNOS Drug History Variables
Current Cocaine Use
For donors in category 1 (only 377/6455 donors had a response for this variable), 64.5% were positive, and 63.1% of transplants involved positive donors (P=0.42). However, in categories 2 and 3, a history of cocaine use was noted in 55.7% and 49.6% of donors versus 52.4% and 44.9% of transplant recipients (P=0.002 and P<0.0001, respectively).
Other Current Drug Use
A similar pattern was seen for category 1 donors, with 74.4% of donors having positive history and 74.1% of transplant utilization in these donors (P=0.16). However, for categories 2 and 3, 74.3% and 68.6% of donors had positive histories but positive history present for 72.6% and 66.5% of transplant recipients (P=0.0005 and P=0.005, respectively).
Past Use of Cocaine
Donor utilization was not different for category 1 donors, with 5.8% of both donors and recipients with a history of cocaine use (P=0.17). However, in categories 2 and 3, a history of prior cocaine use was noted in 19.8% and 24.3% of donors versus 17.3% and 22.6% of transplant recipients (P<0.0001 and P=0.0002, respectively).
Past Use of Intravenous Illicit Drugs
Utilization of such positive donors was lower in all categories. For categories 1 to 3, drug usage history was noted in 2.4%, 15.4%, and 15.5% of donors but 1.9%, 10.2%, and 10.8% of transplant recipients (P=0.004, P<0.0001, and P<0.0001, respectively).
Past Use of Other Drugs
In category 1 donors, such history was associated with marginally increased utilization (40.2% of donors and 41.8% of recipients, P=0.005). In category 2, 57% of donors had this history with 55.2% of such donors utilized (P<0.0001). For category 3, 51.9% of donors had such history, and 50.8% were utilized for transplantation (P=0.12).
Donor Toxicology Data
Cocaine
Utilization was not different for donors in the first category, with 4% of donors testing positive and 3.7% utilization for transplantation (P=0.60). However, in categories 2 and 3, positive cocaine toxicology was noted for 9.6% and 10.7% of donors but 8.3% and 9.1% of positive donors used for transplantation (P<0.0001 for both).
Opiates
Utilization of such positive donors was lower in all categories. For categories 1 to 3, opiate-positive toxicology occurred in 9.3%, 22.1%, and 22.7% of donors and 8.1%, 18.3%, and 19% of donors utilized for transplant (P=0.009, P<0.0001, and P<0.0001, respectively).
Amphetamines
Utilization was not different for donors in category 1, with 4.4% of category 1 donors and recipients with positive toxicology (P=0.87). However, in categories 2 and 3, 8.8% and 11.3% of donors tested positive as compared with 8.3% and 10.2% of hearts utilized for transplant (P=0.02 and 0.003, respectively).
Alcohol
Utilization was not different for donors in category 1, with 7% of donors with positive toxicology and 7.5% of utilized organs with similar positive results (P=0.40). However, in categories 2 and 3, 12.9% and 10.2% of donors were positive for alcohol as compared with 14.6% and 11.9% of donors utilized for transplant (P<0.0001 for both).
Marijuana
Utilization was similar regardless of marijuana toxicology across the age categories. Toxicology was positive in 27.8%, 26.6%, and 19.5% of heart donors, and utilization was 28.6%, 26.7%, and 18.8% in transplant recipients (all P>0.3).
Benzodiazepines
Utilization was similar for categories 1 and 3 with donors positive in 22.3% and 28.1% of donors and 23.2% and 27.4% of transplants, respectively (P=0.22 and P=0.5, respectively). For category 2, 27.3% of donors were positive compared with 25.9% of transplant recipients (P=0.0006).
Measured Toxicology Score
First, MTS was dichotomized into 0 versus ≥1: utilization was not different for donors in category 1, with 51.4% of donors with such a score and 52% of transplants (P=0.31). However, in categories 2 and 3, 65.3% and 63.9% of donors had an MTS ≥1 as compared with 63.8% and 61.5% of transplant recipients (P=0.0003 and P<0.0001, respectively). Next, MTS was dichotomized into 0 versus ≥2: utilization was not different for donors in category 1, with 20.5% of donors with a score of ≥2 and 20.7% of recipients (P=0.68). However, in categories 2 and 3, 33.4% and 32.8% of donors had MTS ≥2 as compared with 30.8% and 30.1% of transplants (P<0.0001 for both).
UNOS Toxicology Score
First, UTS was dichotomized into 0 versus ≥1: utilization was marginally increased for donors in category 1, with 41.1 % of donors with such a score and 42.7 % of transplants (P=0.001). For category 2, 61.5 % of donors had a UTS ≥1 as compared with 59.8% of transplant recipients (P<0.0001). For category 3, the UTS was ≥1 in 61.2% and 60.7% of donors and recipients, respectively (P=0.32). Next, UTS was dichotomized into 0 versus ≥2: utilization was marginally higher for donors in category 1, with 31.1% and 32.2% of recipients, respectively (P=0.02). However, in categories 2 and 3, 48.6% and 46% of donors had a UTS ≥2 as compared with 46.4% and 44.4% of transplants (P<0.0001 and P=0.001, respectively).
Discussion
Improved care for heart failure patients has led to increasing numbers of patients who eventually reach end stage disease, and the need for cardiac replacement therapies is growing. The demand for heart transplants exceeds the supply of donors chronically, and yet prior reports indicate that only a quarter of donors are utilized for transplantation, at least in the United States. The current work builds on the foundation of others7–9 and examines donor factors that are associated with use or nonuse of donors.
There are several key observations:
The number of offered heart donors is rising, particularly beginning in 2014 through the end of the study in 2017 (30% increase between 2007 and 2017), chiefly due to increasing numbers of donors aged ≤39 years.
The overall percentage of donors accepted for transplant has risen marginally from 26.4% in 2007 to 30.1% in 2017. However, the utilization rate is almost double among younger donors.
The percentage of donors classified as PHS-IR has markedly increased from 8% in 2007 to 27.4% in 2017. PHS-IR was associated with lower donor utilization except in the 15- to 19-year-old donor category.
During the study period (2010–2017), hepatitis C antibody–positive donors were rarely utilized for transplantation regardless of donor age.
More than half of donors studied have either a history of drug use (recorded by the UNOS database) or actual toxicology positive for drugs on the terminal hospital stay.
The discretionary factors most associated with usage of donors have not changed, including male sex and donor age.
Among donors aged 15 to 19 years, organ utilization is high, but history of intravenous drug use and toxicology positive for opiates is associated with less utilization for heart transplantation.
The presence of even 1 drug (other than alcohol or marijuana) on toxicology was associated with significantly less utilization of such donors except in the 15- to 19-year-old donor group.
The number of offered donors began to rise significantly in 2014, potentially due to campaigns to increase organ donation awareness, self-identification of donor status on driver’s licenses, or an increasing number of patients dying in association with illicit drug use, particularly opioids.10 The growth in organ donation between 2013 and 2017 was exponential in nature. However, in the current study, the percentage of donors with opioids detected on toxicology increased from 14.2% in 2007 to 18.6% in 2017, which does not fully explain the increase in donors.
The widespread increase in opiate overdoses has led to major changes in the use of these medications in the medical setting, though synthetic analogs such as fentanyl are increasingly noted in overdose cases.3 By leading to respiratory depression, opiates lead to anoxic deaths but do not have a cardiac toxicity. The current analysis shows that donors with opiate-positive toxicology are less likely to be utilized for transplantation, regardless of age category. Previous work has demonstrated that the use of donors with history of drug use or toxicology positive for ≥1 drugs is not associated with long-term differences in mortality.11,12
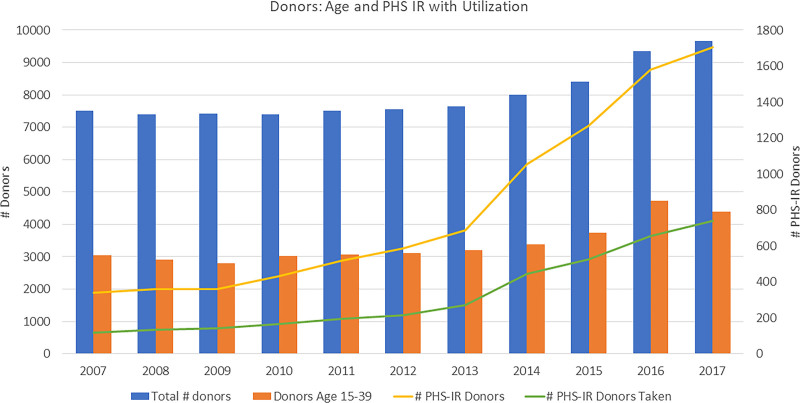
Another factor that was noted in this study is the impact of a donor being labeled as PHS-IR (formerly known as Centers for Disease Control high risk).13 Despite improvements in donor testing, moving from serological assays to nucleic acid transcriptase testing (greatly improving accuracy), the current study shows that the use of these donors is reduced in comparison to standard risk donors except in the youngest group (15–19-year-old donors). Figure 3 illustrates the issue. The total number of donors has increased especially in the last 3 years of the study cohort. However less than half are between the ages of 15 to 39 years where the utilization for heart transplantation is the highest. Focusing only on the 15- to 39-year-old donors, during the same period of time, the incidence of donor PHS-IR status has risen 5-fold (338 PHS-IR donors in 2007 to 1706 in 2017). The utilization of young PHS-IR donors remains significantly below half (34.6% use in 2007 to 43.4% in 2017; P<0.0001). The risk of disease transmission is quite low,14,15 but labeling a donor as PHS-IR may suggest otherwise,16 and the current study demonstrates the negative effect on donor utilization outside of the youngest donor cohort (age, ≤19 years).
Figure 3.
The bar graphs use the primary y axis to the left, and the line plots use the secondary y axis (to the right). The number of donors has increased especially in the last 3 years of the study cohort. However, less than half are between the ages of 15 and 39 years where the utilization for heart transplantation is the highest. Focusing on the 15- to 39-year-old donors only, over the same period of time, the incidence of Public Health Service Increased Risk (PHS-IR) status has risen 5-fold. The utilization of young PHS-IR donors remains significantly below half.
The use of hepatitis C–infected hearts has rapidly expanded in recent years with the availability of direct acting antiretroviral drugs with high sustained virological response (cure) rates.17–19 Usage of these hearts is still not universal with remaining concerns regarding rejection,20,21 allograft vasculopathy,22 cost of the antiviral agents,23 and limited center experience preventing widespread adoption.24,25 Additional experience over time will provide reassurance of the safety of these organs, despite the additional complexity of intentional recipient infection with hepatitis C.
Often, risk factors coexist, and drug use is another common factor leading to brain death and donor availability. The current analysis demonstrates that drug use (either historical or toxicological evidence of drug use) clearly impacts the decision to utilize donors particularly beyond age 19. Certain drugs such as alcohol or marijuana were not associated with differences in usage rates, but most are associated with fewer of these donors (even aged ≤39 years) being utilized for transplants. Previous work has conclusively shown that donor drug history does not impact the posttransplant survival, though that is among donors who are selected for transplant (which leads to significant bias).11,12
To better match the demand for donor hearts with the burgeoning list of patients waiting for transplantation will require fundamental changes in donor acceptance practices. It is notable that male donors are strongly preferred.26,27 Prior reports of inferior survival with female donors may have more to do with a mismatch of heart mass, particularly in transplant recipients with significant pulmonary arterial hypertension.28 In addition, the average accepted donor age is far lower than the average recipient age. It is clear that younger donors are associated with increased long-term survival, but the current allocation schema does not restrict the use of young donors for older recipients, nor the converse.
Donor selection is a fundamental challenge for heart transplant programs, and evidence in the current study suggests that practices have not changed to a significant degree. Some of this may be because of regulatory surveillance with programs held to a high standard of 1-year survival, which functions as a double-edged sword. On one hand, survival rates are improved with strict observation, but this environment may lead to unintended consequences29 as smaller programs may choose to avoid higher risk donors or recipients.9 Prior evidence suggests that factors associated with donor discard do not correlate with worse survival long term.8,30,31 In Europe where the donor shortage is more acute, the use of donors with nonobstructive coronary disease has been reported and outcomes seem to be similar long term.32
In October 2018, UNOS implemented a new prioritization system that emphasizes patients who qualify for temporary mechanical circulatory support over patients with stable durable left ventricular assist devices or inotrope dependence. As well, the artificial use of donor service areas and local organ allocation was dissolved in preference for distance-based concentric circles around the donor hospital to eliminate disparities based on organ procurement organization efficiency or geographic constructs. While this has led to shorter time to transplantation for those acutely ill patients, it has led to fewer patients in the less urgent priority categories receiving a transplant.33,34 A potential solution is to incentivize centers to expand donor selection criteria while reducing the risk of regulatory consequences for resultant outcomes, which may be marginally lower but would result in increased transplantation and ultimately longer survival in aggregate for patients waiting for transplantation.
Conclusions
Despite >50 years of heart transplantation, donor selection remains empirical with wide disparities across programs often based on experience and limited data sets. The current study finds that donor age and sex remain the most important predictors of utilization of heart donors, followed by blood type and size. In addition, drug use (whether history or toxicological confirmation) exerts a substantial negative effect on utilization of such donors, even when older donor populations are excluded. Designation of donors as PHS-IR is a strong predictor of nonuse and the presence of hepatitis C infection. To move forward, we must make progress on policies that facilitate expansion of the current donor pool but also utilization of offered organs, especially for candidates who may not otherwise have an opportunity to receive timely transplantation.
Article Information
Sources of Funding
None.
Disclosures
Dr Baran has consulted for Livanova, Getinge, Abiomed, and Abbott. He is on the Steering Committee for Procyrion and CareDx. He is a speaker for Pfizer. Dr J.G. Copeland has consulted for Syncardia. Dr H. Copeland has received consulting fees from Bridge to Life. The other authors report no conflicts.
Supplemental Material
Table S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- IQR
- interquartile range
- MTS
- Measured Toxicology Score
- PHS-IR
- Public Health Service Increased Risk
- UNOS
- United Network for Organ Sharing
- UTS
- UNOS Toxicology Score
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.122.009547.
For Sources of Funding and Disclosures, see page 695.
Contributor Information
Ashleigh Long, Email: ashleighlong2015@gmail.com.
Justin Lansinger, Email: justinlansinger@gmail.com.
Jack G. Copeland, Email: hannahcopeland411@gmail.com.
Hannah Copeland, Email: hannahcopeland411@gmail.com.
References
- 1.Akintoye E, Alvarez P, Shin D, Egbe A, Panos A, Sellke F, Briasoulis A. Changing demographics, temporal trends in waitlist, and posttransplant outcomes after heart transplantation in the United States: analysis of the UNOS database 1991-2019. Circ Heart Fail. 2021;14:e008764. doi: 10.1161/CIRCHEARTFAILURE.121.008764 [DOI] [PubMed] [Google Scholar]
- 2.Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Jr, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. doi: 10.1016/j.healun.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, Simopoulos TT. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther. 2018;7:13–21. doi: 10.1007/s40122-018-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995-2010. Am J Transplant. 2015;15:642–649. doi: 10.1111/ajt.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khush KK, Menza R, Nguyen J, Zaroff JG, Goldstein BA. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail. 2013;6:300–309. doi: 10.1161/CIRCHEARTFAILURE.112.000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khush KK, Ball RL. Great variability in donor heart acceptance practices across the United States. Am J Transplant. 2020;20:1582–1596. doi: 10.1111/ajt.15760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmavaram N, Hess T, Jaeger H, Smith J, Hermsen J, Murray D, Dhingra R. National trends in heart donor usage rates: are we efficiently transplanting more hearts? J Am Heart Assoc. 2021;10:e019655. doi: 10.1161/JAHA.120.019655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess NR, Seese LM, Sultan I, Wang Y, Thoma F, Kilic A. Impact of center donor acceptance patterns on utilization of extended-criteria donors and outcomes. J Card Surg. 2021;36:4015–4023. doi: 10.1111/jocs.15902 [DOI] [PubMed] [Google Scholar]
- 9.Tran ZK, Nelson DB, Martens TP, Abramov D, Shih W, Chung JS, Razzouk AJ, Rabkin DG. Impact of transplant center volume on donor heart offer utilization rates in the United States. J Card Surg. 2021;36:4527–4532. doi: 10.1111/jocs.16014 [DOI] [PubMed] [Google Scholar]
- 10.Phillips KG, Ranganath NK, Malas J, Lonze BE, Gidea CG, Smith DE, Kon ZN, Reyentovich A, Moazami N. Impact of the opioid epidemic on heart transplantation: donor characteristics and organ discard. Ann Thorac Surg. 2019;108:1133–1139. doi: 10.1016/j.athoracsur.2019.03.076 [DOI] [PubMed] [Google Scholar]
- 11.Vieira JL, Cherikh WS, Lindblad K, Stehlik J, Mehra MR. Cocaine use in organ donors and long-term outcome after heart transplantation: an International Society for Heart and Lung Transplantation Registry analysis. J Heart Lung Transplant. 2020;39:1341–1350. doi: 10.1016/j.healun.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 12.Baran DA, Lansinger J, Long A, Herre JM, Yehya A, Sawey EJ, Badiye AP, Old W, Copeland J, Stelling K, et al. Intoxicated donors and heart transplant outcomes: long-term safety. Circ Heart Fail. 2021;14:e007433. doi: 10.1161/CIRCHEARTFAILURE.120.007433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucirka LM, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Formal policies and special informed consent are associated with higher provider utilization of CDC high-risk donor organs. Am J Transplant. 2009;9:629–635. doi: 10.1111/j.1600-6143.2008.02523.x [DOI] [PubMed] [Google Scholar]
- 14.Gaffey AC, Cucchiara AJ, Goldberg LR, Blumberg EA, Acker MA, Atluri P. Transplantation of center for disease control “high-risk” honor hearts does not adversely impact long-term outcomes in adults. J Card Fail. 2016;22:376–382. doi: 10.1016/j.cardfail.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 15.Gaffey AC, Doll SL, Thomasson AM, Venkataraman C, Chen CW, Goldberg LR, Blumberg EA, Acker MA, Stone F, Atluri P, et al. Transplantation of “high-risk” donor hearts: implications for infection. J Thorac Cardiovasc Surg. 2016;152:213–220. doi: 10.1016/j.jtcvs.2015.12.062 [DOI] [PubMed] [Google Scholar]
- 16.Sapiano MRP, Jones JM, Bowman J, Levi ME, Basavaraju SV. Impact of US public health service increased risk deceased donor designation on organ utilization. Am J Transplant. 2019;19:2560–2569. doi: 10.1111/ajt.15388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, Kusztos AE, Johnson ME, Chen K. et al. ; DONATE HCV Trial Team. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606–1617. doi: 10.1056/NEJMoa1812406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilic A, Hickey G, Mathier M, Sultan I, Gleason TG, Horn E, Keebler ME. Outcomes of adult heart transplantation using hepatitis C-positive donors. J Am Heart Assoc. 2020;9:e014495. doi: 10.1161/JAHA.119.014495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi HK, Schlendorf KH. Hepatitis c positive organ donation in heart transplantation. Cur Transplant Rep. 2021;8:359–367. doi: 10.1007/s40472-021-00350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidea CG, Narula N, Reyentovich A, Fargnoli A, Smith D, Pavone J, Lewis T, Karpe H, Stachel M, Rao S, et al. Increased early acute cellular rejection events in hepatitis c-positive heart transplantation. J Heart Lung Transplant. 2020;39:1199–1207. doi: 10.1016/j.healun.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Woolley AE, Singh SK. The curious phenomenon of early cardiac allograft rejection with hepatitis C–infected donor heart transplants. J Heart Lung Transplant. 2020;39:1208–1209. doi: 10.1016/j.healun.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 22.Madan S, Patel SR, Jorde UP. Cardiac allograft vasculopathy and secondary outcomes of hepatitis c-positive donor hearts at 1 year after transplantation. J Heart Lung Transplant. 2020;39:1318–1321. doi: 10.1016/j.healun.2020.06.021 [DOI] [PubMed] [Google Scholar]
- 23.Logan C, Yumul I, Cepeda J, Pretorius V, Adler E, Aslam S, Martin NK. Cost-effectiveness of using hepatitis c viremic hearts for transplantation into HCV-negative recipients. Am J Transplant. 2021;21:657–668. doi: 10.1111/ajt.16245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moayedi Y, Gulamhusein AF, Ross HJ, Teuteberg JJ, Khush KK. Accepting hepatitis c virus-infected donor hearts for transplantation: multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant. 2018;32:e13308. doi: 10.1111/ctr.13308 [DOI] [PubMed] [Google Scholar]
- 25.Moayedi Y, Gulamhusein AF, Khush KK. Treading lightly as we step into a new era: use of hepatitis c virus-infected organs for transplantation. J Thorac Cardiovasc Surg. 2020;159:505–510. doi: 10.1016/j.jtcvs.2019.05.091 [DOI] [PubMed] [Google Scholar]
- 26.Foster BJ, Zhang X, De Simone A, Dahhou M, Sapir-Pichhadze R, Cardinal H, West L. Differences in heart graft survival by recipient sex. Transplant Direct. 2021;7:e749. doi: 10.1097/TXD.0000000000001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Shudo Y, Lingala B, Joseph Woo Y. The impact of donor sex on heart transplantation outcomes-a study of over 60,000 patients in the United States. J Heart Lung Transplant. 2021;40:814–821. doi: 10.1016/j.healun.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 28.Kawabori M, Critsinelis AC, Hironaka CE, Chen FY, Zhan Y, Thayer KL, Couper GS. Right ventricular undersizing is associated with increased 1-year mortality. J Thorac Cardiovasc Surg. 2021;161:1048–1059.e3. doi: 10.1016/j.jtcvs.2020.11.156 [DOI] [PubMed] [Google Scholar]
- 29.Jay C, Schold JD. Measuring transplant center performance: the goals are not controversial but the methods and consequences can be. Curr Transplant Rep. 2017;4:52–58. doi: 10.1007/s40472-017-0138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aliabadi-Zuckermann AZ, Gokler J, Kaider A, Riebandt J, Moayedifar R, Osorio E, Haberl T, Angleitner P, Laufer G, Forsythe J, et al. Donor heart selection and outcomes: an analysis of over 2,000 cases. J Heart Lung Transplant. 2018;37:976–984. doi: 10.1016/j.healun.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 31.Reul RM, Jr, Saleem AA, Keller CN, Malik TH, Rosengart TK, Goss JA, Rana AA. Allograft discard risk index for heart transplantation. Clin Transplant. 2021;35:e14442. doi: 10.1111/ctr.14442 [DOI] [PubMed] [Google Scholar]
- 32.Lechiancole A, Vendramin I, Sponga S, Sappa R, Zanuttini D, Spedicato L, Ferrara V, Di Nora C, Livi U. Influence of donor-transmitted coronary artery disease on long-term outcomes after heart transplantation - a retrospective study. Transpl Int. 2021;34:281–289. doi: 10.1111/tri.13793 [DOI] [PubMed] [Google Scholar]
- 33.Jani M, Lee S, Acharya D, Hoeksema S, Boeve T, Leacche M, Manandhar-Shrestha NK, Jovinge SV, Loyaga-Rendon RY. Decreased frequency of transplantation and lower post-transplant survival free of re-transplantation in LVAD patients with the new heart transplant allocation system. Clin Transplant. 2022;36:e14493. doi: 10.1111/ctr.14493 [DOI] [PubMed] [Google Scholar]
- 34.Patel JN, Chung JS, Seliem A, Sakr A, Stoletniy L, Rabkin DG, Abramov D. Impact of heart transplant allocation change on competing waitlist outcomes among listing strategies. Clin Transplant. 2021;35:e14345. doi: 10.1111/ctr.14345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.