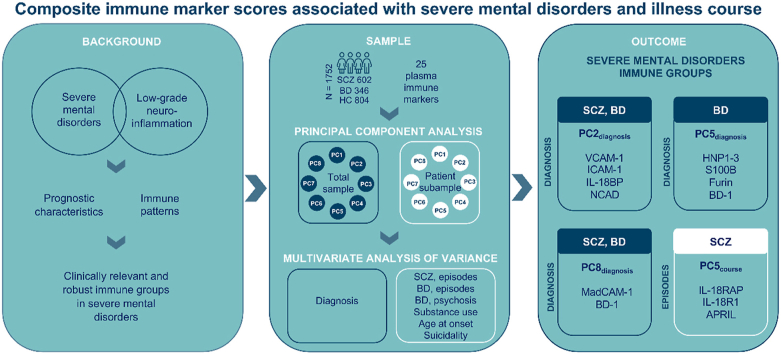
Abstract
Background
Low-grade inflammation has been implicated in the pathophysiology of severe mental disorders (SMDs) and a link between immune activation and clinical characteristics is suggested. However, few studies have investigated how patterns across immune markers are related to diagnosis and illness course.
Methods
A total of 948 participants with a diagnosis of schizophrenia (SCZ, N = 602) or bipolar (BD, N = 346) spectrum disorder, and 814 healthy controls (HC) were included. Twenty-five immune markers comprising cell adhesion molecules (CAMs), interleukin (IL)-18-system factors, defensins, chemokines and other markers, related to neuroinflammation, blood-brain barrier (BBB) function, inflammasome activation and immune cell orchestration were analyzed. Eight immune principal component (PC) scores were constructed by PC Analysis (PCA) and applied in general linear models with diagnosis and illness course characteristics.
Results
Three PC scores were significantly associated with a SCZ and/or BD diagnosis (HC reference), with largest, however small, effect sizes of scores based on CAMs, BBB markers and defensins (p < 0.001, partial η2 = 0.02–0.03). Number of psychotic episodes per year in SCZ was associated with a PC score based on IL-18 system markers and the potential neuroprotective cytokine A proliferation-inducing ligand (p = 0.006, partial η2 = 0.071).
Conclusion
Analyses of composite immune markers scores identified specific patterns suggesting CAMs-mediated BBB dysregulation pathways associated with SMDs and interrelated pro-inflammatory and neuronal integrity processes associated with severity of illness course. This suggests a complex pattern of immune pathways involved in SMDs and SCZ illness course.
Keywords: Principal component analysis, Composite scores, Severe mental disorders, Schizophrenia, Bipolar disorder, Immune marker, Neuroinflammation
Abbreviations: PCA, Principal Component Analysis; SMD, Severe Mental Disorders; SCZ, Schizophrenia; BD, Bipolar Disorder
Graphical abstract
Highlights
-
•
Composite score of VCAM-1, ICAM-1, NCAD and IL-18BP associated with SCZ and BD.
-
•
Composite score of MadCAM-1 and BD-1 associated with SCZ and BD.
-
•
Composite score of S100B, furin, HNP1-3 and BD-1 associated with BD.
-
•
Composite score of APRIL and IL-18R markers associated with psychotic episode rate.
1. Introduction
Schizophrenia and bipolar disorder are severe mental disorders (SMDs) with shared clinical characteristics and genetic underpinnings (McCutcheon et al., 2020; Purcell et al., 2009). SMDs are associated with considerable disability and suffering; however, disease course varies between individuals (Charlson et al., 2018; Ferrari et al., 2016). Up to one third of patients with schizophrenia experience a chronic course with limited effect of therapy and a similar proportion has a benign course (Marder and Cannon, 2019). Likewise, the course of bipolar disorder varies significantly between afflicted individuals (Lagerberg et al., 2021; Turvey et al., 1999). The heterogeneity probably reflects different underlying disease mechanisms.
There are several lines of evidence of immune system involvement in the pathophysiology of SMDs (Miller and Goldsmith, 2017; Munkholm et al., 2013). Large registry studies and meta-analyses have recently shown a strong relationship of SMDs with infections and autoimmune disorders (Benros et al., 2011; Bergink et al., 2014; Cullen et al., 2019; Köhler-Forsberg et al., 2019; Najjar et al., 2018). Genome-wide association studies (GWAS) link the immune-related major histocompatibility complex (MHC) locus with both schizophrenia (Ripke et al., 2014) and bipolar disorder (Mullins et al., 2021). Also, immune genetic associations are found outside the MHC region and there is genetic overlap with immune mediated disorders (Pouget, 2018). Imaging techniques have identified neuroinflammation (Pasternak et al., 2016), in line with findings of elevated microglia cell activity in studies of postmortem brain tissue (Trepanier et al., 2016) and immune marker aberrations in cerebrospinal fluid (Bechter et al., 2010). Nevertheless, knowledge about low-grade inflammation in SMDs is mainly based on reports of altered circulating immune marker levels (Benedetti et al., 2020; Frydecka et al., 2018; Goldsmith et al., 2016; Khoury and Nasrallah, 2018). Such immune markers, constituting an extensive number of cytokines and adhesion molecules (Kroken et al., 2018), are involved in complex interacting immune regulatory mechanisms, potentially related to the increased cardiovascular disease risk in SMDs (De Hert et al., 2018; Reponen et al., 2020). Determinants of immune marker levels are mainly unknown, although a major impact of non-heritable factors is indicated (Brodin et al., 2015), in particular acquired metabolic disturbances (Huet et al., 2021; Makki et al., 2013), and interactions with endocrine systems (Taub, 2008). However, as studies of systemic immune abnormalities in SMDs are based on individual markers (Dickerson et al., 2016; Kroken et al., 2018), common underlying mechanisms have been challenging to uncover, and findings are prone to spurious variation.
A recent systematic review of meta-analyses suggested diagnosis-related as well as state specific immune marker abberration in SMDs (Yuan et al., 2019). However, studies of clinical state showed significant inconsistencies. In general, studies of symptom severity and immune markers are susceptible to interfering effects of stress (Lataster et al., 2013; Segerstrom and Miller, 2004; Tourjman et al., 2013). Only a few studies report associations between immune markers and illness course characteristics (De Picker et al., 2017; Lizano et al., 2020), such as suicidality (Black and Miller, 2015; Isung et al., 2020). To improve clinical relevance and interpretability of SMDs - immune associations, we propose to analyze distinct groups in terms of illness course characteristics not dependent on current clinical state. Severity of illness course is often described quantitatively by number of illness episodes (Coulon et al., 2020; Immonen et al., 2017; Kennedy et al., 2015) together with qualitative markers of comorbid substance use disorder (Kendler et al., 2019; Messer et al., 2017), history of suicidality (Yates et al., 2019), and in bipolar disorders, presence of psychotic episodes (Burton et al., 2018; Keck et al., 2003). A more severe illness course also seems to be related to an earlier age at onset (Cirone et al., 2021; Coulon et al., 2020; Hanssen et al., 2015). To address the complex patterns of the immune system, the current study uses Principal Component Analysis (PCA) of immune markers. By obtaining composite immune scores in an exploratory approach, more of the interplay of the immune pathways underlying the phenotypes relative to single immune marker analyses, might be indicated (Miller and Goldsmith, 2017).
We aim to identify immune marker components associated with diagnosis and illness course characteristics. To be able to indicate the potential small effects of immune components in the complex mechanisms underlying these phenotypes, we apply a large sample of SMDs and healthy controls (HC). Associations of immune markers with complex illness course characteristics are anticipated to be small in these highly complex and multifaceted mechanisms, however the potential immune components might prove meaningful in the long term. On the basis of the inflammatory model of SMDs, a set of generally stable and abundantly expressed immune markers encompassing novel markers and markers with established link to SMDs, representing pathways of potential pathophysiological relevance, including neuroinflammation, blood-brain barrier (BBB) function, inflammasome activation and immune cell orchestration, were chosen. Commonly used illness course characteristics were analyzed, including number of illness episodes (Häfner, 2019; Luciano et al., 2021; Peters et al., 2016; Provenzani et al., 2021), suicide attempts (Black and Miller, 2015; Yates et al., 2019), comorbid substance use disorder (Kendler et al., 2019; Messer et al., 2017) and for bipolar disorder presence of psychotic features (Burton et al., 2018; Keck et al., 2003).
2. Methods
2.1. Study setting
Participants were included through the Thematically Organized Psychosis (TOP)-study at the Norwegian Center for Mental Disorder Research (NORMENT). Recruitment of patients is conducted from the major hospitals in Oslo, currently covering a catchment area of approximately 700,000 inhabitants. Patient inclusion criteria are age range from 18 to 65 years and meeting the Diagnostic Manual of Mental Disorders (DSM)-IV (First, 2013) criteria for schizophrenia spectrum or bipolar spectrum disorders. Exclusion criteria are IQ < 70, severe somatic illness, neurological disorder, or a history of moderate or severe head trauma. HC were randomly selected from the same catchment area and age range, using statistical records. HC were excluded based on the same criteria in addition to symptoms of SMDs, current substance abuse, and history of SMDs in close relatives. Furthermore, only individuals with sufficient Scandinavian language skills to complete the assessments were included. All participants gave informed written consent. For the current study, participants with CRP above 10.0 mg/L were excluded to prevent acute infections influencing the immune markers (Povoa, 2002).
2.2. Sample
Participants of the TOP sample with immune assessments (N = 1762) consisted of N = 602 patients with schizophrenia spectrum disorders (SCZ), here including schizophrenia (N = 345), schizophreniform disorder (N = 36), schizoaffective disorder (N = 86), delusional disorder (N = 40), brief psychotic disorder (N = 8), and psychotic disorder not otherwise specified (NOS, N = 87), N = 346 patients with bipolar spectrum disorders (BD), here including bipolar I disorder (N = 216), bipolar II disorder (N = 112) and bipolar disorder NOS (N = 18), and N = 814 HC. Immune data overlapping with the current study have been published elsewhere (Andreou et al., 2021; Engh et al., 2021; Sheikh et al., 2022; Szabo et al., 2022).
2.3. Clinical assessments
Trained psychologists and physicians conducted interviews of the patient participants. Sociodemographic and medical history were collected, and diagnostic interviews were performed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-1) (Spitzer et al., 1992), including substance use disorders and number of psychotic and affective episodes. The clinical interviewers participated in regular diagnostic meetings and were supervised by senior researchers. Inter-rater reliability of the diagnostics was good, with an overall kappa score of 0.77 (95% CI: 0.60–0.94) (Ringen et al., 2008). Current symptom levels were assessed with Global Assessment of Functioning (GAF) – symptoms scale (Pedersen and Karterud, 2012) and The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Routine blood tests and a physical examination including height and weight for body mass index (BMI) was performed in all participants and within two weeks of symptom assessments for patients. Information about psychopharmacological treatment was obtained from interviews and medical records, and categorized into use of antipsychotics (yes/no), antidepressants (yes/no), and anticonvulsants and/or lithium (yes/no). Details of anti-inflammatory drug use in patients were similarly recorded and is given in Supplementary Table 1.
2.4. Inflammatory markers
Blood samples were drawn from the antecubital vein on EDTA vials and plasma stored at −80 °C for later immunological analyses. The patient subsample had blood withdrawn earlier in the day (average at 10 a.m.) than HC (average at 3 p.m.). The samples were stored on average 5–8 years (range 3–10), with shorter duration in HC. Freezer storage time was controlled for in the analyses. Twenty-five inflammatory markers were analyzed at the Research Institute of Internal Medicine, Oslo University Hospital. Applying enzyme immunoassays (EIA), samples were analyzed in duplicate by use of antibodies available from R&D systems (Minneapolis, MN, USA) in a 384 format, using a combination of a pipetting robot from Selma and a dispenser/washer from Biotek. We used an ELISA plate reader (BIO-RAD, Hercules, CA, USA) read the absorbance at 450 nm with wavelength correction set 540 nm. In all EIAs, intra- and inter-assay coefficients of variation were <10%. See Supplementary Table 5 for immunoassay details and characteristics.
We analyzed a broad range of immune markers with a potential link to SMDs: chemokines, cell adhesion molecules (CAMs), the IL-18 system, defensins, and markers potentially associated with neuroinflammation and BBB integrity; detailed single marker case-control analyses of several of the factors, i.e. CAMs, NSE, BAFF, APRIL and IL-18 markers are reported previously in overlapping samples (Andreou et al., 2021; Engh et al., 2021; Sheikh et al., 2022; Szabo et al., 2022). The chemokines were growth-regulated oncogene alpha (GROα), stromal cell-derived factor 1 alpha (SDF1α), eotaxin, and regulated upon activation, normal T-cell expressed and secreted (RANTES) (Louboutin and Strayer, 2013; Mantyla et al., 2015; Reale et al., 2011; Stuart and Baune, 2014). CAMs were mucosal vascular addressin cell adhesion molecule-1 (MadCAM-1), neural cadherin (NCAD), junctional adhesion molecule-A (JAMA), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and P-selectin (PSEL) (Sakurai, 2017; Sladojevic et al., 2014; Wu et al., 2017). The IL-18 system was assessed by IL-18, IL-18 binding protein (IL-18BP), IL-18 receptor 1 (IL-18R1) and IL-18 receptor accessory protein (IL-18RAP) (Alboni et al., 2010; Bossu et al., 2010; Cherlin et al., 2020; Dinarello et al., 2013). The defensins were human neutrophil peptides 1–3 (HNP1-3), beta defensin 1 (BD-1) and beta defensin 2 (BD-2) (Craddock et al., 2008; Hao et al., 2001; Williams et al., 2012). Markers particularly associated with BBB integrity were S100 calcium binding protein B (S100B) (Michetti et al., 2018), furin (Hou et al., 2018; Thomas, 2002), neuron specific enolase (NSE) (Haque et al., 2018) and glial fibrillary acidic protein (GFAP) (Trepanier et al., 2016), and markers with potential association with neuroinflammation were A proliferation-inducing ligand (APRIL) and B-cell-activating factor belong to the TNF family (BAFF) (Bossen and Schneider, 2006), alpha-2-macroglobulin (A2M) (Yee et al., 2017) and serpin family A member 3 (SERPINA3) (Chiu et al., 1999; Horváth and Mirnics, 2014; Ramsey et al., 2013; Saetre et al., 2007).
2.5. Definition of illness course characteristics
We included the following illness course characteristics:
Number of psychotic episodes per year (lifetime), SCZ: Patients were categorized according to the quartiles of number of psychotic episodes per year of illness duration, and the patients in the lower quartile (below the 25th percentile, 0.18 episodes/year) and in the upper quartile (above the 75th percentile, 1.00 episodes/year) were selected for statistical analysis (Cella et al., 2014; Nopoulos et al., 1998) to compare different groups in terms of prognostic value by the least and most severe illness course in terms of rate of episodes, respectively (Emsley et al., 2013), to facilitate interpretation of results and clinical relevance. Current or previous psychotic episodes were identified by the SCID-1 assessment and use of medical records.
Number of affective episodes per year (lifetime), BD: Patients were categorized according to the quartiles of number of affective episodes per year of illness duration, and the patients in the lower quartile (below the 25th percentile, 0.46 episodes/year) and in the upper quartile (above the 75th percentile, 1.85 episodes/year) were selected for statistical analysis (Cella et al., 2014; Nopoulos et al., 1998) to compare different groups in terms of prognostic value by the least and most severe illness course in terms of rate of episodes, respectively (Peters et al., 2016), to facilitate interpretation of results and clinical relevance. Current and previous affective episodes included hypomanic, manic, major depressive and mixed episodes, and were identified by the SCID-1 assessment and use of medical records.
Suicide attempt (lifetime): Patients were categorized into no history (‘absent’) or history (‘present’) of suicide attempt based on interviews and medical records.
Comorbid substance use disorder (lifetime): Three variables, each with patients dichotomized into no history (current or previous) (‘absent’) or a history (current or previous) (‘present’), of 1) any comorbid substance use disorder, 2) comorbid alcohol use disorder, and 3) comorbid cannabis use disorder, respectively, were made based on the SCID-1 assessment.
BD with psychotic episodes (lifetime): Patients with BD were categorized into no history (current or previous) (‘absent’) or a history (current or previous) (‘present’) of psychotic episodes based on the SCID-1 assessment.
Age at onset: The patient subsample was categorized according to age at onset of first illness episode (psychotic, hypomanic, manic, major depressive or mixed): ‘early onset’ <18 years, ‘adult onset’ ≥18 years <40, and ‘late onset’ ≥40 years.
2.6. Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 27 (SPSS Inc., Chicago, IL, USA). Sample characteristics were analyzed using independent t-test for normally distributed variables, Kruskal-Wallis test and Mann-Whitney U test for non-normally distributed variables and chi-square tests for categorical variables. For correlations we used Pearson's r and Spearman's rho. Normality was assessed by use of histograms, Q-Q-plots and Kolmogorov-Smirnov statistics. The immune marker data was log-transformed followed by repeated removal of residuals more extreme than 3 x IQR or 1.5 x IQR below or above the 25th and 75th percentile, respectively, depending on the distribution of the marker (3 x IQR: BAFF, APRIL, furin, GFAP, SDF1α, eotaxin, JAMA, NCAD, ICAM-1, VCAM-1, SERPINA3, IL-18BP, IL-18R1, BD-1, BD-2; 1.5 x IQR: S100B, GROα, MadCAM-1, IL-18RAP, HNP1-3).
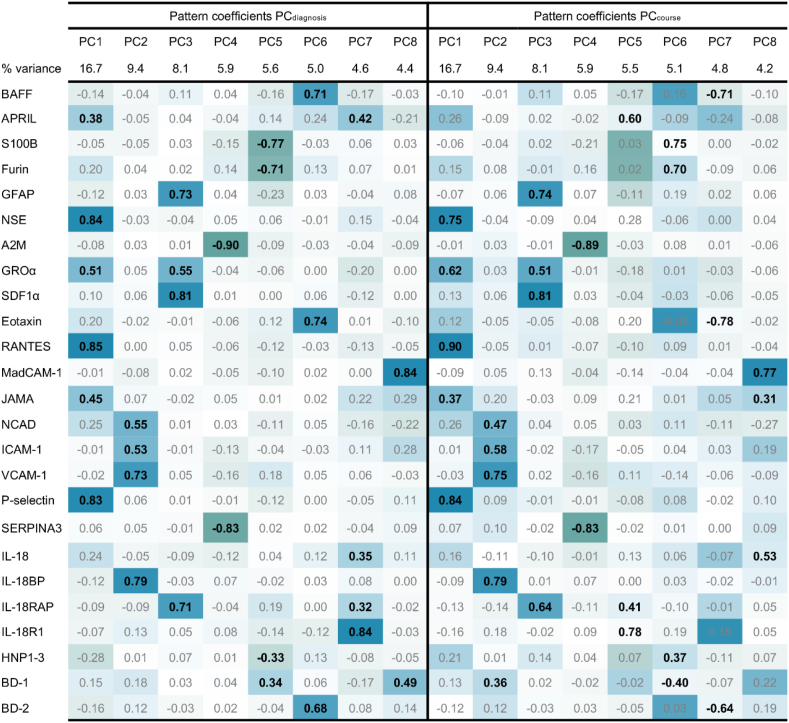
Principal Component Analyses (PCA) of the 25 immune markers were performed to reduce the dataset into fewer variables while retaining the majority of the variance within the sample (Dickerson et al., 2015; Lindqvist et al., 2011; Nguyen et al., 2018; Raymond et al., 2019). PCA was used to identify subgroups of immune markers, principal components (PCs). PC scores (‘composite immune marker scores’) were calculated from the PCA and used in the statistical models, representing the participant's score on each component. PCA of the 25 immune markers were performed in a) the total sample of patients and HC (SCZ + BD + HC) to generate ‘PCdiagnosis’ scores for analysis of diagnoses related immune patterns, and in b) the patient subsample (SCZ + BD) to generate ‘PCcourse’ scores for analysis of illness specific course characteristics with immune patterns. The PCA did not include other factors than the immune markers. All test assumptions (Field, 2018) were met; the Kaiser-Meyer-Olkin value was 0.749 and 0.742 for the total sample and the patient subsample, respectively, exceeding the recommended value of 0.5, indicating that a PCA is appropriate. The Bartlett's Test of Sphericity was statistically significant (p < 0.001) in both the total and the subsample, supporting the factorability of the correlation matrices. Oblimin rotation was applied to allow for correlation between components, and PCs were extracted based on an Eigenvalue cut-off of >1 (Kaiser's criterion). PCs are in the following described by immune markers with component loadings above 0.3. For complete details of immune marker component loadings of each PC, and PC raw scores, see Fig. 1 and Supplementary Table 2, respectively.
Fig. 1.
Principal component analysisa in total sample (PCdiagnosis) and patient subsample (PCcourse), pattern matrix
aOblimin rotation
The pattern matrix contains regression coefficients (loadings), with values ≥ 0.3 as bolded for description of PCs.
Abbreviations: Principal component (PC).
Multivariate analyses of covariance (MANCOVAs) were performed separately for diagnosis (SCZ, BD, HC) and each illness course characteristic (independent variables in separate MANCOVAs) to assess differences in the PC scores (dependent variables) between groups. MANCOVA was applied due to the case of several dependent variables, using an initial MANCOVA omnibus test to indicate an effect (Wilks’ lambda). To include potential confounding factors while keeping model complexity low and ensuring clarity of variable selection, analyses were performed with backward elimination of potential confounders, including age, sex, BMI and freezer storage time, and also SCZ and BD diagnosis and psychopharmacological treatment [antipsychotic use (yes/no), antidepressant use (yes/no), anticonvulsants and/or lithium (yes/no)] in patient subsample analysis. A p-value of >0.05 was used for removal of variables. See Supplementary Table 3 for details of the backward elimination. Differences in individual PC scores of dichotomous illness course characteristics were obtained from the univariate test statistics output and of age at onset and diagnosis (three categories) from the univariate tests by pairwise comparisons based on estimated marginal means. Given the sparsely explored research area, a modest correction of the significance level threshold to p < 0.025 (0.05/2) was applied for the initial MANCOVA omnibus test based on analyzing 1) diagnosis and 2) illness course characteristics, to indicate variables for further analysis while avoiding excessive risk of rejecting hypotheses that merit further investigation. In the follow-up univariate analyses of the indicated variables and each of the eight PCs, the significance level threshold was Bonferroni corrected to p < 0.00625 (0.05/8) due to analyzing eight PCs, and pairwise comparisons were controlled by standard method of substituting p-values lower than the omnibus test p-value, with the omnibus test p-value (Levin et al., 1994). All p-values are reported uncorrected.
2.7. Ethics
The TOP study is approved by the Regional Scientific Ethical Committee and the Norwegian Data Protection Inspectorate. The Biobank is approved by the Norwegian Directorate of Health. Participation is voluntary and written consent is a prerequisite. Information on the study and the possibility to withdraw is given both written and orally.
3. Results
3.1. Demography, symptoms, illness course characteristics, immune markers and principal components
There were more female participants in the BD group as compared to the SCZ and HC groups (both p < 0.001), and participants with SCZ were younger than those with BD and HC (both p < 0.001). Participants with SCZ were more severely ill as measured by GAF and PANSS (both p < 0.001) and had less frequent early onset (p < 0.001) and comorbid alcohol use disorder (p = 0.002) compared to the BD group, see Table 1 for details. For concentrations of the 25 immune markers across groups, see Supplementary Table 4. Immune marker differences in overlapping samples were reported in previous publications (Andreou et al., 2021; Engh et al., 2021; Sheikh et al., 2022; Szabo et al., 2022). Bivariate correlations between sample characteristics and PCs are given in Supplementary Table 6.
Table 1.
Demographic and illness course characteristics.
| Categories | SCZ (N = 602) | BD (N = 346) | HC (N = 814) | p-valuec | Pairwise comparisons | |
|---|---|---|---|---|---|---|
| Sample characteristics | ||||||
| Sex, males, N (%) | 358 (59.5) | 142 (41.0) | 456 (56.0) | <0.001 | BD < SCZ,HC | |
| Age (years), median (IQR) | 27 (22–36) | 31 (23–42) | 32 (26–39) | <0.001 | SCZ < BD,HC | |
| BMI (kg/m2), median (IQR) | 25.1 (22.4–29.1) | 25.1 (22.3–27.7) | 24.1 (22.1–26.4) | <0.001 | HC < SZ,BD | |
| Freezing timea (years), median (IQR) | 8 (5–10) | 8 (3–10) | 5 (3–9) | <0.001 | HC < SZ,BD | |
| GAF-S, median (IQR) | 40 (37–51) | 58 (51–65) | – | <0.001 | – | |
| PANSS, median (IQR) | 61 (51–71) | 43 (38–51) | – | <0.001 | – | |
| Duration of illness (years), median (IQR) | 4.0 (1–10) | 10 (5–19) | – | <0.001 | BD < SCZ | |
| Antidepressant use, N (%) | 169 (28.1) | 115 (33.2) | – | 0.11 | – | |
| Antipsychotic use, N (%) | 508 (84.4) | 192 (55.5) | – | <0.001 | – | |
| Anticonvulsant and lithium use, N (%) | 74 (12.3) | 181 (52.3) | – | <0.001 | – | |
| Illness course characteristics | ||||||
| Age at onset, N (%) | <18/18–39/>40 | 131 (22.5)/422 (72.5)/29 (5.0) | 149 (43.2)/181 (52.5)/15 (4.3) | – | <0.001 | – |
| Psychotic episodes, SCZ, Nb | upper/lower | 120/117 | – | – | – | |
| Affective episodes, BD, Nb | upper/lower | – | 78/79 | – | – | |
| Suicide attempt, N (%) | present/absent | 232 (45.3)/280 (54.7) | 118 (39.5)/181 (60.5) | – | 0.11 | – |
| Any comorbid substance use disorder, N (%) | present/absent | 140 (23.3)/462 (76.7) | 80 (23.1)/266 (76.9) | – | 1.0 | – |
| Comorbid alcohol use disorder, N (%) | present/absent | 23 (4.7)/462 (95.3) | 32 (10.7)/266 (89.3) | – | 0.002 | – |
| Comorbid cannabis use disorder, N (%) | present/absent | 40 (8.0)/462 (92.0) | 20 (7.0)/266 (93.0) | – | 0.72 | – |
| BD with psychotic episodes, N (%) | present/absent | – | 207 (61.4)/130 (38.6) | – | – | |
Missing data (%), sample characteristics: BMI 15.2, duration of illness 2.2; illness course characteristics: psychotic episodes of SCZ 25.6, comorbid alcohol use disorder 17.4, comorbid cannabis disorder 16.9, suicide attempt 14.5, any comorbid substance use disorder 13.1, affective episodes, BD, 9.2, BD with psychotic episodes 2.6, age at onset 2.2.
Abbreviations: Bipolar disorder (BD), Body mass index (BMI), Global Assessment of Functioning - symptoms (GAF-S), Healthy controls (HC), Interquartile range (IQR), Positive and Negative Syndrome Scale (PANSS), Schizophrenia spectrum disorders (SCZ), Severe mental disorders (SMD).
Freezing time of plasma sample used to analyze immune markers. Minimum and maximum values (years): SCZ, 1–15; BD, 1–15; HC 1–14.
Number of patients in the ‘upper’ and in the ‘lower’ quartile of number of psychotic (SCZ) and affective (BD) episodes per year of illness duration.
Chi square test for categorical variables, Kruskal Wallis and Mann-Whitney U Test for variables represented by median (interquartile range).
The PCA reduced the 25 immune markers into different sets of eight PCs (Eigenvalue cut-off of >1) in the total sample and patient subsample, explaining 59.7% of the variances in both samples. Total sample PCs included the following immune markers: RANTES, NSE, PSEL, JAMA, GROα and APRIL (PC1diagnosis); IL-18BP, VCAM-1, NCAD and ICAM-1 (PC2diagnosis); SDF1α, GFAP, IL-18RAP and GROα (PC3diagnosis); A2M and SERPINA3 (PC4diagnosis); S100B, furin, HNP1-3 and BD-1 (PC5diagnosis); eotaxin, BAFF and BD-2 (PC6diagnosis); IL-18R1, APRIL, IL-18 and IL-18RAP (PC7diagnosis); MadCAM-1 and BD-1 (PC8diagnosis). Patient subsample PCs included the following immune markers: RANTES, PSEL, NSE, GROα and JAMA (PC1course); IL-18BP, VCAM-1, ICAM-1, NCAD and BD-1 (PC2course); SDF1α, GFAP, IL-18RAP and GROα (PC3course); A2M and SERPINA3 (PC4course); IL-18R1, APRIL and IL-18RAP (PC5course); S100B, furin, BD-1 and HNP1-3 (PC6course); eotaxin, BAFF and BD-2 (PC7course); MadCAM-1, IL-18 and JAMA (PC8course). For more details, see Fig. 1 and Supplementary Fig. 1.
3.2. Associations between diagnosis, age at onset and illness course characteristics and immune principal components
3.2.1. Diagnosis
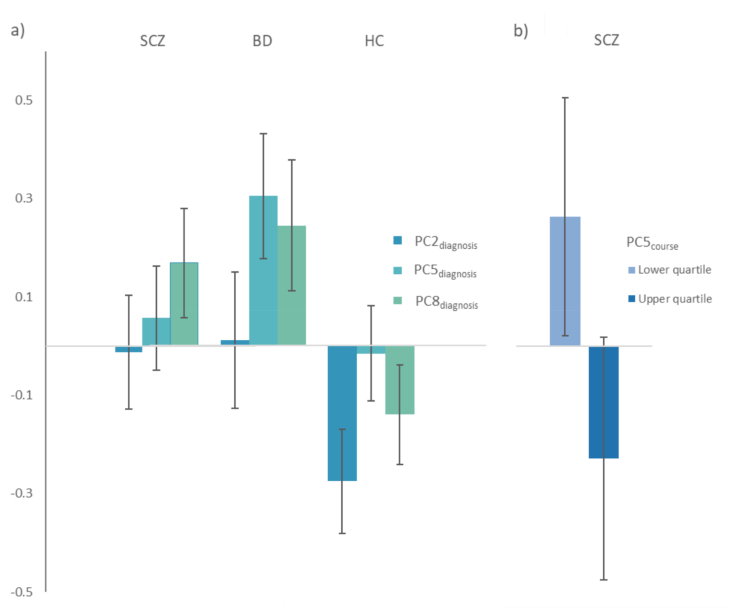
As shown in Table 2, there was a significant effect of diagnosis (SCZ, BD, HC) in the MANCOVA omnibus test of eight PCdiagnosis scores: F (16, 1480) = 4.692, p < 0.001, Wilks’ lambda = 0.906, partial η2 = 0.048. In follow-up analyses of the individual PCs, we found significant differences between diagnostic groups for PC2diagnosis score: SCZ > HC (p = 0.001) and BD > HC (p = 0.001), PC5diagnosis score: BD > HC (p < 0.001) and BD > SCZ (p = 0.004), and PC8diagnosis score: SCZ > HC (p < 0.001) and BD > HC (p < 0.001). See Fig. 2a) and Supplementary Table 7 for details.
Table 2.
MANCOVA omnibus tests of associations between diagnosis and eight principal components (adjustments with backward elimination procedure).
| Model | Wilks' λ | F | df | Error df | p-value | Partial η2 |
|---|---|---|---|---|---|---|
| Diagnosisa | 0.906 | 4.692 | 16 | 1480 | <0.001 | 0.048 |
| Age | 0.942 | 5.714 | 8 | 740 | <0.001 | 0.058 |
| Sex | 0.954 | 4.500 | 8 | 740 | <0.001 | 0.046 |
| BMI | 0.958 | 4.099 | 8 | 740 | <0.001 | 0.042 |
| Freezing time | 0.684 | 42.820 | 8 | 740 | <0.001 | 0.316 |
MANCOVA was performed for diagnosis (SCZ, BD, HC; independent variable) to assess differences in the principal component scores (eight, dependent variables).
Abbreviations: Bipolar spectrum disorder (BD), Multivariate analyses of covariance (MANCOVA), Severe mental disorders (SMD), Schizophrenia spectrum disorder (SCZ).
Total sample (SCZ, BD, HC).
Fig. 2.
Immune principal components (PC) associated with a) diagnosis and b) number of psychotic episodes per year in SCZ
a) Estimated marginal means of PC scores based on Log-10 transformed immune marker values of PC2diagnosis (IL-18BP, VCAM-1, NCAD, ICAM-1), PC5diagnosis (S100B, furin, HNP1-3, BD-1) and PC8diagnosis (MadCAM-1, BD-1). Error bars represent confidence intervals.
b) Estimated marginal means of PC5course (IL-18R1, APRIL and IL-18RAP) scores based on Log-10 transformed immune marker values, of patients with SCZ in the lower and upper quartiles, respectively, of number of psychotic episodes per year.
Error bars represent confidence intervals.
3.2.2. Age at onset and illness course characteristics
There was a significant effect of number of psychotic episodes per year, SCZ in the MANCOVA omnibus test of the eight PCcourse scores: F (8, 96) = 2.340, p = 0.024, Wilks’ lambda = 0.837, partial η2 = 0.163 (Table 3). The univariate test statistics showed a significant effect on PC5course score: lower quartile > upper quartile (mean difference 0.493, p = 0.006). No statistically significant effects were found in MANCOVAs (omnibus test) for age at onset (p = 0.097), number of affective episodes per year, BD (p = 0.398), suicide attempt, lifetime (p = 0.831), any comorbid substance use disorder (p = 0.085), comorbid alcohol use disorder (p = 0.637), comorbid cannabis use disorder (p = 0.203), or BD with psychotic episodes (p = 0.388). See Table 3, Fig. 2b) and Supplementary Table 7 for details.
Table 3.
MANCOVA omnibus tests of associations between illness course characteristics and eight principal components (adjustments with backward elimination procedure).
| Model | Wilks' λ | F | df | Error df | p-value | Partial η2 |
|---|---|---|---|---|---|---|
| Age at onseta | 0.946 | 1.487 | 16 | 848 | 0.097 | 0.027 |
| Age | 0.936 | 3.635 | 8 | 424 | <0.001 | 0.064 |
| Sex | 0.952 | 2.652 | 8 | 424 | 0.008 | 0.048 |
| BMI | 0.942 | 3.255 | 8 | 424 | 0.001 | 0.058 |
| Freezing time | 0.632 | 30.890 | 8 | 424 | <0.001 | 0.368 |
| Psychotic episodes, SCZa,b | 0.837 | 2.340 | 8 | 96 | 0.024 | 0.163 |
| Freezing time | 0.657 | 6.276 | 8 | 96 | <0.001 | 0.343 |
| Affective episodes, BDa,b | 0.902 | 1.062 | 8 | 78 | 0.398 | 0.098 |
| Sex | 0.764 | 3.016 | 8 | 78 | 0.005 | 0.236 |
| Freezing time | 0.644 | 5.380 | 8 | 78 | <0.001 | 0.356 |
| Mood stabilizers | 0.809 | 2.299 | 8 | 78 | 0.029 | 0.191 |
| Suicide attempta,c | 0.989 | 0.534 | 8 | 401 | 0.831 | 0.011 |
| Age | 0.933 | 3.605 | 8 | 401 | <0.001 | 0.067 |
| Sex | 0.945 | 2.909 | 8 | 401 | 0.004 | 0.055 |
| BMI | 0.929 | 3.822 | 8 | 401 | <0.001 | 0.071 |
| Freezing time | 0.667 | 25.029 | 8 | 401 | <0.001 | 0.333 |
| Any comorbid substance use disordera,c | 0.970 | 1.752 | 8 | 448 | 0.085 | 0.030 |
| Age | 0.936 | 3.857 | 8 | 448 | <0.001 | 0.064 |
| Sex | 0.943 | 3.394 | 8 | 448 | <0.001 | 0.057 |
| BMI | 0.940 | 3.552 | 8 | 448 | <0.001 | 0.060 |
| Freezing time | 0.617 | 34.733 | 8 | 448 | <0.001 | 0.383 |
| Comorbid alcohol use disordera,c | 0.983 | 0.761 | 8 | 362 | 0.637 | 0.017 |
| Age | 0.926 | 3.612 | 8 | 362 | <0.001 | 0.074 |
| Sex | 0.932 | 3.313 | 8 | 362 | 0.001 | 0.068 |
| BMI | 0.934 | 3.216 | 8 | 362 | 0.002 | 0.066 |
| Freezing time | 0.619 | 27.835 | 8 | 362 | <0.001 | 0.381 |
| Comorbid cannabis use disordera,c | 0.970 | 1.381 | 8 | 363 | 0.203 | 0.030 |
| Age | 0.920 | 3.921 | 8 | 363 | <0.001 | 0.080 |
| Sex | 0.932 | 3.298 | 8 | 363 | 0.001 | 0.068 |
| BMI | 0.923 | 3.781 | 8 | 363 | <0.001 | 0.077 |
| Freezing time | 0.626 | 27.121 | 8 | 363 | <0.001 | 0.374 |
| BD with psychotic episodesd | 0.951 | 1.068 | 8 | 167 | 0.388 | 0.049 |
| Age | 0.888 | 2.626 | 8 | 167 | 0.010 | 0.112 |
| Sex | 0.906 | 2.162 | 8 | 167 | 0.033 | 0.094 |
| Freezing time | 0.638 | 11.852 | 8 | 167 | <0.001 | 0.362 |
MANCOVA was performed for age at onset and each illness course characteristic (independent variable) separately to assess differences in the principal component scores (eight, dependent variables).
Abbreviations: Bipolar spectrum disorder (BD), Multivariate analyses of covariance (MANCOVA), Severe mental disorders (SMD), Schizophrenia spectrum disorder (SCZ).
Patient subsample (SCZ, BD).
Number of psychotic (SCZ) and affective (BD) episodes per year of illness duration.
Lifetime.
BD subsample.
4. Discussion
PCA was used in a large SMD sample to investigate patterns of immune markers associated with diagnosis and illness course characteristics. After reduction of 25 markers into eight components, three components based on CAMs, BBB markers and defensins were associated with SCZ or BD. For course characteristics in SCZ, number of psychotic episodes per year was associated with a component of APRIL and soluble IL-18 receptor markers. Thus, our study suggests that CAMs assisted BBB dysfunction, pro-inflammatory signaling and dysregulated neuroprotective processes may play a role in the progression of SMDs.
Supporting a role of inflammation in SMDs (Goldsmith et al., 2016; Kroken et al., 2018), several immune patterns (PCdiagnosis) were associated with a diagnosis of SCZ or BD. Using composite scores, the susceptibility of unforeseen variability of single markers is limited. A similar approach was applied by Nguyen et al. (Nguyen et al., 2018) showing an enhanced index of ICAM-1 and VCAM-1 in SCZ compared to HC. Our results in a several times larger sample extend these findings showing an association of SCZ with components strongly influenced by CAMs (PC2diagnosis and PC8diagnosis) with a similar pattern in BD.
Specifically, ICAM-1, VCAM-1 and NCAD loaded positively on PC2diagnosis; however, together with IL-18BP, ICAM-1 accounted for the main difference in increased concentrations in the patient groups. Although conflicting evidence (Futtrup et al., 2020; Kronig et al., 2005; Muller, 2019; Schwarz et al., 2000; Stefanovic et al., 2016), elevated levels of ICAM-1 are previously indicated in both SCZ (Beumer et al., 2012; Cai et al., 2020; Nguyen et al., 2018; Sheikh et al., 2022; Stefanovic et al., 2016) and BD (Pantovic-Stefanovic et al., 2018; Reininghaus et al., 2016; Schaefer et al., 2016; Turan et al., 2014). ICAM-1 has also been associated with increased symptom severity (Muller, 2019; Stefanovic et al., 2016; Turan et al., 2014) and better treatment response (Stefanovic et al., 2016) in SCZ, although not associated with course characteristics in the current study. There is conflicting evidence of VCAM-1 (Futtrup et al., 2020; Nguyen et al., 2018; Stefanovic et al., 2016); however, both ICAM-1 and VCAM-1 may be related to manic episodes (Turan et al., 2014). In a study from our centre of adolescent participants with early-onset psychosis (Wedervang-Resell et al., 2020), reduced levels of PSEL and VCAM-1 and no significant alterations of ICAM-1, MadCAM-1, JAMA or NCAD, were found. While both studies suggest involvement of CAMs in SMD pathophysiology, differences indicate the need of examining temporal patterns as well as potential variations between subgroups. Increased plasma levels of IL-18 and IL-18BP was recently demonstrated in our group, as also shown by others (Palladino et al., 2012), together with higher expression of the inflammasome-related genes NLRP3 and NLRC4 in blood leukocytes in SMDs (Guo et al., 2015; Strowig et al., 2012; Szabo et al., 2022), supporting systemic inflammasome activation in these patients. IL-18BP, regulated by a negative feedback from IL-18 (Dinarello et al., 2013; Palladino et al., 2012), may therefore reflect long standing low-grade inflammasome activation in SMDs. Our finding that IL-18BP loaded positively on PC2diagnosis together with CAMs expressed on vascular cells including the BBB (Marchetti and Engelhardt, 2020), may link NLRP3 and NLRC4 inflammasome activation with adhesion and transmigration of leukocytes to underlying tissues, potentially a mechanism promoting neuroinflammation in SMDs (Herman and Pasinetti, 2018).
Further support linking IL-18 signaling to SCZ was the finding that PC5course with positive loadings of IL-18R1, IL-18RAP and APRIL was negatively associated with severity of illness course as measured by rate of psychotic episodes, in these patients. While the mechanism of IL-18 and IL-18BP are well established, the regulation and function of soluble IL-18R1 and IL-18RAP are less established. Membrane bound IL-18R1 and IL-18R2 promotes IL-18 signaling and is further amplified by IL-18RAP (Yang, 2020). Conversely, their soluble forms act as decoy receptors and inhibit IL-18 signaling (Reznikov et al., 2002). Thus, we speculate that within patients with SCZ, decreased levels of soluble IL-18R1 and IL-18RAP could promote IL-18 signaling at the cellular level (Alboni et al., 2010; Cherlin et al., 2020; Tsutsumi et al., 2014) and influence illness course severity. The positive loading of APRIL, a potential neuroprotective cytokine by mediating production of the anti-inflammatory IL-10 (Baert et al., 2019), in PC5course in relation to less illness severity seem in line with our recently reported association of lower APRIL with increased psychotic symptoms and generally decreased levels in SMD (Engh et al., 2021). Taken together, increases in these markers with anti-inflammatory function seem to be associated with a less severe illness course.
Interestingly, PC5diagnosis score was increased in BD relative to SCZ and HC with negative loadings of BBB-related markers dominating the component. While CAMs regulate migration, the major PC5diagnosis marker, S100B, is an astrocyte-expressed protein indicating BBB disruption (Futtrup et al., 2020) due to e.g. brain trauma and cerebrovascular diseases (Chong et al., 2016). Several studies report elevated S100B in BD (Bartoli et al., 2020; da Rosa et al., 2016) and SCZ (Aleksovska et al., 2014; Hong et al., 2016; Rothermundt et al., 2009). However, there is a positive association with symptom severity (Futtrup et al., 2020; Pollak et al., 2018; van de Kerkhof et al., 2014) and reduced levels in BD after treatment of manic phase, suggesting a transient disruption of BBB that may be clinically dependent (Tsai and Huang, 2017). Importantly, the current patients had relatively low symptom load, enabling putative assessment of the basal underlying BBB integrity of BD. We found that furin and the alpha-defensins HNP1-3 all loaded negatively together with S100B, and BD-1 had a small positive effect. While furin is a convertase activating a range of protein precursors implicated amongst others in BBB disruption (Baumann et al., 2019), alpha-defensins have broad functions including inflammation regulatory effects such as chemotaxis (Fruitwala et al., 2019), and the current HNP1-3 markers have been linked to immunological components of a related illness, Alzheimer's disease (DeMichele-Sweet et al., 2021; Szekeres et al., 2016). Nevertheless, a BD specific BBB integrity, after comparison with SCZ and HC, is suggested by the component, a finding that warrant further investigation given the sparsity of studies of BBB markers in BD (Futtrup et al., 2020).
The defensin BD-1 constituted a positive load together with MadCAM-1 on PC8diagnosis. Beta-defensins have a wide repertoire of mechanisms including pro-inflammatory and anti-inflammatory functions, and may be reduced in patients with inflammatory disorders (Shelley et al., 2020) and associated with neuroinflammation in Alzheimer's disease (Williams et al., 2013). Generally, the underlying mechanisms indicated across BD and SCZ by the component loadings of PC2diagnosis and PC8diagnosis, are pro-inflammatory processes including neuroinflammation mediated by CAMs. While CAMs are involved in various functions (Harjunpaa et al., 2019), they regulate migration of inflammatory cells across the BBB (Kong et al., 2018; Muller, 2019) and thus the current associations are indicative of BBB dysregulation with central inflammatory infiltration.
The use of composite immune variables rather than single immune markers has been proposed as a way to advance the field, both in terms of providing a coherent understanding of the interplay between immune pathways and by improving the quality of immune variable signals (Miller and Goldsmith, 2017). As suggested by the current study, analyzing immune patterns might gain additional information beyond single marker analyses, exemplified here by 1) the indicated mediating mechanism of neuroinflammation of NLRP3 and NLRC4 inflammasome linked CAMs assistance of leucocyte transmigration, 2) displaying links between BBB integrity markers in BD, and 3) suggesting interplay of different anti-inflammatory pathways in illness course severity. Thus, the current study adds novel information regarding interrelationships of immune pathways in SMD to the corresponding detailed studies of single immune markers of our centre demonstrating aberrations of some of the current immune markers (Andreou et al., 2021; Engh et al., 2021; Sheikh et al., 2022; Szabo et al., 2022), as well as generally to immune research in SMD (Kroken et al., 2018). In the long term, more knowledge about these and other immune interplays might enhance our ability to test hypotheses in experimental designs, broaden the targets for intervention, and hopefully repurpose and develop agents of clinical significance.
There are limitations to consider. The cross-sectional design limits interpretation about causality. For several participants the freezing time was rather long and samples from HCs were generally gathered more recently than samples from patients. Although plasma was stored at standard −80 °C and freezing time was included in the statistical models, an influence on the results related to degradation of markers with time, cannot be excluded. The immune markers are measured peripherally, complicating inferences about central mechanisms. However, the study involves centrally expressed factors and a uniquely large sample relative to other immune – SMD samples. Furthermore, given well-evidenced immune alternations in SMDs, we performed two PCAs to allow comparisons across SMDs and HC as well as analyses of illness course characteristics with immune patterns within SMDs. This prevents analyses of case-control differences of PCs associated with illness course characteristics. While the large sample size allows detection of minor effects, the effect of immune factors within the complex interplay of biological and environmental factors is expected to be small. By using immune principal components, novel findings of immune pathway interactions indicating biological mechanisms might be possible, and the PCA provide a well-established and simple method for this purpose. Also, severe illness courses due to longstanding, but few episodes, are not detected by the current course characteristics number of psychotic and affective episodes. A range of confounder variables were included in the analyses, including BMI and psychotropic medication, however, several factors might impact immune marker levels and cause residual confounding, including clinical features, smoking and cardiometabolic conditions and treatments. Moreover, immune molecules are synthesized in various tissues such as in muscles, liver, fat, the vasculature, the brain, lymphoid tissue and white blood cells; generally, this might underlie difficulties of replicating immune results. Lastly, we applied a moderate correction for multiple comparisons of the initial MANCOVA omnibus test due to testing two clinical categories, illness course characteristics and diagnosis, while adjusting for eight PCs in the follow-up univariate analyses. Importantly, SCZ and BD have previously established immune aberrations related to the component markers, and a too strict correction would risk discarding interesting hypotheses that merit further investigation.
In the current study we established immune components based on PCA of a set of immune markers to uncover immune patterns reflecting underlying mechanisms of SMDs. CAMs-mediated dysregulation of BBB integrity was suggested across BD and SCZ, and involvement of pro-inflammatory processes associated with dysregulated neuroprotective mechanisms in illness course severity in SCZ. These findings emphasize the importance of investigating multiple immune markers simultaneously to elucidate the underlying immune dynamics in SMDs.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Ole A. Andreassen reports a relationship with HealthLytix that includes: consulting or advisory. Ole A. Andreassen reports a relationship with Lundbeck that includes: speaking and lecture fees. Ole A. Andreassen reports a relationship with Sunovion that includes: speaking and lecture fees.
Acknowledgements
This study was funded by the Research Council of Norway (grant numbers 223273, 283798, 283799); and the South-Eastern Norway Regional Health Authority (grant numbers 2019-108, 2017-112). The authors thank the participants for their contributions to the study, in addition to colleagues at NORMENT.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100483.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alboni S., Cervia D., Sugama S., Conti B. Interleukin 18 in the CNS. J. Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksovska K., Leoncini E., Bonassi S., Cesario A., Boccia S., Frustaci A. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou D., Steen N.E., Jørgensen K.N., Smelror R.E., Wedervang-Resell K., Nerland S., Westlye L.T., Nærland T., Myhre A.M., Joa I., Reitan S.M.K., Vaaler A., Morken G., Bøen E., Elvsåshagen T., Boye B., Malt U.F., Aukrust P., Skrede S., Kroken R.A., Johnsen E., Djurovic S., Andreassen O.A., Ueland T., Agartz I. Lower circulating neuron-specific enolase concentrations in adults and adolescents with severe mental illness. Psychol. Med. 2021;1–10 doi: 10.1017/S0033291721003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert L., Benkhoucha M., Popa N., Ahmed M.C., Manfroi B., Boutonnat J., Sturm N., Raguenez G., Tessier M., Casez O., Marignier R., Ahmadi M., Broisat A., Ghezzi C., Rivat C., Sonrier C., Hahne M., Baeten D., Vives R.R., Lortat-Jacob H., Marche P.N., Schneider P., Lassmann H.P., Boucraut J., Lalive P.H., Huard B. A proliferation-inducing ligand-mediated anti-inflammatory response of astrocytes in multiple sclerosis. Ann. Neurol. 2019;85(3):406–420. doi: 10.1002/ana.25415. Mar. [DOI] [PubMed] [Google Scholar]
- Bartoli F., Misiak B., Crocamo C., Carra G. Glial and neuronal markers in bipolar disorder: a meta-analysis testing S100B and NSE peripheral blood levels. Prog Neuropsychopharmacol. Biol. Psychiatr. 2020;101 doi: 10.1016/j.pnpbp.2020.109922. Jul 13. [DOI] [PubMed] [Google Scholar]
- Baumann J., Huang S.F., Gassmann M., Tsao C.C., Ogunshola O.O. Furin inhibition prevents hypoxic and TGFbeta-mediated blood-brain barrier disruption. Exp. Cell Res. 2019;383(2) doi: 10.1016/j.yexcr.2019.111503. Oct 15. [DOI] [PubMed] [Google Scholar]
- Bechter K., Reiber H., Herzog S., Fuchs D., Tumani H., Maxeiner H.G. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 2010;44(5):321–330. doi: 10.1016/j.jpsychires.2009.08.008. Apr. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Aggio V., Pratesi M.L., Greco G., Furlan R. Neuroinflammation in bipolar depression. Front. Psychiatr. 2020;11:71. doi: 10.3389/fpsyt.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros M.E., Nielsen P.R., Nordentoft M., Eaton W.W., Dalton S.O., Mortensen P.B. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am. J. Psychiatr. 2011;168(12):1303–1310. doi: 10.1176/appi.ajp.2011.11030516. 2011/12/01. [DOI] [PubMed] [Google Scholar]
- Bergink V., Gibney S.M., Drexhage H.A. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol. Psychiatr. 2014;75(4):324–331. doi: 10.1016/j.biopsych.2013.09.037. 2014/02/15/ [DOI] [PubMed] [Google Scholar]
- Beumer W., Drexhage R.C., De Wit H., Versnel M.A., Drexhage H.A., Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012;37(12):1901–1911. doi: 10.1016/j.psyneuen.2012.04.001. Dec. [DOI] [PubMed] [Google Scholar]
- Black C., Miller B.J. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol. Psychiatr. 2015;78(1):28–37. doi: 10.1016/j.biopsych.2014.10.014. Jul 1. [DOI] [PubMed] [Google Scholar]
- Bossen C., Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. 2006;18(5):263–275. doi: 10.1016/j.smim.2006.04.006. Oct. [DOI] [PubMed] [Google Scholar]
- Bossu P., Ciaramella A., Salani F., Vanni D., Palladino I., Caltagirone C., Scapigliati G. Interleukin-18, from neuroinflammation to Alzheimer's disease. Curr. Pharmaceut. Des. 2010;16(38):4213–4224. doi: 10.2174/138161210794519147. https://www.ncbi.nlm.nih.gov/pubmed/21184660 [DOI] [PubMed] [Google Scholar]
- Brodin P., Jojic V., Gao T., Bhattacharya S., Angel C.J., Furman D., Shen-Orr S., Dekker C.L., Swan G.E., Butte A.J., Maecker H.T., Davis M.M. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. doi: 10.1016/j.cell.2014.12.020. Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C.Z., Ryan K.A., Kamali M., Marshall D.F., Harrington G., McInnis M.G., Tso I.F. Psychosis in bipolar disorder: does it represent a more "severe" illness? Bipolar Disord. 2018;20(1):18–26. doi: 10.1111/bdi.12527. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.Q., Catts V.S., Webster M.J., Galletly C., Liu D., O'Donnell M., Weickert T.W., Weickert C.S. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol. Psychiatr. 2020;25(4):761–775. doi: 10.1038/s41380-018-0235-x. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Swan S., Medin E., Reeder C., Wykes T. Metacognitive awareness of cognitive problems in schizophrenia: exploring the role of symptoms and self-esteem. Psychol. Med. 2014;44(3):469–476. doi: 10.1017/S0033291713001189. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson F.J., Ferrari A.J., Santomauro D.F., Diminic S., Stockings E., Scott J.G., McGrath J.J., Whiteford H.A. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull. 2018;44(6):1195–1203. doi: 10.1093/schbul/sby058. Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlin S., Lewis M.J., Plant D., Nair N., Goldmann K., Tzanis E., Barnes M.R., McKeigue P., Barrett J.H., Pitzalis C., Barton A., Consortium M., Cordell H.J. Investigation of genetically regulated gene expression and response to treatment in rheumatoid arthritis highlights an association between IL18RAP expression and treatment response. Ann. Rheum. Dis. 2020;79(11):1446–1452. doi: 10.1136/annrheumdis-2020-217204. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H.J., Hong C.J., Chen J.Y., Wang Y.C., Lin C.Y., Bai Y.M., Song H.L., Lai H.C., Tsai S.J. Alpha-1-antichymotrypsin polymorphism in schizophrenia: frequency, age at onset and cognitive function. Neuropsychobiology. 1999;40(2):71–74. doi: 10.1159/000026600. [DOI] [PubMed] [Google Scholar]
- Chong Z.Z., Changyaleket B., Xu H., Dull R.O., Schwartz D.E. Identifying S100B as a biomarker and a therapeutic target for brain injury and multiple diseases. Curr. Med. Chem. 2016;23(15):1571–1596. doi: 10.2174/0929867323666160406121117. [DOI] [PubMed] [Google Scholar]
- Cirone C., Secci I., Favole I., Ricci F., Amianto F., Davico C., Vitiello B. What do we know about the long-term course of early onset bipolar disorder? A review of the current evidence. Brain Sci. 2021;11(3) doi: 10.3390/brainsci11030341. Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon N., Godin O., Bulzacka E., Dubertret C., Mallet J., Fond G., Brunel L., Andrianarisoa M., Anderson G., Chereau I., Denizot H., Rey R., Dorey J.M., Lançon C., Faget C., Roux P., Passerieux C., Dubreucq J., Leignier S., Capdevielle D., André M., Aouizerate B., Misdrahi D., Berna F., Vidailhet P., Leboyer M., Schürhoff F. Early and very early-onset schizophrenia compared with adult-onset schizophrenia: French FACE-SZ database. Brain Behav. 2020;10(2) doi: 10.1002/brb3.1495. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock R.M., Huang J.T., Jackson E., Harris N., Torrey E.F., Herberth M., Bahn S. Increased alpha-defensins as a blood marker for schizophrenia susceptibility. Mol. Cell. Proteomics. 2008;7(7):1204–1213. doi: 10.1074/mcp.M700459-MCP200. Jul. [DOI] [PubMed] [Google Scholar]
- Cullen A.E., Holmes S., Pollak T.A., Blackman G., Joyce D.W., Kempton M.J., Murray R.M., McGuire P., Mondelli V. Associations between non-neurological autoimmune disorders and psychosis: a meta-analysis. Biol. Psychiatr. 2019;85(1):35–48. doi: 10.1016/j.biopsych.2018.06.016. 2019/01/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa M.I., Simon C., Grande A.J., Barichello T., Oses J.P., Quevedo J. Serum S100B in manic bipolar disorder patients: systematic review and meta-analysis. J. Affect. Disord. 2016;206:210–215. doi: 10.1016/j.jad.2016.07.030. Dec. [DOI] [PubMed] [Google Scholar]
- De Hert M., Detraux J., Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018;20(1):31–40. doi: 10.31887/DCNS.2018.20.1/mdehert. https://www.ncbi.nlm.nih.gov/pubmed/29946209 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Picker L.J., Morrens M., Chance S.A., Boche D. Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Front. Psychiatr. 2017;8:238. doi: 10.3389/fpsyt.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMichele-Sweet M.A.A., Klei L., Creese B., Harwood J.C., Weamer E.A., McClain L., Sims R., Hernandez I., Moreno-Grau S., Tarraga L., Boada M., Alarcon-Martin E., Valero S., Nia-Load Family Based Study Consortium, A. s. D.G.C. Liu Y., Hooli B., Aarsland D., Selbaek G., Bergh S., Rongve A., Saltvedt I., Skjellegrind H.K., Engdahl B., Stordal E., Andreassen O.A., Djurovic S., Athanasiu L., Seripa D., Borroni B., Albani D., Forloni G., Mecocci P., Serretti A., De Ronchi D., Politis A., Williams J., Mayeux R., Foroud T., Ruiz A., Ballard C., Holmans P., Lopez O.L., Kamboh M.I., Devlin B., Sweet R.A. Genome-wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol. Psychiatr. 2021 doi: 10.1038/s41380-021-01152-8Pubmed. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F., Schroeder J., Stallings C., Origoni A., Bahn S., Yolken R. Multianalyte markers of schizophrenia and bipolar disorder: a preliminary study. Schizophr. Res. 2015;168(1–2):450–455. doi: 10.1016/j.schres.2015.08.003. Oct. [DOI] [PubMed] [Google Scholar]
- Dickerson F., Stallings C., Origoni A., Schroeder J., Katsafanas E., Schweinfurth L., Savage C., Khushalani S., Yolken R. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr. Bull. 2016;42(1):134–141. doi: 10.1093/schbul/sbv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R., Chiliza B., Asmal L., Harvey B.H. The nature of relapse in schizophrenia. BMC Psychiatr. 2013;13:50. doi: 10.1186/1471-244X-13-50. Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh J.A., Ueland T., Agartz I., Andreou D., Aukrust P., Boye B., Boen E., Drange O.K., Elvsashagen T., Hope S., Hoegh M.C., Joa I., Johnsen E., Kroken R.A., Lagerberg T.V., Lekva T., Malt U.F., Melle I., Morken G., Naerland T., Steen V.M., Wedervang-Resell K., Weibell M.A., Westlye L.T., Djurovic S., Steen N.E., Andreassen O.A. Plasma levels of the cytokines B cell-activating factor (BAFF) and A proliferation-inducing ligand (APRIL) in schizophrenia, bipolar, and major depressive disorder: a cross sectional, multisite study. Schizophr. Bull. 2021 doi: 10.1093/schbul/sbab106. Sep. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J., Stockings E., Khoo J.P., Erskine H.E., Degenhardt L., Vos T., Whiteford H.A. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440–450. doi: 10.1111/bdi.12423. Aug. [DOI] [PubMed] [Google Scholar]
- Field A. fifth ed. SAGE Publications; 2018. Discovering Statistics Using IBM SPSS Statistics.https://books.google.co.uk/books?id=0QTyswEACAAJ [Google Scholar]
- First M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013;201(9):727–729. doi: 10.1097/NMD.0b013e3182a2168a. Sep. [DOI] [PubMed] [Google Scholar]
- Fruitwala S., El-Naccache D.W., Chang T.L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019;88:163–172. doi: 10.1016/j.semcdb.2018.02.023. Apr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydecka D., Krzystek-Korpacka M., Lubeiro A., Stramecki F., Stańczykiewicz B., Beszłej J.A., Piotrowski P., Kotowicz K., Szewczuk-Bogusławska M., Pawlak-Adamska E., Misiak B. Profiling inflammatory signatures of schizophrenia: a cross-sectional and meta-analysis study. Brain Behav. Immun. 2018;71:28–36. doi: 10.1016/j.bbi.2018.05.002. Jul. [DOI] [PubMed] [Google Scholar]
- Futtrup J., Margolinsky R., Benros M.E., Moos T., Routhe L.J., Rungby J., Krogh J. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6 doi: 10.1016/j.bbih.2020.100102. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression [Original Article] Mol. Psychiatr. 2016;21:1696. doi: 10.1038/mp.2016.3. 02/23/online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen M., van der Werf M., Verkaaik M., Arts B., Myin-Germeys I., van Os J., Verhey F., Kohler S., Genetic R., Outcome in Psychosis study, g Comparative study of clinical and neuropsychological characteristics between early-, late and very-late-onset schizophrenia-spectrum disorders. Am. J. Geriatr. Psychiatr. 2015;23(8):852–862. doi: 10.1016/j.jagp.2014.10.007. Aug. [DOI] [PubMed] [Google Scholar]
- Hao H.N., Zhao J., Lotoczky G., Grever W.E., Lyman W.D. Induction of human beta-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J. Neurochem. 2001;77(4):1027–1035. doi: 10.1046/j.1471-4159.2001.00305.x. https://www.ncbi.nlm.nih.gov/pubmed/11359868 May. [DOI] [PubMed] [Google Scholar]
- Haque A., Polcyn R., Matzelle D., Banik N.L. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018;8(2) doi: 10.3390/brainsci8020033. Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjunpaa H., Llort Asens M., Guenther C., Fagerholm S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019;10:1078. doi: 10.3389/fimmu.2019.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman F.J., Pasinetti G.M. Principles of inflammasome priming and inhibition: implications for psychiatric disorders. Brain Behav. Immun. 2018;73:66–84. doi: 10.1016/j.bbi.2018.06.010. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Zhao M., Li H., Peng F., Wang F., Li N., Xiang H., Su Y., Huang Y., Zhang S., Zhao G., Zhou R., Mao L., Lin Z., Fang Y., Zhang Q., Xie B. Higher plasma S100B concentrations in schizophrenia patients, and dependently associated with inflammatory markers. Sci. Rep. 2016;6 doi: 10.1038/srep27584. Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth S., Mirnics K. Immune system disturbances in schizophrenia. Biol. Psychiatr. 2014;75(4):316–323. doi: 10.1016/j.biopsych.2013.06.010. 07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Liang W., Zhang J., Li Q., Ou H., Wang Z., Li S., Huang X., Zhao C. Schizophrenia-associated rs4702 G allele-specific downregulation of FURIN expression by miR-338-3p reduces BDNF production. Schizophr. Res. 2018;199:176–180. doi: 10.1016/j.schres.2018.02.040. Sep. [DOI] [PubMed] [Google Scholar]
- Huet L., Delgado I., Dexpert S., Sauvant J., Aouizerate B., Beau C., Forestier D., Ledaguenel P., Magne E., Capuron L. Relationship between body mass index and neuropsychiatric symptoms: evidence and inflammatory correlates. Brain Behav. Immun. 2021;94:104–110. doi: 10.1016/j.bbi.2021.02.031. May. [DOI] [PubMed] [Google Scholar]
- Häfner H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex, age, and other risk factors influence incidence and course of illness. Psychiatry journal. 2019 doi: 10.1155/2019/9804836. 2019, 9804836-9804836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen J., Jääskeläinen E., Korpela H., Miettunen J. Age at onset and the outcomes of schizophrenia: a systematic review and meta-analysis. Early Interv Psychiatry. 2017;11(6):453–460. doi: 10.1111/eip.12412. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isung J., Williams K., Isomura K., Gromark C., Hesselmark E., Lichtenstein P., Larsson H., Fernandez de la Cruz L., Sidorchuk A., Mataix-Cols D. Association of primary humoral immunodeficiencies with psychiatric disorders and suicidal behavior and the role of autoimmune diseases. JAMA Psychiatr. 2020 doi: 10.1001/jamapsychiatry.2020.1260. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. https://www.ncbi.nlm.nih.gov/pubmed/3616518 [DOI] [PubMed] [Google Scholar]
- Keck P.E., Jr., McElroy S.L., Havens J.R., Altshuler L.L., Nolen W.A., Frye M.A., Suppes T., Denicoff K.D., Kupka R., Leverich G.S., Rush A.J., Post R.M. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr. Psychiatr. 2003;44(4):263–269. doi: 10.1016/s0010-440x(03)00089-0. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Ohlsson H., Sundquist J., Sundquist K. Prediction of onset of substance-induced psychotic disorder and its progression to schizophrenia in a Swedish national sample. Am. J. Psychiatr. 2019;176(9):711–719. doi: 10.1176/appi.ajp.2019.18101217. Sep. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K.P., Cullen K.R., DeYoung C.G., Klimes-Dougan B. The genetics of early-onset bipolar disorder: a systematic review. J. Affect. Disord. 2015;184:1–12. doi: 10.1016/j.jad.2015.05.017. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury R., Nasrallah H.A. Inflammatory biomarkers in individuals at clinical high risk for psychosis (CHR-P): state or trait? Schizophr. Res. 2018;199:31–38. doi: 10.1016/j.schres.2018.04.017. Sep. [DOI] [PubMed] [Google Scholar]
- Kong D.H., Kim Y.K., Kim M.R., Jang J.H., Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041057. Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken R.A., Sommer I.E., Steen V.M., Dieset I., Johnsen E. Constructing the immune signature of schizophrenia for clinical use and research; an integrative review translating descriptives into diagnostics. Front. Psychiatr. 2018;9:753. doi: 10.3389/fpsyt.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronig H., Riedel M., Schwarz M.J., Strassnig M., Moller H.J., Ackenheil M., Muller N. ICAM G241A polymorphism and soluble ICAM-1 serum levels: evidence for an active immune process in schizophrenia. Neuroimmunomodulation. 2005;12(1):54–59. doi: 10.1159/000082364. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O., Petersen L., Gasse C., Mortensen P.B., Dalsgaard S., Yolken R.H., Mors O., Benros M.E. A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatr. 2019;76(3):271–279. doi: 10.1001/jamapsychiatry.2018.3428. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerberg T.V., Icick R., Aminoff S.R., Nerhus M., Barrett E.A., Bjella T.D., Olsen S.H., Hoegh M.C., Melle I. Substance misuse trajectories and risk of relapse in the early course of bipolar disorder. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.656912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataster T., Valmaggia L., Lardinois M., van Os J., Myin-Germeys I. Increased stress reactivity: a mechanism specifically associated with the positive symptoms of psychotic disorder. Psychol. Med. 2013;43(7):1389–1400. doi: 10.1017/S0033291712002279. Jul. [DOI] [PubMed] [Google Scholar]
- Levin J.R., Serlin R.C., Seaman M.A. A controlled, powerful multiple-comparison strategy for several situations. 1994. [doi:10.1037/0033-2909.115.1.153]. 115, 153-159. [DOI]
- Lindqvist D., Janelidze S., Erhardt S., Traskman-Bendz L., Engstrom G., Brundin L. CSF biomarkers in suicide attempters--a principal component analysis. Acta Psychiatr. Scand. 2011;124(1):52–61. doi: 10.1111/j.1600-0447.2010.01655.x. Jul. [DOI] [PubMed] [Google Scholar]
- Lizano P., Lutz O., Xu Y., Rubin L.H., Paskowitz L., Lee A.M., Eum S., Keedy S.K., Hill S.K., Reilly J.L., Wu B., Tamminga C.A., Clementz B.A., Pearlson G.D., Gershon E.S., Keshavan M.S., Sweeney J.A., Bishop J.R. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-00914-0. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin J.P., Strayer D.S. Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy. CNS Neurol. Disord.: Drug Targets. 2013;12(6):815–829. doi: 10.2174/18715273113126660173. Sep. [DOI] [PubMed] [Google Scholar]
- Luciano M., Steardo L., Jr., Sampogna G., Caivano V., Ciampi C., Del Vecchio V., Di Cerbo A., Giallonardo V., Zinno F., De Fazio P., Fiorillo A. Affective temperaments and illness severity in patients with bipolar disorder. Medicina. 2021;57(1):54. doi: 10.3390/medicina57010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013 doi: 10.1155/2013/139239. Dec 22. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyla T., Mantere O., Raij T.T., Kieseppa T., Laitinen H., Leiviska J., Torniainen M., Tuominen L., Vaarala O., Suvisaari J. Altered activation of innate immunity associates with white matter volume and diffusion in first-episode psychosis. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti L., Engelhardt B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol. 2020;2(1):H1–H18. doi: 10.1530/VB-19-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S.R., Cannon T.D. Schizophrenia. N. Engl. J. Med. 2019;381(18):1753–1761. doi: 10.1056/NEJMra1808803. Oct 31. [DOI] [PubMed] [Google Scholar]
- McCutcheon R.A., Reis Marques T., Howes O.D. Schizophrenia-an overview. JAMA Psychiatr. 2020;77(2):201–210. doi: 10.1001/jamapsychiatry.2019.3360. Feb 1. [DOI] [PubMed] [Google Scholar]
- Messer T., Lammers G., Müller-Siecheneder F., Schmidt R.F., Latifi S. Substance abuse in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatr. Res. 2017;253:338–350. doi: 10.1016/j.psychres.2017.02.067. Jul. [DOI] [PubMed] [Google Scholar]
- Michetti F., D'Ambrosi N., Toesca A., Puglisi M.A., Serrano A., Marchese E., Corvino V., Geloso M.C. The S100B story: from biomarker to active factor in neural injury. J. Neurochem. 2018 doi: 10.1111/jnc.14574. Aug 24. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Goldsmith D.R. Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2017;42(1):299–317. doi: 10.1038/npp.2016.211. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N. The role of intercellular adhesion molecule-1 in the pathogenesis of psychiatric disorders. Front. Pharmacol. 2019;10:1251. doi: 10.3389/fphar.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N., Forstner A.J., O'Connell K.S., Coombes B., Coleman J.R.I., Qiao Z., Als T.D., Bigdeli T.B., Borte S., Bryois J., Charney A.W., Drange O.K., Gandal M.J., Hagenaars S.P., Ikeda M., Kamitaki N., Kim M., Krebs K., Panagiotaropoulou G., Schilder B.M., Sloofman L.G., Steinberg S., Trubetskoy V., Winsvold B.S., Won H.H., Abramova L., Adorjan K., Agerbo E., Al Eissa M., Albani D., Alliey-Rodriguez N., Anjorin A., Antilla V., Antoniou A., Awasthi S., Baek J.H., Baekvad-Hansen M., Bass N., Bauer M., Beins E.C., Bergen S.E., Birner A., Bocker Pedersen C., Boen E., Boks M.P., Bosch R., Brum M., Brumpton B.M., Brunkhorst-Kanaan N., Budde M., Bybjerg-Grauholm J., Byerley W., Cairns M., Casas M., Cervantes P., Clarke T.K., Cruceanu C., Cuellar-Barboza A., Cunningham J., Curtis D., Czerski P.M., Dale A.M., Dalkner N., David F.S., Degenhardt F., Djurovic S., Dobbyn A.L., Douzenis A., Elvsashagen T., Escott-Price V., Ferrier I.N., Fiorentino A., Foroud T.M., Forty L., Frank J., Frei O., Freimer N.B., Frisen L., Gade K., Garnham J., Gelernter J., Giortz Pedersen M., Gizer I.R., Gordon S.D., Gordon-Smith K., Greenwood T.A., Grove J., Guzman-Parra J., Ha K., Haraldsson M., Hautzinger M., Heilbronner U., Hellgren D., Herms S., Hoffmann P., Holmans P.A., Huckins L., Jamain S., Johnson J.S., Kalman J.L., Kamatani Y., Kennedy J.L., Kittel-Schneider S., Knowles J.A., Kogevinas M., Koromina M., Kranz T.M., Kranzler H.R., Kubo M., Kupka R., Kushner S.A., Lavebratt C., Lawrence J., Leber M., Lee H.J., Lee P.H., Levy S.E., Lewis C., Liao C., Lucae S., Lundberg M., MacIntyre D.J., Magnusson S.H., Maier W., Maihofer A., Malaspina D., Maratou E., Martinsson L., Mattheisen M., McCarroll S.A., McGregor N.W., McGuffin P., McKay J.D., Medeiros H., Medland S.E., Millischer V., Montgomery G.W., Moran J.L., Morris D.W., Muhleisen T.W., O'Brien N., O'Donovan C., Olde Loohuis L.M., Oruc L., Papiol S., Pardinas A.F., Perry A., Pfennig A., Porichi E., Potash J.B., Quested D., Raj T., Rapaport M.H., DePaulo J.R., Regeer E.J., Rice J.P., Rivas F., Rivera M., Roth J., Roussos P., Ruderfer D.M., Sanchez-Mora C., Schulte E.C., Senner F., Sharp S., Shilling P.D., Sigurdsson E., Sirignano L., Slaney C., Smeland O.B., Smith D.J., Sobell J.L., Soholm Hansen C., Soler Artigas M., Spijker A.T., Stein D.J., Strauss J.S., Swiatkowska B., Terao C., Thorgeirsson T.E., Toma C., Tooney P., Tsermpini E.E., Vawter M.P., Vedder H., Walters J.T.R., Witt S.H., Xi S., Xu W., Yang J.M.K., Young A.H., Young H., Zandi P.P., Zhou H., Zillich L., Psychiatry H.A.-I., Adolfsson R., Agartz I., Alda M., Alfredsson L., Babadjanova G., Backlund L., Baune B.T., Bellivier F., Bengesser S., Berrettini W.H., Blackwood D.H.R., Boehnke M., Borglum A.D., Breen G., Carr V.J., Catts S., Corvin A., Craddock N., Dannlowski U., Dikeos D., Esko T., Etain B., Ferentinos P., Frye M., Fullerton J.M., Gawlik M., Gershon E.S., Goes F.S., Green M.J., Grigoroiu-Serbanescu M., Hauser J., Henskens F., Hillert J., Hong K.S., Hougaard D.M., Hultman C.M., Hveem K., Iwata N., Jablensky A.V., Jones I., Jones L.A., Kahn R.S., Kelsoe J.R., Kirov G., Landen M., Leboyer M., Lewis C.M., Li Q.S., Lissowska J., Lochner C., Loughland C., Martin N.G., Mathews C.A., Mayoral F., McElroy S.L., McIntosh A.M., McMahon F.J., Melle I., Michie P., Milani L., Mitchell P.B., Morken G., Mors O., Mortensen P.B., Mowry B., Muller-Myhsok B., Myers R.M., Neale B.M., Nievergelt C.M., Nordentoft M., Nothen M.M., O'Donovan M.C., Oedegaard K.J., Olsson T., Owen M.J., Paciga S.A., Pantelis C., Pato C., Pato M.T., Patrinos G.P., Perlis R.H., Posthuma D., Ramos-Quiroga J.A., Reif A., Reininghaus E.Z., Ribases M., Rietschel M., Ripke S., Rouleau G.A., Saito T., Schall U., Schalling M., Schofield P.R., Schulze T.G., Scott L.J., Scott R.J., Serretti A., Shannon Weickert C., Smoller J.W., Stefansson H., Stefansson K., Stordal E., Streit F., Sullivan P.F., Turecki G., Vaaler A.E., Vieta E., Vincent J.B., Waldman I.D., Weickert T.W., Werge T., Wray N.R., Zwart J.A., Biernacka J.M., Nurnberger J.I., Cichon S., Edenberg H.J., Stahl E.A., McQuillin A., Di Florio A., Ophoff R.A., Andreassen O.A. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021;53(6):817–829. doi: 10.1038/s41588-021-00857-4. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm K., Vinberg M., Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord. 2013;144(1–2):16–27. doi: 10.1016/j.jad.2012.06.010. Jan 10. [DOI] [PubMed] [Google Scholar]
- Najjar S., Steiner J., Najjar A., Bechter K. A clinical approach to new-onset psychosis associated with immune dysregulation: the concept of autoimmune psychosis. J. Neuroinflammation. 2018;15(1):40. doi: 10.1186/s12974-018-1067-y. Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.T., Dev S.I., Chen G., Liou S.C., Martin A.S., Irwin M.R., Carroll J.E., Tu X., Jeste D.V., Eyler L.T. Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur. Arch. Psychiatr. Clin. Neurosci. 2018;268(8):849–860. doi: 10.1007/s00406-017-0842-6. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P., Flaum M., Arndt S., Andreasen N. Morphometry in schizophrenia revisited: height and its relationship to pre-morbid function. Psychol. Med. 1998;28(3):655–663. doi: 10.1017/s0033291797006417. May. [DOI] [PubMed] [Google Scholar]
- Palladino I., Salani F., Ciaramella A., Rubino I.A., Caltagirone C., Fagioli S., Spalletta G., Bossu P. Elevated levels of circulating IL-18BP and perturbed regulation of IL-18 in schizophrenia. J. Neuroinflammation. 2012;9:206. doi: 10.1186/1742-2094-9-206. Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantovic-Stefanovic M., Petronijevic N., Dunjic-Kostic B., Velimirovic M., Nikolic T., Jurisic V., Lackovic M., Damjanovic A., Totic-Poznanovic S., Jovanovic A.A., Ivkovic M. sVCAM-1, sICAM-1, TNF-alpha and IL-6 levels in bipolar disorder type I: acute, longitudinal and therapeutic implications. World J. Biol. Psychiatr. 2018;19(Suppl. 2):S41–S51. doi: 10.1080/15622975.2016.1259498. [DOI] [PubMed] [Google Scholar]
- Pasternak O., Kubicki M., Shenton M.E. In vivo imaging of neuroinflammation in schizophrenia. Schizophr. Res. 2016;173(3):200–212. doi: 10.1016/j.schres.2015.05.034. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G., Karterud S. The symptom and function dimensions of the Global Assessment of Functioning (GAF) scale. Compr. Psychiatr. 2012;53(3):292–298. doi: 10.1016/j.comppsych.2011.04.007. Apr. [DOI] [PubMed] [Google Scholar]
- Peters A.T., West A.E., Eisner L., Baek J., Deckersbach T. The burden of repeated mood episodes in bipolar I disorder: results from the national epidemiological survey on alcohol and related conditions. J. Nerv. Ment. Dis. 2016;204(2):87–94. doi: 10.1097/NMD.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak T.A., Drndarski S., Stone J.M., David A.S., McGuire P., Abbott N.J. The blood-brain barrier in psychosis. Lancet Psychiatr. 2018;5(1):79–92. doi: 10.1016/S2215-0366(17)30293-6. Jan. [DOI] [PubMed] [Google Scholar]
- Pouget J.G. The emerging immunogenetic architecture of schizophrenia. Schizophr. Bull. 2018;44(5):993–1004. doi: 10.1093/schbul/sby038. Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002;28(3):235–243. doi: 10.1007/s00134-002-1209-6. Mar. [DOI] [PubMed] [Google Scholar]
- Provenzani U., Salazar de Pablo G., Arribas M., Pillmann F., Fusar-Poli P. Clinical outcomes in brief psychotic episodes: a systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 2021;30:e71. doi: 10.1017/S2045796021000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O'Donovan M.C., Sullivan P.F., Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J.M., Schwarz E., Guest P.C., van Beveren N.J., Leweke F.M., Rothermundt M., Bogerts B., Steiner J., Bahn S. Distinct molecular phenotypes in male and female schizophrenia patients. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond W.D., Eilertsen G.O., Nossent J. Principal component analysis reveals disconnect between regulatory cytokines and disease activity in Systemic Lupus Erythematosus. Cytokine. 2019;114:67–73. doi: 10.1016/j.cyto.2018.10.013. Feb. [DOI] [PubMed] [Google Scholar]
- Reale M., Patruno A., De Lutiis M.A., Pesce M., Felaco M., Di Giannantonio M., Di Nicola M., Grilli A. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011;12:13. doi: 10.1186/1471-2202-12-13. Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus E.Z., Lackner N., Birner A., Bengesser S., Fellendorf F.T., Platzer M., Rieger A., Queissner R., Kainzbauer N., Reininghaus B., McIntyre R.S., Mangge H., Zelzer S., Fuchs D., Dejonge S., Muller N. Extracellular matrix proteins matrix metallopeptidase 9 (MMP9) and soluble intercellular adhesion molecule 1 (sICAM-1) and correlations with clinical staging in euthymic bipolar disorder. Bipolar Disord. 2016;18(2):155–163. doi: 10.1111/bdi.12380. Mar. [DOI] [PubMed] [Google Scholar]
- Reponen E.J., Dieset I., Tesli M., Morch R.H., Aas M., Vedal T.S.J., Haug E., Drange O.K., Steen N.E., Hope S., Szabo A., Gohar S.M., Wedervang-Resell K., Djurovic S., Melle I., Aukrust P., Andreassen O.A., Ueland T. Atherogenic lipid ratios related to myeloperoxidase and C-reactive protein levels in psychotic disorders. Front. Psychiatr. 2020;11:672. doi: 10.3389/fpsyt.2020.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov L.L., Kim S.H., Zhou L., Bufler P., Goncharov I., Tsang M., Dinarello C.A. The combination of soluble IL-18Ralpha and IL-18Rbeta chains inhibits IL-18-induced IFN-gamma. J. Interferon Cytokine Res. 2002;22(5):593–601. doi: 10.1089/10799900252982070. May. [DOI] [PubMed] [Google Scholar]
- Ringen P.A., Lagerberg T.V., Birkenaes A.B., Engn J., Faerden A., Jonsdottir H., Nesvag R., Friis S., Opjordsmoen S., Larsen F., Melle I., Andreassen O.A. Differences in prevalence and patterns of substance use in schizophrenia and bipolar disorder. Psychol. Med. 2008;38(9):1241–1249. doi: 10.1017/S003329170700236X. Sep. [DOI] [PubMed] [Google Scholar]
- Ripke S., Neale B.M., Corvin A., Walters J.T.R., Farh K.-H., Holmans P.A., Lee P., Bulik-Sullivan B., Collier D.A., Huang H., Pers T.H., Agartz I., Agerbo E., Albus M., Alexander M., Amin F., Bacanu S.A., Begemann M., Belliveau R.A., Jr., Bene J., Bergen S.E., Bevilacqua E., Bigdeli T.B., Black D.W., Bruggeman R., Buccola N.G., Buckner R.L., Byerley W., Cahn W., Cai G., Campion D., Cantor R.M., Carr V.J., Carrera N., Catts S.V., Chambert K.D., Chan R.C.K., Chen R.Y.L., Chen E.Y.H., Cheng W., Cheung E.F.C., Ann Chong S., Robert Cloninger C., Cohen D., Cohen N., Cormican P., Craddock N., Crowley J.J., Curtis D., Davidson M., Davis K.L., Degenhardt F., Del Favero J., Demontis D., Dikeos D., Dinan T., Djurovic S., Donohoe G., Drapeau E., Duan J., Dudbridge F., Durmishi N., Eichhammer P., Eriksson J., Escott-Price V., Essioux L., Fanous A.H., Farrell M.S., Frank J., Franke L., Freedman R., Freimer N.B., Friedl M., Friedman J.I., Fromer M., Genovese G., Georgieva L., Giegling I., Giusti-Rodríguez P., Godard S., Goldstein J.I., Golimbet V., Gopal S., Gratten J., de Haan L., Hammer C., Hamshere M.L., Hansen M., Hansen T., Haroutunian V., Hartmann A.M., Henskens F.A., Herms S., Hirschhorn J.N., Hoffmann P., Hofman A., Hollegaard M.V., Hougaard D.M., Ikeda M., Joa I., Julià A., Kahn R.S., Kalaydjieva L., Karachanak-Yankova S., Karjalainen J., Kavanagh D., Keller M.C., Kennedy J.L., Khrunin A., Kim Y., Klovins J., Knowles J.A., Konte B., Kucinskas V., Ausrele Kucinskiene Z., Kuzelova-Ptackova H., Kähler A.K., Laurent C., Lee Chee Keong J., Hong Lee S., Legge S.E., Lerer B., Li M., Li T., Liang K.-Y., Lieberman J., Limborska S., Loughland C.M., Lubinski J., Lönnqvist J., Macek M., Jr., Magnusson P.K.E., Maher B.S., Maier W., Mallet J., Marsal S., Mattheisen M., Mattingsdal M., McCarley R.W., McDonald C., McIntosh A.M., Meier S., Meijer C.J., Melegh B., Melle I., Mesholam-Gately R.I., Metspalu A., Michie P.T., Milani L., Milanova V., Mokrab Y., Morris D.W., Mors O., Murphy K.C., Murray R.M., Myin-Germeys I., Müller-Myhsok B., Nelis M., Nenadic I., Nertney D.A., Nestadt G., Nicodemus K.K., Nikitina-Zake L., Nisenbaum L., Nordin A., O'Callaghan E., O'Dushlaine C., O'Neill F.A., Oh S.-Y., Olincy A., Olsen L., Van Os J., Pantelis C., Papadimitriou G.N., Papiol S., Parkhomenko E., Pato M.T., Paunio T., Pejovic-Milovancevic M., Perkins D.O., Pietiläinen O., Pimm J., Pocklington A.J., Powell J., Price A., Pulver A.E., Purcell S.M., Quested D., Rasmussen H.B., Reichenberg A., Reimers M.A., Richards A.L., Roffman J.L., Roussos P., Ruderfer D.M., Salomaa V., Sanders A.R., Schall U., Schubert C.R., Schulze T.G., Schwab S.G., Scolnick E.M., Scott R.J., Seidman L.J., Shi J., Sigurdsson E., Silagadze T., Silverman J.M., Sim K., Slominsky P., Smoller J.W., So H.-C., Spencer C.A., Stahl E.A., Stefansson H., Steinberg S., Stogmann E., Straub R.E., Strengman E., Strohmaier J., Scott Stroup T., Subramaniam M., Suvisaari J., Svrakic D.M., Szatkiewicz J.P., Söderman E., Thirumalai S., Toncheva D., Tosato S., Veijola J., Waddington J., Walsh D., Wang D., Wang Q., Webb B.T., Weiser M., Wildenauer D.B., Williams N.M., Williams S., Witt S.H., Wolen A.R., Wong E.H.M., Wormley B.K., Simon Xi H., Zai C.C., Zheng X., Zimprich F., Wray N.R., Stefansson K., Visscher P.M., Trust Case-Control Consortium W., Adolfsson R., Andreassen O.A., Blackwood D.H.R., Bramon E., Buxbaum J.D., Børglum A.D., Cichon S., Darvasi A., Domenici E., Ehrenreich H., Esko T., Gejman P.V., Gill M., Gurling H., Hultman C.M., Iwata N., Jablensky A.V., Jönsson E.G., Kendler K.S., Kirov G., Knight J., Lencz T., Levinson D.F., Li Q.S., Liu J., Malhotra A.K., McCarroll S.A., McQuillin A., Moran J.L., Mortensen P.B., Mowry B.J., Nöthen M.M., Ophoff R.A., Owen M.J., Palotie A., Pato C.N., Petryshen T.L., Posthuma D., Rietschel M., Riley B.P., Rujescu D., Sham P.C., Sklar P., St Clair D., Weinberger D.R., Wendland J.R., Werge T., Schizophrenia Working Group of the Psychiatric Genomics, C., & Psychosis Endophenotypes International, C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. 2014/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermundt M., Ahn J.N., Jorgens S. S100B in schizophrenia: an update. Gen. Physiol. Biophys. 2009;28:F76–F81. https://www.ncbi.nlm.nih.gov/pubmed/20093730 Spec No Focus. [PubMed] [Google Scholar]
- Saetre P., Emilsson L., Axelsson E., Kreuger J., Lindholm E., Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatr. 2007;7:46. doi: 10.1186/1471-244x-7-46. Sep. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The role of cell adhesion molecules in brain wiring and neuropsychiatric disorders. Mol. Cell. Neurosci. 2017;81:4–11. doi: 10.1016/j.mcn.2016.08.005. Jun. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Sarkar S., Schwarz M., Friebe A. Soluble intracellular adhesion molecule-1 in patients with unipolar or bipolar affective disorders: results from a pilot trial. Neuropsychobiology. 2016;74(1):8–14. doi: 10.1159/000446919. [DOI] [PubMed] [Google Scholar]
- Schwarz M.J., Riedel M., Ackenheil M., Muller N. Decreased levels of soluble intercellular adhesion molecule-1 (sICAM-1) in unmedicated and medicated schizophrenic patients. Biol. Psychiatr. 2000;47(1):29–33. doi: 10.1016/s0006-3223(99)00206-1. Jan 1. [DOI] [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M.A., O`Connell K.S., Lekva T., Szabo A., Akkouh I.A., Osete J.R., Agartz I., Engh J.A., Andreou D., Boye B., Bøen E., Elvsåshagen T., Hope S., Werner M.C.F., Joa I., Johnsen E., Kroken R.A., Lagerberg T.V., Melle I., Drange O.K., Morken G., Nærland T., Sørensen K., Vaaler A.E., Weibell M.A., Westlye L.T., Aukrust P., Steen V.M., Djurovic S., Steen N.E., Andreassen O.A., Ueland T. Systemic cell-adhesion molecules (CAM) in severe mental illness-potential role of intracellular CAM-1 in linking peripheral and neuro-inflammation. Biol. Psychiatr. 2022 doi: 10.1016/j.biopsych.2022.06.029. accepted. [DOI] [PubMed] [Google Scholar]
- Shelley J.R., Davidson D.J., Dorin J.R. The dichotomous responses driven by beta-defensins. Front. Immunol. 2020;11:1176. doi: 10.3389/fimmu.2020.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladojevic N., Stamatovic S.M., Keep R.F., Grailer J.J., Sarma J.V., Ward P.A., Andjelkovic A.V. Inhibition of junctional adhesion molecule-A/LFA interaction attenuates leukocyte trafficking and inflammation in brain ischemia/reperfusion injury. Neurobiol. Dis. 2014;67:57–70. doi: 10.1016/j.nbd.2014.03.010. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Arch. Gen. Psychiatr. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. Aug. [DOI] [PubMed] [Google Scholar]
- Stefanovic M.P., Petronijevic N., Dunjic-Kostic B., Velimirovic M., Nikolic T., Jurisic V., Lackovic M., Damjanovic A., Totic-Poznanovic S., Jovanovic A.A., Ivkovic M. Role of sICAM-1 and sVCAM-1 as biomarkers in early and late stages of schizophrenia. J. Psychiatr. Res. 2016;73:45–52. doi: 10.1016/j.jpsychires.2015.11.002. Feb. [DOI] [PubMed] [Google Scholar]
- Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. 2012/01/01. [DOI] [PubMed] [Google Scholar]
- Stuart M.J., Baune B.T. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. May. [DOI] [PubMed] [Google Scholar]
- Szabo A., O'Connell K.S., Ueland T., Sheikh M.A., Agartz I., Andreou D., Aukrust P., Boye B., Boen E., Drange O.K., Elvsashagen T., Engh J.A., Hope S., Collier Hoegh M., Joa I., Johnsen E., Kroken R.A., Vik Lagerberg T., Lekva T., Malt U.F., Melle I., Morken G., Naerland T., Steen V.M., Sorensen K., Wedervang-Resell K., Auten Weibell M., Westlye L.T., Steen N.E., Andreassen O., Djurovic S. Increased circulating IL-18 levels in severe mental disorders indicate systemic inflammasome activation. Brain Behav. Immun. 2022;99:299–306. doi: 10.1016/j.bbi.2021.10.017. Jan. [DOI] [PubMed] [Google Scholar]
- Szekeres M., Ivitz E., Datki Z., Kalman J., Pakaski M., Varhelyi Z.P., Klivenyi P., Zadori D., Somogyvari F., Szolnoki Z., Vecsei L., Mandi Y. Relevance of defensin beta-2 and alpha defensins (HNP1-3) in Alzheimer's disease. Psychiatr. Res. 2016;239:342–345. doi: 10.1016/j.psychres.2016.03.045. May 30. [DOI] [PubMed] [Google Scholar]
- Taub D.D. Neuroendocrine interactions in the immune system. Cell. Immunol. 2008;252(1–2):1–6. doi: 10.1016/j.cellimm.2008.05.006. Mar-Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3(10):753–766. doi: 10.1038/nrm934. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourjman V., Kouassi E., Koue M.E., Rocchetti M., Fortin-Fournier S., Fusar-Poli P., Potvin S. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr. Res. 2013;151(1–3):43–47. doi: 10.1016/j.schres.2013.10.011. Dec. [DOI] [PubMed] [Google Scholar]
- Trepanier M.O., Hopperton K.E., Mizrahi R., Mechawar N., Bazinet R.P. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol. Psychiatr. 2016;21(8):1009–1026. doi: 10.1038/mp.2016.90. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.C., Huang T.L. Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr. Psychiatr. 2017;74:27–34. doi: 10.1016/j.comppsych.2016.12.008. Apr. [DOI] [PubMed] [Google Scholar]
- Tsutsumi N., Kimura T., Arita K., Ariyoshi M., Ohnishi H., Yamamoto T., Zuo X., Maenaka K., Park E.Y., Kondo N., Shirakawa M., Tochio H., Kato Z. The structural basis for receptor recognition of human interleukin-18. Nat. Commun. 2014;5:5340. doi: 10.1038/ncomms6340. Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan C., Kesebir S., Suner O. Are ICAM, VCAM and E-selectin levels different in first manic episode and subsequent remission? J. Affect. Disord. 2014;163:76–80. doi: 10.1016/j.jad.2014.03.052. Jul. [DOI] [PubMed] [Google Scholar]
- Turvey C.L., Coryell W.H., Solomon D.A., Leon A.C., Endicott J., Keller M.B., Akiskal H. Long-term prognosis of bipolar I disorder. Acta Psychiatr. Scand. 1999;99(2):110–119. doi: 10.1111/j.1600-0447.1999.tb07208.x. Feb. [DOI] [PubMed] [Google Scholar]
- van de Kerkhof N.W., Fekkes D., van der Heijden F.M., Verhoeven W.M. BDNF and S100B in psychotic disorders: evidence for an association with treatment responsiveness. Acta Neuropsychiatr. 2014;26(4):223–229. doi: 10.1017/neu.2013.59. Aug. [DOI] [PubMed] [Google Scholar]
- Wedervang-Resell K., Ueland T., Aukrust P., Friis S., Holven K.B., C H.J., Lekva T., Lonning V., Smelror R.E., Szabo A., Andreassen O.A., Myhre A.M., Agartz I. Reduced levels of circulating adhesion molecules in adolescents with early-onset psychosis. NPJ Schizophr. 2020;6(1):20. doi: 10.1038/s41537-020-00112-5. Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W.M., Castellani R.J., Weinberg A., Perry G., Smith M.A. Do beta-defensins and other antimicrobial peptides play a role in neuroimmune function and neurodegeneration? Sci. World J. 2012 doi: 10.1100/2012/905785. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W.M., Torres S., Siedlak S.L., Castellani R.J., Perry G., Smith M.A., Zhu X. Antimicrobial peptide beta-defensin-1 expression is upregulated in Alzheimer's brain. J. Neuroinflammation. 2013;10:127. doi: 10.1186/1742-2094-10-127. Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Liu L., Zhou H. Endothelial cell activation in central nervous system inflammation. J. Leukoc. Biol. 2017;101(5):1119–1132. doi: 10.1189/jlb.3RU0816-352RR. May. [DOI] [PubMed] [Google Scholar]
- Yang C.-Y. Comparative analyses of the conformational dynamics between the soluble and membrane-bound cytokine receptors. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-64034-z. 7399-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates K., Lång U., Cederlöf M., Boland F., Taylor P., Cannon M., McNicholas F., DeVylder J., Kelleher I. Association of psychotic experiences with subsequent risk of suicidal ideation, suicide attempts, and suicide deaths: a systematic review and meta-analysis of longitudinal population studies. JAMA Psychiatr. 2019;76(2):180–189. doi: 10.1001/jamapsychiatry.2018.3514. 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J.Y., Nurjono M., Ng W.Y., Teo S.R., Lee T.S., Lee J. Peripheral blood gene expression of acute phase proteins in people with first episode psychosis. Brain Behav. Immun. 2017;65:337–341. doi: 10.1016/j.bbi.2017.06.006. Oct. [DOI] [PubMed] [Google Scholar]
- Yuan N., Chen Y., Xia Y., Dai J., Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry. 2019;9(1):233. doi: 10.1038/s41398-019-0570-y. 2019/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.